Abstract
Recent research suggests that estrogen is protective against binge eating in adult females, and that pubertal estrogen may be critical for these effects. Nonetheless, to date, no study has examined the role of pubertal estrogen in adult binge eating phenotypes in females, potentially due to difficulties experimentally manipulating estrogen in humans to examine causal effects. We used a novel animal model to examine whether estrogen removal prior to puberty (via pre-pubertal ovariectomy (P-OVX)) increases rates of binge eating prone (BEP) phenotypes in adulthood in females. A total of 77 P-OVX and 79 intact rats were followed from pre-puberty into adulthood and phenotyped for BEP status in adulthood. Results showed significantly increased rates (~2–8x higher) of adult BEP phenotypes in P-OVX as compared to intact rats. Findings confirm that estrogen removal substantially increases later risk for binge eating in females, potentially by disrupting typical adolescent brain development.
Keywords: binge eating, estrogen, progesterone, animals, puberty
Emerging data suggest that estrogen may be protective against binge eating in females in adulthood. Initial indications of significant estrogen effects come from 40+ years of experimental animal studies showing that the removal of estrogen (via ovariectomy (OVX)) in adulthood leads to immediate, sustained increases in food intake (Asarian & Geary, 2013). These increases are reversed with exogenous estradiol administration (Asarian & Geary, 2013). Studies of estrogen effects on binge eating are fewer. However, initial data show increased rates of binge eating on palatable food (PF; foods that are high in fat and sugar) in female rats ovariectomized in adulthood (Klump, Suisman, Culbert, Kashy, Keel, et al., 2011; Micioni Di Bonaventura et al., 2017) that are reversed following exogenous estradiol treatment (Micioni Di Bonaventura et al., 2017; Yu, Geary, & Corwin, 2008).
Studies in humans show the same pattern of results. These studies largely focus on changes in food intake and binge eating across the menstrual cycle as a quasi-experimental design for detecting hormone effects. Natural, menstrual cycle-related changes in hormones predicting changes in binge eating suggest an effect of hormones on food intake rather than the reverse. Lower levels of food intake, binge eating, and emotional eating are observed in the first half of the cycle when estradiol levels are elevated, and higher levels of these behaviors are found in the second half of the cycle when estradiol levels are relatively lower and progesterone levels (that antagonize the protective effects of estrogen – see Asarian & Geary, 2013) are high (Baker, Girdler, & Bulik, 2013; Harden, Kretsch, Moore, & Mendle, 2014; Klump, Culbert, & Sisk, 2017; Klump, Keel, et al., 2013; Klump et al., 2014). Follow-up analyses have confirmed that lower estradiol levels and/or interactions between estradiol and progesterone significantly predict binge eating phenotypes (Edler, Lipson, & Keel, 2007; Klump, Keel, et al., 2013; Klump et al., 2014).
Taken together, animal and human studies converge in suggesting a protective effect of estrogen on binge eating in females in adulthood. However, emerging data indicate that these protective effects are not present earlier in development (Asarian & Geary, 2013; Klump, Culbert, & Sisk, 2017). For example, exogenous estradiol treatment fails to alter chow intake in pre-pubertal female rats (Asarian & Geary, 2013), and differences in PF intake between female rats that are prone versus resistant to binge eating only emerge during mid-late puberty (Culbert, Sinclair, Hildebrandt, Klump, & Sisk, 2018; Klump, Suisman, Culbert, Kashy, & Sisk, 2011). Likewise, although rates of binge eating increase during mid-late puberty in girls, there are minimal associations between estradiol levels and binge eating prior to adulthood (Harden et al., 2014; Klump, 2013; Klump et al., 2018; Klump, Keel, Sisk, & Burt, 2010).
The differing effects of estradiol in pre-pubertal versus adult females suggest differential roles for estrogen in risk for binge eating across development. We believe that these differences can be explained, in part, by organizational (i.e., hormonally-induced effects that permanently alter brain structure/function) versus activational (i.e., temporary changes caused by the activating, modulating, and/or inhibiting effects of circulating hormones) gonadal hormone effects (Culbert, Sisk, & Klump, 2018; Klump, Culbert, & Sisk, 2017; Klump et al., 2018; Sisk & Zehr, 2005). During critical periods of development (i.e., prenatal period, puberty), gonadal hormones permanently organize and sculpt brain structure and/or function to facilitate responsiveness to the activational effects of gonadal hormones later in adulthood (particularly in sexually differentiated behaviors, like binge eating). Because hormonally induced adult behaviors are dependent upon neural organization, hormone-behavior associations are not observed before or during the period of development but emerge after organization has occurred (Schulz, Molenda-Figueira, & Sisk, 2009; Sisk & Zehr, 2005). Critically, if hormones are absent during key periods of development, then organization will not occur, and the adult-typical behavior will be permanently altered (Schulz et al., 2009; Sisk & Zehr, 2005). Alternatively, if hormones are present during the critical period, but absent in adulthood, the level at which the behavior occurs may change, but the behavior itself will have been encoded and will occur in the expected adult or sex-typical manner (Schulz et al., 2009; Sisk & Zehr, 2005).
As noted above, the developmental differences for binge eating in females fit some of these patterns. No significant phenotypic estrogen-binge eating associations are observed before/during puberty in female animals or humans (Asarian & Geary, 2013; Klump et al., 2010, 2018), but significant associations are present in adulthood (Edler et al., 2007; Klump, Keel, et al., 2013; Klump et al., 2014; Yu et al., 2008). Although adult OVX causes increases in binge eating in all female rats (Klump, Suisman, Culbert, Kashy, Keel, et al., 2011), we found previously that binge eating prone (BEP) rats continue to binge eat more relative to binge eating resistant (BER) rats after adult OVX. The maintenance of the BER/BEP group status even after adult OVX suggests that binge eating proneness might be permanently organized during earlier periods of development.
Interestingly, twin data from our lab suggest that estrogen may exert protective organizational effects during puberty in females through its genomic actions in the central nervous system; specifically, higher levels of estradiol were associated with significantly lower heritability of binge eating during puberty, whereas lower levels of estradiol were associated with significantly stronger heritability during puberty in girls (Klump et al., 2018). These data suggest that previously observed increases in the heritability of binge eating during puberty (Klump, Culbert, O’Connor, Fowler, & Burt, 2017) may be driven by subsets of girls who have comparatively lower levels of estradiol during puberty. Once again, phenotypic associations between estradiol and binge eating were minimal (Klump et al., 2018), suggesting that differences in genetic effects between low/high estradiol groups might reflect pubertal organizational changes that predate hormone-binge eating phenotypic associations observed in adulthood. Importantly, these and prior analyses (Klump et al., 2010) failed to find any significant phenotypic or genetic effects of progesterone on risk for binge eating in girls during puberty; thus, although progesterone is important for binge eating in adulthood in women (see Edler et al., 2007; Klump, Keel, et al., 2013; Klump et al., 2014), estrogen appears to be the primary driver of pubertal organizational effects in females.
Clearly, additional tests are needed to confirm the presence of protective organizational effects of pubertal estrogen on binge eating in females. Animal studies are ideal for examining these influences, as ovarian hormones can be directly manipulated to test effects on behavior. One promising model is the binge eating resistant (BER)/binge eating prone (BEP) rat model (Boggiano et al., 2007) that has been used in previous studies of activational/organizational hormone effects (Culbert, Sinclair, et al., 2018; Klump, Suisman, Culbert, Kashy, Keel, et al., 2011; Klump, Suisman, Culbert, Kashy, & Sisk, 2011). This model identifies BER and BEP female rats based on their consumption of intermittently presented, highly PF in adulthood and shows good face validity for the binge eating that occurs in binge eating-related disorders (i.e., binge-eating disorder (BED), bulimia nervosa (BN)) in women (see Methods for additional information on model validity).
Using the BER/BEP model, the aim of this study was to conduct an initial test of the organizational theories described above. A subset of female rats underwent removal of ovarian hormones prior to puberty (via pre-pubertal ovariectomy, P-OVX), and rates of BEP phenotypes were compared between P-OVX versus intact rats in adulthood. In these analyses, a protective, organizational effect of ovarian hormones in females would be suggested if rates of BEP phenotypes were significantly higher in adult rats that underwent pre-pubertal OVX as compared to intact female rats. In combination with findings in adulthood (Klump, Suisman, Culbert, Kashy, Keel, et al., 2011), this would imply a significant effect of pre-pubertal OVX on binge eating phenotypes in females that does not occur when ovarian hormones are simply removed in adulthood.
We were also interested in exploring when any group differences (i.e., P-OVX versus intact) in binge eating emerge across development. If ovarian hormones “organize” protection against BEP phenotypes during adolescent brain development in females, then P-OVX/intact group differences in binge eating should emerge during the adolescent period, with P-OVX rats exhibiting substantially increased rates of binge eating starting in adolescence rather than during pre-adolescence or only in adulthood. These data would highlight the adolescent time period as critical to the effects of P-OVX on binge eating.
Methods
Animals
We conducted our tests in three separate experiments to demonstrate replicability of effects and ensure adequate sample sizes for some of our exploratory analyses (see Statistical Analyses below).
A total of 160 weanling female Sprague-Dawley rats across the three experiments (Experiment 1 n = 60, Experiment 2 n = 40, Experiment 3 n = 60) were obtained from Harlan (Madison, Wisconsin) on postnatal day 21. Animals were singly housed in clear Plexiglas cages (45 × 23 × 21 cm) and given ad lib access to water and chow (Rodent diet 8640; Harlan Teklad Global Diets, Madison, WI). Animals were maintained on a 12/12hr light-dark cycle, and the temperature was maintained at 21 ± 2°C. All animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and the protocol (#06/13-135-00, Development of an Animal Model for the Study of Disordered Eating) was approved by the Michigan State University Institutional Animal Care and Use Committee.
