Abstract
Brief everyday stressors can provoke cardiovascular, hormonal, and immune changes, with considerable variation in the magnitude and duration of these responses. Acute responses to daily stressors can vary widely among individuals experiencing the same stressor, and these physiological responses may not align with stress appraisals. This review highlights individual and dyadic factors that may heighten and prolong stress reactivity, and their implications for health. We discuss depression, rumination, early life adversity, and social evaluation as individual level factors, and interpersonal stress processes and relationship quality as dyadic level factors that may influence physiological stress responses. Heightened and prolonged stress reactivity can provide a gateway to the physiological dysregulation that underlies depression and chronic disease, which themselves alter stress reactivity – a vicious cycle. Interventions that may dampen physiological stress reactivity include yoga, meditation, health behaviors (diet, exercise, and sleep), and cognitive behavioral therapy.
Keywords: Stress reactivity, depression, close relationships, rumination, coregulation
Stress Reactivity: What Pushes Us Higher, Faster, and Longer – and Why It Matters
Brief everyday stressors are inherent in life, but their health consequences vary based on individual differences in physiological stress reactivity. The body’s acute responses to daily stressors can differ widely among individuals experiencing the same stressor, and these physiological responses may not align with stress appraisals (Campbell & Ehlert, 2012; Turner et al., 2020).The hormonal, cardiovascular, and inflammatory responses to stress are biologically adaptive, readying the body for action and possible injury, but a heightened or prolonged response, especially in the context of modern stressors that do not require fighting or fleeing, promotes biological wear and tear that can shape health trajectories (McEwen, 1998). Although we focus on amplified and long-lasting reactivity, low responsiveness can also be problematic (Turner et al., 2020).
A stress response is adaptive when its magnitude aligns with the stressor’s threat and the body can return to a resting state soon after the threat ends. An exaggerated response needlessly taxes the body, preparing it for action that may be inappropriate; for instance, many modern stressors, like receiving critical feedback from a supervisor, are not life-threatening and do not require a “fight-or-flight” response, yet the stress response mobilizes energy reserves (e.g., glucose) for the exaggerated response. The response’s duration matters as well. Prestressor worry and post-stressor rumination prolong the stressor’s effects; worry provokes an anticipatory response, and rumination hinders a return to baseline. In contrast, an adaptive stress response is flexible and short-lived. In the context of a recurring stressor (e.g., parents managing their young child’s tantrums), a failure to habituate, or recognize the relative safety of this repeating scenario, provokes an unnecessary physiological response. Although many people habituate to a repeated stressor, unpredictable and uncontrollable stressors more reliably evoke cardiovascular, neuroendocrine, and inflammatory responses.
Reactivity to stressors yields insights into health-related risks that cannot be gleaned from resting measurements. For example, parasympathetic nervous system (PNS) functioning—the body’s rest and digest system—did not vary between depressed and nondepressed individuals during resting states, but differences emerged when confronting a stressor (Hu, Lamers, de Geus, & Penninx, 2016). The PNS opposes the “fight-or-flight” response of the sympathetic nervous system (SNS), and when PNS activity is low (reflected by lower heart rate variability; HRV), the body’s stress response remains uncontested, facilitating autonomic arousal (sympathetic dominance). Beyond physiological responding, HRV is related to cognitive, emotional, and social processes, e.g., individuals with lower HRV are less able to respond flexibly during stress (Appelhans & Luecken, 2006), and lower HRV can promote inflammation (Williams et al., 2019). Although the immune system’s acute inflammatory response to infection or trauma is beneficial, chronic inflammation characterizes multiple systemic diseases and disorders including cardiovascular disease, metabolic syndrome, diabetes, asthma, arthritis, psoriasis, and chronic pain among others, and each of these also carries an increased risk for depression (Kiecolt-Glaser, Derry, & Fagundes, 2015). In fact, inflammation plays a key role in depression’s pathogenesis for a subset of depressed individuals (Kiecolt-Glaser et al., 2015).
