Abstract
Objectives
Comparative data on glucose disorders using fasting blood samples between people living with HIV (PLWH) and the general population are lacking. The objective of this study was to compare the prevalence and risk factors of obesity and disturbances in glucose homeostasis between PLWH treated with modern antiretroviral therapy and the general population.
Methods
Adjusted prevalence of obesity, features of insulin resistance (triglyceride:high‐density lipoprotein cholesterol ratio and alanine aminotransferase), impaired fasting glucose (IFG), diabetes mellitus (DM) and combined dysglycaemia (presence of IFG or DM) were determined using fasting blood samples among 1041 PLWH and 7047 subjects representing the general population.
Results
People living with HIV had a lower prevalence of obesity [18.2%, 95% confidence interval (CI): 15.1–21.2 vs. 23.9%, 95% CI: 22.4–25.4], but a higher prevalence of insulin resistance and IFG (20.0%, 95% CI: 16.6–23.4 vs. 9.8%, 95% CI: 8.7–10.8) than the general population. Fasting glucose concentration was higher, but glycated haemoglobin (HbA1c) was lower, among PLWH. Prevalence of dysglycaemia for a given body mass index (BMI) was higher in PLWH than in the general population. The prevalence of DM did not differ between PLWH (13.2%, 95% CI: 10.2–15.9) and the general population (14.5%, 95% CI: 13.6–15.4).
Conclusions
The prevalence of obesity was lower, but the risk of dysglycaemia for a given BMI was significantly higher, among PLWH, highlighting the importance of prevention and treatment of obesity among HIV‐infected subjects. Regardless of the increased prevalence of insulin resistance and IFG, DM was surprisingly not more common among PLWH, raising concern about the under‐diagnosis of DM, possibly due to low sensitivity of HbA1c in this patient population.
Keywords: diabetes mellitus, general population, HIV, impaired fasting glucose, obesity
Introduction
The clinical significance of metabolic comorbidities affecting the aging HIV population is of increasing importance. The pathogenesis of many of these comorbidities including diabetes mellitus (DM) is tightly linked to obesity [1].
During recent years, concerns have been raised about increasing obesity rates among people living with HIV (PLWH) [2]. Although there are some data on obesity from ongoing cohort studies with [3] or without [4] HIV‐negative controls, comparative data with the general population are scarce [5]. According to the latest published data from North America, obesity prevalence increased from 9% in 1998 to 18% in 2010 among PLWH and from 22% to 27% in the general population [6]. These data may, however, be outdated due to major changes in treatment guidelines for PLWH during the last 10 years, including the universal treatment recommendation and the preferential use of integrase strand transfer inhibitors (INSTIs) and tenofovir alafenamide (TAF). Of note, both INSTIs and TAF have recently been associated with increased weight gain as compared with the older antiretroviral agents [7].
Increasing obesity prevalence leads to concerns of rising prevalence of diabetes [2]. However, there are no data comparing the prevalence of diabetes among PLWH and the general population using fasting glucose samples. Some studies with PLWH have used true population controls, but the diagnosis of diabetes has been based on administrative data, with their limitations, or blood samples were not collected in the fasting state [5, 8, 9]. Fasting glucose samples also allow the detection of an earlier disturbance in glucose homeostasis: impaired fasting glucose (IFG). This has clinical significance, as subjects with IFG have a five‐fold higher risk of developing diabetes than subjects with normoglycaemia [10]. Therefore, direct comparison of fasting glucose values can give a more comprehensive view on glucose homeostasis between PLWH and the general population than administrative data.
In the present study, we compare the prevalence rates of obesity, diabetes, IFG and surrogate markers of insulin resistance using fasting blood samples, both in PLWH and in the general population. We also investigate HIV‐specific risk factors of these outcomes among PLWH treated with modern antiretroviral therapy (ART), including TAF and INSTI.
Methods
Study population
All PLWH registered at Helsinki University Hospital in 2017 were evaluated for the study. The hospital provides care for c. 60% of all PLWH in Finland. As the number of people of foreign origin was very low in the general population, only PLWH born in Finland were included in the study. The study was approved by Helsinki University Hospital. Ethics committee evaluation is not needed for this type of retrospective chart review.
Data on the general population originate from the FinHealth 2017 Study, which is a population‐based health examination study conducted in 2017. The FinHealth 2017 Study protocol is described in detail elsewhere [11, 12]. Briefly, 10 247 adults over 18 years of age living in Finland were eligible to participate by two‐stage cluster sampling from the population registry. Sixty‐nine percent of the invited subjects participated in at least one part of the study, and 58% participated in the health examinations. As the prevalence of HIV infection among the general population is very low, FinHealth subjects are expected to represent the HIV‐negative population. During the last 10 years, the number of new HIV diagnoses has remained between 2.8 and 3.4 per 100 000 population [13]. The FinHealth 2017 Study was approved by the Coordinating Ethics Committee for the Helsinki‐Uusimaa hospital district. A signed informed consent was obtained from all participants in the FinHealth Study.
Data collection and variables
For PLWH, demographic and HIV‐specific data as well as laboratory results were collected from the clinic database. Fasting blood samples were analysed for glucose, triglycerides, high‐density lipoprotein (HDL) cholesterol, glycated haemoglobin (HbA1c) and alanine aminotransferase (ALT).
For the general population, the FinHealth 2017 Study included a health examination with measurements of height and body weight, and collection of blood samples in fasting state. In addition, the study subjects fulfilled health questionnaires or were interviewed regarding, for example, their current diseases (including diabetes) and medications. Data on age and gender were obtained from the population registry.
