Abstract
Background
Women of lower socioeconomic status (SES) with early‐stage breast cancer are more likely to report poorer physician‐patient communication, lower satisfaction with surgery, lower involvement in decision making, and higher decision regret compared to women of higher SES. The objective of this study was to understand how to support women across socioeconomic strata in making breast cancer surgery choices.
Methods
We conducted a 3‐arm (Option Grid, Picture Option Grid, and usual care), multisite, randomized controlled superiority trial with surgeon‐level randomization. The Option Grid (text only) and Picture Option Grid (pictures plus text) conversation aids were evidence‐based summaries of available breast cancer surgery options on paper. Decision quality (primary outcome), treatment choice, treatment intention, shared decision making (SDM), anxiety, quality of life, decision regret, and coordination of care were measured from T0 (pre‐consultation) to T5 (1‐year after surgery.
Results
Sixteen surgeons saw 571 of 622 consented patients. Patients in the Picture Option Grid arm (n = 248) had higher knowledge (immediately after the visit [T2] and 1 week after surgery or within 2 weeks of the first postoperative visit [T3]), an improved decision process (T2 and T3), lower decision regret (T3), and more SDM (observed and self‐reported) compared to usual care (n = 257). Patients in the Option Grid arm (n = 66) had higher decision process scores (T2 and T3), better coordination of care (12 weeks after surgery or within 2 weeks of the second postoperative visit [T4]), and more observed SDM (during the surgical visit [T1]) compared to usual care arm. Subgroup analyses suggested that the Picture Option Grid had more impact among women of lower SES and health literacy. Neither intervention affected concordance, treatment choice, or anxiety.
Conclusions
Paper‐based conversation aids improved key outcomes over usual care. The Picture Option Grid had more impact among disadvantaged patients.
Lay Summary
The objective of this study was to understand how to help women with lower incomes or less formal education to make breast cancer surgery choices.
Compared with usual care, a conversation aid with pictures and text led to higher knowledge. It improved the decision process and shared decision making (SDM) and lowered decision regret. A text‐only conversation aid led to an improved decision process, more coordinated care, and higher SDM compared to usual care. The conversation aid with pictures was more helpful for women with lower income or less formal education.
Conversation aids with pictures and text helped women make better breast cancer surgery choices.
Keywords: breast cancer disparities, breast cancer surgery, conversation aids, decision support techniques, lower educational attainment, lower health literacy, lower socioeconomic status, pictorial superiority
Short abstract
A paper‐based pictorial conversation aid (pictures plus text) is beneficial to all patients with early‐stage breast cancer and particularly to disadvantaged patients. Between‐surgeon variation suggests that the maximal impact of such interventions requires standardized physician training combined with these interventions.
Introduction
Although overall breast cancer survival is improving, disparities in breast cancer treatment, communication, long‐term health outcomes, and mortality remain. 1 , 2 Among women with early‐stage breast cancer, lower socioeconomic status (SES) is a stronger predictor of poor outcomes and treatment received than race or ethnicity. 3 , 4 Women of lower SES with early‐stage breast cancer report poorer communication with their care team, less breast cancer surgery knowledge, higher uptake of mastectomy, and worse cancer‐related and patient‐centered health outcomes. 1 , 2 , 5 , 6 , 7 , 8 , 9 , 10 , 11 They are also less likely to receive guideline‐based management, including radiation after breast‐conserving surgery. 2 , 10
Breast‐conserving surgery with radiation and mastectomy are equally effective surgical treatment options for early‐stage breast cancer, yet they have distinct tradeoffs that women value differently. 12 Shared decision making (SDM) is recommended, yet only 44% to 51% of women with early‐stage breast cancer across socioeconomic strata achieve the degree of participation that they desire. 5 , 6 , 13 , 14 , 15 , 16 Women of lower SES are more likely to play a passive role in decision making and report higher decision regret after surgery. 2 , 5 , 8 , 9 , 11
Patient decision aids could help to reduce these disparities by providing evidence‐based information about the tradeoffs between options and by facilitating SDM. 17 , 18 However, most patient decision aids require high literacy/health literacy levels 19 and are, therefore, less effective for people with lower education, health literacy, or SES. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 Short, paper‐based conversation aids designed for use in clinical encounters might better meet the needs of disadvantaged patients (with pictures, simpler text, and cost prompts). 18 , 22 , 26
Conversation aids increase patients' knowledge and participation in decision making and improve risk perceptions without increasing the visit duration. 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 They can be integrated into routine care and electronic health records. 34 According to proportionate universalism, care should meet the needs of those who have the greatest burden of disease and address health disparities and priority populations. 37 For example, plain language and pictures can promote understanding, regardless of patients' health literacy. 18 , 38 Picture‐based interventions rely on the concept of pictorial superiority, which is defined as the ability of pictures to improve understanding and recall, with evidence showing increased visual literacy in people of lower textual literacy. 39 , 40 , 41 , 42 , 43 The effect of paper‐based conversation aids that include a pictorial intervention specifically designed to meet the needs of disadvantaged patients has not been evaluated across socioeconomic strata.
Our primary aim was to compare the effectiveness of 2 conversation aids (pictorial and text only) and usual care for decision quality and other secondary outcomes.
Our secondary aim was to measure the effect of the pictorial conversation aid on SES‐based disparities in decision making (decision quality, knowledge, and SDM).
Materials and Methods
This trial follows the 2010 Consolidated Standards of Reporting Trials checklist (Supporting Table 1). 44 , 45 For details, see the published protocol and ClinicalTrials.gov. 46
Dartmouth College CPHS approved the study on June 8, 2017. Montefiore Medical Center Institutional Review Board (IRB) provided authorization agreement to rely on review by Dartmouth College on September 8, 2017. Ethical approval for Washington University in St. Louis was provided by The Washington University in St. Louis IRB on May 9, 2017. NYU School of Medicine IRB provided ethical approval on August 29, 2017.
Study Design
We conducted a 3‐arm, multisite, parallel, controlled superiority trial of surgeons randomized to 1 of 3 arms: text‐only conversation aid, pictorial conversation aid, or usual care. Patients were units of observation. To minimize contamination, we randomized surgeons to 1 of 3 arms nested within 4 cancer centers. We used balanced block randomization to account for the varying number of surgeons at each site. Patients who provided informed consent inherited the arm to which their surgeon was randomized. Although we planned to stratify patients according to SES in statistical analyses, we did not enforce balance with respect to SES when enrolling subjects (see Supporting Table 2 for the SES composite variable definition). In the context of this study, we defined patients of lower SES as having at least 2 of the following: lower income, lower educational attainment, or underinsurance (see Supporting Table 2 for further details).
We used community‐based participatory research methods to ensure partnerships and shared responsibility among stakeholders. 38 , 47 , 48 , 49 , 50 To support patient recruitment and data collection, we used breast cancer survivors at each site as patient associates. 46 They provided continuous input in designing, planning, conducting, and managing the study.
Setting
We conducted the study at 7 clinics within 4 National Cancer Institute–designated cancer centers in urban and rural locations that provided care to diverse SES, ethnic, and racial populations.
Study Participants
We randomized breast surgeons to accrue patients in 1 of 3 trial arms for 18 months. We recruited English‐, Spanish‐, and Mandarin Chinese‐speaking women (18 years old or older) with a biopsy‐confirmed diagnosis of early‐stage breast cancer (stages I‐IIIA) eligible for breast‐conserving surgery and mastectomy according to medical records and participating surgeons' judgment. We excluded men; transgender men and women; patients who had previously undergone prophylactic mastectomy; and patients with visual impairment, inflammatory breast cancer, or severe mental illness or dementia. To complete follow‐up assessments, we recruited patients receiving neoadjuvant therapy only during the first 9 months of the trial.
