Abstract
Chimeric antigen receptor‐T (CAR‐T) cell therapy is a promising treatment for CD19+ B‐cell malignancies. However, elimination of B cells by anti‐CD19 CAR‐T cells may lead to the reactivation of hepatitis B virus (HBV) and related hepatitis in patients with HBV infection. This study aims to evaluate the safety and efficacy of humanized anti‐CD19 CAR‐T (hCAR‐T) therapy in B‐cell malignancies with HBV infection. Twenty relapsed/refractory (r/r) diffuse large B‐cell lymphoma (DLBCL) and acute lymphoblastic leukemia (ALL) patients with HBV infection were treated with hCAR‐T therapy. Among them, five hepatitis B antigen‐positive patients who received antiviral prophylaxis did not develop HBV reactivation, including two patients who received both hCAR‐T and allogeneic hematopoietic stem cell transplantation (allo‐HSCT). Among 15 patients with resolved HBV infection, two received antiviral prophylaxis, and the other 13 did not experience HBV reactivation without antiviral prophylaxis. One patient with resolved HBV infection experienced HBV reactivation 6 months after hCAR‐T therapy and sequential allo‐HSCT. Moreover, HBV infection did not affect in vivo expansion of hCAR‐T cells or increase the risk of severe cytokine release syndrome. In conclusion, hCAR‐T therapy is safe and effective in DLBCL and ALL patients with chronic and resolved HBV infection under proper antiviral prophylaxis.
Keywords: HBV reactivation, humanized anti‐CD19 CAR‐T, leukemia, lymphoma, safety
1. INTRODUCTION
Chimeric antigen receptor‐T (CAR‐T) cell immunotherapy has produced exciting results in relapsed/refractory (r/r) B‐cell non‐Hodgkin lymphoma (NHL) and acute lymphoblastic leukemia (ALL). 1 , 2 , 3 , 4 The administration of CAR‐T targeting CD19 causes a long period of B‐cell aplasia in the peripheral blood (PB), which may lead to reactivation of hepatitis B virus (HBV) and related hepatitis in NHL and ALL patients with HBV infection. 4 China is a hyperendemic country for HBV infection, and many B‐cell malignancies have a significant association with HBV infection. 5 , 6 However, little is known about the risks of HBV reactivation and hepatitis during and after CAR‐T therapy, because patients with a history of acute or chronic active HBV infection have been excluded from past clinical trials of CAR‐T therapy due to concerns about their safety. 1 , 7
HBV reactivation is a common complication in patients undergoing B‐cell‐depleting therapy and allogenic hematopoietic stem cell transplantation (allo‐HSCT). Anti‐CD20‐directed monoclonal antibodies cause a decrease in the circulating anti‐HBV antibodies, and may neutralize antibodies targeting the hepatitis B antigen (HBsAg). Previous studies demonstrated that both NHL patients with chronic and resolved HBV infections were likely to be at high risk of HBV reactivation when they received rituximab with a median 6–12 months after the last dose. 8 , 9 Moreover, patients who underwent allo‐HSCT represent another population with high risk of HBV reactivation. The rate of HBV reactivation ranges from 40% over 2 years in HBV core antibody (anti‐HBc)‐positive patients to 70%–86% over 5 years in HBsAg‐positive recipients. 10 , 11 Allo‐HSCT is often offered to r/r ALL patients to consolidate remission after CAR‐T therapy. Allo‐HSCT is also an important option for r/r NHL patients after CAR‐T therapy. 12 , 13 Both CAR‐T therapy and allo‐HSCT are associated with immunosuppression, and their influence on the incidence of HBV reactivation and related hepatitis events during and after CAR‐T immunotherapy remain unclear.
Although CAR‐T therapy has shown significant therapeutic efficacy in treating r/r ALL, relapse remains the major concern for ALL patients. Immunogenicity derived from the murine single chain variable fragment (scFv) may lead to tumor relapse in complete remission (CR) patients, and it can limit the efficacy of CAR T‐cell therapy in diffuse large B‐cell lymphoma (DLBCL) patients. Therefore, utilizing humanized anti‐CD19 CAR‐T (hCAR‐T) cells instead of murine CARs is emerging as a current trend in CAR‐T immunotherapy. 14
This study reports the safety and efficacy of utilizing hCAR‐T cells to treat r/r ALL and DLBCL patients with HBV infection. In addition, we also analyzed safety and toxicity data from patients undergoing allo‐HSCT after receiving hCAR‐T cell therapy.
2. PATIENTS AND METHODS
2.1. Study design
In this study, 20 patients with r/r DLBCL or B‐ALL were admitted to the Department of Hematology in Tianjin First Central Hospital between July 2018 and May 2019. All of the admitted patients met the diagnostic criteria according to the WHO classification. 15 Additionally, all of the patients were enrolled in a clinical trial of anti‐CD19 CAR‐T‐cell‐expressing humanized anti‐CD19 scFv and 4‐1BB‐CD3ζ costimulatory‐activation domains (ChiCTR1800019622 and ChiCTR1800018059).
Cytokine release syndrome (CRS) and neurologic adverse events were evaluated in accordance with criteria from the American Society for Transplantation and Cellular Therapy 16 The clinical response of CAR‐T cells for ALL patients was defined in accordance with criteria from the National Comprehensive Cancer Network guidelines. DLBCL patients were defined in accordance with 2014 Lugano criteria.
2.2. Eligibility criteria related to HBV infection
Chronic versus acute infection is defined by the presence of HBsAg for at least 6 months. Resolved HBV infection was defined by a negative test for HBsAg and undetectable HBV deoxyribonucleic acid (DNA), but a positive test for anti‐HBc. Patients with detectable HBV DNA diagnosis of ALL/DLBCL in our department were not excluded if HBV DNA were undetectable and alanine aminotransferase (ALT)/aspartate aminotransferase (AST) < 2.5 upper limit of normal under antiviral prophylaxis at the time of CAR‐T therapy. HBV reactivation in HBsAg‐positive, anti‐HBc‐positive patients is reasonably defined as one of the following: (i) a ≥2 log (100‐fold) increase in HBV DNA compared to the baseline level, (ii) HBV DNA ≥ 3 log (1000) IU/ml in a patient with previously undetectable level (since HBV DNA levels fluctuate), or (iii) HBV DNA ≥ 4 log (10,000) IU/ml if the baseline level is not available. For HBsAg‐negative, anti‐HBc‐positive patients, the following criteria are reasonable for HBV reactivation: (i) HBV DNA is detectable, or (ii) reverse HBsAg seroconversion occurs (reappearance of HBsAg). 17 A hepatitis flare is reasonably defined as an ALT increase to ≥3 times the baseline level and >100 U/L. 17
2.3. Quantitative serum HBsAg, HBV DNA, and HBV assay
Serological HBV markers including serum HBeAg, anti‐HBs, anti‐HBe, and anti‐HBc were detected by the Elecsys instrumental platform (Roche Diagnostics). Serum HBsAg titers were measured using an Elecsys HBsAg II quant assay (Roche Diagnostics). HBV DNA was measured in serum using a quantitative, real‐time polymerase chain reaction (PCR) assay (Kehua Bio‐engineering Co. Ltd.,) with a lower limit of quantitation of 50 IU/ml.
