Abstract
To determine if pump therapy with continuous glucose monitoring offering low glucose suspend (LGS) decreases fear of hypoglycemia among children with type 1 diabetes and their parents. The CGM TIME trial is a multicenter randomized controlled trial that enrolled 144 children with type 1 diabetes for at least 1 year (mean duration 3.4 ± 3.1 years) starting pump therapy (MiniMed™ Veo™, Medtronic Canada). CGM (MiniMed™ Enlite™ sensor) offering LGS was introduced simultaneously or delayed for 6 months. Hypoglycemia Fear Scale (HFS) was completed by children ≥10 years old and all parents, at study entry and 12 months later. Simultaneous and Delayed Group participants were combined for all analyses. Subscale scores were compared with paired t‐tests, and individual items with paired Wilcoxon tests. Linear regression examined association with CGM adherence. 121/140 parents and 91/99 children ≥10 years had complete data. Mean Behavior subscale score decreased from 21.1 (SD 5.9) to 17.2 (SD 6.1) (p < .001) for children, and 20.7 (SD 7.5) to 17.4 (7.4) (p < .001) for parents. Mean Worry subscale score decreased from 17.9 (SD 11.9) to 11.9 (SD 11.4) (p < .001) for children, and 23.1 (SD 13.2) to 17.6 (SD 10.4) (p < .001) for parents. Median scores for 10/25 child items and 12/25 parent items were significantly lower at 12 months (p < .001). Linear regression found no association between HFS scores and CGM adherence. Insulin pump therapy with CGM offering LGS significantly reduced fear of hypoglycemia not related to CGM adherence in children with type 1 diabetes and their parents.
Keywords: continuous glucose monitoring, fear of hypoglycemia, pump therapy, type 1 diabetes
1. INTRODUCTION
Fear of hypoglycemia is an important determinant of quality of life for children with type 1 diabetes and their families. 1 , 2 , 3 , 4 An important step in insulin pump technology was the development of the low glucose suspend (LGS) feature which allows low readings on a continuous glucose monitor (CGM) to automatically stop the pump's insulin delivery for a prespecified period of time. There have been multiple studies confirming that LGS is safe in adults 5 , 6 , 7 and children 8 , 9 and can reduce the risk of overnight hypoglycemia without significantly increasing risk of ketosis. 6 , 7 , 8 , 9 , 10 , 11 , 12 Two large trials demonstrated that LGS can significantly decrease frequency and severity of hypoglycemia. 9 , 12
The CGM TIME Trial (Timing of Initiation of continuous glucose Monitoring in Established pediatric diabetes, www.clinicaltrials.gov reg. no. NCT01295788) is a multicenter randomized controlled trial which explored the impact of timing of CGM initiation in relation to initiation of insulin pump therapy (Enlite™ Sensor and Paradigm™ Veo™ Insulin Pump Medtronic Canada) on adherence to CGM and glycemic control. The trial demonstrated significantly greater adherence to CGM (adjusted difference of 2.2 hours per day) in participants who started CGM and pump simultaneously compared to those who started CGM 6 months after pump therapy, with greater proportion of time spent using CGM associated with better glycemic control. 13 This manuscript explores the effect on child and parent fear of hypoglycemia of pump therapy with and without CGM that offered LGS.
The Hypoglycemia Fear Survey (HFS) was developed by Cox et al in 1987. 14 It has been cited in over 200 papers and is the most commonly used instrument for quantifying fear of hypoglycemia in type 1 and type 2 diabetes. Clarke et al. 3 and Marrero et al. 15 modified HFS for use by parents of young children with diabetes. HFS which takes 2 to 4 minutes to complete is divided into two parts. Part 1 addresses behaviors associated with fear of hypoglycemia (10 questions) and worry about hypoglycemia (15 questions). Part 2 collects information about the frequency and severity of hypoglycemia. In questions 1 to 25, participants rate their agreement with each statement (for example, “I keep my sugar higher when I will be alone for a while”) on a 5‐point Likert scale with 0 representing “never” and 4 representing “always”. The parent version of the questionnaire contains 40 items and the child version 32 items; the items on the Behavior and Worry subscales are nearly identical in the parent and child versions.
