Abstract
Spousal bereavement is associated with an elevated risk of morbidity and mortality. Several well-regarded multidisciplinary research teams have sought to understand the biopsychosocial processes underlying why widow(er)s are at elevated physical health risk. In this paper, we review research from multiple investigatory teams, including our own, showing that, on average, widow(er)s exhibit maladaptive patterns of autonomic, neuroendocrine, and immune activity compared to matched comparisons. Widow(er)s also exhibit poorer health behaviors than they did before their spouse’s death. There is considerable variation in post-loss psychological adjustment trajectories among widow(er)s, which likely corresponds to physical health risk trajectories. Yet, there is little biobehavioral research on patterns of change in physical health risk after the death of a spouse. We summarize recently published work demonstrating the utility of attachment theory to characterize and predict individual differences in physical health biomarkers; we highlight the need for a biopsychosocial approach to understand and characterize post-loss trajectory patterns. We conclude by discussing the possibility that this line of inquiry could help researchers, and ultimately providers, identify adjustment trajectories earlier and thus deliver appropriate interventions when they are most needed.
Keywords: health, bereavement, attachment theory, immune system, heart disease
When friends and family members use the phrase “broken-hearted,” to describe a widow(er), they are usually referencing the intense emotional pain experienced after the death of a spouse (Stroebe, Schut, & Stroebe, 2007). Indeed, the loss of a spouse takes a considerable emotional toll, ranking first on the Social Readjustment Rating Scale (Holmes & Rahe, 1967). In addition to the mental health toll, “broken-hearted” widow(er)s are at risk for premature morbidity and mortality, especially as it relates to cardiovascular events (Moon, Glymour, Vable, Liu, & Subramanian, 2014). The focus of our recent work has been to understand why those who are spousally bereaved are at heightened risk of morbidity and mortality (Chirinos, Ong, Garcini, Alvarado, & Fagundes, 2019; Fagundes et al., 2019, 2018; Knowles, Ruiz, & O’Connor, 2019).
Health Behaviors
Bereavement is associated with negative changes in routine health behaviors, one probable mechanism underlying the link between grief and poor health. Poor dietary behaviors characterize many who are widowed (Stahl & Schulz, 2014). Widow(er)s consistently report eating alone, skipping meals, and eating more commercial meals in the first year post-loss; there is a strong positive relationship between bereavement and nutritional risk (Stahl & Schulz, 2014). Alcohol consumption also increases among bereaved spouses (Stahl & Schulz, 2014). During the first year of bereavement, compared with matched comparison, widow(er)s also report sleep problems, including difficulty falling asleep and staying asleep; they are also more sedentary (Stahl & Schulz, 2014).
Autonomic and Neuroendocrine Dysregulation
Psychological stress promotes the fight-or-flight response resulting in the autonomic nervous system (ANS) and hypothalamic-pituitary-adrenal axis (HPA) activation. When the sympathetic nervous system is activated as part of a coordinated stress-response, the catecholamines, epinephrine, and norepinephrine boost heart rate, blood pressure, and blood glucose levels. Generally, in response to stress, parasympathetic nervous system activity decreases (Gianaros & Wager, 2015).
Autonomic Activity.
The autonomic nervous system has two main divisions: the sympathetic and parasympathetic branches. The sympathetic nervous system regulates the fight or flight response, while the parasympathetic division helps maintain homeostasis and conserve resources (Thayer & Lane, 2000). Lower resting parasympathetic activation is associated with worse physical and mental health and is assessed by measuring the variability in heart rate. Heart rate rises and falls with one’s breath; an oscillatory pattern called respiratory sinus arrhythmia. The parasympathetic nervous system controls respiratory sinus arrhythmia and partially controls heart rate because the vagus innervates the sinoatrial node, which is the heart’s pacemaker (see Figure 1, left side, vagus nerve in blue). We can capture a vagally mediated heart rate by measuring heart rate variability (HRV) at the frequency of people’s respiration rate (8 to 25 cycles per min) or by evaluating beat-to-beat variability in heart rate. Although there are other sources of heart rate variability that are not attributed to vagally mediated parasympathetic influences, when referencing HRV below, we are referring to indices of vagally mediated HRV because of its links to mental and physical health (Thayer & Lane, 2000).
Figure 1.
