Abstract
Background
Non‐bullous pemphigoid (NBP) is a pemphigoid variant which frequently resembles other pruritic skin diseases. In contrast with bullous pemphigoid (BP), blisters are absent. In BP, previous studies showed that IgE autoantibodies may be involved in its pathogenesis. IgE‐activated mast cells, basophils and eosinophils may participate in BP by inducing pruritus and possibly blister formation, although the differential role of IgE in NBP compared with BP has not yet been described.
Objective
To assess IgE in serum and skin of NBP and BP patients.
Methods
We examined total IgE and pemphigoid‐specific IgE in the serum of 68 NBP and 50 BP patients by enzyme‐linked immunosorbent assay (ELISA). Sera of 25 pemphigus patients and 25 elderly patients with pruritus were included as controls. Skin biopsies of 14 NBP and 14 BP patients with the highest IgE titres to NC16A were stained for IgE by immunofluorescence techniques.
Results
Total IgE was elevated in 63% of NBP and 60% of BP patients, and in 20% of pemphigus controls, as well as 60% of elderly controls. IgE ELISAs were more frequently positive in BP than in NBP (NC16A 18% vs. 9%, P = 0.139; BP230 34% vs. 22%, P = 0.149). IgE ELISAs for NC16A and BP230 were positive in 8% and 20% of elderly controls, respectively, while all pemphigus controls were negative. Two of 28 biopsies (7%; one NBP, one BP) showed linear IgE along the basement membrane zone, while in most biopsies (71% NBP; 86% BP) IgE was bound to dermal cells.
Conclusion
Since IgE was present in the serum and skin of both NBP and BP patients, this supports IgE‐dependent mechanisms common to both diseases, such as pruritus. However, it remains to be elucidated whether IgE contributes to blister formation in BP.
Short abstract
Linked Commentary: I. Kortekaas Krohn. J Eur Acad Dermatol Venereol 2021; 35: 781–782. https://doi.org/10.1111/jdv.17164.
Introduction
Bullous pemphigoid (BP) is the most common autoimmune bullous disease, mainly affecting elderly patients aged over 70. 1 , 2 Most patients present with severe pruritus and skin blisters. 1 However, one in five patients lacks blisters, a subtype termed non‐BP (NBP). 3 , 4 , 5 While BP is well characterized and well studied, comparatively little is known about NBP.
The pathogenesis of BP involves IgG autoantibodies targeting hemidesmosome proteins BP180 and BP230. 6 BP230 (also BP antigen 1) is a 230 kDa intracellular protein. 7 BP180 (also BP antigen 2, or type XVII collagen) is a transmembrane protein of 180 kDa. 7 The extracellular non‐collagenous 16A (NC16A) domain of BP180 contains immunodominant epitopes, and anti‐NC16A IgG antibodies correlate with disease activity. 7 , 8 , 9 Interestingly, IgE autoantibodies targeting pemphigoid antigens were demonstrated in serum and skin of BP patients as well. 8
IgE is a key mediator of allergic responses by inducing degranulation of mast cells and basophils through crosslinking of IgEs bound to high‐affinity IgE receptors (FcɛRI). 10 Elevated total IgE levels in BP patients (70%) were first reported in 1974. 11 Circulating IgE antibodies to BP180 were observed in 30–77% of BP patients and correlated with disease activity in several studies. 8 , 12 , 13 , 14 , 15 , 16 , 17 , 18 IgE antibodies to BP230, though less extensively studied, were detected in 22–67% of BP patients. 15 , 16 , 19 , 20 In the skin, linear IgE deposits were reported along the basement membrane zone (BMZ) 21 , 22 , 23 , 24 , whereas other reports detected IgE bound to eosinophils and mast cells in the dermis. 18 , 25 Recently, Ben Mordehai et al. 26 assessed total IgE levels in NBP and reported significantly higher levels in the serum of NBP compared to BP patients. However, pemphigoid‐specific IgE levels and skin biopsies were not assessed.
Omalizumab, a monoclonal antibody targeting IgE, has been used therapeutically in several BP patients. 27 , 28 A meta‐analysis found complete responses in approximately 84% of patients, with decreased pruritus and blister development. 28 These results imply an important role for IgE in the disease mechanism of BP, and potentially in blister formation.
The aim of this study was to assess whether IgE is implicated in blister formation, by comparing the presence of IgE antibodies in the serum and skin of patients with NBP and BP.
Materials and methods
Selection of patients and controls
Patients diagnosed with pemphigoid at our Center for Blistering Diseases were retrospectively selected. Patients were diagnosed with NBP or BP if two of the following three diagnostic criteria were met: (i) compatible clinical features of pruritus and/or cutaneous blisters, (ii) positive linear IgG or C3c staining by direct immunofluorescence (DIF) microscopy and (iii) positive IgG staining on the epidermal side of salt‐split skin by in DIF microscopy. 3
Sixty‐eight NBP patients were included, of whom clinical, diagnostic and prognostic features have been published separately. 5 Fifty consecutive BP patients diagnosed between 2014 and 2015 were selected retrospectively for comparison. Leftover diagnostic material was used, consisting of serum samples taken for immunoserology and biopsies taken for DIF microscopy, both stored at minus 80 degrees Celsius. Patient characteristics, and data concerning peripheral eosinophilia, and eosinophils in lesional haematoxylin–eosin stained skin biopsies were noted if available.
