Abstract
Risk assessment is critical to determine the timing of elective surgeries and preserve valuable resources in time of pandemic. This study was undertaken to better understand the potential value of molecular testing to risk‐stratify thyroid nodules with malignant cytology (Bethesda VI). Systematic review of the literature contributed 21 studies representing 2036 preoperative specimens. The BRAF p.V600E substitution was detected in 46% to 90% of cases with a pooled positivity rate of 70% (95% confidence intervals: 64%‐76%). None of the studies used comprehensive oncogene panels. Retrospective analysis of 531 clinical specimens evaluated with the next‐generation sequencing ThyGeNEXT Thyroid Oncogene Panel identified a total of 436 gene alterations. BRAF mutation rate was 64% in specimens tested as part of standard clinical care and 75% in specimens from cross‐sectional research studies (P = .022). Testing for additional actionable gene alterations such as TERT promoter mutations or RET and NTRK gene rearrangements further increased the diagnostic yield to 78%‐85% and up to 95% when including the ThyraMIR Thyroid miRNA Classifier. These data support the role of molecular cytopathology in surgical and therapeutic decision‐making and warrant additional studies.
Keywords: BRAF, miRNA, molecular testing, risk assessment, thyroid nodule
1. INTRODUCTION
Multiple surgical societies have issued recommendations for a safe and responsible return to practice amid the COVID‐19 pandemic. 1 , 2 , 3 As elective surgeries resume, surgeons face a major backlog of cases and must prioritize patients based on individual's risk, local and state requirements and available resources. Preoperative risk stratification of thyroid nodules typically involves a combination of blood work, imaging and fine needle aspiration biopsy (FNAB), as well as molecular testing for nodules with indeterminate cytology. 4 , 5 , 6 These testing modalities are also offered clinically to aid surgical and therapeutic decisions for advanced or metastatic thyroid cancers and for nodules with high‐risk cytopathologic features. Yet, little information is available on the type and distribution of gene alterations present in FNAB positive for malignancy (Bethesda category VI). To address this point, we conducted a systematic review of the literature and analyzed molecular data generated in two distinct sets of preoperative FNAB: (1) Representative clinical cases submitted to a CLIA‐certified laboratory for evaluation with the next‐generation sequencing ThyGeNEXT Thyroid Oncogene Panel; and (2) Cross‐sectional cohort of cases previously collected and tested during the development and validation of the ThyraMIR Thyroid miRNA Classifier. The data underscore the value of comprehensive, actionable molecular results to further the risk assessment of thyroid nodules with malignant cytology and inform clinical decision‐making.
2. MATERIALS AND METHODS
The biomedical literature database from the National Center for Biotechnology Information (https://pubmed.ncbi.nlm.nih.gov/) was searched to identify relevant studies published after 2010 and reporting more than 5 positive cases. Studies limited to RNA biomarkers were not included in the analysis. Retrospective review of cases previously tested as part of standard clinical care in Interpace Biosciences' laboratory or as part of clinical research studies was performed exclusively on deidentified molecular data. The analyses did not involve any clinical data or protected health information that could be linked to individual subjects and did not constitute human subjects research as defined in 45 CFR 46.102. Pooled rates and I2 heterogeneity tests were calculated using fixed or random effect models and the Cochran Q statistic. Proportions were compared using Pearson chi‐square tests.
3. RESULTS
Review of the literature identified 21 studies from 12 countries involving n = 2036 preoperative thyroid specimens with malignant/Bethesda VI cytology (Table 1). 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 BRAF p.V600E (c.1799T>A) positivity rate ranged from 46% to 90% with a median rate of 68%. Heterogeneity across studies was moderate. The pooled BRAF rate was 75% (95% confidence intervals: 71% to 78%, I2 = 46%) for a fixed effect model and 70% (95% confidence intervals: 64% to 76%, I2 = 0%) for a random effect model (Figure 1). The numbers of positive cases and reporting studies were too low to calculate pooled statistics for other gene alterations. Only six studies (n = 339 cases) used multi‐gene panels interrogating various combinations of BRAF, RAS, RET and PAX8 gene alterations. Four of these studies were from the same group. 9 , 10 , 14 , 19 RET‐PTC rearrangements were detected in four studies (n = 13 cases, 3.8%), RAS mutations in three studies (n = 6 cases, 1.8%) and PAX‐PPARG in a single study (n = 2 cases, 0.6%).