Laboratory stress paradigms provide standardized methods for examining stress reactivity. For example, the Trier Social Stress Test (TSST) evokes potent cardiovascular, endocrine, and inflammatory stress responses in most people (Fagundes, Glaser, Hwang, Malarkey, & Kiecolt-Glaser, 2013). During an anticipatory stress phase, participants are asked to prepare a speech for a 2–3 person “hiring committee” about why they are the best candidate for a job, followed by a 5-minute presentation which is framed as part of a job interview. Immediately following the presentation, participants complete a challenging 5-minute mental arithmetic task requiring them to start at the beginning of the task when a mistake is made. The evaluators maintain neutral expressions throughout to maximize social evaluative threat.
Depression, work stress, and low socioeconomic status reliably predict heightened inflammatory responses to the TSST (Steptoe, Hamer, & Chida, 2007). Not surprisingly, personally relevant daily stressors can evoke much stronger and more sustained physiological responses than laboratory stressors. Below we review both individual and dyadic psychosocial factors that push stress reactivity higher, faster, and longer, as well as interventions that promote a more adaptive response.
Stress Reactivity at the Individual Level
Traveling Companions: Stress and Depression
Depression and stress reactivity have an unhealthy reciprocal relationship (Kiecolt-Glaser et al., 2015). This bidirectional relationship contributes to greater physiological reactivity, including larger and longer-lasting inflammatory responses. Larger responses to stressors increase the likelihood of heightened depressive symptoms and inflammation, as well as the possibility of clinically significant depression. Inflammation can increase depression vulnerability by heightening amygdala reactivity to threat, reducing ventral striatal reward responding, and reducing serotonin availability within the brain (Dantzer, 2016; Eisenberger et al., 2010; Inagaki, Muscatell, Irwin, Cole, & Eisenberger, 2012).
Although greater reactivity can influence depression onset, current and past depression can also heighten stress responses. Even in the absence of a current depressive episode, individuals with a history of depression may have greater emotional and physiological reactivity to stressors than those without prior depression (Fagundes et al., 2013; Hammen, 1991). For example, individuals with remitted depression had lower HRV in response to stressors than controls (Hu et al., 2016). The scarring hypothesis helps to explain depression’s long-lasting effects in that latent depressive symptoms may emerge at times of high stress (Wichers, Geschwind, Van Os, & Peeters, 2010). Relatedly, even mild depressive symptoms heightened and prolonged inflammatory activity during the TSST (Fagundes et al., 2013). Therefore, even subclinical or remitted depression can impact stress reactivity. Taken together, these findings demonstrate bidirectional pathways between stress responses and depression.
Work from our lab assessed the individual and joint contributions of a mood disorder history and an interpersonal stressor, a marital conflict discussion, on obesity-related metabolic responses to high-fat meals (Kiecolt-Glaser, 2018). Participants with a mood disorder history had steeper rises in glucose and interleukin-6 (IL-6, an important inflammatory marker) than those without such a history. Additionally, those with a mood disorder history who also used more hostile behaviors during the conflict discussion had lower post-meal resting energy expenditure (REE), higher insulin, and higher peak triglyceride responses than other participants. If the lowered REE were sustained over a year, it would add a weight gain of 7.6 pounds. These data illustrate additional pathways through which interpersonal stressors coupled with a mood disorder history could synergistically heighten the risk for obesity, cardiovascular disease, and metabolic syndrome.
Worry and Rumination
Anticipatory stress, worry, and post-event rumination extend the physiological, cognitive, and emotional impact of acute stressors. Worry and rumination, collectively termed perseveration, raise baseline levels of physiological arousal and also facilitate ongoing reactivity, even in the absence of imminent threat (Ottaviani et al., 2016). For example, rumination promoted larger and more long-lasting cortisol, blood pressure, heart rate, and HRV responses, and worry predicted lower HRV and diastolic and systolic blood pressure reactivity (Ottaviani et al., 2016). Anticipatory cortisol responses have been reported in 20–40% of healthy individuals preceding the TSST; in fact, the anticipatory stress response explained 67% of the variance in cortisol reactivity during the stressor (Engert et al., 2013). Heightened reactivity among worriers and ruminators may extend to other physiological systems. For instance, because low HRV is associated with greater inflammation, worriers’ and ruminators’ elevated autonomic responses to stressors may contribute to inflammation, and, ultimately, poorer health and disease outcomes (Turner et al., 2020).