Control sera included 25 consecutive pemphigus patients with high levels of autoantibodies to desmoglein 1 and/or 3, and 25 consecutive elderly patients with pruritus who tested negative for pemphigoid. The study was approved by the local Ethics Committee (University Medical Center Groningen, the Netherlands).
Laboratory tests
Enzyme‐linked immunosorbent assay
IgE antibodies against BP180 and BP230 were measured by enzyme‐linked immunosorbent assay (ELISA) using NC16A and BP230 coated plates from MBL (Nagoya, Japan). Optimization was performed by testing serial dilutions of serum (1 : 25, 1 : 50, 1 : 100) and secondary antibody (1 : 125, 1 : 250, 1 : 500). Samples were diluted 1 : 25 with phosphate‐buffered saline (PBS) with 0.05% Tween‐20 and 0.5% bovine serum albumin, and incubated for 1 h at room temperature. Anti‐human IgE‐horseradish peroxidase (Acris, Herford, Germany) was diluted 1 : 250 and used as conjugate with 1 h incubation time. A substrate was added for 30 min after which the enzyme reaction was stopped. Optical density values were measured with a spectrophotometer, set to 450 nm.
IgG cross‐reactivity was tested by protein G column chromatography, running a patients serum with high titres for anti‐NC16A IgE and IgG through a protein G column. The IgG‐depleted flow‐through and IgG‐rich fraction were both collected. The flow‐through showed a complete negative result on the IgG ELISA and remained equally positive for IgE. Conversely, the IgG‐rich fraction was positive by IgG ELISA, and completely negative for IgE, showing no cross‐reactivity.
Previous studies with similar methodologies reported that antigen‐specific IgE antibodies in sera of BP and epidermolysis bullosa acquisita could be effectively measured without preadsorption of IgG, and showed no significant difference in OD values between pre‐ and postadsorption of IgG. 17 , 29
The diagnostic accuracy of the novel IgE ELISA was examined by receiver operating characteristic curve analysis. The area under the curve for the anti‐NC16A IgE ELISA was 0.507, and 0.553 for the anti‐BP230 IgE ELISA, indicating that they were unsuitable as diagnostic tests for pemphigoid. The ELISA cut‐off values were set on the mean plus three times the standard deviation of sera of the pemphigus control population (A450 0.620 for anti‐NC16A ELISA; 0.929 for anti‐BP230 ELISA).
Serum total IgE was measured by Phadia immunoCap technology (cut‐off value ≥115 kU/L).
Immunofluorescent IgE staining of skin biopsies
Skin biopsies taken for DIF microscopy of 14 NBP and 14 BP patients with the highest anti‐NC16A IgE titres were additionally stained for IgE. According to our standard protocol for DIF microscopy, biopsies were kept in saline overnight at room temperature before storage at minus 80 degrees Celsius.
We used polyclonal rabbit anti‐human IgE, ɛ‐chain specific (Dako, Glostrup, Denmark) diluted 1 : 200 with PBS containing 1% ovalbumin, and incubated for 30 min. Slides were washed for 15 min with PBS. In a second step, polyclonal donkey anti‐rabbit IgG was used with a fluorescein isothiocyanate label (Jackson, Ely, UK) diluted 1 : 100 with PBS containing 1% ovalbumin and incubated for 30 min. Biopsies were washed for 15 min with PBS and viewed independently by two authors (AL and GD). Any discrepancies between ratings were discussed.
To study IgE staining patterns in a larger sample of control skin, diagnostic biopsies received by our immunodermatology laboratory of patients without an autoimmune blistering disease were additionally stained for IgE during a period of 3 months.
Statistical analysis
Non‐normally distributed data are presented as median with interquartile range (IQR). Correlations between binary data were analysed by the chi‐squared test, or the Fisher's exact test when required. The Mann–Whitney U‐test was used for comparing non‐normally distributed data. A P‐value < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics 23 software (IBM, Armonk, NY, USA).
Results
Patient characteristics are described in Table 1. The median age of pemphigoid patients and elderly controls corresponded (79 years, IQR = 14 vs. 76 years, IQR = 10).
Table 1.
