Abstract
Background and Aim
Endoscopic resection for early gastric cancer (EGC) is widely performed. However, there is still a paucity of strong evidence regarding long‐term outcomes after endoscopic submucosal dissection (ESD) for the expanded indication criteria of the Japanese guidelines (ver. 2010).
Methods
Endoscopic submucosal dissection was performed in patients with EGC that met the expanded indication criteria: (i) cT1a, differentiated‐type EGC of 2 to 5 cm, ulcer negative or (ii) cT1a, differentiated‐type EGC of ≤3 cm, ulcer positive. Patients whose pathological examination fulfilled the curative resection criteria were then enrolled in this cohort study: negative vertical margin, negative lymphovascular invasion, and (i) pT1a, differentiated‐type, and ulcer negative; (ii) pT1a, differentiated‐type, ≤3 cm, and ulcer positive; or (iii) pT1b1 (<500‐μm submucosal invasion), differentiated‐type, and ≤3 cm. Patients with only a positive horizontal margin as a noncurative factor were included for follow‐up.
Results
From September 2003 to February 2012, a total of 356 patients underwent ESD, and 214 were enrolled in the survival analysis. One hundred twenty patients (56%) had >2 cm in diameter and ulcer‐negative lesions, and 94 (44%) had ≤3 cm and ulcer‐positive lesions. The vital status at 5 years after ESD was confirmed in all (100%) patients. No local or metastatic recurrence was detected; however, 26 metachronous gastric cancers developed, and 1 patient died of metachronous gastric cancer. The 5‐year disease‐specific and overall survival rates were 99.5% (95% confidence interval [CI], 97.2%–100%) and 93.9% (95% CI, 89.8%–96.4%), respectively.
Conclusion
ESD for EGC that fulfills the expanded criteria is feasible and shows favorable long‐term outcomes.
Keywords: Endoscopic submucosal dissection, Gastric cancer, Guideline, Survival
Introduction
Gastric cancer is one of the most common malignancies worldwide; 1 million cases were reported in 2018, as mentioned at GLOBOCAN 2018. 1 For the treatment of early gastric cancer (EGC), both endoscopic mucosal resection 2 and endoscopic submucosal dissection (ESD) enable complete local removal of lesion with less invasiveness than surgery. 3 , 4 , 5 , 6 Therefore, endoscopic resection is indicated for EGC with a negligible risk of lymph node metastasis. 7 In the Japanese Gastric Cancer Treatment Guidelines 2010 (ver. 3), 8 endoscopic resection is absolutely indicated for macroscopically intramucosal (cT1a) differentiated‐type EGC of ≤2 cm in diameter with an ulcer‐negative status. For lesions that meet the criteria of (i) cT1a differentiated‐type EGC of >2 cm, ulcer negative; (ii) cT1a differentiated‐type EGC of ≤3 cm, ulcer positive; and (iii) cT1a undifferentiated‐type EGC of ≤2 cm, ulcer negative, ESD can be performed as an expanded indication (Table 1). After ESD, a resected specimen is histologically examined for the degree of likelihood of cure. If histological findings fulfill the curative criteria (Table 2), the patient is followed up with solely endoscopic treatment; otherwise, the patient undergoes surgery.
Table 1.
Endoscopic indication criteria for endoscopic submucosal dissection in JGCA2010 (ver. 3)
| Indication | Histological type (biopsy) | Depth (endoscopy) | Size (endoscopy) (cm) | Ulcer/Scar (endoscopy) |
|---|---|---|---|---|
| Absolute | Diff | cT1a | ≤2 | cUL0 |
| Expanded | Diff | cT1a | >2 | cUL0 |
| Diff | cT1a | ≤3 | cUL1 | |
| Undiff | cT1a | ≤2 | cUL0 |
Gray cells correspond to patients in this study. cT1a, clinically intramucosal; cUL, clinical finding of ulcer or scar; Diff, differentiated type; Undiff, undifferentiated.
Table 2.
Histological curability criteria for endoscopic submucosal dissection in JGCA2010 (ver. 3)
| Indication | Histological type | Depth (histology) | Size (histology) (cm) | Ulcer/Scar (histology) | HM, VM | ly, v |
|---|---|---|---|---|---|---|
| Absolute | Diff | pT1a | ≤2 | pUL0 | 0 | 0 |
| Expanded | Diff | pT1a | Any | pUL0 | 0 | 0 |
| Diff | pT1a | ≤3 | pUL1 | 0 | 0 | |
| Diff | pT1b1 (<500 μm) | ≤3 | pUL0 or pUL1 | 0 | 0 | |
| Undiff | pT1a | ≤2 | pUL0 | 0 | 0 |
Gray cells correspond to patients in this study. Diff, differentiated; HM, horizontal margin; ly, lymphatic involvement; pT1a, histologically intramucosal; pT1b, histologically submucosal; pUL, histological finding of ulcer or scar; Undiff, undifferentiated; v, venous involvement; VM, vertical margin.