Outcome definitions
Obesity and other body mass index (BMI) categories were classified as follows: obesity, ≥ 30 kg/m2; overweight, 25.0–29.9 kg/m2; normal weight, 18.5–24.9 kg/m2; underweight, < 18.5 kg/m2.
Diabetes mellitus was defined according to the following criteria: (1) DM notified by the systematic manual review of the medical records for PLWH or by the questionnaire/interview for FinHealth subjects; (2) use of antidiabetic medication; (3) fasting plasma glucose ≥ 7.0 mmol/L (126 mg/dL), or (4) HbA1c > 48 mmol/mol (6.5%). IFG was defined as fasting glucose level of 6.1–6.9 mmol/L (110–125 mg/dL) in a non‐diabetic subject. A combination outcome variable of dysglycaemia was defined as the presence of DM or IFG.
We used fasting plasma triglycerides‐to‐HDL cholesterol ratio (TG:HDL‐C) and ALT as surrogate markers for insulin resistance. The conventional cut‐off values indicating insulin resistance with TG:HDL‐C ratio (mg/dL) are ≥ 3.75 for men and ≥ 3.0 for women [14, 15]. As uncontrolled HIV infection increases TG and decreases HDL‐C concentrations, we performed a sensitivity analysis limited to subjects with viral load < 50 HIV RNA copies/mL. Elevated ALT was defined as > 33 IU/L for men and > 25 IU/L for women [16]. For ALT, we conducted a sensitivity analysis excluding subjects with positive hepatitis B surface antigen or hepatitis C antibody test. Knowing that hepatitis C is a risk factor for diabetes, we also compared the prevalence of IFG and DM between hepatitis C‐negative PLWH and the general population.
Statistical analysis
The sampling design is structured to represent the Finnish adult population in 2017. Inverse probability weights were used in the FinHealth data analysis to adjust for differences in selection probability, to correct the effects of non‐participation and to provide nationally representative results [11]. For the analyses, each HIV‐positive subject was assessed with a weight coefficient of 1. In all data analyses, a complex survey sampling technique was used to include weights in the analysis.
Characteristics of PLWH and the general population were compared using a Mann–Whitney U‐test for continuous data and χ 2 test for categorical data.
Crude and adjusted predictive margins were calculated for outcome variables. Crude predictive margins were assessed to present the actual prevalence of each outcome. Multivariable logistic regression analyses were used to calculate adjusted predictive margins representing adjusted prevalence rates. In the regression models, we included the following covariates: age, gender, BMI, smoking and HIV status. Interactions between the covariates were tested in all analyses.
To assess the effect of HIV‐specific covariates on obesity and dysglycaemia, we conducted two multivariable regression analyses. In both analyses, we included age, gender, BMI, smoking status, mode of HIV transmission and CD4 nadir count. The first analysis also included past use of selected old nucleoside reverse transcriptase inhibitors (NRTIs), didanosine, stavudine or zidovudine. The second analysis included selected current NRTIs [abacavir (ABC), tenofovir disoproxil fumarate (TDF), TAF] and drugs from the current third antiretroviral agent class [nonnucleoside reverse transcriptase inhibitor (NNRTI), protease inhibitor (PI) and INSTI]. In the latter analysis, patients who received more than one of the selected NRTIs or third agents were excluded.
Statistical analyses were performed using STATA v.15 (StataCorp. 2017, College Station, TX, USA).
Results
The demographic characteristics of the study groups are shown in Table 1. The majority of PLWH were male (81%) as compared with half of the subjects in the general population. The median age did not differ significantly between the study groups, but there were fewer PLWH in the youngest and the oldest age groups. The median BMI was significantly lower among PLWH than in the general population.
Table 1.
Demographic and clinical characteristics of people living with HIV (PLWH) and the general population (FinHealth 2017 Study)
| PLWH | General population | |
|---|---|---|
| Number of subjects | 1041 | 7047 |
| Gender [% (95% CI)] | ||
| Male | 81 (79–83) | 49 (48–51) |
| Female | 19 (17–21) | 51 (49–52) |
| Age (years) [median (IQR; 95% CI)] | 50.4 (43.2–57.1; 49.8–51.1) | 51.4 (35.7–66.5; 50.3–52.6) |
| Age group [% (95% CI)] | ||
| 18–34 years | 9 (8–11) | 24 (22–26) |
| 35–49 years | 38 (35–41) | 24 (22–25) |
| 50–64 years | 43 (40–46) | 25 (24–26) |
| 65+ years | 9 (8–11) | 28 (26–29) |
| BMI (kg/m2) [median (IQR; 95% CI)] | 25.3 (22.7–28.4; 25.0–25.6) | 26.4 (23.5–29.8; 26.1–26.6) |
| BMI groups [% (95% CI)] | ||
| BMI < 18.5 | 2 (1–3) | 1 (1–1) |
| BMI ≥ 18.5 to < 25 | 46 (42–49) | 37 (35–39) |
| BMI ≥ 25 to < 30 | 36 (33–39) | 38 (37–40) |
| BMI ≥ 30 | 17 (15–20) | 24 (23–26) |
| Smoking status [% (95% CI)] | ||
| Current smoker | 40 (37–43) | 17 (15–18) |
| Ex‐smoker | 29 (27–32) | 24 (23–26) |
| Never smoker | 31 (28–34) | 59 (57–61) |
CI, confidence interval; IQR, interquartile range; BMI, body mass index.