Patient characteristics and results of IgE ELISA in pemphigoid patients and controls
| Study group | Control group | ||||
|---|---|---|---|---|---|
|
NBP n = 68 |
BP n = 50 |
P‐value NBP vs. BP |
Pemphigus disease n = 25 |
Elderly subjects with pruritus n = 25 |
|
| Median age, years (IQR) | 79.0 (16) | 78.0 (14) | 0.794 | 60.0 (29) | 76.0 (10) |
| Gender | |||||
| Male, n (%) | 29 (42) | 15 (30) | 0.160 | 17 (68) | 12 (48) |
| Female, n (%) | 40 (58) | 35 (70) | 8 (32) | 13 (52) | |
| Eosinophilia, n (%) (>0.40 109/L) | 26 of 58 (44.8) | 10 of 27 (37.0) | 0.499 | 1 of 13 (7.7) | 6 of 10 (60) |
| Lesional histopathology biopsy | 37 of 54 (68.5) | 18 of 20 (90.0) | |||
| Eosinophils in dermal infiltrate, n (%) | 3 of 54 (5.6) | 6 of 20 (30.0) | 0.123 | ||
| Eosinophilic spongiosis, n (%) | 0.014 | ||||
| DIF positive for linear IgG and/or C3c along the BMZ, n (%) | 40 of 67 (59.7) | 44 (88.0) | 0.001 | 0 | 0 |
| IIF on SSS, positive for IgG (roof), n (%) | 45 (66.2) | 44 (88.0) | 0.007 | 0 | 0 |
| IgG ELISA | |||||
| Positive NC16A IgG ELISA, n (%) | 21 (30.9) | 33 (66) | <0.001 | 0 | 1 of 18 (4) |
| Positive BP230 IgG ELISA, n (%) | 30 (46.9) | 27 (54) | 0.450 | 0 | 1 of 9 (4) |
| If positive, ELISA titre NC16A, median (IQR) | 32.0 (40) | 65.0 (94) | 0.016 | 21 | |
| If positive, ELISA titre BP230, median (IQR) | 29.50 (28) | 51.0 (44) | 0.067 | 15 | |
| Study results | |||||
| Elevated total IgE, (≥115 kU/L) | 42 (62.7) | 28 (59.6) | 0.737 | 5 (20) | 15 (60) |
| Median total IgE (IQR), kU/L | 243 (970.1) | 164 (554.8) | 0.350 | 40 (83.2) | 137 (300.7) |
| IgE ELISA | |||||
| OD value anti‐NC16A IgE, median (IQR) | 213.0 (120) | 354.5 (225) | <0.001 | 288 (97) | 294 (70) |
| OD value anti‐BP230 IgE, median (IQR) | 402.5 (567) | 559.5 (611) | <0.001 | 475 (203) | 545 (272) |
| Positive NC16A IgE ELISA†, n (%) | 6 (8.8) | 9 (18.0) | 0.139 | 0 | 2 (8) |
| Positive BP230 IgE ELISA†, n (%) | 15 (22.1) | 17 (34.0) | 0.149 | 0 | 5 (20) |
| Positive NC16A and BP230 IgE ELISA, n (%) | 2 (2.9) | 3 (6.0) | 0.649 | 0 | 0 |
Bold values were statistically signifcant (P<0.05).
BMZ, basement membrane zone; DIF, direct immunofluorescent microscopy; ELISA, enzyme‐linked immunosorbent assay; IIF, indirect immunofluorescent microscopy; IQR, interquartile range; kU, kilo unit; OD, optical density; SSS, salt‐split skin.
Cut‐off 620 (3xSD pemphigus). ‡Cut‐off 929 (3xSD pemphigus).
IgE in serum
Total and specific IgE against NC16A and BP230
Total IgE and IgE ELISA results are shown in Table 1. Pemphigoid‐specific IgE autoantibodies were more commonly detected in BP (NC16A 18%, BP230 34%) than in NBP (NC16A 9%, BP230 22%); however, no significant difference was found (P = 0.139 and P = 0.149, respectively). Overall, median IgE ELISA titres were significantly higher in BP compared with NBP (P < 0.001).
In general, elevated total IgE was associated with specific IgE to NC16A (P = 0.004) and BP230 (P < 0.001). In NBP, elevated total IgE levels were associated with specific IgE to BP230 (P < 0.001) but not to NC16A (P = 0.339). Conversely, in BP a strong association was found between elevated total IgE and specific IgE to NC16A (P = 0.007), but not to BP230 (P = 0.058). In elderly controls with pruritus, no associations were observed between either elevated total IgE levels or specific IgE to NC16A or BP230 (P = 1.000 and P = 0.061).
Associations between serum IgE and IgG autoantibodies
Specific IgE autoantibodies to NC16A did not correlate with IgG autoantibodies to NC16A in serum of NBP and BP patients (P = 0.363 and P = 0.141). However, when analysing pemphigoid as one group, we did find an association between specific IgE and IgG autoantibodies to NC16A (P = 0.009). Specific IgE antibodies to BP230 were strongly associated with IgG antibodies to BP230 in both NBP and BP patients (P < 0.001 and P = 0.001), and in pemphigoid patients as one group (P < 0.001).
Specific IgE autoantibodies to NC16A were not associated with linear IgG along the BMZ by DIF in NBP and BP (P = 1.000; P = 1.000). Specific IgE antibodies to BP230 were associated with a negative DIF result in NBP (P = 0.013), but not in BP (P = 0.162).
IgE in the skin
Biopsies for DIF microscopy of 14 patients of each pemphigoid phenotype were stained for IgE. NBP biopsies were taken from lesional (n = 6), perilesional (n = 6) and healthy skin (n = 1), and from an unknown location in one patient. BP biopsies were taken from perilesional (n = 9), lesional (n = 2) or healthy skin (n = 2), and from the buccal mucosa in one patient.
IgE on the surface of cells in the dermis
IgE in skin was mainly observed on the surface of cells in the upper dermis (NBP 71% and BP 86%; Figs 1a–c and 2a–c; Table 2). Two of 14 BP biopsies lacked IgE‐expressing cells: one taken from buccal mucosa and one from healthy skin. Four of 14 NBP biopsies showed no IgE‐expressing cells: three taken from perilesional skin and one from healthy skin. The latter NBP healthy skin biopsy did, however, show linear IgE staining along the BMZ.
Figure 1.
