Abstract
Acetone is one of the most abundant carbonyl compounds in the atmosphere and it plays an important role in atmospheric chemistry. The role of the ocean in the global atmospheric acetone budget is highly uncertain, with past studies reaching opposite conclusions as to whether the ocean is a source or sink. Here we use a global 3-D chemical transport model (GEOS-Chem) simulation of atmospheric acetone to evaluate the role of air-sea exchange in the global budget. Inclusion of updated (slower) photolysis loss in the model means that a large net ocean source is not needed to explain observed acetone in marine air. We find that a simulation with a fixed seawater acetone concentration of 15 nM based on observations can reproduce the observed global patterns of atmospheric concentrations and air-sea fluxes. The Northern Hemisphere oceans are a net sink for acetone while the tropical oceans are a net source. On a global scale the ocean is in near-equilibrium with the atmosphere. Prescribing an ocean concentration of acetone as a boundary condition in the model assumes that ocean concentrations are controlled by internal production and loss, rather than by air-sea exchange. An implication is that the ocean plays a major role in controlling atmospheric acetone. This hypothesis needs to be tested by better quantification of oceanic acetone sources and sinks.
1. Introduction
Acetone is the most abundant carbonyl compound in the atmosphere after formaldehyde. In the upper troposphere, acetone photolysis is an important source of OH, the main atmospheric oxidant [Singh et al., 1995]. Acetone also affects the budget of nitrogen oxides by serving as a precursor for peroxyacetyl nitrate (PAN) [Singh et al., 1995], with complex implications for tropospheric ozone. Acetone is directly emitted by vegetation [Schade and Goldstein, 2001], biomass burning [Andreae and Merlet, 2001], and industry [Singh et al., 1994], and is also produced in the atmosphere by oxidation of biogenic and anthropogenic volatile organic compounds (VOCs) [Pozzer et al., 2010]. It is lost through oxidation by OH, photolysis, and surface uptake (Table 1).
Table 1.
Global Budget of Atmospheric Acetone
| This Work | Jacob et al. [2002] | Other Estimatesa | |
|---|---|---|---|
| Inventory, Tg | 5.6 | 3.8 | 3.8–7.2 |
| Sources, Tg a−1 | |||
| Emissions | |||
| Anthropogenic | 0.73b | 1.1 ± 0.5 | 1.1–2 |
| Open biomass burning | 2.8 | 4.5 ± 1.6 | 2.4–9 |
| Terrestrial Biosphere | 32 | 35 ± 10 | 20–172 |
| Atmospheric Production | |||
| Oxidation of isoalkanes (mainly anthropogenic) | 26c | 21 ± 5 | 1–28 |
| Oxidation of biogenic VOCs | 5 | 7 ± 3 | 7 |
| Sinks, Tg a−1 | |||
| Oxidation by OH | 33 | 27 | 18–27 |
| Photolysis | 19 | 46 | 9–22 |
| Land uptake | 12 | 9 | 9–19 |
| Net Ocean exchange, Tg a−1 d | −2 | 13 ± 6 | −62–2.5 |
| Gross source | 80 | NA | NA |
| Gross sink | 82 | NA | NA |
Arnold et al. [2005], Singh et al. [2003, 2004], Potter et al. [2003], Marandino et al. [2006], Sinha et al. [2007], Pozzer et al. [2010], and Elias et al. [2011].
Including biofuel use.
Including 22 Tg a−1 from propane and 4 Tg a−1 from the higher alkanes.
Positive values indicate upward fluxes.
A major uncertainty in the budget of atmospheric acetone is the role of the ocean. Photochemical and biological processes in the ocean both produce and consume acetone [Nemecek-Marshall et al., 1995; Sinha et al., 2007; Zhou and Mopper, 1997]. Atmospheric acetone concentrations over the remote oceans are in the range of 200–500 pptv [Singh et al., 2000], only a factor of 3–4 lower than over continental source regions. To account for this weak gradient in the face of a relatively short (~15 days) atmospheric lifetime, Jacob et al. [2002] hypothesized a large oceanic emission of acetone in simulations with the GEOS-Chem global chemical transport model (CTM). Since then, measurements of the quantum yield for acetone photolysis have led to upward revision of the atmospheric lifetime for acetone [Blitz et al., 2004]. In addition, field observations have shown that the ocean can be a either a source or a sink of acetone [Marandino et al., 2005; Sinha et al., 2007; Taddei et al., 2009; Williams et al., 2004]. Here we use GEOS-Chem to integrate these new experimental data into an improved understanding of the role of the ocean in the global budget of atmospheric acetone.