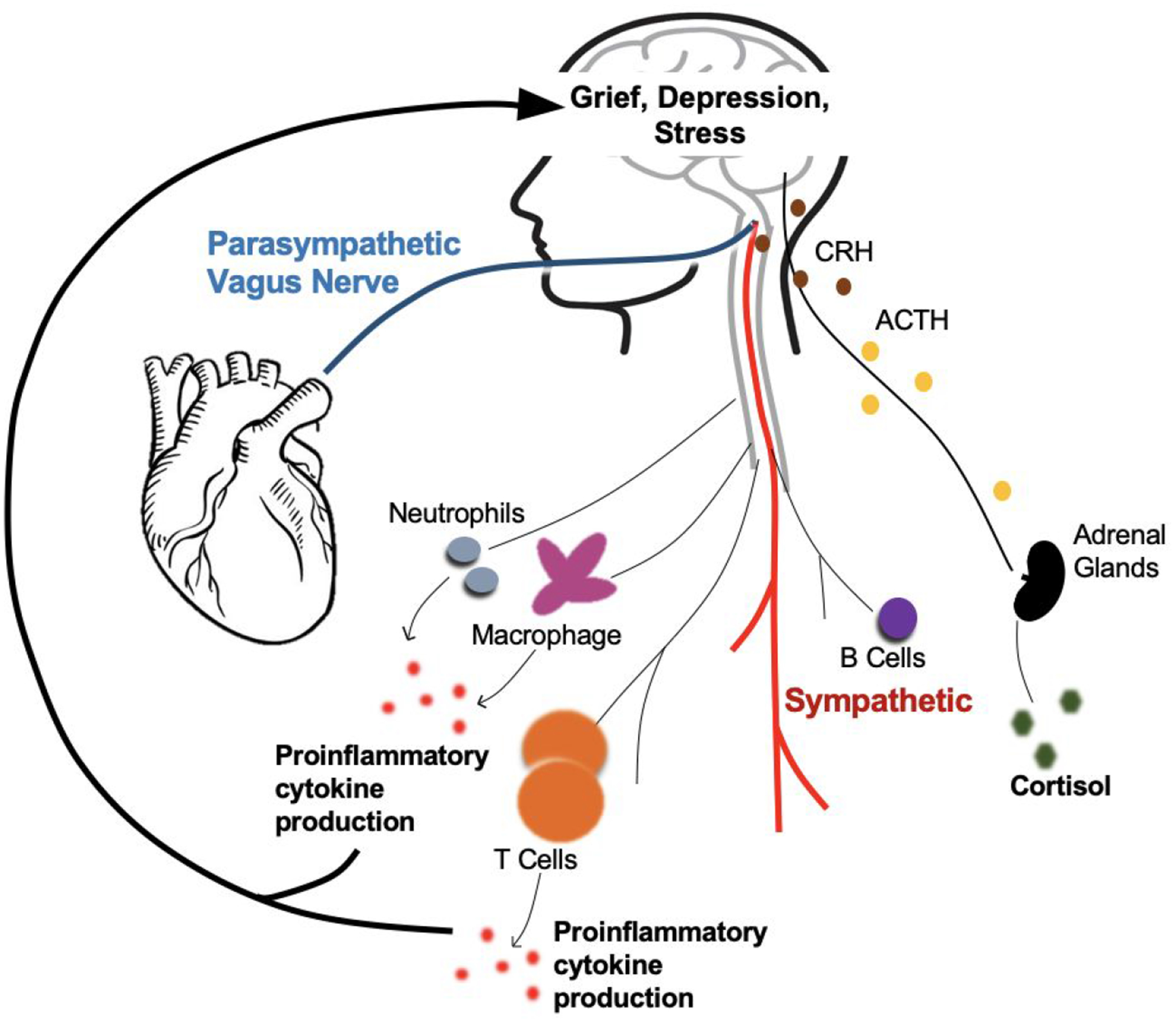
Autonomic, neuroendocrine, and immunological mechanisms underlying the association between bereavement and heart disease.