Experimental Design
With few exceptions (see below), the experimental design was identical across the three experiments.
Pre-pubertal OVX and sham surgeries.
Three days following arrival in the lab (i.e., postnatal day 24), half of the rats in each experiment were randomized to receive bilateral, pre-pubertal OVX surgery (“P-OVX” rats) while the other half received a sham surgery (“intact” rats). Surgeries were performed under isofluorane anesthesia, and animals received 5mg/kg of ketoprofen analgesia during surgery and 24 hours after surgery. All animals were given at least three days to recover prior to feeding tests. Three P-OVX and one intact rat died before feeding tests began. Thus, final sample sizes were 77 P-OVX rats (Experiment 1 n = 29, Experiment 2 n = 20, Experiment 3 n = 28) and 79 intact rats (Experiment 1 n = 30, Experiment 2 n = 20, Experiment 3 n = 29).
Definitions of puberty and adulthood.
All intact and P-OVX rats were monitored daily for vaginal opening following OVX/sham surgeries. The age at which vaginal opening occurred was used as the indicator of puberty for the intact rats; P-OVX rats were monitored for vaginal opening to expose all rats to similar handling procedures. Definitions of pre-early puberty (no vaginal opening) and mid-late puberty (vaginal opening had occurred) were therefore specific to each intact rat. All intact rats were “pre-pubertal” until postnatal day 31, at which time 17.7% of intact rats experienced vaginal opening. Vaginal opening occurred in all remaining intact rats (82.3%) between postnatal days 31 and 35. Because vaginal opening is an estrogen-dependent process that does not occur in P-OVX rats, P-OVX rats were categorized as “pre-pubertal” until the day on which approximately half of the intact rats from their experiment had experienced vaginal opening. On that day, all of the P-OVX rats in the experiment were categorized as “pubertal”.
There are no physical indicators of the end of puberty in female rats; thus, we used standard classifications of adulthood (Spear, 2000) to define the end of puberty/start of adulthood. We used an earlier day in Experiment 2 (i.e., postnatal day 58) than in the other experiments (i.e., postnatal day 60) to ensure an adequate number of adult feeding tests for defining BER/BEP status, as Experiment 2 experienced truncated data collection due to availability of animal cages/lab equipment.
BER/BEP model and procedures.
BER/BEP model.
Several aspects of the BER/BEP model mimic features of binge eating in humans. BEP rats binge eat more on highly PF compared to BER rats, but they do not differ from BER rats in their intake of chow (Boggiano et al., 2007; Oswald, Murdaugh, King, & Boggiano, 2011). BEP rats are not significantly different from BER rats in body weight or rates of diet-induced obesity (Boggiano et al., 2007). BEP rats seem to experience a lack of control over their binge eating episodes, as they endure increasingly high levels of pain (via foot shock) in order to consume PF (Oswald et al., 2011). In women, binge eating episodes are intermittent and discrete (i.e., lasting only a few hours); the BER/BEP framework models this time course by alternating feeding test days with chow only days and focusing on the first 4 hours of PF access (Boggiano et al., 2007). The distribution of binge eating in the BER/BEP model also resembles that observed in women, as there is a continuum of binge eating (from low to high levels), but the model also identifies rats that are particularly resistant or prone to binge eating (Boggiano et al., 2007). Finally, more female than male rats are categorized BEP, and BEP phenotypes emerge during puberty (Culbert, Sinclair, et al., 2018; Klump, Suisman, Culbert, Kashy, & Sisk, 2011).
Procedures.
Feeding tests.
Feeding tests followed standard protocols (Boggiano et al., 2007). A premeasured amount of PF was provided to all rats just prior to lights out. Water and standard rat chow (~70–80 grams; 29% protein, 17% fat, 54% carbohydrates; Rodent diet 8640; Harlan Teklad Global Diets, Madison, Wisconsin) remained freely available for the duration of the feeding test. After 4 hours of PF exposure1, all remaining PF and chow were weighed, and cage bedding was searched to ensure that all PF and chow were included in the measurement. Body weight was assessed just prior to lights out, and chow/water was provided ad lib and measured prior to lights out on all non-feeding test days.
The type of PF used during the feeding tests differed across the three experiments. In Experiment 1, ~25 grams of Betty Crocker Vanilla Frosting (4.24 kcal/g, 4% protein kcal, 18% fat kcal, 82% carbohydrate kcal; General Mills Inc., Minneapolis, MN) was placed in a petri dish that was secured to a wire hook hung over the cage wall. This is the same PF that has been used in past BER/BEP experiments in our lab (e.g., Klump, Suisman, Culbert, Kashy, & Sisk, 2011). In Experiments 2 and 3, we placed ~30 grams of high-fat pellets (Experiment 2; Research Diets #D12451, 4.73 kcal/g, 20% protein kcal, 45% fat kcal, 35% carbohydrate kcal; Research Diets Inc., New Brunswick, NJ) or high fat/high sugar pellets (Experiment 3; Research Diets D01120301B, custom-made, 4.58 kcal/g, 4% protein kcal, 40% fat kcal, 56% carbohydrate kcal) in a ceramic dish on the floor of each rat’s cage to determine if the type of PF used significantly affected BEP status. PF amounts were converted from grams to total kcals to equate intake across experiments. As described below, results were consistent across experiments/PF type, a finding that has been observed in previous studies of the BER/BEP phenotypes in adulthood (Boggiano et al., 2007).
Feeding tests were conducted 3x/week beginning on postnatal day 28. Because of individual differences in the onset of puberty and data collection length, the number of feeding tests per developmental stage differed somewhat across rats, with rats receiving 2–3 feeding tests in pre-puberty, 10–12 feeding tests in mid-late puberty, and 5–7 feeding tests in adulthood.
BER/BEP definitions.
We followed methods previously described (Boggiano et al., 2007) and used in our lab (e.g., Klump, Suisman, Culbert, Kashy, & Sisk, 2011) to identify BER/BEP phenotypes in adulthood. We calculated tertiles of 4-hour PF intake for each adult feeding test. After establishing tertiles for PF intake on each feeding test day, we identified BER rats as those that consistently ate in the lowest tertile of PF intake and never ate in the highest tertile for any feeding test. By contrast, BEP rats were those that consistently ate in the highest tertile of PF intake on testing days, and never ate in the lowest tertile during any feeding test.
Past studies frequently used a 50–67% criterion for defining “consistent” intake (e.g., BEP rats had to consume in the highest tertile in 3/6 (50%) feeding tests, and never in the lowest tertile) (Culbert, Sinclair, et al., 2018; Hildebrandt, Klump, Racine, & Sisk, 2014; Klump, Racine, Hildebrandt, & Sisk, 2013; Klump, Suisman, Culbert, Kashy, & Sisk, 2011; Sinclair et al., 2015). We used a similar definition and required that rats consume in the highest or lowest tertile of PF intake on 57–67% of the adult feeding tests to meet our BEP and BER criteria, respectively. Because number of adult feeding tests varied somewhat across the three experiments, we calculated tertiles separately within each experiment and used a cut-off of 4/7 tests (57%) for Experiment 1, 3/5 tests (60%) for Experiment 2, and 4/6 tests (67%) for Experiment 3 for defining BER/BEP phenotypes.
Notably, categorical BER/BEP groupings result in loss of data, as rats that score intermediate to these extremes are excluded from analyses. We therefore also calculated a continuous “binge proneness” variable (see Culbert, Sinclair, et al., 2018; Klump, Suisman, Culbert, Kashy, Keel, et al., 2011; Klump, Suisman, Culbert, Kashy, & Sisk, 2011) that counts the number of times all rats scored in the top tertile of PF intake during the adult tests. We controlled for the varying number of adult feeding tests across experiments by calculating “proportion” variables (i.e., BEP proportion = number of times the rat scored in the top tertile/number of feeding tests) separately within each experiment. Because these analyses are confirmatory, data tables and figures are included in the Supplemental Material available online, but the overall findings are discussed in the Results section below.
Statistical Analyses
Analyses in adulthood: Rates of BER/BEP phenotypes across P-OVX/intact status.
We compared rates of BER versus BEP categorical phenotypes in adulthood in P-OVX versus intact rats using Z tests of proportions and odds ratios (ORs). The ORs indexed the extent to which rats in the P-OVX group were at increased risk of having a BEP phenotype. T-tests were then used to compare the binge proneness proportion variable across P-OVX/intact groups.
We conducted these analyses separately in rats from each of the three experimental groups, as well as in a “Combined” sample that aggregated samples across the three experiments. Rates of BER/BEP phenotypes in adulthood can be quite low (i.e., ranging from ~10–30%; Boggiano et al., 2007; Culbert, Sinclair, et al., 2018; Hildebrandt, Klump, Racine, & Sisk, 2014; Klump, Racine, Hildebrandt, & Sisk, 2013; Klump, Suisman, Culbert, Kashy, Keel, et al., 2011; Klump, Suisman, Culbert, Kashy, & Sisk, 2011; Sinclair et al., 2015), which is particularly problematic in the current study where BER/BEP rats are further subdivided by P-OVX/intact status. Our approach of conducting analyses in combined and individual experimental groups allowed us to maximize statistical power to detect group differences while also demonstrating replicability of effects.
Exploratory analyses: Developmental emergence of binge eating prone phenotypes.
We used mixed linear models (MLM) to conduct exploratory analyses to examine when during development BER/BEP and P-OVX/intact group differences emerged. These models predicted PF consumption as a function of pubertal status (i.e., pre-puberty versus puberty), BER/BEP group, P-OVX/intact status, and all 2- and 3-way interactions. Sample sizes would be too small to conduct these exploratory models in each experiment separately – thus, we used the “Combined” sample only for all of these exploratory models. However, we included the raw data for each individual experiment in the Supplemental Material available online (see below and Supplemental Material) to show general replicability of effects, and we included a categorical “experiment” variable as a covariate in all MLMs to control for potential differences in PF intake across experiments (see Table S1 in the Supplemental Material for PF intake by experiment). We also included “study day” and the experiment x study day interaction in all models to control for expected increases in PF intake across adolescent development. Because BER/BEP groups and the binge proneness proportion variable were defined using adult PF values, we did not include the adult PF data in these analyses. Including the adult data could artificially increase the chances of detecting significant group differences and/or obscure differences across pre-puberty and puberty.