2. Methods
We use the GEOS-Chem global 3-D CTM including detailed ozone-NOx-VOC-aerosol chemistry (version 9.01.01, www.geos-chem.org) to simulate the global atmospheric distribution of acetone. GEOS-Chem is driven by NASA/GEOS-5 assimilated meteorological data with 0.5° × 0.67° horizontal resolution, 47 levels in the vertical, and 3–6 hour temporal resolution. We degrade the horizontal resolution to 2° × 2.5° for input into GEOS-Chem. We use a 1-year simulation for 2006, with a 1-year spin-up to remove the effect of initial conditions.
Sources of acetone include direct emissions from biogenic, anthropogenic, and biomass burning sources, as well as the atmospheric oxidation of organic precursors (Table 1). The ensemble of sources is described by Jacob et al. [2002] and updates are described below. We use the RETRO (Reanalysis of the TROpospheric chemical composition) emission inventory for anthropogenic emissions of acetone and of the isoalkane precursors [van het Bolscher et al., 2008]. The RETRO emission inventory is for 2000, and we scale it to 2006 with activity factors from van Donkelaar et al. [2008]. We use 2006 GFED2 monthly biomass burning emissions for acetone and the isoalkanes [van der Werf et al., 2009]. Terrestrial biogenic emissions of acetone from metabolism and decay are calculated locally using the Model of Emissions of Gases and Aerosols from Nature (MEGAN v2.0) [Guenther et al., 2006]. Acetone production from biogenic monoterpenes and 2-methyl-3-buten-2-ol is calculated using MEGAN emissions for these VOCs and fixed yields from Jacob et al. [2002].
Losses for acetone in the model include oxidation by OH, photolysis, and deposition. Exchange with the ocean is discussed below. We use a rate constant k = 1.33 × 10−13 + 3.28 × 10−11exp[2000/T] cm3 molecule−1 s−1 for the oxidation of acetone by OH [Sander et al., 2011]. Photolysis of acetone is computed using absorption cross sections and pressure-dependent quantum yields from Blitz et al. [2004], and local actinic fluxes from the Fast-JX radiative transfer scheme [Wild et al., 2000] as implemented in GEOS-Chem by Mao et al. [2010]. We assume a dry deposition velocity of 0.1 cm s−1 for ice-free land as in Jacob et al. [2002].
There is evidence that the ocean mixed layer is a large acetone reservoir, containing ∼5 times the atmospheric burden [Williams et al., 2004]. We compile in Table 2 literature values for observed surface seawater concentrations and air-sea fluxes. The processes controlling acetone concentrations in seawater are uncertain. Production can take place by photo-degradation of dissolved organic matter [Zhou and Mopper, 1997] and directly by phytoplankton and bacteria [Nemecek-Marshall et al., 1995]. Loss can take place by photo-degradation or biotic consumption [Mopper and Stahovec, 1986; Nemecek-Marshall et al., 1995]. Two recent field campaigns support a biologically mediated acetone source in seawater. Sinha et al. [2007] measured the air-sea flux of acetone while also monitoring phytoplankton abundance. They found systematic upward fluxes in the presence of both strong sunlight and biological activity. Taddei et al. [2009] also observed upward acetone fluxes in phytoplankton bloom areas in the South Atlantic.
Table 2.
Measurements of Acetone Concentrations in Surface Seawater and Air-Sea Fluxesa
| Period | Location | Concentration (nM) | Fluxb (ng m−2 s−1) | Reference |
|---|---|---|---|---|
| Mar 1989 | Bahamas | 3 – 15c | −2.8 – 1.0*c | Zhou and Mopper [1997] |
| Oct – Nov 2002 | Tropical Atlantic | 17.6 ± 8.1 | 7.8* | Williams et al. [2004] |
| May -Jul 2004 | W. Pacific (mid-latitude) | 13.6 ± 3.0 | −10.2d, −7.4* | Marandino et al. [2005] |
| May -Jul 2004 | W. Pacific (equatorial) | 13.9 ± 11.7 | −2.6d, 1.1* | Marandino et al. [2005] |
| Jun – Jul 2004 | North Atlantic | <9.6 | ——— | Hudson et al. [2007] |
| May – Jun 2005 | Norwegian fjord | ——— | 0.21 | Sinha et al. [2007] |
| Jan – Feb 2007 | S. Atlantic (bloom) | ——— | 0.67 | Taddei et al. [2009] |
| Jan 2007 | S. Atlantic (non-bloom) | ——— | ∼−1.0 – 0.2e | Taddei et al. [2009] |
| Jul – Aug 2008 | Northwest Pacific | 19.0 ± 4.4 | ——— | Kameyama et al. [2010] |
Values are means standard deviation unless otherwise noted.