The guideline states that ESD for lesions that fulfill the expanded indication criteria is an investigational treatment and that the standard treatment is surgery because sufficient evidence regarding the prognosis after ESD is lacking. Several reports have focused on the long‐term outcome after gastric ESD, 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 but most such studies were retrospective. One recent multicenter prospective cohort study demonstrated excellent long‐term survival after ESD for patients with expanded indication lesions 15 ; however, there is still a paucity of strong evidence to support the excellent long‐term outcomes of ESD. We herein report the results of our prospective cohort study investigating the long‐term outcome after ESD in patients with EGC that fulfilled the differentiated‐type expanded indication criteria.
Methods
Study design and setting
This was a single‐center prospective cohort study conducted in a cancer referral hospital, Osaka International Cancer Institute (formerly Osaka Medical Center for Cancer and Cardiovascular Diseases), Japan. From September 2003 to February 2012, patients with differentiated‐type EGC that met the expanded indication criteria underwent ESD, and the curability of resection was assessed by histological examination. The patients whose resection fulfilled the curative resection criteria were registered and followed up for 5 years.
Participants
Patients with differentiated‐type EGC that fulfilled the expanded indication criteria were assessed for eligibility for this prospective cohort study if ESD was planned (Fig. 1). The indication criteria of ESD in this study were as follows: (i) cT1a differentiated‐type EGC of 2 to 5 cm in diameter, ulcer negative or (ii) cT1a differentiated‐type EGC of ≤3 cm, ulcer positive. According to a request from our institutional review board, the size of the ulcer‐negative lesions was limited to ≤5 cm because of the unestablished feasibility of ESD for such a large (>5 cm) lesion at the time of protocol submission and the low generalizability of the results. The preoperative diagnosis was based on the endoscopic findings and the histological diagnosis by forceps biopsy. Computed tomography or abdominal ultrasonography was used to evaluate lymph node and distant metastases. Synchronous multiple gastric cancers were defined as lesions existed at the day of ESD, while metachronous gastric cancers were defined as other lesions detected at surveillance esophagogastroduodenoscopy (EGD) at a >2‐month interval. 17
Figure 1.
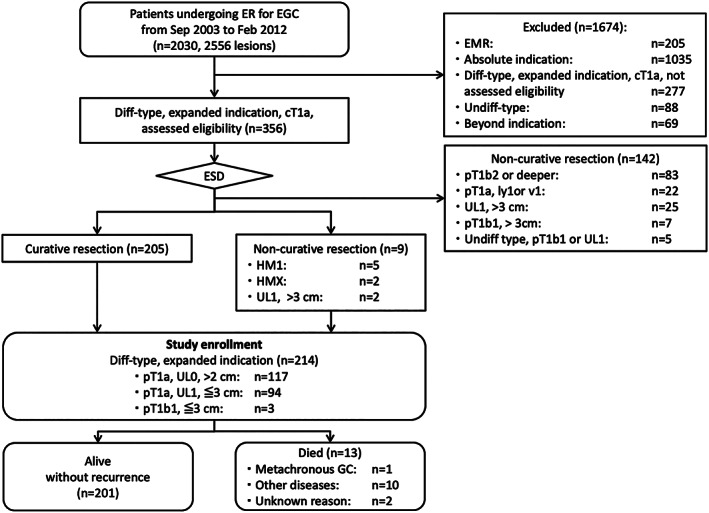
Diagram of patient enrollment and follow‐up. Diff, differentiated; ER, endoscopic resection; EGC, early gastric cancer; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; GC, gastric cancer; HM, horizontal margin; pT1b1, submucosal invasion of <500 μm; pT1b2, submucosal invasion of ≥500 μm; UL, ulceration or scar; Undiff, undifferentiated.
This study was approved by the Institutional Review Board of the Osaka International Cancer Institute on September 5, 2003, and followed the Declaration of Helsinki. Written informed consent for the ESD procedures and study participation was obtained from all patients.
Endoscopic submucosal dissection procedure
The ESD procedure consisted of marking around the lesion, submucosal injection of solution, circumferential mucosal incision, and submucosal dissection. 6 The ESD procedures were mainly performed by experts who had experience in performing more than 100 gastric ESDs, and some were performed by trainees under the supervision of the experts. The main device was the IT knife (Olympus, Tokyo, Japan) until 2006; the IT knife2 (Olympus) was used after January 2007.