2. RESEARCH DESIGN AND METHODS
The protocol for the CGM TIME Trial has been published. 16 The trial included 144 children age 5 to 18 years old with type 1 diabetes for at least 1 year (mean diabetes duration 3.4 ± 3.1 years), treated with insulin injections. Children were randomized to start CGM when they switched from injections to an insulin pump (Simultaneous Group) or 6 months after starting pump therapy (Delayed Group). The primary outcomes of CGM adherence and change in HbA1c 6 months after CGM initiation were reported separately. 13 Children ≥10 years of age, as well as parents of all participants, completed the HFS questionnaire at the time of their pump start (after randomization) and at the 3, 6, 9, and 12 month follow‐up visits. There were minor differences in wording between the parent and child versions, and between the initial and follow‐up questionnaires. Children were included in this analysis if they completed HFS at study entry and 12 month study visit. One parent per child was included in the parent analysis if the same parent completed HFS at study entry and 12 month visit. If more than one parent per child completed HFS at both time points, an index parent was chosen according to a priori order of mother, father, and other parent. Each item in Part 1 of HFS consists of 25 questions, which are scored from 0 (“never”) to 4 (“always”). Scores on the Behavior subscale (items 1–10) and the Worry subscale (items 11–25) were calculated separately for children and parents at each time point. Participants were excluded from the analysis if more than 2 HFS items were missing. CONSORT standards for design and reporting of clinical trials were followed.
Data on CGM adherence were uploaded weekly to CareLink™. CGM hours in the 21‐day period just before the 12 month study visit were used to determine CGM adherence. Sometimes CareLink™ did not collect data on all 7 days each week due to technical errors, however in each 21 day period, the total number of hours CGM was available and number of days data were reported. An adjustment was made where the number of CGM usage hours in a projected 21 day period was calculated as (total hours/total days × 21 days).
2.1. Statistics
This is an exploratory study with multiple statistical tests, so p‐values should be interpreted with caution. Participants from the two randomization groups were combined for this analysis. HFS subscale means and standard deviations were calculated separately for parents and children. When subscale means were calculated, missing items were handled with simple imputation, with the mean score of other items on the subscale for that individual imputed for the missing score. Comparisons between subscale scores at study entry and at 12 months were evaluated using paired t‐tests. Mean scores for each HFS item at each time point were calculated by adding the scores and dividing by the number of participants in the group who answered the question (without imputation). Metrics to summarize scores at each visit were expressed as medians and interquartile ranges, and paired Wilcoxon tests were used to test for statistical significance.
A multivariable linear regression model was used to examine association between CGM adherence (mean hours per day) in the 3 weeks prior to the 12 month questionnaire, adjusted for child's age and gender, duration of diabetes, and randomization group. For the parent analysis, parent gender was also included. Statistical analysis was performed by the CHEO Research Institute using R version 3.4.2. 17
3. RESULTS
Ninety‐seven of 98 eligible children age 10 and over completed HFS at study entry. Of these, 95 also completed HFS at the 12 month visit. Four of these children were excluded for missing items on HFS, leaving a sample size of 91 children, 48 in the Simultaneous Group and 43 in the Delayed Group. One hundred and forty out of 141 participants had at least one parent who completed HFS at the first study visit. Of these, 122 had the same parent complete the questionnaire at the 12 month visit. One parent was excluded due to missing items on HFS. A total of 121 parents were included, 59 in the Simultaneous Group and 62 in the Delayed Group.
Scores on the Behavior and Worry subscales were different between study entry and the 12 month follow‐up visit, for both parents and children (Figure 1). The mean Behavior subscale score decreased from 21.1 (SD 5.9) to 17.2 (SD 6.1) (p < .001) for children, and decreased from 20.7 (SD 7.5) to 17.4 (7.4) (p < .001) for parents. The mean Worry subscale score decreased from 17.9 (SD 11.9) to 11.9 (SD 11.4) (p < .001) for children, and 23.1 (SD 13.2) to 17.6 (SD 10.4) (p < .001) for parents.
FIGURE 1.
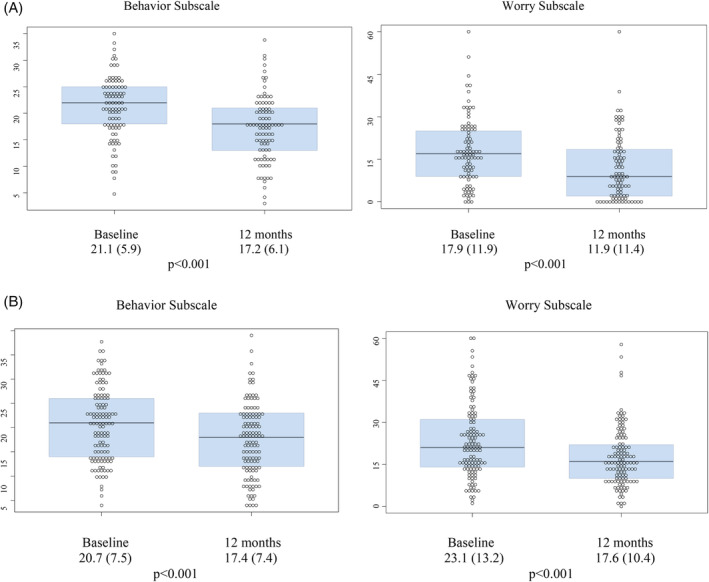
A, Hypoglycemia Fear Scale Subscale Scores for Children and B, Parents
An exploratory analysis is shown in Table 1, which lists the first 25 questions of HFS and the p‐values comparing the baseline and 12 month mean scores for parents and children.