TABLE 1.
Rates of BRAF p.V600E substitution in preoperative thyroid nodule specimens with malignant/Bethesda VI cytology reported in the literature 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27
| Study | Year | Country | Method | Cases, No. | BRAF rate (%) |
|---|---|---|---|---|---|
| Hemalatha et al. 7 | 2018 | India | Sanger sequencing | 37 | 46 |
| Cañadas‐Garre et al. 8 | 2012 | Spain | PCR RFLP | 12 | 50 |
| Eszlinger et al. a , 9 | 2014 | Denmark | Pyrosequencing | 42 | 52 |
| Krane et al. a , 10 | 2015 | United States | Pyrosequencing | 42 | 52 |
| Park et al. 11 | 2013 | South Korea | Real‐time PCR | 35 | 60 |
| Biron et al. 12 | 2018 | Canada | Digital droplet PCR | 15 | 60 |
| Yeo et al. 13 | 2012 | South Korea | Pyrosequencing | 136 | 63 |
| Eszlinger et al. a , 14 | 2015 | Italy | Pyrosequencing | 30 | 63 |
| Danilovic et al. 15 | 2014 | Brazil | Real‐time PCR | 35 | 66 |
| Johnson et al. 16 | 2014 | United Kingdom | PCR melting curve | 18 | 67 |
| Diggans et al. 17 | 2015 | United States | Real‐time PCR | 172 | 69 |
| Beaudenon et al. a , 18 | 2014 | United States | PCR hybridization | 28 | 68 |
| Eszlinger et al. a , 19 | 2017 | Germany | Pyrosequencing | 32 | 72 |
| Zhao et al. 20 | 2015 | China | Sanger sequencing | 119 | 74 |
| Kloos et al. 21 | 2013 | United States | Real‐time PCR | 48 | 75 |
| Bellevicine et al. a , 22 | 2020 | Italy | Real‐time PCR | 165 | 76 |
| Beiša et al. 23 | 2016 | Lithuania | Real‐time PCR | 49 | 80 |
| Lee et al. 24 | 2012 | South Korea | MEMO sequencing | 876 | 85 |
| Kim et al. 25 | 2018 | South Korea | Real‐time PCR | 41 | 85 |
| Zhang et al. 26 | 2015 | China | Real‐time PCR | 37 | 86 |
| Chang et al. 27 | 2012 | South Korea | PCR melting curve | 67 | 90 |
Abbreviations: PCR, polymerase chain reaction; RFLP, restriction fragment length polymorphism; MEMO, mutant enrichment with 3′‐modified oligonucleotide.
Studies evaluating BRAF, RAS, RET and PAX8 gene alterations.
FIGURE 1.
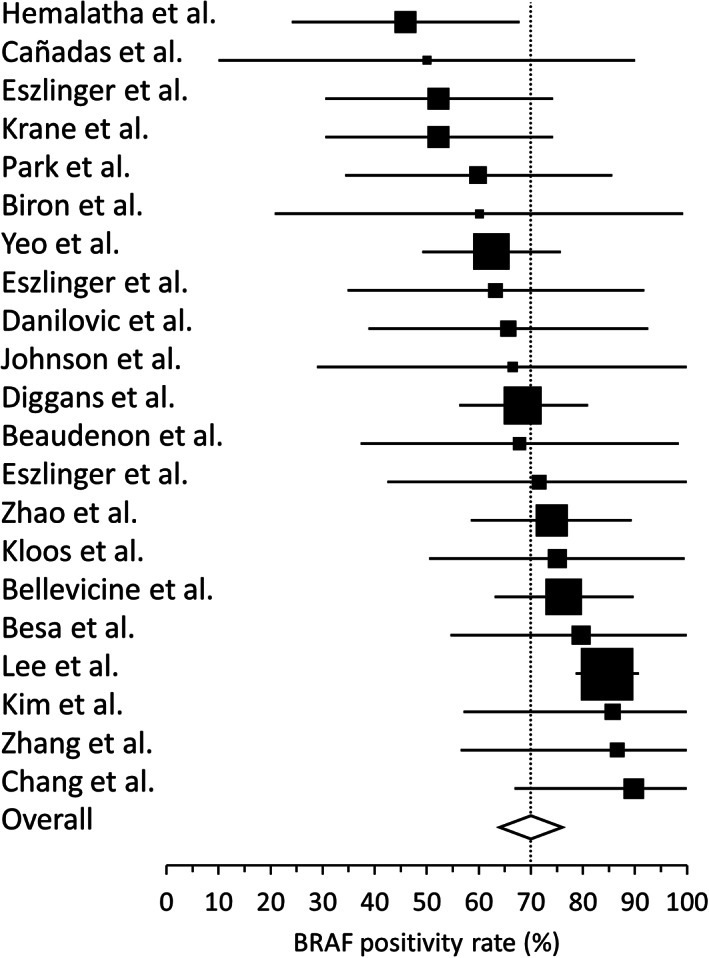
Forest plot for BRAF p.V600E positivity rates reported in 21 peer‐reviewed publications. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 Black boxes represent the relative weight of each study, whiskers represent the 95% confidence intervals for each reported BRAF rate and the diamond represents the calculated pooled rate assuming a random effect model (70% pooled rate, 95% confidence intervals: 64%‐76%, I2 = 0%)