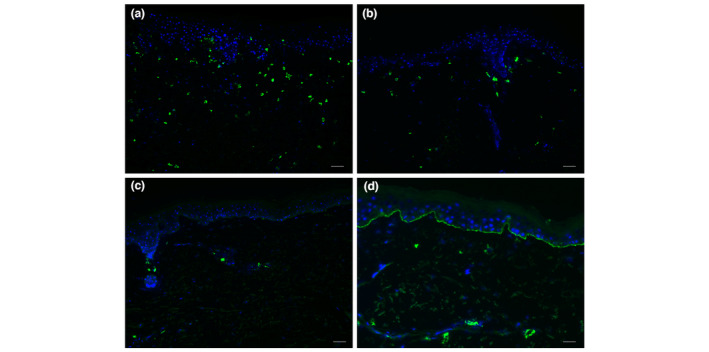
Immunofluorescent staining of IgE in the skin of bullous pemphigoid (BP) patients. (a) Many IgE‐positive cells in the dermis (++), representative for five of 14 BP skin samples. (b) Multiple IgE‐positive cells in the dermis (+), representative for one of 14 BP skin samples. (c) Few IgE‐positive cells in the dermis (+/−), representative for six of 14 BP skin samples. (d) One BP skin sample showed linear IgE along the basement membrane zone, while 13 other skin samples did not. White bar is 50 μm.
Figure 2.
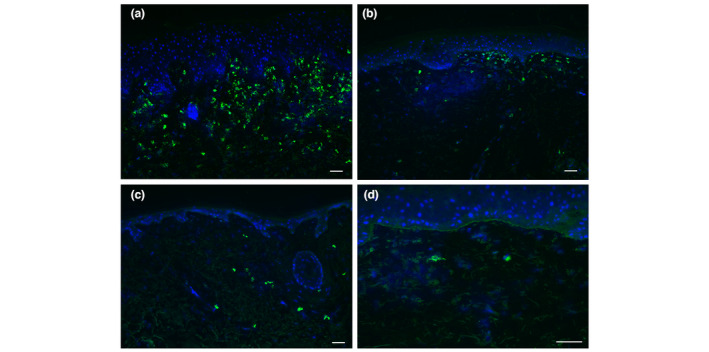
Immunofluorescent staining of IgE in the skin of non‐bullous pemphigoid (NBP) patients. (a) Many IgE‐positive cells in the dermis (++), representative for two of 14 NBP skin samples. (b) Multiple IgE‐positive cells in the dermis (+), representative for four of 14 NBP skin samples. (c) Few IgE‐positive cells in the dermis (+/−), representative for four of 14 NBP skin samples. (d) One BP skin sample showed linear IgE along the basement membrane zone, while 13 other skin samples did not. White bar is 50 μm.
Table 2.
IgE in the skin of non‐bullous and bullous pemphigoid
| Immunofluorescence findings | Non‐bullous pemphigoid (n = 14) | Bullous pemphigoid (n = 14) |
|---|---|---|
| n (%) | n (%) | |
| IgE deposits linear along the BMZ | 1 (7.1) | 1 (7.1) |
| Positive, ++ | 0 (0) | 1 (7.1) |
| Positive, + | 1 (7.1) | 0 (0) |
| Negative | 12 (85.7) | 12 (85.7) |
| IgE on cells in the dermis | 10 (71.4) | 12 (85.7) |
| Positive, ++ many cells | 2 (14.3) | 5 (35.7) |
| Positive, + multiple cells | 4 (28.6) | 1 (7.1) |
| Positive, +/− only few cells | 4 (28.6) | 6 (42.9) |
| Negative | 4 (28.6) | 2 (14.3) |
BMZ, basement membrane zone.
The presence of IgE on cells was not associated with circulating specific IgE antibodies to NC16A and BP230 (P = 0.648 and P = 1.000). However, the cell‐surface expression of IgE did correlate with circulating IgG antibodies to NC16A (P = 0.038), and with positive linear IgG staining along the BMZ (P = 0.021) by DIF microscopy. No correlation was found with circulating IgG antibodies to BP230 (P = 0.673).
In BP patients, median total IgE levels were significantly higher in patients with IgE‐expressing cells in the upper dermis (659 vs. 87; P = 0.048). Such a correlation was not found in NBP patients (P = 0.777). No significant differences in median titres of specific IgE to NC16A and BP230 were observed in pemphigoid patients with or without IgE‐positive cells present in the skin.
Linear IgE along the basement membrane zone
In total, two biopsies (7%, 1 NBP and 1 BP) showed IgE deposits along the BMZ (Figs 1d and 2d; Table 2). Both biopsies with linear IgE staining were taken from healthy skin. The NBP patient had no specific IgE against NC16A and BP230, but showed IgG antibodies to NC16A. The BP patient was positive for IgE and IgG antibodies to BP230, but not to NC16A. Both patients had linear IgG along the BMZ by DIF microscopy.
Additional IgE staining of diagnostic skin biopsies
In total, 122 biopsies of subjects suspected of autoimmune blistering diseases, but with negative routine IgG diagnostic pemphigoid tests, were stained for IgE. None of the biopsies showed IgE in a linear pattern along the BMZ. Binding of IgE on a few cells was seen occasionally, but less frequently and extensively as in either BP or NBP biopsies.
Eosinophils and IgE in serum and histopathologic skin biopsies
Data on eosinophils in serum and lesional histopathologic skin biopsies of pemphigoid patients are shown in Table 1.