In the MLMs, we used an autoregressive (lag 1) error structure to model the residual covariance from one feeding trial to the next. The upper-level unit of analysis was the rat, and the lower-level unit of analysis was the feeding test. In analyses of the BER/BEP categorical variable, P-OVX/intact status and BER/BEP groups were upper-level predictors, and pre-puberty/puberty was a lower-level predictor. Each predictor was treated as categorical, and results are presented as estimated marginal means and F-tests. In analyses of the binge proneness proportion variable, P-OVX/intact status was an upper-level predictor, and pre-puberty/puberty was the lower-level predictor. P-OVX/intact status and pre-puberty/puberty were effect coded, the binge proneness proportion variable was grand mean centered, and results are presented as regression coefficients and t-tests.
Results
Analyses in Adulthood: Rates of BER/BEP Phenotypes across P-OVX/Intact Status
Rates of adult BEP phenotypes were significantly higher in P-OVX than in intact rats and rates of BER phenotypes were significantly lower in the Combined sample (BEP OR = 8.20; BER OR = 0.18) as well as in all three experiments (see Table 1). Identical findings were obtained for the binge proneness proportion variable, as the P-OVX rats scored significantly higher than the intact rats in all samples/experiments (all p’s < .01; see Table S2 in Supplemental Material). Notably, in analyses of the Combined sample, we included the “experiment” variable as a covariate (via ANCOVAs); consequently, these group differences were independent of any experiment differences in PF intake. As a final check on the robustness of these results, we followed procedures in previous studies (e.g., Klump, Racine, et al., 2013) and conducted post hoc analyses using more lenient (i.e., bottom/top tertile in 40–50% of tests) and more strict (i.e., bottom/top tertile in 71–83% of tests) definitions of the categorical BER/BEP variable. We again found significantly higher rates of BEP, and lower rats of BER, phenotypes in P-OVX rats (see Table 1).
Table 1.
Rates of Adult BER/BEP Phenotypes in Intact versus Pre-Pubertal Ovariectomy (P-OVX) Rats.
| Primary Definition (57–67%) |
Less Strict Definition (40–50%) |
More Strict Definition (71–83%) |
||||
|---|---|---|---|---|---|---|
| Groups | BER | BEP | BER | BEP | BER | BEP |
| Combined Sample (N = 79 Intact, 77 P-OVX) | ||||||
| Raw PF Values | ||||||
| Intact | 35% (28/79) |
8% (6/79) |
46% (36/79) |
10% (8/79) |
28% (22/79) |
6% (5/79) |
| P-OVX | 9% (7/77) |
40% (31/77) |
12% (9/77) |
47% (37/77) |
5% (4/77) |
31% (24/77) |
| Z Test of Proportions | 3.70 | 4.35 | 4.71 | 4.82 | 3.42 | 3.63 |
| p value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| P-OVX Odds Ratio (95% CI) |
0.18 (0.07–0.45) |
8.20 (3.17–21.18) |
0.16 (0.07–0.36) |
8.21 (3.48–19.34) |
0.14 (0.05–0.44) |
6.70 (2.40–18.70) |
| Weight-Standardized Values | ||||||
| Intact | 24% (19/79) |
11% (9/79) |
30% (24/79) |
18% (14/79) |
19% (15/79) |
8% (6/79) |
| P-OVX | 15% (12/77) |
23% (18/77) |
21% (16/77) |
32% (25/77) |
14% (11/77) |
22% (17/77) |
| Z Test of Proportions | 1.32 | 1.94 | 1.37 | 2.10 | 0.79 | 2.45 |
| p value | .19 | .05 | .17 | .04 | .79 | .01 |
| P-OVX Odds Ratio (95% CI) |
0.58 (0.26–1.30) |
2.37 (0.99–5.67) |
0.60 (0.29–1.25) |
2.23 (1.06–4.72) |
0.71 (0.30–1.66) |
3.45 (1.28–9.29) |
| Experiment #1 (N = 30 Intact, 29 P-OVX) | ||||||
| Raw PF Values | ||||||
| Intact | 33% (10/30) |
13% (4/30) |
37% (11/30) |
17% (5/30) |
33% (10/30) |
13% (4/30) |
| P-OVX | 7% (2/30) |
37% (11/30) |
13% (4/30) |
40% (12/30) |
3% (1/30) |
33% (10/30) |
| Z Test of Proportions | 2.58 | 2.09 | 2.09 | 2.01 | 3.00 | 1.83 |
| p value | <.001 | .04 | .04 | .04 | .003 | .06 |
| P-OVX Odds Ratio (95% CI) |
0.14 (0.03–0.72) |
3.76 (1.04–13.65) |
0.27 (0.07–0.97) |
3.16 (0.95–10.50) |
0.07 (0.01–0.58) |
3.25 (0.89–11.90) |
| Weight-Standardized Values | ||||||
| Intact | 27% (8/30) |
13% (4/30) |
30% (9/30) |
17% (5/30) |
23% (7/30) |
13% (4/30) |
| P-OVX | 20% (6/30) |
23% (7/30) |
23% (7/30) |
27% (8/30) |
20% (6/30) |
23% (7/30) |
| Z Test of Proportions | 0.61 | 1.00 | 0.58 | 0.94 | 0.31 | 1.00 |
| p value | .54 | .32 | .56 | .35 | .76 | .32 |
| P-OVX Odds Ratio (95% CI) |
0.69 (0.21–2.30) |
1.97 (0.51–7.64) |
0.71 (0.22–2.25) |
1.82 (0.52–6.38) |
0.82 (0.24–2.81) |
2.13 (0.55–8.19) |
| Experiment #2 (N = 20 Intact, 20 P-OVX) | ||||||
| Raw PF Values | ||||||
| Intact | 45% (9/20) |
0% (0/20) |
60% (12/20) |
5% (1/20) |
30% (6/20) |
0% (0/20) |
| P-OVX | 10% (2/20) |
45% (9/20) |
15% (3/20) |
60% (12/20) |
5% (1/20) |
40% (8/20) |
| Z Test of Proportions | 2.48 | 3.41 | 2.94 | 3.71 | 2.08 | 3.16 |
| p value | .01 | <.001 | .003 | .002 | .04 | .002 |
| P-OVX Odds Ratio (95% CI) |
0.14 (0.02–0.75) |
33.87 (1.80–636.91) |
0.12 (0.03–0.54) |
28.50 (3.15–257.45) |
0.12 (0.01–1.14) |
27.88 (1.48–526.14) |
| Weight-Standardized Values | ||||||
| Intact | 30% (6/20) |
10% (2/20) |
45% (9/20) |
20% (4/20) |
20% (4/20) |
0% (0/20) |
| P-OVX | 15% (3/20) |
35% (7/20) |
20% (2/20) |
50% (10/20) |
10% (2/20) |
35% (7/20) |
| Z Test of Proportions | 1.14 | 1.89 | 2.48 | 1.99 | 0.89 | 2.91 |
| p value | .25 | .05 | .01 | .04 | .37 | .004 |
| P-OVX Odds Ratio (95% CI) |
0.41 (0.09–1.95) |
4.85 (0.86–27.22) |
0.14 (0.02–0.75) |
4.00 (0.98–16.27) |
0.44 (0.07–2.76) |
22.78 (1.20–432.61) |
| Experiment #3 (N = 29 Intact, 28 P-OVX) | ||||||
| Raw PF Values | ||||||
| Intact | 31% (9/29) |
7% (2/29) |
45% (13/29) |
7% (2/29) |
21% (6/29) |
3% (1/29) |
| P-OVX | 11% (3/28) |
39% (11/28) |
14% (2/28) |
46% (13/28) |
7% (2/28) |
21% (6/28) |
| Z Test of Proportions | 1.88 | 2.91 | 3.23 | 3.39 | 1.47 | 2.07 |
| p value | .06 | .004 | .001 | <.001 | .14 | .04 |
| P-OVX Odds Ratio (95% CI) |
0.35 (0.08–1.41) |
5.70 (1.16–28.04) |
0.16 (0.03–0.77) |
6.73 (1.39–32.58) |
0.35 (0.06–1.86) |
6.21 (0.70–54.96) |
| Weight-Standardized Values | ||||||
| Intact | 17% (5/29) |
10% (3/29) |
21% (6/29) |
17% (5/29) |
14% (4/29) |
7% (2/29) |
| P-OVX | 11% (3/28) |
14% (4/28) |
25% (7/28) |
25% (7/28) |
11% (3/28) |
11% (3/28) |
| Z Test of Proportions | 0.71 | 0.45 | 0.39 | 0.72 | 0.35 | 0.51 |
| p value | .48 | .65 | .70 | .47 | .73 | .61 |
| P-OVX Odds Ratio (95% CI) |
0.58 (0.12–2.68) |
1.44 (0.29–7.13) |
1.28 (0.37–4.42) |
1.60 (0.44–5.80) |
0.75 (0.15–3.70) |
1.62 (0.25–10.51) |
Note. BER = binge eating resistant; BEP = binge eating prone; Intact = ovaries intact; Primary Definition = rats had to score in the lowest/highest tertile in 57–67% of feeding tests to be categorized as BER/BEP; Less Strict Definition = rats had to score in the lowest/highest tertile in 40–50% of the tests to be categorized as BER/BEP; More Strict Definition = rats had to score in the lowest/highest tertile in 71–83% of the feeding tests to be categorized as BER/BEP; PF = palatable food; 95% CI = 95% confidence interval. Significant and trend-level Z tests are bolded.