The sympathetic nervous system increases heart rate and blood pressure. The parasympathetic nervous system counteracts the sympathetic nervous system via the vagus nerve. The HPA axis releases cortisol through a cascade of hormones (CRH and ACTH) to suppress the immune system. But under chronic stress conditions, the HPA axis can promote inflammation. The innate (macrophage, neutrophils, natural killer cells) and adaptive immune system (T cells) release proinflammatory cytokines that promote inflammation. Feedback mechanisms link peripheral systems to the brain, increasing grief and depressive symptoms and emotional sensitivity to future stressors which perpetuate the cycle again. Chronic stress as a result of bereavement dysregulate these systems and creates an internal environment susceptible to heart disease and mental illness. Abbreviations: CRH=Corticotropin releasing hormone; ACTH=Adrenocorticotropic hormone; HPA axis = hypothalamic-pituitary-adrenal axis
Because lower HRV is prognostic for disease, we surmised HRV could be a potential mechanism linking bereavement and cardiovascular risk (Thayer & Sternberg, 2006). We recently demonstrated that spousally bereaved older adults have lower resting HRV than age-matched controls (Fagundes et al., 2018). Excellent work from other groups has provided additional evidence for the role of HRV in the context of bereavement. In a study examining within-person differences in depression among bereaved individuals, O’Connor, Allen, & Kaszniak (2002) showed that those who exhibited more depressive symptoms had lower HRV than widow(er)s who reported less depressive symptoms, suggesting that heart rate variability may serve as a biomarker of poor mental health among widow(er)s (O’Connor, Allen, & Kaszniak, 2002). Bereavement has also been linked to elevated catecholamines and higher blood pressure, showing that sympathetic activity is elevated among widow(er)s (Buckley et al., 2011). One condition that is of considerable interest to cardiologists and grief researchers alike is Takotsubo. Known colloquially as “broken heart syndrome,” Takotsubo is characterized by considerable emotional distress. Both the sympathetic and parasympathetic nervous system has been linked to the pathogenesis of Takotsubo syndrome (Norcliffe-Kaufmann et al., 2016).
Neuroendocrine Activity.
Cortisol is a glucocorticoid produced in the adrenal glands in response to stress or low blood glucose (Gianaros & Wager, 2015). As seen on the right-hand side of Figure 1, the corticotropin-release hormone is secreted when the brain signals a stress-response, which stimulates the release of adrenocorticotropic hormone from the anterior pituitary gland. In turn, the adrenal cortex secretes cortisol. Compared to those with non-clinical levels of grief, participants with complicated grief, a former psychiatric diagnosis, show lower levels of morning cortisol and a flatter cortisol slope across the day, two indicators of dysregulated neuroendocrine function (O’Connor, Wellisch, Stanton, Olmstead, & Irwin, 2012). Morning cortisol levels are higher among women grieving the death of their spouse compared with nongrieving women (Irwin, Daniels, Craig Risch, Bloom, & Weiner, 1988). Chronic secretion of cortisol impairs immune cell’s ability to kill pathogens (Gianaros & Wager, 2015); furthermore, although generally anti-inflammatory, chronically high cortisol levels can sometimes lead to glucocorticoid insensitivity, thereby raising systemic inflammation (Miller, Cohen, & Ritchey, 2002).
Immune Activity
Some of the foundational studies in the field of psychoneuroimmunology, the discipline concerned with brain-immune interactions, compared bereaved individuals to matched controls. A psychoneuroimmunologist often performs functional assays to measure how immune cells in both the innate and adaptive immune system react to “challenge.” White blood cells mediate the innate immune response, which plays an initial role when the body is exposed to a pathogen (Kusnecov & Anisman, 2013). The innate immune system is activated within minutes to hours after exposure to an antigen; however, it is non-specific and thus is not always adequate to eliminate a pathogen. The adaptive immune system takes much longer to respond effectively but is antigen-specific, and develops a memory for long-term protection from reinfection. Functional methods are used to show that stressors, such as bereavement, impair responses from T and B Cells (an aspect of the adaptive immune system), as well as Natural Killer Cells and neutrophils (components of the innate immune system illustrated on the bottom left of Figure 1)(Kusnecov & Anisman, 2013). A recent meta-analysis showed that functional markers of both the innate immune system and the adaptive immune system are impaired among those who are bereaved (Knowles et al., 2019).
Proinflammatory cytokines (bottom of Figure 1, represented in pink) such as interleukin(IL)-6 and tumor necrosis factor (TNF)-α are chemical signals that increase immune cell trafficking to infection sites. Acute local inflammation in response to an initial infection or trauma can be beneficial; however, chronic low-grade inflammation can contribute to a variety of diseases in older adulthood such as type 2 diabetes, Alzheimer’s disease, osteoporosis, rheumatoid arthritis, periodontal disease, some cancers, and cardiovascular disease. In the context of bereavement, the link between cardiovascular disease and inflammation is of particular interest.