2.4. CAR‐T cell manufacturing and monitoring
The procedures for CAR‐T cell manufacturing and quality‐control assays have been described in our previous study. 18 Expansion and persistence of CAR‐T cells in patient PB and bone marrow were assessed by quantitative polymerase chain reaction at different time points. 18
2.5. Allo‐HSCT
ALL and DLBCL patients received total body irradiation/cyclophosphamide (CY)‐based preparative regimens: cyclosporine A, mycophenolate mofetil, and short‐term methotrexate were used to prevent graft‐versus‐host disease (GVHD).
2.6. Statistical analysis
Follow‐up was either to death or January 1st, 2020. All of the statistical analyses were performed using the statistical software GraphPad Prism 7. Non‐normal distribution data were expressed as a median and interquartile range. Baseline variables were compared between cohorts using the χ2 test for categorical variables and the Mann‐Whitney U test for continuous variables. Overall survival (OS) and progression‐free survival (PFS) were calculated using the Kaplan‐Meier method and compared using the log‐rank test. p value < 0.05 was considered to be significant.
3. RESULTS
3.1. Patient characteristics and antiviral therapy
The virological serum parameters at diagnosis of ALL/DLBCL and at the time of CAR‐T cell infusion are shown in Figure 1A and Table S1. This trial included seven cases of r/r ALL and 13 cases of r/r DLBCL. HBsAg was positive in five patients at the time of hCAR‐T cell infusion, and anti‐HBc was positive in the other 15 patients. Within five HBsAg‐positive patients, three were HBsAg‐positive chronic HBV infection and the other two were HBsAg‐negative chronic HBV infection. Three had detectable HBV DNA at diagnosis before antiviral treatment (533 IU/ml, 620 IU/ml, and 890 IU/ml for P1, P17, and P19, respectively), and these patients achieved a complete virological response (undetectable HBV DNA levels) at the time of CAR‐T cell infusion. All of the five patients with chronic HBV infection received 300 mg tenofovir (TDF) and/or 0.5 mg entecavir (ETV) daily at least one month before hCAR‐T cell infusion and continued this therapy to the end of the last follow‐up (Figure 1B).
FIGURE 1.
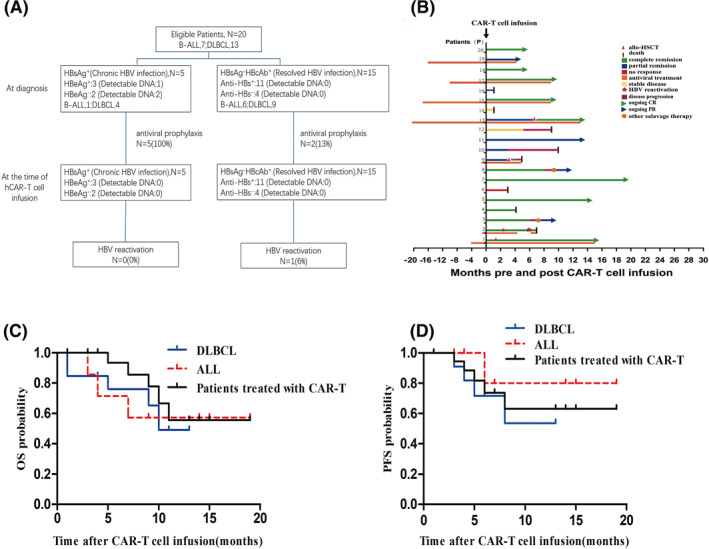
The enrollment of patients, clinical course and outcome for 20 patients treated with hCAR‐T cell therapy (A), Status of enrolled patients and schematic of study stages on which patients were treated. The detectable HBV DNA of HBsAg positive patients at diagnosis of the disease were 533 IU/ml, 620 IU/ml, and 890 IU/ml for P1, P17, and P19, respectively. Within the HBsAg‐positive patients, P1, P13, P15, P17, and P19 received antiviral prophylaxis for 4, 20, 18, 10, and 16 months prior to hCAR‐T cell infusion, respectively. Within the anti‐HBc positive patients, only P2 and P9, who proceed to allo‐HSCT, received antiviral prophylaxis for 1 month before hCAR‐T cell infusion (B). The prophylactic antiviral treatment and clinical response for 20 patients. Among the patients, P1 and P13 underwent allo‐HSCT post hCAR‐T cell infusion. The duration of responses was shown. Long‐term remissions (>12 months) were observed in P1, P5, P7, and P13. (C and D) OS and PFS of the patients treated with hCAR‐T therapy. For (C and D), black lines indicate all the patients who were treated with hCAR‐T cells (n = 20), blue lines indicate diffuse large B‐cell lymphoma patients (n = 13), and red broken lines indicate acute lymphoblastic leukemia patients (n = 7). ALL, acute lymphoblastic leukemia; allo‐HSCT, allogeneic hematopoietic stem cell transplantation; CAR‐T, chimeric antigen receptor; CR, complete remission; DLBCL, diffuse large B‐cell lymphoma; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; hCAR‐T, humanized CAR‐T; OS, overall survival; PFS, progression‐free survival; PR, partial response.