Early Life Adversity
Early life adversity primes physiological systems in ways that alter stress reactivity across the lifespan. The Adaptive Calibration Model emphasizes the plastic nature of stress response systems, in that they can recalibrate even after birth to align with unique environmental demands (Del Giudice, Ellis, & Shirtcliff, 2011). Similarly, the Biological Embedding Model suggests that stressful experiences early in life are programmed into cells that regulate inflammation, thus promoting greater psychological and biological stress reactivity throughout the life span (Miller, Chen, & Parker, 2011). Within this model, primed immune cells are more reactive to stress and unrestrained due to deficient inhibitory signaling (Miller et al., 2011). Research on daily stressors corroborated these findings: IL-6 levels were 2.35 times greater in individuals with a childhood abuse history who experienced multiple stressors in the prior day compared to participants with the same number of prior day stressors but no abuse history (Gouin, Glaser, Malarkey, Beversdorf, & Kiecolt-Glaser, 2012).
Social Evaluation
In laboratory settings, participants’ cortisol rose when others evaluated their performance during the TSST, and, in particular, when the task felt uncontrollable while others were present (Dickerson & Kemeny, 2004). Receiving negative feedback and making negative social comparisons also amplified physiological stress reactivity (Dickerson & Kemeny, 2004). Stress recovery after completing the task was prolonged when individuals felt it was uncontrollable, slowing the return to baseline cortisol levels (Dickerson & Kemeny, 2004).
Stress Reactivity at the Dyadic Level
Interpersonal Stress Processes
Couples shape each other’s stress reactivity—for better or worse. Three distinct but interrelated processes help to explain associations between partners’ reactivity, as well as partners’ effects on each other’s reactivity (Butler, 2015). First, partners’ responses to external experiences become more similar over time. One reason partners’ stress converges is because stress can be contagious. For example, when one partner is stressed, the other partner is more likely to be stressed (Kiecolt-Glaser & Wilson, 2017). In this way, partners pick up and “catch” each other’s stress. Partners influence how they react to each other, another component of couples’ interconnected stress reactivity. For example, stress hormones rise in response to one’s own or a partner’s hostile behavior during conflict. Partners’ stress can also synchronize while they interact, such that their reactivity rises and falls together, thus impacting both partners’ psychological and biological responses. For instance, when couples’ cardiovascular reactivity synchronized during a marital disagreement, both partners had greater negative affect reactivity and higher inflammation (Wilson et al., 2018). Lastly, couples regulate each other’s emotional responses to stress (Butler, 2015). This process is complex because partners attempt to convey their own emotions while also trying to understand their partners’ emotions. Coregulation can be beneficial, such as when partners share positive emotions and become less stressed. However, coregulation can come at a cost when sharing negative emotions, such as extensively discussing their worries (i.e., co-rumination), thus heightening their stress reactivity.
Relationship Quality
Positive relationship perceptions and interactions including support and validation can lessen stress responses, whereas frequent conflict and hostile interactions exacerbate stress responses (Kiecolt-Glaser, 2018). Distressed couples use more hostile behaviors such as sarcasm, disgust, and eye rolling during stressful interactions, and these may heighten their stress responses and prime greater reactivity to future stress. Couples may become trapped in this negative cycle. Indeed, distressed partners are more likely to provoke one another, increasing their sensitivity and susceptibility to stress, which ultimately drives elevated and prolonged stress responses over time (Butler, 2015). Thus it is not surprising that unhappy marriages provide fertile grounds for increasing the risk of depressive symptoms and clinical depression (Kiecolt-Glaser, 2018).
Work from our lab showed that newlyweds who used more hostile behaviors during a marital problem discussion had larger acute increases in stress hormones (epinephrine, norepinephrine, and adrenocorticotropic hormone) as well as bigger negative changes in immune function 24 hours later compared to the less hostile (Kiecolt-Glaser, 2018). These data, collected in couples’ first year of marriage, foreshadowed marital satisfaction and divorce 10 years later. Post-conflict epinephrine in year 1 was 34% higher in couples who subsequently divorced than those who were still married 10 years later. During the first year of marriage hostile partners’ epinephrine and norepinephrine were also higher throughout the day and night in those who would later divorce compared to the still-married, with similar findings in those who were satisfied compared to the dissatisfied (but still married) 10 years later (Kiecolt-Glaser, 2018). Norepinephrine and epinephrine have half-lives of 1–2 minutes, and thus these 24-hour differences did not simply reflect responses to the conflict—these data demonstrated heightened and sustained sympathetic nervous system responsiveness. Of note, many potential confounds were accounted for in that these couples had no current or past mental or physical disorder, and they had low depressive symptoms; moreover, initially happy newlywed couples whose marriages were later troubled did not differ from their untroubled counterparts on individual difference trait variables or cardiovascular reactivity to a nonmarital experimental stressor as newlyweds. These findings show how some couples remain distressed following a marital disagreement, while others find a way to cool down during and long after this potent interpersonal stressor; these data also highlight potential mechanistic pathways from marital distress to ill health (Kiecolt-Glaser, 2018).
