Abstract
Technical advances in CT have enabled implementation of dual-energy CT into routine clinical practice. By acquiring images at two different energy spectra, dual-energy CT enables material decomposition, allowing generation of material- and energy-specific images. Material-specific images include virtual nonenhanced images and iodine-specific images (iodine maps). Energy-specific images include virtual monoenergetic images. The reconstructed images can provide unique qualitative and quantitative information about tissue composition and contrast media distribution. In thoracic oncologic imaging, dual-energy CT provides advantages in characterization of thoracic malignancies and lung nodules, determination of extent of disease, and assessment of response to therapy. An especially important feature in children is that dual-energy CT does not come at a higher radiation exposure.
Keywords: CT, CT-Quantitative, Lung, Mediastinum, Neoplasms-Primary, Pediatrics, Thorax, Treatment Effects
© RSNA, 2021
Summary
Dual-energy CT has the potential to improve characterization of primary and secondary lesions, evaluation of tumor extent, and assessment of therapy response at a radiation dose that is comparable to or less than that of single-energy CT imaging, which is particularly important for pediatric patients.
Essentials
■ In pediatric thoracic oncologic CT, the postprocessing technique that best exploits the advantages of dual-energy CT is the material-decomposition algorithm.
■ Material decomposition allows generation of material- and energy-specific data sets, which can expand the ability of CT to differentiate materials with similar attenuation values, thus providing more information on tissue composition than single-energy CT.
■ Radiation exposure from dual-energy CT is less than or equal to that of a comparable single-energy CT.
■ In the setting of pediatric oncologic imaging, iodine-specific images could have a role in lesion characterization, evaluation of tumor extent, and response assessment.
■ In the setting of pediatric oncologic imaging, iodine-specific images may be a potential surrogate method for assessing treatment response and tumor viability.
Introduction
Single-energy CT has been a cornerstone in oncologic imaging. At conventional single-energy CT, tissue differentiation is based on differences in x-ray photon attenuation between adjacent structures. The challenge of conventional CT is that different materials, such as iodine and calcium (bone), can show identical attenuation values, although they have different elemental compositions. Therefore, conventional imaging provides limited information on tissue composition.
Dual-energy CT (also referred to as multienergy or spectral CT) acquires data at two different energy peaks, which enables the differentiation and quantification of materials with similar attenuation values and allows generation of material-decomposition images. Material decomposition allows for the creation of additional images, such as virtual nonenhanced images (iodine subtracted from the image), iodine-specific images (pure iodine or iodine overlay images), and virtual monoenergetic images. Thus, dual-energy CT provides more information on tissue composition than does single-energy CT (1–6).
The clinical applications in the pediatric thorax that have been previously reported include use of the bone removal software in CT angiography for assessment of the arterial system and use of perfused blood volume and lung vessel algorithms to assess pulmonary embolism, pulmonary atresia, pulmonary hypertension, and arteriovenous malformations (7–9). Data on the use of dual-energy CT in thoracic oncologic imaging have not been as well described in children (10).
In this article, dual-energy CT principles, scanner equipment, workflow parameters, material decomposition postprocessing techniques, radiation exposures, and applications of dual-energy CT material-decomposition imaging in pediatric thoracic oncology are discussed. Specifically, the role of dual-energy CT in lesion characterization, evaluation of disease extent, and assessment of treatment response are addressed.
Dual-Energy CT Systems
Several dual-energy hardware systems are available for acquiring dual-energy images. Dual-source dual-energy CT (Siemens Healthineers) uses two x-ray tubes operating independently at low and high energies and two detectors to obtain simultaneous data sets. Second- and third-generation dual-source systems have a tin (Sn) filter mounted in front of the high-kilovoltage-peak x-ray tube that attenuates lower-energy photons, thereby increasing the mean spectral energy. The gantry speeds for the first-, second-, and third-generation scanners are 0.33 second, 0.28 second, and 0.25 second, respectively. Kilovoltage combinations for thoracic imaging are: Sn80/140 for the first- and second-generation scanners and Sn70/150 and Sn80/150 for the third-generation scanner. The Sn70/150 kVp is used for smaller patients, reserving the Sn80/150 kVp for larger patients. The two tubes have different fields of view (FOV). The FOV of the low-energy tube (tube A) is 50 cm, whereas the FOV of the high-energy tube (tube B) is 26 cm, 33 cm, and 35 cm on the first-, second-, and third-generation CT systems, respectively. A potential disadvantage of the smaller FOV of the high-energy tube is that peripherally located structures may be excluded from the FOV in very large or obese patients, although in children this has not been a limitation.
Single-source, rapid kilovoltage switching dual-energy CT (GE Healthcare) uses a single x-ray tube that rapidly alternates between low (80 kVp) and high (140 kVp) energies and a single detector that quickly captures the data from both energies, enabling near-simultaneous acquisition of the two data sets. The gantry speed is 0.5 second and the FOV is 50 cm. A potential disadvantage of the single-source system is limited spectral separation related to lack of independent tube filtration.
The dual-layer detector dual-energy CT (IQon spectral CT; Philips Healthcare) uses a single x-ray beam operating at 120 or 140 kVp and a single detector made of two layers: a superficial layer that absorbs lower-energy x-ray photons and a deeper layer that absorbs higher-energy photons (11). There is simultaneous acquisition of the two energy spectra. Gantry rotation time is 0.27 second, and there is a 50-cm FOV. A potential limitation is decreased spectral separation.
Twin-beam dual-energy CT (Siemens Healthineers) uses a high-energy tube operating at 120 kVp and a filter composed of gold and tin that splits the beam into high- and low-energy spectra before it reaches the patient (12,13). Gantry rotation times range from 0.28 to 0.5 second, and there is a full FOV of 50 cm. Similar to other single-source systems, a potential limitation is decreased spectral separation.
CT Image Workflow
In the dual-source system, low-energy (70 or 80 kVp) images, high-energy (140 or 150 kVp) images, and a blended image that combines the high- and low-energy data (50% from each) and has an appearance similar to that of a conventional 120-kVp CT image are generated at the CT console. In the fast kilovoltage switching technology, a 140-kVp image is generated. In the dual-layer system, 120-kVp or 140-kVp images are generated, with the 120-kVp image preferred in children. In the split-filter (twin-beam) technology, 120-kVp images are generated.
The low- and high-energy and blended images are sent to a picture archiving and communication system (PACS) for a clinical interpretation and to a thin-client server for future processing if needed. For oncologic imaging, additional images are generated using material-decomposition algorithms and sent to the PACS. These include virtual nonenhanced (or water), iodine-specific (or material density), and virtual monoenergetic (also called virtual monochromatic) images.
Material-Decomposition Images
Virtual nonenhanced images subtract iodine content from tissues to yield simulated nonenhanced images. These simulated images can depict small foci of calcium, fat, and hemorrhage, similar to true nonenhanced images, which can help in lesion characterization (14,15). Generating virtual nonenhanced images also has the potential to reduce radiation dose by eliminating the need for acquiring true unenhanced images (16). A potential limitation of virtual nonenhanced images is that very small or faint calcification may not be seen (14,15).
Iodine-specific images show the distribution and amount of iodine in tissue, providing a measurement of enhancement. These images can be displayed as a pure iodine or material-density image or as an iodine overlay image, which superimposes the iodine content on the virtual nonenhanced image. The iodine content also can be assessed quantitatively in Hounsfield units and milligrams per milliliter. Research published in 2017 using thoracic phantoms suggests that iodine concentration can be accurately quantified with latest-generation dual-source and dual-layer CT systems (17). In the setting of pediatric oncology, iodine-specific images may have a role in lesion characterization, evaluation of tumor extent, and response assessment.