Researchers evaluate levels of pro-inflammatory cytokines by either assessing “circulating” levels of cytokines or by “stimulating” certain immune cells (e.g., monocytes/macrophages and T cells) (Knowles et al., 2019). Most commonly, serum or plasma levels of “circulating” proinflammatory cytokines are evaluated. There is now considerable work showing that bereaved individuals have higher levels of circulating inflammatory biomarkers, and other markers of immune dysregulation than age-matched comparisons. Widow(er)s had higher plasma levels of specific cytokines if they also carried a specific proinflammatory gene polymorphism (Schultze-Florey et al., 2012). Although our review is focused on spousal bereavement, some of the studies on circulating levels of inflammation and bereavement combined different forms of bereavement into one group; these studies show similar results (Cohen, Granger, & Fuller-Thomson, 2015).
Another approach to measuring inflammation that is more time-consuming, but likely more indicative of a typical pro-inflammatory response is to stimulate (challenge) specific immune cells such as monocytes/macrophages and T cells. By exposing the white blood cells to a bacterial pathogen, a procedure called “ex vivo stimulation,” we recently demonstrated that bereavement is associated with the capacity of immune cells to produce inflammatory cytokines when challenged (Fagundes et al., 2018). This method more likely represents the in vivo context where the immune system produces cytokines in response to stress or infection (Korenromp et al., 2011). Specifically, widow(er)s showed enhanced proinflammatory cytokine production by in vitro lipopolysaccharide-stimulated peripheral blood leukocytes compared to matched controls (Fagundes et al., 2018). When examining between-persons differences among widows and widowers, we found that individuals with higher grief severity had higher levels of proinflammatory T-cell-derived cytokines than those with less grief severity (Fagundes et al. 2019). We also examined the association between depressive symptoms and inflammation because we wanted to know if the well-established relationship between elevated inflammation and greater depressive symptoms existed among widow(er)s (Kiecolt-Glaser, Derry, & Fagundes, 2015). We found that those who experienced higher levels of depression exhibited elevated levels of T-cell derived proinflammatory cytokines compared with those who had lower levels of depressive symptoms. However, depression did not explain the relationships with grief and proinflammatory cytokines (Fagundes et al., 2019).
In the broader psychoneuroimmunology literature, there is evidence that levels of inflammation predict future psychopathology. Because depression and inflammation interact through a bidirectional feedback loop (Kiecolt-Glaser et al., 2015), widow(er)s’ levels of inflammation at one-time point may predict future post-loss adjustment, at another time point, an important area for future investigation.
Individual Differences
In the bereavement literature, the vast majority of research on biomarkers of risk and health has focused on differences between bereaved and matched comparisons. Yet there is considerable adjustment variation among widow(er)s. Widow(er)s consistently fall under one of five different psychological adjustment trajectories over the first two years after the death (Bonanno, 2004) (see Figure 2). Thus, bereavement poses unique problems for interventionists (Bonanno et al., 2002), because they have no way of knowing which trajectory profile a widow(er) will most likely fall into until the trajectory unfolds with time, making intervention efforts problematic (Robbins & Kubiak, 2014). Research that identifies a widow(er)’s most probable long-term trajectory shortly after the loss would be a major advance. Stable individual differences in personality constructs are likely sources of information to predict future grief trajectories.