Interventions and Comparator
The principal investigator trained surgeons in SDM and how to use their assigned intervention before recruitment. 46 Surgeons in the intervention arms introduced the paper‐based conversation aids during the first surgical encounter (see Fig. 1). The text‐only conversation aid (Option Grid) was a 1‐page, tabular evidence‐based summary of available options. It was written in plain language (Flesch‐Kincaid grade level of 6.6). 51 The 4‐page pictorial conversation aid (Picture Option Grid) used the same evidence as the Option Grid but included images and simpler text (Flesch‐Kincaid grade level of 6.5). It was designed with and for women of lower SES and health literacy. 38 It included a prompt for surgeons to discuss treatment costs. We developed, tested, and validated both conversation aids according to user‐centered design principles. 29 , 38 , 52 , 53 , 54 In the usual‐care arm, surgeons provided their standard information about breast cancer (see Supporting Table 3). We translated the interventions into Spanish and Mandarin Chinese (see Supporting Table 4). 46
Figure 1.
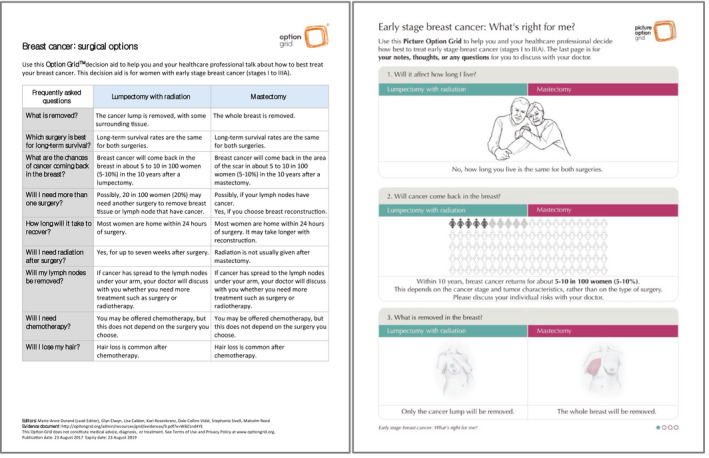
Paper‐based conversation aids used in the trial.
Outcomes
We used validated, short‐form questionnaires whenever available. We measured outcomes at the baseline (T0), during the surgical visit (T1), immediately after the visit (T2), 1 week after surgery or within 2 weeks of the first postoperative visit (T3), and 12 weeks after surgery or within 2 weeks of the second postoperative visit (T4; Supporting Table 2). Whenever possible, we measured patient outcomes 1 year after surgery (T5). At T1, we audio‐recorded encounters when patients consented.
The primary outcome was the revised 19‐item Decision Quality Instrument for breast cancer, 55 which was adapted for patients of lower SES. 56 The Decision Quality Instrument has 3 subscales that measure the extent to which patients are informed about treatment options (knowledge score), receive surgery aligned with their preferences (concordance score), and are involved in decision making (decision process score).
Secondary outcomes included treatment choice, treatment intention, collaboRATE (a validated brief measure of SDM), 57 , 58 Patient‐Reported Outcomes Measurement Information System 8A (a validated short‐form anxiety measure), 59 the 5‐level EuroQol 5D (a validated quality of life measure), 60 the Decision Regret Scale (validated decision regret scale), 61 and integRATE (a patient‐reported measure of coordination of care). 62 We used Observer OPTION‐5 to assess the extent to which SDM occurred during encounters by analyzing the T1 audio recordings. 63 In addition, we collected sociodemographic information about the participants, including health literacy. 46 We used the Single‐Item Literacy Screener (a validated health literacy assessment). 64
Data Management, Statistical Analysis, and Power
We used REDCap for data management, Stata for data cleaning, and R for data analyses. 46 Our data and safety monitoring board met every 6 months. We used intention‐to‐treat analysis for primary analyses. Although we followed patients over time, primary analyses and study power were determined by our primary cross‐sectional analysis plan. 46 The statistical analyst was blinded to site and arm assignment.
Analyses Corresponding to the Primary Aim
Our primary aim was to compare the effectiveness of 2 conversation aids (pictorial and text‐only) and usual care for decision quality and other secondary outcomes. The analysis plan was guided by the following hypotheses:
Hypothesis 1.1. Both conversation aids would increase SDM in clinic visits and improve decision quality (primary outcome), knowledge, and quality of life among patients of higher and lower SES in comparison with usual care. They would also reduce decision regret and improve patients' perceived coordination of care.
Hypothesis 1.2. The Picture Option Grid would be more effective than the Option Grid at improving primary and secondary outcomes in patients of lower SES. There would be no difference between the effects of the 2 conversation aids in patients of higher SES.
Using linear and logistic regression models for outcomes represented as continuous and binary, respectively, we first performed separate analyses for each time period (the time periods over which outcome analyses were possible were T0, T1, T2, T3, T4, and T5, with treatment intent indicated at T2 such that T2 to T5 constituted the follow‐up time period). The primary outcome (decision quality) was measured only at T2 and T3. We also analyzed outcomes measured multiple times after T0 (decision quality, anxiety, quality of life, and coordination of care) by using a longitudinal model (Supporting Table 2) because this allowed for the possibility of obtaining more precise estimates; these outcomes could change the further removed a patient was from surgery. We assessed surgeons' learning effects by including their number of conversation aid uses. We accounted for clustering (patients within surgeons and surgeons within clinics) by using mixed‐effect regression models (surgeons and clinics were treated as random effects). For multiple observations across time, we accounted for repeated within‐patient measurements. We were concerned about a confounding bias due to chance occurrences because the number of randomization units was relatively small (16 surgeons). We conducted propensity score analyses of patient treatment group membership to identify a set of potential confounders to include as adjuster variables. The variables with the strongest associations with the intervention groups were those that were least balanced between the study arms. Those variables were thus prioritized for inclusion in the statistical models for the outcomes. We also included the start date and the baseline counterpart of the outcome variable (if measured at the baseline) as additional covariates to further lessen the reliance of cluster randomization in order to interpret the findings causally. Controlling for cancer center reduced the amount of clustering evident at the clinic level. For more details about scoring methods, data collection points, and the SES composite score, see Supporting Table 2.
Analyses Corresponding to the Secondary Aim
Our secondary aim was to measure the effect of the pictorial conversation aid on SES‐based disparities in decision making (decision quality, knowledge, and SDM). The analysis plan was guided by the following hypothesis:
Hypothesis 2.1. Compared with the Option Grid and usual‐care arms, the Picture Option Grid would reduce disparities in decision quality, knowledge, quality of life, and participation in SDM between patients of lower and higher SES. It is also likely to reduce disparities in treatment choice.
We performed treatment‐effect modification analyses with respect to patients' insurance, education, SES, health literacy, and language (measured at T0; see Supporting Table 2). For each, we created binary variables to directly compare the intervention's effect across higher and lower levels of the modifying predictor and included data from all follow‐up times (T2‐T5) at which the given outcome was measured. We used a linear regression model to test for differences between each of the Picture Option Grid, usual care, and Option Grid groups in the continuously valued outcomes (eg, scale variables such as decision quality) and analogous logistic regression models for binary‐valued outcomes across subpopulations (see the protocol for details) while accounting for the repeated measurements on patients over time as well as clustering due to clinic and physician. Other predictors analogous to those used in the primary analysis were included as covariates in the models. A logistic mixed‐effect regression analysis was used to compare the effect of the intervention group on the likelihood that treatment intent and treatment received were the same.
For both aims, we evaluated the model's adequacy by using residual analysis and other model fit diagnostics. 65 We recorded reasons for dropout and missing data and examined whether any observed variables were associated with a propensity to drop out. We then used multiple imputation to account for missing baseline and outcome data. 66 We conducted heterogeneity of treatment effects analyses in clinically relevant subgroups.
Patient and Public Involvement
We engaged patients and stakeholders as equal members of the research team from study planning to dissemination (see Supporting Table 5). Patients and stakeholders participated in the monthly meetings, quarterly community advisory board meetings, and quarterly trial steering group meetings. In addition, a unique patient associate role was created: a breast cancer survivor at each study site assisted with the recruitment, consent, and data collection and analysis processes. We heard consistently from the research teams at all sites that working with the patient associates was a unique and rewarding aspect of the trial.
