Abstract
Purpose
To apply previously published benefit-to-risk ratio methods for mammography and molecular breast imaging (MBI) risk estimates to an expanded range of mammographic screening techniques, compressed breast thicknesses, and screening views.
Materials and Methods
Only previously published estimates were used; therefore, this study was exempt from the requirement to obtain institutional review board approval. Benefit-to-risk ratios were calculated as the ratio of breast cancer deaths averted and lives lost to screening over 10-year intervals starting at age 40 years for MBI, two-dimensional (2D) full-field digital mammography (FFDM) alone, 2D FFDM with synthetic mammography, and 2D FFDM with tomosynthesis for two-, four-, and five-view screening mammography and compressed breast thicknesses of 20–29 mm, 50–59 mm, and 80–89 mm.
Results
Central estimates of the benefit-to-risk ratios ranged from 3 to 179 for screening mammography and from 5 to 9 for MBI. Benefit-to-risk ratios for MBI were inferior to those for mammography for most scenarios, but MBI may be performed at an equal or superior benefit-to-risk ratio for women aged 40–59 years with a compressed breast thickness of at least 80 mm and for those undergoing mammographic screening examinations with four or five views per breast. The benefit-to-risk ratios across all ages with use of tomosynthesis plus 2D FFDM as a screening examination were 45% lower than those for tomosynthesis plus synthetic mammography.
Conclusion
Benefit-to-risk ratios for MBI are within the lower range of those for mammography when accounting for variation in mammography technique, compressed breast thickness, and age. Benefit-to-risk ratios of synthetic mammography plus tomosynthesis are superior to those of tomosynthesis plus 2D FFDM.
Keywords: Breast, Mammography, Molecular Imaging, Molecular Imaging-Cancer, Radiation Safety, Radionuclide Studies, Screening, Tomosynthesis
© RSNA, 2019
See also the commentary by Hruska in this issue.
Summary
Benefit-to-risk ratios with molecular breast imaging are within the lower range of corresponding mammography ratios when accounting for variation in mammographic technique, compressed breast thickness, and age.
Key Points
■ Benefit-to-risk ratios of mammography are superior to those of molecular breast imaging (MBI) for most mammographic screening techniques, compressed breast thicknesses, and patient age ranges.
■ The benefit-to-risk ratios of MBI overlap and, in a few scenarios, exceed the lower range of benefit-to-risk ratios for mammography; use of MBI should therefore not be precluded solely based on these estimates.
■ The use of tomosynthesis plus two-dimensional full-field digital mammography for screening should be carefully considered given the lower benefit-to-risk ratios compared with tomosynthesis plus synthetic mammography.
Introduction
In molecular breast imaging (MBI), intravenous injection of technetium 99m (99mTc)–sestamibi is used to image breasts with dedicated dual-head cadmium zinc telluride detectors, with drawn doses of 99mTc-sestamibi of approximately 240–300 MBq (6.5–8 mCi) (1). Concerns regarding systemic radiation exposure have limited the widespread use of MBI as an adjunct breast imaging modality (1–3). Prior studies estimating the benefit-to–radiation risk of MBI and prior-generation breast-specific gamma imaging have concluded that the benefit-to–radiation risk ratios for breast imaging with 99mTc-sestamibi are inferior to those with two-dimensional (2D) full-field digital screening mammography (FFDM) (4,5). However, the utility of such theoretical estimates has been questioned (1,6,7).
Prior benefit-to–radiation risk ratio estimates considered only two-view screening mammography, comparing 2D FFDM with MBI (4,5). Currently, screening mammography is performed with a variety of techniques that include 2D FFDM alone, tomosynthesis plus 2D FFDM, and tomosynthesis plus synthetic mammography, with radiation dose varying among these techniques (8–10). Radiation dose at screening mammography also varies according to compressed breast thickness and number of views obtained (11,12).
The primary objective of our study was to apply previously published methods for estimating benefit-to-risk ratios for 2D FFDM and MBI (4,5) to estimates of benefit-to-risk ratios for tomosynthesis plus 2D FFDM and tomosynthesis plus synthetic mammography. We calculated benefit-to-risk ratios for various compressed breast thicknesses and number of views to include a standard two-view screening examination in addition to four- and five-view screening examinations, as used for women with breast implants or, potentially, those with large breasts (13). We hypothesized that benefit-to-risk estimates for mammography will demonstrate wide variation according to imaging technique, compressed breast thickness, and number of views and that the benefit-to-risk ratios for MBI may be equivalent to those for mammography in certain scenarios.