Fluxes were either directly measured by eddy covariance or mesocosms, or were inferred from the measured saturation ratios. The latter are indicated by asterisks. Positive fluxes are upward.
Ranges of concentrations and inferred fluxes. The authors reported a flux of 15.7 ng m−2 s−1 based on an acetone concentration of 55 nM in the surface micro-layer. We repeated their flux calculations using their measured bulk seawater concentrations.
The authors reported a discrepancy between the fluxes measured by eddy covariance and inferred from saturation ratios. Both are shown here. All measured fluxes were downward, but the range of saturation ratios (0.35–6.5), implied both upward and downward fluxes.
Taddei et al. [2009] report “near zero, negative or highly variable low acetone fluxes” in non-bloom conditions. They do not report a mean value for non-bloom conditions, thus a range is given here based on DOY 28 – 30 in Figure 4 of Taddei et al. [2009].
We assume in GEOS-Chem a fixed seawater acetone concentration of 15 nM, based on the data ensemble in Table 2. The data do not show evident seasonal or spatial patterns that would warrant a more detailed treatment. We also note that the two studies that report the lowest bulk seawater acetone concentrations, Zhou and Mopper [1997] and Hudson et al. [2007], rely on the smallest number of samples. By setting a fixed seawater concentration we assume implicitly that acetone in the ocean is controlled by internal sources and sinks, rather than by exchange with the atmosphere. There is some support for this argument based on analogy with methanol [Millet et al., 2008]. Williams et al. [2004] reported a sharper vertical gradient for acetone than methanol in the ocean mixed layer, suggesting a shorter lifetime for acetone. Heikes et al. [2002] estimated an ocean mixed lifetime for methanol of 3 days against bacterial uptake, and Williams et al. [2004] estimated an ocean mixed layer burden of 15.9 Tg for acetone. Assuming an upper limit of 3 days for the acetone lifetime in the ocean mixed layer, a minimum source of 2000 Tg a−1 would be needed to sustain the ocean mixed layer burden. This is much larger than the atmospheric source of acetone (Table 1), and thus the acetone concentration in the ocean is likely internally controlled.
We calculate air-sea fluxes of acetone locally in GEOS-Chem by applying the two-film model of Liss and Slater [1974], with liquid and gas-phase transfer velocities from Nightingale et al. [2000] and Johnson [2010] respectively, and a Henry’s law equilibrium constant for acetone of 27 M atm−1 [Benkelberg et al., 1995]. Equilibrium with a seawater concentration of 15 nM implies atmospheric acetone mixing ratios of 220 pptv and 550 pptv at 283 K and 298 K respectively. Observed acetone mixing ratios in the marine boundary layer are typically in that range [Singh et al., 2000], so the ocean can be either a net source or sink for acetone depending on the local atmospheric concentration and surface temperature.
3. Global Atmospheric Distribution of Acetone
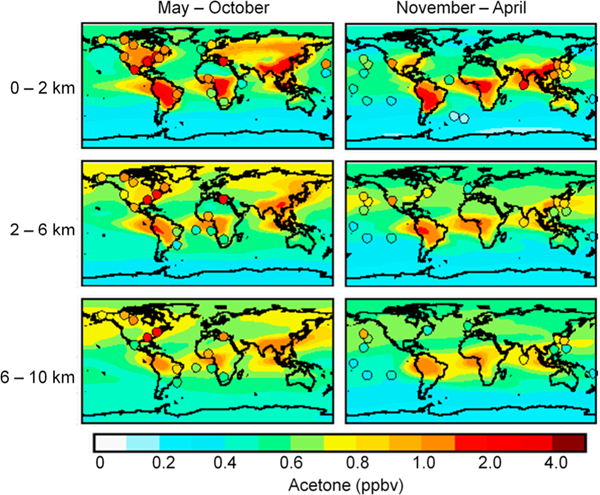
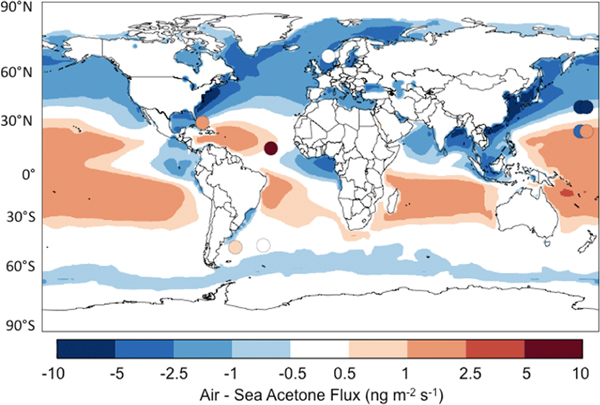
Figure 1 shows the simulated acetone mixing ratios in three altitude ranges and for two seasons. Mean observations from aircraft missions, ship cruises, and surface sites are overlaid as circles. The observations are generally for years other than 2006 but we expect that interannual variability is small relative to other aspects of variability. Table S1 in the auxiliary material gives details of the observations.1 Figures S1 and S2 show comparisons with the aircraft vertical profiles. Figure 2 presents the simulated annual mean air-sea acetone fluxes. The ocean parameterization produces a weak net global sink of 2 Tg a−1, but the net flux varies geographically and seasonally (Figure S3). The ocean is a net sink at northern latitudes but a net source between 20°N and 40°S. At high southern latitudes the atmosphere and ocean are near equilibrium during summer and the ocean is a weak sink during winter. Comparison with observed fluxes is discussed below (see auxiliary material for further details).