Altering Stress Reactivity: Yoga, Meditation, Health Behaviors, and Cognitive-Behavioral Therapy
A central axiom of meditation, yoga, and related disciplines is that regular practice reduces stress while also providing health benefits. A regular practice may “reboot” the SNS-dominated nervous system by increasing PNS activity, essentially combatting the body’s tendency to respond to stress with an overabundance of inflammation. The evidence, although limited, is provocative. For example, expert yoga practitioners’ serum IL-6 levels were 41% lower than well-matched novice practitioners, and experts had smaller lipopolysaccharide-stimulated IL-6 responses to lab stressors (cold pressor and mental arithmetic) than their novice counterparts (Kiecolt-Glaser et al., 2010).
Meditation may reduce the psychological and physiological impact of stress (Pascoe, Thompson, Jenkins, & Ski, 2017). Indeed, participants who were randomized to meditation interventions had lower levels of inflammatory markers, cortisol, resting heart rate, triglycerides, and blood pressure post-intervention compared to control subjects (Pascoe et al., 2017). Seasoned meditators also had smaller cortisol rises in response to the TSST than nonmeditators (Rosenkranz et al., 2016). After 6 weeks of training in a Tibetan Buddhist-based compassion meditation, more frequent practice predicted lower inflammatory responses to the TSST (Pace et al., 2009). Mindfulness meditation can hasten recovery from stressors; when viewing stressful images; participants’ amygdala activation levels were lower when instructed to use a mindfulness skill compared to distraction (Kral et al., 2018). These findings suggest that meditation and related contemplative interventions might prove useful in reducing stress reactivity and its subsequent physiological impact.
Although beyond the scope of this brief review, growing evidence suggests that exercise, a heathy diet low in sugar and saturated fats, and regular high-quality sleep may also reduce stress reactivity as well as depressive symptoms (Molendijk, Molero, Sánchez-Pedreño, Van der Does, & Martínez-González, 2018; Vargas & Lopez-Duran, 2017; West et al., 2010). More comprehensive research is needed, but improving these health behaviors may provide an important intervention point for reducing stress reactivity.
Central to cognitive-behavioral therapy (CBT), cognitive reappraisal seeks to better align a person’s stress appraisals with the stressor’s actual threat. However, research addressing CBT’s ability to reduce physiological stress reactivity has been mixed. Larger samples with longer treatment trials and study follow-ups may be necessary to better evaluate CBT’s effects on stress reactivity.
Health Implications
Heightened and prolonged stress reactivity is a gateway to the physiological dysregulation that underlies depression and chronic disease, which themselves alter stress reactivity – a vicious cycle. For example, individuals with heightened reactivity and a prolonged recovery from stress have a greater risk for cardiovascular morbidity and mortality, especially older adults (Turner et al., 2020). Individuals who had larger cortisol responses to an acute stressor also had shorter telomeres, suggesting accelerated cellular aging (Tomiyama et al., 2012). Premature aging and chronic diseases such as cardiovascular disease are associated with higher rates of depression (Kiecolt-Glaser et al., 2015), which then loops back and further upsets multiple biological systems (endocrine, immune, cardiovascular, metabolic, and neurocognitive). These alterations are sufficient to worsen chronic disease, potentially resulting in premature mortality (Hughes, Connor, & Harkin, 2016). Although we have focused on heightened and prolonged stress reactivity, blunted reactivity also has health consequences (Turner et al., 2020), suggesting that a mid-range stress response that is in line with the current situational demands and returns to baseline after the threat resolves, is optimal. As research continues to uncover the health implications of heightened and prolonged stress reactivity, another significant line of inquiry probes how to best intervene on individuals’ physiological stress responses. Early identification and treatment of individuals whose stress reactivity is higher and persists longer compared to their peers could provide a window of opportunity to influence health outcomes.