Materials and Methods
We applied the benefit-to-risk ratio method for mammography and MBI used by Hendrick and Tredennick (4) to 2D FFDM alone, 2D FFDM plus tomosynthesis, and tomosynthesis plus synthetic mammography at various compressed breast thickness ranges. To account for advances in mammography equipment, radiation doses for these mammographic techniques were obtained from a 2018 article by Østerås et al (10). Radiation dose estimates for the studies by Hendrick and colleagues were based on the 2010 American College of Radiology Imaging Network Digital Mammographic Imaging Screening Trial (4,5,14). Only previously published estimates of detection rates, radiation dose, and radiation risks were used in this study; therefore, this study was exempt from the requirement to obtain institutional review board approval.
Estimation of screening benefit, risks, and corresponding benefit-to-risk ratios will be provided as a function of annual screening over 10-year intervals from 40–49 years, 50–59 years, 60–69 years, and 70–79 years of age. Benefit is calculated as breast cancer deaths averted with screening (DA) by considering the breast cancer detection rate per 1000 women screened (DR), the probability that a woman with breast cancer will die of that breast cancer in the absence of screening (Pdas), and the probability that, once detected, earlier detection will avert a breast cancer death (Pls). These variables were considered for each year of screening and were summed for each 10-year interval of interest, as follows:
where the summation over e is from e = b, the beginning of the age of the screening interval, to e = b + 9, with b = 40, 50, 60, or 70 years of age. S(e) is the number of women surviving at age e, and the factor S(e)/S(b) is the fraction of 100 000 women who were alive at age b and are still alive at age e. Survival for each age is derived from the Human Mortality Database (15).
The MBI cancer detection rate is based on the 2015 study by Rhodes et al (16) on the use of MBI for supplemental screening in women with dense breast tissue. For tomosynthesis screening, the detection rate from Rhodes et al was modified to reflect the higher detection rate for mammography plus tomosynthesis compared with digital mammography alone, assuming an incremental yield of 1.2 cancers per 1000 women screened (17). Because detection rates are per 1000 women screened, the sum was multiplied by 100 to convert to deaths averted per 100 000 women screened.
In accordance with the study by Hendrick and Tredennick (4), we used a central estimate of 20% (range, 15%–25%) for the probability of a woman with breast cancer dying from that cancer in the absence of screening based on estimates from the Cancer Intervention and Surveillance Modeling Network in 2009 and 2016 (18,19). We similarly used a central estimate of 20% (range, 15%–40%) for the probability that early detection with screening will avert a breast cancer death.
Radiation-induced mortality secondary to screening was calculated as lives lost to screening (LLS). This is found by considering the product of the organ-specific estimates of radiation dose and the organ- and age-specific lifetime attributable risk for cancer mortality (LARi[e], where i refers to the organ and e to the age at exposure). These variables are considered for each year of screening and were summed for each 10-year interval of interest, as follows:
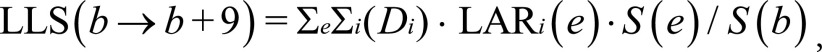 |
where the summation over e is from e = b, the beginning of the age of screening interval, to e = b + 9, with b = 40, 50, 60, or 70 years of age. The summation over i includes all organs receiving a nonnegligible radiation dose.
For 2D FFDM and tomosynthesis, the breast is the only organ exposed to a substantial radiation dose. For standard screening mammography, two views of each breast are considered. Estimates for four and five views of each breast will also be considered, according to standard breast implant screening protocols (13). For all mammographic screening, 2D FFDM alone, 2D FFDM plus tomosynthesis, and tomosynthesis with synthetic mammography are considered. Synthetic mammography is assumed to add no additional radiation dose to a screening study.
As in the study by Hendrick and Tredennick (4), for MBI we assumed that the administered dose was 240 MBq (6.4 mCi) of 99mTc-sestamibi summed over all internal organs stated to receive a radiation dose.