Peripheral eosinophilia was significantly associated with elevated total IgE levels in NBP (P = 0.028), but not in BP (P = 0.208). No associations were found between peripheral eosinophilia and specific IgE antibodies to NC16A and BP230 (P = 0.516 and P = 0.326). Peripheral eosinophilia was also not associated with IgE on cells (P = 0.594), or IgE in a linear pattern along the BMZ (P = 0.481).
No correlations were found between the presence of eosinophils in histopathologic lesional skin biopsies, and results of total IgE, specific IgE to NC16A/BP230, IgE in the skin on cells in the upper dermis, or IgE in the skin in a linear pattern along the BMZ (data not shown).
Discussion
IgE autoantibodies were clearly present in the serum and skin of both NBP and BP patients. Although not statistically significant, circulating specific IgE to NC16A and BP230 was more often detected in BP (18% and 34%) than in NBP (9% and 22%). In the skin, IgE was most frequently observed on dermal cells (NBP 71% and BP 86%), and only two biopsies (7%; 1 NBP, 1 BP) showed linear IgE depositions along the BMZ. These findings imply that IgE autoantibodies could have indirect effects on blistering by binding to immune cells, likely eosinophils and mast cells, but other factors presumably play a more conclusive role in whether pemphigoid patients form blisters or not.
In contrast with the findings of Ben Mordehai et al., our study did not detect significantly higher total IgE levels in NBP compared with BP serum. 26 Overall, pemphigoid‐specific IgE was more frequently directed to BP230 than to NC16A, in line with several BP studies. 15 , 30 Predominant reactivity of IgE to intracellular BP230 might account for the low occurrence of linear IgE staining by DIF. Hashimoto et al. 17 reported an association between specific IgE to BP230 and a pemphigoid nodularis phenotype. Interestingly, subanalysis of previously published clinical NBP data 5 showed a similar trend, with 47% of the NBP patients with specific IgE to BP230 presenting with papules and nodules (P = 0.121; data not shown). This suggests that specific IgE to BP230 might be pathognomonic for a prurigo nodularis‐like pemphigoid phenotype. In NBP, specific IgE to NC16A or BP230 was not associated with an urticarial phenotype, similar to previous reports on BP. 13 , 17 , 31
Overall, we found a lower percentage of IgE reactivity to NC16A and BP230 by ELISA in BP patients, compared with other studies. 8 , 20 This might be dependent on methodological differences of established IgE assays, such as serum dilutions, or determined cut‐off values. Another explanation could be differences between the BP study cohorts, since the percentage of IgG reactivity to BP180 and BP230 in our consecutive BP patients was lower (66% and 44%, respectively) compared with other studies (90% and 60%, respectively) that might have selected their patients differently. 32
We observed linear IgE along the BMZ in only two of 24 pemphigoid biopsies (7%), while others reported linear IgE in 0%, 3%, 18%, 25% and 44% of BP biopsies, indicating that linear IgE is not commonly observed in pemphigoid skin. 18 , 21 , 22 , 23 , 24 , 25 Differences in methodology and biopsy location may account for the discrepancies between these studies.
Interestingly, IgE on the surface of dermal cells was associated with IgG reactivity to NC16A (82%; P = 0.038), and linear IgG deposits along the BMZ by DIF (86%; P = 0.021), but not with pemphigoid‐specific IgE. Our study did not specify the cell types expressing IgE. However, previous studies identified IgE on mast cells and eosinophils in perilesional BP skin. 18 , 25 It has been hypothesized that IgE could induce mast cell and eosinophil degranulation, releasing proteolytic enzymes matrix metalloprotease‐9 and neutrophil elastase, substances that are known to cleave the extracellular domain of BP180 in vitro. 33 , 34 , 35 This has recently been supported by a murine BP model demonstrating eosinophil‐dependent blister formation following sensitization with NC16A‐specific IgE. 36 Furthermore, since omalizumab (anti‐IgE) therapy reduces both pruritus and blisters, a role for IgE in blister formation is supported. 28 In contrast, our data do not support the hypothesis that IgE plays an essential role in blister formation in pemphigoid, since we observed IgE on cells in the dermis in 71% of NBP patients that clinically lack blisters.
Based on our findings, it is likely that factors other than IgE are more important for blister development. Several studies designate eosinophils as the most important regulators of blister formation, since activated eosinophils were shown to accumulate in serum, skin and blister fluids of BP, and were able to induce dermal–epidermal separation upon activation by IL‐5. 37 , 38 , 39 Beside FcɛRI, eosinophils also express receptors for complement anaphylatoxins C3a and C5a. 40 , 41 , 42 These complement anaphylatoxins not only orchestrate eosinophil migration, but were also capable to evoke eosinophil degranulation. 42 Previous studies demonstrated that complement activation might be important for blister formation in BP. 43 , 44 , 45 Nonetheless, others found evidence for blister induction via complement‐independent pathways by IgG and IgE antibody induced internalization of the complete BP180 molecule. 46 , 47 , 48 , 49
Surprisingly, a subset of elderly controls with pruritus showed specific IgE antibodies to NC16A and BP230 in the absence of IgG autoantibodies to these antigens. In contrast, none of the pemphigus controls had pemphigoid‐specific IgE. In accordance, Freire et al. 25 reported specific IgE antibodies to BP180 in one‐third of healthy control sera. Previously, IgG autoantibodies to NC16A and BP230 by ELISA were detected in elderly individuals, chronic pruritus patients, healthy controls and dermatology patients, all without pemphigoid. 50 , 51 , 52 , 53 , 54 It has been suggested that repeated cell injury, for example by scratching, could expose new and otherwise hidden antigens to the immune system, a phenomenon termed epitope spreading. 55 Moreover, ageing of the immune system can result in a pro‐inflammatory immune status, increasing the risk of autoimmunity. 56 Both events could conceivably contribute to the development of IgE and IgG autoantibodies in elderly controls with pruritus. Yet, the relevance of these autoantibodies in individuals without pemphigoid remains unknown. Neurodegenerative diseases, such as multiple sclerosis and Parkinson’s disease, may precede BP, and the expression of BP180 and BP230 in both skin and brain tissue suggests that cross‐reactivity could initiate BP. 57 However, further research is necessary to demonstrate whether BP should be seen as a neuro‐cutaneous disease rather than a skin disease.