Among the 15 patients with resolved HBV infection, HBV DNA was undetectable, and 11 of these patients were positive for antibody to HBsAg (anti‐HBs) at the time of hCAR‐T cell infusion (Figure 1A). Only two patients (P2 and P9), who proceeded to allo‐HSCT after hCAR‐T therapy, received ETV for prophylaxis beginning 1 month before hCAR‐T cell infusion (Figure 1B). In 13 patients with resolved HBV infection, HBsAg and/or HBV DNA monitoring was performed prospectively every month until at least 1 year after hCAR‐T cell infusion. ETV or TDF treatment was given as a preemptive therapy in the case of either detectable HBV DNA or HBsAg seroconversion.
All of the patients received a lymphodepleting regimen containing fludarabine (30 mg/m2/day for 3 days) plus CY (400 mg/m2/day for 3 days). The median dosage of hCAR‐T cells was 1.5 × 106/kg for ALL patients and 4 × 106/kg for DLBCL patients (Table 1).
TABLE 1.
Hepatitis characteristics pre and post hCAR‐T cell infusion, including the degradation of CRS and ICANS
| At the time of CAR‐T cell infusion | Post CAR‐T cell infusion | Follow‐up (months) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt No | Conditioning regimen | CAR‐T cells (106/kg) | HBsAg status a /levels (IU/ml) | Anti‐HBs status | HBeAg status | Anti‐HBe status | Anti‐HBc status | ALT b (U/L) | HBV DNA c (IU/ml) | Prophylaxis | Time to reactivation (months) | Peak HBsAg status/levels (IU/ml) | Peak ALT (U/L) | Peak DNA (IU/ml) | Response | CRS | ICANS | |
| 1 | FC | 2 | +/130 | ‐ | + | ‐ | + | 15.7 | <50 | TDF | +/138.5 | 103.4 | 72 | CR | 2 | 0 | 15 | |
| 2 | FC | 1 | ‐ | + | ‐ | + | + | 10.4 | <50 | ETV | 6 | +/168 | 141. 4 | 7.2 × 108 | CR | 1 | 0 | 7 |
| 3 | FC | 1.5 | ‐ | + | ‐ | ‐ | + | 23.6 | <50 | ‐ | 23.6 | <50 | CR | 1 | 0 | 9 | ||
| 4 | FC | 1 | + | ‐ | ‐ | + | 30.6 | <50 | ‐ | 52 | <50 | CR | 2 | 0 | 4 | |||
| 5 | FC | 1 | ‐ | + | ‐ | + | + | 8 | <50 | ‐ | 40.7 | <50 | CR | 2 | 0 | 14 | ||
| 6 | FC | 1.6 | ‐ | + | ‐ | + | + | 24.2 | <50 | ‐ | 54 | <50 | NR | 2 | 0 | 3 | ||
| 7 | FC | 6 | ‐ | + | ‐ | ‐ | + | 16.2 | <50 | ‐ | 20 | <50 | CR | 1 | 2 | 19 | ||
| 8 | FC | 3.8 | ‐ | ‐ | ‐ | + | + | 17.5 | <50 | ‐ | 18.2 | <50 | CR | 2 | 0 | 11 | ||
| 9 | FC | 4.1 | ‐ | + | ‐ | + | + | 35 | <50 | ETV | ‐ | 39 | <50 | PR | 1 | 0 | 5 | |
| 10 | FC | 2 | ‐ | ‐ | ‐ | ‐ | + | 12.4 | <50 | ‐ | 88 | <50 | PR | 1 | 0 | 10 | ||
| 11 | FC | 7 | ‐ | + | ‐ | + | + | 18.4 | <50 | ‐ | 22.8 | <50 | PR | 1 | 0 | 13 | ||
| 12 | FC | 5.8 | ‐ | + | ‐ | ‐ | + | 12 | <50 | ‐ | 36.2 | <50 | SD | 1 | 0 | 9 | ||
| 13 | FC | 3 | +/150 | ‐ | + | ‐ | + | 16 | <50 | TDF | +/145 | 20.9 | <50 | PR | 1 | 0 | 13 | |
| 14 | FC | 5 | ‐ | + | ‐ | + | + | 19 | <50 | ‐ | 26.5 | <50 | SD | 2 | 0 | 1 | ||
| 15 | FC | 6 | +/71 | ‐ | + | ‐ | + | 17.7 | <50 | ETV | +/69 | 22.6 | <50 | CR | 2 | 0 | 9 | |
| 16 | FC | 4 | ‐ | + | ‐ | ‐ | + | 25.4 | <50 | ‐ | 61 | <50 | PR | 3 | 0 | 15 | ||
| 17 | FC | 2 | +/86 | ‐ | ‐ | + | + | 15 | <50 | ETV | +/82 | 19.6 | <50 | CR | 1 | 0 | 7 | |
| 18 | FC | 2 | ‐ | ‐ | ‐ | + | 10.6 | <50 | ‐ | 10.6 | <50 | CR | 1 | 0 | 9 | |||
| 19 | FC | 2 | +/57 | ‐ | ‐ | + | + | 36.7 | <50 | ETV + TDF | +/55 | 105 | <50 | PR | 1 | 0 | 4 | |
| 20 | FC | 6 | ‐ | + | ‐ | + | 4.5 | <50 | TDF | ‐ | 25 | <50 | CR | 3 | 1 | 14 | ||
Abbreviations: ALT, alanine aminotransferase; Anti‐HBe, hepatitis B e antibody; Anti‐HBc, hepatitis B core antibody; Anti‐HBs, hepatitis B surface antibody; CAR‐T, chimeric antigen receptor; CR, complete remission; CRS, cytokine release syndrome; ETV, entecavir; FC, fludarabine/cyclophosphamide; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; hCAR‐T, humanized CAR‐T; ICANS, immune effector cell‐associated neurotoxicity syndrome; NR, not remission; PR, partial remission; SD, stable disease; TDF, tenofovir.
HBsAg‐negative: HBsAg levels ranged from 0 to 0.05 IU/ml.
The upper limit of normal ALT was 50 U/L.
The lower limit of quantitation (LLQ) of HBV DNA was 50 IU/ml.
3.2. HBV reactivation and hepatitis
Details of hepatitis and liver function at the time of hCAR‐T cell infusion and post hCAR‐T therapy are listed in Table 1. Overall, HBV reactivation only occurred in one patient (P2, 5%) with resolved HBV infection. Among five patients with chronic HBV infection, none had developed HBV reactivation or HBV‐related hepatitis. Among 13 patients with resolved HBV infection (85% anti‐HBs seropositivity) who did not receive allo‐HSCT after hCAR‐T therapy, none had HBV reactivation or HBV‐related hepatitis during the trial.