The HIV‐related characteristics of PLWH are shown in Table 2. The most common mode of HIV transmission was male‐to‐male sexual contact (53%). Nearly all patients (97%) were receiving ART. More than half of the patients were currently receiving TAF (51%) and INSTI (59%). The majority of PLWH had achieved treatment goals, with 93% having a viral load < 50 copies/mL and 90% with their most recent CD4 count ≥ 350 cells/μL. The prevalence of hepatitis C antibody positivity was 96% among those reporting, and 5% among those not reporting, intravenous drug use.
Table 2.
HIV‐related characteristics of people living with HIV (PLWH)
| Number of patients | 1041 |
| HIV transmission mode [n (%)] | |
| Heterosexual contact | 310 (29.8) |
| Male‐to‐male sexual contact | 548 (52.6) |
| Intravenous drug use | 140 (13.4) |
| Other/unknown | 43 (4.1) |
| Time since HIV diagnosis (years) [median (IQR)] | 11.6 (6.9–17.5) |
| Duration of ART (years) [median (IQR)] | 9.0 (4.6–13.5) |
| Subjects receiving ART [n (%)] | 1009 (96.9) |
| Characteristics of the present ART [n (%)] | |
| Subjects taking TDF | 167 (16.0) |
| Subjects taking TAF | 534 (51.3) |
| Subject taking ABC | 270 (25.9) |
| Subjects taking NNRTI | 285 (27.4) |
| Subjects taking PI | 203 (19.5) |
| Subjects taking INSTI | 612 (58.8) |
| Most recent CD4 count (cells/μL) [median (IQR)] | 656 (496–851) |
| CD4 count < 200 [n (%)] | 7 (0.7) |
| CD4 count ≥ 200 to < 350 [n (%)] | 63 (6.1) |
| CD4 count ≥ 350 to < 500 [n (%)] | 184 (17.7) |
| CD4 count ≥ 500 [n (%)] | 751 (72.1) |
| Most recent HIV‐1 RNA [n (%)] | |
| Subjects with < 50 copies/mL | 971 (93.4) |
| Subjects with 50–400 copies/mL | 24 (2.3) |
| Subjects with > 400 copies/mL | 4 (0.4) |
| CD4 nadir count (cells/μL) [median (IQR)] | 266 (160–356) |
| CD4 nadir count < 200 [n (%)] | 335 (32.2) |
| CD4 nadir count ≥ 200 to < 350 [n (%)] | 403 (38.7) |
| CD4 nadir count ≥ 350 to < 500 [n (%)] | 170 (16.3) |
| CD4 nadir count ≥ 500 [n (%)] | 91 (8.7) |
IQR, interquartile range; ART, antiretroviral therapy; TDF, tenofovir disoproxil fumarate; TAF, tenofovir alafenamide; ABC, abacavir; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; INSTI, integrase strand transfer inhibitor.
The weighted crude and adjusted prevalence rates of the outcome variables are shown in Table 3. Obesity was less prevalent among PLWH than in the general population. All markers of insulin resistance were significantly more prevalent among PLWH than among the general population. In a sub‐analysis limited to subjects with viral load < 50 copies/mL, the weighted adjusted prevalence of increased TG:HDL‐C ratio remained significantly higher among PLWH [26.1%, 95% confidence interval (CI): 23.2–29.1] than among the general population (17.8%, 95% CI: 16.8–18.8). Similarly, in a sensitivity analysis excluding patients with chronic hepatitis B and C, the weighted adjusted prevalence of elevated ALT was still significantly higher in PLWH (40.0%, 95% CI: 36.3–43.6) than in the general population (27.3%, 95% CI: 25.6–29.1).
Table 3.
Weighted crude and adjusted prevalence rates of the outcome variables
| Weighted crude prevalence [% (95 % CI)] | Weighted adjusted prevalence* [% (95% CI)] | |||
|---|---|---|---|---|
| PLWH | General population | PLWH | General population | |
| Obesity (BMI ≥ 30 kg/m2) | 16.3 (14.1–18.6) | 24.0 (22.5−25.5) | 18.2 (15.1–21.2) | 23.9 (22.4–25.4) |
| Elevated ALT level † | 44.0 (41.0−47.1) | 27.3 (25.6–29.1) | 43.1 (39.6–46.5) | 27.3 (25.6–29.1) |
| Elevated TG:HDL‐C ratio ‡ | 29.0 (26.2–31.8) | 17.8 (16.8–18.8) | 27.0 (24.2–29.8) | 17.8 (16.8–18.8) |
| IFG § | 22.0 (19.4–24.6) | 9.7 (8.7–10.7) | 20.0 (16.6–23.4) | 9.8 (8.7–10.8) |
| Diabetes ¶ | 13.3 (11.2–15.5) | 14.9 (13.9–16.0) | 13.2 (10.5–15.9) | 14.5 (13.6–15.4) |
CI, confidence interval; PLWH, people living with HIV; BMI, body mass index; ALT, alanine aminotransferase; TG:HDL‐C ratio, triglyceride‐to‐high‐density lipoprotein cholesterol ratio; IFG, impaired fasting glucose.
Calculated using predictive margins adjusted for sex, age, BMI (not included for obesity), smoking status, HIV status and statistically significant interactions between these variables.
Elevated ALT level was defined as ALT > 33 U/L for men and > 25 U/L for women.
The cut‐off value for TG:HDL‐C ratio was defined as ≥ 3.75 in men and ≥ 3.0 in women.
