Abstract
Chronic cough, defined as a cough lasting > 8 weeks, is a common medical condition that exerts a substantial physical, mental, and social burden on patients. A subset of patients with chronic cough are troubled with a cough that persists despite optimal treatment of presumed associated common and uncommon conditions (refractory chronic cough; RCC) or in which no diagnosable cause for cough can be identified despite extensive assessment (unexplained chronic cough; UCC). Many of these patients exhibit clinical features of cough hypersensitivity, including laryngeal paresthesia, hypertussia, and allotussia. Over‐the‐counter cough remedies are ineffective and can lead to intolerable side effects when used for RCC/UCC, and the lack of approved treatments indicated for these conditions reflects a major unmet need. An increased understanding of the anatomy and neurophysiology of protective and pathologic cough has fostered a robust clinical development pipeline of several targeted therapies for RCC/UCC. This manuscript reviews the mechanisms presumed to underly RCC/UCC together with the rationale and clinical evidence for several targeted therapies currently under clinical investigation, including transient receptor potential channel antagonists, P2X3‐receptor antagonists, voltage‐gated sodium channel blockers, neuromodulators, and neurokinin‐1–receptor antagonists. Finally, we provide an overview of targets that have been investigated in preclinical models of cough and other airway diseases that may hold future promise for clinical studies in RCC/UCC. Development of targeted therapies with different sites of action may foster a precision medicine approach to treat this heterogeneous, underserved patient population.
Chronic cough (CC), defined as a cough lasting > 8 weeks, is a common medical condition with a global prevalence of approximately 10% and a higher prevalence in Oceania, Europe, and the United States. 1 , 2 The burden of CC can be severe, as patients with CC experience substantial physical effects (e.g., stress urinary incontinence, sleep disturbance, and chest pain), psychological consequences (e.g., frustration, anxiety, and depression), and social impairments (e.g., social distress/isolation and inability to carry out daily activities), leading to a reduced quality of life. 3 , 4 , 5 Moreover, the burden of CC is often long‐lasting, as some patients with CC experience symptoms that persist for several years despite numerous doctor visits, empirical treatment trials, and frequent medical testing. 4 , 5 , 6
Although CC is often associated with underlying medical conditions (e.g., asthma, gastroesophageal reflux disease (GERD), nonasthmatic eosinophilic bronchitis, and upper‐airway cough syndrome), a subset of patients with CC have a cough that is extremely difficult to control. These cases may be characterized by either a cough that persists despite optimal treatment of presumed associated common and uncommon conditions according to best practice guidelines in an adherent patient (refractory chronic cough, RCC) or circumstances in which no diagnosable cause for cough can be identified despite extensive assessment for common and uncommon causes (unexplained chronic cough, UCC). 2 , 7 , 8 , 9 There is a significant unmet need for patients with RCC/UCC, as there are no treatments that have approved indications for these conditions. Over‐the‐counter, symptomatic cough suppressants (e.g., codeine, dextromethorphan, and benzonatate) have been used to treat CC but are associated with limited efficacy or prominent drug‐related adverse events (AEs), including sedation, risk of abuse, or overdose. 10 , 11 Furthermore, treatments that successfully resolve CC associated with a specific condition may have no effect in RCC/UCC. For example, proton pump inhibitor therapy can be effective in relieving cough in patients with GERD with pathologic esophageal acid exposure by blocking gastric acid secretion, but proton pump inhibitor therapy has limited efficacy in patients with normal esophageal acid exposure and in patients with UCC. 12 , 13 Similarly, there is evidence that treatments such as inhaled corticosteroids and montelukast can be effective in reducing cough, but typically only in patients with evidence of an eosinophilic phenotype. 14 , 15 There is an imperative need for targeted therapies that can successfully block the pathways associated with pathologic cough responses that underly RCC and UCC while maintaining protective cough.
Although nonpharmacologic approaches have been investigated for management of CC, this review provides an overview of pharmacologic treatments in development for RCC and UCC that selectively target pathways implicated in the dysregulation of the cough response. This review begins with a summary of current knowledge regarding the neurobiological and neurophysiological processes that are dysregulated in CC, followed by an overview of the most recent preclinical and clinical data for therapies that are in development for RCC/UCC. Finally, we briefly discuss novel targets that have been identified in preclinical studies which may be potentially relevant for future treatment of CC.
NEUROBIOLOGY OF PROTECTIVE AND PATHOLOGIC COUGH
Protective cough
Cough is needed to prevent aspiration and facilitate the clearance of foreign bodies, irritants, and excess secretions from the airways. 16 , 17 This typically occurs reflexively by activation of airway sensory fibers that arise from the nodose (inferior) or jugular (superior) ganglia of the vagus nerve and terminate peripherally near the epithelium in the larynx, trachea, and large intrapulmonary bronchi and centrally in the brain stem (Figure 1a–c ). 16 , 17 , 18 Two distinct subtypes of vagal afferent fibers are thought to be the primary mediators of the cough response. Aδ‐fibers, originally referred to as “cough receptors,” are axons of myelinated neurons that are sensitive to punctate mechanical stimulation (touch) and rapid acidification but relatively insensitive to other chemical stimuli. 16 , 17 , 18 Because of this responsiveness to mechanical stimuli, Aδ‐fibers are thought to play a critical role in mediating the protective cough reflex in response to foreign body aspiration and excessive secretions. In contrast to Aδ‐fibers, C‐fibers are unmyelinated and express receptors that enable them to respond to a wide array of chemical stimuli, including capsaicin, bradykinin, ozone, allyl isothiocyanate, prostaglandin E2 (PGE2), adenosine, adenosine triphosphate (ATP), nicotine, and many cytokines. 17 Characterization of airway Aδ‐fibers and C‐fibers has predominantly been performed in preclinical species (e.g., guinea pigs and mice), though immunohistochemical research using biopsies from humans have revealed comparable nerve terminal structures in the airway mucosa. 19 Additionally, the ability of humans to functionally respond to mechanical and chemical stimuli suggests that functionally equivalent receptors are most likely also present in human airways. 20
Figure 1.
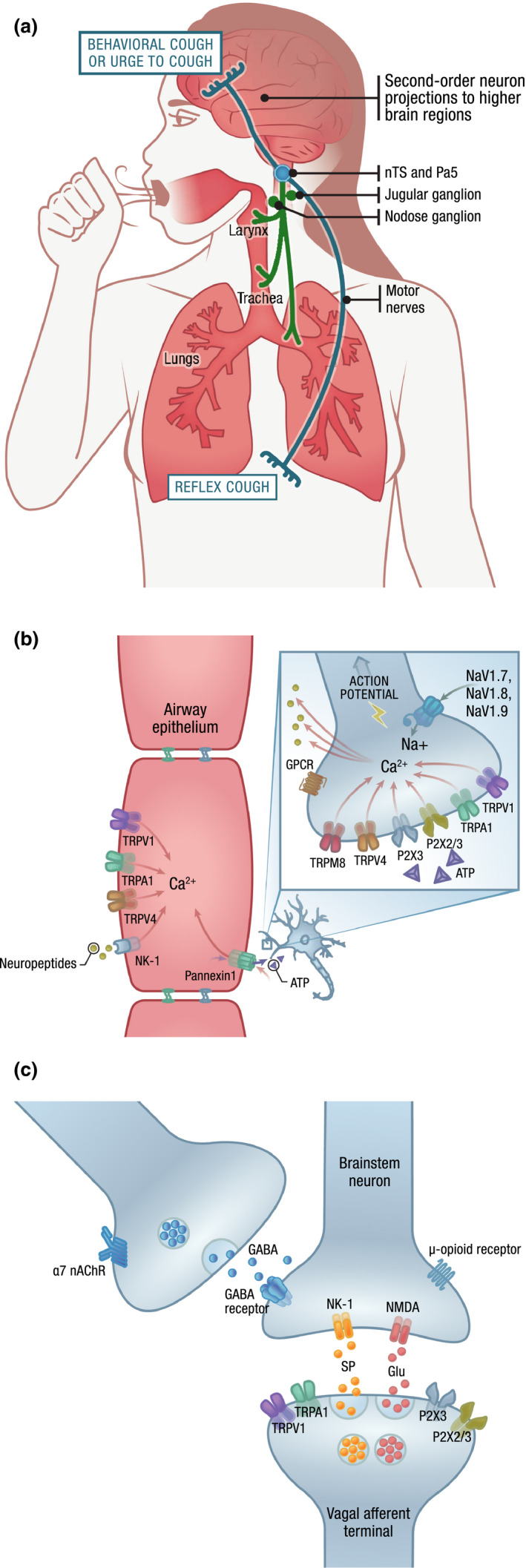
The anatomical and molecular mediators of cough. (a) The cough cascade can be triggered in the airway by activation of vagal sensory neurons originating from the jugular and nodose ganglia. Airway sensory nerve activation results in an action potential being carried along the vagus nerve to the central nervous system via projections to the brain stem, specifically at the nTS and the Pa5. Release of neurotransmitters by these primary afferents can activate reflex pathways controlling respiratory muscles to evoke involuntary cough and/or second‐order neurons projecting to higher brain regions that evoke behavioral cough or sensations of an urge to cough. (b) Stimulation and activation of airway sensory neurons. Ion channels expressed by vagal sensory nerves respond to various chemical and mechanical stimuli to trigger depolarization of the plasma membrane. Influx of calcium may trigger sensory nerves to release neuropeptides. One action of neuropeptides may be to bind to receptors on epithelial cells (e.g., NK‐1), inducing release of proinflammatory mediators. Ion channels can also be found on airway epithelial cells, where stimulation can lead to calcium influx that triggers release of inflammatory mediators that may perpetuate further sensory nerve activation. Although the underlying mechanisms are not precisely known, ATP can be released through pannexin channels and activate P2X3 channels, leading to cell depolarization. Cumulative depolarization of a sufficient magnitude can trigger the opening of NaVs expressed by sensory nerves, leading to generation of an action potential carried through the vagus nerve to the central nervous system. (c) Action potentials carried by vagal afferents can trigger the release of glutamate to activate postsynaptic brain stem neurons via NMDA receptors. These afferent neurons may also release substance P, which can bind to NK‐1 receptors and propagate the signal leading to cough. α7 nAChR agonists may play a role in cough via expression on separate neurons which, when activated, release GABA to inhibit the postsynaptic brain stem neuron, thereby halting the cough cascade. The actual CNS sites of action of therapies for chronic cough may be different and more complex than depicted, and the mechanism of action in chronic cough for some therapies (e.g., gabapentin, amitriptyline/nortriptyline) are not currently well known. ATP, adenosine triphosphate; Ca2+, calcium; CNS, central nervous system; GABA, γ‐aminobutyric acid; Glu, glutamate; GPCR, G protein–coupled receptor; nAChR, nicotinic acetylcholine receptor; NaV, voltage‐gated sodium channel; NA+, sodium; NK‐1, neurokinin‐1 receptor; NMDA, N‐methyl‐D‐aspartate receptor; nTS, nucleus of the solitary tract; Pa5, paratrigeminal nucleus; P2X, ATP‐gated (purine) cation channel subtype 3 and 2/3; SP, substance P; TRP, transient receptor potential; TRPA, TRP ankyrin; TRPM, TRP melastatin; TRPV, TRP vanilloid.