Histological evaluation
The resected specimen was adequately stretched, pinned onto a hard Styrofoam plate (Dekopane; Koyo Sangyo Co. Ltd., Tokyo, Japan), fixed in 20% formalin, and serially sectioned at 2‐mm intervals. Histological diagnosis was made with hematoxylin and eosin staining, and immunohistochemical staining such as Elastica van Gieson or D2‐40 was added in case of muscularis mucosae or deeper invasion. Board‐certified pathologists of the Japanese Society of Pathology or under the supervision of the board‐certified pathologists established the diagnosis of the resected specimens in accordance with the Japanese classification of gastric carcinomas. 18 , 19
The curative resection criteria are no lymphovascular invasion, negative surgical margins, and (i) histologically differentiated‐type, any size, pT1a, and ulcer negative; (ii) histologically differentiated‐type, ≤3 cm, pT1a, and ulcer positive; or (iii) histologically differentiated‐type, ≤3 cm, and pT1b1 (<500 μm). The size of ulcer‐negative pT1a lesions in the curative resection criteria was initially defined as ≤5 cm, but it was repealed at the time of the survival analysis because of inconsistency with real clinical practice based on the Japanese Gastric Cancer Treatment guideline. 8 Patients with only a positive horizontal margin as a noncurative factor were carefully followed up without additional surgery because a very low risk of harboring lymph node metastasis was suggested in the guideline 8 and a favorable outcome of ESD was reported in cases of local recurrence. 20 , 21
Follow‐up and collection of long‐term outcome data
Esophagogastroduodenoscopy was performed 3, 6, and 12 months after ESD and annually thereafter to detect local recurrence and metachronous EGC. Annual abdominal ultrasonography or computed tomography and measurement of tumor marker concentrations were performed to check for lymph node and distant metastases for at least 5 years after ESD. The long‐term outcome data were confirmed in the medical records at the institute and in the hospital cancer registry, which is linked to the Osaka Cancer Registry. 22 In cases of loss to follow‐up, letters of inquiry were sent to the referral physicians or the patients until February 2017.
Outcome measurement
The primary outcome was 5‐year disease‐specific survival. The secondary outcomes were 5‐year overall survival, the relative survival ratio, and metachronous gastric cancer development.
Sample size and statistical methods
The sample size was calculated as follows. Because the 5‐year disease‐specific survival rate of 171 patients with absolute‐indication EGC that fulfilled the curative resection criteria at the Osaka International Cancer Institute was 99% (170 of 171), the incidence of death as a result of gastric cancer was calculated as 1.17 of 1000 person‐years. In total, 205 patients were required to ensure that the upper limit of the 95% confidence interval (CI) of the incidence of events did not exceed 1.17. The sample size was therefore set at 205 patients.
The patients' vital status 5 years after ESD was examined as the survival parameter. The Kaplan–Meier method was used to analyze 5‐year survival and metachronous EGC development after ESD. The relative survival ratio, which defines survival after elimination of the influence of other causes of death using the Ederer II method, was calculated. 23 It was the ratio of the observed survival (overall survival) and expected survival of the general population obtained from the complete (single‐year‐of‐age) national population life tables by sex. Statistical analyses were performed using stata software version 13 (StataCorp, College Station, TX, USA).
Results
Participants and descriptive data
During the study period, 2030 patients with 2556 EGC lesions underwent endoscopic resection for EGC in our institution. Among them, 205 patients who underwent EMR, 1035 patients with absolute‐indication lesions, 277 patients with differentiated‐type expanded‐indication lesions seen by doctors who were not involved in this study, 88 patients with undifferentiated‐type EGC, and 69 patients with beyond‐indication lesions were excluded. Accordingly, 356 patients with differentiated‐type EGC that fulfilled the expanded indication criteria underwent ESD as an investigational treatment (Fig. 1). Histological examination revealed that 205 patients (57.6%) fulfilled the curative resection criteria. The horizontal margin was positive in five patients and indeterminate in two patients. The two ulcer‐positive lesions were >3 cm (35 and 40 mm, respectively), but the patients refused surgery and the operators regarded the risk of lymph node metastasis as low. In consequence, 214 patients were registered for follow‐up. The remaining 142 patients who were determined to have undergone noncurative resection were referred to the department of surgery.
The demographics of all 214 enrolled patients (mean age, 66 years; 170 men and 44 women) are listed in Table 3. Six (3%) patients had a history of endoscopic resection for EGC, and four (2%) had a history of partial gastrectomy. Thirty‐three (15%) patients had a total of 37 synchronous multiple EGCs. Among the 214 main lesions, 120 (56%) were >2 cm in diameter and ulcer negative, and 94 (44%) were ≤3 cm and ulcer positive. Two hundred twelve lesions were resected en bloc (99%). The adverse events included 13 (6.1%) intraprocedural perforations; all of which were closed successfully by endoscopic clipping. One (0.5%) delayed perforation required emergency surgery. Twenty‐nine (13.5%) patients developed delayed bleeding at 0 to 13 days after the ESD procedure, and the bleeding was managed by endoscopic hemostasis. Two (0.9%) stenoses required repeated endoscopic balloon dilation.
Table 3.