IFG was defined as fasting plasma glucose level of 6.1–6.9 mmol/L (110–125 mg/dL) in a non‐diabetic subject.
Diabetes mellitus (DM) was defined as a physician/study subject‐reported diagnosis of DM, current use of antidiabetic medication, HbA1c ≥ 48 mmol/mol (6.5%) or fasting glucose level ≥ 7.0 mmol/L (126 mg/dL).
The prevalence of IFG was significantly higher among PLWH, yet that of diabetes did not differ significantly between PLWH and the general population (Table 3). Also, the sub‐analysis limited to hepatitis C negative PLWH showed a higher weighted adjusted prevalence of IFG among PLWH than in the general population (19.5%, 95% CI: 15.8–23.2 vs 9.8%, 95% CI: 8.7–10.8), but there was no statistically significant difference in the prevalence of diabetes (11.9%, 95% CI: 8.7–15.1 vs 14.5%, 95% CI: 13.6–15.4, respectively). Fasting glucose concentration was higher (median 5.8 mmol/l, IQR 5.4–6.3 mmol/l vs 5.6 mmol/l, IQR 5.3–6.0 mmol/l, P < 0.001), but HbA1c lower (median 34 mmol/mol, IQR 31–36 mmol/mol vs. 36 mmol/mol, IQR 32–37 mmol/mol, P < 0.001) in PLWH than in FinHealth subjects.
The relationship between age and prevalence of obesity is shown in Fig. 1a and the relationship between BMI and prevalence of dysglycemia in Fig. 1b. PLWH had significantly lower prevalence of obesity than general population between 40–70 years of age. For any BMI value between 20 and 40 kg/m2, PLWH had significantly higher prevalence of dysglycemia than the general population.
Fig. 1.
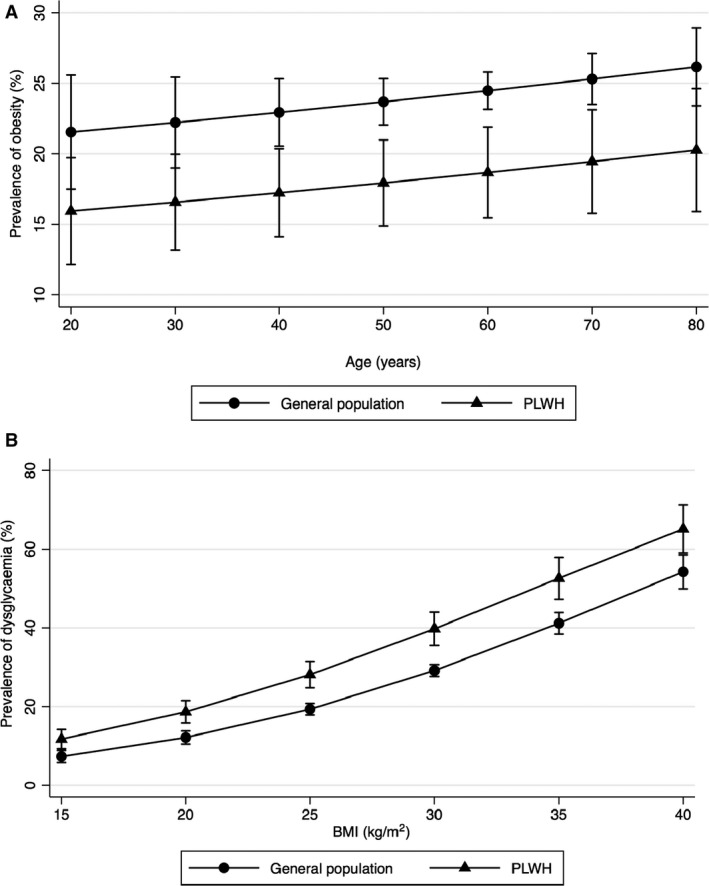
(a, b) Relationship between age and weighted prevalence of obesity adjusted for sex, age and smoking status (a) and between body mass index (BMI) and weighted prevalence of dysglycaemia, defined as presence of impaired fasting glucose or diabetes, adjusted for sex, age, BMI and smoking status (b). Error bars indicate 95% confidence interval. PLWH, people living with HIV.
The results of the first multivariable logistic regression analysis of the factors associated with obesity and dysglycemia among PLWH are shown in Table 4. Regarding obesity, being ex‐smoker was associated with an increased risk, whereas male‐to‐male sexual transmission mode and CD4 nadir > 350 cells/μL were associated with a decreased risk of obesity. Male gender, increasing age and BMI, and intravenous drug use were associated with an increased risk of dysglycaemia.
Table 4.