Changes to Methods After Trial Commencement
After experiencing reduced recruitment because fewer than expected patients met the eligibility criterion (eligible for breast‐conserving surgery and mastectomy according to medical records and participating surgeons' judgment), we revised our target sample size to 600. The revised sample was based on the average accrual rate and power considerations for primary analyses. Furthermore, we revised our inclusion criteria to include all women 18 years old or older, regardless of recurrence status (at the surgeon's discretion). For other minor changes, see Supporting Table 6.
Results
We invited 17 surgeons to take part in the trial: 16 (94.1%) were enrolled and randomly assigned to trial arms (see Supporting Table 7 for surgeon characteristics). Between September 2017 and February 2019, we screened 2057 patients (target, 2200 patients); 1031 patients remained eligible (see Fig. 2). We approached 812 eligible patients, and 622 (76.6%) consented. Table 1 displays consented patients' baseline characteristics: 32.6% were of lower SES. Propensity score analyses revealed statistically significant baseline differences between groups based on site, race and ethnicity, SES, health literacy, and education. At the baseline, the number of patients with missing data for the Decision Quality Instrument knowledge subscale was 30 (5.7%). At T2, the number of patients with missing data ranged from 11 (2.2%) for concordance to 25 (5.1%) for decision process. At T3, the number of patients with missing data ranged from 89 (19.9%) for knowledge to 98 (21.9%) for decision process. Multiple imputation analyses suggested minimally different estimates when data were imputed for most outcomes in comparison with no imputation. We can thus be assured that current findings are very unlikely to be overturned by accounting for missing data via multiple imputation.
Figure 2.
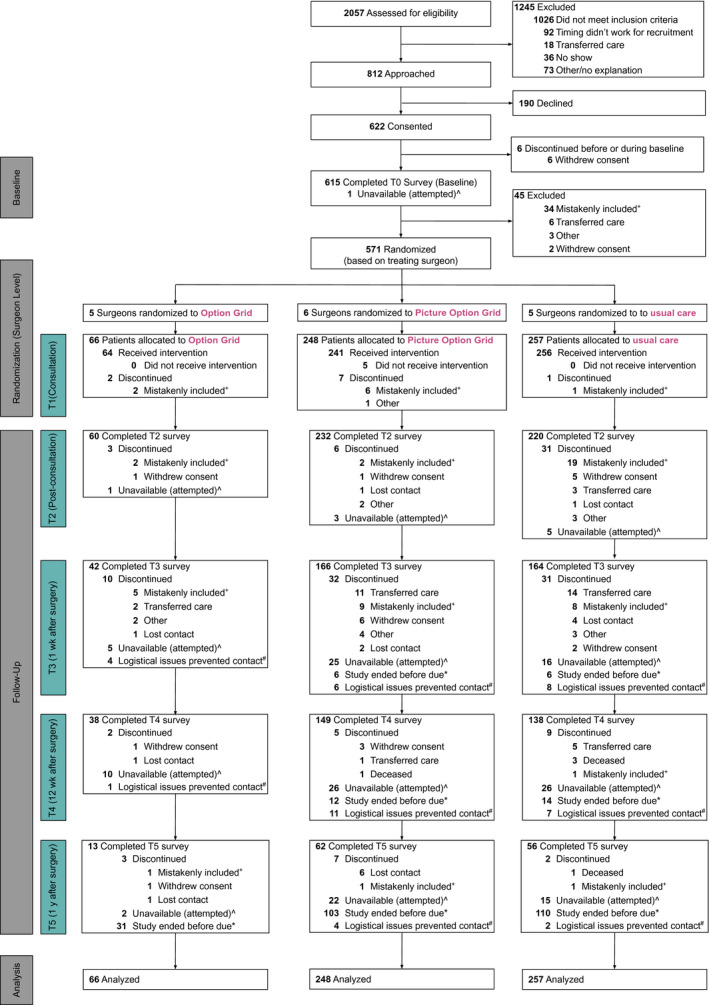
Study flow diagram. ^Unavailable (attempted) indicates that we reached out to patients a maximum of 5 times via phone or email (according to patient preference). *Study ended before due indicates that we collected follow‐up data through June 1, 2019; not all patients received surgery within a timeframe that allowed for follow‐up before this date. #Logistical issues prevented contact included research team turnover, issues with follow‐up reminders, and holidays. +Mistakenly included indicates that the participant was deemed ineligible after consent. This was most often the case because the cancer stage changed or the patient did not have a choice between breast‐conserving surgery and mastectomy.
TABLE 1.
Demographic Characteristics of the Consenting Participants
| Characteristic | Option Grid (n = 69) | Picture Option Grid (n = 276) | Usual Care (n = 271) | Total (n = 616) |
|---|---|---|---|---|
| Age, mean (SD), y | 60.1 (11.5) | 58.9 (13.0) | 60.4 (12.2) | 59.7 (12.5) |
| Race and ethnicity, No. (%) a | ||||
| Black | 19 (27.5) | 37 (13.4) | 40 (14.8) | 96 (15.6) |
| Hispanic | 5 (7.3) | 41 (14.9) | 32 (11.8) | 78 (12.7) |
| Asian | 2 (2.9) | 13 (4.7) | 4 (1.5) | 19 (3.1) |
| White | 41 (59.4) | 177 (64.1) | 176 (64.9) | 394 (64.0) |
| Other | 1 (1.5) | 4 (1.5) | 8 (3.0) | 13 (2.1) |
| Missing | 1 (1.5) | 4 (1.5) | 11 (4.1) | 16 (2.6) |
| Education, No. (%) a | ||||
| Never attended high school | 1 (1.5) | 10 (3.6) | 4 (1.5) | 15 (2.4) |
| Some high school, no diploma received | 6 (8.7) | 21 (7.6) | 21 (7.6) | 48 (7.8) |
| High school diploma or equivalent | 22 (31.9) | 48 (17.4) | 55 (20.3) | 125 (20.3) |
| Some college, no degree received | 9 (13.0) | 46 (16.7) | 57 (21.0) | 112 (18.2) |
| 2‐y degree | 7 (10.1) | 37 (13.4) | 23 (8.5) | 67 (10.9) |
| 4‐y degree or higher | 24 (34.8) | 114 (41.3) | 104 (38.4) | 242 (39.3) |
| Missing | 0 (0.0) | 0 (0.0) | 7 (2.6) | 7 (1.1) |
| Language, No. (%) a | ||||
| English | 64 (92.8) | 235 (85.1) | 236 (87.1) | 535 (86.9) |
| Spanish | 5 (7.3) | 34 (12.3) | 22 (8.1) | 61 (9.9) |
| Mandarin | 0 (0.0) | 5 (1.8) | 0 (0.0) | 5 (0.8) |
| Other | 0 (0.0) | 2 (0.7) | 6 (2.2) | 8 (1.3) |
| Missing | 0 (0.0) | 0 (0.0) | 7 (2.6) | 7 (1.1) |
| Insurance, No. (%) b | ||||
| Public or uninsured | 24 (34.8) | 91 (33.0) | 79 (29.2) | 194 (31.5) |
| Private | 45 (65.2) | 184 (66.7) | 192 (70.9) | 421 (68.3) |
| Missing/other | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Income, No. (%) a , c | ||||
| Above 138% FPL | 27 (39.1) | 147 (53.3) | 154 (56.8) | 328 (53.3) |
| Below 138% FPL | 26 (37.7) | 62 (22.5) | 60 (21.1) | 148 (24.0) |
| Missing | 16 (23.2) | 67 (24.3) | 57 (21.0) | 140 (22.7) |
| Health literacy, No. (%) d | ||||
| Low | 40 (58.0) | 112 (40.6) | 124 (45.8) | 276 (44.8) |
| Not low | 29 (42.0) | 162 (58.7) | 142 (52.4) | 333 (54.1) |
| Missing | 0 (0.0) | 2 (0.7) | 5 (1.9) | 7 (1.1) |
| SES, No. (%) e | ||||
| Lower SES | 30 (43.5) | 90 (32.6) | 83 (30.6) | 203 (33.0) |
| Higher SES | 39 (56.5) | 186 (67.4) | 188 (69.4) | 413 (67.1) |
Abbreviations: FPL, federal poverty level; SES, socioeconomic status.