Figure 1. Stress Duration and Bodily Responses are Multiply.
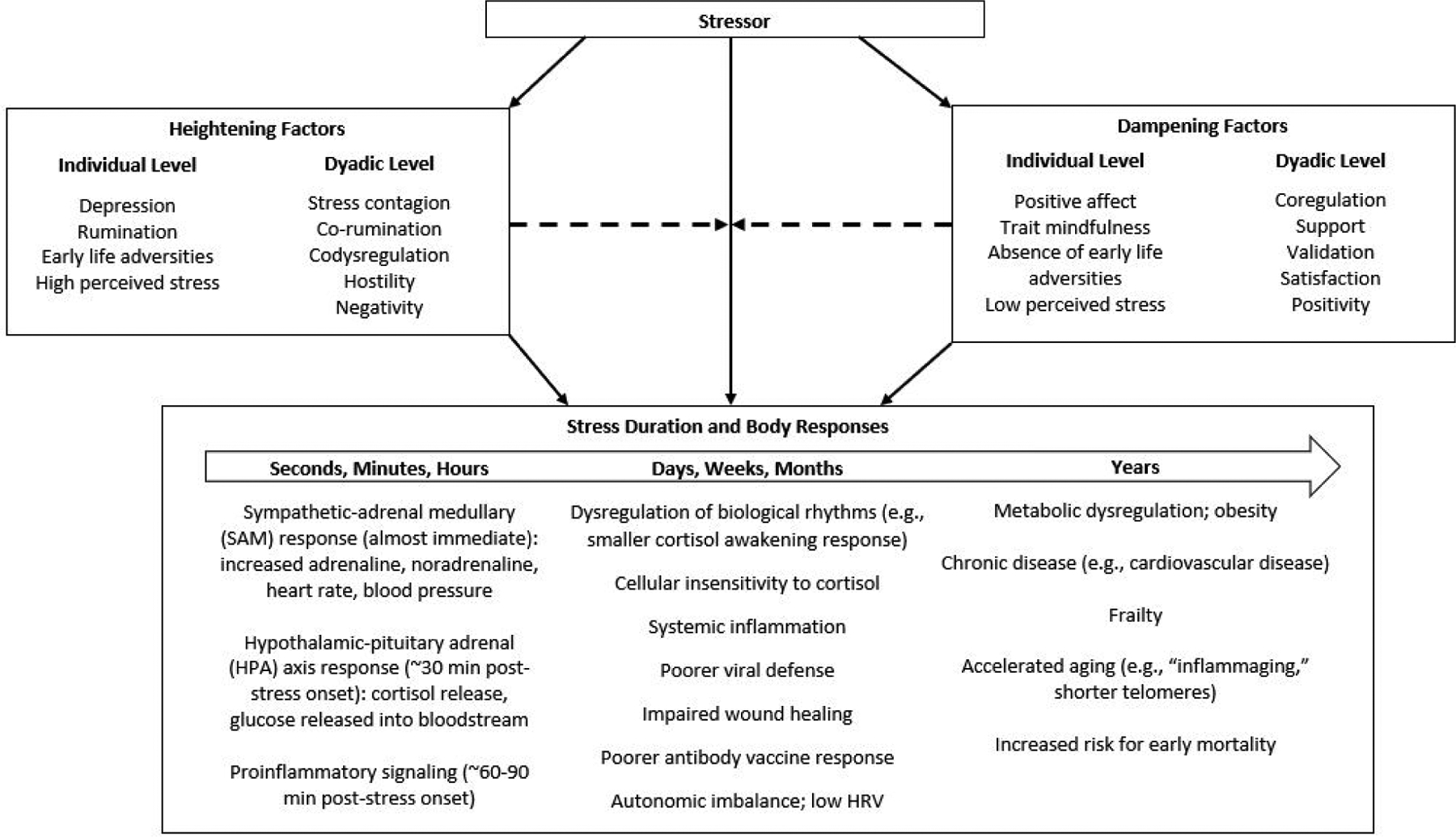
Determined Many individual and dyadic level factors determine the duration and magnitude of the stress response. These factors can moderate (i.e., heighten and dampen) the effect of the stressor on the body, as well as mechanistically link the stressor to the stress response. Factors that influence the stress response duration are not as well-characterized as those that influence the stress response magnitude. The duration and magnitude of a stressor determines the bodily response. Short-term stress lasting seconds, minutes, and hours provokes autonomic, endocrine, and immunological changes that are part of an adaptive response and do not cause lasting physiological dysregulation. However, chronic stressors can dysregulate multiple physiological systems and lead to accelerated aging.