Virtual monoenergetic reconstructions are gray-scale images that simulate the Hounsfield unit values that could be achieved by using a true monoenergetic x-ray source. The x-ray energies are displayed in kiloelectron volts. The range of energies extends between 40 and 200 keV. Images reconstructed at low kiloelectron volts (40–60 keV) have a higher contrast-to-noise ratio and more noise (10). Low-energy images have the potential to improve conspicuity of subtle lesions in solid organs, such as the liver. Images reconstructed at higher energies (100–140 keV) have less noise but also less contrast. They have a role in reducing beam-hardening and metal artifacts, which potentially can obscure pathologic findings.
Dual-Energy CT Protocol
CT examinations in this article were performed using the Siemens SOMATOM Definition Flash (Siemens Healthineers). All dual-energy CT examinations were performed using a dedicated dual-energy protocol for the thorax recommended by the manufacturer, which included Sn80/140 kVp. The examinations were performed with automatic tube current modulation (CARE Dose 4D; Siemens Healthineers), detector collimation of 128 × 0.6 mm, pitch of 1.2, rotation time of 0.28 second, reconstruction thickness of 3 mm, and iterative reconstruction. Quality reference tube current–time product was 275 mAs for 80-kVp tube and 106 mAs for Sn140-kVp tube.
Contrast material was tailored according to the patient’s weight in accordance with our pediatric practice parameters. Patients received nonionic contrast medium (320 mg I/mL) at a volume of 2 mL per kilogram of body weight (maximum 100 mL). Images of the chest from the lung apices to the adrenal glands were acquired 25 to 30 seconds after initiation of contrast agent administration. Contrast agent was injected by a power injector if a 22-gauge catheter could be placed in the antecubital area (18–20). A manual injection was used in patients with smaller gauge catheters or if the catheter was located in a site other than the antecubital area.
From the dual-energy CT data set, 80-kVp, Sn140-kVp, and blended images were generated at the scanner console. All images were reconstructed using a commercial iterative reconstruction technique (Admire; Siemens Healthineers) at 1- and 3-mm section thickness and interval of 2.0 mm. In addition, 3-mm coronal and sagittal multiplanar images were created.
Radiation Exposure
Radiation exposures at dual-energy angiography and contrast-enhanced chest, abdominal, and pelvic CT in children are less than or equal to those of a comparable single-energy CT scan (7–9,21–23). In large part, this reflects the fact that the dose-reduction technologies available with single-energy CT also can be used with dual-energy CT. Automated tube current modulation for dose reduction is available on all systems except the rapid kilovolt peak–switching scanner. The absence of tube current modulation on the latter system can be associated with higher radiation doses. All vendors offer iterative reconstruction techniques that have the potential to lower radiation dose by lowering tube current. High-pitch coverage is also available. An additional advantage of the dual-source CT is the tin filter mounted in front of the high kilovolt peak x-ray tube, which attenuates lower-energy photons, thereby decreasing radiation exposure and increasing spectral separation.
Role of Dual-Energy CT in Tissue Characterization
Mediastinal Masses
Dual-energy CT has differences in contrast enhancement between tumor and normal parenchyma, and iodine concentration values may be useful in differentiating between benign and malignant lesions (24–26). Quantitative iodine concentration measurements provide a quantitative parameter of lesion enhancement. Additionally, nonenhanced images may provide supplementary information about presence of calcification in a lesion, which can help in lesion characterization.
Lee et al found significant differences in iodine concentrations between benign and malignant tumors both in the early contrast phase (1.38 mg/mL vs 2.41 mg/mL, P = .001) and in the delayed phase (1.52 mg/mL vs 2.84 mg/mL, P = .001), while there was no significant difference in attenuation values (27). The best cutoff iodine value for differentiating benign from malignant tumors was 1.40 mg/mL for the early phase dual-energy CT and 1.58 mg/mL for the delayed phase. The benign tumors in this series included neurogenic tumors and a leiomyoma, and the malignant tumors included lymphomas, thymic tumors, metastases, and small cell cancer.
In children, common mediastinal masses include lymphoma, teratoma, neurogenic tumors (neuroblastoma, ganglioneuroma, and neurofibroma), and foregut duplication cysts. Mediastinal duplication cysts typically have homogeneous, near-water attenuation contents. On occasion, the attenuation may be equal to that of soft tissue because the cyst fluid contains mucoid, proteinaceous or hemorrhagic debris, or milk of calcium, mimicking a solid lesion. Conversely, some neurogenic tumors have low attenuation due to the presence of cystic degeneration, fatty components, or areas of myxoid stroma, thus mimicking a cystic mass. Iodine-enhanced images using only a single-phase dual-energy scan allow differentiation of solid and cystic masses. Demonstration of a non–iodine-containing lesion supports the diagnosis of a complex cyst or benign solid mass (Figs 1, 2), while demonstration of iodine within mediastinal mass increases the concern for malignancy (10) (Fig 3). Our experience suggests that malignant tumors, such as lymphoma and neuroblastoma, have an iodine concentration greater than or equal to 1 mg/mL, although more studies are needed to confirm this threshold level.
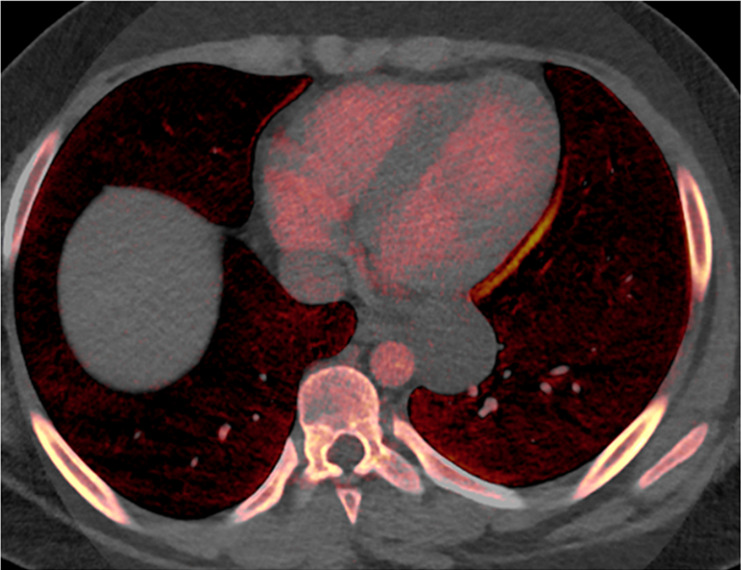
Figure 1a:
Mediastinal cyst characterization using iodine-specific CT images in a 13-year-old boy with a mediastinal mass detected at chest radiography. (a) Blended image demonstrates a fluid-filled middle mediastinal mass (arrow). The contents measured approximately 30 HU (attenuation slightly above water), suggesting a complex cyst. (b) Iodine-specific image shows absent iodine content confirming diagnosis of a cyst. The absence of iodine is based on comparison to chest wall musculature, which normally has no iodine content. Pathologically proven enteric duplication cyst.
Figure 2a:
Benign mediastinal mass in a 16-year-old boy. (a) Coronal 40-keV CT image shows a posterior mediastinal mass. (b) Sagittal iodine-specific CT image shows trace iodine content, measured at 0.3 mg/mL. Circle indicates the region of interest. Note the iodine content in the mass is similar to that of muscle. Ganglioneuroma confirmed on surgical resection. App = application, CM = contrast material, Stddev = standard deviation, VNC = virtual noncontrast.