Chemical, mechanical, and thermal stimuli can trigger the opening of specific ion channels expressed by the terminals of vagal sensory nerves, which in turn induces a membrane depolarization known as a “generator potential.” 21 Generator potentials of sufficient magnitude stimulate the opening of voltage‐gated sodium channels, leading to action potentials and sensory nerve activation. The majority of chemical mediators that evoke generator potentials act via ionotropic receptors (e.g., transient receptor potential vanilloid‐1 (TRPV1) and P2X receptors), which consist of ion channels that are opened in response to binding specific ligands such as capsaicin or ATP. 21 , 22 Sensory nerve excitability can also be affected by ligands binding to G protein–coupled receptors (GPCRs), which then gate ion channels or increase or decrease the responsiveness to ionotropic‐receptor activation through complex signaling pathways. 21 , 22
Experiments in preclinical species have demonstrated that airway sensory nerve activation results in an action potential being carried along the vagus nerve to the central nervous system (CNS) via projections to the brain stem nucleus of the solitary tract (nTS) and paratrigeminal nucleus. 23 , 24 Release of neurotransmitters at synapses between these projecting neurons and second‐order brain stem neurons can lead to activation of projections to other brain stem nuclei involved in cough generation or higher brain regions involved in cough perception and descending modulatory control (Figure 1a,c ). 16 , 17 , 24 , 25 , 26 Central neurotransmission by vagal afferent neurons is predominantly mediated by glutamatergic signaling through non–N‐methyl‐d‐aspartate (NMDA) receptors, although other transmitters (e.g., substance P, neurokinin A, γ‐aminobutyric acid (GABA), and ATP) and NMDA receptors can also play a role. 17 , 23 , 25 Ultimately, activation of these pathways can lead to involuntary cough reflexes, an urge to cough that may evoke voluntary cough, or behavioral controls that may lead to cough facilitation or suppression. 24 , 26
Pathologic cough responses
Chronic cough is thought to arise from dysregulation of the vagal sensory nerves that mediate cough. The causative factors that contribute to this dysregulation are the subject of intense investigation and could include both pathologic processes and genetic risk factors. Here, we focus our discussion on the pathologic processes as the potential roles of genetic factors that may influence this dysregulation are not currently well understood and are considered outside the scope of the current review.
The clinical characteristics of patients with CC have been previously described. Many patients with CC may exhibit features of hypersensitivity that are analogous to mechanisms that underly chronic pain. 17 , 24 , 27 For example, many patients with CC exhibit laryngeal paresthesia, often reported as an abnormal sensation or irritability in the throat. 27 , 28 , 29 Hypertussivity, which could be analogous to hyperalgesia, is characterized by stronger cough reactions in response to tussive agents. 24 , 27 Allotussia occurs when low‐level stimuli that do not usually evoke cough (e.g., talking, singing, and laughing) lead to a strong urge to cough, a feature similar to allodynia. 27 , 28 , 29 Such features are sufficiently typical in CC that the term “cough hypersensitivity syndrome” has been endorsed to describe this condition. 24
Multiple interrelated mechanisms have been hypothesized to contribute to cough hypersensitivity syndrome, although direct evidence for neural dysfunction in humans is currently limited. 24 , 29 These mechanisms include increased activation or excitability of sensory neurons to chemical stimulation in the periphery or the heighted responsivity of CNS cough networks to peripheral inputs, respectively termed peripheral and central sensitization. The current state‐of‐the art mechanistic understanding of these two processes are described below.
Increased activation or excitability of sensory neurons
Some airway sensory neurons express chemically sensitive receptors (e.g., TRPV1, TRP ankyrin‐1 (TRPA1), or P2X3) that enable them to respond to both endogenous stimuli (including protons, lipid mediators, oxidant chemicals, and ATP) produced by mucosal inflammation and exogenous irritants. 17 , 18 Some patients with CC have an increased sensitivity to cough challenges with the tussive agents capsaicin, citric acid, and ATP compared with healthy volunteers. 30 , 31 Previous research has suggested that endogenous inflammatory mediators are associated with an increased sensitivity to irritants, 16 , 17 and it has been proposed that the excessive cough noted in patients with CC may be in part due to increased levels of endogenous inflammatory mediators (e.g., histamine, PGE2, and ATP). 32 , 33 However, the mechanisms underlying this enhanced sensitivity in humans are not precisely known. One possible mechanism for increased excitability via inflammatory mediators is through GPCR signaling. For example, sensory neurons express receptors for many cytokines and inflammatory mediators, which, when activated, can augment the activity of downstream excitatory ion channels or inhibit potassium‐leak currents, both of which can increase depolarization to stimuli. 21 Examples of these receptors can be found in Table S1 .
Increased activity of airway sensory nerves can also be driven by changes in receptor expression or phenotypic switches that allow these nerves to release new mediators. Preclinical research in models of lung inflammation has demonstrated there is an increased expression of ion channels or receptors (e.g., TRPV1 and tachykinin receptors) by airway sensory nerves, thereby modifying their sensitivity to chemical stimuli, 17 which is consistent with the increase in cough sensitivity to many cough challenge agents seen in humans with RCC. These findings are supported by the overexpression of TRPV1, a key mediator of chemical‐induced cough, in patients with CC compared with healthy volunteers. 34 Vagal neurons may also develop the ability to express neuropeptides (e.g., substance P and calcitonin gene–related peptide) in response to prolonged inflammation, which could play a role in the development of central sensitization. 17 , 35 Of note, inflammatory airway and systemic inflammatory disease are not always associated with cough as a primary or major symptom, which suggests increased expression of inflammatory mediators alone may not be sufficient to drive CC.
Neuroplasticity and changes in central transmission (central sensitization)
It has also been proposed that changes in central transmission, rather than peripheral transmission alone, may promote CC. 17 , 36 Imaging in humans has revealed differences in central processing, particularly in the midbrain, in these patients in response to chemical stimuli. 36 Patients with CC exhibit some features consistent with central sensitization, a process also observed in patients with chronic pain. 16 , 24 Central sensitization may underly the chronicity of CC, as central sensitization can persist long beyond the original injury and inflammation that induced the sensitization. However, the exact role of central sensitization and any underlying mechanisms in humans with CC are currently unclear. Substance P is thought to play a role in the increased excitability of CNS neurons in airway disease, including pathologic cough. 35 However, it is unlikely that a single CNS target underlies this central dysregulation, as evidence in chronic pain indicates more complex changes in central glial cells, second‐order neuron sensitivity, and altered descending central pathways may all be involved. 37 , 38
TARGETS FOR TREATMENT OF RCC AND UCC
A number of pharmacologic targets have been proposed and investigated as treatment of RCC/UCC. In this section, we describe the underlying rationale for assessing these targets and review clinical data published or presented in the past 5 years. Manuscripts and abstracts describing clinical studies in RCC/UCC were identified using common disease state terms (i.e., chronic cough, refractory cough, unexplained cough, idiopathic cough, unresolved cough, and troublesome cough) over a time span from January 1, 2014, through February 29, 2020. Because of the underlying role of neuronal hypersensitivity in RCC/UCC, many of the investigational therapies described below are also being investigated in other conditions related to neuronal hypersensitivity, including pain or itch. However, disease states other than RCC/UCC are outside the scope of this review.