Figure 1.
Simulated and observed global distribution of atmospheric acetone for two seasons and three altitude bins. Simulated mixing ratios are for 2006 and are shown as solid contours. Filled circles are vertically binned mean observations in different years from aircraft missions, ship cruises and surface sites [de Gouw et al., 2001, 2006; Hornbrook et al., 2011; Jacob et al., 1996; Lelieveld et al., 2002; Lewis et al., 2005; Mao et al., 2006; Marandino et al., 2005; Murphy et al., 2010; Singh et al., 2000, 2009, 2001, 1994, 2004; Warneke and de Gouw, 2001; Williams et al., 2004]. Data for aircraft missions have been averaged over regionally coherent regions as in Jacob et al. [2002] with the addition of more recent missions. Data from ship cruises are primarily the averages reported in the literature. Circles are placed at the mean latitude and longitude of the observations. Table S1 gives details of the observations. Figures S1 and S2 show comparisons with the aircraft vertical profiles.
Figure 2.
Simulated annual mean air-sea fluxes of acetone for 2006, and mean observed air-sea fluxes (Table 2, third column). Positive values indicate a net flux from the sea to the air. Marandino et al. [2005] report a discrepancy between the flux calculated from the saturation ratio and that measured by eddy covariance. Both are shown here over the western Pacific, with the measured fluxes plotted to the west of the calculated fluxes. There is seasonal variation to the net air-sea fluxes, and Figure S3 presents the fluxes for January and July.
The atmospheric simulation shows no systematic bias compared to the observations in Figure 1 and provides in general a good representation of observed latitudinal and seasonal variability as well as land-ocean gradients. The acetone mixing ratios in Figure 1 based on PTR-MS and API-CIMS instruments are upper limits because of interference from propanal and glyoxal; however, this is expected to be a minor issue [de Gouw et al., 2003], especially in the pristine marine boundary layer where propanal mixing ratios are typically <20% of acetone mixing ratios [Singh et al., 2003]. Our simulation performs similarly to the simulation of Jacob et al. [2002], which used Bayesian optimization to match observations. A major difference is that we do not need to invoke a large global ocean source in order to explain the observations in marine air. This is because our mean atmospheric lifetime of acetone against chemical sinks (photolysis and reaction with OH) is 39 days, compared to 19 days of Jacob et al. [2002], reflecting the lower photolysis quantum yields from Blitz et al. [2004]. Thus we have more transport of continental acetone to the oceans. The tropical oceans remain a net source of acetone in our simulation, which is consistent with the limited data of Table 2 [Williams et al., 2004] and can account for the relatively high acetone concentrations observed over the tropical Pacific [Singh et al., 2001]. Unlike at northern mid-latitudes, aircraft vertical profiles over the tropical oceans do not show depletion of acetone in the marine boundary layer relative to the free troposphere above [Singh et al., 2001].
