Abstract
Aim
To assess growth plate fusion by magnetic resonance imaging (MRI) and evaluate the correlation with sex, age, pubertal development, physical activity and BMI.
Methods
Wrist, knee and ankle of 958 healthy subjects aged 14.0‐21.5 years old were examined using MRI and graded by two radiologists. Correlations of growth plate fusion score with age, pubertal development, physical activity and BMI were assessed.
Results
Complete growth plate fusion occurred in 75%, 85%, 97%, 98%, 98% and 90%, 97%, 95%, 97%, 98% (radius, femur, proximal‐ and distal tibia and calcaneus) in 17‐year‐old females and 19‐year‐old males, respectively. Complete fusion occurs approximately 2 years earlier in girls than in boys. Pubertal development correlated with growth plate fusion score (ρ = 0.514‐0.598 for the different growth plate sites) but regular physical activity did not. BMI also correlated with growth plate fusion (ρ = 0.186‐0.384). Stratified logistic regression showed increased odds ratio (OR F: 2.65‐8.71; M: 1.71‐4.03) for growth plate fusion of obese or overweight subects versus normal‐weight subjects. Inter‐observer agreement was high (Κ = 0.87‐0.94).
Conclusion
Growth plate fusion can be assessed by MRI; occurs in an ascending order, from the foot to the wrist; and is significantly influenced by sex, pubertal development and BMI, but not by physical activity.
Keywords: growth plate, magnetic resonance imaging, maturation process, obesity, puberty
Abbreviations
- κ
Kappa value
- BA
Bone age
- BMI
Body mass index
- Cm
Centimetre
- GH
Growth hormone
- GP
Greulich & Pyle
- IGF‐1
Insulin growth factor 1
- MRI
Magnetic resonance imaging
- N
Number of participants
- OR
Odds ratio
- Rho
Spearman correlation coefficient
- SD
Standard deviation
- SHBG
Sex‐binding hormone protein
- TW
Tanner & Whitehouse
- YO
Years old
Key notes.
The growth plates fuse in an ascending order, from the foot to the wrist, and females mature significantly earlier than males.
Overweight individuals are significantly more skeletally mature than their peers.
Physical activity does not significantly affect growth plate fusion.
1. INTRODUCTION
Evaluation of growth and maturation of children and adolescents often entails a bone age, assessed from plain radiographs of the left hand and wrist using the methods of Greulich and Pyle (GP) 1 based on Caucasian children in Ohio, and Tanner and Whitehouse (TW) 2 based on children in the United Kingdom. These methods depict the appearance of mineralisation and ossification using ionising radiation yet bone itself is not driving the skeletal maturation process. Instead, skeletal maturation is controlled by the hyaline cartilage of the growth plate 3 which is not visualised on a radiograph and complete maturity is reached first when the growth plate has been completely transformed into bone. In forensic medicine, skeletal age assessment has been applied to assess the age of young athletes in international competitions, and more recently to assess the age of refugees lacking valid date of birth documents. 4 , 5 , 6 , 7 , 8 , 9 However, traditional methods of skeletal age assessment make use of ionising radiation, raising ethical concerns about application outside the context of clinical medicine.
In this context, magnetic resonance imaging (MRI) has emerged as an alternative method to depict the growth plate, avoiding the use of ionising radiation. MRI depicts accurately bone and soft tissues not only anatomically but also physiopathologically. MRI can depict the cartilaginous growth plate and sometimes even its individual sublayers: the resting, proliferative and hypertrophic zones. 10 MRI has also been used to confirm that endochondral ossification has been reached, and skeletal maturation is complete. 11
In clinical practice, the onset and progress of puberty in adolescents and young adults are carefully assessed, with the five‐stage system of secondary sexual characteristics (breast development, pubis hair growth and testicular volume) developed by Marshall and Tanner 12 , 13 as a standard for pubertal assessments.
During puberty, sex steroids, primarily oestradiol, accelerate skeletal maturation and hasten growth plate fusion, 14 but other hormonal and nutritional factors may also play important roles. For example, increased adipose tissue may affect puberty, and studies have attempted to assess how hormonal differences in obese vis‐à‐vis non‐obese adolescents may affect skeletal‐ and chronological age. 15 , 16 This has become increasingly relevant with the increase of obesity among children and youths. 17
Another point of contention is the issue of correct chronological age in youth sports and whether physical activity with repetitive micro trauma to a certain growth plate hastens the closure of the growth plate (for example in soccer) or delays it (for example in gymnastics). 18 Studies have been performed both on elite athletes and on recreational youth athletes and their peers, 19 , 20 but none have, to our knowledge, studied the association between physical activity and skeletal maturation by MR imaging.
The aims of the study were (a) to evaluate with MRI the timing of closure between different bones in the axial skeleton in relation to the subjects' chronological age and (b) to use MRI to assess whether factors such as self‐assessment of pubertal development (Tanner scale), body mass index (BMI) and physical activity correlate with the fusion of the different growth plates at different chronological ages in a healthy population of adolescents and young adults.
2. PATIENTS AND METHODS
This cross‐sectional study consisted of 958 healthy volunteers (481 females and 477 males) between 14.0 and 21.5 years old. The study was performed from 2017 to 2018 in accordance with the Declaration of Helsinki and was approved by the ethics committee of Stockholm, Sweden. Written consent was obtained from all volunteers and their legal guardians if necessary, according to ethical guidelines. Inclusion criteria were as follows: (a) Birth in the country in which the study was conducted; (b) Age verified by birth certificate from national authorities. Exclusion criteria were as follows: (a) History of residency outside the country in which the study was conducted for a period exceeding 6 months; (b) History of bilateral trauma in close proximity to the growth plate; (c) Medical history of chronic disease; (d) Long‐term medication that might affect the growth plate; (e) Pregnancy, past or current (all female volunteers were tested); and (f) Non‐compliance during MRI examination.