Acknowledgments
Work on this paper was supported in part by NIH grants T32 CA229114, R01 CA186720, R01 CA186251, and R01 AG057032.
References
- Appelhans BM, & Luecken LJ (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10(3), 229–240. [Google Scholar]
- Butler EA (2015). Interpersonal affect dynamics: It takes two (and time) to tango. Emotion Review, 7(4), 336–341. [Google Scholar]
- Campbell J, & Ehlert U (2012). Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology, 37(8), 1111–1134. [DOI] [PubMed] [Google Scholar]
- Dantzer R (2016). Role of the kynurenine metabolism pathway in inflammation-induced depression: Preclinical approaches. In Inflammation-Associated Depression: Evidence, Mechanisms and Implications (pp. 117–138): Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, & Shirtcliff EA (2011). The Adaptive Calibration Model of stress responsivity. Neuroscience and Biobehavioral Reviews, 35(7), 1562–1592. doi: 10.1016/j.neubiorev.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, & Irwin MR (2010). Inflammation-Induced Anhedonia: Endotoxin Reduces Ventral Striatum Responses to Reward. Biological Psychiatry, 68(8), 748–754. doi: 10.1016/j.biopsych.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert V, Efanov SI, Duchesne A, Vogel S, Corbo V, & Pruessner JC (2013). Differentiating anticipatory from reactive cortisol responses to psychosocial stress. Psychoneuroendocrinology, 38(8), 1328–1337. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Hwang BS, Malarkey WB, & Kiecolt-Glaser JK (2013). Depressive symptoms enhance stress-induced inflammatory responses. Brain, Behavior, and Immunity, 31, 172–176. doi: 10.1016/j.bbi.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin J-P, Glaser R, Malarkey WB, Beversdorf D, & Kiecolt-Glaser JK (2012). Childhood abuse and inflammatory responses to daily stressors. Annals of Behavioral Medicine, 44(2), 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C (1991). Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology, 100(4), 555. [DOI] [PubMed] [Google Scholar]
- Hu MX, Lamers F, de Geus EJ, & Penninx BW (2016). Differential autonomic nervous system reactivity in depression and anxiety during stress depending on type of stressor. Psychosomatic Medicine, 78(5), 562–572. [DOI] [PubMed] [Google Scholar]
- Hughes MM, Connor TJ, & Harkin A (2016). Stress-related immune markers in depression: implications for treatment. International Journal of Neuropsychopharmacology, 19(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, & Eisenberger NI (2012). Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage, 59(4), 3222–3226. doi: 10.1016/j.neuroimage.2011.10.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK (2018). Marriage, divorce, and the immune system. American Psychologist, 73(9), 1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Christian L, Preston H, Houts CR, Malarkey WB, Emery CF, & Glaser R (2010). Stress, inflammation, and yoga practice. Psychosomatic Medicine, 72(2), 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Derry HM, & Fagundes CP (2015). Inflammation: depression fans the flames and feasts on the heat. American Journal of Psychiatry, 172(11), 1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, & Wilson SJ (2017). Lovesick: How couples’ relationships influence health. Annual Review of Clinical Psychology, 13, 421–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral TR, Schuyler BS, Mumford JA, Rosenkranz MA, Lutz A, & Davidson RJ (2018). Impact of short-and long-term mindfulness meditation training on amygdala reactivity to emotional stimuli. Neuroimage, 181, 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840(1), 33–44. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137(6), 959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk M, Molero P, Sánchez-Pedreño FO, Van der Does W, & Martínez-González MA (2018). Diet quality and depression risk: a systematic review and dose-response meta-analysis of prospective studies. Journal of Affective Disorders, 226, 346–354. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, & Brosschot JF (2016). Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychological bulletin, 142(3), 231. [DOI] [PubMed] [Google Scholar]