P < .05 when a comparison was made across the 3 arms (chi‐square tests were used for dichotomous and categorical outcomes; t tests were used for continuous outcomes).
Public indicates Medicaid or Medicare without supplemental insurance; private indicates private/employer insurance (including Tricare) or Medicare with supplemental insurance.
Income was dichotomized as above or below 138% FPL in the year of recruitment and was based on household size.
Measured with Chew's Single‐Item Literacy Screener and dichotomized with the top score (highest option vs all others).
SES was calculated with the annual household income, insurance status, and education. If 1 item was missing, the remaining 2 were used, with higher SES conservatively assumed.
Of the 622 consenting patients, 615 completed baseline assessments. According to the arm to which their surgeon was allocated, 64 patients received the Option Grid, 241 received the Picture Option Grid, and 256 received usual care (see Fig. 2). The imbalanced allocation to arms was due to differences in the number of patients seen by each surgeon or changes in their role or attendance. We collected 311 usable recordings from the 440 patients who consented to recording (70.7%). No adverse events related to trial participation were reported. Usual care varied between sites (Supporting Table 3). There was important between‐surgeon variation that inflated the standard errors of estimated treatment effects (see Fig. 3).
Figure 3.
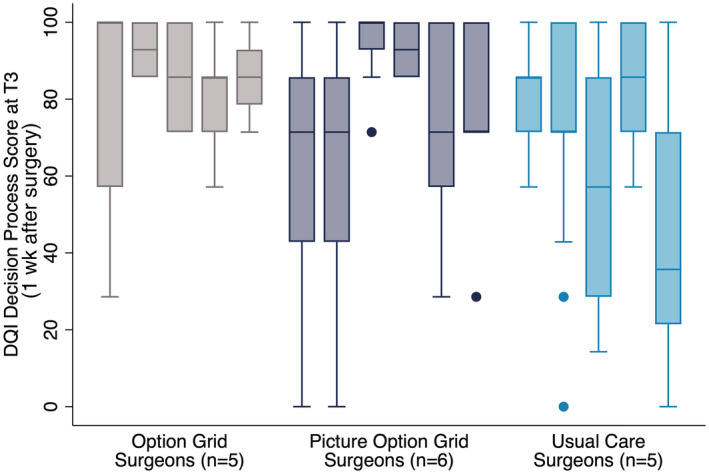
Surgeon variation observed on the decision process subscale. DQI indicates Decision Quality Instrument.
Primary Outcome
Decision Quality Instrument knowledge subscale
Compared with patients in the usual‐care arm, patients in the Picture Option Grid arm reported greater knowledge across T2 and T3 (estimate, 0.27; 95% CI, 0.01‐0.53; P = .04; see Fig. 4). There were no statistically significant differences in knowledge between the Option Grid and usual‐care arms or between the Picture Option Grid and Option Grid arms (Table 2). See Supporting Table 8 for unadjusted scores.
Figure 4.
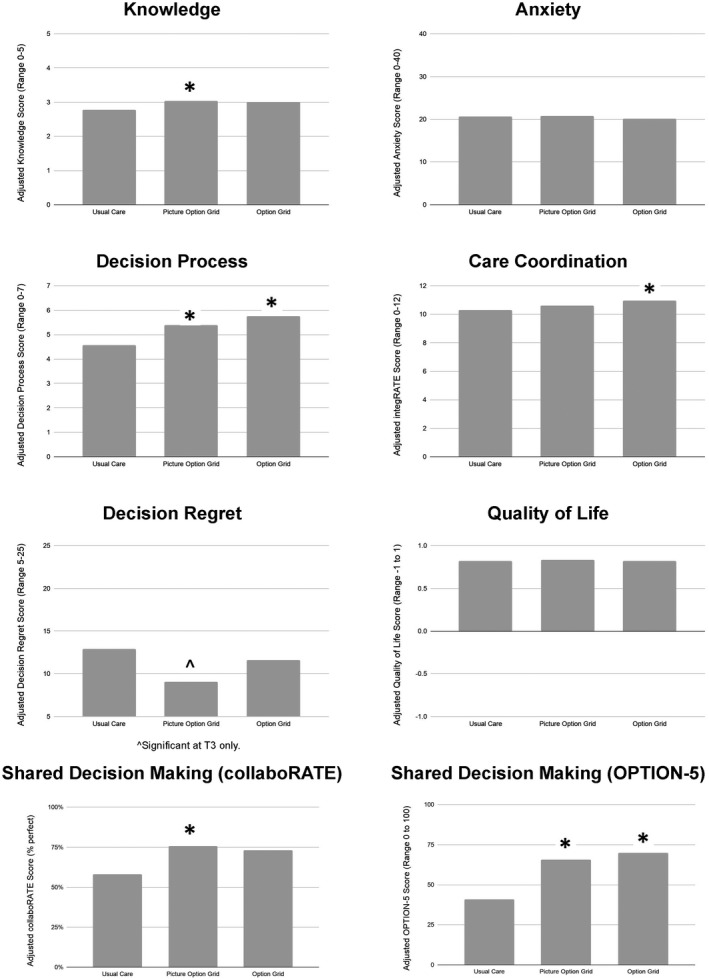
Bar graphs of adjusted scores for primary and secondary outcome measures by arm and across time points. An asterisk denotes statistical significance at P < .05.
TABLE 2.
Adjusted Coefficients Across Time Points for Primary and Secondary Outcome Measures Across Follow‐Up: Results From Multilevel Regression Models
| Outcome | Option Grid (vs Usual Care) | Picture Option Grid (vs Usual Care) | ||||
|---|---|---|---|---|---|---|
| Adjusted Coefficient (95% CI) a | Main Effect P | Subgroup Effects | Coefficient (95% CI) | Main Effect P | Subgroup Effects | |
| Decision quality | ||||||
| Value concordance (T2, T3) | 0.04 (–0.77 to 0.83) | .15 | –0.36 (–0.86 to 0.12) | .24 | ||
| Knowledge (T0, T2, T3) | 0.23 (–0.12 to 0.58) | .20 | 0.27 (0.01 to 0.53) | .04 | Higher for lower SES b | |
| Decision process (T2, T3) c | 1.18 (0.23 to 2.13) | .01 | 0.82 (0.01 to 1.62) | .05 | ||
| Shared decision making | ||||||
| Patient‐reported (T2) | 0.15 (–0.04 to 0.34) | .12 | 0.17 (0.03 to 0.32) | .01 | ||
| Observed (T1) | 28.9 (8.0 to 49.9) | .01 | 24.7 (5.9 to 43.5) | .01 | ||
| Decision regret (T3, T4, T5) c | –6.5 (–34.5 to 21.5) | .65 | Higher with lower education b | –19.2 (–39.5 to 1.2) | .07 | |
| Quality of life (T0, T4) | 0.003 (–0.04 to 0.05) | .89 | 0.017 (–0.01 to 0.05) | .25 | Higher for lower health literacy b | |
| Anxiety (T0, T2, T3, T4) | –0.53 (–1.77 to 0.72) | .41 | 0.14 (–0.62 to 0.91) | .72 | ||
| Integration of health care delivery (T0, T4) | 0.66 (0.04 to 1.28) | .04 | 0.32 (–0.07 to 0.71) | .11 | ||
Abbreviations: SES, socioeconomic status; T0, presurgical visit (baseline); T1, during the surgical visit; T2, immediately after the surgical visit; T3, approximately 1 week after surgery; T4, approximately 12 weeks after surgery; T5, 1 year after surgery.
Adjusted for site, insurance, socioeconomic status, race, health literacy, and start date.
The subgroup effect was significant, even in the absence of significance in the adjusted coefficient.
Decision process scores were significant for the Picture Option Grid arm only at T2, and decision regret scores were significant for the Picture Option Grid arm only at T3.
Subgroup analyses for the Decision Quality Instrument knowledge subscale
Consistent with hypothesis 2.1, the difference in knowledge between patients of lower SES and those of higher SES was smaller for patients in the Picture Option Grid arm than patients in the usual‐care arm (estimate, 0.36; 95% CI, 0.09‐0.63; P = .01). This implies that the Picture Option Grid reduced disparities in knowledge between patients of lower and higher SES in comparison with usual care.