Transient receptor potential channels
Transient receptor potential channels comprise a large family of ion channels that, upon opening, induce depolarization of sensory nerves to a variety of stimuli (such as chemical mediators, temperature, stretching, and pH changes). 39 , 40 Depending on the type of channel, TRP channels can be expressed both in the airways (on smooth muscle, epithelial cells, and sensory nerves) and in the CNS, in addition to other body tissues and organs. 39 , 40
TRPV1: rationale for targeting in CC
TRPV1 is a calcium (Ca2+)‐permeable channel directly activated by capsaicin, an agent commonly used for inhaled challenge–evoked cough, as well as pH changes, increased temperature, and various endogenous mediators. 40 , 41 TRPV1 channels can also be indirectly activated by signaling that occurs downstream of GPCR activation by mediators associated with airway inflammation and cough (e.g., bradykinin and PGE2). 40 , 42 TRPV1 is expressed by both nodose and jugular neurons; although TRPV1 gating predominantly activates C‐fibers, it has been suggested that stimuli of these receptors may directly or indirectly activate some Aδ‐fibers. 43 In addition to the ability of TRPV1 activators to evoke cough in preclinical species and humans, the potential role of TRPV1 in CC in humans is supported by the roughly fivefold greater expression of TRPV1 channels in patients with CC compared with healthy volunteers, as well as a greatly potentiated response to capsaicin in patients with CC. 34 , 44 Preclinical evidence supports the potential therapeutic use of TRPV1 antagonism, as TRPV1 antagonism in guinea pigs sufficiently inhibited vagal nerve activation and cough induced by various chemical stimuli, including capsaicin, PGE2, bradykinin, and pH changes. 42 , 45
TRPV1: clinical evidence in CC
Two selective TRPV1 antagonists have been investigated in patients with RCC. The TRPV1 antagonist SB‐705498 was investigated in a randomized, double‐blind, placebo‐controlled trial of 21 patients with RCC (Table 1 ). 46 Although SB‐705498 decreased the responsiveness to capsaicin challenge, there was no significant improvement in objective cough frequency or patient‐reported measures compared with placebo. The authors of this study suggested that increasing TRPV1‐receptor occupancy (e.g., by using a more potent TRPV1 antagonist or prolonging dosing) may yield better efficacy. XEN‐D0501, a TRPV1 antagonist with 1,000‐fold greater potency vs. SB‐705498 in blocking capsaicin‐induced activation in isolated vagus nerves from guinea pigs and humans, was subsequently investigated in 20 patients with RCC (Table 1 ). 45 Treatment with XEN‐D0501 for 14 days reduced sensitivity to capsaicin but did not significantly improve objective or most subjective measures of cough in these patients. These studies suggest that capsaicin cough sensitivity may not translate to a reduced objective cough frequency in patients with RCC and that TRPV1 channels may not play an important role in the etiology of CC. In addition to the limited efficacy observed with TRPV1 antagonists, TRPV1 inhibition is also associated with thermoregulation issues and insensitivity to thermal pain due to the role of TRPV1 in thermal regulation. 45 Ultimately, the limited efficacy and safety profile of TRPV1 antagonists to date may hinder further clinical work in this area.
Table 1.
Data from prospective clinical studies investigating targeted therapies in RCC/UCC a
| Drugs | Study design | Patient population | Efficacy | Safety |
|---|---|---|---|---|
| TRPV1‐targeting agents | ||||
| SB‐705498 |
|
|
|
|
| XEN‐D0501 |
|
|
|
|
| Capsaicin (desensitization) |
|
|
|
|
| TRPV4‐channel blockers | ||||
| GSK2798745 |
|
|
|
Not reported |
| P2X3‐receptor antagonists | ||||
| Gefapixant |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| BLU‐5937 |
|
|
NA |
|
| S‐600918 |
|
|
|
|
| BAY 1817080 |
|
|
|
|
| NaV1.7 blockers | ||||
| GSK2339345 |
|
|
|
|
| Neuromodulators | ||||
| Gabapentin, amitriptyline, or nortriptyline |
|
|
|
|
| Pregabalin and SPT |
|
|
|
|
| Morphine |
|
|
|
|
| NK‐1 receptor antagonists | ||||
| Orvepitant |
|
|
|
|
| NMDA‐receptor antagonists | ||||
| Memantine |
|
|
|
|
| Other targets | ||||
| PA101 (inhaled sodium cromoglicate) |
|
|
|
|
ACCP, American College of Chest Physicians; ACE, angiotensin‐converting enzyme; ACF, awake cough frequency; AE, adverse event; ATP, adenosine triphosphate; b.i.d., twice daily; BTS, British Thoracic Society; CC, chronic cough; Cn, concentration of capsaicin inducing at least “n” coughs after capsaicin inhalation; CQLQ, Cough‐Specific Quality‐of‐Life Questionnaire; CSD, cough severity diary; Emax, maximal capsaicin cough response over four inhalations; EudraCT, European Union Drug Regulating Authorities Clinical Trials Database; GRC, global rating of change; HARQ, Hull Airway Reflux Questionnaire; IQR, interquartile range; LCQ, Leicester Cough Questionnaire; MAD, multiple‐ascending dose; MCID, minimal clinically important difference; MTD, maximum tolerated dose; NA, not applicable; NK‐1, neurokinin 1; NMDA, N‐methyl‐D‐aspartate; PK, pharmacokinetic; PRO, patient‐reported outcome; pt, patient; P2X3, ATP‐gated (purine) cation channel subtype 3; q.d., once daily; RCC, refractory chronic cough; RCT, randomized controlled trial; RTI, respiratory tract infection; SAD, single‐ascending dose; SD, standard deviation; SPT, skin‐prick test; TCA, tricyclic antidepressant; TEAE, treatment‐emergent AE; t.i.d., three times daily; TRPV, transient receptor potential vanilloid; UCC, unexplained chronic cough; VAS, visual analog scale.
Includes prospective studies presented or published from January 2014 to February 2020.
In addition to TRPV1 antagonism, the ability of oral capsaicin to induce desensitization of the cough reflex through TRPV1 has also been investigated in 24 patients with UCC and 15 healthy control patients in a crossover, randomized, double‐blind study. 44 Regular intake of oral capsaicin decreased sensitivity to capsaicin in patients with UCC and healthy volunteers and improved cough symptoms in patients with UCC. This study was limited by the lack of a washout period between crossover arms, the use of a non–cough‐specific questionnaire, and the absence of objective cough measurements. Moreover, orally administered capsaicin is rapidly metabolized before reaching general circulation, suggesting minimal systemic absorption of intact capsaicin. 47 This suggests that the capsaicin‐induced desensitization observed in this study was potentially mediated by an unknown local action in the gastrointestinal tract or nonspecific effects. Ultimately, additional research is needed to assess whether capsaicin‐induced desensitization is an effective treatment of RCC/UCC. An ongoing phase II clinical trial will assess the effects of oral capsaicin vs. placebo on cough reflex and symptoms in patients with UCC (NCT04125563; Table 2 ).
Table 2.
Ongoing and recently completed clinical trials investigating targeted therapies for RCC/UCC (data not yet presented or published) a
| Drug | Trial identifier | Study design | Key eligibility criteria | Outcome measures | Estimated completion a |
|---|---|---|---|---|---|
| Oral capsaicin (TRPV1 agonist) | NCT04125563 |
|
|
|
|
| AX‐8 (TRPM8 agonist) | EudraCT identifier, 2017‐003108‐27 |
|
|
|
Completed (data posted online in EudraCT.gov but not yet presented or published) |
| Gefapixant (P2X3 antagonist) | NCT03449134 (COUGH‐1) |
|
|
|
Completed (data yet to be presented or published) |
| NCT03449147 (COUGH‐2) |
|
|
|
Completed (data yet to be presented or published) | |
| BLU‐5937 (P2X3 antagonist) | NCT03979638 (RELIEF) |
|
|
|
Terminated early (data yet to be presented or published) |
| BAY1902607 (P2X3 antagonist) | NCT03535168 |
|
|
|
Completed (data yet to be published) |
| S‐600918 (P2X3 antagonist) | NCT04110054 |
|
|
|
|
| Orvepitant (NK‐1 antagonist) | NCT02993822 (VOLCANO‐2) |
|
|
|
Completed (data yet to be published) |
| Bradanicline (ATA‐101) | NCT03622216 |
|
|
|
Completed (data yet to be published) |
| Indomethacin | NCT03662269 |
|
|
|
|
ACCP, American College of Chest Physicians; ACEI, angiotensin‐converting enzyme inhibitor; ACF, awake cough frequency; AE, adverse event; b.i.d., twice daily; BTS, British Thoracic Society; CSD, cough severity diary; CT, computed tomography; EUDRA‐CT, European Union Drug Regulating Authorities Clinical Trials Database; HARQ‐S, Hull Airway Reflex Questionnaire–Swedish version; ICIQ‐SF, International Consultation on Incontinence Questionnaire Short Form; LCQ, Leicester Cough Questionnaire; LogCn, logarithmic values of inhaled capsaicin threshold concentration needed to reach n coughs; P2X3, ATP‐gated (purine) cation channel subtype 3; PGIC, patient global impression of change; PK, pharmacokinetic; pts, patients; q.d., once daily; RCC, refractory chronic cough; RCT, randomized controlled trial; SF‐36, Short Form (36) Health Survey; TRPM, transient receptor potential melastatin; TRPV, TRP vanilloid; UCC, unexplained chronic cough; VAS, visual analog scale.
Includes trial information available in clinical trial registries (e.g., ClinicalTrials.gov).
TRPA1: rationale for targeting in CC
TRPA1 is a Ca2+‐permeable channel activated by cold temperatures in addition to several chemical stimuli, including natural products (e.g., cinnamaldehyde, and allyl isothiocyanate), products of oxidative stress, and environmental irritants (e.g., ozone). 39 , 40 Similar to TRPV1 receptors, TRPA1 channels may also be indirectly activated by signaling that occurs downstream of GPCR activation by endogenous tussive agents (e.g., bradykinin and PGE2). 42 Preclinical evidence suggests TRPA1 channels may activate C‐fibers and nonneuronal airway cells that express TRPA1 channels (e.g., airway epithelial and smooth muscle cells), but not Aδ‐fibers. 40 TRPA1 agonists can evoke coughing in preclinical guinea pig models, and the selective TRPA1 antagonist GRC 17536 can inhibit citric acid–induced cough in guinea pigs. 48 , 49 Moreover, the TRPA1 agonist cinnamaldehyde can induce cough in healthy human volunteers. 48 These data suggest that TRPA1 could play a role in CC.
TRPA1: clinical evidence in CC
Despite promising preclinical data, an unpublished double‐blind, placebo‐controlled study assessing GRC 17536 found no evidence of objective or subjective efficacy in patients with CC. 50 However, it is unclear if dosing in this study was adequate, as no data from target engagement functional assays were provided; thus, TRPA1 antagonism cannot yet be ruled out as a potential antitussive approach for RCC/UCC.