Lifetime radiation-induced mortality for all modalities was estimated by using the U.S. National Academy of Sciences Biologic Effects of Ionizing Radiation VII report (20). In this report, age-dependent cancer incidence and mortality risks are estimated on the basis of data from high-level ionizing radiation exposure. Extrapolation to the lower organ doses used in diagnostic imaging, including mammography and MBI, assumed a linear, no-threshold model.
Benefit-to-risk ratios are the ratio of estimated cancer deaths averted per 100 000 women screened to the estimated radiation-caused cancer deaths per 100 000 women screened. It was calculated over 10-year intervals, starting at age 40, 50, 60, and 70 years for MBI, mammography plus tomosynthesis, 2D FFDM alone, and tomosynthesis with synthetic mammography.
Results
Table 1 shows the estimated mortality benefit for annual screening over 10-year age ranges in terms of breast cancer deaths averted per 100 000 women screened and mortality risk in terms of radiation-caused cancer deaths per 100 000 women screened for each of the specified screening methods when considering pooled compressed breast thickness. Lifetime risk for radiation-caused mortality decreased with age for all screening methods, with approximately 50% reduction in risk for tomography plus FFDM for each decade and approximately 50% reduction in risk for MBI over 4 decades. The difference in rate of risk decrease is a result of radiation exposure to organs other than breast tissue with MBI. Mortality benefit and mortality risk for MBI are greater than those for mammography regardless of age and mammographic technique.
Table 1:
Estimated Mortality Benefit and Mortality Risk for Annual Screening according to Age Range
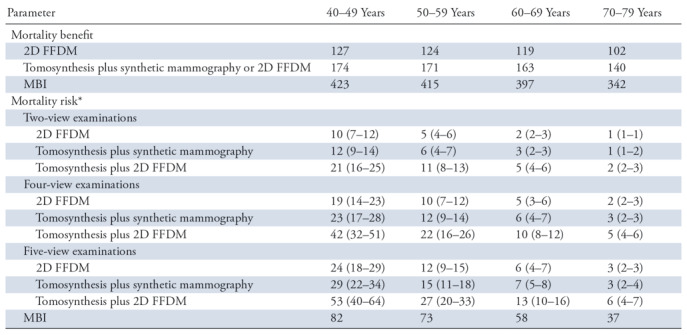
Note.—Data are for pooled compressed breast thickness values. Mortality benefit is the number of breast cancer deaths averted per 100 000 women screened, and mortality risk is the number of radiation-caused cancer deaths per 100 000 women screened. FFDM = full-field digital mammography, MBI = molecular breast imaging, 2D = two dimensional.
*Numbers in parentheses are the range of mortality risk estimates based on the 25th–75th-percentile mammography dose ranges.
Table 2 shows the benefit-to-risk ratios for each screening method when considering pooled compressed breast thickness. Results are again shown according to age range. Benefit-to-risk ratios increased with age for all screening modalities due to the more rapid decrease in radiation risks compared with the decrease in benefits. For the scenarios considered, the central estimates of the benefit-to-risk ratios for screening mammography ranged from 3 to 110. The same ratios for MBI ranged from 5 to 9. For the youngest age range of 40–49 years, the benefit-to-risk ratio of mammography ranged from 3 to 15; the risk-to-benefit ratio with MBI was 5. For the oldest age range of 70–79 years, the risk-to-benefit ratio for mammography ranged from 24 to 110; the MBI ratio was 9. The use of both tomosynthesis and 2D FFDM for a screening examination resulted in the lowest benefit-to-risk ratios for all age ranges owing to the approximate doubling of radiation dose due to the dual radiation exposures. The mean benefit-to-risk ratio with tomosynthesis plus 2D FFDM across all ages was 39% and 46% lower than that for 2D FFDM alone and tomosynthesis plus synthetic mammography, respectively. The benefit-to-risk ratios for MBI were inferior to those for mammography for all methods and age ranges when considering a two-view screening examination. When considering a four-view examination in patients aged 40–49 years, the benefit-to-risk ratio with MBI was superior to that with tomosynthesis plus 2D FFDM. With regard to five-view examinations, the benefit-to-risk ratio with MBI was equivalent to that of 2D FFDM alone in patients aged 40–49 years and superior to that of tomosynthesis plus 2D FFDM for patients aged 40–49 and 50–59 years.