Nonetheless, rats that have undergone OVX have previously been found to consume significantly more chow and have higher body weights than intact rats (Asarian & Geary, 2013). Thus, we re-calculated tertiles and BER/BEP groups using weight standardized PF values (intake/exp(0.66 * ln(BW); see Babbs, Wojnicki, & Corwin, 2012). Odds ratios continued to show rates of BEP phenotypes in the P-OVX group that were substantially higher than in the intact group (e.g., Combined sample ORs = 2.37–3.45; see Table 1). This pattern of findings was replicated in analyses of the binge proneness proportion variable, although effects were somewhat attenuated (e.g., d = .15, p = .34 in the Combined sample) and more variable (see Table S2 in Supplemental Material). Notably, in all analyses moving forward, we used weight standardized PF values for defining the BER/BEP groups and the binge proneness proportion variable. This approach ensured that our BER/BEP definitions used the most conservative, weight-standardized values. It was also important to do given the strong correlations between BER/BEP categorical group and P-OVX/intact status when using raw PF values to define groups (r = .65) as compared to weight-standardized BER/BEP groups (r = .28). However, in terms of the PF outcome variables, we included results for both the raw and weight-standardized data to show replicability of effects.
Taken together, data in adulthood suggested that P-OVX shifts the distribution of PF intake toward a BEP phenotype and away from a BER phenotype in adulthood. The stronger results for the BEP versus the BER phenotype and the categorical (versus proportion) variables further suggested that this effect might be potentiated in BEP rats. An important check for this possibility was to examine whether P-OVX produces greater increases in PF intake in BEP than in BER rats, and whether this effect is specific to PF (rather than PF and chow).
Table S3 includes results from MLMs examining the effects of P-OVX/intact status, BER/BEP group, and their interaction on average PF intake, chow intake, and body weight in adulthood. Because of the need to examine interactions in these analyses (and the corresponding need for increased statistical power), MLMs were only conducted in the Combined sample, but raw data by experiment are provided in Table S4 in Supplemental Material. There were significant P-OVX x BER/BEP group interactions for raw (F(1, 103) = 11.48; p < .001) and weight-standardized (F(1, 122) = 5.70; p < .05) PF intake, with P-OVX BEP rats consuming more raw and weight-standardized PF than any other group (Raw PF [M (SD)]: BEP P-OVX = 49.65 (0.79), BEP Intact = 41.30 (1.10), BER P-OVX = 28.17 (0.96), BER Intact = 25.94 (0.76); Weight-Standardized PF [M (SD)]: BEP P-OVX = 1.34 (0.02), BEP Intact = 1.27 (0.02), BER P-OVX = 0.79 (0.02), BER Intact = 0.82 (0.02)). Importantly, the P-OVX effect was specific to BEP rats; P-OVX BER and intact BER rats consumed comparable amounts of PF (see Table S3 in Supplemental Material). There were no significant P-OVX x BER/BEP group interactions for chow intake or body weight (all p’s > .05), suggesting that the effects of P-OVX on chow intake and body weight did not vary by BER/BEP status. Overall, these findings confirmed that pre-pubertal OVX has a specific effect on PF intake in adult BEP rats that is not observed for chow intake or body weight.
Exploratory Analyses: Developmental Emergence of Binge Eating Prone Phenotypes
Findings from the MLMs exploring the developmental emergence of BEP phenotypes are presented in Table 2 and Figures 1 and 2. In terms of raw PF intake, there were no significant main effects of P-OVX or pubertal status, although the main effect of BER/BEP status was significant (BEP rats consumed more PF overall than BER rats; see Table 2 and Figure 12). However, significant pubertal status x BER/BEP phenotype interactions were observed (F(1, 684) = 13.18, p < .001, d = .28; see Table 2), such that there was a small but significant difference in raw PF intake between BER and BEP rats before puberty (Mdiff = 2.11, se = .79, F(1, 385) = 7.11, p = .008, d = .27), but after puberty, this difference was much more substantial (Mdiff = 5.01, se = .53, F(1, 117) = 90.86, p < .001, d = 1.76). This finding was also present for the weight-standardized PF values (see Table 2) and replicates previous work showing a pubertal emergence of BEP phenotypes in female rats (Culbert, Sinclair, et al., 2018; Klump, Suisman, Culbert, Kashy, & Sisk, 2011).
Table 2.
Mixed Linear Models Examining Differences in Palatable Food Intake, Chow Intake, and Body Weight across Pre-Pubertal Ovariectomy (P-OVX) Status, Pubertal Status, and BER/BEP Phenotypes.
| Pre-Puberty | Puberty | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Intact M (SE) | P-OVX M (SE) | Intact M (SE) | P-OVX M (SE) | P-OVX Status F (df) | Pub Status F (df) | BER/BEP Status F (df) | Pub x P-OVX F (df) | P-OVX x BER/BEP F (df) | Pub x BER/BEP F (df) | Pub x P-OVX x BER/BEP F (df) |
| Raw Intake Values (kcals) | |||||||||||
| Palatable Food | |||||||||||
| BER | 22.66 | 22.91 | 21.70 | 20.69 | |||||||
| (1.38) | (169) | (0.89) | (112) | 2.38 | 1.99 | 43.49** | 2.01 | 3.56 | 13.18** | 4.85* | |
| BEP | 26.59 | 27.41 | 27.91 | 34.51 | (155) | (780) | (155) | (686) | (155) | (684) | (686) |
| (197) | (1.41) | (128) | (0.92) | ||||||||
| Chow | |||||||||||
| BER | 4.05 | 6.35 | 8.12 | 10.58 | |||||||
| (0.87) | (102) | (0.38) | (0.48) | 15.96** | 53.47** | .00 | 2.89 | .30 | 1.81 | 2.38 | |
| BEP | 4.44 | 4.57 | 8.30 | 11.78 | (337) | (528) | (337) | (454) | (336) | (453) | (453) |
| (122) | (0.88) | (0.54) | (0.39) | ||||||||
| Weight-Standardized Values (kcals) | |||||||||||
| Palatable Food | |||||||||||
| BER | 0.92 | 0.89 | 0.89 | 0.80 | |||||||
| (0.05) | (0.06) | (0.03) | (0.04) | <.001 | 1.67 | 40.45** | 1.01 | 2.84 | 13.93** | 4.19* | |
| BEP | 1.04 | 1.01 | 1.10 | 1.26 | (201) | (750) | (200) | (606) | (200) | (604) | (605) |
| (0.07) | (0.05) | (0.04) | (0.03) | ||||||||
| Chow | |||||||||||
| BER | 0.17 | 0.29 | 0.34 | 0.40 | |||||||
| (0.03) | (0.04) | (0.02) | (0.02) | 10.73** | 44.90** | .07 | .14 | 1.10 | 2.47 | 3.47 | |
| BEP | 0.19 | 0.19 | 0.35 | 0.44 | (348) | (512) | (348) | (449) | (347) | (448) | (448) |
| (0.05) | (0.03) | (0.02) | (0.02) | ||||||||
| Body Weight (g) | |||||||||||
| BER | 124.66 | 135.88 | 125.16 | 141.64 | |||||||
| (2.57) | (3.23) | (2.35) | (2.97) | 26.35** | 9.68** | .24 | 5.40* | .01 | .08 | .07 | |
| BEP | 125.22 | 137.44 | 126.81 | 143.26 | (58) | (706) | (58) | (770) | (58) | (770) | (769) |
| (3.72) | (2.65) | (3.40) | (2.42) | ||||||||
Note. Intact = ovaries intact; BER = binge eating resistant; BEP = binge eating prone. Sample sizes: Intact BER N = 19, Intact BEP N = 9, P-OVX BER N = 12, P-OVX BEP N = 18. Values are estimated marginal means and standard errors (SE). “Experiment”, “study day”, and “experiment x study day” interactions were included as covariates in all models. Significant effects are bolded.
p ≤ .05,
p < .01
Figure 1. Mean Raw PF Intake (top panel), Raw Chow Intake (middle panel) and Body Weight (bottom panel) as a function of Pre-Pubertal Ovariectomy (P-OVX) and Binge Eating Resistant (BER) and Binge Eating Prone (BEP) Status across Feeding Test Days in Adolescence and Adulthood.