Decision Quality Instrument concordance subscale
There was no effect of the interventions on the Decision Quality Instrument concordance subscale in comparison with usual care, and there were no statistically significant differences between intervention arms (Fig. 5).
Figure 5.
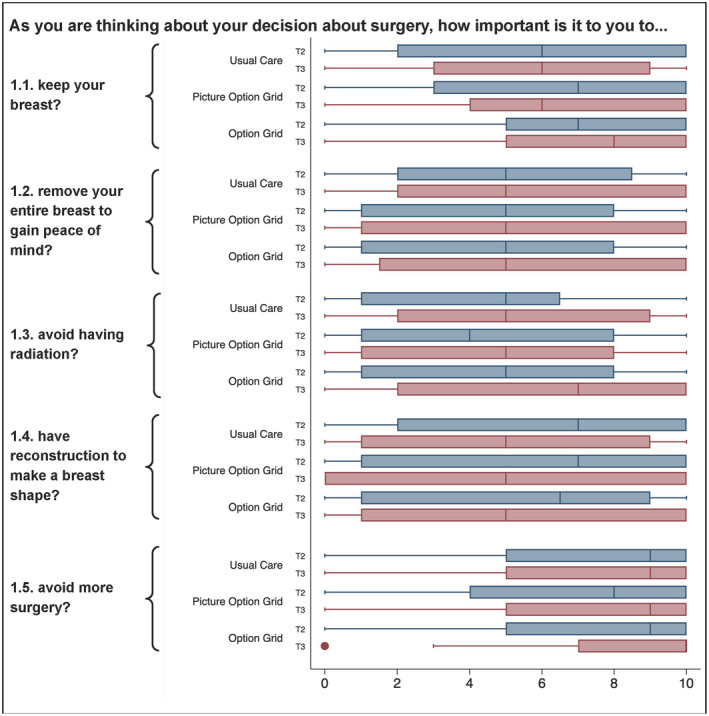
Box plots of responses to the Decision Quality Instrument concordance subscale at T2 and T3 by item number and study arm. T2 indicates immediately after the visit; T3, 1 week after surgery.
Subgroup analyses for the Decision Quality Instrument concordance subscale
In contrast to hypothesis 2.1, no difference was noted for concordance for any element of SES, and this implies that the Picture Option Grid did not reduce disparities in concordance between patients of lower and higher SES in comparison with usual care.
Decision Quality Instrument decision process subscale
Compared with patients in the usual‐care arm, patients in the Option Grid arm reported higher decision process scores across T2 and T3 (estimate, 1.18; 95% CI, 0.23‐2.13; P = .01). In comparison with usual care, patients in the Picture Option Grid arm reported higher decision process scores (estimate, 0.82; 95% CI, 0.01‐1.62; P = .05). There were no statistically significant differences between interventions.
Subgroup analyses for the Decision Quality Instrument decision process subscale
In contrast to hypothesis 2.1, no difference was noted for decision process for any element of SES, and this implies that the Picture Option Grid did not reduce disparities in decision process between patients of lower and higher SES in comparison with usual care.
Secondary Outcomes
Compared with patients in the usual‐care arm, patients in the Picture Option Grid arm reported higher SDM as measured by collaboRATE at T2 (estimate, 0.17; 95% CI, 0.03‐0.32; P = .01; see Table 2). There was no effect of the Option Grid on SDM as measured by collaboRATE, and there were no statistically significant differences between the interventions. Both the Option Grid and the Picture Option Grid led to more observed SDM as measured by Observer OPTION‐5 at T1 (Option Grid estimate, 28.93; 95% CI, 7.98‐49.87; P = .01; Picture Option Grid estimate, 24.71; 95% CI, 5.93‐43.49; P = .01). In comparison with usual care, patients in the Option Grid arm reported more coordinated care at T4 (estimate, 0.66; 95% CI, 0.04‐1.28; P = .04). There were no statistically significant differences in coordinated care between intervention arms or between the Picture Option Grid and usual‐care arms. In comparison with usual care, patients in the Picture Option Grid arm reported lower decision regret at T3 (estimate, –23.16; 95% CI, –45.28 to –1.04; P = .04). There was no effect on decision regret at T4 or T5, and there were no differences between interventions. There was no effect of the interventions on anxiety or treatment choice.
Subgroup analyses for secondary outcomes
The difference in decision regret between patients of lower education and those of higher education was greater for patients in the Option Grid arm than patients in the usual‐care arm (estimate, 50.56; 95% CI, 17.03‐84.08; P = .003). The difference in quality of life between patients of higher health literacy and patients of lower health literacy was smaller in the Picture Option Grid arm than the usual‐care arm (estimate, 0.05; 95% CI, 0.01‐0.09; P = .03). This implies that the Picture Option Grid reduced disparities in quality of life between patients of lower and higher SES in comparison with usual care.
However, in contrast to hypothesis 2.1, no such difference was noted for SDM or treatment choice for any element of SES, and this implies that the Picture Option Grid did not reduce disparities in SDM or treatment choice between patients of higher and lower SES in comparison with usual care.
Discussion
The Picture Option Grid resulted in greater knowledge (T2 and T3) and an improved decision process (T2 and T3; primary outcome), lower decision regret (T3), and higher self‐reported (T2) and observed SDM (T1) in comparison with usual care. The Option Grid resulted in an improved decision process (T2 and T3; primary outcome) and more coordinated care (T4) and observed SDM (T1) in comparison with usual care. Neither intervention affected preference concordance (third subscale of the Decision Quality Instrument). There were no statistically significant differences between the interventions for all outcomes measured. In agreement with our secondary hypothesis, compared with usual care, the Picture Option Grid had more impact on knowledge and quality of life among disadvantaged patients. There was insufficient evidence to suggest that the interventions affected treatment choice or anxiety.
When used by clinicians, paper‐based conversation aids that pay attention to health literacy appear effective across socioeconomic strata. 37 They offer a practical, accessible, and inexpensive solution to promote patient participation in decision making during clinical encounters without relying on high levels of health, textual, or computer literacy. 19 The Picture Option Grid improved more outcomes than the Option Grid and had a positive impact on disadvantaged patients, likely by leveraging pictorial superiority. 38 , 41 , 42 , 67 , 68 The pictorial intervention's greater impact for disadvantaged patients is consistent with a recent meta‐analysis. 69 This review showed that pictorial health information improved understanding and recall in comparison with text alone across populations but largely increased understanding for lower health literacy populations. This confirms a differential impact of pictorial health interventions for disadvantaged groups. On the basis of our findings, the Picture Option Grid could reduce disparities in knowledge and quality of life among disadvantaged groups in agreement with past work. 18
In a recent systematic review and meta‐analysis of conversation aids evaluated in 23 randomized controlled trials and 30 nonrandomized studies, the interventions increased knowledge and observed SDM in agreement with our findings. 36 The meta‐analysis also showed improved satisfaction with the decision‐making process and reduced decisional conflict without increasing visit duration (not measured in the current trial). Systematic reviews of patient decision aids for early‐stage breast cancer have suggested that these tools support treatment decisions, increase the uptake of breast‐conserving surgery, reduce decisional conflict, increase knowledge and satisfaction with the decision‐making process, and, in some instances, improve quality of life. 20 , 21 , 70 The conversation aids evaluated in our trial, though, had no impact on treatment decisions or the uptake of breast‐conserving surgery. The lack of impact on treatment decisions is, however, consistent with Scalia et al's review of conversation aids. 36 It is possible that conversation aids such as the Option Grid and Picture Option Grid, primarily used during the consultation, have less impact on the treatment decision than pre‐encounter decision aids while improving other key decision outcomes. Knowledge and decision process (equivalent to a measure of satisfaction with the decision‐making process) were improved in the Picture Option Grid arm, with an effect on quality of life shown only in the subgroup analysis.