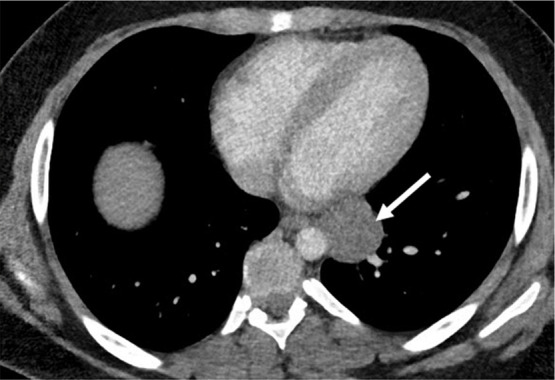
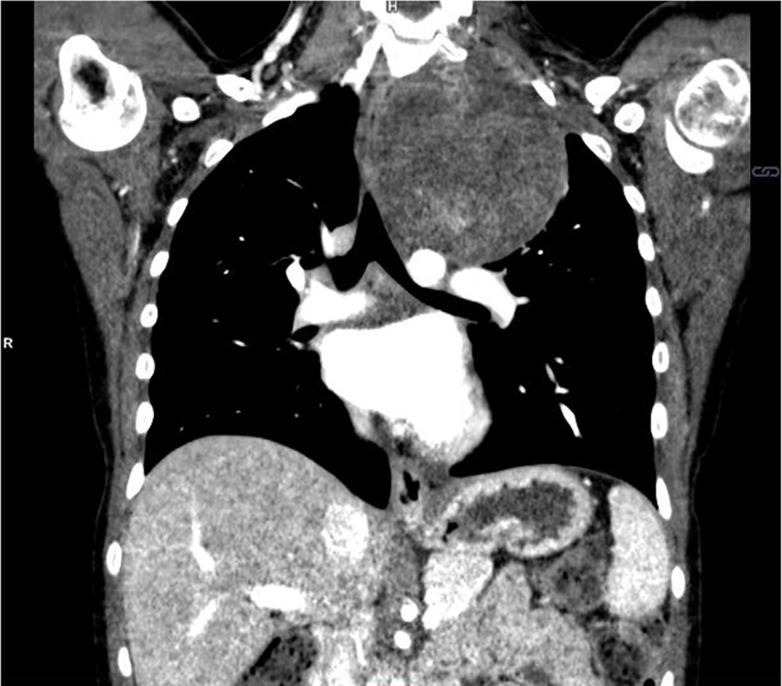
Figure 3a:
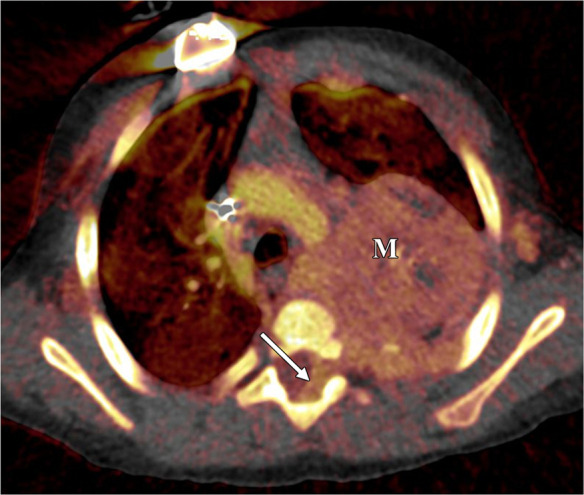
Neuroblastoma in a 1-year-old boy. (a) Axial iodine-specific CT image shows iodine content (2.8 mg/mL) within a posterior mediastinal mass (M), as well as iodine content within an area of intraspinal extension (arrow). The iodine content is greater than that of adjacent muscle. (b) Iodine-specific sagittal CT image shows the full extent of the intraspinal tumor extent (arrows).
Figure 1b:
Mediastinal cyst characterization using iodine-specific CT images in a 13-year-old boy with a mediastinal mass detected at chest radiography. (a) Blended image demonstrates a fluid-filled middle mediastinal mass (arrow). The contents measured approximately 30 HU (attenuation slightly above water), suggesting a complex cyst. (b) Iodine-specific image shows absent iodine content confirming diagnosis of a cyst. The absence of iodine is based on comparison to chest wall musculature, which normally has no iodine content. Pathologically proven enteric duplication cyst.
Figure 2b:
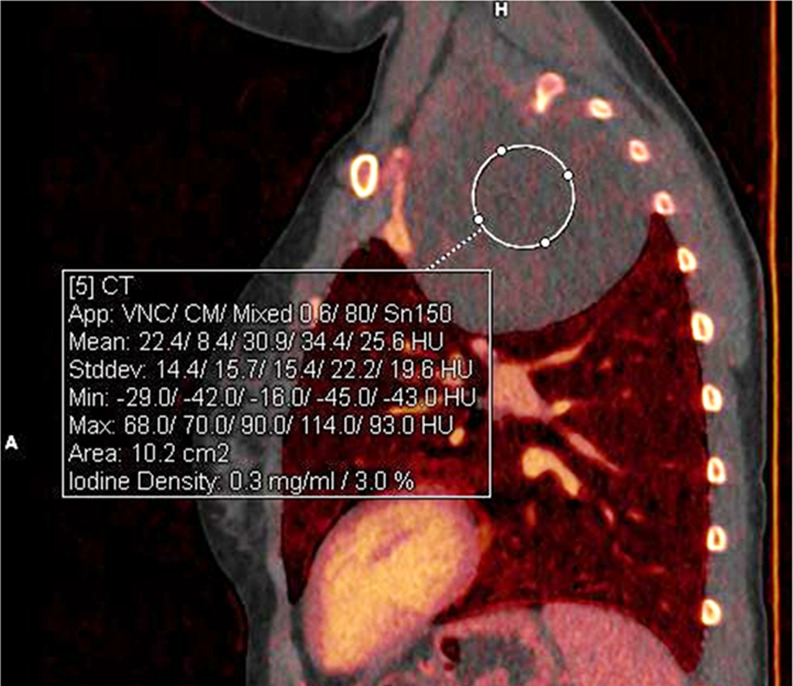
Benign mediastinal mass in a 16-year-old boy. (a) Coronal 40-keV CT image shows a posterior mediastinal mass. (b) Sagittal iodine-specific CT image shows trace iodine content, measured at 0.3 mg/mL. Circle indicates the region of interest. Note the iodine content in the mass is similar to that of muscle. Ganglioneuroma confirmed on surgical resection. App = application, CM = contrast material, Stddev = standard deviation, VNC = virtual noncontrast.
Figure 3b:
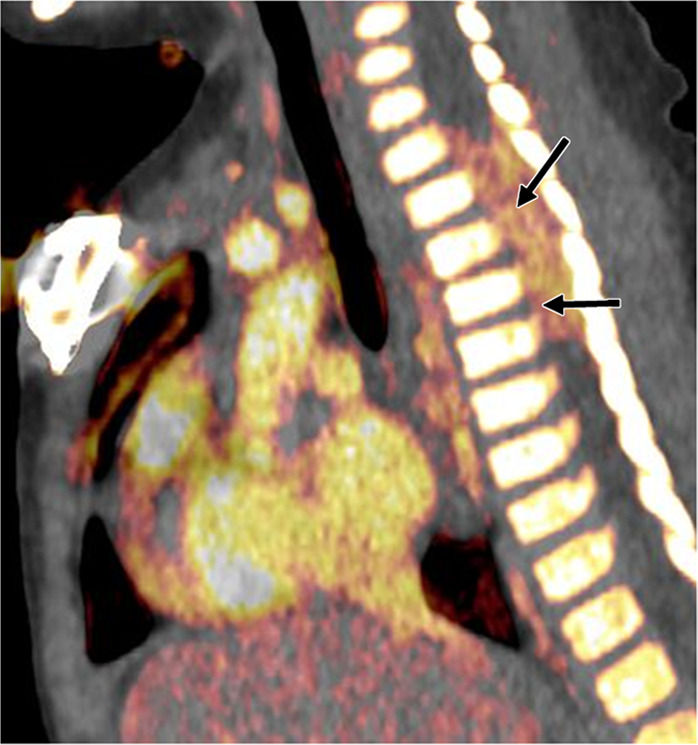
Neuroblastoma in a 1-year-old boy. (a) Axial iodine-specific CT image shows iodine content (2.8 mg/mL) within a posterior mediastinal mass (M), as well as iodine content within an area of intraspinal extension (arrow). The iodine content is greater than that of adjacent muscle. (b) Iodine-specific sagittal CT image shows the full extent of the intraspinal tumor extent (arrows).