TRPV4: rationale for targeting in CC
TRPV4 is a Ca2+‐permeable ion channel that responds to both exogenous and endogenous stimuli, including hypoosmolarity, arachidonic acid (and its metabolites), and mechanical stress. 39 , 40 TRPV4 channels are seldom expressed on vagal sensory neurons and therefore plausibly promote sensory nerve activation through indirect means. 43 For example, TRPV4 activation on airway macrophages and epithelial cells is associated with ATP release, which may activate sensory neurons through P2X3 receptors. 51
TRPV4: clinical evidence in CC
Despite TRPV4 receptors playing a putative role in indirect activation of sensory nerves, there are no ongoing trials assessing TRPV4‐targeted agents for treatment of CC, and a phase I/II trial investigating the TRPV4 antagonist GSK2798745 in patients with RCC/UCC (NCT03372603) was terminated early because of a lack of efficacy. 52
TRPM8: rationale for targeting in CC
TRP melastatin‐8 (TRPM8) channels are Ca2+‐permeable channels that are activated by cold temperatures and cooling compounds (e.g., menthol, icilin, and eucalyptol). 39 , 40 TRPM8 channels are expressed by ~ 60% of nasal trigeminal afferent neurons and have lower expression on bronchopulmonary vagal sensory neurons. 53 Although the TRPM8 agonist menthol activates some vagal sensory neurons, it exerts antitussive effects that are seemingly due to activation of the nasal trigeminal neuronal pathway, 53 which suggests completing sensory inputs to the brain can lead to cough modulation.
TRPM8: clinical evidence in CC
Inhaled menthol has been demonstrated to reduce cough sensitivity in cough challenges in both healthy human volunteers and in patients with CC. 54 , 55 However, the effects of menthol on objective cough frequency and subjective measures of CC have yet to be investigated in clinical studies. The selective TRPM8 agonist AX‐8 was recently investigated in an uncontrolled pilot study in patients with RCC (European Union Drug Regulating Authorities Clinical Trials Database (EudraCT) identifier, 2017‐003108‐27). The study was announced to have met its primary end point by significantly reducing awake cough frequency (compared with that of baseline), and results have been posted in the European Union Clinical Trials Register; however, study data have yet to be published in peer‐reviewed literature (Table 2 ). Initial data suggest treatment with AX‐8 led to numerical reductions in objective awake cough frequency, cough severity, urge to cough, and throat irritation; however, the implications of these data are currently unclear because of the small sample size, lack of a control cohort, and lack of a reported statistical analysis.
P2X3 receptors
The purinergic P2X family comprises ATP‐gated ion channels that consist of homotrimers or heterotrimers of P2X subunits P2X1 to P2X7. 56 Homotrimeric P2X3 and heterotrimeric P2X2/3 receptors are the most well‐studied P2X receptors on vagal sensory neurons, which are expressed by vagal sensory neurons at both peripheral terminals in the airways and central terminals in the CNS. 56 , 57 Preclinical studies have suggested that stimulation of the P2X3 homotrimeric receptor (expressed by jugular vagal afferent neurons) leads to a transient, rapidly inactivating current, whereas stimulation of P2X2/3 heterotrimeric receptor (expressed by nodose vagal afferent neurons) leads to a longer‐lasting, slowly inactivating current, suggesting that stimulation of P2X2/3 receptors may be required to elicit action potentials. 57
The role of P2X3 and P2X2/3 receptors in CC is also supported by the involvement of these receptors’ principal endogenous ligand, extracellular ATP, in airway diseases and inflammation. 58 ATP can be released from airway epithelial cells through pannexin‐1 channels in response to several stimuli and mechanisms, including injury, inflammation, and environmental stimuli (e.g., allergens and pollutants). 59 , 60 , 61 Once released, extracellular ATP acts as a damage signal that can drive further inflammation by stimulating the release of proinflammatory cytokines. 59 , 60 , 61 ATP may play a direct role in cough, as challenge with exogenous ATP is sufficient to evoke cough in healthy volunteers and, to a greater extent, in patients with CC. 30 Moreover, evidence in animals suggests that ATP potentiates acid‐evoked cough in animals, and P2X3‐receptor antagonists can reduce this effect. 62 However, it remains unclear whether the role of P2X2/3 and P2X3 receptors in pathologic CC is driven by an enhanced sensitivity to ATP, increased ATP in the airways potentially driven by the TRPV4/pannexin axis, reduced ATP degradation, or a combination of these factors. 30 , 43 , 63
Gefapixant/AF‐219: clinical evidence in CC
Gefapixant (formerly AF‐219) is a P2X3 antagonist with moderate selectivity for the P2X3 receptor over the P2X2/3 receptor. 64 The efficacy of gefapixant has been investigated in multiple clinical trials to date. The efficacy of gefapixant was originally demonstrated in a double‐blind, placebo‐controlled, crossover study in 24 patients with RCC or UCC (Table 1 ). 65 A large reduction in placebo‐adjusted objective daytime cough frequency was observed at a dosage of 600 mg twice daily for 2 weeks. Although gefapixant was generally well tolerated, all patients experienced mild or moderate taste disturbances while receiving gefapixant and six patients discontinued because of these effects. As a result, lower doses have been investigated in subsequent clinical trials. In a phase II dose‐escalation trial, treatment with gefapixant 15, 30, or 50 mg twice daily resulted in significant reductions in awake cough frequency, and fewer taste disturbances were reported at doses of ≤ 50 mg twice daily compared with the rate observed with the supratherapeutic 600‐mg twice‐daily dose. 66 The ability of gefapixant to treat CC at lower doses was most recently investigated in a phase IIb, double‐blind, parallel‐group trial in 253 patients with CC (Table 1 ). 67 Significant reductions in objective cough frequency (awake and 24‐hour) were observed at the 50‐mg twice‐daily dose. As with the original study, gefapixant was generally well tolerated, and taste disturbances (specifically dysgeusia) were the most common treatment‐related AEs. Gefapixant was recently investigated in two phase III clinical trials (COUGH‐1/NCT03449134 and COUGH‐2/NCT03449147) in patients with RCC/UCC (Table 2 ). It was announced that both trials met the primary efficacy end point for the higher dose (45 mg twice daily); primary data for these trials have not yet been posted or published.
Gefapixant has also been investigated for its ability to reduce cough‐reflex sensitivity in a phase II, randomized, double‐blind, crossover, placebo‐controlled study in patients with RCC/UCC and healthy volunteers (Table 1 ). 31 Treatment with a single dose of gefapixant 100 mg reduced sensitivity to ATP and distilled water cough challenges; however, no effects were observed on capsaicin or citric acid cough challenges, suggesting that these tussive agents may activate cough through distinct pathways. Patients treated with gefapixant also reported improvements in patient‐reported cough severity, urge to cough, and cough frequency.
On the basis of preclinical data, it has been hypothesized that taste disturbances are related to the role of P2X2/3 receptors in signaling between taste buds and gustatory sensory nerves. 68 Consequently, multiple P2X3 antagonists have been developed with higher selectivity for P2X3 heterotrimers with the aim of reducing the incidence of taste disturbances.
BLU‐5937: clinical evidence in CC
The P2X3 antagonist BLU‐5937 has an approximately 1500‐fold higher affinity for P2X3 compared with the P2X2/3 receptor. 62 A phase I study in healthy volunteers reported a low incidence (4.2%) of taste alterations at doses proposed to be potentially therapeutic on the basis of preclinical pharmacokinetic modeling (Table 1 ). 69 A phase II dose‐escalation trial was conducted to assess whether these doses are efficacious in patients with RCC (NCT03979638; Table 2 ). Per ClinicalTrials.gov (https://clinicaltrials.gov/), this trial was terminated early owing to the impact of COVID‐19 on trial activities; top‐line data released by the sponsor reported that the trial failed to meet its primary end point for any tested dose, though the drug was reported as well tolerated (with taste effects reported in 6.5–10.0% with BLU‐5937 compared with 4.9% with placebo), and significant reductions in awake cough frequency were observed in a prespecified subgroup analysis of patients with high cough counts at baseline.
S‐600918: clinical evidence in CC
S‐600918 was recently investigated in a phase II, double‐blind, randomized, crossover trial in 31 Japanese patients with RCC/UCC (registry identifier, JapicCTI‐184027). 70 Although the study did not meet its primary end point of significantly reducing daytime cough frequency, a trend toward improvement in daytime cough frequency was observed after 2 weeks of treatment, and a significant improvement in 24‐hour cough frequency was reported. The study reported a low incidence (3.2%) of taste changes during S‐600918 treatment that was comparable with that of placebo. S‐600918 will be further investigated in a phase IIb trial in approximately 372 patients with RCC/UCC (NCT04110054).
Other P2X3 antagonists: clinical evidence in CC
BAY1817080 was recently investigated in a phase I/II proof‐of‐concept trial in patients with RCC (NCT03310645). 71 Significant reductions in 24‐hour cough frequency were observed at higher doses, with the frequency of taste‐related AEs ranging from 5% to 21% across dose levels (Table 1 ).
A separate P2X3 antagonist—BAY1902607—is also being investigated in a phase I/II proof‐of‐concept trial (NCT03535168; Table 2 ). This study has been reported as completed on ClinicalTrials.gov, but data have not yet been posted or published.