Review of cases submitted for molecular testing as part of standard clinical care between April 2015 and June 2020 yielded 381 unique Bethesda VI specimens with molecular data (Table 2). One or several genetic alterations were detected in 78% (162/209) of the specimens tested with ThyGeNEXT and in 73% (125/172) of the specimens tested with previous versions of the test (P = .28). The exact nucleotide variations are described in Supplemental Table S1. Overall, 75% (287/381) of the specimens were positive by molecular testing, 270 for mutations in the BRAF, PIK3CA, RAS and/or TERT genes (94% of positive cases) and 17 for PAX8, NTRK or RET gene rearrangements (6% of positive cases). The most frequent alterations were BRAF p.V600E (64%) and TERT promoter mutations (11%).
TABLE 2.
Distribution of molecular results in n = 381 CLIA specimens
| Molecular result | Pre‐ThyGeNEXT | ThyGeNEXT | Combined |
|---|---|---|---|
| BRAF | 111 | 120 | 231 |
| PIK3CA | 1 | 0 | 1 |
| RAS | 7 | 7 | 14 |
| CCDC6‐RET | 4 | 7 | 11 |
| NCOA4‐RET | 1 | 2 | 3 |
| PAX8‐PPARG | 1 | 0 | 1 |
| BRAF + TERT a | n/a | 14 | 14 |
| PIK3CA + TERT a | n/a | 1 | 1 |
| RAS + TERT a | n/a | 5 | 5 |
| TERT a | n/a | 4 | 4 |
| ETV6‐NTRK a | n/a | 1 | 1 |
| TRIM24‐RET a | n/a | 1 | 1 |
| Negative | 47 | 47 | 94 |
| Total | 172 | 209 | 381 |
Gene alteration not interrogated in previous versions of the ThyGeNEXT test.
Review of cases collected and tested as part of clinical research studies yielded 150 Bethesda VI specimens with molecular data (Table 3). There were 127 specimens (85%) positive for one or several gene alterations, 117 for mutations in the BRAF, RAS and/or TERT genes (92% of positive cases) and 10 for PAX8, NTRK or RET gene rearrangements (8% of positive cases). BRAF p.V600E was detected in 112 specimens (75% of all cases). The ThyraMIR test classified 137 specimens (91%) as high risk for malignancy, including the majority of mutation positive cases (121/127 or 95%) (Figure 2). Out of 23 mutation negative specimens, the miRNA classifier identified 16 cases (70%) as positive/high risk, further increasing the yield of molecular testing to 95% overall (143/150).
TABLE 3.
Distribution of molecular results in n = 150 research specimens
| Molecular result | Pre‐ThyGeNEXT | ThyGeNEXT | Combined |
|---|---|---|---|
| BRAF | 88 | 22 | 110 |
| RAS | 5 | 0 | 5 |
| CCDC6‐RET | 5 | 1 | 6 |
| NCOA4‐RET | 1 | 0 | 1 |
| PAX8‐PPARG | 1 | 0 | 1 |
| BRAF + TERT a | n/a | 2 | 2 |
| ETV6‐NTRK a | n/a | 2 | 2 |
| ThyraMIR high risk | 13 | 3 | 16 |
| Negative | 5 | 2 | 7 |
| Total | 118 | 32 | 150 |
Gene alteration not interrogated in previous versions of the ThyGeNEXT test.
FIGURE 2.