Table 2:
Estimated Benefit-to-Risk Ratios for Annual Screening according to Age Range
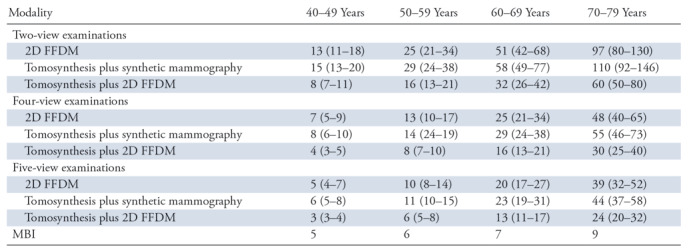
Note.—Data are for pooled compressed breast thickness values. Numbers in parentheses are the range of benefit-to-risk ratio estimates based on the 25th–75th-percentile mammography dose ranges. FFDM = full-field digital mammography, MBI = molecular breast imaging, 2D = two dimensional.
Table 3 shows the benefit-to-risk ratios for each of the screening methods and age intervals when considering compressed breast thickness ranges of 20–29 mm, 50–59 mm, and 80–89 mm. Risk estimates decreased across all screening methods with increases in compressed breast thickness, with the benefit-to-risk ratio of MBI showing equivalence or superiority in the 40–49-year-old range for one scenario (five-view tomosynthesis plus 2D FFDM) at a compressed breast thickness of 20–29 mm, three scenarios when considering four- or five-view mammography at a compressed breast thickness of 50–59 mm, and all considered scenarios except two-view FFDM and two-view tomosynthesis plus synthetic mammography at a compressed breast thickness of 80–89 mm. For patients aged 50–59 years, benefit-to-risk ratios of MBI were equivalent or superior to five-view tomosynthesis plus 2D FFDM at a compressed breast thickness of 50–59 mm and to multiple four- or five-view scenarios at a compressed breast thickness of 80–89 mm. Depending on compressed breast thickness and age range, benefit-to-risk ratios for mammography ranged from 2 to 179; benefit-to-risk ratios for MBI ranged from 5 to 9.
Table 3:
Estimated Benefit-to-Risk Ratios for Annual Screening according to Compressed Breast Thickness and Age Range
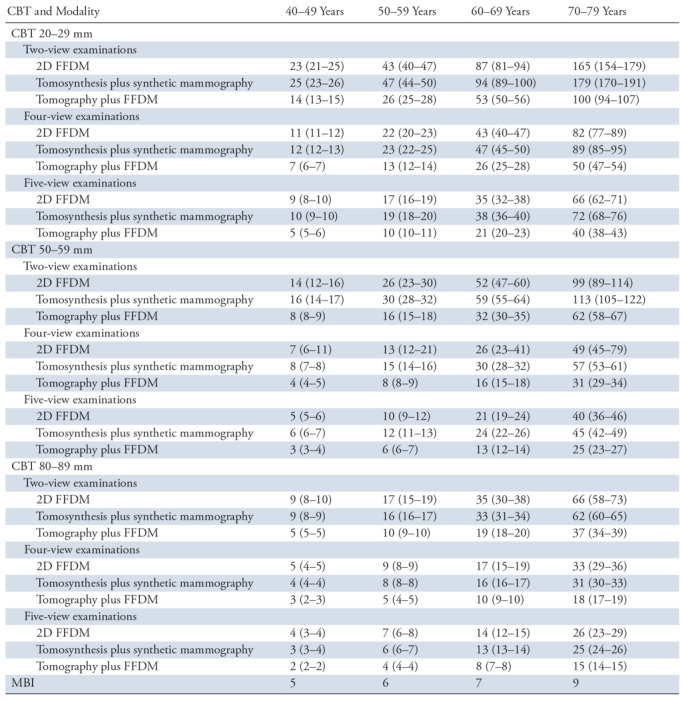
Note.—Numbers in parentheses are the range of benefit-to-risk ratio estimates based on the 25th–75th-percentile mammography dose ranges. Note that estimated benefit-to-risk ratios for MBI are similar for all breast thicknesses. CBT = compressed breast thickness, FFDM = full-field digital mammography, MBI = molecular breast imaging, 2D = two dimensional.