Another important finding of our study was the large between‐surgeon variation observed across outcomes, which significantly inflated the standard errors of our estimated treatment effects. The duration of surgeon training was roughly 1 hour. Conversation aids are not standalone and rely on the clinician's willingness and ability to use them. It, therefore, seems necessary to take baseline skill levels into account in studies of this nature. 26 A meta‐analysis of trials revealed that clinicians' fidelity to suggested use of conversation aids was partial and inconsistent. 26 Additional analyses of audio recordings will assess the fidelity of using the conversation aids and explore reasons for this variation.
Furthermore, the number of patients eligible for both surgical options was significantly lower than indicated by initial feasibility assessments. Field notes and discussions with surgeons suggested that surgeons may have differed in opinions regarding patient eligibility for breast‐conserving surgery and mastectomy: some were more open than others to offering both options to patients with similar cancer presentations. Surgeons' possible procedural preferences may have reduced the pool of potentially eligible patients. Clinicians' attitudes toward SDM are a known barrier to the delivery and implementation of conversation aids. 71 In one study, surgeon behavior was influenced by perceived low patient interest in SDM or by their own preferences for a specific option. 72 Surgeons also reported withholding options that they deemed less suitable on the basis of intuitive risk assessments. 72
A strength of this randomized controlled trial was the focus on comparing 2 formats of a conversation aid, with one specifically designed with and for women of lower SES. Detailed attention was paid to the use of the primary outcome measure, the Decision Quality Instrument, and to all other measures. The goal was to design as brief a survey as possible and to ensure that each measure was presented in plain language. Other strengths of the study included the continuous stakeholder involvement (clinicians, patients, and community advocates), including the involvement of a breast cancer survivor at each site in all aspects of enrollment and follow‐up. We also recruited National Cancer Institute–designated cancer centers (including 1 public hospital) in urban and rural settings of the United States to diversify the study population and maximize generalizability. Following a community‐based participatory research approach throughout the trial facilitated the conduct of the study and significantly strengthened the applicability and dissemination of the results.
Several limitations need to be considered. Randomization at the surgeon level led to chance imbalance between arms, with surgeons in the Option Grid arms having lower volumes of eligible patients and different distributions of the patient characteristics. Attempts to modify these patterns were unsuccessful. Findings related to the Option Grid intervention and particularly the subgroup analysis showing an increase in decision regret should be interpreted with caution. We recruited approximately 50% fewer patients than planned. Attrition of the number of eligible patients between T0 and T3 occurred as patients became ineligible after additional examinations revealed that their stage or surgical options had changed. Multiple efforts, including translations, standardized interviews, in‐person recruitment, and close collaboration with community advocates, were made to improve recruitment into our lower SES group. 46 This mirrors the documented underrepresentation of disadvantaged populations in clinical trials. 73 , 74 , 75
In conclusion, paper‐based conversation aids used in breast cancer surgical encounters improved outcomes for all patients. The Picture Option Grid may be most effective, especially for disadvantaged populations. It has the potential to reduce disparities in knowledge and quality of life while improving other outcomes across socioeconomic strata. Significant variation between surgeons highlighted the importance of robust SDM training and the evaluation of optimal training and implementation strategies.
Funding Support
The research reported in this article was funded through an award from the Patient‐Centered Outcomes Research Institute (1511‐32875). The statements presented in this article are solely the responsibility of the authors and do not necessarily represent the views of the Patient‐Centered Outcomes Research Institute, its board of governors, or its methodology committee. The funder had no role in any aspects of the setup or execution of this study. The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health (grant P30 CA016672).
Conflict of Interest Disclosures
Glyn Elwyn and Marie‐Anne Durand have developed the Option Grid patient decision aids, which are licensed to EBSCO Health; they receive consulting income from EBSCO Health and may receive royalties in the future. A. James O’Malley reports grants from the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Patient‐Centered Outcomes Research Institute. Mary C. Politi reports grants from Merck outside the submitted work. Catherine H. Saunders holds a copyright in the consideRATE suite of tools. Karen Sepucha received salary support from 2014 to 2018 as a member of the scientific advisory board for Healthwise, a not‐for‐profit foundation that develops and distributes patient education and decision support materials; she also reports grants from the Agency for Healthcare Research and Quality, the Patient‐Centered Outcomes Research Institute, and the Patrick and Catherine Weldon Donaghue Medical Research Foundation outside the submitted work. Richard J. Barth reports grants and other from CairnSurgical, Inc, and grants from the National Institutes of Health outside the submitted work; in addition, Barth has a patent licensed to Dartmouth College. The other authors made no disclosures.
Author Contributions
Marie‐Anne Durand: Study plan and design, drafting of the manuscript, support beyond recruitment and follow‐up to help to manage all dissemination activities, and review and approval of the final version of the manuscript. Renata W. Yen: Research project manager (operational management of the project and responsibility for managing all trial activities, including site enrollment, institutional review board approval, trial registration data management, interim analyses for the data safety monitoring board, and all data cleaning after data collection ended), drafting of the manuscript (support), support beyond recruitment and follow‐up to help to manage all dissemination activities, and review and approval of the final version of the manuscript. A. James O’Malley: Study plan and design (support), senior trial statistician (responsibility for all statistical analyses involving model estimation, generation of statistical inferences, and statements of statistical uncertainty as well as oversight of analytical aspects of the study), detailed missing data analysis (including multiple imputation analyses), drafting of the manuscript (support), and review and approval of the final version of the manuscript. Danielle Schubbe: Research assistant/research coordinator (facilitation of study recruitment, follow‐up, data management, and analysis at each participating cancer center), support beyond recruitment and follow‐up to help to manage all dissemination activities, and review and approval of the final version of the manuscript. Mary C. Politi: Study plan and design (support), site principal investigator at Washington University in Saint Louis, and review and approval of the final version of the manuscript. Catherine H. Saunders: Research assistant/research coordinator (facilitation of study recruitment, follow‐up, data management, and analysis at each participating cancer center), support beyond recruitment and follow‐up to help to manage all dissemination activities, and review and approval of the final version of the manuscript. Shubhada Dhage: Study plan and design (support), clinical site principal investigator, and review and approval of the final version of the manuscript. Kari Rosenkranz: Participating surgeon, study plan and design (support), clinical site principal investigator, and review and approval of the final version of the manuscript. Julie Margenthaler: Study plan and design (support), clinical site principal investigator, and review and approval of the final version of the manuscript. Anna N. A. Tosteson: Study plan and design (support) and review and approval of the final version of the manuscript. Eloise Crayton: Study plan and design (involvement), patient partner and/or patient associate, and review and approval of the final version of the manuscript. Sherrill Jackson: Study plan and design (involvement), patient partner and/or patient associate, and review and approval of the final version of the manuscript. Ann Bradley: Study plan and design (involvement), patient partner and/or patient associate, and review and approval of the final version of the manuscript. Linda Walling: Study plan and design (involvement), patient partner and/or patient associate, and review and approval of the final version of the manuscript. Christine M. Marx: Patient partner and/or patient associate and review and approval of the final version of the manuscript. Robert J. Volk: Expertise and advice (study design, study procedures, management, and analysis) and review and approval of the final version of the manuscript. Karen Sepucha: Expertise and advice (study design, study procedures, management, and analysis) and review and approval of the final version of the manuscript. Elissa Ozanne: Expertise and advice (study design, study procedures, management, and analysis) and review and approval of the final version of the manuscript. Sanja Percac‐Lima: Expertise and advice (study design, study procedures, management, and analysis) and review and approval of the final version of the manuscript. Emily Bergin: Research assistant/research coordinator (facilitation of study recruitment, follow‐up, data management, and analysis at each participating cancer center) and review and approval of the final version of the manuscript. Courtney Goodwin: Research assistant/research coordinator (facilitation of study recruitment, follow‐up, data management, and analysis at each participating cancer center) and review and approval of the final version of the manuscript. Caity Miller: Research assistant/research coordinator (facilitation of study recruitment, follow‐up, data management, and analysis at each participating cancer center) and review and approval of the final version of the manuscript. Camille Harris: Research assistant/research coordinator (facilitation of study recruitment, follow‐up, data management, and analysis at each participating cancer center) and review and approval of the final version of the manuscript. Richard J. Barth, Jr: Participating surgeon and review and approval of the final version of the manuscript. Rebecca Aft: Participating surgeon and review and approval of the final version of the manuscript. Sheldon Feldman: Participating surgeon and review and approval of the final version of the manuscript. Amy E. Cyr: Participating surgeon and review and approval of the final version of the manuscript. Christina V. Angeles: Participating surgeon and review and approval of the final version of the manuscript. Shuai Jiang: Detailed missing data analysis (including multiple imputation analyses) and review and approval of the final version of the manuscript. Glyn Elwyn: Study plan and design (support), drafting of the manuscript (support), support beyond recruitment and follow‐up to help to manage all dissemination activities, and review and approval of the final version of the manuscript.