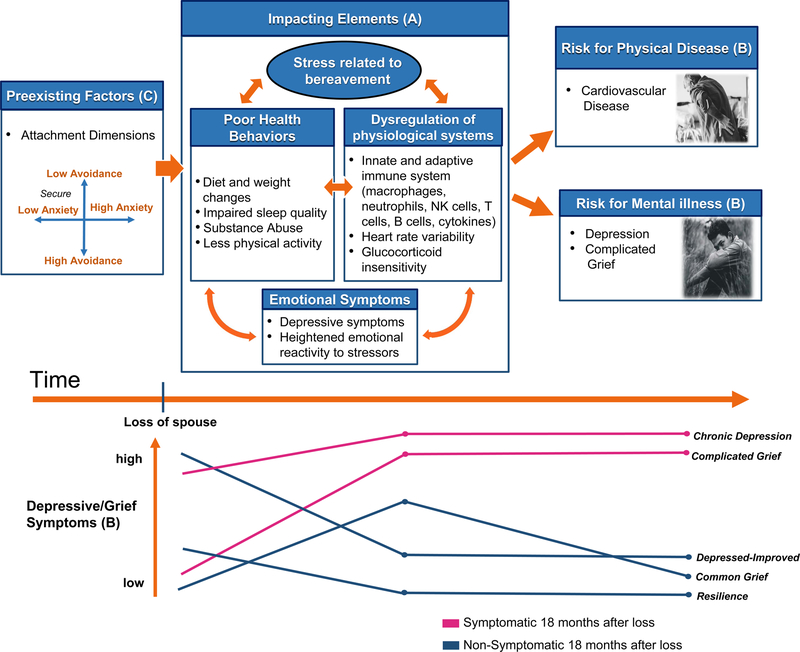
Figure 2.
Factors influencing physical and mental health outcomes in spousally bereaved individuals over time.
After the death of the spouse, bereaved individuals experience a number of emotional, behavioral, and physiological changes. Stress related to bereavement (a), including loss-oriented and restoration-oriented stressors, may encourage negative health behavior, disrupt homeostasis within physiological systems, or create emotional instability. All of these impacting elements affect and are affected by each other. Months or years later (b), individuals who still exhibit high levels of depressive or grief symptoms are at increased risk for cardiovascular disease, depression, or grief-related disorders. Preexisting factors (c) such as attachment patterns influence how susceptible individuals are to physiological, behavioral, and emotional dysregulation as a result of bereavement-related stress. High attachment anxiety is a risk factor for poor emotional and physical health. The five grief trajectories identified by Bonanno et al. (2004) are represented in (d), with pink and blue representing individuals who are symptomatic and nonsymptomatic 18 months after their loss.
Attachment theory provides a theoretical framework for understanding individual differences in grief reactions after the death of a spouse (Shaver & Tancredy, 2001). There are two patterns of attachment insecurity: attachment anxiety and attachment avoidance. People with high attachment anxiety use “hyperactivating” emotional coping strategies that accentuate the stress response. People with high attachment avoidance are uncomfortable depending on others and use “deactivating” coping strategies in an attempt to inhibit stress. In the adult attachment literature, those who are low on both attachment anxiety and attachment avoidance are considered securely attached (LeRoy et al. 2020). We cross-sectionally assessed widow(er)s, within approximately three months after the death of their spouse, to determine if individual differences in attachment patterns predicted self-reported health and inflammation (as evaluated by ex vivo cytokine production). Attachment anxiety was associated with increased ex vivo stimulated monocyte production of pro-inflammatory cytokines. Likewise, attachment anxiety was associated with poorer self-reported mental and physical health. Bereaved spouses (death of spouse within approximately 3 months) who had higher levels of attachment anxiety reported poorer mental and physical health (i.e., less energy, poorer emotional functioning, poorer social functioning, and poorer self-rated health) (LeRoy et al. 2020). Attachment anxiety was also associated with greater ex vivo cytokine production. In contrast, attachment avoidance was not associated with inflammation; however, compared with those low on attachment avoidance, those high on attachment avoidance reported better self-reported mental and physical health (LeRoy et al. 2020). Importantly, this data was cross-sectional, it will be important for future work to determine if these individual differences predict trajectories in grief, self-reported health, and biomarkers of physical health risk over one to two years after the loss.
In addition to identifying stable individual difference characteristics of risk, a more fine-grained understanding of widow(er)s “lived experiences” would inform prediction and intervention efforts. Our understanding of the behaviors, emotions, and contextual influences underlying physical and mental health after the death of a spouse is limited because the methods used have relied on “snapshots” of grief (often assessed retrospectively) and stress physiology. Yet according to the dual-process model of coping with bereavement, grief is a dynamic process during which widow(er)s vacillate between coping with “restoration oriented stressors” and “loss oriented stressors” (Stroebe & Schut, 2010). Mobile health sensor technologies can now collect contextual and physiological data in one’s natural environment non-invasively and with little burden; these methods may be particularly useful for evaluating the dynamic processes associated with grief. Based on this data, inference algorithms could then generate estimates of context, behavior, and stress physiology to better predict a widow(er)’s health risk and future adjustment trajectory. This information would be particularly helpful to intervention scientists because of the now well-established literature showing that widow(er)s have different intervention needs based on their characteristics and adjustment trajectories (Bonanno et al., 2002). For example, we now know that the vast majority of widow(er)s should not receive psychotherapy focused on “grief work,” but a minority of widow(er)s do benefit from this treatment (Bonanno et al., 2002). Further, because widow(er)s must attend to new daily tasks and cope with new stressors, interventions focused on specific skills training, health behavior change, or stress-reduction is likely warranted. We argue that a personalized approach to care during this difficult period is an important next step.