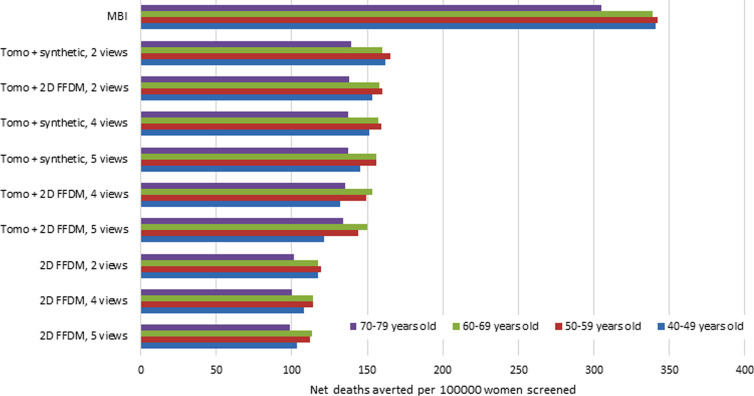
The Figure shows the estimated net lives saved from screening per 100 000 women. This was calculated by subtracting the estimated lives lost from screening from the number of deaths averted, as provided in Table 1. Estimated net lives saved from screening per 100 000 women ranged from 99 to 165 for mammography and from 305 to 342 for MBI.
Discussion
Previous benefit-to–radiation risk estimates demonstrated inferiority of MBI compared with 2D FFDM but do not account for screening tomosynthesis plus 2D FFDM or synthetic mammography and do not provide subgroup analysis according to compressed breast thickness and number of views obtained (4,5). When considering these variables, the range of benefit-to-risk ratios for MBI overlapped with the lower range of ratios for mammography in all cases. For select scenarios, the estimated ratios for MBI exceeded those for mammography.
Caution is advised in using our benefit-to-risk ratios to discourage the use of MBI for supplemental screening. If MBI ratios of 5–9 are unacceptable, then 42% (15 of 36) of the considered scenarios for mammography in patients aged 40–49 years and 26% (28 of 108) of all scenarios for mammography in Tables 2 and 3 could receive equal scrutiny. Given the net benefit for both MBI and mammography, if the goal of screening is to maximize net lives saved, our data, and by extension findings from the study by Hendrick and Tredennick (4), support the use of MBI for supplemental screening.
Given that MBI is proposed for supplemental screening and not as a replacement for screening mammography, comparing benefit-to-risk ratios between these modalities is of uncertain clinical significance. However, based on comparing these modalities, the American College of Radiology Appropriateness Criteria for Breast Cancer Screening has rated MBI as usually not appropriate for screening due to radiation risk (21,22). Comparing MBI performance characteristics directly with those of US, abbreviated MRI, and other supplemental screening options may be preferable.
For mammography, benefit-to-risk ratios favor 2D FFDM; however, 2D FFDM also has the lowest net lives saved from screening (Figure), further illustrating the limitation of only considering these ratios when evaluating screening options. In comparison to tomosynthesis plus synthetic mammography, tomosynthesis plus 2D FFDM demonstrates an overall decrease in benefit-to-risk ratios of 45.3% and a 5% reduction in net lives saved. Use of synthetic mammography instead of tomosynthesis plus 2D FFDM for screening has been advocated for dose reduction and is supported by our results (23,24).
Figure:
Chart shows estimated net deaths averted per 100 000 women screened. Data were calculated by subtracting the estimated lives lost from screening from the estimated deaths averted with screening. FFDM = full-field digital mammography, MBI = molecular breast imaging, synthetic = synthetic mammography, tomo = tomosynthesis, 2D = two-dimensional.
Compressed breast thickness accounts for 76% of the dose variability between women (11). An average compressed breast thickness of 5.4 cm (range, 2.0–11.5 cm) was reported in the Digital Mammographic Imaging Screening Trial based on 49 528 women (14). Using those data, Miglioretti et al (25) estimated that a compressed breast thickness of at least 7.5 cm corresponded to a mean of 8.4 total views for both breasts and a mean dose of 10.0 mGy. Assuming this compressed breast thickness and number of views for women aged 40–49 years, we estimated a benefit-to-risk ratio of 5 for MBI, 4.6 for 2D FFDM, and 3.2 for tomosynthesis plus 2D FFDM. This supports our estimates for a compressed breast thickness of 80–89 mm and demonstrates that the benefit-to-risk ratios for mammography, unlike MBI, are profoundly affected by compressed breast thickness and number of views obtained. These factors should be considered when evaluating screening options for subpopulations of women.