Lymph Nodes
In adults, iodine concentrations have been reported to be useful in the characterization of mediastinal lymph nodes and differentiation between benign and metastatic lymph nodes. In a study of patients with metastatic non–small cell lung cancer, Li et al reported that an iodine content of 29.32 mg/cm and normalized iodine value (nodal iodine content or aortic iodine content) of 0.4328 in a lymph node represented the optimal threshold to separate metastatic from nonmetastatic lymph nodes (sensitivity, 80% and 75%; specificity, 65% and 75%; positive predictive value, 70% and 75%; negative predictive value, 76% and 75%; accuracy, 73% and 75%, respectively) (28). In another study of patients with intrathoracic nodal metastases from non–small cell lung cancer, Yang et al reported that normalized iodine concentrations were higher in metastatic than in nonmetastatic lymph nodes (P < .05) (29). The difference was attributed to an increase of the number of small vascular beds in metastatic lymph nodes, which resulted in increased tumor perfusion. The authors also reported that quantitative parameters showed higher accuracy than size measurements for the preoperative diagnosis of metastatic lymph nodes.
In children, common causes of intrathoracic lymphadenopathy are lymphoma, metastases, and granulomatous disease. Location, size criteria, and attenuation values are not specific enough to determine presence or absence of malignancy. Our clinical experience suggests that iodine-enhanced images and iodine concentration measurements may be able to differentiate malignant from benign nodes due to granulomatous infection and inflammatory disease. The iodine content on qualitative and quantitative images is higher in malignant than in nonmalignant nodes (Figs 4, 5).
Figure 4:
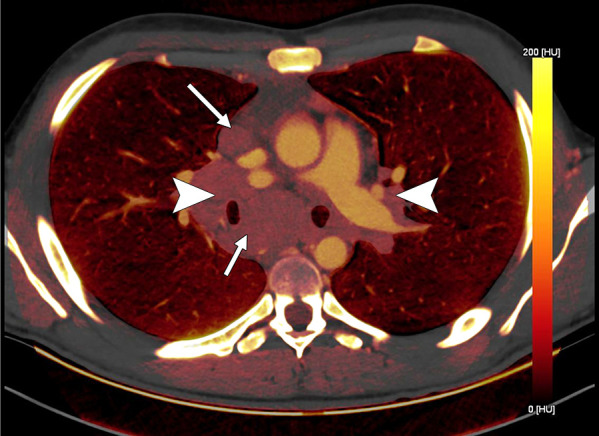
Lymph node characterization in a 16-year-old boy with lymphoma. The iodine-specific CT image shows increased iodine content within mediastinal nodes (arrows) and both hilar areas (arrowheads). The measured iodine content was 1.5 mg/mL.
Figure 5:
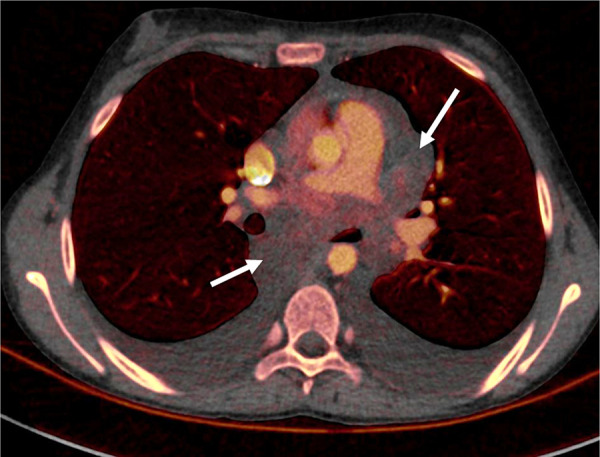
Inflammatory myofibroblastic tumor in a 15-year-old girl. Dual-energy CT iodine-specific image shows virtually no iodine content within this benign mediastinal lymphadenopathy (arrows). The iodine content was 0.4 mg/mL. Biopsy showed myofibroblastic and fibroblastic spindle cells with inflammatory infiltrate of lymphocytes and plasma cells consistent with myofibroblastic tumor.
Lung Nodules
Dual-energy iodine-specific images also may help characterize solitary pulmonary nodules (24–26,30–34). Chae et al evaluated the role of dual-energy CT for classification in 49 solitary pulmonary nodules (55.6% prevalence of malignancy).The size of the nodules ranged from 5 to 70 mm, and the average size was 24.8 mm in diameter ± 11.8 standard deviation. CT attenuation values on iodine-enhanced images and degree of enhancement on blended images were compared (30). Using a cutoff value of 20 HU for malignant nodules, the CT attenuation values on iodine-enhanced images had higher sensitivity and accuracy than did the number using degree of enhancement (sensitivity, 92% and 72%; specificity, 70% and 70%; and accuracy, 82.2% and 71.1%, respectively).
In children with malignant solid tumors, pulmonary nodules are more often malignant than benign (35), and not surprisingly, conventional single-energy CT cannot reliably differentiate benign from malignant nodules (36). In our evaluation of lung nodules, we noted that iodine content is different and higher in malignant lesions than in benign lesions, with malignant lesions having an iodine concentration of greater than or equal to 1 mg/mL (Fig 6).
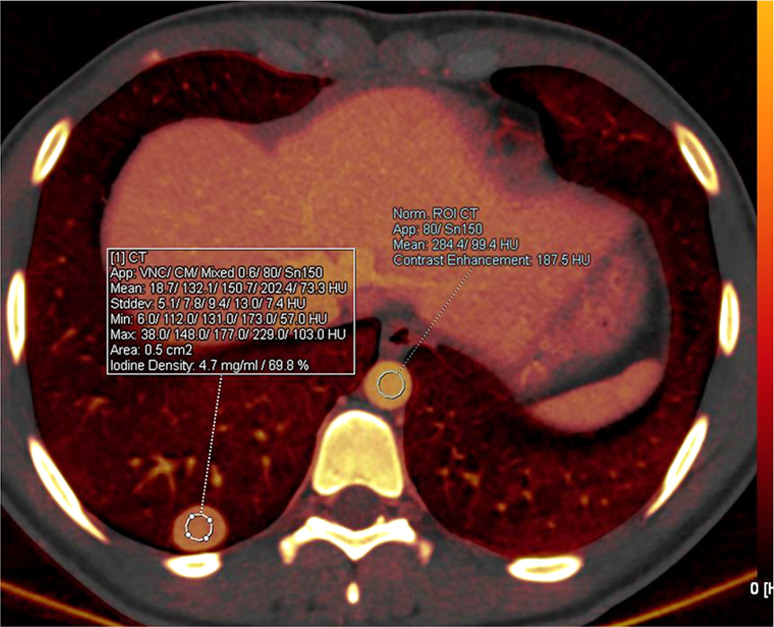
Figure 6a:
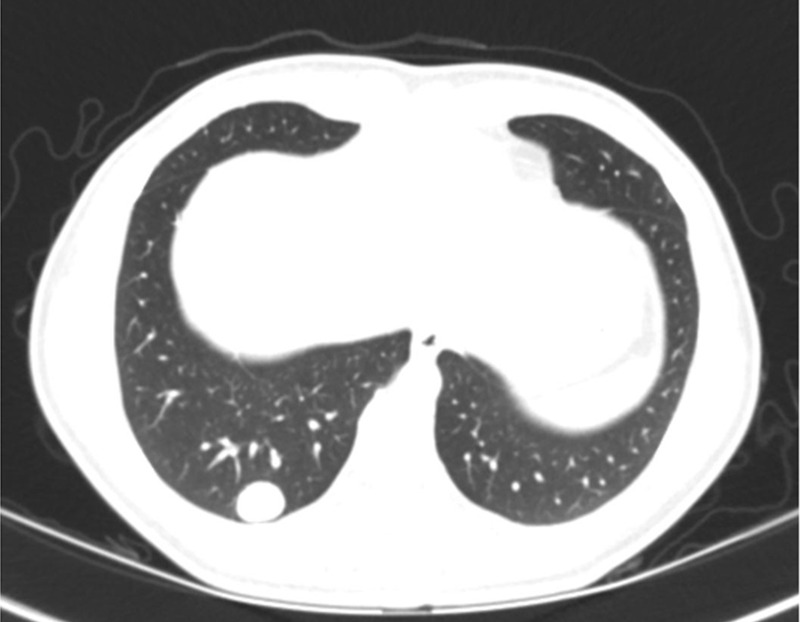
Malignant lung nodule in a 17-year-old girl with history of paraganglioma of the left neck. (a) Lung window CT image shows a well-defined right lower lobe nodule. (b) Axial iodine-specific CT image shows an iodine-containing right lower lobe nodule with an iodine density of 4.7 mg/mL. The nodule was surgically proven to be metastatic paraganglioma. Circles are ROIs on long nodule and aorta. App = application, CM = contrast material, Norm = normal, ROI = region of interest, Stddev = standard deviation, VNC = virtual noncontrast.