Multivariable regression analysis of the predictor variables for obesity and dysglycaemia among people living with HIV (PLWH)
| Predictor variable | Obesity (BMI ≥ 30 kg/m2) | P value | Dysglycaemia* | P‐value | ||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Gender | ||||||
| Male | Reference | Reference | ||||
| Female | 0.88 | 0.54–1.44 | 0.611 | 0.33 | 0.20–0.54 | < 0.001 |
| Age (years) | 1.00 | 0.99–1.02 | 0.643 | 1.04 | 1.03–1.06 | < 0.001 |
| Smoking | ||||||
| Never smoker | Reference | Reference | ||||
| Current smoker | 0.82 | 0.50–1.33 | 0.419 | 1.07 | 0.73–1.58 | 0.721 |
| Ex‐smoker | 1.76 | 1.13–2.74 | 0.013 | 0.93 | 0.64–1.36 | 0.714 |
| Body mass index (kg/m2) | N/A | 1.12 | 1.08–1.16 | < 0.001 | ||
| HIV transmission mode | ||||||
| Male‐to‐male sex | Reference | Reference | ||||
| Heterosexual | 2.69 | 1.74–4.16 | < 0.001 | 1.38 | 0.94–2.03 | 0.105 |
| Intravenous drug use | 2.85 | 1.61–5.04 | < 0.001 | 3.18 | 1.83–5.54 | < 0.001 |
| Unknown/other | 1.27 | 0.30–5.40 | 0.744 | 0.43 | 0.12–1.59 | 0.206 |
| CD4 nadir ≥ 350 cells/μL | 0.60 | 0.38–0.94 | 0.026 | 1.40 | 0.97–2.01 | 0.072 |
| Use of selected NRTIs in the past † | 0.95 | 0.66–1.38 | 0.806 | 1.26 | 0.92–1.73 | 0.153 |
BMI, body mass index; OR, odds ratio; CI, confidence interval; NRTI, nucleoside reverse transcriptase inhibitor.
Dysglycaemia was defined as the presence of diabetes or impaired fasting glucose.
Didanosine, zidovudine or stavudine.
In the second multivariable logistic regression analysis, the current use of TAF (odds ratio, OR = 1.79, 95% CI: 1.12–2.86, P = 0.015) and TDF (OR = 1.96, 95% CI: 1.01–3.80, P = 0.047) were associated with a significantly higher risk of obesity than was the case with ABC. There was no statistically significant difference between TDF and TAF (P = 0.6). The current use of NNRTIs as compared with INSTIs was associated with a lower risk of obesity (OR = 0.52, 95% CI: 0.31–0.87, P = 0.013), but there were no statistically significant differences between the current use of PIs vs. INSTIs (P = 0.6) or PIs vs. NNRTIs (P = 0.09). The use of ABC (OR = 0.56, 95% CI: 0.33–0.97, P = 0.038) was associated with a significantly lower risk, and the use of TAF (OR = 0.62, 95% CI: 0.38–1.00, P = 0.051) with a borderline lower risk, of dysglycaemia compared with TDF. There was no significant difference between the use of ABC and the use of TAF (P = 0.6), or between any of the third agents (data not shown), in relation to the risk of dysglycaemia.
Discussion
To the best of our knowledge, the current study presents the first comparison of adjusted prevalence rates of insulin resistance, IFG and diabetes using fasting blood samples between PLWH and the general population. Despite the significantly lower prevalence of obesity, the adjusted prevalence rates of insulin resistance and IFG were significantly higher in PLWH than in the general population. These higher prevalence rates did not translate into higher diabetes prevalence, potentially implying under‐diagnosis of DM among PLWH.
Even though obesity rates are increasing among PLWH [2], we still found a significantly lower adjusted prevalence of obesity among PLWH than among the general population, which is in line with data from the COCOMO study in Denmark [5]. The obesity rates among PLWH vary greatly even within the European context. The prevalence rates of obesity among PLWH both in our study (18%) and the COCOMO (11%) study are two to three times higher than that reported in the large EuroSIDA cohort (6%) [4]. These differences probably reflect different obesity rates in general populations in different European countries [17], different patient characteristics and possibly different use of ART. The most recent data comparing PLWH and the general population from North America are similar to our results demonstrating significantly less obesity among PLWH (18–25.5%) than in the general population (27–36%) [6, 18].
Knowing the relatively stable increase of 0.3–1 kg/year in body weight in the general population [19, 20, 21], we wanted to explore whether PLWH would have a steeper slope between age and prevalence of obesity as a feature of a premature ageing [22]. For any given age, the probability of obesity was steadily 6–7% lower for PLWH than for the general population, contradicting enhanced weight gain among PLWH. However, there is still concern about the effect of ageing, as Gelpi et al. [23] recently reported that the probability of abdominal obesity, measured by waist‐to‐hip ratio, is more enhanced by age in PLWH than in population controls.
Recent data have associated increased weight gain with the use of INSTIs and TAF [24, 25, 26, 27, 28]. In our study, almost 60% of the participants were receiving an INSTI‐based regimen and over 50% a TAF‐based regimen. The current users of INSTIs had a significantly higher risk of obesity compared with the users of NNRTIs. Similarly, those taking TAF or TDF had a higher risk of obesity compared with subjects taking ABC. As opposed to the earlier data [24, 25], we did not observe a significant difference between the current users of TDF and TAF. Although these results must be interpreted with caution, because of the non‐randomized, cross‐sectional design of the study, our real‐world data support the accumulating evidence of weight gain with INSTIs and TAF [24, 25, 26, 27, 28]. We also identified a CD4 nadir count > 350 cells/μL to be associated with a smaller risk of obesity, which is in agreement with a recent review demonstrating that a high CD4 nadir is a protective factor against obesity [7].
Regardless of the lower obesity rate, the adjusted prevalence of insulin resistance was significantly higher in PLWH than in the general population. We used TG:HDL‐C ratio and plasma ALT as surrogate markers of insulin resistance, as both predict type 2 diabetes in non‐HIV populations [29, 30, 31, 32]. Also among PLWH, a high TG:HDL‐C ratio predicted new‐onset DM independently of traditional risk factors [14], and elevated ALT has been associated with a higher risk of diabetes [33]. In addition, the adjusted prevalence of IFG among PLWH (20.0%) was double that in the general population (9.8%) in our study, again implying a higher degree of insulin resistance. We were only able to identify a single previous study comparing prevalence of IFG between PLWH and HIV‐negative controls: the Women’s Interagency HIV Study (WIHS) reported an unadjusted IFG prevalence of 9% for both HIV‐positive and HIV‐negative women [34]. The lower prevalence of IFG among PLWH in the WIHS cohort than in our study can at least partially be explained by the gender difference; all WIHS subjects were women and also in our study female gender was an independent protective factor for dysglycaemia.