This study has several limitations, of which the most important is its retrospective nature. Consequently, diagnostic skin samples were taken from different locations by different healthcare professionals. Moreover, our study only assessed IgE targeting NC16A; therefore, no conclusions could be drawn on IgE antibodies to other BP180 epitopes. Previous epitope mapping studies of BP180 IgE in BP sera mainly detected NC16A reactivity; however, reactivity to other extracellular and intracellular domains of BP180 has been reported. 25 , 58 , 59 No epitope mapping data of NBP are available.
In conclusion, IgE in NBP and BP skin was more often found on the surface of immune cells rather than deposited linear along the BMZ. The IgE ELISAs for NC16A and BP230 were slightly more often positive in BP than in NBP patients, however, are unsuitable as a diagnostic pemphigoid test due to low specificity. Our main finding is that IgE autoantibodies are present in serum and skin of both NBP and BP patients, supporting the notion that IgE potentially modulates pruritus associated with these pemphigoid diseases rather than being centrally involved in blister formation per se. Further studies, however, are needed to determine why blisters are absent in NBP, and to understand the exact role of IgE in the disease mechanism of pemphigoid.
Conflict of interest None declared.
Funding source None.
References
- 1. Schmidt E, Zillikens D. Pemphigoid diseases. Lancet (London, England) 2013; 381: 320–332. [DOI] [PubMed] [Google Scholar]
- 2. Hubner F, Recke A, Zillikens D et al. Prevalence and age distribution of pemphigus and pemphigoid diseases in germany. J Invest Dermatol 2016; 136: 2495–2498. [DOI] [PubMed] [Google Scholar]
- 3. Meijer JM, Diercks GFH, de Lang EWG et al. Assessment of diagnostic strategy for early recognition of bullous and nonbullous variants of pemphigoid. JAMA Dermatol 2019; 155: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamberts A, Meijer JM, Jonkman MF. Nonbullous pemphigoid: a systematic review. J Am Acad Dermatol 2018; 78: 989–995.e2. [DOI] [PubMed] [Google Scholar]
- 5. Lamberts A, Meijer JM, Pas HH et al. Nonbullous pemphigoid: insights in clinical and diagnostic findings, treatment responses and prognosis. J Am Acad Dermatol 2019; 81: 355–363. [DOI] [PubMed] [Google Scholar]
- 6. Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Annu Rev Pathol 2016; 11: 175–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goletz S, Zillikens D, Schmidt E. Structural proteins of the dermal‐epidermal junction targeted by autoantibodies in pemphigoid diseases. Exp Dermatol 2017; 26: 1154–1162. [DOI] [PubMed] [Google Scholar]
- 8. Dopp R, Schmidt E, Chimanovitch I et al. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in Bullous pemphigoid: serum levels of these immunoglobulins reflect disease activity. J Am Acad Dermatol 2000; 42: 577–583. [PubMed] [Google Scholar]
- 9. Daneshpazhooh M, Ghiasi M, Lajevardi V et al. BPDAI and ABSIS correlate with serum anti‐BP180 NC16A IgG but not with anti‐BP230 IgG in patients with bullous pemphigoid. Arch Dermatol Res 2018; 310: 255–259. [DOI] [PubMed] [Google Scholar]
- 10. Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol 2008; 8: 205–217. [DOI] [PubMed] [Google Scholar]
- 11. Arbesman CE, Wypych JI, Reisman RE, Beutner EH. IgE levels in sera of patients with pemphigus or bullous pemphigoid. Arch Dermatol 1974; 110: 378–381. [PubMed] [Google Scholar]
- 12. Messingham KAN, Noe MH, Chapman MA et al. A novel ELISA reveals high frequencies of BP180‐specific IgE production in bullous pemphigoid. J Immunol Methods 2009; 346: 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Beek N, Luttmann N, Huebner F et al. Correlation of serum levels of IgE autoantibodies against BP180 with bullous pemphigoid disease activity. JAMA Dermatol 2017; 153: 30–38. [DOI] [PubMed] [Google Scholar]
- 14. Bing L, Xiping Z, Li L et al. Levels of anti‐BP180 NC16A IgE do not correlate with severity of disease in the early stages of bullous pemphigoid. Arch Dermatol Res 2015; 307: 849–854. [DOI] [PubMed] [Google Scholar]
- 15. Ishiura N, Fujimoto M, Watanabe R et al. Serum levels of IgE anti‐BP180 and anti‐BP230 autoantibodies in patients with bullous pemphigoid. J Dermatol Sci 2008; 49: 153–161. [DOI] [PubMed] [Google Scholar]
- 16. Iwata Y, Komura K, Kodera M et al. Correlation of IgE autoantibody to BP180 with a severe form of bullous pemphigoid. Arch Dermatol 2008; 144: 41–48. [DOI] [PubMed] [Google Scholar]
- 17. Hashimoto T, Ohzono A, Teye K et al. Detection of IgE autoantibodies to BP180 and BP230 and their relationship to clinical features in bullous pemphigoid. Br J Dermatol 2017; 177: 141–151. [DOI] [PubMed] [Google Scholar]