TABLE 1.
Hypoglycemia fear scale individual question analysis for children and Parents. Mean scores for all questions except #8 and #17 for parents were lower at the 12 month visit compared to study entry for both parents and children
| Hypoglycemia Fear Scale Questions (Wording from Child Version) | Baseline mean score and SD (Children) | 12 month mean score and SD (Children) | Baseline mean score and SD (Parents) | 12 month mean score and SD (Parents) |
|---|---|---|---|---|
| Behavior Scale ‐ “How often do you… | ||||
| 1) Eat large snacks at bedtime” | 1.82 (1.10) | 1.56 (1.09) | 1.64 (1.11) | 1.09 (1.06) a |
| 2) Try not to be by myself when my sugar is likely to be low” | 1.99 (1.18) | 1.18 (1.20) a | 2.55 (1.47) | 2.08 (1.39) a |
| 3) Keep blood sugars to be a little high to be on the safe side” | 1.68 (0.92) | 1.22 (1.05) a | 1.75 (1.11) | 1.23 (1.00) |
| 4) Keep my sugar higher when I will be alone for a while” | 1.18 (1.04) | 0.88 (1.00) | 1.23 (1.26) | 0.88 (1.03) |
| 5) Eat something as soon as I feel the first sign of a low blood sugar” | 2.41 (1.22) | 1.66 (1.26) a | 2.34 (1.39) | 2.08 (1.41) |
| 6) Take less insulin when I think my sugar might get too low” | 2.39 (1.16) | 2.11 (1.20) | 2.23 (1.25) | 1.89 (1.20) |
| 7) Keep my blood sugar higher when I am going to be away from my parents” | 1.30 (1.09) | 0.97 (1.12) | 1.36 (1.37) | 0.92 (1.11) a |
| 8) Carry some kind of sugar, drink, or food with me” | 3.66 (0.64) | 3.31 (0.93) | 3.76 (0.62) | 3.78 (0.54) |
| 9) Try not to do exercise when I think my sugar is low” | 2.36 (1.38) | 2.34 (1.20) | 1.52 (1.34) | 1.47 (1.28) |
| 10) Check my sugar often when I am away from home | 2.31 (1.08) | 1.92 (1.05) | 2.35 (1.26) | 1.99 (1.22) |
| Worry Scale ‐ “How often to you worry about… | ||||
| 11) Not recognizing that my blood sugar is low” | 1.34 (1.13) | 1.04 (0.94) | 1.93 (1.27) | 1.68 (1.12) |
| 12) Not having food, fruit, or juice with me when my blood sugar gets low” | 1.42 (1.20) | 1.18 (1.01) | 1.75 (1.25) | 1.44 (1.07) |
| 13) Feeling dizzy or passing out in public because of low blood sugar” | 1.25 (1.22) | 0.82 (1.01) | 1.64 (1.30) | 1.16 (0.94) a |
| 14) Having a reaction while asleep” | 1.52 (1.16) | 1.01 (1.10) a | 2.44 (1.22) | 1.99 (1.02) a |
| 15) Embarrassing myself because of a low blood sugar” | 0.60 (0.93) | 0.58 (0.82) | 0.44 (0.84) | 0.40 (0.83) |
| 16) Having a reaction while I am by myself” | 1.36 (1.15) | 0.81 (0.92) a | 2.17 (1.29) | 1.60 (1.01) a |
| 17) Appearing to be 'stupid' or clumsy in front of other people” | 0.85 (1.07) | 0.65 (0.90) | 0.33 (0.83) | 0.36 (0.72) |
| 18) Losing control because of low blood sugar” | 1.10 (1.07) | 0.67 (0.82) a | 1.13 (1.31) | 0.68 (0.91) a |
| 19) No one being around to help me during a reaction” | 1.33 (1.14) | 0.88 (0.87) a | 2.14 (1.39) | 1.57 (1.09) a |
| 20) Making a mistake or having an accident at school because of a low sugar” | 1.10 (1.12) | 0.70 (0.92) | 0.99 (1.12) | 0.75 (0.92) |
| 21) Getting in trouble at school because of something that happens when my sugar is low” | 0.67 (0.96) | 0.60 (0.87) | 0.88 (1.16) | 0.82 (1.02) |
| 22) Having seizures” | 1.03 (1.29) | 0.60 (0.93) a | 1.60 (1.47) | 0.93 (1.01) a |
| 23) Getting long‐term complications from frequent low blood sugars” | 1.20 (1.23) | 0.86 (1.10) | 1.90 (1.45) | 1.58 (1.18 |
| 24) Feeling dizzy or woozy when my sugar is low” | 1.62 (1.17) | 0.77 (0.98) a | 1.79 (1.21) | 1.19 (0.88) a |
| 25) Having a reaction” | 1.51 (1.26) | 0.71 (0.99) a | 2.05 (1.22) | 1.42 (1.04) a |
Denotes p value <.001.