Voltage‐gated sodium channels
Voltage‐gated sodium channels: rationale for targeting in CC
Voltage‐gated sodium channels, or NaVs, are critical for action‐potential induction in neurons, including sensory nerves mediating cough. 21 , 72 These channels are ultimately responsible for action‐potential generation in response to generator potentials arising from the opening of other ion channels. 21 As such, NaVs are natural targets for potentially blocking the sensory nerve activation that leads to activation of the cough cascade. Although nonselective systemic blockade of NaVs can be lethal, some specific NaV isoforms (i.e., NaV 1.7, 1.8, and 1.9) appear to be selectively expressed on vagal sensory neurons in guinea pigs and have therefore been proposed as promising therapeutic targets for CC. 72 Further supporting this hypothesis, selective inhibition of NaV1.7 via gene silencing in nodose sensory neurons reduces the excitability of these neurons and inhibits mechanically induced and citric acid–induced cough in guinea pigs. 73 , 74 Although NaV1.8 and NaV1.9 have been studied in preclinical pain models, relatively little information is available regarding the role of these isoforms in CC. 72 Another approach to block NaVs while avoiding systemic exposure and toxicity is the use of inhaled, nebulized lidocaine to treat patients with CC. 75 However, this approach does not appear to have been investigated in randomized, placebo‐controlled trials to date.
NaV blockade: clinical evidence in CC
The selective NaV1.7 inhibitor GSK2339345 was investigated in a randomized, double‐blind, placebo‐controlled crossover study in 16 patients with RCC (Table 1 ). 76 Paradoxically, GSK2339345 significantly increased 8‐hour cough counts compared with placebo and had a protussive effect in all tested patients, leading to termination of the trial. The authors suggested that induction of transient cough by the study drug may have been due to activation and sensitization of TRPV1‐expressing sensory neurons. However, the quality of evidence for this hypothesis was low as GSK2339345 had no effect on capsaicin‐induced cough. Moreover, it was unclear whether GSK2339345 adequately reached the target NaV on the basis of inconclusive findings in the capsaicin and citric acid challenges employed in the study.
An additional compound described as a charged sodium channel blocker (NTX‐1175) is reported as being in development for RCC; however, to our knowledge no preclinical or clinical data have yet been published.
Neuromodulators
Several different neuromodulators have been investigated for treatment of RCC/UCC on the basis of preclinical research as well as the use of neuromodulators in treatment of other neuropathic disorders.
GABA‐related agents: rationale and clinical evidence in CC
Some patients with CC present with central sensitization, which suggests that drugs with a central site of action may be appropriate for these patients. 17 , 24 As central sensitization is also a feature of chronic pain and CC exhibits shared qualities with neuropathic disorders, neuromodulators that have been used to treat chronic neuropathic pain (e.g., gabapentin) have also been investigated for treatment of CC. 24 , 27 , 77 Although the precise mechanism of action in CC remains unclear, the GABA‐related neuromodulators gabapentin and pregabalin are both believed to act by blocking a subset of central voltage‐gated calcium channels that contain the α2δ subunit and do not appear to act directly at GABAA or GABAB receptors. 78 Gabapentin was demonstrated to be effective for treatment of RCC in a randomized, double‐blind, placebo‐controlled trial, with a greater effect in patients with features of central sensitization. 79 Treatment guidelines for CC have suggested the use of gabapentin for adults with RCC/UCC, although recent European Respiratory Society (ERS) guidelines have provided a conditional recommendation based on the level of available evidence. 7 , 8
A recent systematic review was conducted regarding the efficacy and safety of gabapentin for patients with CC. 80 Seven retrospective and prospective studies were identified that included a total of 159 patients treated with gabapentin at varying dosages. Gabapentin generally demonstrated efficacy in reducing both objective and subjective cough measures in these trials, although measured outcomes and results differed by study and treatment effects tended to wear off after treatment discontinuation. The most common side effects were CNS symptoms (e.g., fatigue, somnolence, and dizziness). The authors concluded that considerable variations in study design, dosage, and outcomes precluded performing a formal meta‐analysis, and that further randomized controlled trials should be conducted to identify the optimal dose and duration of gabapentin therapy for RCC/UCC. A retrospective review conducted in 38 patients treated at a tertiary clinic with either gabapentin (n = 9) or pregabalin (n = 29) reported a high discontinuation rate and adverse safety profile, and the authors concluded that gabapentin and pregabalin are effective in a subset of patients but are significantly limited by their safety profile. 81
The GABA analogue pregabalin has also been investigated in combination with speech therapy compared with speech therapy alone in a randomized, double‐blind, placebo‐controlled trial in patients with RCC/UCC (Table 1 ). 82 Addition of pregabalin to speech therapy improved subjective, but not objective, measures of cough. The authors proposed that this discrepancy may be due to the psychoactive properties of pregabalin, which may alter the perception of cough (i.e., subjective cough measures such as cough severity or cough‐related quality of life) but not objective cough frequency.
Antidepressants: rationale and clinical evidence in CC
Similar to gabapentin, tricyclic antidepressants (e.g., amitriptyline and nortriptyline), which can treat chronic neuropathic pain, have been investigated for treatment of CC because of shared similarities between CC and chronic neuropathic pain. 24 , 27 , 77 The mechanism of action of tricyclic antidepressants is not well understood in CC, but these agents are known to exert effects on serotoninergic, noradrenergic, adrenergic, histaminergic, and muscarinic signaling. 83 A retrospective chart review of 48 patients with RCC/UCC revealed that the vast majority of patients treated with amitriptyline reported a subjective improvement in their cough. 84 An anonymous survey deployed 2–3 years after initiation of amitriptyline treatment revealed that many patients still reported an improvement in their cough; however, 64% of patients who responded to the survey had stopped taking amitriptyline, with the most commonly cited reasons for discontinuation being side effects (e.g., sedation and dry mouth) and lack of improvement.
Short‐term and long‐term effects of GABA‐related agents and tricyclic antidepressants in RCC/UCC
A recent study investigated the short‐term and long‐term effects of gabapentin, amitriptyline, and nortriptyline in 28 patients with UCC (Table 1 ). 85 Most patients in this study received gabapentin, and some patients received tricyclic antidepressants after discontinuing gabapentin therapy (and vice versa). Significant improvements in subjective cough measures were observed with both gabapentin and the tricyclic antidepressants after 2 months of therapy. Improvements in Leicester Cough Questionnaire scores were sustained after 6 months of treatment with gabapentin, but too many patients discontinued the tricyclic antidepressants to reliably analyze efficacy. Most treatment failures were due to tachyphylaxis, defined as a diminishing therapeutic effect of the neuromodulator, and the lack of perceived benefit was the most common cause for drug discontinuation.
The incidence of tachyphylaxis and dependence with neuromodulator therapy was further investigated by the same group in a retrospective study of 68 patients with RCC/UCC treated with various neuromodulators (amitriptyline, desipramine, gabapentin, nortriptyline, and tramadol). 86 Most patients in this study group were initially prescribed amitriptyline. Most patients experienced a treatment success after one or more lines of neuromodulator treatment, and the concomitant use of behavioral cough suppression therapy significantly improved the success rate. Over half of the patients in this study exhibited at least one incident of tachyphylaxis or dependence. Side effects led to drug discontinuation in 26% of patients treated with amitriptyline and > 40% of patients treated with gabapentin or nortriptyline, with sedation being the most commonly reported side effect for all 3 treatments.
Morphine: rationale and clinical evidence in CC
Morphine is a selective μ‐opioid–receptor agonist, although the exact role of opioid receptors in cough regulation in humans is unclear. 87 Morphine has been demonstrated to inhibit capsaicin‐ and citric acid–induced cough in preclinical species, with its antitussive activity attributed to both central and peripheral sites of action. 87 , 88 , 89 In humans, low‐dose morphine was previously suggested to be effective for CC treatment on the basis of a randomized, double‐blind, placebo‐controlled study that demonstrated improvements in subjective cough measures in 27 patients with chronic treatment‐resistant cough, although objective cough reflex to citric acid cough challenges was not significantly altered. 90 The most frequently observed AEs were constipation (40%) and drowsiness (25%). More recently, the objective efficacy of low‐dose morphine was investigated in a double‐blind, placebo‐controlled, crossover study in 22 patients with RCC (Table 1 ). 91 Treatment with morphine 5–10 mg twice daily significantly reduced daytime, nighttime, and 24‐hour objective cough frequency, as well as patient‐reported measures of cough. The most recent ERS guidelines recommend low‐dose, slow‐release morphine as the preferred neuromodulatory agent to treat RCC/UCC, although this approach is not uniformly endorsed by other society guidelines. 7 , 8
Neurokinin‐1 receptors
Neurokinin‐1 receptors: rationale for targeting in CC
Tachykinins, such as substance P, play a role in the periphery neurogenic inflammation and also act centrally as neuromodulators via neurokinin (NK) receptors expressed in the brain. 17 Tachykinins have been suggested to play both a peripheral and central role in the cough cascade, 92 and infusion of substance P into the nTS augments cough responses, potentially via augmentation of glutamatergic signaling in the nTS. 35 Selective antagonism of the NK‐1 receptor has been demonstrated to block capsaicin‐induced and citric acid–induced cough in preclinical species. 93 , 94
NK‐1–receptor antagonists: clinical evidence in CC
The highly CNS‐penetrable NK‐1–receptor antagonist orvepitant has been investigated in 13 patients with RCC in an open‐label pilot study. 95 Treatment with orvepitant led to a significant improvement in objective and subjective cough measures, with some improvements maintained 4 weeks after treatment discontinuation (Table 1 ). A phase IIb, randomized, placebo‐controlled, dose‐ranging study (NCT02993822) was recently completed (Table 2 ); although it has been announced that there were significant improvements in patient‐reported (but not objective) measures of cough compared with placebo, the results of this trial have not yet been published.
A different NK‐1–receptor antagonist, serlopitant, was investigated in a phase II trial (NCT03282591). The study did not meet its primary end points and the compound is no longer in development. The results of this trial are unpublished but are available on ClinicalTrials.gov.