Figure 2 shows the strongest ocean sink downwind of the Northern Hemisphere continents where high atmospheric acetone concentrations are coupled with strong wind speeds and cold temperatures. We explain in this manner the observed acetone depletion in the marine boundary layer relative to the free troposphere over the North Pacific [Singh et al., 2003] and data over the Northwest Atlantic showing the ocean to be a sink for acetone in continental outflow [Mao et al., 2006]. Based on acetone / CO ratios from the INDOEX and MINOS field campaigns, de Reus et al. [2003] speculated that that the ocean serves a sink for acetone in polluted regions and a source in clean air masses. Our global results are consistent with this view. Marandino et al. [2005] find a stronger ocean sink than the model over the northwest Pacific (Figure 2) but their atmospheric acetone observations are very high (averaging 1.05 ppbv) and anomalous relative to the other data in Figure 1.
The lowest tropospheric concentrations simulated by the model are over the Southern Ocean, reflecting the remoteness from terrestrial sources and the cold ocean temperatures. There the mean concentration is 0.2 ppbv and the ocean is a weak net sink. These results are consistent with cruise data in the Southern Ocean between South Africa and Chile [Taddei et al., 2009].
4. Atmospheric Budget
Table 1 summarizes our global atmospheric budget of acetone and compares it to previous estimates. We separate the ocean source and sink terms since we view them as independent. We find a global source of acetone of 146 Tg a−1 including 80 Tg a−1 from the oceans, 33 Tg a−1 from the terrestrial biosphere, and 26 Tg a−1 from the atmospheric oxidation of isoalkanes. We calculate a mean tropospheric lifetime of 14 days with ocean uptake contributing 56% of the sink; reaction with OH, photolysis, and land uptake contribute 23%, 13%, and 8% respectively.
The updated acetone photolysis rates triple the tropospheric lifetime of acetone against photolysis relative to Jacob et al. [2002] and reverse the relative importance of oxidation versus photolysis. We estimate an atmospheric burden of 5.6 Tg, which is larger than the estimate by Jacob et al. [2002] (3.8 Tg), because our weaker photolysis sink increases the burden in the upper troposphere. This is consistent with observations, as shown in Figure 1. Arnold et al. [2005] previously found with the TOMCAT CTM that the updated (slower) photolysis loss increases the acetone tropospheric burden by 50%. Our results are highly dependent on the Blitz et al. [2004] quantum yields for acetone photolysis, which represent the only direct measurements to date. Arnold et al. [2005] show that the Blitz et al. [2004] quantum yields reduce model bias relative to aircraft profiles from the remote Pacific, but Arnold et al. [2005] assumed a net zero acetone air-sea flux. Given the implications for constraining other budget terms, independent verification of the quantum yields for acetone photolysis would be very useful.
Separation of the ocean source and sink in our budget suggests that the ocean exerts a major control on the abundance of atmospheric acetone. Using a seawater concentration of 15 nM, we find that on a global scale the atmosphere is in near equilibrium with the ocean. The net ocean sink of 2 Tg a−1, is within the uncertainty of the other source and sink terms. However there are coherent regions where the ocean is either a net sink or a net source, depending on the atmospheric acetone concentration and temperature. Marandino et al. [2005] proposed that the ocean is a large global net sink based on extrapolation of their measurements in the North Pacific Ocean, but as pointed out above their measurements were taken under conditions of anomalously high acetone concentrations in surface air. The other flux measurements in Table 2 do not support this extrapolation.
We explored the sensitivity of our findings to seawater acetone concentration through simulations with fixed seawater concentrations of 10 and 20 nM, values near the low and high ends of the data ensemble in Table 2. Both options produce atmospheric acetone distributions inconsistent with observations. With a seawater acetone concentration of 10 nM, the model underestimates acetone mixing ratios by a factor of 2 in the marine boundary layer at northern mid-latitudes. Under this scenario, the ocean is a net sink (13 Tg a−1) as the tropical oceans shift to a net acetone sink. With the seawater acetone concentration set to 20 nM, the atmosphere remains near equilibrium with the ocean on a global scale, but atmospheric acetone over the remote tropical oceans is overestimated by approximately a factor of 2 compared to observations. Seawater acetone concentrations clearly vary spatially and seasonally, but there is presently insufficient information to constrain this variability in models. Better quantification of the sources and sinks of acetone in the oceans is critical to improve our understanding of the budget of atmospheric acetone.
Supplementary Material
Acknowledgments.
This work was supported by the NASA Atmospheric Composition Modeling and Analysis Program. Support for Emily V. Fischer was provided by the NOAA Climate and Global Change Postdoctoral Fellowship Program, administered by the University Corporation for Atmospheric Research, and by a Harvard University Center for the Environment Postdoctoral Fellowship. Helpful discussions with Joost de Gouw and Jonathan Williams are gratefully acknowledged.
Footnotes
The Editor thanks two anonymous reviewers for their assistance in evaluating this paper.