Baseline characteristics of the 214 patients
| Characteristics | |
| Age (years) | 66.4 ± 7.8 |
| Sex (%) | |
| Male | 170 (79) |
| Female | 44 (21) |
| Past history of endoscopic resection for EGC (%) | 6 (2.8) |
| Past history of partial gastrectomy (%) | 4 (1.9) |
| Synchronous multiple EGC (%) | |
| Absent | 181 (85) |
| Present | 33 (15) |
| Longitudinal location of the main tumor | |
| Upper third | 55 (26) |
| Middle third | 113 (53) |
| Lower third | 46 (22) |
| Circumferential location of the main tumor (%) | |
| Lesser curvature | 115 (54) |
| Posterior wall | 50 (23) |
| Anterior wall | 34 (16) |
| Greater curvature | 15 (7) |
| Macroscopic type (%) | |
| Depressed | 117 (55) |
| Protruded | 88 (41) |
| Mixed | 9 (4.2) |
| Ulcer or scar in the main lesion (%) | |
| Negative | 120 (56) |
| Positive | 94 (44) |
| Tumor diameter (mm, ±SD) | 24.5 ± 11.5 |
| Ulcer positive | 15.2 ± 7.0 |
| Ulcer negative | 31.9 ± 8.7 |
| Resection | |
| En bloc | 212 (99) |
| Piecemeal | 2 (9.3) |
| Adverse events (%) | |
| Interprocedural perforation | 13 (6.1) |
| Delayed perforation | 1 (0.5) |
| Delayed bleeding | 29 (13.5) |
| Stenosis | 2 (0.9) |
EGC, early gastric cancer; SD, standard deviation.
Survival data
The vital status of all patients (100%) was confirmed at 5 years after ESD during the median follow‐up period of 7.4 years (range, 0.6–12.7 years). No local or metastatic recurrence was detected in any patients. Thirteen patients died during the study period. One died of metachronous gastric cancer, 10 of diseases other than gastric cancer, and 2 of unconfirmed causes (Fig. 1). The 5‐year disease‐specific survival rate was 99.5% (95% CI, 97.2%–100%) if neither of the two unconfirmed deaths was caused by gastric cancer and 98.5% (95% CI, 95.7%–99.7%) if both were caused by gastric cancer. The 5‐year overall survival rate was 93.9% (95% CI, 89.8%–96.4%) (Fig. 2). The relative survival ratio was 1.04 (0.99–1.07) (Fig. 3).
Figure 2.
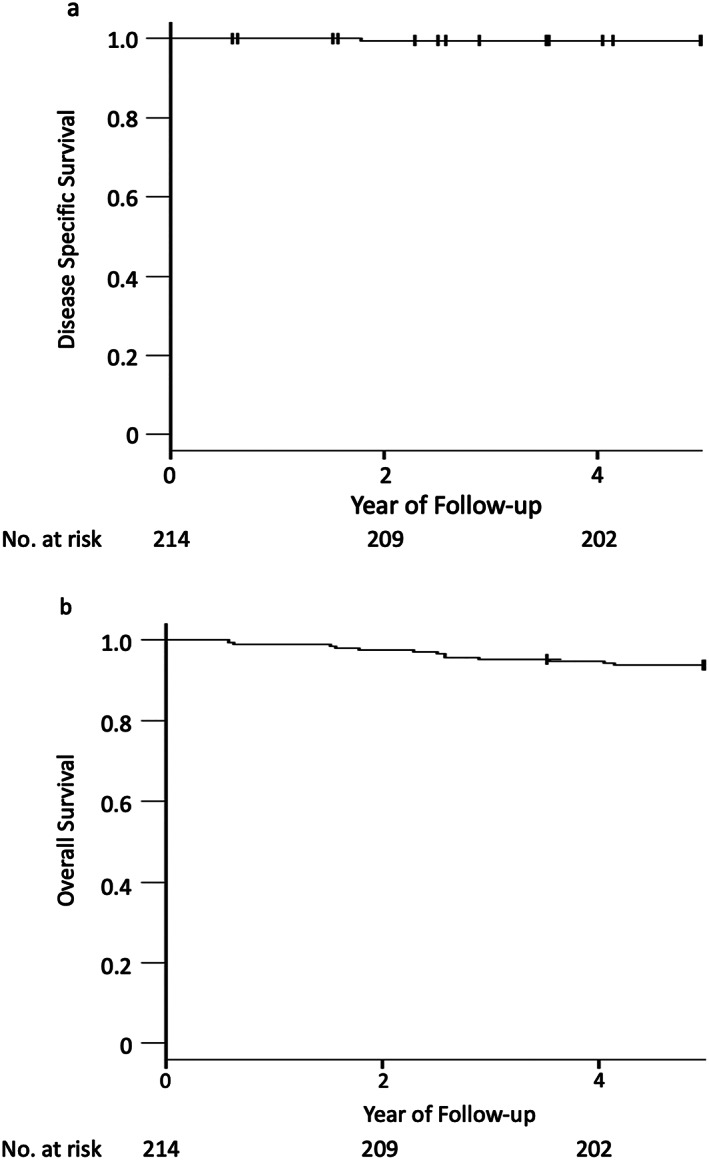
Kaplan–Meier analysis of (a) disease‐specific survival and (b) overall survival. The 5‐year overall survival rate was 93.9% (95% confidence interval, 89.8%–96.4%). The follow‐up rate at 5 years was 99.5%.