Figure 6b:
Malignant lung nodule in a 17-year-old girl with history of paraganglioma of the left neck. (a) Lung window CT image shows a well-defined right lower lobe nodule. (b) Axial iodine-specific CT image shows an iodine-containing right lower lobe nodule with an iodine density of 4.7 mg/mL. The nodule was surgically proven to be metastatic paraganglioma. Circles are ROIs on long nodule and aorta. App = application, CM = contrast material, Norm = normal, ROI = region of interest, Stddev = standard deviation, VNC = virtual noncontrast.
However, a potential pitfall is that inflammatory nodules can demonstrate increased iodine content similar to malignant nodules. The combined review of blended and iodine images should help reduce misdiagnosis. Infectious pathologies often have poorly defined margins and may show a halo sign, allowing differentiation from metastases (Fig 7).
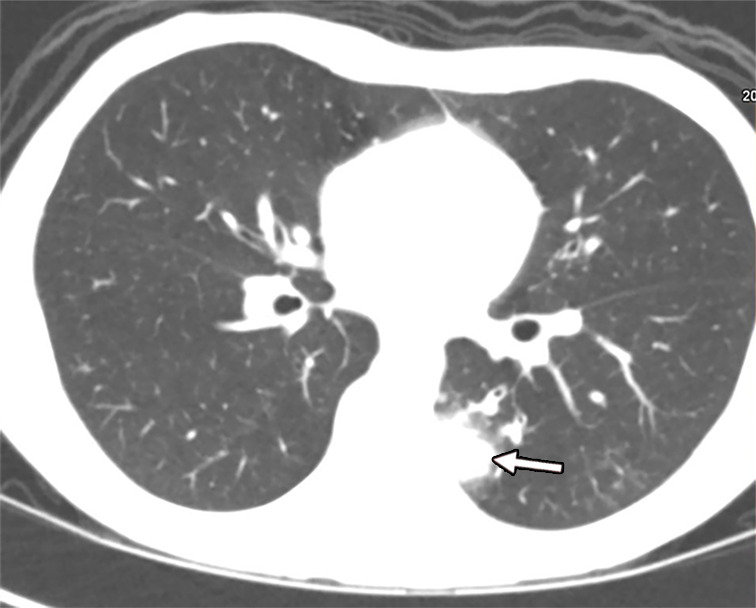
Figure 7a:
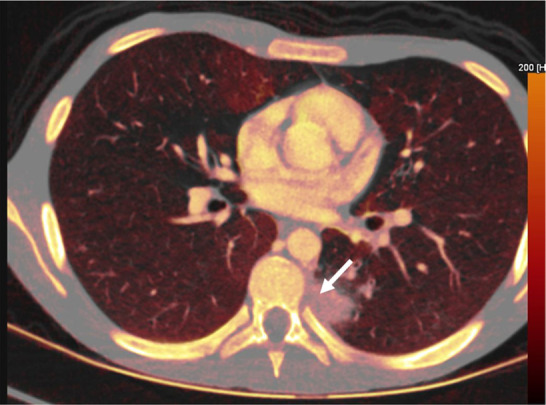
Fungal infection in a 15-year-old boy being treated for lymphoma. (a) Iodine-specific CT image shows iodine content within a left lower lobe nodule (arrow).The iodine concentration was 2.4 mg/mL. (b) Lung window CT image shows an ill-defined lung nodule with a surrounding halo (arrow). Biopsy yielded Aspergillus.
Figure 7b:
Fungal infection in a 15-year-old boy being treated for lymphoma. (a) Iodine-specific CT image shows iodine content within a left lower lobe nodule (arrow).The iodine concentration was 2.4 mg/mL. (b) Lung window CT image shows an ill-defined lung nodule with a surrounding halo (arrow). Biopsy yielded Aspergillus.
Dual-Energy CT in Determination of Disease Extent
Incidental hepatic lesions are not as common in children as in adults, but they can occur and generally are presumed to be benign in asymptomatic patients. However, in oncologic patients undergoing CT, incidental lesions can raise concern for metastases. The tumors that most often metastasize to liver are Wilms tumor, neuroblastoma, sarcomas, and lymphoma.
Low-energy monoenergetic and iodine-specific images can help improve lesion conspicuity and detection rate of subtle hypo- and hyperattenuating lesions by increasing attenuation and contrast differences between hepatic metastases and normal hepatic parenchyma (14,37,38). The suggested optimal monoenergetic energies are 40–60 keV (10,14,37). In addition, iodine-specific images can be helpful in differentiating between cysts and metastases. Metastases show iodine contrast, whereas cysts do not (Figs 8, 9).
Figure 8a:
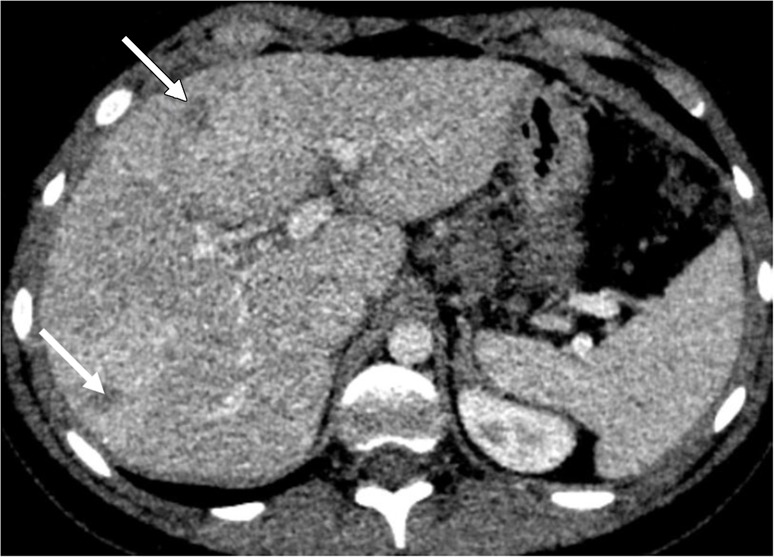
Liver metastases in a 10-year-old boy with desmoplastic small round cell tumor metastatic to chest and liver. (a) Blended CT image shows poorly defined hypoattenuating lesions in the right hepatic lobe (arrows). (b) Iodine-enhanced CT image increases lesion conspicuity and also shows some iodine content within the lesion (arrows).