Conclusion
Spousal bereavement increases the risk for poor health outcomes, as it is accompanied by alterations in mood, behavior, and disruptions to physiological and immunological systems. We surmise these systems impact and are affected by negative health behaviors and emotional distress; together, these interacting factors potentiate risk for heart disease and depression. Individual differences among widow(ers)s may help to identify individuals that require formal intervention. Future work should distinguish comprehensive patterns of risk and reslience to target individualized treatments toward vulnerable individuals.
ACKNOWLEDGEMENTS
This work was supported by the National Heart, Lung, and Blood Institute (1R01HL127260-01).
REFERENCES
- Bonanno GA, Wortman CB, Nesse RM (2004). Prospective patterns of resilience and maladjustment during widowhood. Psychology and Aging, 19, 260–271. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Wortman CB, Lehman DR, Tweed RG, Haring M, Sonnega J, Carr D, & Nesse RM (2002). Resilience to loss and chronic grief: A prospective study from preloss to 18-months postloss. Journal of Personality and Social Psychology, 83(5), 1150. 10.1037/0022-3514.83.5.1150 [DOI] [PubMed] [Google Scholar]; A historical classic, one of the first papers to show different trajectory profiles of grief.
- Buckley T, Mihailidou AS, Bartrop R, McKinley S, Ward C, Morel-Kopp M-C, Spinaze M, & Tofler GH (2011). Haemodynamic changes during early bereavement: Potential contribution to increased cardiovascular risk. Heart, Lung and Circulation, 20(2), 91–98. 10.1016/j.hlc.2010.10.073 [DOI] [PubMed] [Google Scholar]
- Chirinos DA, Ong JC, Garcini LM, Alvarado D, & Fagundes C (2019). Bereavement, self-reported sleep disturbances, and inflammation: Results from Project HEART. Psychosomatic Medicine, 81(1), 67. 10.1097/PSY.0000000000000645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Granger S, & Fuller-Thomson E (2015). The association between bereavement and biomarkers of inflammation. Behavioral Medicine, 41(2), 49–59. 10.1080/08964289.2013.866539 [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Brown RL, Chen MA, Murdock KW, Saucedo L, LeRoy A, Wu EL, Garcini LM, Shahane AD, Baameur F, & Heijnen C (2019). Grief, depressive symptoms, and inflammation in the spousally bereaved. Psychoneuroendocrinology, 100, 190–197. 10.1016/j.psyneuen.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Murdock KW, LeRoy A, Baameur F, Thayer JF, & Heijnen C (2018). Spousal bereavement is associated with more pronounced ex vivo cytokine production and lower heart rate variability: Mechanisms underlying cardiovascular risk? Psychoneuroendocrinology, 93, 65–71. 10.1016/j.psyneuen.2018.04.010 [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, & Wager TD (2015). Brain-Body Pathways Linking Psychological Stress and Physical Health. Current Directions in Psychological Science, 24(4), 313–321. 10.1177/0963721415581476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes T, & Rahe R (1967). The social readjustment rating scale. Journal of Psychosomatic Research, 11, 213–218. [DOI] [PubMed] [Google Scholar]
- Irwin M, Daniels M, Craig Risch S, Bloom E, & Weiner H (1988). Plasma cortisol and natural killer cell activity during bereavement. Biological Psychiatry, 24(2), 173–178. 10.1016/0006-3223(88)90272-7 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Derry HM, & Fagundes CP (2015). Inflammation: Depression fans the flames and feasts on the heat. American Journal of Psychiatry, 172(11), 1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles LM, Ruiz JM, & O’Connor M-F (2019). A systematic review of the association between bereavement and biomarkers of immune function. Psychosomatic Medicine, 81(5), 415. 10.1097/PSY.0000000000000693 [DOI] [PubMed] [Google Scholar]; A clearly written, user-friendly, and comprehensive meta-analysis for readers who wish to expand their knowledge on biomarkers of immune function associated with bereavement.