- 18. Dimson OG, Giudice GJ, Fu CL et al. Identification of a potential effector function for IgE autoantibodies in the organ‐specific autoimmune disease bullous pemphigoid. J Invest Dermatol 2003; 120: 784–788. [DOI] [PubMed] [Google Scholar]
- 19. Delaporte E, Dubost‐Brama A, Ghohestani R et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol 1996; 157: 3642–3647. [PubMed] [Google Scholar]
- 20. Koga H, Ishii N, Hashimoto T, Nakama T. Case of shift from linear immunoglobulin A bullous dermatosis to pemphigus herpetiformis for a short period of time. J Dermatol 2017; 44: 189–193. [DOI] [PubMed] [Google Scholar]
- 21. Yayli S, Pelivani N, Beltraminelli H et al. Detection of linear IgE deposits in bullous pemphigoid and mucous membrane pemphigoid: a useful clue for diagnosis. Br J Dermatol 2011; 165: 1133–1137. [DOI] [PubMed] [Google Scholar]
- 22. Provost TT, Tomasi TBJ. Immunopathology of bullous pemphigoid. Basement membrane deposition of IgE, alternate pathway components and fibrin. Clin Exp Immunol 1974; 18: 193–200. [PMC free article] [PubMed] [Google Scholar]
- 23. Moriuchi R, Nishie W, Ujiie H et al. In vivo analysis of IgE autoantibodies in bullous pemphigoid: a study of 100 cases. J Dermatol Sci 2015; 78: 21–25. [DOI] [PubMed] [Google Scholar]
- 24. Kamata A, Kurihara Y, Funakoshi T et al. Basement membrane zone IgE deposition is associated with bullous pemphigoid disease severity and treatment results. Br J Dermatol 2019; 182: 1221–1227. [DOI] [PubMed] [Google Scholar]
- 25. Freire PC, Munoz CH, Stingl G. IgE autoreactivity in bullous pemphigoid: eosinophils and mast cells as major targets of pathogenic immune reactants. Br J Dermatol 2017; 177: 1644–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ben Mordehai Y, Faibish H, Astman N et al. Characteristics of patients with bullous pemphigoid: comparison of classic bullous pemphigoid to non‐bullous pemphigoid. J Eur Acad Dermatol Venereol 2019; 34: 161–165. [DOI] [PubMed] [Google Scholar]
- 27. Fairley JA, Baum CL, Brandt DS, Messingham KAN. Pathogenicity of IgE in autoimmunity: successful treatment of bullous pemphigoid with omalizumab. J Allergy Clin Immunol 2009; 123: 704–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kremer N, Snast I, Cohen ES et al. Rituximab and omalizumab for the treatment of bullous pemphigoid: a systematic review of the literature. Am J Clin Dermatol 2018; 20: 209–216. [DOI] [PubMed] [Google Scholar]
- 29. Koga H, Teye K, Yamashita K et al. Detection of anti‐type VII collagen IgE antibodies in epidermolysis bullosa acquisita. Br J Dermatol 2019; 180: 1107–1113. [DOI] [PubMed] [Google Scholar]
- 30. Ghohestani RF, Cozzani E, Delaporte E et al. IgE antibodies in sera from patients with bullous pemphigoid are autoantibodies preferentially directed against the 230‐kDa epidermal antigen (BP230). J Clin Immunol 1998; 18: 202–209. [DOI] [PubMed] [Google Scholar]
- 31. Saniklidou AH, Tighe PJ, Fairclough LC, Todd I. IgE autoantibodies and their association with the disease activity and phenotype in bullous pemphigoid: a systematic review. Arch Dermatol Res 2018; 310: 11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Di Zenzo G, Della Torre R, Zambruno G, Borradori L. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol 2012; 30: 3–16. [DOI] [PubMed] [Google Scholar]
- 33. Verraes S, Hornebeck W, Polette M et al. Respective contribution of neutrophil elastase and matrix metalloproteinase 9 in the degradation of BP180 (type XVII collagen) in human bullous pemphigoid. J Invest Dermatol 2001; 117: 1091–1096. [DOI] [PubMed] [Google Scholar]
- 34. Stahle‐Backdahl M, Inoue M, Guidice GJ, Parks WC. 92‐kD gelatinase is produced by eosinophils at the site of blister formation in bullous pemphigoid and cleaves the extracellular domain of recombinant 180‐kD bullous pemphigoid autoantigen. J Clin Invest 1994; 93: 2022–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amber KT, Valdebran M, Kridin K, Grando SA. The role of eosinophils in bullous pemphigoid: a developing model of eosinophil pathogenicity in mucocutaneous disease. Front Med 2018; 5: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin L, Hwang B‐J, Culton DA et al. Eosinophils mediate tissue injury in the autoimmune skin disease bullous pemphigoid. J Invest Dermatol 2018; 138: 1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Engmann J, Rüdrich U, Behrens G et al. Increased activity and apoptosis of eosinophils in blister fluids, skin and peripheral blood of patients with bullous pemphigoid. Acta Derm Venereol 2017; 97: 464–471. [DOI] [PubMed] [Google Scholar]