- Pace TW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, … Raison CL (2009). Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology, 34(1), 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe MC, Thompson DR, Jenkins ZM, & Ski CF (2017). Mindfulness mediates the physiological markers of stress: Systematic review and meta-analysis. Journal of Psychiatric Research, 95, 156–178. [DOI] [PubMed] [Google Scholar]
- Rosenkranz MA, Lutz A, Perlman DM, Bachhuber DR, Schuyler BS, MacCoon DG, & Davidson RJ (2016). Reduced stress and inflammatory responsiveness in experienced meditators compared to a matched healthy control group. Psychoneuroendocrinology, 68, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, & Chida Y (2007). The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, Behavior, and Immunity, 21(7), 901–912. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, O’Donovan A, Lin J, Puterman E, Lazaro A, Chan J, … Blackburn E (2012). Does cellular aging relate to patterns of allostasis?: An examination of basal and stress reactive HPA axis activity and telomere length. Physiology & Behavior, 106(1), 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AI, Smyth N, Hall SJ, Torres SJ, Hussein M, Jayasinghe SU, … Clow AJ (2020). Psychological stress reactivity and future health and disease outcomes: A systematic review of prospective evidence. Psychoneuroendocrinology, 114, 104599. [DOI] [PubMed] [Google Scholar]
- Vargas I, & Lopez-Duran N (2017). Investigating the effect of acute sleep deprivation on hypothalamic-pituitary-adrenal-axis response to a psychosocial stressor. Psychoneuroendocrinology, 79, 1–8. [DOI] [PubMed] [Google Scholar]
- West SG, Krick AL, Klein LC, Zhao G, Wojtowicz TF, McGuiness M, … Kris-Etherton PM (2010). Effects of diets high in walnuts and flax oil on hemodynamic responses to stress and vascular endothelial function. Journal of the American College of Nutrition, 29(6), 595–603. doi:29/6/595 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers M, Geschwind N, Van Os J, & Peeters F (2010). Scars in depression: is a conceptual shift necessary to solve the puzzle? Psychological Medicine, 40(3), 359–365. [DOI] [PubMed] [Google Scholar]
- Williams DP, Koenig J, Carnevali L, Sgoifo A, Jarczok MN, Sternberg EM, & Thayer JF (2019). Heart rate variability and inflammation: a meta-analysis of human studies. Brain, Behavior, and Immunity. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Bailey BE, Jaremka LM, Fagundes CP, Andridge R, Malarkey WB, … Kiecolt-Glaser JK (2018). When couples’ hearts beat together: Synchrony in heart rate variability during conflict predicts heightened inflammation throughout the day. Psychoneuroendocrinology, 93, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Recommended Readings
- Brosschot JF, Gerin W, & Thayer JF (2006). The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research, 60(2), 113–124. DOI: 10.1016/j.jpsychores.2005.06.074 [DOI] [PubMed] [Google Scholar]; A seminal article on how rumination prolongs and heightens physiological stress reactivity.
- Butler EA (2015). Interpersonal affect dynamics: It takes two (and time) to tango. Emotion Review, 7(4), 336–341. DOI: 10.1177/1754073915590622 [DOI] [Google Scholar]; This review summarizes three processes that give rise to interpersonal affective patterns, including synchrony, transmission, and coregulation and codysregulation.
- Cohen S, Murphy ML, & Prather AA (2019). Ten surprising facts about stressful life events and disease risk. Annual Review of Psychology, 70, 577–597. DOI: 10.1146/annurev-psych-010418-102857 [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides a valuable perspective on how and when stress does (and does not) affect disease risk.
- Kiecolt-Glaser JK, & Wilson SJ (2017). Lovesick: How couples’ relationships influence health. Annual Review of Clinical Psychology, 13, 421–443. DOI: 10.1146/annurev-clinpsy-032816-045111 [DOI] [PMC free article] [PubMed] [Google Scholar]; This review highlights recent advances in research addressing the pathways from romantic relationships to physiological functioning and health.
- Rohleder N (2019). Stress and inflammation – The need to address the gap in the transition between acute and chronic stress effect. Psychoneuroendocrinology, 105, 164–171. DOI: 10.1016/j.psyneuen.2019.02.021. [DOI] [PubMed] [Google Scholar]; This article summarizes the effects of acute and chronic stress on inflammation, and then discusses the importance of assessing the transitional phase between the two.