Figure 3.
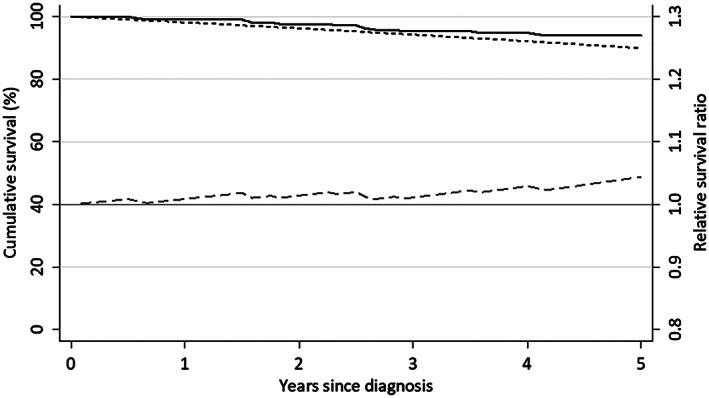
Overall survival (%), expected survival (%), and relative survival ratio.  , Overall survival (OS);
, Overall survival (OS);  , Expected survival on the lifetable of general population (ES);
, Expected survival on the lifetable of general population (ES);  , Relative survival ratio (the ratio of OS/ES).
, Relative survival ratio (the ratio of OS/ES).
Metachronous gastric cancer development
In total, 26 metachronous gastric cancers were detected in 26 patients by endoscopy during the 5‐year follow‐up period (Fig. 4). All metachronous EGCs developed in patients who had undergone curative resection. Of 24 lesions treated by ESD, 23 met the curative resection criteria, and 1 underwent noncurative resection (>3 cm and ulcer positive). The patient who underwent noncurative resection did not receive additional surgery because of concomitant pancreatic cancer, which led to the patient's death. The other two metachronous lesions were advanced cancers detected on annual surveillance EGD. One of these patients was diagnosed with type 3 gastric cancer with distant lymph node metastasis 1 year after ESD and died of gastric cancer despite chemotherapy. The other patient was found to have metachronous cancer 17 months after ESD and died of an unconfirmed cause at 77 years of age.
Figure 4.
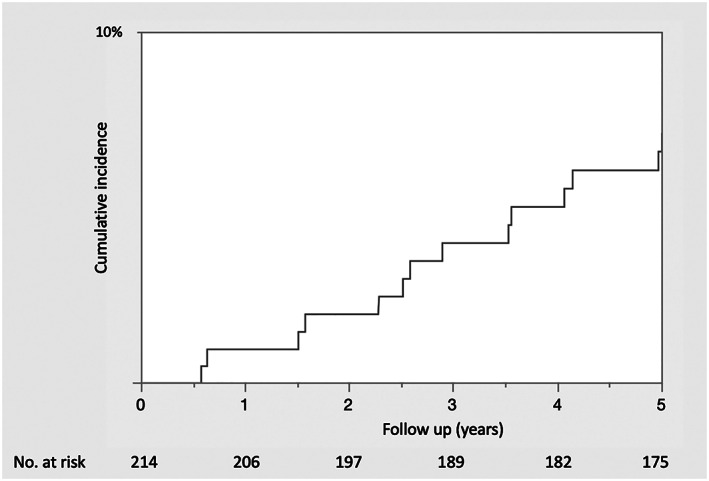
Kaplan–Meier analysis of metachronous gastric cancer development.
Discussion
We conducted a prospective cohort study to investigate long‐term survival after ESD for differentiated‐type EGC that fulfilled the expanded indication criteria and for which curative resection was histologically confirmed. The 5‐year disease‐specific and overall survival rates were excellent at 99.5% (95% CI, 97.2%–100%) and 93.9% (95% CI, 89.8%–96.4%), respectively.
Excellent long‐term outcomes of ESD for expanded‐criteria lesions have been reported in many studies. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 However, because most of these previous reports were retrospective, the net survival rate was not prospectively evaluated. In a retrospective study, bias may be introduced because of unavailable data or conflicting data sources, which may not be the case for a prospective study. In the present study, the vital status at 5 years after surgery was confirmed in all patients, while most retrospective studies do not describe the follow‐up rate of the study subjects. Because of the quite low mortality from EGC, it is important to achieve a high follow‐up rate in a cohort study to investigate long‐term survival of patients with EGC. A prospective cohort study that enrolled 470 patients with EGC who met the expanded indication criteria from 29 institutions throughout Japan and were followed up for 5 years showed favorable outcomes with an overall survival rate of 97.0%. 15 In that study, 317 patients satisfied the curative resection criteria, and the 5‐year follow‐up rate was 95%. The outcome of the present study is compatible with the outcome of that multicenter prospective study. Our study showed not only an excellent 5‐year survival rate but also a good relative survival ratio of 1.04 (0.99–1.07). This indicates that even large or ulcer‐positive differentiated‐type EGC does not shorten the patient's lifespan if it is treated with ESD. Our study results strengthen the evidence that ESD for EGC that meets the expanded criteria can be a standard treatment for EGC.