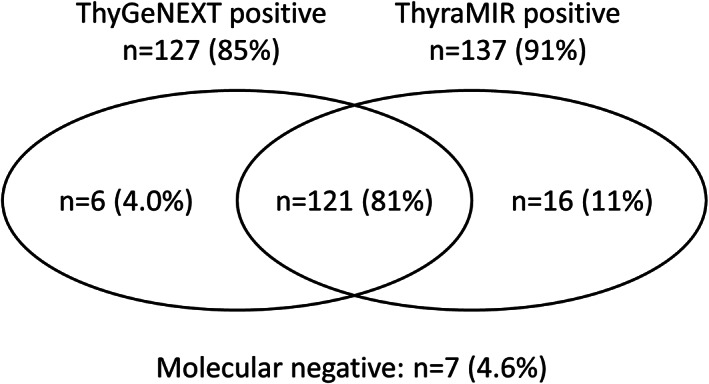
Venn diagram showing the relationship between ThyGeNEXT and ThyraMIR molecular results in n = 150 research specimens
4. DISCUSSION
In our clinical experience analysis, a total of 436 gene alterations were identified in 414 of 531 preoperative specimens with malignant cytology. There were significant differences between the two cohorts and their respective collection schemes. The clinical research cohort was a representative cross‐sectional sampling of thyroid nodules at the time of FNAB diagnosis while the CLIA cohort consisted of more challenging cases that required molecular testing as part of their routine clinical management. Predictably, the positivity rate was lower among CLIA specimens relative to research specimens (75% vs 85%, P = .019). The majority of positive cases harbored a BRAF p.V600E substitution in both cohorts (67% overall), 64% for CLIA specimens and 75% for research specimens (P = .022). The second most frequent gene alteration was TERT promoter mutations (26/241 or 11%), highlighting the limitation of molecular strategies that only interrogate exome variants. For example, the Afirma Xpression Atlas, based on transcriptome RNA sequencing, has a reported false negative rate of 26% relative to targeted DNA sequencing and cannot assess variants outside the fraction of exonic sequences that are actually transcribed into mRNA. 28
The BRAF positivity rates observed in the two clinical cohorts were consistent with the 70% pooled rate estimated from 21 published studies (95% confidence intervals: 64% to 76%). The use of different molecular methods, reagents and protocols probably contributed to the broad range of reported rates (46% to 90%). Methods with poor analytical sensitivity, for example, Sanger sequencing, or with clinical cutoffs set at high percent variant, are expected to have a lower detection rate. In one of the reviewed studies, Lee et al 24 evaluated 876 Bethesda VI specimens using three distinct molecular methods and reported BRAF detection rates of 63%, 79% and 85% for methods with an increasing analytical sensitivity of 20%, 2% and 0.1% variant, respectively. Another parameter likely contributing to the heterogeneity of BRAF rates was regional variations in the prevalence of BRAF mutation and/or papillary thyroid carcinoma. For example, three out of the five studies with the highest reported BRAF rates (80% to 90%) were conducted in South Korea, a region where BRAF p.V600E is particularly prevalent. 24 , 25 , 27 In a series of 200 resected conventional papillary carcinomas from South Korea, Seo et al 29 showed that 93% of the surgical specimens were positive for BRAF p.V600E and that 96% of these cases could be detected in the corresponding malignant FNAB.
The high frequency of oncogenic BRAF mutations in preoperative thyroid FNAB has important clinical implications. Multiple studies have shown that BRAF p.V600E correlates with aggressive features of thyroid carcinoma such as extrathyroidal extensions, vascular invasion, larger nodule size, advanced staging, lymph node metastasis and recurrence. 4 , 5 , 6 A large multicenter study recently demonstrated that BRAF is associated with mortality in older patients, independently of other clinicopathologic risk factors. 30 Other studies have suggested that knowledge of the BRAF mutational status may be useful to guide the extent of thyroid surgery. 31 , 32 However, the association of BRAF p.V600E with worse prognosis independent of other risk factors remains debated and requires additional investigation. Testing with a comprehensive oncogene panel that includes relevant and actionable gene alterations such as TERT promoter mutations or RET and NTRK gene rearrangements can also aid surgical decision‐making and speed up individualized patient care. Molecular insights may change a patient's risk profile when multiple markers are detected in the same nodule, identify carcinomas that are refractory to radioactive iodine treatment or facilitate the selection of targeted therapies. 6 , 33 , 34 , 35 Combination testing with a miRNA expression classifier may further raise the risk profile of nodules positive for weaker driver mutations such as RAS and increase the positive diagnostic yield up to 95%. Because of their unique biology, miRNAs are practical surrogate markers to identify altered oncogenic pathways in mutation negative cells and in heterogenous thyroid nodules where only a small fraction of tumor cells may carry a somatically acquired gene alteration. 36 , 37
In summary, our systematic review of the literature indicates that 70% to 75% of FNAB with malignant/Bethesda VI cytology are expected to be positive for the oncogenic BRAF p.V600E substitution. The majority of these studies (71%) assessed only BRAF mutational status. Our analysis of 531 representative clinical specimens is the first to report the potential value of a comprehensive oncogene panel combined with a miRNA expression classifier. Additional work is required to fully assess the role of molecular testing for the preoperative risk stratification of malignant FNAB, clinical decision‐making, timing of surgery and optimal utilization of valuable healthcare resources.