Multivariable linear regression did not show a significant association between change in HFS scores from baseline to 12 months and CGM adherence measured in the 21 days prior to completing HFS (p = .26 for children and p = .37 for parents). This analysis was adjusted for the child's age, child's gender, parent gender, and diabetes duration. (Results not shown).
4. CONCLUSIONS
This is, to our knowledge, the first trial measuring fear of hypoglycemia in children with type 1 diabetes with at least 1 year duration and their parents in the context of starting pump therapy with CGM incorporating the option of the low glucose suspend feature. During the 12‐month study period, there were eight episodes of severe hypoglycemia among study participants; five of the eight episodes occurred while participants were using CGM with LGS active. As the number of episodes of severe hypoglycemia was quite low, we do not feel that this affected the HFS scores in a significant way. In addition, the CGM TIME trial found no association between average CGM adherence hours and the proportion of time spent with blood glucose below 3.9 mmol/L. The lack of association between CGM adherence and change in Hypoglycemia Fear Scale scores is interesting, and likely reflects a variety of psychological factors. The CGM adherence in this sample was highly variable between participants, ranging from 0 hours per day to almost 100% adherence. Because we only measured CGM adherence in the 21 days leading up to the completion of the Hypoglycemia Fear Scale, it is possible that the use of CGM earlier in the trial had helped alleviate fear of hypoglycemia, for example, by reassuring parents that their child did not have overnight hypoglycemia, or by facilitating adjustments of basal rates so that the child's glycemic control was less variable. It is also possible that the insulin delivery method (pump versus injections) led to less fear of hypoglycemia as basal insulin dosing could be adjusted more precisely. LGS was introduced as part of the trial, but its use was optional. We were not able to calculate the percentage of time that LGS was used.
This was an exploratory trial and due to multiple testing we are unable to draw definitive conclusions from our results, however they do suggest that pump therapy with CGM that incorporated the option of the low glucose suspend feature might be helpful in alleviating some fear of hypoglycemia, which is a distressing aspect of diabetes management for both children with diabetes and their parents.
AUTHOR CONTRIBUTIONS
Margaret L. Lawson, Jennilea M. Courtney, Brenda J. Bradley, Karen McAssey, Cheril Clarson, Susan Kirsch, Jacqueline R Curtis, Farid H Mahmud, Christine Richardson, Tammy Cooper designed the study. Margaret L. Lawson, Jennilea M. Courtney, Brenda J. Bradley, Karen McAssey, Cheril Clarson, Susan Kirsch, Jacqueline R Curtis, Farid H Mahmud, Christine Richardson, Tammy Cooper performed the research. Maria Esther Perez Trejo, Ken Tang, Jason Chan analyzed the data. Kate C. Verbeeten and Margaret L. Lawson wrote the paper.
CONFLICT OF INTERTEST
This is an investigator‐initiated trial. Pumps and CGM supplies were purchased by the Study Group from Medtronic Canada at a discounted price. Margaret L. Lawson has been a speaker, without honorarium, at educational events sponsored by Medtronic and Animas with travel reimbursement to attend these events. Cheril Clarson has been a speaker with honorarium at educational events sponsored by Medtronic. Karen McAssey and Susuan E. Kirsch have been speakers with honorarium at educational events sponsored by Medtronic, Dexcom, and Animas. For the other authors, no competing financial interests exist.
5.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pedi.13150.