We consider that achieving an excellent outcome of ESD for expanded‐indication lesions is dependent upon a careful histological examination for curability. Several recent studies investigating the lymph node metastasis rate of surgically resected EGC suggested that application of the expanded indication criteria of ESD to Western patients was difficult because the lymph node metastasis rate of T1a EGC was higher than that in Eastern reports. 16 , 24 , 25 Data from the Surveillance, Epidemiology, and End Results database analyzing 545 T1a EGCs in the USA indicated that the lymph node metastasis rates of 2 to 3 cm, 3 to 4 cm, and ≥4 cm T1a lesions were 4.5%, 4.1%, and 20.0%, respectively. These studies suggest that a high lymph node metastasis rate may be associated with genetic or environmental differences between the East and West. However, we suspect that these differences are most likely attributed to different tissue handling methods in different countries. 26 Submucosal invasion and lymphovascular involvement often exist in a small area of the T1 lesion. Japanese surgeons and pathologists usually section a specimen meticulously (1‐cm width), which may increase the probability of detecting deep submucosal invasion or lymphovascular involvement. In Western countries, however, where specimens are often sectioned in one line, the depth of tumor invasion or presence of lymphovascular involvement (i.e. risk of lymph node metastasis) might be missed. The ESD specimens in this study were cut into 2‐mm intervals; therefore, the histological findings associated with lymph node metastasis were carefully evaluated.
Intraoperative perforation occurred in 6.1% of patients (13 of 214); this rate is relatively high compared with our previous report (2.8%) 27 and other reports (1.2%–8.2%). 28 , 29 , 30 , 31 However, all cases could be treated conservatively. Risk factors for perforation include a large tumor size, ulcer positivity, an upper tumor location, and a longer operation time, 28 , 30 , 31 and ESD for differentiated‐type EGC according to the expanded criteria (i.e. ulcer positive or large diameter of >2 cm) is thought to increase the risk. Indeed, perforation occurred in eight patients with ulcer‐positive lesions, and the tumor diameter was ≥3 cm in five patients with ulcer‐negative lesions. With such a high incidence of perforation even in experienced endoscopists' hands, ESD for expanded‐criteria lesions should ideally be performed by experienced endoscopists. As we previously reported, 32 one delayed perforation required emergency surgery. Delayed bleeding occurred in 29 patients (14%), and this rate is also relatively high compared with previous reports. 31 A recent systematic review indicated that the expanded indication criteria increase the risk of delayed bleeding (odds ratio of 2.03). 33 The use of histamine 2 receptor blockers instead of Proton pump inhibitor (PPI) also increases risk of delayed bleeding. 33 , 34 Until 2005, most patients were administered a histamine 2 receptor blocker after ESD in this study. These factors contributed to the high incidence of delayed bleeding.
This long‐term cohort study revealed that ESD had high curability for EGC that fulfilled the expanded indication criteria. However, 12.1% of patients (26 of 214) were found to have metachronous gastric cancer. This frequency is consistent with that of previous reports (13.9%–15.3%). 35 , 36 , 37 , 38 Although annual surveillance endoscopy usually enables detection of metachronous lesions at an endoscopically treatable stage, some patients may require surgery. A Japanese multicenter prospective study showed that during follow‐up of 317 patients with expanded‐indication EGC after curative resection, 9 patients underwent gastrectomy because of metachronous gastric cancer. 15 In the present study, two metachronous advanced cancers developed at 12 and 17 months after ESD, respectively, and one patient died of the metachronous gastric cancer. This was the only cause of disease‐specific mortality in this study. Abe et al. 38 indicated that among 1526 patients with EGC who underwent curative ESD, 7 patients died of metachronous gastric cancer, and 5 of these 7 died within 5 years after initial ESD. Surveillance endoscopy is mandatory after ESD to achieve good long‐term outcome.
This study has several limitations. First, although the patients were followed up prospectively, some patients were lost to follow‐up because of referral to other hospitals; as a result, the causes of two deaths are unconfirmed. Second, we did not investigate the status of Helicobacter pylori infection, which is known to affect the incidence of metachronous gastric cancer development. 39 , 40 , 41 H. pylori eradication therapy for post‐ESD patients has been covered by the Japanese social insurance since 2010, so most patients neither underwent diagnostics for H. pylori infection nor received eradication therapy at the time of the index ESD. Third, we included patients with a history of endoscopic resection and surgery for EGC. However, these patients accounted for six (2.8%) and four (1.9%), respectively, of all study subjects and therefore might not have substantially affected the overall results. Fourth, we did not compare the long‐term outcome of endoscopic resection with that of surgery. 8 However, without treatment‐related death or local recurrence, the long‐term outcome after ESD was considered to be comparable with that after surgery.