Figure 9:
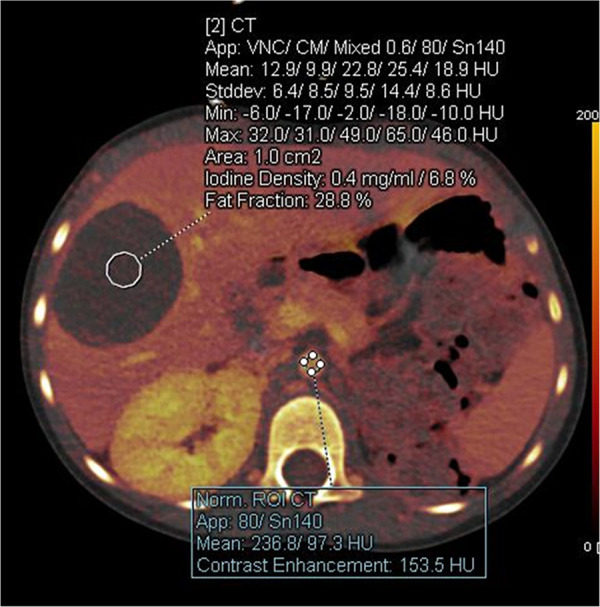
Incidentally detected hepatic lesion in a 3-year-old girl who had undergone a left nephrectomy for Wilms tumor. Axial iodine-enhanced CT image shows a hepatic mass with minimal iodine content (0.4 mg/mL). Circles are ROIs placed on liver and aorta. MRI confirmed a hepatic cyst. App = application, CM = contrast material, ROI = region of interest, Stddev = standard deviation, VNC = virtual noncontrast.
Figure 8b:
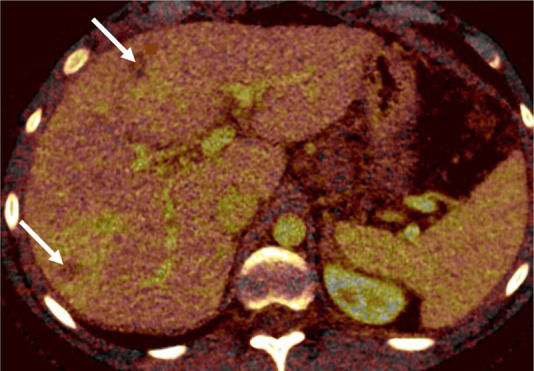
Liver metastases in a 10-year-old boy with desmoplastic small round cell tumor metastatic to chest and liver. (a) Blended CT image shows poorly defined hypoattenuating lesions in the right hepatic lobe (arrows). (b) Iodine-enhanced CT image increases lesion conspicuity and also shows some iodine content within the lesion (arrows).
By increasing lesion conspicuity, iodine-specific images may also allow better delineation of tumor invasion into soft tissues, such as chest wall and pleura. In patients with neuroblastoma, iodine-specific images may allow for better delineation of intraspinal extension (Fig 3).
Assessment of Treatment Response
The traditional criteria to monitor response to chemotherapy are based on serial measurements of tumor size, including the Response Evaluation Criteria in Solid Tumors and World Health Organization criteria. However, some chemotherapies, particularly the new antiangiogenesis and immunotherapy drugs, may not alter tumor size but instead may cause a dramatic change in attenuation or iodine content. In this setting, iodine-specific dual-energy CT may be a potential surrogate method for assessing treatment response and tumor viability (7–10).
In a comparison of pre- and postchemotherapy CT studies, Baxa et al reported that iodine uptake quantification in dual-phase dual-energy CT has advantages in assessment of treatment response in patients with non–small cell lung cancer. They showed a significant decrease in the arterial enhancement fraction (ratio of iodine enhancement in arterial and venous phases) in responding nodal metastases and a significant increase in nonresponding nodes (39). In another study assessing response changes in the primary lung tumor, Baxa et al demonstrated a significant decrease in iodine content and arterial enhancement fraction in responding tumors, while no significant change was observed in nonresponding tumors (40). Another study by Ren et al compared iodine uptake from dual-energy CT with fluorine 18 (18F) fluorodeoxyglucose PET/CT metabolic parameters in patients with lung cancer prior to and during treatment (41). Iodine-related quantitation correlated well with metabolism-based measurements at 18F fluorodeoxyglucose PET/CT, decreasing with treatment response, suggesting that iodine quantitation might be a feasible substitute for assessment of lung cancer response to treatment.
Our experience is similar in children with lymphoma and neuroblastoma. For example, Figures 10 and 11 show examples of tumors with decreased iodine concentration that responded to treatment. The use of iodine-enhanced images to assess treatment response requires standardization of threshold values, but if these can be validated, dual-energy CT could be a modality used to assess therapy responses prior to the tumor reducing in size.
Figure 10a:
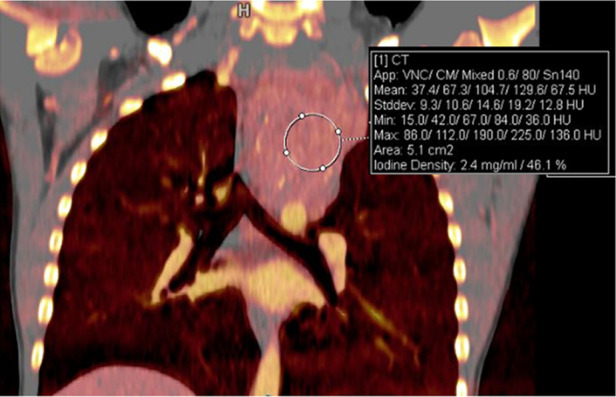
Treatment response in a 6-year-old girl with neuroblastoma. (a) Baseline iodine-specific CT image shows increased iodine content in a posterior mediastinal mass. Circle indicates region of interest over mediastinal mass. The iodine concentration was 2.4 mg/mL. (b) Iodine-specific CT image 4 months later shows decreased iodine content within the mass. Circle indicates region of interest over mediastinal mass. Findings are consistent with partial treatment response. Note that tumor size did not change between the two time points. Iodine 123 meta-iodobenzylguanidine study showed minimal residual metabolic activity and improved from baseline. App = application, CM = contrast material, Stddev = standard deviation, VNC = virtual noncontrast.
Figure 11a:
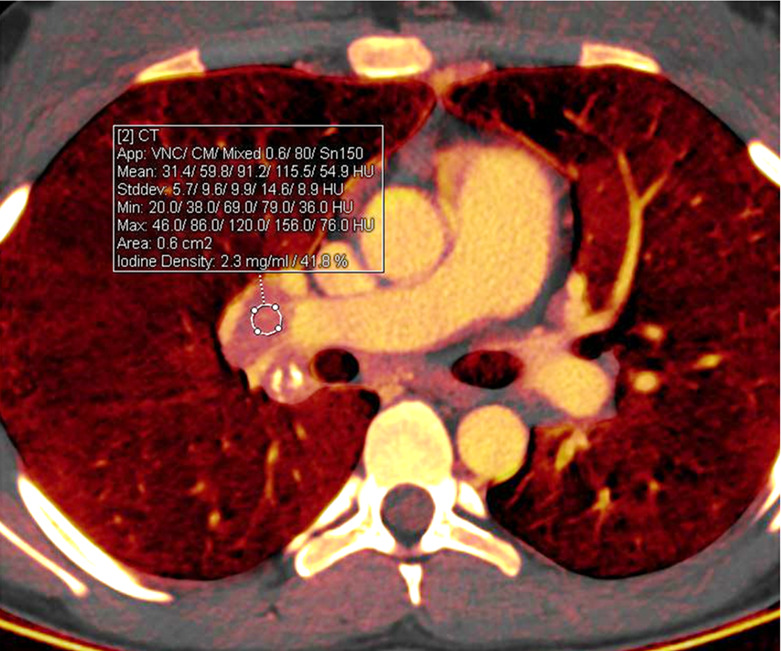
Treatment response in a 16-year-old girl with lymphoma. (a) Baseline iodine-specific CT image shows iodine content in a right hilar node. Circle indicates region of interest. The iodine concentration was 2.3 mg/mL. (b) Iodine-specific CT image 2 months later shows decreased iodine content. Circles indicate regions of interest within hilar node and pulmonary artery. The iodine concentration was 0.6 mg/mL. Findings are consistent with complete treatment response. Complete response confirmed at PET/CT. App = application, CM = contrast material, Stddev = standard deviation, VNC = virtual noncontrast.