CONFLICT OF INTEREST
E.L. is a consultant for Interpace Biosciences Inc.
Supporting information
Table S1 Distribution of DNA mutations in n = 270 CLIA specimens positive with the ThyGeNEXT Thyroid Oncogene Panel.
ACKNOWLEDGMENTS
The authors would like to thank Gyanendra Kumar, Venkata A. Timmaraju, Keith Haugh and the CLIA Laboratory team from Interpace Biosciences for providing the deidentified molecular data used in the clinical experience analysis.
Labourier E, Fahey TJ III. Preoperative molecular testing in thyroid nodules with Bethesda VI cytology: Clinical experience and review of the literature. Diagnostic Cytopathology. 2021;49:E175–E180. 10.1002/dc.24637
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. American Academy of Otolaryngology–Head and Neck Surgery . Guidance for Return to Practice for Otolaryngology‐Head and Neck Surgery. Accessed August 26, 2020. https://www.entnet.org/content/guidance-return-practice-otolaryngology-head-and-neck-surgery
- 2. Society of Surgical Oncology . Resource for Management Options of Endocrine/Head and Neck Cancer During COVID‐19. Accessed August 26, 2020. https://www.surgonc.org/wp-content/uploads/2020/03/Endocrine-Head-and-Neck-Resource-during-COVID-19-3.30.20.pdf
- 3. American College of Surgeons . Roadmap for Resuming Elective Surgery after COVID‐19 Pandemic. Accessed August 26, 2020. https://www.facs.org/covid-19/clinical-guidance/roadmap-elective-surgery
- 4. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1‐133. 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel KN, Yip L, Lubitz CC, et al. The American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in adults. Ann Surg. 2020;271(3):e21‐e93. 10.1097/SLA.0000000000003580. [DOI] [PubMed] [Google Scholar]
- 6. NCCN Clinical Guidelines in Oncology. Thyroid Carcinoma Version 1.2020. https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf. Accessed August 26, 2020.
- 7. Hemalatha R, Pai R, Manipadam MT, et al. Presurgical screening of fine needle aspirates from thyroid nodules for BRAF mutations: a prospective single center experience. Indian J Endocrinol Metab. 2018;22(6):785‐792. 10.4103/ijem.IJEM_126_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cañadas‐Garre M, Becerra‐Massare P, López de la Torre‐Casares M, et al. Reduction of false‐negative papillary thyroid carcinomas by the routine analysis of BRAF(T1799A) mutation on fine‐needle aspiration biopsy specimens: a prospective study of 814 thyroid FNAB patients. Ann Surg. 2012;255(5):986‐992. 10.1097/SLA.0b013e31824e8d70. [DOI] [PubMed] [Google Scholar]
- 9. Eszlinger M, Krogdahl A, Münz S, et al. Impact of molecular screening for point mutations and rearrangements in routine air‐dried fine‐needle aspiration samples of thyroid nodules. Thyroid. 2014;24(2):305‐313. 10.1089/thy.2013.0278. [DOI] [PubMed] [Google Scholar]
- 10. Krane JF, Cibas ES, Alexander EK, Paschke R, Eszlinger M. Molecular analysis of residual ThinPrep material from thyroid FNAs increases diagnostic sensitivity. Cancer Cytopathol. 2015;123(6):356‐361. 10.1002/cncy.21546. [DOI] [PubMed] [Google Scholar]
- 11. Park SJ, Sun JY, Hong K, et al. Application of BRAF, NRAS, KRAS mutations as markers for the detection of papillary thyroid cancer from FNAB specimens by pyrosequencing analysis. Clin Chem Lab Med. 2013;51(8):1673‐1680. 10.1515/cclm-2012-0375. [DOI] [PubMed] [Google Scholar]