More specially, P2 was the only case that had HBV reactivation. P2 achieved CR after one cycle of hCAR‐T therapy, and 2 months later, haploidentical HSCT was carried out (donor HBV profile: HBsAg negative, anti‐HBs positive with 600 IU/L, anti‐HBc positive). The patient discontinued antiviral treatment against medical advice on day +135 post hCAR‐T therapy, and HBV reactivation occurred on day +180 post hCAR‐T therapy with detectable HBV DNA (7.1 × 108 IU/ml), HBsAg seroconversion (HBsAg 168 IU/ml), and HBeAg positivity (Figure 2A). The first elevation of ALT occurred on day +150 was due to cytomegalovirus (CMV) infection after allo‐HSCT and her CMV DNA was undetectable on day +180 post hCAR‐T therapy. Antiviral treatment was restarted with 0.5 mg ETV plus 300 mg TDF when HBV reactivation occurred. Coagulopathy, which is a common complication of HBV‐associated liver failure, occurred on day +195 post CAR‐T infusion. A CT scan of the brain was performed to identify the reason for her sudden disorder of limb activity and progressive deterioration in consciousness on day +198 post CAR‐T cell infusion, and it showed massive basal ganglion hemorrhage. On day +200 post hCAR‐T therapy, the viral titers declined to 9 × 106 IU/ml. However, enzyme bilirubin separation was observed and plasma exchange was begun (Figure 2A). Ultimately, the patient died of cerebral hemorrhage and hepatitis flare on day +205 post hCAR‐T therapy. P1 had slightly elevated serum HBV DNA on day +45 post CAR‐T cell infusion, without increased levels of ALT, AST, or TBIL (Figure 2B). HBV DNA returned to normal on day +60 post CAR‐T cell infusion and remained undetectable to the end of the last follow‐up. Because HBV DNA levels can fluctuate during antiviral treatment, P1 had not experienced HBV reactivation during or after hCAR‐T therapy in our trial.
FIGURE 2.
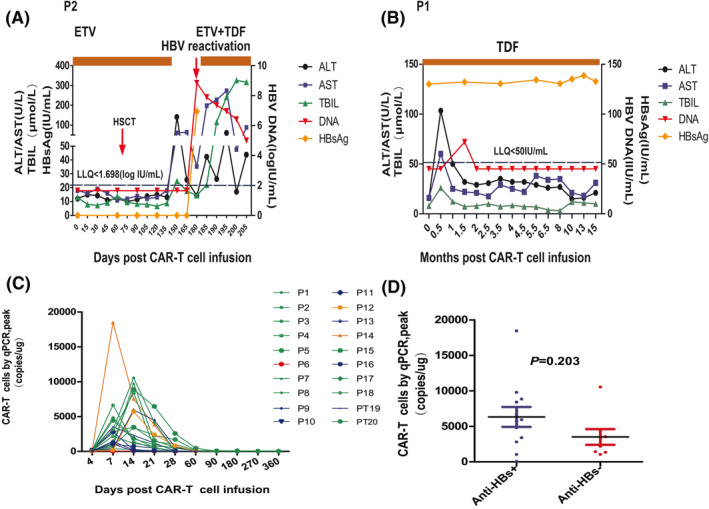
(A and B) The dynamic changes in ALT, AST, TBIL, HBV‐DNA copies, and HBsAg levels post CAR‐T cell therapy in P2 and P1. The LLQ of HBV DNA was 50 IU/ml, which shown in P2 and P1. For P2, on day +150 after CAR‐T cell infusion, serum ALT levels rose to the 141.4 U/L. Meanwhile, serum CMV PCR analysis was positive for 4500 copies/ml. She subsequently recovered with ganciclovir induction therapy for two weeks and had normal ALT levels without detectable CMV DNA in the serum on day +165. (C and D) hCAR‐T cell expansion and persistence in vivo. For (C), the CAR gene copy number was evaluated in 20 ng total DNA from PB mononuclear cells using quantitative PCR at different time points post hCAR‐T cell infusion. For (D), the median peak levels of CAR gene copy numbers in anti‐HBs‐positive patients were not significantly different from those of anti‐HBs‐negative patients (5833 [IQR: 2953–8748] vs. 2830 [IQR: 1384–10,560] copies/μg, p = 0.203). For (C), the green lines, blue lines, yellow lines, and red lines represent patients who obtained a complete remission, partial remission, stable disease, and no remission, respectively. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAR‐T, chimeric antigen receptor; ETV, entecavir; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IQR, interquartile range; LLQ, lower limit of quantitation; PCR, polymerase chain reaction; TBIL, total bilirubin
3.3. hCAR‐T therapy bridging to consolidative allo‐HSCT
Transplantation characteristics and complications were listed in Table S2. Before allo‐HSCT, two ALL patients (P1 and P2) had a morphological CR, and minimal residual disease was found to be negative by flow cytometry. P9 and P13 remained in partial remission at three and 7 months after hCAR‐T cell infusion, respectively. P13 received radiation therapy before hCAR‐T cell infusion. By the median follow‐up at 10 months (range, 5–15 months) post transplantation, three of the patients who were treated with TDF or ETV had not experienced HBV reactivation, and the HBV DNA levels remained undetectable at the time of the last follow‐up. P9 experienced disease progression at day 30 post allo‐HSCT and died of GVHD at 60 days post allo‐HSCT.
3.4. Expansion and persistence of hCAR‐T cells
To investigate whether HBV status affected hCAR‐T expansion, we analyzed the dynamics of hCAR‐T cells. The peak levels of hCAR‐T cells in PB were observed 7–14 days following the initial hCAR‐T cell infusion (Figure 2C). The median peak levels of hCAR‐T cells were 3540 copies/µg for HBsAg‐positive patients and 4801 copies/µg for anti‐HBc positive patients. Moreover, patients with positive anti‐HBs prior to hCAR‐T infusion showed comparable hCAR‐T peak levels to anti‐HBs negative patients (p = 0.203; Figure 2D). Among nine patients who remained in CR without bridging to allo‐HSCT, five patients had consistently detectable levels of CAR DNA between 6 and 9 months after infusion of hCAR‐T cells (Figure 2C).