Nicotinic receptors
Nicotinic receptors: rationale for targeting in CC
Activation of nicotinic acetylcholine receptors (nAChRs) via nicotine has been demonstrated to stimulate rat vagal pulmonary sensory neurons. 96 However, nicotine has been previously demonstrated to reduce the urge to cough following capsaicin challenge in healthy smokers and nonsmokers. 97 The ability of nicotine to reduce citric acid–induced cough seems to be dependent on α7 but not α4β2 receptors, and the CNS‐penetrant, α7 nAChR–selective agonist bradanicline (ATA‐101) has been demonstrated to reduce sensitivity to citric acid cough challenges in guinea pigs. 98
α7 nAChR: clinical evidence in CC
A phase II, randomized, double‐blind, placebo‐controlled, crossover, dose‐escalation trial investigating bradanicline in patients with RCC (NCT03622216) was recently completed, though results have not been announced or published to date (Table 2 ).
NMDA receptors
NMDA receptors: rationale for targeting in CC
NMDA receptors expressed in the brain stem are thought to transduce cough signals from vagal afferent nerves and therefore are a potential therapeutic target for alleviating CC. 23 , 25 Preclinical experiments have demonstrated that NMDA‐receptor antagonism by different administration routes (i.e., microinjection into the nTS, intraperitoneal injection, or oral administration) is sufficient to inhibit bradykinin‐evoked or citric acid–evoked coughing. 23 , 25 , 99 Moreover, the NMDA‐receptor antagonist memantine inhibits capsaicin‐induced cough in healthy human volunteers. 100
NMDA receptors: clinical evidence in CC
Memantine was investigated in an open‐label, dose‐escalation trial in 14 patients with RCC (Table 1 ). 101 Memantine did not significantly improve objective or subjective cough after up to 4 weeks of treatment and was poorly tolerated.
Additional investigated targets
An inhaled formulation of sodium cromoglicate (PA101), a therapy reported to reduce activity of sensory C‐fibers via an orphan GPCR, was investigated in a randomized, double‐blind, placebo‐controlled trial that included two separate cohorts of patients with idiopathic pulmonary fibrosis or RCC (Table 1 ). 102 Although PA101 reduced cough frequency in the cohort of patients with idiopathic pulmonary fibrosis, PA101 did not improve objective cough frequency or subjective cough‐related outcomes in patients with RCC.
The cyclooxygenase inhibitor indomethacin is currently being investigated in a randomized, double‐blind, placebo‐controlled trial in patients with RCC on the basis of the hypothesis that indomethacin may reduce levels of airway prostaglandins that can induce cough (NCT03662269; Table 2 ). Data for this trial have not yet been reported.
POTENTIAL NOVEL TARGETS FOR TREATMENT OF CC
Several additional targets investigated in preclinical research to date may be promising in the future development of targeted therapies for RCC/UCC. These include neuromodulatory enzymes (e.g., ATPase), ion channels (e.g., chloride, potassium sodium, and other TRPs), and receptors (e.g., GABA receptors, receptors for cytokines, interferons, and other inflammatory mediators), which have been identified on the basis of their role in airway processes that are thought to be dysregulated in pathologic cough. A list of these potential targets on the horizon is included in Table S1 , along with a brief summary of preclinical research to date that may support investigation of these targets in clinical trials.
FUTURE DIRECTIONS
A wide range of targets have been investigated for treatment of RCC/UCC. Successfulness of recent early‐phase clinical trials has varied, with P2X3‐receptor antagonists perhaps showing the most promise for treatment of patients with RCC or UCC. However, it is already evident that not all patients with CC respond to even the most promising compounds, highlighting the importance of continued research in this field. Improved understanding of the cellular and molecular drivers of CC could pave the way for a precision medicine approach to CC, whereby treatment is based on the clinical and molecular characteristics of individual patients. Indeed, accumulating evidence suggests that patients can express different endotypes of CC and cough hypersensitivity as a function of respiratory comorbidities and heterogeneity in underlying neural dysregulation. 103 Although the concept of precision medicine for CC is currently aspirational, the heterogeneity and multifactorial nature of cough supports the adaptation of such an approach, including identification of patient subgroups that respond to specific treatments. Additionally, different end points and assessments have been used in CC clinical trials, including objective assessment of cough frequency using cough‐monitoring devices and subjective cough‐related patient‐reported outcomes. 104 Well‐designed clinical trials including objective cough monitoring will be important to support regulatory approval of novel antitussives for CC.
Ultimately, a multidisciplinary approach may be the most viable strategy for the management of patients with RCC/UCC. An example of this strategy is the NEUROCOUGH Clinical Research Collaboration, which has been established to facilitate partnership between clinicians, academic researchers, and the pharmaceutical industry, with the aims of improving our mechanistic understanding of cough; developing a robust infrastructure for CC clinical trials and patient identification; enhancing public engagement and recognition of CC; and fostering training and clinical research capacity within CC. 105 NEUROCOUGH and similar initiatives may provide a platform to develop CC treatments that can help these underserved patients in the future.
CONCLUSION
CC is a burdensome condition that is thought to be characterized by dysregulation of the vagal sensory neurons that mediate cough. Several molecular targets have been investigated on the basis of their potential roles in the underlying pathophysiology of RCC and UCC. Although results to date have varied between investigative approaches and there are currently no new approved treatments with indications for these conditions, promising results have been reported for some investigated pharmacologic treatments. Owing to the heterogeneity underlying chronic cough, it is likely that the best therapeutic approach will vary by patient and further multidisciplinary research is needed to establish the best treatment pathways for this patient population.
Funding
Funding for writing and editorial assistance for this manuscript was provided by Merck Sharpe & Dohme Corp., a subsidiary of Merck & Co, Inc., Kenilworth, NJ, USA.
Conflicts of Interest
S.M. declares personal fees from Merck and NeRRe Therapeutics and grant support from Merck. L.M. reports personal fees from Chiesi, GSK, Merck, NeRRe Therapeutics, and Shionogi Inc; grant support from Merck; and other support from AstraZeneca, Boehringer Ingelheim, and Chiesi.
Supporting information
Table S1
Acknowledgments
Medical writing and editorial assistance were provided under the direction of the authors by Nathan Rodeberg, PhD, and Jenna Lewis, MA, ELS, of MedThink SciCom.
Contributor Information
Stuart B. Mazzone, Email: stuart.mazzone@unimelb.edu.au.
Lorcan McGarvey, Email: l.mcgarvey@qub.ac.uk.
References
- 1. Song, W.J. et al. The global epidemiology of chronic cough in adults: a systematic review and meta‐analysis. Eur. Respir. J. 45, 1479–1481 (2015). [DOI] [PubMed] [Google Scholar]
- 2. Irwin, R.S. et al. Classification of cough as a symptom in adults and management algorithms: CHEST Guideline and Expert Panel Report. Chest 153, 196–209 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. French, C.L. , Crawford, S.L. , Bova, C. & Irwin, R.S. Change in psychological, physiological, and situational factors in adults after treatment of chronic cough. Chest 152, 547–562 (2017). [DOI] [PubMed] [Google Scholar]
- 4. Chamberlain, S.A.F. et al. The impact of chronic cough: a cross‐sectional European survey. Lung 193, 401–408 (2015). [DOI] [PubMed] [Google Scholar]
- 5. Kuzniar, T.J. , Morgenthaler, T.I. , Afessa, B. & Lim, K.G. Chronic cough from the patient's perspective. Mayo. Clin. Proc. 82, 56–60 (2007). [DOI] [PubMed] [Google Scholar]
- 6. Koskela, H.O. , Lätti, A.M. & Pekkanen, J. Risk factors for repetitive doctor's consultations due to cough: a cross‐sectional study in a Finnish employed population. BMJ Open 9, e030945 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gibson, P. et al. Treatment of unexplained chronic cough: CHEST Guideline and Expert Panel report. Chest 149, 27–44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morice, A.H. et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur. Respir. J. 55, 1901136 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGarvey, L. & Gibson, P.G. What is chronic cough? Terminology. J. Allergy Clin. Immunol. Pract. 7, 1711–1714 (2019). [DOI] [PubMed] [Google Scholar]
- 10. Yancy, W.S. Jr et al. Efficacy and tolerability of treatments for chronic cough: a systematic review and meta‐analysis. Chest 144, 1827–1838 (2013). [DOI] [PubMed] [Google Scholar]
- 11. Burns, J.M. & Boyer, E.W. Antitussives and substance abuse. Subst. Abuse Rehabil. 4, 75–82 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kahrilas, P.J. , Howden, C.W. , Hughes, N. & Molloy‐Bland, M. Response of chronic cough to acid‐suppressive therapy in patients with gastroesophageal reflux disease. Chest 143, 605–612 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shaheen, N.J. et al. Randomised clinical trial: high‐dose acid suppression for chronic cough – a double‐blind, placebo‐controlled study. Aliment. Pharmacol. Ther. 33, 225–234 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yi, F. et al. Validity of fractional exhaled nitric oxide in diagnosis of corticosteroid‐responsive cough. Chest 149, 1042–1051 (2016). [DOI] [PubMed] [Google Scholar]