Figure 10b:
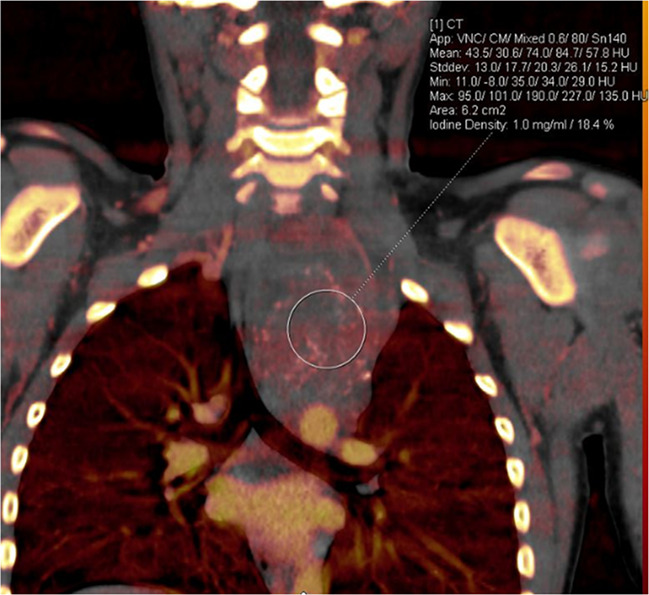
Treatment response in a 6-year-old girl with neuroblastoma. (a) Baseline iodine-specific CT image shows increased iodine content in a posterior mediastinal mass. Circle indicates region of interest over mediastinal mass. The iodine concentration was 2.4 mg/mL. (b) Iodine-specific CT image 4 months later shows decreased iodine content within the mass. Circle indicates region of interest over mediastinal mass. Findings are consistent with partial treatment response. Note that tumor size did not change between the two time points. Iodine 123 meta-iodobenzylguanidine study showed minimal residual metabolic activity and improved from baseline. App = application, CM = contrast material, Stddev = standard deviation, VNC = virtual noncontrast.
Figure 11b:
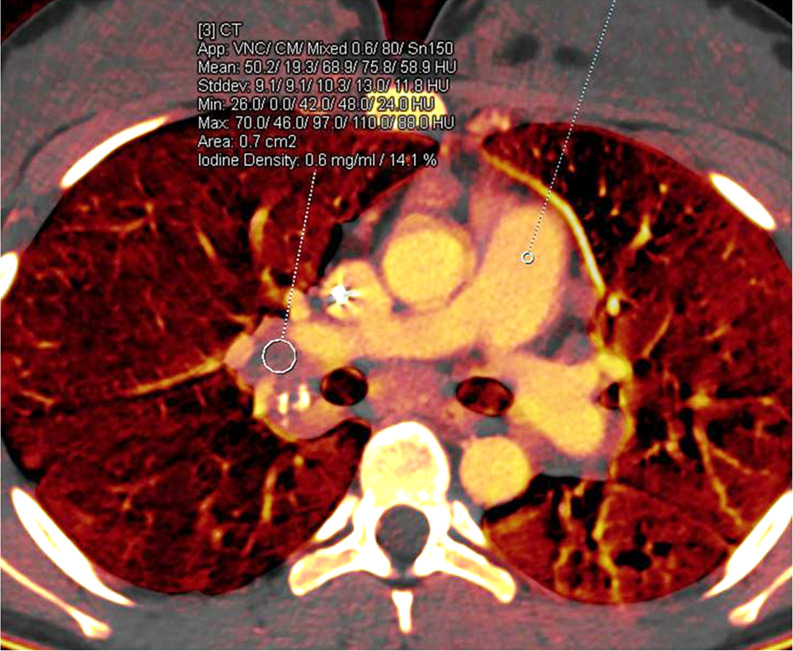
Treatment response in a 16-year-old girl with lymphoma. (a) Baseline iodine-specific CT image shows iodine content in a right hilar node. Circle indicates region of interest. The iodine concentration was 2.3 mg/mL. (b) Iodine-specific CT image 2 months later shows decreased iodine content. Circles indicate regions of interest within hilar node and pulmonary artery. The iodine concentration was 0.6 mg/mL. Findings are consistent with complete treatment response. Complete response confirmed at PET/CT. App = application, CM = contrast material, Stddev = standard deviation, VNC = virtual noncontrast.
Conclusion
Dual-energy CT with material- and energy-specific imaging offers exciting applications previously unavailable with single-energy CT in the evaluation of pediatric thoracic malignancies. This technology opens up the possibility of improving detection and characterization of primary and secondary lesions, improved evaluation of tumor extent, and assessment of therapy response at a radiation dose that is comparable to or less than that of single-energy CT imaging.
Authors declared no funding for this work.
Disclosures of Conflicts of Interest: M.J.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author from Siemens Healthcare for travel and honorarium for lectures, including service on speakers bureaus. Other relationships: disclosed no relevant relationships. S.B. disclosed no relevant relationships. M.C. disclosed no relevant relationships.
Abbreviations:
- FOV
- field of view
- PACS
- picture archiving and communication system
References
- 1.Goo HW, Goo JM. Dual-energy CT: New Horizon in medical imaging. Korean J Radiol 2017;18(4):555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCollough CH, Leng S, Yu L, Fletcher JG. Dual- and multi-energy CT: principles, technical approaches, and clinical applications. Radiology 2015;276(3):637–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Megibow AJ, Kambadakone A, Ananthakrishnan L. Dual-Energy Computed Tomography: Image Acquisition, Processing, and Workflow. Radiol Clin North Am 2018;56(4):507–520. [DOI] [PubMed] [Google Scholar]
- 4.Rajiah P, Parakh A, Kay F, Baruah D, Kambadakone A, Leng S. Update on Multienergy CT: Physics, Principles, and Applications. RadioGraphics 2020;40(5):1284–1308. [DOI] [PubMed] [Google Scholar]
- 5.Siegel MJ, Kaza RK, Bolus DN, et al. White Paper of the Society of Computed Body Tomography and Magnetic Resonance on Dual-Energy CT, Part 1: Technology and Terminology. J Comput Assist Tomogr 2016;40(6):841–845. [DOI] [PubMed] [Google Scholar]
- 6.Patino M, Prochowski A, Agrawal MD, et al. Material separation using dual-energy CT: current and emerging applications. RadioGraphics 2016;36(4):1087–1105. [DOI] [PubMed] [Google Scholar]
- 7.Goo HW. Dual-energy lung perfusion and ventilation CT in children. Pediatr Radiol 2013;43(3):298–307. [DOI] [PubMed] [Google Scholar]
- 8.Siegel MJ, Curtis WA, Ramirez-Giraldo JC. Effects of Dual-Energy Technique on Radiation Exposure and Image Quality in Pediatric Body CT. AJR Am J Roentgenol 2016;207(4):826–835. [DOI] [PubMed] [Google Scholar]
- 9.Zhang LJ, Wang ZJ, Zhou CS, Lu L, Luo S, Lu GM. Evaluation of pulmonary embolism in pediatric patients with nephrotic syndrome with dual energy CT pulmonary angiography. Acad Radiol 2012;19(3):341–348. [DOI] [PubMed] [Google Scholar]
- 10.Siegel MJ, Ramirez-Giraldo JC. Dual-Energy CT in Children: Imaging Algorithms and Clinical Applications. Radiology 2019;291(2):286–297. [DOI] [PubMed] [Google Scholar]
- 11.Rassouli N, Etesami M, Dhanantwari A, Rajiah P. Detector-based spectral CT with a novel dual-layer technology: principles and applications. Insights Imaging 2017;8(6):589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almeida IP, Schyns LEJR, Öllers MC, et al. Dual-energy CT quantitative imaging: a comparison study between twin-beam and dual-source CT scanners. Med Phys 2017;44(1):171–179. [DOI] [PubMed] [Google Scholar]
- 13.Euler A, Obmann MM, Szucs-Farkas Z, et al. Comparison of image quality and radiation dose between split-filter dual-energy images and single-energy images in single-source abdominal CT. Eur Radiol 2018;28(8):3405–3412. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal MD, Pinho DF, Kulkarni NM, Hahn PF, Guimaraes AR, Sahani DV. Oncologic applications of dual-energy CT in the abdomen. RadioGraphics 2014;34(3):589–612. [DOI] [PubMed] [Google Scholar]
- 15.De Cecco CN, Darnell A, Macías N, et al. Virtual unenhanced images of the abdomen with second-generation dual-source dual-energy computed tomography: image quality and liver lesion detection. Invest Radiol 2013;48(1):1–9. [DOI] [PubMed] [Google Scholar]
- 16.Parakh A, Macri F, Sahani D. Dual-energy computed tomography: dose reduction, series reduction, and contrast load reduction in dual-energy computed tomography. Radiol Clin North Am 2018;56(4):601–624. [DOI] [PubMed] [Google Scholar]
- 17.Pelgrim GJ, van Hamersvelt RW, Willemink MJ, et al. Accuracy of iodine quantification using dual energy CT in latest generation dual source and dual layer CT. Eur Radiol 2017;27(9):3904–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ACR-ASER-SCBT-MR-SPR practice parameter for the performance of pediatric computed tomography (CT). https://www.acr.org/-/media/ACR/Files/Practice-Parameters/CT-Ped.pdf. Revised 2019. Accessed December 18, 2020.