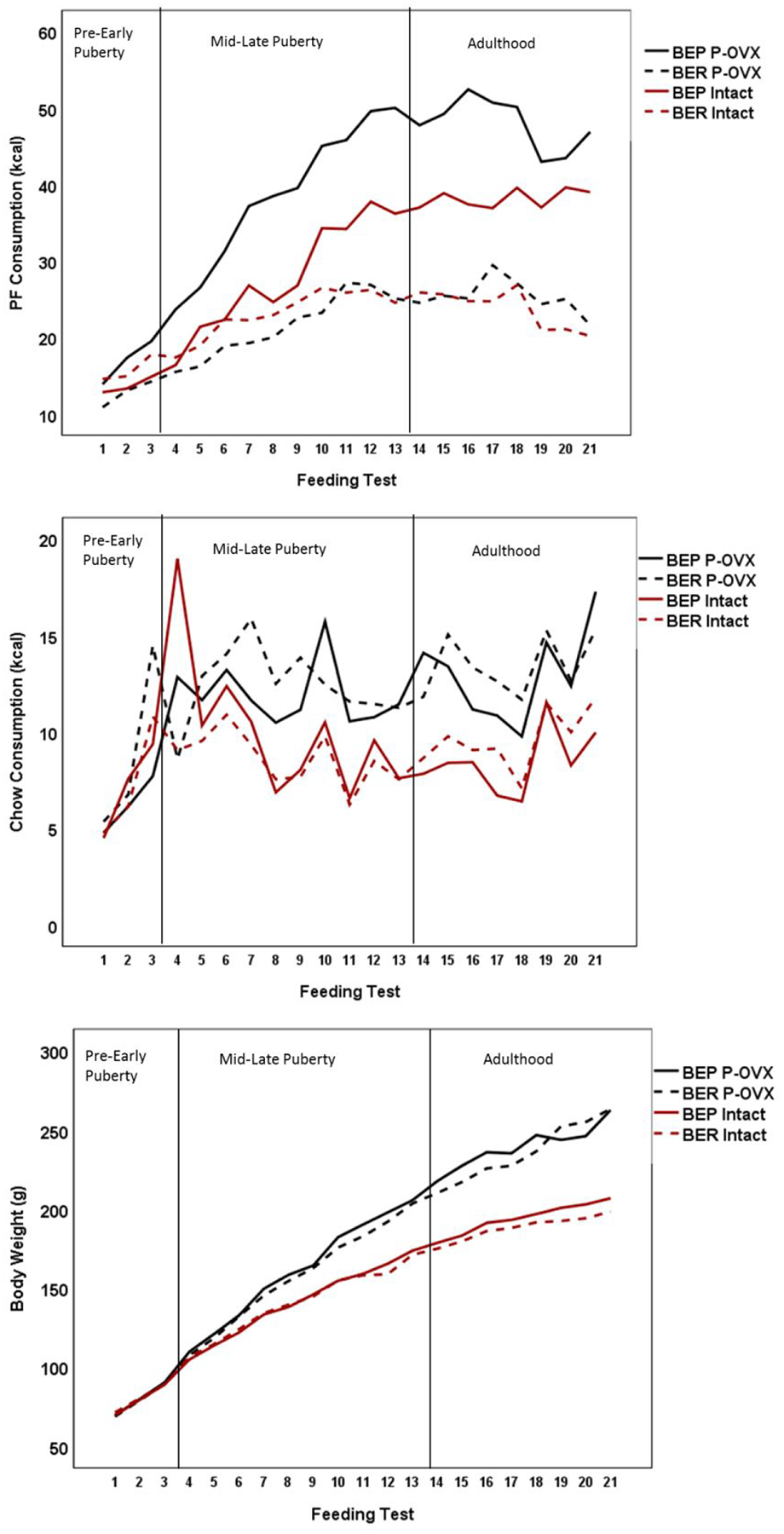
Sample sizes: Intact BER N = 19, Intact BEP N = 9, P-OVX BER N = 12, P-OVX BEP N = 18. The first reference line in the figure denotes the onset of puberty, which had occurred by feeding test 3 for 61% of the rats, and by feeding test 4 for 100% of the rats. The higher chow value for the intact BEP group during feeding test 4 was checked for errors. A subsample of rats consumed a large amount of chow on that day for reasons that are unclear – thus, the recorded values were retained to provide a conservative estimate of chow intake on that day.
Figure 2. Mean Palatable Food Intake by Pre-Pubertal Ovariectomy (P-OVX) and Binge Eating Resistant (BER) and Binge Eating Prone (BEP) Status in Adolescence.
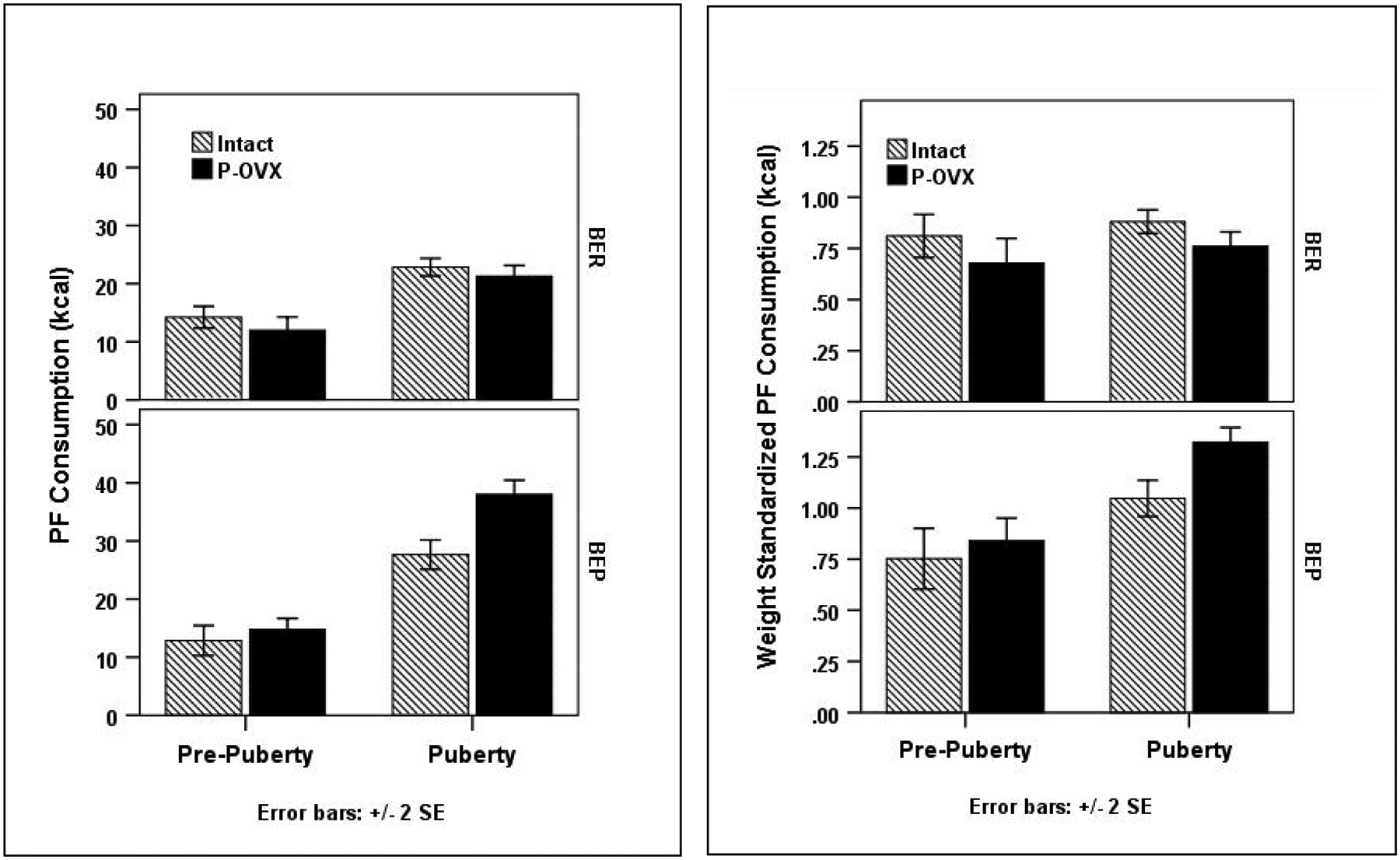
Sample sizes: Intact BER N = 19, Intact BEP N = 9, P-OVX BER N = 12, P-OVX BEP N = 18. Data for raw PF values appear in the left panel; data for weight standardized PF values are in the right panel.
More importantly, there was a significant three-way P-OVX status, pubertal status, and BER/BEP phenotype interaction for raw PF intake (p = .028; see Table 2 and Figure 2). A simple interaction analysis showed that before puberty, there was no evidence of a P-OVX x BER/BEP interaction (F(1, 383) = .033, p = .857, d = .02), but during puberty, there was a significant interaction (F(1, 117) = 12.98, p < .001, d = .67). During puberty, the estimated marginal means showed only a 1 kcal difference between intact versus P-OVX BER rats (intact M = 21.70, P-OVX M = 20.69, d = .13); these findings suggest that P-OVX has minimal effects on PF intake in BER rats. Remarkably, however, a 6.6 kcal difference was present for intact versus P-OVX BEP rats (intact M = 27.91, P-OVX M= 34.51, d = .78), suggesting a significant potentiation of PF intake in BEP rats that underwent P-OVX. Moreover, during puberty, intact BER versus BEP rats showed a 6.21 kcal difference in PF intake (d = .75), but P-OVX BER versus BEP rats showed a 13.82 kcal difference (d = 1.77). Nearly identical findings for the three-way interaction were obtained for the weight-standardized PF values (see Table 2 and Figure 2). Finally, although chow intake and body weight increased across puberty in all rats and were significantly higher in P-OVX as compared to intact rats, there were no significant BER/BEP main effects, puberty x BER/BEP group interactions, or 3-way interactions for the raw or weight-standardized values (see Table 2 and Figure 1). These findings suggested that the interactive effects of puberty, P-OVX, and BEP phenotype were specific to PF intake rather than overall food intake.
An identical pattern of results was observed when examining the binge proneness proportion variable. There was a significant three-way interaction between P-OVX status, pubertal status, and the binge proneness proportion variable for raw PF intake (see Table S6 and Figure S1 in Supplemental Material). For P-OVX rats prior to puberty, the effect of the binge proneness proportion variable on raw PF intake was small and non-significant (b = 2.112, t(1232) = 1.23, p = .220, d = .07), but during puberty, there was a strong effect of the binge proneness proportion variable (b = 13.43, t(434) = 13.16, p < .001, d = 1.27). Mirroring this result, a test of the interaction between pubertal status and the binge proneness proportion variable was significant for the P-OVX rats (t(1637) = 6.14, p <.001, d = .30). For intact rats, the same pattern was present, but less pronounced (see Figure S1), and the interaction between pubertal status and binge proneness proportion was non-significant (t(1645) = 1.63, p =.103, d = .08). As before, findings for the 3-way interaction replicated using the weight-standardized PF values (see Table S6 and Figure S1 in Supplemental Material). Finally, in terms of body weight and chow intake, there were the expected main effects of P-OVX (i.e., P-OVX > intact rats) and pubertal status (i.e., puberty > pre-puberty), and a significant interaction between the two (i.e., greater increases in chow intake and body weight across puberty in P-OVX as compared to intact rats) (see Table S6). However, there was no significant main effect of the binge proneness proportion variable, and no significant interactions involving this variable, confirming that the effects of the binge proneness proportion variable were specific to PF.