3.5. CRS and neurotoxicity of hCAR‐T cells
The most prominent adverse effects associated with CAR‐T cell infusion were fever (100%), accompanied by decreased appetite (60%), chills (55%), and fatigue (55%; Table 2). Grade 3 neutropenia was observed in 13 patients (13/20, 65%), anemia was measured in seven patients (7/20, 35%) and thrombocytopenia in 10 patients (10/20, 50%). Only 10% (2 out of 20) of the patients developed Grade 3 CRS. P16 and P20, who experienced Grade 3 CRS, received tocilizumab and dexamethasone. P7 and P20, with resolved HBV infection, experienced Grade 2 and Grade 1 neurotoxicity, respectively. P7 was treated with tocilizumab. Aside from those three patients, all other patients received antipyretic drugs and supportive treatment without any immunosuppressive drugs. The median time from infusion to the onset of CRS was 3 days (range, 1–8 days), and the median duration was 6 days (range, 2–30 days).
TABLE 2.
All adverse events possibly associated with hCAR‐T therapy a
| Pt no | Sinus tachycardia | Dyspnea | Cough | Pleural effusion | Nausea | Vomiting | Decreased appetite | Increased ALT and/or AST | Hypokalaemia | Hypocalcaemia | Hypoalbuminemia | Dysphasia | Depressed level of consciousness | Neutropenia | Anemia | Thrombocytopenia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 1 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 3 | 3 |
| 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 3 | 3 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 3 | 3 |
| 4 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 3 | 1 | 3 |
| 5 | 1 | 0 | 0 | 0 | 2 | 2 | 2 | 0 | 2 | 1 | 1 | 0 | 0 | 3 | 3 | 3 |
| 6 | 1 | 1 | 0 | 0 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 3 | 3 | 3 |
| 7 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 2 | 3 | 1 | 3 |
| 8 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 3 | 1 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 3 | 3 |
| 10 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 1 | 0 |
| 12 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 |
| 14 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 0 | 3 | 3 | 3 |
| 15 | 0 | 1 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 1 | 3 |
| 16 | 1 | 2 | 0 | 2 | 3 | 3 | 3 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| 17 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| 19 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 2 | 0 |
| Total | 10/20 (50%) | 6/20 (30%) | 4/20 (20%) | 2/20 (10%) | 7/20 (35%) | 7/20 (35%) | 12/20 (60%) | 0/20 (0%) | 7/20 (35%) | 9/20 (45%) | 9/20 (45%) | 2/20 (10%) | 2/20 (10%) | 13/20 (65%) | 18/20 (90%) | 10/20 (50%) |
| Pt no | Fever (temperature ≥38°C) | Chills | Hypotension | Hypoxia | Fatigue | Weight loss | Treatmen |
|---|---|---|---|---|---|---|---|
| 1 | 3 | 1 | 2 | 0 | 2 | 0 | Acetaminophen, ibuprofen, fluid bolus, supportive care |
| 2 | 1 | 1 | 0 | 0 | 1 | 0 | Acetaminophen, supportive care |
| 3 | 1 | 0 | 0 | 0 | 1 | 0 | Acetaminophen, supportive care |
| 4 | 2 | 1 | 2 | 0 | 1 | 0 | Acetaminophen, fluid bolus, supportive care |
| 5 | 3 | 1 | 2 | 0 | 2 | 0 | Acetaminophen, ibuprofen, fluid bolus, supportive care |
| 6 | 3 | 1 | 2 | 2 | 2 | 0 | Acetaminophen, fluid bolus, low‐flow nasal cannula, supportive care |
| 7 | 1 | 1 | 0 | 0 | 0 | 0 | Acetaminophen, tocilizumab, supportive care |
| 8 | 1 | 1 | 2 | 2 | 0 | 0 | Acetaminophen, fluid bolus, low‐flow nasal cannula, supportive care |
| 9 | 1 | 0 | 0 | 0 | 0 | 0 | Acetaminophen, supportive care |
| 10 | 1 | 0 | 0 | 0 | 1 | 0 | Acetaminophen, supportive care |
| 11 | 1 | 0 | 0 | 0 | 1 | 0 | Acetaminophen, supportive care |
| 12 | 1 | 0 | 0 | 0 | 1 | 0 | Acetaminophen, supportive care |
| 13 | 1 | 0 | 0 | 0 | 0 | 0 | Acetaminophen, supportive care |
| 14 | 2 | 1 | 2 | 2 | 0 | 0 | Acetaminophen, fluid bolus, low‐flow nasal cannula, supportive care |
| 15 | 2 | 0 | 2 | 2 | 2 | 0 | Acetaminophen, fluid bolus, low‐flow nasal cannula, supportive care |
| 16 | 2 | 1 | 3 | 3 | 2 | 1 | Tocilizumab, dexamethasone, vasopressor, face mask, supportive care |
| 17 | 1 | 1 | 0 | 0 | 0 | 0 | Acetaminophen, supportive care |
| 18 | 1 | 0 | 0 | 0 | 0 | 0 | Acetaminophen, supportive care |
| 19 | 1 | 0 | 0 | 0 | 0 | 0 | Acetaminophen, supportive care |
| 20 | 1 | 1 | 3 | 3 | 0 | 1 | Tocilizumab, dexamethasone, vasopressor, face mask, supportive care |
| Total | 20/20 (100%) | 11/20 (55%) | 9/20 (45%) | 6/20 (30%) | 11/20 (55%) | 2/20 (10%) |
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAR‐T, chimeric antigen receptor; hCAR‐T, humanized CAR‐T.
Data are n (%). All 20 patients who received treatment are shown. The AEs are according to the National Cancer Institute's Common Terminology Criteria for adverse events (version 5.0.) within 1 month post CAR‐T cell infusion.
To analyze the severity of CRS clearly, patients in our study were divided into two groups (Grade 0–1 vs. 2–5) according to the CRS grading system. The fold changes of interleukin (IL)‐6, interferon‐γ, IL‐10, and tumor necrosis factor‐a were lower in the Grade 0–1 CRS group than in the Grade 2 or greater CRS group (p = 0.001, p = 0.0004, p = 0.022, and p = 0.033, respectively; Figure 3A–D). However, the fold change in the IL‐2R level was not significantly different between these two groups (p = 0.287, Figure 3E). There was no correlation between the peak levels of ALT, AST, and total bilirubin (TBIL) post hCAR‐T cell infusion and the severity of CRS (p = 0.829, p = 0.447, and p = 0.704, respectively; Figure 4A–C). The incidences of Grade 2–5 CRS in patients who were HBsAg‐positive and anti‐HBc positive were 46.7% and 40%, respectively. Moreover, no significant difference was observed in anti‐HBs titers between patients with Grade 0–1 CRS and Grade 2–5 CRS (p = 0.239, Figure 4D).