Our study included screening examinations with four and five views per breast, primarily to account for breast implant screening protocols that require a four-view-per-breast examination and an optional five-view-per-breast examination (13). Women with breasts that exceed the detector size may also require four or five views to completely image the breast. When considering all compressed breast thickness ranges, estimated benefit-to-risk ratios for a four- or five-view examination are 50% and 60.4% lower, respectively, than that for a two-view examination, with estimated reduction in net lives saved of 4.8% and 7.1%, respectively. Although screening mammography would still be recommended for these women, mammographic dose reduction for women with breast implants or large breasts may be desirable.
On the basis of our results, the maximal net deaths averted occurred in women aged 40–69 years; MBI may therefore have the most net benefit for women in this age range. Because breast density typically decreases with age, women in their 1st decades of screening are also more likely to be those for whom supplemental screening with MBI may be considered given the presence of dense breast tissue. If supplemental screening with MBI is performed, the use of synthetic mammography plus tomosynthesis rather than tomosynthesis plus 2D FFDM as the primary screening technique should be strongly considered. Lowering the cumulative mammography radiation dose by using synthetic mammography instead of 2D FFDM could offset some of the added dose of supplemental MBI screening and maximize the overall net benefit of screening. Given higher net deaths averted when using tomosynthesis compared with 2D FFDM alone, our results also support the use of tomosynthesis for all women, regardless of age.
Our study had some limitations. First, we combined estimates of radiation dose, cancer detection rate, and survival benefit for MBI and mammography. This introduces uncertainty into our estimates. Risk estimates assume that a linear-no-threshold model applies to low radiation doses found in MBI and mammography; this assumption has been questioned (1,6,26). As in the study by Hendrick and Tredennick (4), MBI cancer detection rates were obtained from a single-site study comparing incidence 2D FFDM with prevalence MBI (16). The incidence cancer detection rate of MBI is unknown but expected to be lower. Our estimates assume annual MBI paired with annual mammography, but other intervals such as biennial MBI could be considered. Biennial MBI would lower radiation risk by half and, if a similar MBI cancer detection rate is assumed, an approximate doubling of MBI benefit-to-risk ratios would be expected.
In summary, when considering differences in mammographic technique, patient age, and compressed breast thickness, the benefit-to–radiation risk ratios of mammography are superior to those of MBI for most, but not all, scenarios. Given that the benefit-to-risk ratios for MBI are in the lower range of those of mammography and that MBI is associated with superior net lives saved, use of MBI for supplemental screening should not be precluded based on benefit-to-risk estimates. The use of tomosynthesis plus 2D FFDM for screening should be carefully considered given the lower benefit-to-risk ratios with this technique compared with tomosynthesis plus synthetic mammography.
Authors declared no funding for this work.
Disclosures of Conflicts of Interest: M.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is employed by Barnes-Jewish Healthcare; received travel/accommodations/meeting expenses from Northwest Radiology Network. Other relationships: disclosed no relevant relationships. M.F.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a paid consultant for Hologic; receives payment for lectures including service on speakers bureaus from Hologic. Other relationships: disclosed no relevant relationships.