- 15. Takemura, M. et al. Clinical, physiological and anti‐inflammatory effect of montelukast in patients with cough variant asthma. Respiration 83, 308–315 (2012). [DOI] [PubMed] [Google Scholar]
- 16. Canning, B.J. et al. Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest 146, 1633–1648 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mazzone, S.B. & Undem, B.J. Vagal afferent innervation of the airways in health and disease. Physiol. Rev. 96, 975–1024 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Canning, B.J. , Mazzone, S.B. , Meeker, S.N. , Mori, N. , Reynolds, S.M. & Undem, B.J. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea‐pigs. J. Physiol. 557, 543–558 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. West, P.W. , Canning, B.J. , Merlo‐Pich, E. , Woodcock, A.A. & Smith, J.A. Morphologic characterization of nerves in whole‐mount airway biopsies. Am. J. Respir. Crit. Care Med. 192, 30–39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Canning, B.J. The cough reflex in animals: relevance to human cough research. Lung 186(suppl. 1), S23–S28 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor‐Clark, T. & Undem, B.J. Transduction mechanisms in airway sensory nerves. J. Appl. Physiol. 1985(101), 950–959 (2006). [DOI] [PubMed] [Google Scholar]
- 22. Kollarik, M. & Undem, B.J. Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild‐type and TRPV1‐/‐ mice. J. Physiol. 555, 115–123 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Canning, B.J. & Mori, N. Encoding of the cough reflex in anesthetized guinea pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R369–R377 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chung, K.F. , McGarvey, L. & Mazzone, S.B. Chronic cough as a neuropathic disorder. Lancet Respir. Med. 1, 414–422 (2013). [DOI] [PubMed] [Google Scholar]
- 25. Canning, B.J. & Mori, N. An essential component to brainstem cough gating identified in anesthetized guinea pigs. FASEB J. 24, 3916–3926 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mazzone, S.B. et al. Sensorimotor circuitry involved in the higher brain control of coughing. Cough 9, 7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vertigan, A.E. & Gibson, P.G. Chronic refractory cough as a sensory neuropathy: evidence from a reinterpretation of cough triggers. J. Voice. 25, 596–601 (2011). [DOI] [PubMed] [Google Scholar]
- 28. Hilton, E. , Marsden, P. , Thurston, A. , Kennedy, S. , Decalmer, S. & Smith, J.A. Clinical features of the urge‐to‐cough in patients with chronic cough. Respir. Med. 109, 701–707 (2015). [DOI] [PubMed] [Google Scholar]
- 29. Song, W.J. & Morice, A.H. Cough hypersensitivity syndrome: a few more steps forward. Allergy Asthma Immunol. Res. 9, 394–402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fowles, H.E. , Rowland, T. , Wright, C. & Morice, A. Tussive challenge with ATP and AMP: does it reveal cough hypersensitivity? Eur. Respir. J. 49, 1601452 (2017). [DOI] [PubMed] [Google Scholar]
- 31. Morice, A.H. et al. The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo‐controlled study. Eur. Respir. J. 54, 1900439 (2019). [DOI] [PubMed] [Google Scholar]
- 32. Turner, R.D. & Birring, S.S. Chronic cough: ATP, afferent pathways and hypersensitivity. Eur. Respir. J. 54, 1900889 (2019). [DOI] [PubMed] [Google Scholar]
- 33. Birring, S.S. , Parker, D. , Brightling, C.E. , Bradding, P. , Wardlaw, A.J. & Pavord, I.D. Induced sputum inflammatory mediator concentrations in chronic cough. Am. J. Respir. Crit. Care Med. 169, 15–19 (2004). [DOI] [PubMed] [Google Scholar]
- 34. Groneberg, D.A. et al. Increased expression of transient receptor potential vanilloid‐1 in airway nerves of chronic cough. Am. J. Respir. Crit. Care Med. 170, 1276–1280 (2004). [DOI] [PubMed] [Google Scholar]
- 35. Mazzone, S.B. , Mori, N. & Canning, B.J. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea‐pigs. J. Physiol. 569, 559–573 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Farrell, M.J. & Mazzone, S.B. Are neural pathways processing airway inputs sensitized in patients with cough hypersensitivity? Pulm. Pharmacol. Ther. 57, 101806 (2019). [DOI] [PubMed] [Google Scholar]
- 37. von Hehn, C.A. , Baron, R. & Woolf, C.J. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73, 638–652 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spaziano, G. et al. Exposure to allergen causes changes in NTS neural activities after intratracheal capsaicin application, in endocannabinoid levels and in the glia morphology of NTS. Biomed. Res. Int. 2015, 980983 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moran, M.M. , McAlexander, M.A. , Bíró, T. & Szallasi, A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug. Discov. 10, 601–620 (2011). [DOI] [PubMed] [Google Scholar]
- 40. Bonvini, S.J. & Belvisi, M.G. Cough and airway disease: the role of ion channels. Pulm. Pharmacol. Ther. 47, 21–28 (2017). [DOI] [PubMed] [Google Scholar]
- 41. Caterina, M.J. , Schumacher, M.A. , Tominaga, M. , Rosen, T.A. , Levine, J.D. & Julius, D. The capsaicin receptor: a heat‐activated ion channel in the pain pathway. Nature 389, 816–824 (1997). [DOI] [PubMed] [Google Scholar]
- 42. Grace, M. , Birrell, M.A. , Dubuis, E. , Maher, S.A. & Belvisi, M.G. Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax 67, 891–900 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonvini, S.J. et al. Transient receptor potential cation channel, subfamily V, member 4 and airway sensory afferent activation: role of adenosine triphosphate. J. Allergy Clin. Immunol. 138, 249–261 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ternesten‐Hasséus, E. , Johansson, E.‐L. & Millqvist, E. Cough reduction using capsaicin. Respir. Med. 109, 27–37 (2015). [DOI] [PubMed] [Google Scholar]
- 45. Belvisi, M.G. et al. XEN‐D0501, a novel transient receptor potential vanilloid 1 antagonist, does not reduce cough in patients with refractory cough. Am. J. Respir. Crit. Care Med. 196, 1255–1263 (2017). [DOI] [PubMed] [Google Scholar]
- 46. Khalid, S. et al. Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double‐blind randomized controlled trial. J. Allergy Clin. Immunol. 134, 56–62 (2014). [DOI] [PubMed] [Google Scholar]
- 47. Chaiyasit, K. , Khovidhunkit, W. & Wittayalertpanya, S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J. Med. Assoc. Thai. 92, 108–113 (2009). [PubMed] [Google Scholar]
- 48. Birrell, M.A. et al. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am. J. Respir. Crit. Care Med. 180, 1042–1047 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mukhopadhyay, I. et al. Transient receptor potential ankyrin 1 receptor activation in vitro and in vivo by pro‐tussive agents: GRC 17536 as a promising anti‐tussive therapeutic. PLOS One 9, e97005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chung, K.F. Advances in mechanisms and management of chronic cough: the Ninth London International Cough Symposium 2016. Pulm. Pharmacol. Ther. 47, 2–8 (2017). [DOI] [PubMed] [Google Scholar]
- 51. Baxter, M. et al. Role of transient receptor potential and pannexin channels in cigarette smoke‐triggered ATP release in the lung. Thorax 69, 1080–1089 (2014). [DOI] [PubMed] [Google Scholar]
- 52. Ludbrook, V.J. et al. A placebo‐controlled, double‐blind, randomised, crossover study to assess the efficacy, safety, and tolerability of TRPV4 inhibitor GSK2798745 in participants with chronic cough. Thorax 74, A33 (2019). [Google Scholar]
- 53. Plevkova, J. et al. The role of trigeminal nasal TRPM8‐expressing afferent neurons in the antitussive effects of menthol. J. Appl. Physiol. 115, 268–274 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Millqvist, E. , Ternesten‐Hasséus, E. & Bende, M. Inhalation of menthol reduces capsaicin cough sensitivity and influences inspiratory flows in chronic cough. Respir. Med. 107, 433–438 (2013). [DOI] [PubMed] [Google Scholar]
- 55. Morice, A.H. , Marshall, A.E. , Higgins, K.S. & Grattan, T.J. Effect of inhaled menthol on citric acid induced cough in normal subjects. Thorax 49, 1024–1026 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gever, J.R. , Cockayne, D.A. , Dillon, M.P. , Burnstock, G. & Ford, A.P.D.W. Pharmacology of P2X channels. Pflugers Arch. 452, 513–537 (2006). [DOI] [PubMed] [Google Scholar]
- 57. Kwong, K. , Kollarik, M. , Nassenstein, C. , Ru, F. & Undem, B.J. P2X2 receptors differentiate placodal vs. neural crest C‐fiber phenotypes innervating guinea pig lungs and esophagus. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L858–L865 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Burnstock, G. , Brouns, I. , Adriaensen, D. & Timmermans, J.‐P. Purinergic signaling in the airways. Pharmacol. Rev. 64, 834–868 (2012). [DOI] [PubMed] [Google Scholar]
- 59. Makarenkova, H.P. , Shah, S.B. & Shestopalov, V.I. The two faces of pannexins: new roles in inflammation and repair. J. Inflamm. Res. 11, 273–288 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Idzko, M. et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat. Med. 13, 913–919 (2007). [DOI] [PubMed] [Google Scholar]