- 12. Biron VL, Matkin A, Kostiuk M, et al. Analytic and clinical validity of thyroid nodule mutational profiling using droplet digital polymerase chain reaction. J Otolaryngol Head Neck Surg. 2018;47(1):60. 10.1186/s40463-018-0299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeo MK, Liang ZL, Oh T, et al. Pyrosequencing cut‐off value identifying BRAFV600E mutation in fine needle aspiration samples of thyroid nodules. Clin Endocrinol (Oxf). 2011;75(4):555‐560. 10.1111/j.1365-2265.2011.04115.x. [DOI] [PubMed] [Google Scholar]
- 14. Eszlinger M, Piana S, Moll A, et al. Molecular testing of thyroid fine‐needle aspirations improves presurgical diagnosis and supports the histologic identification of minimally invasive follicular thyroid carcinomas. Thyroid. 2015;25(4):401‐409. 10.1089/thy.2014.0362. [DOI] [PubMed] [Google Scholar]
- 15. Danilovic DL, Lima EU, Domingues RB, Brandão LG, Hoff AO, Marui S. Pre‐operative role of BRAF in the guidance of the surgical approach and prognosis of differentiated thyroid carcinoma. Eur J Endocrinol. 2014;170(4):619‐625. 10.1530/EJE-13-0944. [DOI] [PubMed] [Google Scholar]
- 16. Johnson SJ, Hardy SA, Roberts C, Bourn D, Mallick U, Perros P. Pilot of BRAF mutation analysis in indeterminate, suspicious and malignant thyroid FNA cytology. Cytopathology. 2014;25(3):146‐154. 10.1111/cyt.12125. [DOI] [PubMed] [Google Scholar]
- 17. Diggans J, Kim SY, Hu Z, et al. Machine learning from concept to clinic: reliable detection of BRAF V600E DNA mutations in thyroid nodules using high‐dimensional RNA expression data. Pac Symp Biocomput. 2015;20:371‐382. [PubMed] [Google Scholar]
- 18. Beaudenon‐Huibregtse S, Alexander EK, Guttler RB, et al. Centralized molecular testing for oncogenic gene mutations complements the local cytopathologic diagnosis of thyroid nodules. Thyroid. 2014;24(10):1479‐1487. 10.1089/thy.2013.0640. [DOI] [PubMed] [Google Scholar]
- 19. Eszlinger M, Böhme K, Ullmann M, et al. Evaluation of a two‐year routine application of molecular testing of thyroid fine‐needle aspirations using a seven‐gene panel in a primary referral setting in Germany. Thyroid. 2017;27(3):402‐411. 10.1089/thy.2016.0445. [DOI] [PubMed] [Google Scholar]
- 20. Zhao H, Zhang ZH, Zhou B, Xiao T, Pan QJ, Guo HQ. Detection of BRAF c.1799T > A (p.V600E) mutation using residual routine fine‐needle aspiration specimens of papillary thyroid carcinoma. Diagn Cytopathol. 2015;43(10):786‐790. 10.1002/dc.23302. [DOI] [PubMed] [Google Scholar]
- 21. Kloos RT, Reynolds JD, Walsh PS, et al. Does addition of BRAF V600E mutation testing modify sensitivity or specificity of the Afirma Gene Expression Classifier in cytologically indeterminate thyroid nodules? J Clin Endocrinol Metab. 2013;98(4):E761‐E768. 10.1210/jc.2012-3762. [DOI] [PubMed] [Google Scholar]
- 22. Bellevicine C, Migliatico I, Sgariglia R, et al. Evaluation of BRAF, RAS, RET/PTC, and PAX8/PPARg alterations in different Bethesda diagnostic categories: a multicentric prospective study on the validity of the 7‐gene panel test in 1172 thyroid FNAs deriving from different hospitals in South Italy. Cancer Cytopathol. 2020;128(2):107‐118. 10.1002/cncy.22217. [DOI] [PubMed] [Google Scholar]
- 23. Beiša A, Beiša V, Stoškus M, Ostanevičiūtė E, Griškevičius L, Strupas K. The value of the repeated examination of BRAF V600E mutation status in diagnostics of papillary thyroid cancer. Endokrynol pol. 2016;67(1):35‐40. 10.5603/EP.2016.0005. [DOI] [PubMed] [Google Scholar]
- 24. Lee ST, Kim SW, Ki CS, et al. Clinical implication of highly sensitive detection of the BRAF V600E mutation in fine‐needle aspirations of thyroid nodules: a comparative analysis of three molecular assays in 4585 consecutive cases in a BRAF V600E mutation‐prevalent area. J Clin Endocrinol Metab. 2012;97(7):2299‐2306. 10.1210/jc.2011-3135. [DOI] [PubMed] [Google Scholar]