FIGURE 3.
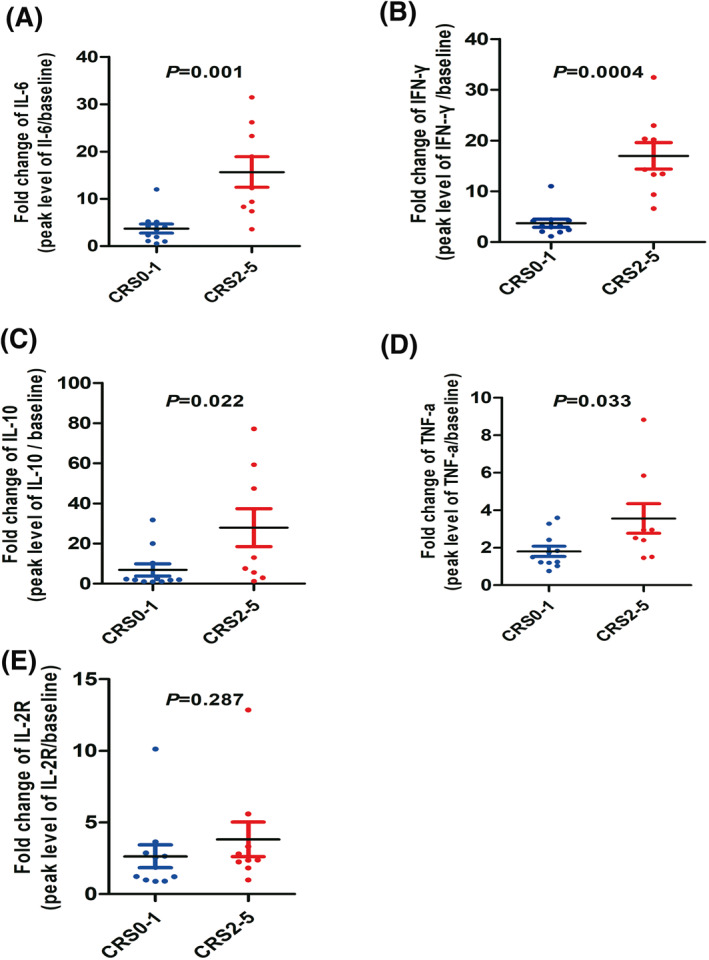
Serum cytokine detection and correlation with CRS. (A–E) Fold changes in IL‐2R, IFN‐γ, IL‐6, IL‐10, and TNF‐a following CAR T‐cell infusion. Dot plots depicting fold changes in cytokine levels from prior to the first day of CAR T‐cell infusion to the peak within 1 month of infusion in 20 evaluable patients with ALL or DLBCL who received conditioning chemotherapy and CAR T‐cell infusion, stratified by Grade 0–1 CRS (n = 11, blue dots) and Grade 2–5 CRS (n = 9, red dots). ALL, acute lymphoblastic leukemia; CAR‐T, chimeric antigen receptor; CRS, cytokine release syndrome; DLBCL, diffuse large B‐cell lymphoma; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor
FIGURE 4.
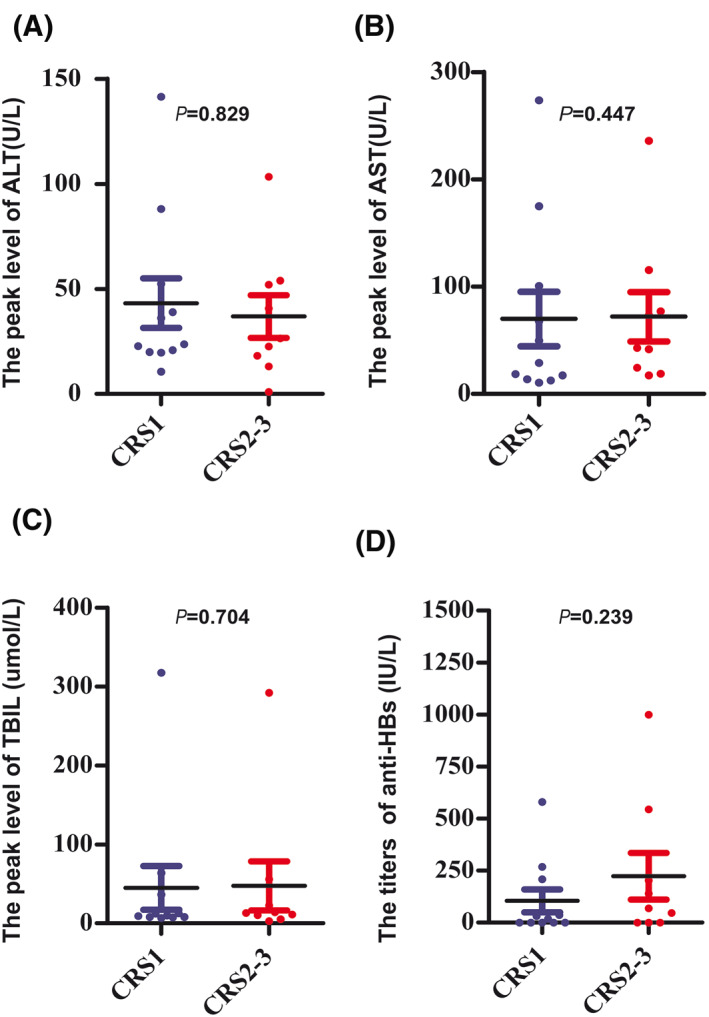
Kinetics of liver function post hCAR‐T cell infusion. The median peak levels of ALT (23.6 [IQR: 20–52.5] vs. 26.5 [IQR: 15.7–53] U/L) (A) AST (29.1 [IQR: 10.7–100.7] vs. 43 [21.8–96.3] U/L) (B) and TBIL (9.9 [IQR: 8.0–36.7] vs. 13.2 [IQR: 7.8–39.4] μmol/L) (C) post hCAR‐T cell infusion were comparable in patients with Grade 0–1 and Grade 2–5 CRS (p = 0.827, p = 0.447, and p = 0.704, respectively) (D), There were no statistically significant differences in the detectable anti‐HBs levels prior to hCAR‐T cell infusion in patients with Grade 0–1 or Grade 2–5 CRS (18.3 [IQR: 0.8–209] vs. 68.7 [IQR: 1.2–373.8] IU/L, p = 0.239). ALT, alanine aminotransferase; Anti‐HBs, hepatitis B surface antibody; AST, aspartate aminotransferase; CRS, cytokine release syndrome; hCAR‐T, humanized CAR‐T; IQR, interquartile range; TBIL, total bilirubin
3.6. Efficacy and survival
The overall response rate was 85%, with 55% the patients achieving CR (6 of 7 with ALL, and 5 of 13 with DLBCL) and 30% of patients achieving a partial response (Figure 1B, Table 1). Among the 13 DLBCL patients, the median follow‐up time for survivors was 10 months, and the median OS was 10 months (95% confidence interval [CI], 7–12 months); the estimated probabilities of OS and PFS at 12 months were 49 ± 17.8% and 57.3 ± 17%, respectively (Figure 1C,D). Among the seven patients with ALL, the median OS and median PFS were not reached (Figure 1C,D).