Post Hoc Analyses: Confirming a Unique Role for Pubertal Hormones
Overall, our primary and exploratory findings suggested a consistent and substantial role for P-OVX in adult risk for BEP phenotypes. These findings are in contrast to what has been observed previously for OVX conducted in adulthood. Specifically, our group found that binge eating increased in all rats following adult OVX, but BEP rats continued to binge eat significantly more than BER rats (Klump, Suisman, Culbert, Kashy, Keel, et al., 2011). These data suggested minimal effects of adult OVX on BEP status.
Nonetheless, in preparing the current report, we realized that our earlier study failed to conduct a key test – namely, examining whether the extreme BEP phenotype itself (rather than consumption of PF per se) is immutable to OVX performed in adulthood. Thus, we conducted post hoc analyses on those earlier data to examine whether the extreme BEP phenotype remains stable and is relatively immutable to ovarian hormone removal in adulthood. Methods for this earlier study were nearly identical to those used herein, including methods for OVX surgeries (in adulthood), feedings tests (using vanilla frosting as the PF), and defining BER/BEP status (using tertiles) (see Klump, Suisman, Culbert, Kashy, Keel, et al., 2011 for full details on the sample and experimental procedures). Across the two experiments in the report, the overall rate of BEP phenotypes was 31% (18/57 rats) prior to OVX; post-OVX, the rate was highly similar at 31%–36% (18–21/57) (Z test of proportions = 0.00–0.79, p’s = .43–1.00), depending on the tertile definition used (see Table S7 in Supplemental Material for information on definitions). Critically, the BEP phenotype also showed within-rat stability from pre-OVX to post-OVX. Of the 18 adult rats categorized as BEP prior to OVX, 67–78% of them continued to meet criteria for BEP status after OVX (see Table S7). These stability rates are very similar to those obtained in a previous study (Hildebrandt, Sinclair, Sisk, & Klump, 2018) of intact adult female rats where 60% of the BEP rats retained their BEP status across extended feeding tests in adulthood (12 feeding tests in Hildebrandt et al., 2018, as compared to 9 feeding tests in Klump, Suisman, Culbert, Kashy, Keel, et al., 2011). Thus, OVX in adulthood does not appear to affect the rate of BEP phenotypes in the full rat population or the overall stability of the BEP phenotype within rats.
Discussion
This is the first study to examine the role of pubertal ovarian hormones in risk for binge eating in females in adulthood. Findings showed substantially increased rates of adult BEP phenotypes in P-OVX rats as compared to intact rats. Results were generally consistent across raw and weight-standardized variables and were present even after controlling for differences in PF composition across experiments. Importantly, group differences in PF intake were minimal in pre-early puberty, but during mid-late puberty, the P-OVX BEP group showed a dramatic and sustained increase in PF intake. This increase in PF intake was significantly larger than that observed in any other group and was not present for chow intake or body weight. Taken together, these data suggest that pubertal estrogen may be protective against the development of binge eating in females, and that the absence of ovarian hormones during adolescent/pubertal development may have a particularly deleterious effect on BEP risk in adulthood in females.
The consistency of results across raw versus weight-standardized variables and different types of PF deserves note. These data suggest that the effects of P-OVX on BEP occur regardless of differences in body weight across P-OVX and intact groups and the macronutrient content of the PF. Nonetheless, the strength of the findings differed somewhat across these variables. The P-OVX versus intact group differences in BEP phenotypes were generally larger for the raw than for the weight-standardized PF values. However, it could be argued that our control for body weight via the standardized scores was an over-correction, given that definitions of binge eating, BED, and BN do not include any control or standardization for body weight (e.g., criteria can be met regardless of the individual’s weight status; American Psychiatric Association, 2013). Thus, the raw PF values may more closely approximate the effects of lower estradiol on binge eating in humans. In terms of the PF used, rates of BEP rats were highest in Experiment 2, which included PF with higher fat and lower carbohydrate content (20% protein, 45% fat, 35% carbohydrates) than the PF used in Experiments 1 (4% protein, 18% fat, 82% carbohydrates) and 3 (4% protein, 40% fat, 56% carbohydrates). This could suggest that the BEP phenotype is particularly responsive to foods that are higher in the overall proportion of fat. The PF used in Experiment 2 also included a larger proportion of calories due to protein, but the lack of over-consumption of chow (29% protein, 17% fat, 54% carbohydrates) by the BEP rats in any experiment suggests that it was the difference in fat content, and/or the ratio of fats to carbohydrates, that likely contributed to the higher BEP rates in Experiment 2. Future research should vary the macronutrient content of the PF to identify differential macronutrient preferences in BEP rats that could inform future neurobiological studies.
Despite variability in the strength of some effects, across all controls and experimental manipulations, the overall pattern of findings strongly suggested that the absence of ovarian hormones during adolescent/pubertal development increased risk for BEP in adulthood in females. These findings were in stark contrast to what we observed for female rats exposed to OVX in adulthood. Following adult OVX, binge eating increased in all rats, but BEP rats showed within-rat stability in BEP status (see post hoc analyses above) and continued to binge eat significantly more than BER rats (Klump, Suisman, Culbert, Kashy, Keel, et al., 2011). In other words, adult OVX appears to have minimal effects on BEP status. These data, coupled with the current findings in adolescence, suggest a specific effect of pubertal hormones on adult risk for binge eating in females and potentially different mechanisms of hormone effects across development. Specifically, adult estrogen appears to be activational in nature in females, in that levels of binge eating acutely change following estrogen removal in adulthood, but individual BEP status is stable. On the other hand, pubertal estrogen appears to be organizational in nature in females, as pre-pubertal estradiol removal (i.e., P-OVX) appears to induce substantially greater rates of BEP phenotypes in adulthood.
The timing and specificity of pre-pubertal OVX effects provide additional insight into the nature of these ovarian hormone effects. Significant differences in PF intake across P-OVX status were not present in pre-early puberty, but emerged during mid-late puberty/adolescence in BEP (but not BER) rats. Strikingly, the absence of ovarian hormones in P-OVX BEP rats resulted in PF intake increases across puberty that were substantially larger than those observed in P-OVX BER rats or any of the intact groups. This specific effect of P-OVX on PF intake (but not chow intake or body weight) in BEP rats (but not BER rats) suggests the possible presence of gene x hormone interactions in risk for BEP in females during puberty. Although to date, no studies have examined genetic differences between BER and BEP female rats, the existence of natural individual differences in binge eating proneness in an outbred strain of rats (i.e., Sprague-Dawley) implies the presence of genetic variation that predicts BEP phenotypes (Hildebrandt et al., 2014). Data from our study suggest that pre-pubertal OVX interacts with this phenotypic/genetic risk to enhance PF intake and the expression of BEP phenotypes during adolescence and into adulthood in females. Although additional studies are needed, our data imply that the absence of ovarian hormones during puberty may permanently alter brain structure and function in female rats with a presumed BEP “genotype” to be more permissive for binge eating in adulthood. These theories are broadly consistent with a new study of gene x hormone effects in girls where higher heritability of binge eating was observed in girls who have lower levels of estrogen during puberty (Klump et al., 2018). The stronger genetic effects in the presence of a “riskier” hormonal milieu (i.e., low estradiol levels) represent the type of gene x hormone effects implied by our data. Importantly, estradiol exerts its effects via nuclear estrogen receptors (ERs), ERα and ERβ, which regulate gene transcription (Asarian & Geary, 2006). Estradiol’s influence over food intake appear to be mediated by ERα (Geary, Asarian, Korach, Pfaff, & Ogawa, 2001), although more recently, GPCR-30, Gq-mER, and ERX have been identified as membrane-bound estrogen receptors (Micevych & Dominguez, 2019), and putative activation of these receptors similarly reduces food intake in OVX rats (Santollo, Marshall, & Daniels, 2013). All of these receptors are candidates for possible gene x hormone effects on binge eating in females across development.
Importantly, these theories are speculative, as additional studies are needed to replicate our results, provide further tests of organizational hormone effects in females, and confirm that BEP phenotypes reflect genetic differences between rats. Next steps for examining these hypotheses include exogenous administration of estrogen during both puberty and adulthood in P-OVX animals. Decreased rates of BEP phenotypes in adulthood in the P-OVX rats exposed to pubertal estrogen, but not adult estrogen, would provide additional data in support of an organizational effect of pubertal estrogen on risk for BEP in females. Genotyping of female BEP rats and studies of neural gene expression are also needed to confirm that underlying mechanisms reflect gene x hormone effects across development. It would be useful in these studies to also examine psychosocial factors (e.g., stress, food restriction; Boggiano & Chandler, 2006; Hagan et al., 2002; Fowler, Vo, Sisk, & Klump, 2019) that may interact with hormonal manipulations to produce long-lasting changes in BEP. Binge eating is multifactorial, and ovarian hormones are only one of many etiologic factors that are likely to contribute to dysregulated eating in females. Transdiagnostic studies that examine binge eating and other phenotypes (e.g., substance use, mood) are also needed to understand the broad and interrelated relevance of pubertal estradiol for a range of psychiatric phenotypes in females. Our findings are consistent with theories and animal studies of other phenotypes (e.g., substance use; see Becker, 2009; Jackson, Robinson, & Becker, 2006; Perry, Westenbroek, & Becker, 2013) that suggest that pubertal estradiol may have long-lasting and organizational effects on a broad range of behaviors and disorders that are comorbid with binge eating.