- 61. Kouzaki, H. , Iijima, K. , Kobayashi, T. , O'Grady, S.M. & Kita, H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL‐33 release and innate Th2‐type responses. J. Immunol. 186, 4375–4387 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Garceau, D. & Chauret, N. BLU‐5937: a selective P2X3 antagonist with potent anti‐tussive effect and no taste alteration. Pulm. Pharmacol. Ther. 56, 56–62 (2019). [DOI] [PubMed] [Google Scholar]
- 63. Belvisi, M.G. & Smith, J.A. ATP and cough reflex hypersensitivity: a confusion of goals? Eur. Respir. J. 50, 1700579 (2017). [DOI] [PubMed] [Google Scholar]
- 64. Richards, D. , Gever, J.R. , Ford, A.P. & Fountain, S.J. Action of MK‐7264 (gefapixant) at human P2X3 and P2X2/3 receptors and in vivo efficacy in models of sensitisation. Br. J. Pharmacol. 176, 2279–2291 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Abdulqawi, R. et al. P2X3 receptor antagonist (AF‐219) in refractory chronic cough: a randomised, double‐blind, placebo‐controlled phase 2 study. Lancet 385, 1198–1205 (2015). [DOI] [PubMed] [Google Scholar]
- 66. Smith, J.A. et al. Gefapixant in two randomised dose‐escalation studies in chronic cough. Eur. Respir. J. 55, 1901615 (2020). [DOI] [PubMed] [Google Scholar]
- 67. Smith, J.A. et al. Gefapixant, a P2X3 receptor antagonist, for the treatment of refractory or unexplained chronic cough: a randomised, double‐blind, controlled, parallel‐group, phase 2b trial. Lancet Respir. Med. 8, 775–785 (2020). [DOI] [PubMed] [Google Scholar]
- 68. Finger, T.E. et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310, 1495–1499 (2005). [DOI] [PubMed] [Google Scholar]
- 69. Garceau, D. , Chauret, N. & Harvey, L. BLU‐5937 a highly selective P2X3 homotrimeric receptor antagonist with improved taste safety profile in healthy subjects. Am. J. Respir. Crit. Care Med. 199, A7396 (2019). [Google Scholar]
- 70. Niimi, A. , Ishihara, H. , Hida, H. & Miyazaki, S. Late breaking abstract — phase 2a randomised, double‐blind, placebo‐controlled, crossover study of a novel P2X3 receptor antagonist S‐600918 in patients with refractory chronic cough. Eur. Respir. J. 54, RCT452 (2019). [Google Scholar]
- 71. Morice, A.H. et al. Safety and efficacy of BAY 1817080, a P2X3 receptor antagonist, in patients with refractory chronic cough (RCC). Am. J. Respir. Crit. Care Med. 201, A7648 (2020). [Google Scholar]
- 72. Muroi, Y. & Undem, B.J. Targeting voltage gated sodium channels NaV1.7, Na V1.8, and Na V1.9 for treatment of pathological cough. Lung 192, 15–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Muroi, Y. , Ru, F. , Chou, Y.‐L. , Carr, M.J. , Undem, B.J. & Canning, B.J. Selective inhibition of vagal afferent nerve pathways regulating cough using Nav 1.7 shRNA silencing in guinea pig nodose ganglia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R1017–R1023 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Muroi, Y. et al. Selective silencing of Na(V)1.7 decreases excitability and conduction in vagal sensory neurons. J. Physiol. 589, 5663–5676 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lim, K.G. , Rank, M.A. , Hahn, P.Y. , Keogh, K.A. , Morgenthaler, T.I. & Olson, E.J. Long‐term safety of nebulized lidocaine for adults with difficult‐to‐control chronic cough: a case series. Chest 143, 1060–1065 (2013). [DOI] [PubMed] [Google Scholar]
- 76. Smith, J.A. et al. Effects of a novel sodium channel blocker, GSK2339345, in patients with refractory chronic cough. Int. J. Clin. Pharmacol. Ther. 55, 712–719 (2017). [DOI] [PubMed] [Google Scholar]
- 77. Ryan, N.M. A review on the efficacy and safety of gabapentin in the treatment of chronic cough. Expert Opin. Pharmacother. 16, 135–145 (2015). [DOI] [PubMed] [Google Scholar]
- 78. Patel, R. & Dickenson, A.H. Mechanisms of the gabapentinoids and alpha 2 delta‐1 calcium channel subunit in neuropathic pain. Pharmacol. Res. Perspect. 4, e00205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ryan, N.M. , Birring, S.S. & Gibson, P.G. Gabapentin for refractory chronic cough: a randomised, double‐blind, placebo‐controlled trial. Lancet 380, 1583–1589 (2012). [DOI] [PubMed] [Google Scholar]
- 80. Shi, G. , Shen, Q. , Zhang, C. , Ma, J. , Mohammed, A. & Zhao, H. Efficacy and safety of gabapentin in the treatment of chronic cough: a systematic review. Tuberc. Respir. Dis. (Seoul.) 81, 167–174 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Al‐Sheklly, B. , Badri, H. , Satia, I. , Woodcock, A. & Smith, J.A. The use of gabapentin and pregabalin for the management of chronic cough in a tertiary cough clinic. Thorax 72, A140–A142 (2017). [Google Scholar]
- 82. Vertigan, A.E. , Kapela, S.L. , Ryan, N.M. , Birring, S.S. , McElduff, P. & Gibson, P.G. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest 149, 639–648 (2016). [DOI] [PubMed] [Google Scholar]
- 83. Gillman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 151, 737–748 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ryan, M.A. & Cohen, S.M. Long‐term follow‐up of amitriptyline treatment for idiopathic cough. Laryngoscope 126, 2758–2763 (2016). [DOI] [PubMed] [Google Scholar]
- 85. Bowen, A.J. et al. Short‐ and long‐term effects of neuromodulators for unexplained chronic cough. Otolaryngol. Head Neck Surg. 159, 508–515 (2018). [DOI] [PubMed] [Google Scholar]
- 86. Bowen, A.J. et al. Tachyphylaxis and dependence in pharmacotherapy for unexplained chronic cough. Otolaryngol. Head Neck Surg. 159, 705–711 (2018). [DOI] [PubMed] [Google Scholar]
- 87. Takahama, K. & Shirasaki, T. Central and peripheral mechanisms of narcotic antitussives: codeine‐sensitive and ‐resistant coughs. Cough 3, 8 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kamei, J. , Iwamoto, Y. , Suzuki, T. , Misawa, M. , Nagase, H. & Kasuya, Y. The role of the mu 2‐opioid receptor in the antitussive effect of morphine in mu 1‐opioid receptor‐deficient CXBK mice. Eur. J. Pharmacol. 240, 99–101 (1993). [DOI] [PubMed] [Google Scholar]
- 89. Karlsson, J.A. , Lanner, A.S. & Persson, C.G. Airway opioid receptors mediate inhibition of cough and reflex bronchoconstriction in guinea pigs. J. Pharmacol. Exp. Ther. 252, 863–868 (1990). [PubMed] [Google Scholar]
- 90. Morice, A.H. et al. Opiate therapy in chronic cough. Am. J. Respir. Crit. Care Med. 175, 312–315 (2007). [DOI] [PubMed] [Google Scholar]
- 91. Al‐Sheklly, B. et al. Randomised control trial quantifying the efficacy of low dose morphine in a responder group of patients with refractory chronic cough. Thorax 72, A24–A25 (2017). [Google Scholar]
- 92. Morice, A.H. , Lowry, R. , Brown, M.J. & Higenbottam, T. Angiotensin‐converting enzyme and the cough reflex. Lancet. 2, 1116–1118 (1987). [DOI] [PubMed] [Google Scholar]
- 93. El‐Hashim, A.Z. , Wyss, D. & Lewis, C. Effect of a novel NK1 receptor selective antagonist (NKP608) on citric acid induced cough and airway obstruction. Pulm. Pharmacol. Ther. 17, 11–18 (2004). [DOI] [PubMed] [Google Scholar]
- 94. Bolser, D.C. , DeGennaro, F.C. , O'Reilly, S. , McLeod, R.L. & Hey, J.A. Central antitussive activity of the NK1 and NK2 tachykinin receptor antagonists, CP‐99,994 and SR 48968, in the guinea‐pig and cat. Br. J. Pharmacol. 121, 165–170 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Smith, J. et al. The neurokinin‐1 receptor antagonist orvepitant is a novel antitussive therapy for chronic refractory cough: results from a phase 2 pilot study (VOLCANO‐1). Chest 157, 111–118 (2020). [DOI] [PubMed] [Google Scholar]
- 96. Xu, J. , Yang, W. , Zhang, G. , Gu, Q. & Lee, L.‐Y. Calcium transient evoked by nicotine in isolated rat vagal pulmonary sensory neurons. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L54–L61 (2007). [DOI] [PubMed] [Google Scholar]
- 97. Davenport, P.W. , Vovk, A. , Duke, R.K. , Bolser, D.C. & Robertson, E. The urge‐to‐cough and cough motor response modulation by the central effects of nicotine. Pulm. Pharmacol. Ther. 22, 82–89 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dicpinigaitis, P. et al. The antitussive effects of alpha7 (α7) nicotinic receptor agonists. Eur. Respir. J. 50, OA4409 (2017). [Google Scholar]
- 99. Smith, J.A. , Hilton, E.C.Y. , Saulsberry, L. & Canning, B.J. Antitussive effects of memantine in guinea pigs. Chest 141, 996–1002 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dicpinigaitis, P.V. , Canning, B.J. , Garner, R. & Paterson, B. Effect of memantine on cough reflex sensitivity: translational studies in guinea pigs and humans. J. Pharmacol. Exp. Ther. 352, 448–454 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Abdulqwai, R. , Dockry, R. , Kimberley, H. , Sen, S. , Hilton, E. & Smith, J. An open‐label pilot study of memantine, an N‐methyl‐D‐aspartate receptor antagonist in chronic cough. Eur. Respir. J. 44, P949 (2014). [Google Scholar]
- 102. Birring, S.S. et al. A novel formulation of inhaled sodium cromoglicate (PA101) in idiopathic pulmonary fibrosis and chronic cough: a randomised, double‐blind, proof‐of‐concept, phase 2 trial. Lancet Respir. Med. 5, 806–815 (2017). [DOI] [PubMed] [Google Scholar]
- 103. Mazzone, S.B. , Chung, K.F. & McGarvey, L. The heterogeneity of chronic cough: a case for endotypes of cough hypersensitivity. Lancet Respir. Med. 6, 636–646 (2018). [DOI] [PubMed] [Google Scholar]
- 104. Cho, P.S.P. , Birring, S.S. , Fletcher, H.V. & Turner, R.D. Methods of cough assessment. J. Allergy Clin. Immunol. Pract. 7, 1715–1723 (2019). [DOI] [PubMed] [Google Scholar]
- 105. McGarvey, L. et al. New understanding in the treatment of cough (NEUROCOUGH) ERS Clinical Research Collaboration: improving care and treatment for patients with cough. Eur. Respir. J. 53, 1900787 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