Supporting information
Supplementary Material
Durand M‐A, Yen RW, O’Malley AJ, Schubbe D, Politi MC, Saunders CH, Dhage S, Rosenkranz K, Margenthaler J, Tosteson ANA, Crayton E, Jackson S, Bradley A, Walling L, Marx CM, Volk RJ, Sepucha K, Ozanne E, Percac‐Lima S, Bergin E, Goodwin C, Miller C, Harris C, Barth RJ Jr, Aft R, Feldman S, Cyr AE, Angeles CV, Jiang S, Elwyn G. What matters most: Randomized controlled trial of breast cancer surgery conversation aids across socioeconomic strata. Cancer 2021:127:422‐436. 10.1002/cncr.33248
This trial is registered at ClinicalTrials.gov (NCT03136367).
References
- 1. Wheeler SB, Reeder‐Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hurd TC, James T, Foster JM. Factors that affect breast cancer treatment: underserved and minority populations. Surg Oncol Clin N Am. 2005;14:119‐130, vii. [DOI] [PubMed] [Google Scholar]
- 3. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490‐496. [DOI] [PubMed] [Google Scholar]
- 4. Cross CK, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the U.S: what have we learned from clinical studies. Cancer. 2002;95:1988‐1999. [DOI] [PubMed] [Google Scholar]
- 5. Chen JY, Diamant AL, Thind A, Maly RC. Determinants of breast cancer knowledge among newly diagnosed, low‐income, medically underserved women with breast cancer. Cancer. 2008;112:1153‐1161. [DOI] [PubMed] [Google Scholar]
- 6. Hawley ST, Lantz PM, Janz NK, et al. Factors associated with patient involvement in surgical treatment decision making for breast cancer. Patient Educ Couns. 2007;65:387‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mac Bride MB, Neal L, Dilaveri CA, et al. Factors associated with surgical decision making in women with early‐stage breast cancer: a literature review. J Womens Health (Larchmt). 2013;22:236‐242. [DOI] [PubMed] [Google Scholar]
- 8. McVea KLSP, Minier WC, Palensky JEJ. Low‐income women with early‐stage breast cancer: physician and patient decision‐making styles. Psychooncology. 2001;10:137‐146. [DOI] [PubMed] [Google Scholar]
- 9. Polacek GN, Ramos MC, Ferrer RL. Breast cancer disparities and decision‐making among U.S. women. Patient Educ Couns. 2007;65:158‐165. [DOI] [PubMed] [Google Scholar]
- 10. Richardson LC. Treatment of breast cancer in medically underserved women: a review. Breast J. 2004;10:2‐5. [DOI] [PubMed] [Google Scholar]
- 11. Siminoff LA, Graham GC, Gordon NH. Cancer communication patterns and the influence of patient characteristics: disparities in information‐giving and affective behaviors. Patient Educ Couns. 2006;62:355‐360. [DOI] [PubMed] [Google Scholar]
- 12. National Cancer Institute . Breast cancer treatment. Published 2016. Accessed January 11, 2016. http://www.cancer.gov/types/breast/patient/breast‐treatment‐pdq#link/_229_toc
- 13. Degner LF, Kristjanson LJ, Bowman D, et al. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277:1485‐1492. [PubMed] [Google Scholar]
- 14. Keating NL, Guadagnoli E, Landrum MB, Borbas C, Weeks JC. Treatment decision making in early‐stage breast cancer: should surgeons match patients' desired level of involvement? J Clin Oncol. 2002;20:1473‐1479. [DOI] [PubMed] [Google Scholar]
- 15. Fagerlin A, Lakhani I, Lantz PM, et al. An informed decision? Breast cancer patients and their knowledge about treatment. Patient Educ Couns. 2006;64:303‐312. [DOI] [PubMed] [Google Scholar]
- 16. Lee CN, Chang Y, Adimorah N, et al. Decision making about surgery for early‐stage breast cancer. J Am Coll Surg. 2012;214:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. [DOI] [PubMed] [Google Scholar]
- 18. Durand MA, Carpenter L, Dolan H, et al. Do interventions designed to support shared decision‐making reduce health inequalities? A systematic review and meta‐analysis. PLoS One. 2014;9:e94670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomson MD, Hoffman‐Goetz L. Readability and cultural sensitivity of web‐based patient decision aids for cancer screening and treatment: a systematic review. Med Inform Internet Med. 2007;32:263‐286. [DOI] [PubMed] [Google Scholar]
- 20. Obeidat R, Finnell DS, Lally RM. Decision aids for surgical treatment of early stage breast cancer: a narrative review of the literature. Patient Educ Couns. 2011;85:e311‐e321. [DOI] [PubMed] [Google Scholar]
- 21. Waljee JF, Rogers MA, Alderman AK. Decision aids and breast cancer: do they influence choice for surgery and knowledge of treatment options? J Clin Oncol. 2007;25:1067‐1073. [DOI] [PubMed] [Google Scholar]
- 22. Politi MC, Adsul P, Kuzemchak MD, Zeuner R, Frosch DL. Clinicians' perceptions of digital vs. paper‐based decision support interventions. J Eval Clin Pract. 2015;21:175‐179. [DOI] [PubMed] [Google Scholar]
- 23. McCaffery KJ, Holmes‐Rovner M, Smith SK, et al. Addressing health literacy in patient decision aids. BMC Med Inform Decis Mak. 2013;13(suppl 2):S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCaffery KJ, Smith SK, Wolf M. The challenge of shared decision making among patients with lower literacy: a framework for research and development. Med Decis Making. 2010;30:35‐44. [DOI] [PubMed] [Google Scholar]
- 25. Smith SK, Nutbeam D, McCaffery KJ. Insights into the concept and measurement of health literacy from a study of shared decision‐making in a low literacy population. J Health Psychol. 2013;18:1011‐1022. [DOI] [PubMed] [Google Scholar]
- 26. Wyatt KD, Branda ME, Anderson RT, et al. Peering into the black box: a meta‐analysis of how clinicians use decision aids during clinical encounters. Implement Sci. 2014;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hess EP, Knoedler MA, Shah ND, et al. The Chest Pain Choice decision aid: a randomized trial. Circ Cardiovasc Qual Outcomes. 2012;5:251‐259. [DOI] [PubMed] [Google Scholar]
- 28. Montori VM, Shah ND, Pencille LJ, et al. Use of a decision aid to improve treatment decisions in osteoporosis: the Osteoporosis Choice randomized trial. Am J Med. 2011;124:549‐556. [DOI] [PubMed] [Google Scholar]
- 29. Elwyn G, Pickles T, Edwards A, et al. Supporting shared decision making using an Option Grid for osteoarthritis of the knee in an interface musculoskeletal clinic: a stepped wedge trial. Patient Educ Couns. 2016;99:571‐577. [DOI] [PubMed] [Google Scholar]
- 30. Mullan RJ, Montori VM, Shah ND, et al. The Diabetes Mellitus Medication Choice decision aid: a randomized trial. Arch Intern Med. 2009;169:1560‐1568. [DOI] [PubMed] [Google Scholar]
- 31. Mann DM, Ponieman D, Montori VM, Arciniega J, McGinn T. The Statin Choice decision aid in primary care: a randomized trial. Patient Educ Couns. 2010;80:138‐140. [DOI] [PubMed] [Google Scholar]
- 32. Weymiller AJ, Montori VM, Jones LA, et al. Helping patients with type 2 diabetes mellitus make treatment decisions: Statin Choice randomized trial. Arch Intern Med. 2007;167:1076‐1082. [DOI] [PubMed] [Google Scholar]
- 33. Scalia P, Elwyn G, Durand MA. “Provoking conversations”: case studies of organizations where Option Grid™ decision aids have become ‘normalized’. BMC Med Inform Decis Mak. 2017;17:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Inselman J, Branda M, Castaneda‐Guarderas A, et al. Uptake and documentation of the use of an encounter decision aid in usual practice: a retrospective analysis of the use of the Statin/Aspirin Choice decision aid. Med Decis Making. 2016;36:557‐561. [DOI] [PubMed] [Google Scholar]
- 35. Fay M, Grande SW, Donnelly K, Elwyn G. Using Option Grids: steps toward shared decision‐making for neonatal circumcision. Patient Educ Couns. 2015;99:236‐242. [DOI] [PubMed] [Google Scholar]