- 25. Kim DS, Kim DW, Heo YJ, et al. Utility of including BRAF mutation analysis with ultrasonographic and cytological diagnoses in ultrasonography‐guided fine‐needle aspiration of thyroid nodules. PLoS One. 2018;13(8):e0202687. 10.1371/journal.pone.0202687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang YZ, Xu T, Cui D, et al. Value of TIRADS, BSRTC and FNA‐BRAF V600E mutation analysis in differentiating high‐risk thyroid nodules. Sci Rep. 2015;5:16927. 10.1038/srep16927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang H, Lee H, Yoon SO, Kim H, Kim A, Kim BH. BRAF(V600E) mutation analysis of liquid‐based preparation‐processed fine needle aspiration sample improves the diagnostic rate of papillary thyroid carcinoma. Hum Pathol. 2012;43(1):89‐95. 10.1016/j.humpath.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 28. Angell TE, Wirth LJ, Cabanillas ME, et al. Analytical and clinical validation of expressed variants and fusions from the whole transcriptome of thyroid FNA samples. Front Endocrinol (Lausanne). 2019;10:612. 10.3389/fendo.2019.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seo JY, Choi JR, Moon HJ, et al. Clinical implication of highly sensitive detection of the BRAFV600E mutation in fine‐needle aspirations according to the thyroid Bethesda system in patients with conventional papillary thyroid carcinoma. Ann Otol Rhinol Laryngol. 2015;124(5):392‐399. 10.1177/0003489414560433. [DOI] [PubMed] [Google Scholar]
- 30. Shen X, Zhu G, Liu R, et al. Patient age‐associated mortality risk is differentiated by BRAF V600E status in papillary thyroid cancer. J Clin Oncol. 2018;36(5):438‐445. 10.1200/JCO.2017.74.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yip L, Nikiforova MN, Yoo JY, et al. Tumor genotype determines phenotype and disease‐related outcomes in thyroid cancer: a study of 1510 patients. Ann Surg. 2015;262(3):519‐525. 10.1097/SLA.0000000000001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krasner JR, Alyouha N, Pusztaszeri M, et al. Molecular mutations as a possible factor for determining extent of thyroid surgery. J Otolaryngol Head Neck Surg. 2019;48(1):51. 10.1186/s40463-019-0372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu R, Bishop J, Zhu G, Zhang T, Ladenson PW, Xing M. Mortality risk stratification by combining BRAF V600E and TERT promoter mutations in papillary thyroid cancer: genetic duet of BRAF and TERT promoter mutations in thyroid cancer mortality. JAMA Oncol. 2017;3(2):202‐208. 10.1001/jamaoncol.2016.3288. [DOI] [PubMed] [Google Scholar]
- 34. Liu J, Liu R, Shen X, Zhu G, Li B, Xing M. The genetic duet of BRAF V600E and TERT promoter mutations robustly predicts loss of radioiodine avidity in recurrent papillary thyroid cancer. J Nucl Med. 2020;61(2):177‐182. 10.2967/jnumed.119.227652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cabanillas ME, Ryder M, Jimenez C. Targeted therapy for advanced thyroid cancer: kinase inhibitors and beyond. Endocr Rev. 2019;40(6):1573‐1604. 10.1210/er.2019-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451‐5465. 10.1002/jcp.27486. [DOI] [PubMed] [Google Scholar]
- 37. Wylie D, Beaudenon‐Huibregtse S, Haynes BC, Giordano TJ, Labourier E. Molecular classification of thyroid lesions by combined testing for miRNA gene expression and somatic gene alterations. J Pathol Clin Res. 2016;2(2):93‐103. 10.1002/cjp2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Distribution of DNA mutations in n = 270 CLIA specimens positive with the ThyGeNEXT Thyroid Oncogene Panel.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