Auxiliary materials are available in the HTML. doi:10.1029/2011GL050086.
References
- Andreae MO, and Merlet P. (2001), Emission of trace gases and aerosols from biomass burning, Global Biogeochem. Cycles, 15(4), 955–966, doi: 10.1029/2000GB001382. [DOI] [Google Scholar]
- Arnold SR, et al. (2005), A three-dimensional model study of the effect of new temperature-dependent quantum yields for acetone photolysis, J. Geophys. Res, 110, D22305, doi: 10.1029/2005JD005998. [DOI] [Google Scholar]
- Benkelberg HJ, et al. (1995), Henry’s law coefficients for aqueous solutions of acetone, acetaldehyde and acetonitrile, and equilibrium constants for the addition compounds of acetone and acetaldehyde with bisulfite, J. Atmos. Chem, 20(1), 17–34, doi: 10.1007/BF01099916. [DOI] [Google Scholar]
- Blitz MA, Heard DE, Pilling MJ, Arnold SR, and Chipper-field MP (2004), Pressure and temperature-dependent quantum yields for the photodissociation of acetone between 279 and 327.5 nm, Geophys. Res. Lett, 31, L06111, doi: 10.1029/2003GL018793. [DOI] [Google Scholar]
- de Gouw JA, et al. (2001), Overview of the trace gas measurements on board the Citation aircraft during the intensive field phase of INDOEX, J. Geophys. Res, 106(D22), 28,453–28,467, doi: 10.1029/2000JD900810. [DOI] [Google Scholar]
- de Gouw JA, Goldan PD, Warneke C, Kuster WC, Roberts JM, Marchewka M, Bertman SB, Pszenny AAP, and Keene WC (2003), Validation of proton transfer reaction-mass spectrometry (PTR-MS) measurements of gas phase organic compounds in the atmosphere during the New England Air Quality Study (NEAQS) in 2002, J. Geophys. Res, 108(D21), 4682, doi: 10.1029/2003JD003863. [DOI] [Google Scholar]
- de Gouw JA, et al. (2006), Volatile organic compounds composition of merged and aged forest fire plumes from Alaska and western Canada, J. Geophys. Res, 111, D10303, doi: 10.1029/2005JD006175. [DOI] [Google Scholar]
- de Reus M, et al. (2003), On the relationship between acetone and carbon monoxide in different air masses, Atmos. Chem. Phys, 3(5), 1709–1723, doi: 10.5194/acp-3-1709-2003. [DOI] [Google Scholar]
- Elias T, et al. (2011), Acetone variability in the upper troposphere: Analysis of CARIBIC observations and LMDz-INCA chemistry-climate model simulations, Atmos. Chem. Phys, 11(15), 8053–8074, doi: 10.5194/acp11-8053-2011. [DOI] [Google Scholar]
- Guenther A, et al. (2006), Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature), Atmos. Chem. Phys, 6(11), 3181–3210, doi: 10.5194/acp-6-3181-2006. [DOI] [Google Scholar]
- Heikes BG, et al. (2002), Atmospheric methanol budget and ocean implication, Global Biogeochem. Cycles, 16(4), 1133, doi: 10.1029/2002GB001895. [DOI] [Google Scholar]
- Hornbrook RS, et al. (2011), Observations of volatile organic compounds during ARCTAS Part 1: Biomass burning emissions and plume enhancements, Atmos. Chem. Phys, 11(21), 11,103–11,130, doi: 10.5194/acp-11-11103-2011. [DOI] [Google Scholar]
- Hudson ED, et al. (2007), Determination of acetone in seawater using derivatization solid-phase microextraction, Anal. Bioanal. Chem, 388, 1275–1282, doi: 10.1007/s00216-007-1324-x. [DOI] [PubMed] [Google Scholar]
- Jacob DJ, et al. (1996), Origin of ozone and NOx in the tropical troposphere: A photochemical analysis of aircraft observations over the South Atlantic basin, J. Geophys. Res, 101(D19), 24,235–24,250, doi: 10.1029/96JD00336. [DOI] [Google Scholar]
- Jacob DJ, Field BD, Jin EM, Bey I, Li Q, Logan JA, Yantosca RM, and Singh HB (2002), Atmospheric budget of acetone, J. Geophys. Res, 107(D10), 4100, doi: 10.1029/2001JD000694. [DOI] [Google Scholar]
- Johnson MT (2010), A numerical scheme to calculate temperature and salinity dependent air-water transfer velocities for any gas, Ocean Sci, 6(4), 913–932, doi: 10.5194/os-6-913-2010. [DOI] [Google Scholar]