- 19.Rigsby CK, Gasber E, Seshadri R, Sullivan C, Wyers M, Ben-Ami T. Safety and efficacy of pressure-limited power injection of iodinated contrast medium through central lines in children. AJR Am J Roentgenol 2007;188(3):726–732. [DOI] [PubMed] [Google Scholar]
- 20.Amaral JG, Traubici J, BenDavid G, Reintamm G, Daneman A. Safety of power injector use in children as measured by incidence of extravasation. AJR Am J Roentgenol 2006;187(2):580–583. [DOI] [PubMed] [Google Scholar]
- 21.Gottumukkala RV, Kalra MK, Tabari A, Otrakji A, Gee MS. Advanced CT Techniques for Decreasing Radiation Dose, Reducing Sedation Requirements, and Optimizing Image Quality in Children. RadioGraphics 2019;39(3):709–726. [DOI] [PubMed] [Google Scholar]
- 22.Tabari A, Gee MS, Singh R, et al. Reducing radiation dose and contrast medium volume with application of dual-energy CT in children and young adults. AJR Am J Roentgenol 2020;214(6):1199–1205. [DOI] [PubMed] [Google Scholar]
- 23.Yu T, Gao J, Liu ZM, et al. Contrast Dose and Radiation Dose Reduction in Abdominal Enhanced Computerized Tomography Scans with Single-phase Dual-energy Spectral Computerized Tomography Mode for Children with Solid Tumors. Chin Med J (Engl) 2017;130(7):823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canellas R, Digumarthy SR, Otrakji A, Kalra MK. Applications of DECT in thoracic oncology: evidence so far. Clin Oncol 2016;1:1148. http://www.clinicsinoncology.com/full-text/cio-v1-id1148.php. [Google Scholar]
- 25.Odisio EG, Truong MT, Duran C, de Groot PM, Godoy MC. Role of Dual-Energy Computed Tomography in Thoracic Oncology. Radiol Clin North Am 2018;56(4):535–548. [DOI] [PubMed] [Google Scholar]
- 26.Zhang LJ, Yang GF, Wu SY, Xu J, Lu GM, Schoepf UJ. Dual-energy CT imaging of thoracic malignancies. Cancer Imaging 2013;13(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SH, Hur J, Kim YJ, Lee HJ, Hong YJ, Choi BW. Additional value of dual-energy CT to differentiate between benign and malignant mediastinal tumors: an initial experience. Eur J Radiol 2013;82(11):2043–2049. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Meng X, Ye Z. Iodine quantification to characterize primary lesions, metastatic and non-metastatic lymph nodes in lung cancers by dual energy computed tomography: An initial experience. Eur J Radiol 2016;85(6):1219–1223. [DOI] [PubMed] [Google Scholar]
- 29.Yang F, Dong J, Wang X, Fu X, Zhang T. Non-small cell lung cancer: Spectral computed tomography quantitative parameters for preoperative diagnosis of metastatic lymph nodes. Eur J Radiol 2017;89:129–135. [DOI] [PubMed] [Google Scholar]
- 30.Chae EJ, Song JW, Seo JB, Krauss B, Jang YM, Song KS. Clinical utility of dual-energy CT in the evaluation of solitary pulmonary nodules: initial experience. Radiology 2008;249(2):671–681. [DOI] [PubMed] [Google Scholar]
- 31.Ko JP, Brandman S, Stember J, Naidich DP. Dual-energy computed tomography: concepts, performance, and thoracic applications. J Thorac Imaging 2012;27(1):7–22. [DOI] [PubMed] [Google Scholar]
- 32.Lu GM, Zhao Y, Zhang LJ, Schoepf UJ. Dual-energy CT of the lung. AJR Am J Roentgenol 2012;199(5 Suppl):S40–S53. [DOI] [PubMed] [Google Scholar]
- 33.Otrakji A, Digumarthy SR, Lo Gullo R, Flores EJ, Shepard JA, Kalra MK. Dual-energy CT: spectrum of thoracic abnormalities. RadioGraphics 2016;36(1):38–52. [DOI] [PubMed] [Google Scholar]
- 34.Simons D, Kachelriess M, Schlemmer HP. Recent developments of dual-energy CT in oncology. Eur Radiol 2014;24(4):930–939. [DOI] [PubMed] [Google Scholar]
- 35.McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology 2006;239(2):514–520. [DOI] [PubMed] [Google Scholar]
- 36.Silva CT, Amaral JG, Moineddin R, Doda W, Babyn PS. CT characteristics of lung nodules present at diagnosis of extrapulmonary malignancy in children. AJR Am J Roentgenol 2010;194(3):772–778. [DOI] [PubMed] [Google Scholar]
- 37.Marin D, Boll DT, Mileto A, Nelson RC. State of the art: dual-energy CT of the abdomen. Radiology 2014;271(2):327–342. [DOI] [PubMed] [Google Scholar]
- 38.Kamps SE, Otjen JP, Stanescu AL, Mileto A, Lee EY, Phillips GS. Dual-energy CT of pediatric abdominal oncology imaging: private tour of new applications of CT technology. AJR Am J Roentgenol 2020;214(5):967–975. [DOI] [PubMed] [Google Scholar]
- 39.Baxa J, Vondráková A, Matoušková T, et al. Dual-phase dual-energy CT in patients with lung cancer: assessment of the additional value of iodine quantification in lymph node therapy response. Eur Radiol 2014;24(8):1981–1988. [DOI] [PubMed] [Google Scholar]
- 40.Baxa J, Matouskova T, Krakorova G, et al. Dual-phase dual-energy CT in patients treated with erlotinib for advanced non-small cell lung cancer: possible benefits of iodine quantification in response assessment. Eur Radiol 2016;26(8):2828–2836. [DOI] [PubMed] [Google Scholar]
- 41.Ren Y, Jiao Y, Zhang L, Zheng X. Dual-Energy CT-Based Iodine Quantitation for Response Evaluation of Lung Cancers to Chemoradiation Therapy/Radiation Therapy: A Comparison with 18FDG–PET/CT-based PERCIST. Int J Radiol Oncol Biol Phys 2017;99(2 Suppl):S48–S49. [Google Scholar]