Abbreviations:
- FFDM
- full-field digital mammography
- MBI
- molecular breast imaging
- 2D
- two dimensional
References
- 1.Hruska CB. Molecular breast imaging for screening in dense breasts: state of the art and future directions. AJR Am J Roentgenol 2017;208(2):275–283. [DOI] [PubMed] [Google Scholar]
- 2.Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol 2018;15(3,3 Pt A):408–414. [DOI] [PubMed] [Google Scholar]
- 3.Holbrook A, Newel MS. Alternative screening for women with dense breasts: breast-specific gamma imaging (molecular breast imaging). AJR Am J Roentgenol 2015;204(2):252–256. [DOI] [PubMed] [Google Scholar]
- 4.Hendrick RE, Tredennick T. Benefit to radiation risk of breast-specific gamma imaging compared with mammography in screening asymptomatic women with dense breasts. Radiology 2016;281(2):583–588. [DOI] [PubMed] [Google Scholar]
- 5.Hendrick RE. Radiation doses and cancer risks from breast imaging studies. Radiology 2010;257(1):246–253. [DOI] [PubMed] [Google Scholar]
- 6.Hruska CB, O’Connor MK., Curies, and Grays, and Sieverts, Oh My: A Guide for Discussing Radiation Dose and Risk of Molecular Breast Imaging. J Am Coll Radiol 2015;12(10):1103–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor MK, Li H, Rhodes DJ, Hruska CB, Clancy CB, Vetter RJ. Comparison of radiation exposure and associated radiation-induced cancer risks from mammography and molecular imaging of the breast. Med Phys 2010;37(12):6187–6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofvind S, Hovda T, Holen ÅS, et al. Digital Breast Tomosynthesis and Synthetic 2D Mammography versus Digital Mammography: Evaluation in a Population-based Screening Program. Radiology 2018;287(3):787–794. [DOI] [PubMed] [Google Scholar]
- 9.Caumo F, Zorzi M, Brunelli S, et al. Digital breast tomosynthesis with synthesized two-dimensional images versus full-field digital mammography for population screening: outcomes from the Verona screening program. Radiology 2018;287(1):37–46. [DOI] [PubMed] [Google Scholar]
- 10.Østerås BH, Skaane P, Gullien R, Martinsen ACT. Average glandular dose in paired digital mammography and digital breast tomosynthesis acquisitions in a population based screening program: effects of measuring breast density, air kerma and beam quality. Phys Med Biol 2018;63(3):035006. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen JV, Williams MB, Patrie JT, Harvey JA. Do women with dense breasts have higher radiation dose during screening mammography? Breast J 2018;24(1):35–40. [DOI] [PubMed] [Google Scholar]
- 12.Helvie MA, Chan HP, Adler DD, Boyd PG. Breast thickness in routine mammograms: effect on image quality and radiation dose. AJR Am J Roentgenol 1994;163(6):1371–1374. [DOI] [PubMed] [Google Scholar]
- 13.Eklund GW, Busby RC, Miller SH, Job JS. Improved imaging of the augmented breast. AJR Am J Roentgenol 1988;151(3):469–473. [DOI] [PubMed] [Google Scholar]
- 14.Hendrick RE, Pisano ED, Averbukh A, et al. Comparison of acquisition parameters and breast dose in digital mammography and screen-film mammography in the American College of Radiology Imaging Network digital mammographic imaging screening trial. AJR Am J Roentgenol 2010;194(2):362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Human Mortality Database website, University of California, Berkeley, USA and Max Planck Institute for Demographic Research, Germany. http://www.mortality.org. Accessed September 17, 2018.
- 16.Rhodes DJ, Hruska CB, Conners AL, et al. Journal club: molecular breast imaging at reduced radiation dose for supplemental screening in mammographically dense breasts. AJR Am J Roentgenol 2015;204(2):241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014;311(24):2499–2507. [DOI] [PubMed] [Google Scholar]
- 18.Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med 2009;151(10):738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different U.S. breast cancer screening strategies. Ann Intern Med 2016;164(4):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Research Council of the National Academies . Health risks from exposure to low levels of ionizing radiation: BEIR VII, phase 2—Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Washington, DC: National Academies Press, 2006. [PubMed] [Google Scholar]
- 21.Mainiero MB, Lourenco A, Mahoney MC, et al. ACR Appropriateness Criteria Breast Cancer Screening. J Am Coll Radiol 2016;13(11,11S):R45–R49. [DOI] [PubMed] [Google Scholar]
- 22.Covington MF, Rhodes DJ, Pizzitola VJ. Molecular breast imaging and the 2016 update to the ACR appropriateness criteria for breast cancer screening. J Am Coll Radiol 2016;13(12 Pt A):1408. [DOI] [PubMed] [Google Scholar]
- 23.Freer PE, Winkler N. Synthesized digital mammography imaging. Radiol Clin North Am 2017;55(3):503–512. [DOI] [PubMed] [Google Scholar]
- 24.Freer PE, Riegert J, Eisenmenger L, et al. Clinical implementation of synthesized mammography with digital breast tomosynthesis in a routine clinical practice. Breast Cancer Res Treat 2017;166(2):501–509. [DOI] [PubMed] [Google Scholar]
- 25.Miglioretti DL, Lange J, van den Broek JJ, et al. Radiation-induced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med 2016;164(4):205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doss M. Are we approaching the end of the linear no-threshold era? J Nucl Med 2018;59(12):1786–1793. [DOI] [PubMed] [Google Scholar]