4. DISCUSSION
Previously, our group had reported the use of anti‐CD19 hCAR‐T therapy in patients with r/r DLBCL and ALL, demonstrating safety, efficacy, and in vivo expansion of CAR‐T cells. 18 , 19 Because anti‐CD19 CAR‐T cells deplete normal B cells as well as malignant cells, B‐cell aplasia is common following CAR‐T therapy. To the best of our knowledge, there have been few studies that have reported the safety of CAR‐T cells in lymphoma patients with HBV or hepatitis C virus infection, and very few patients were included in these studies. 20 , 21 , 22 Thus, it remains unclear if anti‐CD19 CAR‐T immunotherapy is associated with increased risk of HBV reactivation in patients with HBV infection. In our study, none of the HBsAg‐positive patients experienced HBV reactivation or HBV‐related hepatitis during or after hCAR‐T immunotherapy by prophylactic antiviral treatment. Only one patient with resolved HBV infection experienced HBV reactivation 6 months after hCAR‐T therapy and sequential allo‐HSCT.
To date, there has been no standard guideline for the management of HBV reactivation during or after CAR‐T immunotherapy. We treated our patients by referring to the guidelines for other B‐cell‐depleting immunosuppression regimens, such as rituximab. 17 , 23 HBV DNA was monitored routinely every month after hCAR‐T therapy, and antiviral therapy was started at least 4 weeks before hCAR‐T therapy. As reactivation events have previously been observed up to 2 years after the last dose of rituximab, we recommend antiviral therapy for at least 24 months after hCAR‐T therapy. 24 ETV and TDF were chosen for administration to our patients based on previous studies that suggested they were more effective than lamivudine in preventing HBV reactivation. Therefore, our findings suggest that it is safe for ALL or DLBCL patients with chronic HBV infection to receive hCAR‐T therapy under antiviral prophylaxis.
The management of B‐cell malignancies with resolved HBV infection is still not standardized, and there are no set guidelines for decisions such as preemptive therapy versus antiviral prophylaxis. The risk of reactivation and management depends primarily on the immunosuppression regimen used. It is appealing to use CAR‐T cells as a bridge to allo‐HSCT in ALL patients who achieve CR after CAR‐T therapy and in selected r/r DLBCL patients. 12 HBV reactivation occurred in patients with resolved HBV infection who underwent chemotherapy and/or HSCT at a rate of up to 50%. 25 , 26 Therefore, we used prophylactic antiviral therapy to treat two patients with resolved HBV infection who received allo‐HSCT after hCAR‐T. However, one patient developed HBV reactivation and HBV‐related hepatitis because of self‐discontinuation of antiviral therapy. A number of factors increased the risk of HBV reactivation and hepatitis flare in this patient, including the discontinuation of antiviral therapy, the pre‐HSCT conditioning regimen, the intense and repeated cycles of chemotherapy, and the co‐occurrence of acute GVHD that required long‐term immunotherapy. In contrast, Strati et al. found that one DLBCL patient with resolved HBV infection experienced HBV reactivation at 13 months after CAR‐T therapy because she stopped antiviral treatment. Later on, HBV reactivation was controlled by re‐initiation of ETV. 20 In addition, we found that there was a decline in anti‐HBs titers post hCAR‐T therapy (Figure S1), which indicates a high risk for HBV reactivation. It is important to note that HBV reactivation may hit patients with resolved HBV infection undergoing hCAR‐T therapy, and thus, prophylactic antiviral therapy should also be recommended to these patients.
CRS and neurotoxicity are the most common toxicities associated with CAR‐T therapy. CRS occurs in a variable fraction of patients after administration of anti‐CD19 CAR‐T cells, with incidences ranging from 54% to 91% and severe CRS ranging from 8.3% to 43%. 17 , 27 Onset of CRS commonly occurs within the first week, peaking between 1 to 2 weeks after. 1 , 2 Consistent with previous datasets, all of our patients experienced CRS with a median onset of 3 days, and the peak expansion of hCAR‐T cells was observed within 2 weeks after infusion. However, only 10% of our patients experienced severe CRS, and the symptoms were relieved with tocilizumab and corticosteroids. In addition, only two (10%) of our patients experienced mild neurotoxicity. Thus, compared with previous studies about HBV‐negative patients, our study showed that HBV infection did not affect the expansion of hCAR‐T cells or increase the incidence of severe CRS and neurotoxicity in ALL and DLBCL patients.
In conclusion, our study suggests that hCAR‐T is a safe and effective treatment for DLBCL and ALL patients with chronic and resolved HBV infection, even in patients who will proceed to allo‐HSCT post hCAR‐T immunotherapy. Our study also demonstrates that prophylactic antiviral treatment is an appropriate option for patients with chronic and resolved HBV infection during and after hCAR‐T therapy. Further studies including more cases are needed to verify these findings in the future.