- Kameyama S, et al. (2010), High-resolution measurement of multiple volatile organic compounds dissolved in seawater using equilibrator inlet-proton transfer reaction-mass spectrometry (EI-PTR-MS), Mar. Chem, 122, 59–73, doi: 10.1016/j.marchem.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Lelieveld J, et al. (2002), Global air pollution crossroads over the Mediterranean, Science, 298(5594), 794–799, doi: 10.1126/science.1075457. [DOI] [PubMed] [Google Scholar]
- Lewis AC, et al. (2005), Sources and sinks of acetone, methanol, and acetaldehyde in North Atlantic marine air, Atmos. Chem. Phys, 5(7), 1963–1974, doi: 10.5194/acp-5-1963-2005. [DOI] [Google Scholar]
- Liss PS, and Slater PG (1974), Flux of gases across the air-sea interface, Nature, 247, 181–184, doi: 10.1038/247181a0. [DOI] [Google Scholar]
- Mao H, Talbot R, Nielsen C, and Sive B. (2006), Controls on methanol and acetone in marine and continental atmospheres, Geophys. Res. Lett, 33, L02803, doi: 10.1029/2005GL024810. [DOI] [Google Scholar]
- Mao J, et al. (2010), Chemistry of hydrogen oxide radicals (HOx) in the Arctic troposphere in spring, Atmos. Chem. Phys, 10(13), 5823–5838, doi: 10.5194/acp-10-5823-2010. [DOI] [Google Scholar]
- Marandino CA, De Bruyn WJ, Miller SD, Prather MJ, and Saltzman ES (2005), Oceanic uptake and the global atmospheric acetone budget, Geophys. Res. Lett, 32, L15806, doi: 10.1029/2005GL023285. [DOI] [Google Scholar]
- Marandino CA, De Bruyn WJ, Miller SD, Prather MJ, and Saltzman ES (2006), Correction to “Oceanic uptake and the global atmospheric acetone budget,” Geophys. Res. Lett, 33, L24801, doi: 10.1029/2006GL028225. [DOI] [Google Scholar]
- Millet DB, et al. (2008), New constraints on terrestrial and oceanic sources of atmospheric methanol, Atmos. Chem. Phys, 8(23), 6887–6905, doi: 10.5194/acp-8-6887-2008. [DOI] [Google Scholar]
- Mopper K, and Stahovec WL (1986), Sources and sinks of low molecular weight organic carbonyl compounds in seawater, Mar. Chem, 19(4), 305–321, doi: 10.1016/0304-4203(86)90052-6. [DOI] [Google Scholar]
- Murphy JG, et al. (2010), Measurements of volatile organic compounds over West Africa, Atmos. Chem. Phys, 10(12), 5281–5294, doi: 10.5194/acp-10-5281-2010. [DOI] [Google Scholar]
- Nemecek-Marshall M, et al. (1995), Marine Vibrio species produce the volatile organic compound acetone, Appl. Environ. Microbiol, 61(1), 44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale PD, Malin G, Law CS, Watson AJ, Liss PS, Liddicoat MI, Boutin J, and Upstill-Goddard RC (2000), In situ evaluation of air-sea gas exchange parameterizations using novel conservative and volatile tracers, Global Biogeochem. Cycles, 14(1), 373–387, doi: 10.1029/1999GB900091. [DOI] [Google Scholar]
- Potter C, et al. (2003), Model terrestrial biogenic sources of oxygenated organic emissions, Earth Interact, 7(7), 1–15, doi:. [DOI] [Google Scholar]
- Pozzer A, et al. (2010), Observed and simulated global distribution and budget of atmospheric C2-C5 alkanes, Atmos. Chem. Phys, 10(9), 4403–4422, doi: 10.5194/acp-10-4403-2010. [DOI] [Google Scholar]
- Sander SP, et al. (2011), Chemical kinetics and photochemical data for use in atmospheric studies, JPL Publ, 06-02, 684 pp. [Google Scholar]
- Schade GW, and Goldstein AH (2001), Fluxes of oxygenated volatile organic compounds from a ponderosa pine plantation, J. Geophys. Res, 106(D3), 3111–3123, doi: 10.1029/2000JD900592. [DOI] [Google Scholar]
- Singh HB, O’Hara D, Herlth D, Sachse W, Blake DR, Bradshaw JD, Kanakidou M, and Crutzen PJ (1994), Acetone in the atmosphere: Distribution, sources, and sinks, J. Geophys. Res, 99(D1), 1805–1819, doi: 10.1029/93JD00764. [DOI] [Google Scholar]