Supporting information
Appendix: CGM TIME Trial Study Group Members
ACKNOWLEDGMENTS
The authors acknowledge the funding provided by JDRF Canada and the Federal Economic Development Agency for Southern Ontario (FedDev Ontario) through the JDRF Canadian Clinical Trial Network (JDRF CCTN).
Verbeeten KC, Perez Trejo ME, Tang K, et al. Fear of hypoglycemia in children with type 1 diabetes and their parents: Effect of pump therapy and continuous glucose monitoring with option of low glucose suspend in the CGM TIME trial. Pediatr Diabetes. 2021;22:288–293. 10.1111/pedi.13150
Ethics approval from the Institutional Ethics Boards of each participating site. This study was an oral presentation at the Canadian Pediatric Endocrine Group Annual Scientific Meeting on 24 February 2019
Funding information JDRF Canada and Southern Ontario Federal Economic Development Agency, Grant/Award Number: 80‐2010‐585
REFERENCES
- 1. Shepard JA, Vajda K, Nyer M, Clarke W, Gonder‐Frederick L. Understanding the construct of fear of hypoglycemia in pediatric type 1 diabetes. J Pediatr Psychol. 2014;39(10):1115‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson SR, Cooper MN, Davis EA, Jones TW. Hypoglycaemia, fear of hypoglycaemia and quality of life in children with type 1 diabetes and their parents. Diabet Med. 2013;30(9):1126‐1131. [DOI] [PubMed] [Google Scholar]
- 3. Clarke WL, Gonder‐Frederick A, Snyder AL, Cox DJ. Maternal fear of hypoglycemia in their children with insulin dependent diabetes mellitus. J Pediatr Endocrinol Metab. 1998;11(1):189‐194. [DOI] [PubMed] [Google Scholar]
- 4. Barnard K, Thomas S, Royle P, Noyes K, Waugh N. Fear of hypoglycaemia in parents of young children with type 1 diabetes: a systematic review. BMC Pediatr. 2010;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ly TT, Nicholas JA, Retterath A, Davis EA, Jones TW. Analysis of glucose responses to automated insulin suspension with sensor‐augmented pump therapy. Diabetes Care. 2012;35(7):1462‐1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buckingham BA, Cameron F, Calhoun P, et al. Outpatient safety assessment of an in‐home predictive low‐glucose suspend system with type 1 diabetes subjects at elevated risk of nocturnal hypoglycemia. Diabetes Technol Ther. 2013;15(8):622‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maahs DM, Calhoun P, Buckingham BA, et al. A randomized trial of a home system to reduce nocturnal hypoglycemia in type 1 diabetes. Diabetes Care. 2014;37(7):1885‐1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buckingham BA, Raghinaru D, Cameron F, et al. Predictive low‐glucose insulin suspension reduces duration of nocturnal hypoglycemia in children without increasing ketosis. Diabetes Care. 2015;38(7):1197‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor‐augmented insulin pump therapy and automated insulin suspension vs. standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA. 2013;310(12):1240‐1247. [DOI] [PubMed] [Google Scholar]
- 10. Beck RW, Raghinaru D, Wadwa RP, et al. Frequency of morning ketosis after overnight insulin suspension using an automated nocturnal predictive low glucose suspend system. Diabetes Care. 2014;37(5):1224‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sherr JL, Palau Collazo M, Cengiz E, et al. Safety of nighttime 2‐hour suspension of basal insulin in pump‐treated type 1 diabetes even in the absence of low glucose. Diabetes Care. 2014;37(3):773‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold‐based insulin pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224‐232. [DOI] [PubMed] [Google Scholar]
- 13. Lawson ML, Verbeeten KC, Courtney JM, et al. Timing of CGM initiation in pediatric diabetes ‐ the CGM TIME Trial. Pediatric Diabetes. 2020. 10.1111/pedi.13144.(): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cox DJ, Irvine A, Gonder‐Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care. 1987;10(5):617‐621. [DOI] [PubMed] [Google Scholar]
- 15. Marrero DG, Guare JC, Vandagriff JL, Fineberg NS. Fear of hypoglycemia in the parents of children and adolescents with diabetes: maladaptive or healthy response? Diabetes Educ. 1997;23(3):281‐286. [DOI] [PubMed] [Google Scholar]
- 16. Lawson ML, Bradley B, McAssey K, et al. Timing of Initiation of continuous glucose Monitoring in Established pediatric type 1 diabetes: study protocol, recruitment and baseline characteristics. BMC Pediatr. 2014;14:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix: CGM TIME Trial Study Group Members