- 38. de Graauw E, Sitaru C, Horn M et al. Evidence for a role of eosinophils in blister formation in bullous pemphigoid. Allergy Eur J Allergy Clin Immunol 2017; 72: 1105–1113. [DOI] [PubMed] [Google Scholar]
- 39. Lo Schiavo A, Ruocco E, Brancaccio G et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol 2013; 31: 391–399. [DOI] [PubMed] [Google Scholar]
- 40. Messingham KN, Wang JW, Holahan HM et al. Eosinophil localization to the basement membrane zone is autoantibody‐ and complement‐dependent in a human cryosection model of bullous pemphigoid. Exp Dermatol 2016; 25: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: the role of mast cells in autoimmunity and tolerance. Annu Rev Immunol 2008; 26: 705–739. [DOI] [PubMed] [Google Scholar]
- 42. DiScipio RG, Schraufstatter IU. The role of the complement anaphylatoxins in the recruitment of eosinophils. Int Immunopharmacol 2007; 7: 1909–1923. [DOI] [PubMed] [Google Scholar]
- 43. Nelson KC, Zhao M, Schroeder PR et al. Role of different pathways of the complement cascade in experimental bullous pemphigoid. J Clin Invest 2006; 116: 2892–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heimbach L, Li Z, Berkowitz P et al. The C5a receptor on mast cells is critical for the autoimmune skin‐blistering disease bullous pemphigoid. J Biol Chem 2011; 286: 15003–15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Romeijn TR, Jonkman MF, Knoppers C et al. Complement in bullous pemphigoid: results from a large observational study. Br J Dermatol 2017; 176: 517–519. [DOI] [PubMed] [Google Scholar]
- 46. Messingham KN, Srikantha R, DeGueme AM, Fairley JA. FcR‐independent effects of IgE and IgG autoantibodies in bullous pemphigoid. J Immunol 2011; 187: 553–560. [DOI] [PubMed] [Google Scholar]
- 47. Kitajima Y, Nojiri M, Yamada T et al. Internalization of the 180 kDa bullous pemphigoid antigen as immune complexes in basal keratinocytes: an important early event in blister formation in bullous pemphigoid. Br J Dermatol 1998; 138: 71–76. [DOI] [PubMed] [Google Scholar]
- 48. Iwata H, Kamio N, Aoyama Y et al. IgG from patients with bullous pemphigoid depletes cultured keratinocytes of the 180‐kDa bullous pemphigoid antigen (type XVII collagen) and weakens cell attachment. J Invest Dermatol 2009; 129: 919–926. [DOI] [PubMed] [Google Scholar]
- 49. Ujiie H, Sasaoka T, Izumi K et al. Bullous pemphigoid autoantibodies directly induce blister formation without complement activation. J Immunol 2014; 193: 4415–4428. [DOI] [PubMed] [Google Scholar]
- 50. van Beek N, Dohse A, Riechert F et al. Serum autoantibodies against the dermal‐epidermal junction in patients with chronic pruritic disorders, elderly individuals and blood donors prospectively recruited. Br J Dermatol 2014; 170: 943–947. [DOI] [PubMed] [Google Scholar]
- 51. Meijer JM, Lamberts A, Pas HH, Jonkman MF. Significantly higher prevalence of circulating bullous pemphigoid‐specific IgG autoantibodies in elderly patients with a nonbullous skin disorder. Br J Dermatol 2015; 173: 1274–1276. [DOI] [PubMed] [Google Scholar]
- 52. Rieckhoff‐Cantoni L, Bernard P, Didierjean L et al. Frequency of bullous pemphigoid‐like antibodies as detected by western immunoblot analysis in pruritic dermatoses. Arch Dermatol 1992; 128: 791–794. [PubMed] [Google Scholar]
- 53. Hofmann SC, Tamm K, Hertl M, Borradori L. Diagnostic value of an enzyme‐linked immunosorbent assay using BP180 recombinant proteins in elderly patients with pruritic skin disorders. Br J Dermatol 2003; 149: 910–912. [DOI] [PubMed] [Google Scholar]
- 54. Feliciani C, Caldarola G, Kneisel A et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol 2009; 161: 306–312. [DOI] [PubMed] [Google Scholar]
- 55. Didona D, Di Zenzo G. Humoral epitope spreading in autoimmune bullous diseases. Front Immunol 2018; 9: 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pawelec G. Age and immunity: what is ‘immunosenescence’? Exp Gerontol 2018; 105: 4–9. [DOI] [PubMed] [Google Scholar]
- 57. Försti AK, Jokelainen J, Ansakorpi H et al. Psychiatric and neurological disorders are associated with bullous pemphigoid ‐ A nationwide Finnish Care Register study. Sci Rep 2016; 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dresow SK, Sitaru C, Recke A et al. IgE autoantibodies against the intracellular domain of BP180. Br J Dermatol 2009; 160: 429–432. [DOI] [PubMed] [Google Scholar]
- 59. Fairley JA, Fu CL, Giudice GJ. Mapping the binding sites of anti‐BP180 immunoglobulin E autoantibodies in bullous pemphigoid. J Invest Dermatol 2005; 125: 467–472. [DOI] [PubMed] [Google Scholar]