- Singh HB, et al. (1995), High concentrations and photochemical fate of oxygenated hydrocarbons in the global troposphere, Nature, 378, 50–54, doi: 10.1038/378050a0. [DOI] [Google Scholar]
- Singh H, et al. (2000), Distribution and fate of selected oxygenated organic species in the troposphere and lower stratosphere over the Atlantic, J. Geophys. Res, 105(D3), 3795–3805, doi: 10.1029/1999JD900779. [DOI] [Google Scholar]
- Singh HB, et al. (2001), Evidence from the southern Pacific troposphere for large global abundances and sources of oxygenated organic compounds, Nature, 410, 1078–1081, doi: 10.1038/35074067. [DOI] [PubMed] [Google Scholar]
- Singh HB, Tabazadeh A, Evans MJ, Field BD, Jacob DJ, Sachse G, Crawford JH, Shetter R, and Brune WH (2003), Oxygenated volatile organic chemicals in the oceans: Inferences and implications based on atmospheric observations and air-sea exchange models, Geophys. Res. Lett, 30(16), 1862, doi: 10.1029/2003GL017933. [DOI] [Google Scholar]
- Singh HB, et al. (2004), Analysis of the atmospheric distribution, sources, and sinks of oxygenated volatile organic chemicals based on measurements over the Pacific during TRACE-P, J. Geophys. Res, 109, D15S07, doi: 10.1029/2003JD003883. [DOI] [Google Scholar]
- Singh HB, et al. (2009), Chemistry and transport of pollution over the Gulf of Mexico and the Pacific: Spring 2006 INTEX-B campaign overview and first results, Atmos. Chem. Phys, 9(7), 2301–2318, doi: 10.5194/acp-92301-2009. [DOI] [Google Scholar]
- Sinha V, et al. (2007), Air-sea fluxes of methanol, acetone, acetaldehyde, isoprene and DMS from a Norwegian fjord following a phytoplankton bloom in a mesocosm experiment, Atmos. Chem. Phys, 7(3), 739–755, doi: 10.5194/acp-7-739-2007. [DOI] [Google Scholar]
- Taddei S, et al. (2009), Carbon dioxide and acetone air-sea fluxes over the southern Atlantic, Environ. Sci. Technol, 43(14), 5218–5222, doi: 10.1021/es8032617. [DOI] [PubMed] [Google Scholar]
- van der Werf GR, et al. (2009), Estimates of fire emissions from an active deforestation region in the southern Amazon based on satellite data and biogeochemical modelling, Biogeosciences, 6(2), 235–249, doi: 10.5194/bg-6-235-2009. [DOI] [Google Scholar]
- van Donkelaar A, et al. (2008), Analysis of aircraft and satellite measurements from the Intercontinental Chemical Transport Experiment (INTEX-B) to quantify long-range transport of East Asian sulfur to Canada, Atmos. Chem. Phys, 8(11), 2999–3014, doi: 10.5194/acp-8-2999-2008. [DOI] [Google Scholar]
- van het Bolscher M, et al. (2008), REanalysis of the TROpospheric chemical composition over the past 40 years: A long-term global modeling study of tropospheric chemistry funded under the 5th EU framework programme, Rep. EVK2-CT-2002–00170, 77 pp., Max Planck Inst. for Meteorol., Hamburg, Germany. [Google Scholar]
- Warneke C, and de Gouw JA (2001), Organic trace gas composition of the marine boundary layer over the northwest Indian Ocean in April 2000, Atmos. Environ, 35(34), 5923–5933, doi: 10.1016/S1352-2310(01)00384-3. [DOI] [Google Scholar]
- Wild O, et al. (2000), Fast-J: Accurate simulation of in- and below-cloud photolysis in tropospheric chemical models, J. Atmos. Chem, 37, 245–282, doi: 10.1023/A:1006415919030. [DOI] [Google Scholar]
- Williams J, Holzinger R, Gros V, Xu X, Atlas E, and Wallace DWR (2004), Measurements of organic species in air and seawater from the tropical Atlantic, Geophys. Res. Lett, 31, L23S06, doi: 10.1029/2004GL020012. [DOI] [Google Scholar]
- Zhou X, and Mopper K. (1997), Photochemical production of low-molecular-weight carbonyl compounds in seawater and surface microlayer and their air-sea exchange, Mar. Chem, 56(3–4), 201–213, doi: 10.1016/S0304-4203(96)00076-X. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.