- Korenromp IHE, Grutters JC, van den Bosch JMM, Zanen P, Kavelaars A, & Heijnen CJ (2011). Reduced Th2 cytokine production by sarcoidosis patients in clinical remission with chronic fatigue. Brain, Behavior, and Immunity, 25(7), 1498–1502. 10.1016/j.bbi.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Kusnecov AW, & Anisman H (2013). The Wiley-Blackwell handbook of psychoneuroimmunology. Wiley Online Library. [Google Scholar]
- LeRoy AS, Gabert Tess, Garcini LM, Murdock KW, Heijnen CJ, & Fagundes CP (2020). Attachment orientations and loss adjustment among bereaved spouses. Psychoneuroendocrinology [DOI] [PMC free article] [PubMed] [Google Scholar]; A thorough, far-reaching theoretical analysis that takes a biopsychosocial approach to our understanding of attachment and grief trajectories.
- Miller GE, Cohen S, & Ritchey AK (2002). Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology, 21(6), 531. 10.1037/0278-6133.21.6.531 [DOI] [PubMed] [Google Scholar]
- Moon JR, Glymour MM, Vable AM, Liu SY, & Subramanian SV (2014). Short- and long-term associations between widowhood and mortality in the United States: Longitudinal analyses. Journal of Public Health, 36(3), 382–389. 10.1093/pubmed/fdt101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcliffe-Kaufmann L, Kaufmann H, Martinez J, Katz SD, Tully L, & Reynolds HR (2016). Autonomic findings in Takotsubo cardiomyopathy. The American Journal of Cardiology, 117(2), 206–213. 10.1016/j.amjcard.2015.10.028 [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Allen JJB, & Kaszniak AW (2002). Autonomic and emotion regulation in bereavement and depression. Journal of Psychosomatic Research, 52(4), 183–185. 10.1016/S0022-3999(02)00292-1 [DOI] [PubMed] [Google Scholar]; Grief: A Brief History of Research on How Body, Mind, and Brain Adapt. Psychosomatic medicine. This paper is an assessable historical account of biopsychosocial processes related to grief.
- O’Connor M-F, Wellisch DK, Stanton AL, Olmstead R, & Irwin MR (2012). Diurnal cortisol in complicated and non-complicated grief: Slope differences across the day. Psychoneuroendocrinology, 37(5), 725–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins ML, & Kubiak T (2014). Ecological momentary assessment in behavioral medicine. In The handbook of behavioral medicine (pp. 429–446). John Wiley & Sons, Ltd. [Google Scholar]
- Schultze-Florey CR, Martínez-Maza O, Magpantay L, Breen EC, Irwin MR, Gündel H, & O’Connor M-F (2012). When grief makes you sick: Bereavement induced systemic inflammation is a question of genotype. Brain, Behavior, and Immunity, 26(7), 1066–1071. 10.1016/j.bbi.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaver PR, & Tancredy CM (2001). Emotion, attachment, and bereavement: A conceptual commentary. In Handbook of bereavement research: Consequences, coping, and care (pp. 63–88). 10.1037/10436-003 [DOI] [Google Scholar]
- Stahl ST, & Schulz R (2014). Changes in routine health behaviors following late-life bereavement: A systematic review. Journal of Behavioral Medicine, 37(4), 736–755. 10.1007/s10865-013-9524-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroebe M, & Schut H (2010). The Dual Process Model of Coping with Bereavement: A Decade on. OMEGA - Journal of Death and Dying, 61(4), 273–289. 10.2190/OM.61.4.b [DOI] [PubMed] [Google Scholar]
- Stroebe M, Schut H, & Stroebe W (2007). Health outcomes of bereavement. The Lancet, 370(9603), 1960–1973. 10.1016/S0140-6736(07)61816-9 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. 10.1016/S0165-0327(00)00338-4 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Sternberg E (2006). Beyond heart rate variability: Vagal regulation of allostatic systems. Annals of the New York Academy of Sciences, 1088(1), 361–372. 10.1196/annals.1366.014 [DOI] [PubMed] [Google Scholar]