- 36. Scalia P, Durand MA, Berkowitz JL, et al. The impact and utility of encounter patient decision aids: systematic review, meta‐analysis and narrative synthesis. Patient Educ Couns. 2019;102:817‐841. [DOI] [PubMed] [Google Scholar]
- 37. Carey G, Crammond B, De Leeuw E. Towards health equity: a framework for the application of proportionate universalism. Int J Equity Health. 2015;14:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Durand MA, Alam S, Grande SW, Elwyn G. ‘Much clearer with pictures’: using community‐based participatory research to design and test a Picture Option Grid for underserved patients with breast cancer. BMJ Open. 2016;6:e010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alberto PA, Frederick L, Hughes M, McIntosh L, Cihak D. Components of visual literacy: teaching logos. Focus Autism Other Dev Disabl. 2007;22:234‐243. [Google Scholar]
- 40. Hockley WE. The picture superiority effect in associative recognition. Mem Cognit. 2008;36:1351‐1359. [DOI] [PubMed] [Google Scholar]
- 41. Delp C, Jones J. Communicating information to patients: the use of cartoon illustrations to improve comprehension of instructions. Acad Emerg Med. 1996;3:264‐270. [DOI] [PubMed] [Google Scholar]
- 42. Michielutte R, Bahnson J, Dignan MB, Schroeder EM. The use of illustrations and narrative text style to improve readability of a health education brochure. J Cancer Educ. 1992;7:251‐260. [DOI] [PubMed] [Google Scholar]
- 43. Houts PS, Doak CC, Doak LG, Loscalzo MJ. The role of pictures in improving health communication: a review of research on attention, comprehension, recall, and adherence. Patient Educ Couns. 2006;61:173‐190. [DOI] [PubMed] [Google Scholar]
- 44. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Rev Panam Salud Publica. 2015;38:506‐514. [PMC free article] [PubMed] [Google Scholar]
- 45. Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Durand MA, Yen RW, O’Malley AJ, et al. What matters most: protocol for a randomized controlled trial of breast cancer surgery encounter decision aids across socioeconomic strata. BMC Public Health. 2018;18:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grande SW, Durand MA, Fisher ES, Elwyn G. Physicians as part of the solution? Community‐based participatory research as a way to get shared decision making into practice. J Gen Intern Med. 2014;29:219‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wallerstein NB, Duran B. Using community‐based participatory research to address health disparities. Health Promot Pract. 2006;7:312‐323. [DOI] [PubMed] [Google Scholar]
- 49. Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community‐based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173‐202. [DOI] [PubMed] [Google Scholar]
- 50. Crocker JC, Ricci‐Cabello I, Parker A, et al. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta‐analysis. BMJ. 2018;363:k4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Elwyn G, Lloyd A, Joseph‐Williams N, et al. Option Grids: shared decision making made easier. Patient Educ Couns. 2013;90:207‐212. [DOI] [PubMed] [Google Scholar]
- 52. Alam S, Elwyn G, Percac Lima S, Grande SW, Durand MA. Assessing the acceptability and feasibility of encounter decision aids for early stage breast cancer targeted at underserved patients. BMC Med Inform Decis Mak. 2016;16:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sivell S, Edwards A, Manstead AS, et al. Increasing readiness to decide and strengthening behavioral intentions: evaluating the impact of a web‐based patient decision aid for breast cancer treatment options (BresDex: www.bresdex.com). Patient Educ Couns. 2012;88:209‐217. [DOI] [PubMed] [Google Scholar]
- 54. Sivell S, Marsh W, Edwards A, et al. Theory‐based design and field‐testing of an intervention to support women choosing surgery for breast cancer: BresDex. Patient Educ Couns. 2012;86:179‐188. [DOI] [PubMed] [Google Scholar]
- 55. Sepucha KR, Belkora JK, Chang Y, et al. Measuring decision quality: psychometric evaluation of a new instrument for breast cancer surgery. BMC Med Inform Decis Mak. 2012;12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Durand MA, Song J, Yen RW, et al. Adapting the Breast Cancer Surgery Decision Quality Instrument for lower socioeconomic status: improving readability, acceptability, and relevance. MDM Policy Pract. 2018;3:2381468318811839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barr PJ, Thompson R, Walsh T, Grande SW, Ozanne EM, Elwyn G. The psychometric properties of CollaboRATE: a fast and frugal patient‐reported measure of the shared decision‐making process. J Med Internet Res. 2014;16:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Elwyn G, Barr PJ, Grande SW, Thompson R, Walsh T, Ozanne EM. Developing CollaboRATE: a fast and frugal patient‐reported measure of shared decision making in clinical encounters. Patient Educ Couns. 2013;93:102‐107. [DOI] [PubMed] [Google Scholar]
- 59. Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the Patient‐Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18:263‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res. 2011;20:1727‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23:281‐292. [DOI] [PubMed] [Google Scholar]
- 62. Elwyn G, Thompson R, John R, Grande SW. Developing IntegRATE: a fast and frugal patient‐reported measure of integration in health care delivery. Int J Integr Care. 2015;15:e008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barr PJ, O’Malley AJ, Tsulukidze M, Gionfriddo MR, Montori VM, Elwyn G. The psychometric properties of Observer OPTION5, an observer measure of shared decision making. Patient Educ Couns. 2015;98:970‐976. [DOI] [PubMed] [Google Scholar]
- 64. Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med. 2008;23:561‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3rd ed. Routledge; 2003. [Google Scholar]
- 66. Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85‐95. [Google Scholar]
- 67. Schubbe D, Cohen S, Yen RW, et al. Does pictorial health information improve health behaviours and other outcomes? A systematic review protocol. BMJ Open. 2018;8:e023300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Katz MG, Kripalani S, Weiss BD. Use of pictorial aids in medication instructions: a review of the literature. Am J Health Syst Pharm. 2006;63:2391‐2397. [DOI] [PubMed] [Google Scholar]
- 69. Schubbe D, Scalia P, Yen RW, et al. Using pictures to convey health information: A systematic review and meta‐analysis of the effects on patient and consumer health behaviors and outcomes. Patient Educ Couns. 2020;103:1935‐1960. [DOI] [PubMed] [Google Scholar]
- 70. Collins ED, Moore CP, Clay KF, et al. Can women with early‐stage breast cancer make an informed decision for mastectomy? J Clin Oncol. 2009;27:519‐525. [DOI] [PubMed] [Google Scholar]
- 71. Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13(suppl 2):S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kannan S, Seo J, Riggs KR, Geller G, Boss EF, Berger ZD. Surgeons' views on shared decision‐making. J Patient Cent Res Rev. 2020;7:8‐18. [PMC free article] [PubMed] [Google Scholar]
- 73. Bonevski B, Randell M, Paul C, et al. Reaching the hard‐to‐reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Durant RW, Davis RB, St George DM, Williams IC, Blumenthal C, Corbie‐Smith GM. Participation in research studies: factors associated with failing to meet minority recruitment goals. Ann Epidemiol. 2007;17:634‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104:e16‐e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