In conclusion, ESD for EGC that fulfilled the expanded criteria for differentiated‐type EGC is feasible, and the long‐term outcome is favorable.
Acknowledgments
The authors thank the physicians who referred the patients to our department and the doctors in our institution who were involved in management of the study participants. The authors also thank Angela Morben, DVM, ELS, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
Shichijo, S. , Uedo, N. , Kanesaka, T. , Ohta, T. , Nakagawa, K. , Shimamoto, Y. , Ohmori, M. , Arao, M. , Iwatsubo, T. , Suzuki, S. , Matsuno, K. , Iwagami, H. , Inoue, S. , Matsuura, N. , Maekawa, A. , Nakahira, H. , Yamamoto, S. , Takeuchi, Y. , Higashino, K. , Ishihara, R. , Fukui, K. , Ito, Y. , Narahara, H. , Ishiguro, S. , and Iishi, H. (2021) Long‐term outcomes after endoscopic submucosal dissection for differentiated‐type early gastric cancer that fulfilled expanded indication criteria: A prospective cohort study. Journal of Gastroenterology and Hepatology, 36: 664–670. 10.1111/jgh.15182.
Declaration of conflict of interest: The authors declare no conflict of interest.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Uedo N, Iishi H, Tatsuta M et al. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer 2006; 9: 88–92. [DOI] [PubMed] [Google Scholar]
- 3. Yamamoto S, Uedo N, Ishihara R et al. Endoscopic submucosal dissection for early gastric cancer performed by supervised residents: assessment of feasibility and learning curve. Endoscopy 2009; 41: 923–928. [DOI] [PubMed] [Google Scholar]
- 4. Sugimoto T, Okamoto M, Mitsuno Y et al. Endoscopic submucosal dissection is an effective and safe therapy for early gastric neoplasms: a multicenter feasible study. J. Clin. Gastroenterol. 2012; 46: 124–129. [DOI] [PubMed] [Google Scholar]
- 5. Yamashina T, Uedo N, Dainaka K et al. Long‐term survival after endoscopic resection for early gastric cancer in the remnant stomach: comparison with radical surgery. Ann. Gastroenterol. 2015; 28: 66–71. [PMC free article] [PubMed] [Google Scholar]
- 6. Takeuchi Y, Uedo N, Iishi H et al. Endoscopic submucosal dissection with insulated‐tip knife for large mucosal early gastric cancer: a feasibility study (with videos). Gastrointest. Endosc. 2007; 66: 186–193. [DOI] [PubMed] [Google Scholar]
- 7. Gotoda T, Yanagisawa A, Sasako M et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3: 219–225. [DOI] [PubMed] [Google Scholar]
- 8. Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14: 113–123. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki H, Oda I, Abe S et al. High rate of 5‐year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19: 198–205. [DOI] [PubMed] [Google Scholar]
- 10. Fukunaga S, Nagami Y, Shiba M et al. Long‐term prognosis of expanded‐indication differentiated‐type early gastric cancer treated with endoscopic submucosal dissection or surgery using propensity score analysis. Gastrointest. Endosc. 2017; 85: 143–152. [DOI] [PubMed] [Google Scholar]
- 11. Ahn JY, Jung HY, Choi KD et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest. Endosc. 2011; 74: 485–493. [DOI] [PubMed] [Google Scholar]
- 12. Min BH, Kim ER, Kim KM et al. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy 2015; 47: 784–793. [DOI] [PubMed] [Google Scholar]
- 13. Gotoda T, Iwasaki M, Kusano C, Seewald S, Oda I. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br. J. Surg. 2010; 97: 868–871. [DOI] [PubMed] [Google Scholar]
- 14. Kosaka T, Endo M, Toya Y et al. Long‐term outcomes of endoscopic submucosal dissection for early gastric cancer: a single‐center retrospective study. Dig. Endosc. 2014; 26: 183–191. [DOI] [PubMed] [Google Scholar]
- 15. Hasuike N, Ono H, Boku N et al. A non‐randomized confirmatory trial of an expanded indication for endoscopic submucosal dissection for intestinal‐type gastric cancer (cT1a): the Japan Clinical Oncology Group study (JCOG0607). Gastric Cancer 2018; 21: 114–123. [DOI] [PubMed] [Google Scholar]
- 16. Bausys R, Bausys A, Maneikis K, Belogorceva V, Stratilatovas E, Strupas K. Safety of expanded criteria for endoscopic resection of early gastric cancer in a Western cohort. BMC Surg. 2018; 18: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. SEER program coding and staging manual 2004, revision 1. Cited 20 Jun 2020. Available from URL: https://seer.cancer.gov/archive/manuals/2004Revision1/SPM_2004_maindoc.r1.pdf
- 18. Japanese Gastric Cancer Association . Japanese classification of gastric carcinoma—2nd English edition. Gastric Cancer 1998; 1: 10–24. [DOI] [PubMed] [Google Scholar]