A novel finding in our study is the discrepancy in the prevalence rates of insulin resistance and IFG vs. DM among PLWH. Surprisingly, the adjusted prevalence of DM was not higher among PLWH than in the general population, despite the higher prevalence of IFG and insulin resistance. These data raise concerns of under‐diagnosis of DM, possibly due to lower sensitivity of HbA1c among PLWH [35, 36, 37, 38, 39]. Our finding of higher glucose concentration yet lower HbA1c among PLWH than among FinHealth subjects supports this hypothesis. False low HbA1c values lead directly to under‐diagnosis of DM based on HbA1c criteria. In addition, in clinical practice false low HbA1c may delay the treatment of DM even when fasting glucose values fulfil the criteria of diabetes. Unfortunately, we could not compare the proportions of DM diagnoses made by HbA1c criteria between the study groups, as the criteria for diagnosis of DM made before 2017 was not available.
The prevalence of DM among PLWH (13%) in our study was higher than that reported from the EuroSIDA cohort (5%) [4] or the COCOMO study (4%) [5]. These differences probably reflect different DM prevalence rates in different European countries [40], as, in line with our results, the prevalence of DM among PLWH has not been significantly different from that of local HIV‐negative controls [3, 5]. These data are in contrast to a large US study from 2009–2010 which found a significantly higher predicted marginal prevalence of DM in PLWH (11.8%) than in the general population (8.0%) [18]. However, this study was hampered by the different definition of DM in the study groups, and laboratory criteria were not included. In the other earlier studies comparing the prevalence of DM in PLWH and the general population, the diagnosis of DM has been based on administrative data or registries [8, 9, 41]. There are some data using fasting blood samples for the diagnosis of DM in large HIV populations, but in these studies the HIV‐negative controls did not represent the general population but clinical cohorts [34, 42] or, for example, employers of a single company [43]. Furthermore, the applicability of these older data may be limited in today’s clinic population, as the use of older PIs and thymidine analogue NRTIs – known risk factors for DM [44] – has significantly decreased.
To evaluate risk factors for disturbances in glucose homeostasis, we used the combination outcome of dysglycaemia. When comparing the relationship between dysglycaemia and BMI, PLWH had 5–10% higher prevalence of dysglycaemia compared with general population for any given BMI. As equal body weight is associated with more harm on glucose homeostasis among PLWH, prevention and treatment of obesity among PLWH must be emphasized. Multiple causes may contribute to this increased risk of dysglycaemia among PLWH, e.g. increased waist‐to‐hip ratio despite lower BMI [23], certain ART agents, lipodystrophy and inflammatory status [2]. Unfortunately, we could not study these risk factors in detail, as waist‐to‐hip ratios and presence or absence of lipodystrophy were not systematically recorded. Intravenous drug use was associated with a higher risk of dysglycaemia. Chronic hepatitis C may explain this finding, but we cannot differentiate the specific effect of hepatitis C from intravenous drug use as such, as hepatitis C positivity was almost a universal finding in this transmission group. Regarding the HIV‐specific risk factors, we observed a decreased risk of dysglycaemia in subjects receiving ABC, which has not been reported earlier. We consider a channelling bias the most likely explanation for this finding in our non‐randomized study setting, i.e. ABC was avoided among subjects with high risk of cardiovascular disease, which often coincides with high risk of dysglycaemia.
The main strength and novelty of our study are the first‐time use of fasting blood samples for the comparisons between PLWH and the general population. The use of fasting blood samples allows features of glucose homeostasis to be compared in a more objective manner than does the use of administrative data alone. Furthermore, the HIV‐infected group represents the HIV population of today, in whom there is a common use of INSTIs and TAF. The data between the groups are temporally comparable, as all data were collected during the same year. Lastly, because of the universal health coverage in the present study setting, we could exclude the healthcare payer being a significant confounding factor as has been demonstrated for comorbidities in the United States [45].
Our study has also limitations. Even though we adjusted the study groups for multiple factors, we did not have information, for example, on dietary or exercise habits, or family history of DM. Smoking status was based on self‐report in both groups and was not confirmed by exhaled carbon monoxide. Both of our study groups were of white ethnicity, so our data may not be generalizable to other ethnic groups. For the diagnosis of DM we used only a single value of fasting glucose ≥ 7.0 mmol/L (126 mg/dL) instead of the conventional repeated fasting glucose ≥ 7.0 mmol/L (126 mg/dL). This may increase the prevalence of DM in both groups to some extent but should not interfere with their comparison. As we did not have serum insulin values, we could not calculate more extensively validated measures of insulin resistance, such as Homeostatic Model Assessment index, but had to limit our analyses to less optimal surrogates of insulin resistance (plasma ALT, TG:HDL‐C ratio).
In conclusion, our data do not support increased obesity rates among PLWH treated with modern ART as compared with the general population, but insulin resistance and IFG were significantly more prevalent among PLWH. Nevertheless, the prevalence of DM was not higher, raising a concern for under‐diagnosis of DM among PLWH. Male gender, increasing age and body weight remain the main risk factors for developing dysglycaemia. The enhanced relationship between BMI and dysglycaemia highlights the need also to prevent and treat obesity among PLWH.