CONFLICT OF INTEREST
None of the authors have any conflicts of interest to report.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Medical Ethics Committee of the Department of Hematology, Tianjin First Center Hospital (Tianjin, China) (Approved No. of ethic committee: 2015 0002X and 2018 N105KY). The patients gave their written informed consent in accordance with the Declaration of Helsinki. This clinical trial is registered at http://www.chictr.org.cn/index.aspx as ChiCTR1800018059, and ChiCTR1800019622. The patients agreed to the use of their specimens and data for our study.
AUTHOR CONTRIBUTIONS
Rui Cui collected the data, analyzed the data, and wrote the manuscript; Yanyu Jiang, Nan Mou, and Zhenxing Yang performed laboratory work for this study; Cuicui Lye and Qing Li were responsible for acquisition and analysis of clinical data and statistical analysis; Xuxiang Liu and Lanfang Li coordinated the study and revised it critically for important intellectual content; and Qi Deng was responsible for conception and design of this study and revised the manuscript. All of the authors approved the final manuscript.
5.
TRANSPARENT PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/hon.2807.
Supporting information
Supplementary Material 1
Supplementary Material 2
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (81900186) and The Development Program of Tianjin First Central Hospital (2019CM04).
Contributor Information
Qi Deng, Email: kachydeng@126.com.
Lanfang Li, Email: lilanfangmeng@163.com.
References
REFERENCES
- 1. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T‐cell therapy in refractory large B‐cell lymphoma. N Engl J Med. 2017;377(26):2531‐2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B‐cell lymphoma. N Engl J Med. 2019;380(1):45‐56. [DOI] [PubMed] [Google Scholar]
- 3. Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR‐T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li M, Gan Y, Fan C, et al. Hepatitis B virus and risk of non‐Hodgkin lymphoma: an updated meta‐analysis of 58 studies. J Viral Hepat. 2018;25(8):894‐903. [DOI] [PubMed] [Google Scholar]
- 6. Chen CJ, Wang LY, Yu MW. Epidemiology of hepatitis B virus infection in the Asia‐Pacific region. J Gastroenterol Hepatol. 2000;15(Suppl):E3‐E6. [DOI] [PubMed] [Google Scholar]
- 7. Locke FL, Ghobadi A, Jacobson CA, et al. Long‐term safety and activity of axicabtagene ciloleucel in refractory large B‐cell lymphoma (ZUMA‐1): a single‐arm, multicentre, phase 1‐2 trial. Lancet Oncol. 2019;20(1):31‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skrabs C, Muller C, Agis H, Mannhalter C, Jager U. Treatment of HBV‐carrying lymphoma patients with Rituximab and CHOP: a diagnostic and therapeutic challenge. Leukemia. 2002;16(9):1884‐1886. [DOI] [PubMed] [Google Scholar]
- 9. Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27(4):605‐611. [DOI] [PubMed] [Google Scholar]
- 10. Seto WK, Chan TS, Hwang YY, et al. Hepatitis B reactivation in occult viral carriers undergoing hematopoietic stem cell transplantation: a prospective study. Hepatology. 2017;65(5):1451‐1461. [DOI] [PubMed] [Google Scholar]
- 11. Lindemann M, Koldehoff M, Fiedler M, et al. Control of hepatitis B virus infection in hematopoietic stem cell recipients after receiving grafts from vaccinated donors. Bone Marrow Transpl. 2016;51(3):428‐431. [DOI] [PubMed] [Google Scholar]
- 12. Shadman M, Gauthier J, Hay KA, et al. Safety of allogeneic hematopoietic cell transplant in adults after CD19‐targeted CAR T‐cell therapy. Blood Adv. 2019;3(20):3062‐3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hay KA, Gauthier J, Hirayama AV, et al. Factors associated with durable EFS in adult B‐cell ALL patients achieving MRD‐negative CR after CD19 CAR T‐cell therapy. Blood. 2019;133(15):1652‐1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sommermeyer D, Hill T, Shamah SM, et al. Fully human CD19‐specific chimeric antigen receptors for T‐cell therapy. Leukemia. 2017;31(10):2191‐2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cazzola M. Introduction to a review series: the 2016 revision of the WHO classification of tumors of hematopoietic and lymphoid tissues. Blood. 2016;127(20):2361‐2364. [DOI] [PubMed] [Google Scholar]
- 16. Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl. 2019;25(4):625‐638. [DOI] [PubMed] [Google Scholar]
- 17. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang J, Deng Q, Jiang YY, et al. CAR‐T 19 combined with reduced‐dose PD‐1 blockade therapy for treatment of refractory follicular lymphoma: a case report. Oncol Lett. 2019;18(5):4415‐4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang J, Mou N, Yang Z, et al. Efficacy and safety of humanized anti‐CD19‐CAR‐T therapy following intensive lymphodepleting chemotherapy for refractory/relapsed B acute lymphoblastic leukaemia. Br J Haematol. 2020. 10.1111/bjh.16623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strati P, Nastoupil LJ, Fayad LE, Samaniego F, Adkins S, Neelapu SS. Safety of CAR T‐cell therapy in patients with B‐cell lymphoma and chronic hepatitis B or C virus infection. Blood. 2019;133(26):2800‐2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei J, Zhu X, Mao X, Huang L, Meng F, Zhou J. Severe early hepatitis B reactivation in a patient receiving anti‐CD19 and anti‐CD22 CAR T cells for the treatment of diffuse large B‐cell lymphoma. J Immunother Cancer. 2019;7(1):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abbasi A, Peeke S, Shah N, et al. Axicabtagene ciloleucel CD19 CAR‐T cell therapy results in high rates of systemic and neurologic remissions in ten patients with refractory large B cell lymphoma including two with HIV and viral hepatitis. J Hematol Oncol. 2020;13(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152(6):1297‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strati P, Adkins S, Nastoupil LJ, et al. Hematopoietic recovery and immune reconstitution after axi‐cel CAR T‐cell therapy in patients with relapsed/refractory large. B‐cell lymphoma. 2019;37(15_Suppl):7545. [Google Scholar]
- 25. Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49(4):652‐657. [DOI] [PubMed] [Google Scholar]
- 26. Vigano M, Serra G, Casella G, Grossi G, Lampertico P. Reactivation of hepatitis B virus during targeted therapies for cancer and immune‐mediated disorders. Expert Opin Biol Ther. 2016;16(7):917‐926. [DOI] [PubMed] [Google Scholar]
- 27. Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor‐modified T‐cell therapy. Blood. 2017;130(21):2295‐2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1
Supplementary Material 2