Finally, it will be important to translate our findings to humans to understand the impact of lower estradiol levels on later risk for binge eating in women. Our theories would predict that girls with lower estradiol levels during puberty, who are also at increased genetic risk for binge eating, would be particularly vulnerable for developing binge eating during post-puberty and early adulthood. Lower estradiol levels could be innate and reflect natural individual differences in estradiol levels (Albin, Niklasson, Westgren, & Norjavaara, 2012; Norjavaara, Ankarberg, & Albertsson-Wikland, 1996); in this case, girls who have naturally lower estradiol levels, and who are at genetic risk for binge eating, would be the most likely to experience organizational changes during puberty/adolescence and increased risk for binge eating in adulthood. Alternatively, lower estradiol levels could be environmentally mediated. As a concrete example, dieting often begins during adolescence in girls (von Soest & Wichstrm, 2009; Field et al., 2003) and precedes the development of binge eating and binge-related disorders (particularly BN) (Fairburn, Welch, Doll, Davies, & O’Connor, 1997; Stice, Davis, Miller, & Marti, 2008). More extreme dieting is known to depress estradiol levels (Misra et al., 2004; Pirke, Schweiger, Lemme, Krieg, & Berger, 1985), and these lower estradiol levels may then interact with genetic risk in vulnerable girls and lead to organizational neural changes that increase the chances of binge eating in post-puberty and early adulthood. This process could be one (of many) reasons why a history of dieting often precedes the development of binge eating in females.
Moving forward, it will be important for studies to begin testing these theories and ideas in humans. Longitudinal studies across adolescence and into adulthood are needed that collect measures of dieting (and other critical risk factors, e.g., stress), hormone levels, and binge-related phenotypes. Ideally, these studies would be conducted in genetically informative samples (e.g., twins) or utilize polygenic genetic risk scores (e.g., for AN (Watson et al., 2019), body weight (Peterson et al., 2011), or binge eating (when they become available)) in order to examine the key hormone x gene interactions that are thought to underlie the processes described herein. These studies could also incorporate neural measures that are available in humans (e.g., fMRI) to identify the specific neurobiological pathways that are altered by lower estradiol levels. Imaging studies thus far are clear in showing changes in brain structure and function during puberty/adolescence in girls by estradiol levels (Herting et al., 2014; Peper et al., 2009). Studies linking these neural changes with later binge eating risk are needed. Finally, it might be useful to specifically study populations that are known to be at risk for lower estradiol levels during puberty (e.g., adolescent elite athletes or dancers; Kapczuk, 2017) to examine their later/adult risk for binge eating and binge-related disorders. In combination with additional animal studies, these longitudinal investigations may provide the strongest evidence in favor of estradiol-mediated organizational changes during puberty on later risk for binge eating.
Before ending, some key limitations of our study should be noted. First, to maximize sample sizes to examine categorical phenotypes and interactions across groups, we collapsed across three experiments for a subset of analyses. Although most experimental procedures were identical, different PFs were used, data collection length varied, and rates of BEP phenotypes in intact rats were somewhat lower in some of the experiments (% BEP = 10–13%, see Table 1) than in past work (% BEP = 15–33%; e.g., Culbert, Sinclair, et al., 2018; Hildebrandt et al., 2014; Klump, Suisman, Culbert, Kashy, & Sisk, 2011; LeMon et al., 2019). Although we controlled for potential experiment differences in analyses, replication of our findings in large and independent samples is needed.
Second, we did not exogenously administer estrogen and progesterone across development to confirm that pre-pubertal OVX effects are due to estrogen per se. Past animal studies have focused primarily on estrogen effects on food intake and binge eating (Asarian & Geary, 2013), and studies in girls suggest estrogen is more important than progesterone for potentiating genetic influences on binge eating during puberty (Klump et al., 2018). Nevertheless, data in adult women show an important role for progesterone in binge eating and emotional eating across the menstrual cycle (e.g., Edler et al., 2007; Klump, Keel, et al., 2013). Additional studies that exogenously administer estrogen and progesterone in P-OVX and adult OVX rats across development (i.e., in puberty and adulthood) are needed to extend/replicate our results and confirm the presence of organizational estrogen effects.
Third, we focused on hormonal processes that may be important for females and did not examine these processes in males or examine male-specific etiologic pathways. Binge eating is much more prevalent in females than in males in both humans (Klump et al., 2017) and animals (Culbert, Sinclair, et al., 2018; Klump, Racine, et al., 2013), with sex ratios of 2:1 up to 6:1. Nonetheless, males do engage in binge eating and suffer from binge-related disorders, and we currently have a limited understanding of the etiology of binge eating in males. Prior data suggest that more than half of the genetic risk for disordered eating is not shared between males and females but is instead sex-specific (Baker et al., 2009). We suspect that the ovarian hormone x gene interactions described herein are one set of genetic risk factors that are specific to females. We have also theorized and shown in prior data that androgens (including testosterone, the predominant gonadal hormone in males), are likely important for organizational and activational risk for binge eating in males (Culbert, Burt, Sisk, Nigg, & Klump, 2014; Culbert, Sinclair, et al., 2018). Future animal and human studies are needed to examine sex-specific hormone x gene interactions for binge eating and binge-related disorders in males.
Fourth, animal models of binge eating (and other phenotypes) are unable to model all aspects of the behavior as it appears in humans. Although the BER/BEP model exhibits many parallels to human binge eating (e.g., sex differences in binge eating, pubertal emergence, preference for palatable food during binges), it does not include other core psychological and psychosocial processes (e.g., concerns about body weight and shape) that are key to binge eating behaviors. These limitations underscore the need for the types of studies described above that can translate our findings to the human condition.
Despite these limitations, our findings strongly suggest that the absence of ovarian hormones during puberty has a pronounced and lasting impact on risk for binge eating in females that is qualitatively different from the effects of OVX in adulthood. Effects appear particularly significant for female BEP rats, which may have latent genetic risk for binge eating that is exacerbated by a risky pubertal hormonal milieu. While further research is needed, these results, in combination with recent data showing that estradiol substantially moderates genetic influences on binge eating in girls (Klump et al., 2018), provide important evidence of puberty and estrogen as key organizational agents in risk for binge eating phenotypes across development in females.
Supplementary Material
Acknowledgments
This research was supported by funding from Michigan State University and a grant from the National Institute of Mental Health (R01 MH111715). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH.
Footnotes
A 24-hour measurement of PF consumption was also taken in Experiments 1 and 3 to ensure that BER and BEP rats showed consistent differences in PF intake after both shorter (4-hour) and longer (24-hour) periods of exposure to PF. Significant group differences in PF intake (BEP rats > BER rats) in the Combined sample were present at the 24-hour measurement (F(1, 122) = 16.79, p < .001, d = .74).
Adulthood is included in Figure 1 to show developmental patterns across time, but as noted in Methods, the MLMs were conducted in pre-early puberty and mid-late puberty only.
References
- Albin AK, Niklasson A, Westgren U, & Norjavaara E (2012). Estradiol and pubertal growth in girls. Hormone Research in Paediatrics, 78(4), 218–225. 10.1159/000343076 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Asarian L, & Geary N (2006). Modulation of appetite by gonadal steroid hormones. Philosophical Transactions of the Royal Society B: Biological Sciences, 361, 1251–1263. 10.1098/rstb.2006.1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, & Geary N (2013). Sex differences in the physiology of eating. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 305(11), R1215–R1267. 10.1152/ajpregu.00446.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs RK, Wojnicki FHE, & Corwin RLW (2012). Assessing binge eating: An analysis of data previously collected in bingeing rats. Appetite, 59(2), 478–482. 10.1016/j.appet.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JH, Girdler SS, & Bulik CM (2013). The role of reproductive hormones in the development and maintenance of eating disorders. Expert Rev Obstet Gynecol, 7(6), 573–583. 10.1586/eog.12.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JH, Maes HH, Lissner L, Aggen SH, Lichtenstein P, & Kendler KS (2009). Genetic risk factors for disordered eating in adolescent males and females. Journal of Abnormal Psychology, 118(3), 576–586. 10.1037/a0016314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB (2009). Sexual differentiation of motivation: A novel mechanism? Hormones and Behavior, 55(5), 646–654. 10.1016/j.yhbeh.2009.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, & Eldridge AJ (2007). High intake of palatable food predicts binge-eating independent of susceptibility to obesity: An animal model of lean vs obese binge-eating and obesity with and without binge-eating. International Journal of Obesity, 31, 1357–1367. 10.1038/sj.ijo.0803614 [DOI] [PubMed] [Google Scholar]
- Boggiano MM, & Chandler PC (2006). Binge eating in rats produced by combining dieting with stress. Current Protocols in Neuroscience, 36(1), 9–23. 10.1002/0471142301.ns0923as36 [DOI] [PubMed] [Google Scholar]
- Culbert KM, Burt SA, Sisk CL, Nigg JT, & Klump KL (2014). The effects of circulating testosterone and pubertal maturation on risk for disordered eating symptoms in adolescent males. Psychological Medicine, 44(11), 2271–2286. 10.1017/S0033291713003073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Sinclair EB, Hildebrandt BA, Klump KL, & Sisk CL (2018). Perinatal testosterone contributes to mid-to-post pubertal sex differences in risk for binge eating in male and female rats. Journal of Abnormal Psychology, 127(2), 239–250. 10.1037/abn0000334 [DOI] [PubMed] [Google Scholar]