Author contributions
AH, KJK, PKi and JS designed the study; AH, KJK and PKo collected the data; AH, KJK, PKi, JO and JS performed data analysis; PJ, KL, PKi, PKo and JS interpreted the results; AH, KJK and JS wrote the manuscript; and PKi, JO, PJ, KL and PKo reviewed and edited the manuscript. All authors approved the final version of the manuscript.
Acknowledgements
Financial disclosure: This work was supported by the Finnish Subsidy for Health Science Research. Part of the data collection of PLWH was supported by a grant from the Gilead Sciences Nordic Fellowship programme.
Conflict of interest: AH received conference support from Merck and Gilead and a lecture fee from Merck. PKi received honoraria, lecture fees and conference support from Gilead, Merck and GSK/ViiV, and a research grant from Gilead. JS received honoraria, lecture fees and conference support from Gilead, Merck and GSK/ViiV, and a research grant from Gilead and Merck. All other authors have no conflicts of interest to declare.
The first two authors contributed equally to this work.
References
- 1. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840–846. [DOI] [PubMed] [Google Scholar]
- 2. Kumar S, Samaras K. The impact of weight gain during HIV treatment on risk of pre‐diabetes, diabetes mellitus, cardiovascular disease, and mortality. Front Endocrinol 2018; 9: 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schouten J, Wit FW, Stolte IG et al. Cross‐sectional comparison of the prevalence of age‐associated comorbidities and their risk factors between hiv‐infected and uninfected individuals: the AGEhIV Cohort Study. Clin Infect Dis 2019; 59: 1787–1797. [DOI] [PubMed] [Google Scholar]
- 4. Pelchen‐Matthews A, Ryom L, Borges ÁH et al. Aging and the evolution of comorbidities among HIV‐positive individuals in a European cohort. AIDS 2018; 32: 2405–2416. [DOI] [PubMed] [Google Scholar]
- 5. Knudsen AD, Gelpi M, Afzal S et al. Brief Report: Prevalence of peripheral artery disease is higher in persons living with HIV compared with uninfected controls. JAIDS 2018; 79: 381–385. [DOI] [PubMed] [Google Scholar]
- 6. Koethe JR, Jenkins CA, Lau B et al. Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 2016; 32: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sax PE, Erlandson KM, Lake JE et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2019; 71: 1379–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rasmussen LD, Mathiesen ER, Kronborg G, Pedersen C, Gerstoft J, Obel N. Risk of diabetes mellitus in persons with and without HIV: a Danish nationwide population‐based cohort study. PLoS One 2012; 7: e44575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tripathi A, Liese AD, Jerrell JM et al. Incidence of diabetes mellitus in a population‐based cohort of HIV‐infected and non‐HIV‐infected persons: the impact of clinical and therapeutic factors over time. Diabet Med 2014; 31: 1185–1193. [DOI] [PubMed] [Google Scholar]
- 10. Richter B, Hemmingsen B, Metzendorf M, Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev 2018; 10: CD012661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borodulin K, Sääksjärvi K. Finnish Institute for Health and Welfare. FinHealth 2017 Study Methods. Available at: http://www.julkari.fi/handle/10024/139084 (accessed 20 July 2020).
- 12. Finnish Institute for Health and Welfare . National FinHealth Study 2017. Available at: https://thl.fi/en/web/thlfi‐en/research‐and‐expertwork/population‐studies/national‐finhealth‐study (accessed 20 July 2020).
- 13. ECDC: HIV/AIDS surveillance in Europe 2019 (2018 data). Available at: https://www.ecdc.europa.eu/sites/default/files/documents/hiv‐surveillance‐report‐2019.pdf (accessed 20 July 2020).
- 14. Squillace N, Lorenzini P, Lapadula G et al. Triglyceride/HDL ratio and its impact on the risk of diabetes mellitus development during ART. J Antimicrob Chemother 2016; 71: 2663–2669. [DOI] [PubMed] [Google Scholar]
- 15. Wakabayashi I, Daimon T. Comparison of discrimination for cardio‐metabolic risk by different cut‐off values of the ratio of triglycerides to HDL cholesterol. Lipids Health Dis 2019; 18: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 2017; 112: 18–35. [DOI] [PubMed] [Google Scholar]
- 17. Body Mass Index (BMI) by sex, age and educational attainment level. Eurostat: European Health Interview Survey (EHIS) conducted in 2014. Available at: https://ec.europa.eu/eurostat/statistics‐explained/index.php?title=Overweight_and_obesity_‐_BMI_statistics (accessed 20 July 2020).