- 19. Japanese Gastric Cancer Association . Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14: 101–112. [DOI] [PubMed] [Google Scholar]
- 20. Sekiguchi M, Suzuki H, Oda I et al. Favorable long‐term outcomes of endoscopic submucosal dissection for locally recurrent early gastric cancer after endoscopic resection. Endoscopy 2013; 45: 708–713. [DOI] [PubMed] [Google Scholar]
- 21. Hoteya S, Iizuka T, Kikuchi D et al. Secondary endoscopic submucosal dissection for residual or recurrent tumors after gastric endoscopic submucosal dissection. Gastric Cancer 2014; 17: 697–702. [DOI] [PubMed] [Google Scholar]
- 22. Tabuchi T, Ito Y, Ioka A, Miyashiro I, Tsukuma H. Incidence of metachronous second primary cancers in Osaka, Japan: update of analyses using population‐based cancer registry data. Cancer Sci. 2012; 103: 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl. Cancer Inst. Monogr. 1961; 6: 101–121. [PubMed] [Google Scholar]
- 24. Choi AH, Nelson RA, Merchant SJ, Kim JY, Chao J, Kim J. Rates of lymph node metastasis and survival in T1a gastric adenocarcinoma in Western populations. Gastrointest. Endosc. 2016; 83: 1184–1192.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pokala SK, Zhang C, Chen Z et al. Lymph node metastasis in early gastric adenocarcinoma in the United States of America. Endoscopy 2018; 50: 479–486. [DOI] [PubMed] [Google Scholar]
- 26. Abdelfatah MM, Barakat M, Othman MO, Grimm IS, Uedo N. The incidence of lymph node metastasis in submucosal early gastric cancer according to the expanded criteria: a systematic review. Surg. Endosc. 2019; 33: 26–32. [DOI] [PubMed] [Google Scholar]
- 27. Ohta T, Ishihara R, Uedo N et al. Factors predicting perforation during endoscopic submucosal dissection for gastric cancer. Gastrointest. Endosc. 2012; 75: 1159–1165. [DOI] [PubMed] [Google Scholar]
- 28. Mannen K, Tsunada S, Hara M et al. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J. Gastroenterol. 2010; 45: 30–36. [DOI] [PubMed] [Google Scholar]
- 29. Chung IK, Lee JH, Lee SH et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest. Endosc. 2009; 69: 1228–1235. [DOI] [PubMed] [Google Scholar]
- 30. Watari J, Tomita T, Toyoshima F et al. Clinical outcomes and risk factors for perforation in gastric endoscopic submucosal dissection: a prospective pilot study. World J Gastrointest Endosc. 2013; 5: 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig. Endosc. 2013; 25: 71–78. [DOI] [PubMed] [Google Scholar]
- 32. Hanaoka N, Uedo N, Ishihara R et al. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy 2010; 42: 1112–1115. [DOI] [PubMed] [Google Scholar]
- 33. Libânio D, Costa MN, Pimentel‐Nunes P, Dinis‐Ribeiro M. Risk factors for bleeding after gastric endoscopic submucosal dissection: a systematic review and meta‐analysis. Gastrointest. Endosc. 2016; 84: 572–586. [DOI] [PubMed] [Google Scholar]
- 34. Uedo N, Takeuchi Y, Yamada T et al. Effect of a proton pump inhibitor or an H2‐receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am. J. Gastroenterol. 2007; 102: 1610–1616. [DOI] [PubMed] [Google Scholar]
- 35. Kato M, Nishida T, Yamamoto K et al. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut 2013; 62: 1425–1432. [DOI] [PubMed] [Google Scholar]
- 36. Hanaoka N, Uedo N, Shiotani A et al. Autofluorescence imaging for predicting development of metachronous gastric cancer after Helicobacter pylori eradication. J. Gastroenterol. Hepatol. 2010; 25: 1844–1849. [DOI] [PubMed] [Google Scholar]
- 37. Sugimoto T, Yamaji Y, Sakitani K et al. Neutrophil infiltration and the distribution of intestinal metaplasia is associated with metachronous gastric cancer following endoscopic submucosal dissection. Can. J. Gastroenterol. Hepatol. 2015; 29: 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abe S, Oda I, Suzuki H et al. Long‐term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47: 1113–1118. [DOI] [PubMed] [Google Scholar]
- 39. Yoon SB, Park JM, Lim CH, Cho YK, Choi MG. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumors: a meta‐analysis. Helicobacter 2014; 19: 243–248. [DOI] [PubMed] [Google Scholar]
- 40. Fukase K, Kato M, Kikuchi S et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open‐label, randomised controlled trial. Lancet 2008; 372: 392–397. [DOI] [PubMed] [Google Scholar]
- 41. Lee YC, Chiang TH, Chou CK et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta‐analysis. Gastroenterology 2016; 150: 1113–1124.e5. [DOI] [PubMed] [Google Scholar]