- Culbert KM, Sisk CL, & Klump KL (2018). Sex steroid hormones and differential risk for eating pathology: A review of genetic and phenotypic effects across development. Current Opinion in Behavioral Sciences, 23, 124–130. 10.1016/j.cobeha.2018.06.005 [DOI] [Google Scholar]
- Edler C, Lipson SF, & Keel PK (2007). Ovarian hormones and binge eating in bulimia nervosa. Psychological Medicine, 37(1), 131–141. 10.1017/S0033291706008956 [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Welch SL, Doll HA, Davies BA, & O’Connor ME (1997). Risk factors for bulimia nervosa: A community-based case-control study. Archives of General Psychiatry, 54(6), 509–517. 10.1001/archpsyc.1997.01830180015003 [DOI] [PubMed] [Google Scholar]
- Field AE, Austin SB, Taylor CB, Malspeis S, Rosner B, Rockett HR,…Colditz GA (2003). Relation between dieting and weight change among preadolescents and adolescents. Pediatrics, 112(4), 900–906. 10.1542/peds.112.4.900 [DOI] [PubMed] [Google Scholar]
- Fowler N, Vo P, Sisk CL, & Klump KL (2019). Ovarian hormone influences on binge eating in women: A potential moderating role of stress. F1000Research, 8. 10.12688/f1000research.16895.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N, Asarian L, Korach KS, Pfaff DW, & Ogawa S (2001). Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology, 142(11), 4751–4757. 10.1210/endo.142.11.8504 [DOI] [PubMed] [Google Scholar]
- Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, & Blackburn K (2002). A new animal model of binge eating: Key synergistic role of past caloric restriction and stress. Physiology & Behavior, 77(1), 45–54. 10.1016/S0031-9384(02)00809-0 [DOI] [PubMed] [Google Scholar]
- Harden KP, Kretsch N, Moore SR, & Mendle J (2014). Descriptive review: Hormonal influences on risk for eating disorder symptoms during puberty and adolescence. International Journal of Eating Disorders, 47, 718–726. 10.1002/eat.22317 [DOI] [PubMed] [Google Scholar]
- Herting M, Gautam P, Spielberg JM, Kan E, Dahl RE, & Sowell ER (2014). The role of testosterone and estradiol in brain volume changes across adolescence: A longitudinal structural MRI study. Human Brain Mapping, 35(11), 5633–5645. 10.1002/hbm.22575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt BA, Klump KL, Racine SE, & Sisk CL (2014). Differential strain vulnerability to binge eating behaviors in rats. Physiology & Behavior, 127, 81–86. 10.1016/j.physbeh.2014.01.012 [DOI] [PubMed] [Google Scholar]
- Hildebrandt BA, Sinclair EB, Sisk CL, & Klump KL (2018). Exploring reward system responsivity in the nucleus accumbens across chronicity of binge eating in female rats. International Journal of Eating Disorders, 51(8), 989–993. 10.1002/eat.22895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, & Becker JB (2006). Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology, 31(1), 129–138. 10.1038/sj.npp.1300778 [DOI] [PubMed] [Google Scholar]
- Kapczuk K (2017). Elite athletes and pubertal delay. Minerva Pediatrica, 69(5), 415–426. 10.23736/s0026-4946.17.05044-7 [DOI] [PubMed] [Google Scholar]
- Klump KL (2013). Puberty as a critical risk period for eating disorders: A review of human and animal studies. Hormones and Behavior, 64(2), 399–410. 10.1016/j.yhbeh.2013.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, O’Connor S, Fowler N, & Burt SA (2017). The significant effects of puberty on the genetic diathesis of binge eating in girls. International Journal of Eating Disorders, 50(8), 984–989. 10.1002/eat.22727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, & Sisk CL (2017). Sex differences in binge eating: Gonadal hormone effects across development. Annual Review of Clinical Psychology, 13, 183–207. 10.1146/annurev-clinpsy-032816 [DOI] [PubMed] [Google Scholar]
- Klump KL, Fowler N, Mayhall L, Sisk CL, Culbert KM, & Burt SA (2018). Estrogen moderates genetic influences on binge eating during puberty: Disruption of normative processes? Journal of Abnormal Psychology, 127(5), 458–470. 10.1037/abn0000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Culbert KM, & Edler C (2008). Ovarian hormones and binge eating: Exploring associations in community samples. Psychological Medicine, 38(12), 1749–1757. 10.1017/S0033291708002997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, … Hu JY (2013). The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. Journal of Abnormal Psychology, 122(1), 131–137. 10.1037/a0029524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Sisk C, & Burt SA (2010). Preliminary evidence that estradiol moderates genetic influences on disordered eating attitudes and behaviors during puberty. Psychological Medicine, 40(10), 1745–1753. 10.1017/S0033291709992236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Racine SE, Hildebrandt B, Burt SA, Neale M, Sisk CL, … Keel PK (2014). Influences of ovarian hormones on dysregulated eating: A comparison of associations in women with versus women without binge episodes. Clinical Psychological Science, 2(5), 545–559. 10.1177/2167702614521794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Racine S, Hildebrandt B, & Sisk CL (2013). Sex differences in binge eating patterns in male and female adult rats. International Journal of Eating Disorders, 46(7), 729–736. 10.1002/eat.22139 [DOI] [PubMed] [Google Scholar]
- Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, & Sisk CL (2011). The effects of ovariectomy on binge eating proneness in adult female rats. Hormones and Behavior, 59(4), 585–593. 10.1016/j.yhbeh.2011.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Suisman JL, Culbert KM, Kashy DA, & Sisk CL (2011). Binge eating proneness emerges during puberty in female rats: A longitudinal study. Journal of Abnormal Psychology, 120(4), 948–955. 10.1037/a0023600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMon JV, Sisk CL, Klump KL, & Johnson AW (2019). Reduced sensitivity to devaluation for instrumental but not consummatory behaviors in binge eating prone rats. Physiology & Behavior, 206, 13–21. 10.1016/j.physbeh.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, & Dominguez R (2019). Membrane estradiol signaling in the brain. Frontiers in Neuroendocrinology, 30(3), 315–327. 10.1016/j.yfrne.2009.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micioni Di Bonaventura MV, Lutz TA, Romano A, Pucci M, Geary N, Asarian L, & Cifani C (2017). Estrogenic suppression of binge-like eating elicited by cyclic food restriction and frustrative-nonreward stress in female rats. International Journal of Eating Disorders, 50, 624–635. 10.1002/eat.22687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Aggarwal A, Miller KK, Almazan C, Worley M, Soyka LA,…Klibanski A (2004). Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics, 114(6), 1574–1583. 10.1542/peds.2004-0540 [DOI] [PubMed] [Google Scholar]
- Norjavaara E, Ankarberg C, & Albertsson-Wikland K (1996). Diurnal rhythm of 17 beta-estradiol secretion throughout pubertal development in healthy girls: Evaluation by a sensitive radioimmunoassay. The Journal of Clinical Endocrinology & Metabolism, 81(11), 4095–4102. 10.1210/jcem.81.11.8923866 [DOI] [PubMed] [Google Scholar]
- Oswald KD, Murdaugh DL, King VL, & Boggiano MM (2011). Motivation for palatable food despite consequences in an animal model of binge eating. International Journal of Eating Disorders, 44, 203–211. 10.1002/eat.20808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM,…Hulshoff Pol HE (2009). Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology, 34(3), 332–342. 10.1016/j.psyneuen.2008.09.012 [DOI] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, & Becker JB (2013). Impact of pubertal and adult estradiol treatments on cocaine self-administration. Hormones and Behavior, 64(4), 573–578. 10.1016/j.yhbeh.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RE, Maes HH, Holmans P, Sanders AR, Levinson DF, Shi J…Webb BT (2011). Genetic risk sum score comprised of common polygenic variation is associated with body mass index. Human Genetics, 129(2), 221–230. 10.1007/s00439-010-0917-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirke KM, Schweiger U, Lemme W, Krieg JC, & Berger M (1985). The influence of dieting on the menstrual cycle of healthy young women. The Journal of Clinical Endocrinology & Metabolism, 60(6), 1174–1179. 10.1210/jcem-60-6-1174 [DOI] [PubMed] [Google Scholar]
- Santollo J, Marshall A, & Daniels D (2013). Activation of membrane-associated estrogen receptors decreases food and water intake in ovariectomized rats. Neuroendocrinology, 154(1), 320–329. 10.1210/en.2012-1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, & Sisk CL (2009). Back to the future: The organizational–activational hypothesis adapted to puberty and adolescence. Hormones and Behavior, 55(5), 597–604. 10.1016/j.yhbeh.2009.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair EB, Culbert KM, Gradl DR, Richardson KA, Klump KL, & Sisk CL (2015). Differential mesocorticolimbic responses to palatable food in binge eating prone and binge eating resistant female rats. Physiology & Behavior, 152, 249–256. 10.1016/j.physbeh.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, & Zehr JL (2005). Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology, 26(3–4), 163–174. 10.1016/j.yfrne.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews, 24, 417–463. 10.1016/S0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Stice E, Davis K, Miller NP, & Marti CN (2008). Fasting increases risk for onset of binge eating and bulimic pathology: A 5-year prospective study. Journal of Abnormal Psychology, 117(4), 941–946. 10.1037/a0013644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Soest T, & Wichstrøm L (2009). Gender differences in the development of dieting from adolescence to early adulthood: A longitudinal study. Journal of Research on Adolescence, 19(3), 509–529. 10.1111/j.1532-7795.2009.00605.x [DOI] [Google Scholar]
- Wade G (1976). Sex hormones, regulatory behaviors, and body weight. In Rosenblatt JS, Hinde RA, & Shaw E (Eds.), Advances in the Study of Behavior (pp. 201–279). New York: Academic Press, Inc. [Google Scholar]
- Watson HJ, Yilmaz Z, Thornton LM, Hübel C, Coleman JR, Gaspar HA,… Medland SE (2019). Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nature Genetics, 51(8), 1207–1214. 10.1038/s41588-019-0439-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Geary N, & Corwin RL (2008). Ovarian hormones inhibit fat intake under binge-type conditions in ovariectomized rats. Physiology and Behavior, 95(3), 501–507. 10.1016/j.physbeh.2008.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