- 18. Hernandez‐Romieu AC, Garg S, Rosenberg ES, Thompson‐Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV‐infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care 2017; 5: e000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pajunen P, Vartiainen E, Männistö S, Jousilahti P, Laatikainen T, Peltonen M. Intra‐individual changes in body weight in population‐based cohorts during four decades: the Finnish FINRISK study. Eur J Public Health 2012; 22: 107–112. [DOI] [PubMed] [Google Scholar]
- 20. Malhotra R, Ostbye T, Riley CM, Finkelstein EA. Young adult weight trajectories through midlife by body mass category. Obesity (Silver Spring) 2013; 21: 1923–1934. [DOI] [PubMed] [Google Scholar]
- 21. Hutfless S, Maruthur NM, Wilson RF et al. Strategies to Prevent Weight Gain Among Adults. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. Available at https://www.ncbi.nlm.nih.gov/books/NBK133218/pdf/Bookshelf_NBK133218.pdf (accessed 20 July 2020). [PubMed] [Google Scholar]
- 22. Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci 2014; 69: 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gelpi M, Afzal S, Lundgren J et al. Higher risk of abdominal obesity, elevated low‐density lipoprotein cholesterol, and hypertriglyceridemia, but not of hypertension, in people living with human immunodeficiency virus (HIV): results from the Copenhagen Comorbidity in HIV Infection Study. Clin Infect Dis 2018; 67: 579–586. [DOI] [PubMed] [Google Scholar]
- 24. Gomez M, Seybold U, Roider J, Härter G, Bogner JR. A retrospective analysis of weight changes in HIV‐positive patients switching from a tenofovir disoproxil fumarate (TDF)‐ to a tenofovir alafenamide fumarate (TAF)‐containing treatment regimen in one German university hospital in 2015–2017. Infection 2019; 47: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schafer JJ, Sassa KN, O'Connor JR, Shimada A, Keith SW, DeSimone JA. Changes in body mass index and atherosclerotic disease risk score after switching from tenofovir disoproxil fumarate to tenofovir alafenamide. Open Forum . Infect Dis 2019; 6: ofz414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bourgi K, Rebeiro PF, Turner M et al. Greater weight gain in treatment naïve persons starting dolutegravir‐based antiretroviral therapy. Clin Infect Dis 2020; 70: 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kerchberger AM, Sheth AN, Angert CD et al. Weight gain associated with integrase stand transfer inhibitor use in women. Clin Infect Dis 2019; 71: 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eckard AR, McComsey GA. Weight gain and integrase inhibitors. Curr Opin Infect Dis 2020; 33: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin D, Qi Y, Huang C et al. Associations of lipid parameters with insulin resistance and diabetes: a population‐based study. Clin Nutr 2018; 37: 1423–1429. [DOI] [PubMed] [Google Scholar]
- 30. Kim‐Dorner S, Deuster PA, Zeno SA, Remaley AT, Poth M. Should triglycerides and the triglycerides to high‐density lipoprotein cholesterol ratio be used as surrogates for insulin resistance? Metab Clin Exp 2010; 59: 299–304. [DOI] [PubMed] [Google Scholar]
- 31. Abbasi F, Shiffman D, Tong CH, Devlin JJ, McPhaul MJ. Insulin Resistance probability scores for apparently healthy individuals. J Endocr Soc 2018; 2: 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lallukka S, Yki‐Järvinen H. Non‐alcoholic fatty liver disease and risk of type 2 diabetes. Best Pract Res Clin Endocrinol Metab 2016; 30: 385–395. [DOI] [PubMed] [Google Scholar]
- 33. Sabin CA, Ryom L, Kovari H et al. Association between ALT level and the rate of cardio/cerebrovascular events in HIV‐positive individuals: the D: A: D study. J Acquir Immune Defic Syndr 2013; 63: 456–463. [DOI] [PubMed] [Google Scholar]
- 34. Galaviz KI, Schneider MF, Tien PC et al. Predicting diabetes risk among HIV‐positive and HIV‐negative women. AIDS 2018; 32: 2767–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monroe AK, Glesby MJ, Brown TT. Diagnosing and managing diabetes in HIV‐infected patients: current concepts. Clin Infect Dis 2015; 60: 453–462. [DOI] [PubMed] [Google Scholar]
- 36. Kim PS, Woods C, Georgoff P et al. A1C underestimates glycemia in HIV infection. Diabetes Care 2009; 32: 1591–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eckhardt BJ, Holzman RS, Kwan CK, Baghdadi J, Aberg JA. Glycated Hemoglobin A(1c) as screening for diabetes mellitus in HIV‐infected individuals. AIDS Patient Care STDS 2012; 26: 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slama L, Palella FJ Jr, Abraham AG et al. Inaccuracy of haemoglobin A1c among HIV‐infected men: effects of CD4 cell count, antiretroviral therapies and haematological parameters. J Antimicrob Chemother 2014; 69: 3360–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coelho AR, Moreira FA, Santos AC et al. Diabetes mellitus in HIV‐infected patients: fasting glucose, A1c, or oral glucose tolerance test ‐ which method to choose for the diagnosis? BMC Infect Dis 2018; 18: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. International Diabetes Federation: Diabetes Atlas. Available at: https://diabetesatlas.org/data/en/region/3/eur.html (accessed 20 July 2020).
- 41. Kendall CE, Wong J, Taljaard M et al. A cross‐sectional, population‐based study measuring comorbidity among people living with HIV in Ontario. BMC Public Health 2014; 14: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brown TT, Cole SR, Li X et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005; 165: 1179–1184. [DOI] [PubMed] [Google Scholar]
- 43. Galli L, Salpietro S, Pellicciotta G et al. Risk of type 2 diabetes among HIV‐infected and healthy subjects in Italy. Eur J Epidemiol 2012; 27: 657–665. [DOI] [PubMed] [Google Scholar]
- 44. Noubissi EC, Katte J, Sobngwi E. Diabetes and HIV. Curr Diab Rep 2018; 18: 125. [DOI] [PubMed] [Google Scholar]
- 45. Gallant J, Hsue PY, Shreay S, Meyer N. comorbidities among US patients with prevalent HIV infection‐a trend analysis. J Infect Dis 2017; 216: 1525–1533. [DOI] [PubMed] [Google Scholar]