2.1. MRI examinations
MRI was performed on a 1.5‐T whole‐body scanner with dedicated coils. Growth plates of the distal radius, distal femur, proximal and distal tibia, and calcaneus were examined with cartilage sequences. The non‐dominant side of the subject was favoured for the examination unless there was a history of trauma in close proximity to the growth plate, in which case the dominant side was examined. All examinations were done within 0‐6 months of the subject's most recent birthday.
Protocol settings varied according to the manufacturer recommendations. We used the most commonly available MR scanners at our hospitals: Signa (General Electric Healthcare), Achieva (Philips Healthcare) and Magnetom Avanto Fit (Siemens Healthcare GmbH). Each acquisition took approximately 4‐5 minutes, and the total examination time for all three joints was around 30 minutes. Before imaging, height and weight were measured and self‐assessments of physical activity and pubertal development on the Tanner scale 12 , 13 were completed by the subjects. MRI studies were coded and anonymised prior to evaluation.
2.2. Image assessment
Images were examined and rated according to a system based on Kellinghaus' modified version of Schmeling's developmental stages 6 , 7 , and the developmental stages by Dedouit et al 8 with minor alterations. Our scale was introduced in a previous study 21 in which we defined the scale as follows:
Stage 1. Continuous, stripe‐like, cartilage signal intensity is present between the metaphysis and the epiphysis with a thickness greater than 1.5 mm with a multilaminar appearance.
Stage 2. Continuous cartilage signal intensity is present between the metaphysis and the epiphysis with a thickness greater than 1.5 mm with increased signal intensity but without a multilaminar appearance.
Stage 3. Continuous cartilage signal intensity is present between the metaphysis and the epiphysis with a thickness less than 1.5 mm with increased signal intensity.
Stage 4a. The cartilage is not continuous. A hazy area involving one third or less of the growth plate is present between the metaphysis and the epiphysis, representing epiphyseal‐metaphyseal fusion.
Stage 4b. The cartilage is not continuous. A hazy area involving between one third and two thirds of the growth plate is present between the metaphysis and the epiphysis, representing epiphyseal‐metaphyseal fusion.
Stage 4c. The cartilage is not continuous. A hazy area involving more than two thirds of the growth plate is present between the metaphysis and the epiphysis, representing epiphyseal‐metaphyseal fusion.
Stage 5. The epiphyseal cartilage has fused completely, with or without an epiphyseal scar. (Figure 1)
Figure 1.
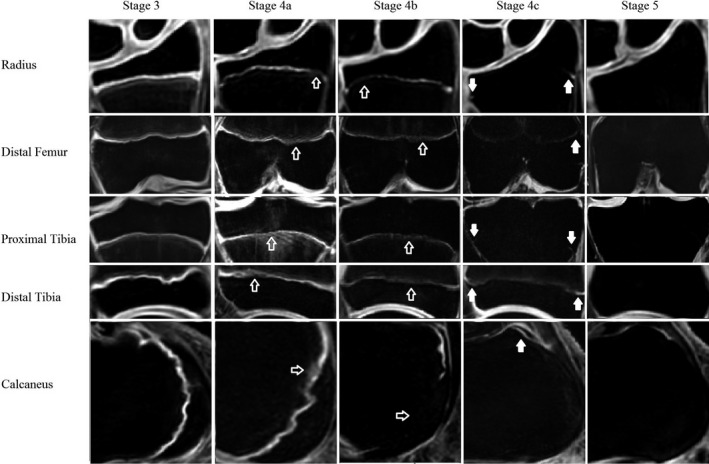
Each row is one of the five growth plates studied, and each column represents a different stage of closure in our study population. The open arrows in stage 4a and 4b indicate areas where bone bridging is present while the solid white arrows in stage 4c indicate areas where the growth plate still is unfused
The image with the highest degree of closure was considered the most developed and was graded according to the modified staging system. Stage 5 grading required that the growth plate be completely closed on all images. All subjects were graded by two paediatric radiologists with 25 and 3 years of experience in paediatric radiology, respectively. The radiologists were blinded to the age and sex of the subjects as well as to the assessments of the other radiologist. In cases of disagreement, a third independent reading was performed by a paediatric radiologist with 13 years of experience.
2.3. Statistical analysis
Weighted Cohen's kappa (Κ) was calculated from the results from the paediatric radiologists to evaluate inter‐ and intra‐observership agreement. 22 Each growth plate was calculated separately. There was an excellent inter‐observer agreement (Radius Κ = 0.91‐0.92; Femur Κ = 0.87‐0.91; Proximal Tibia Κ = 0.89‐0.92; Distal Tibia Κ = 0.87‐0.91; Calcaneus Κ = 0.92‐0.94), validating our method. 23
Spearman's rank correlation coefficient was calculated to evaluate whether BMI, pubertal development or physical activity affected the maturity of the five growth plates included in this study.
Calculations were also performed to evaluate at what age growth plate closure was complete (stage 5) by logistic regression. Multiple logistic regressions were performed to calculate the odds ratio for each growth plate. Stage 5 was used as a dependent variable, and BMI, age, and sex were used as independent variables. Conditional logistic regression stratified for age and sex was also performed to further evaluate whether BMI had an impact on the rate of maturity. Age‐ and sex‐adjusted BMI was computed using the Swedish growth reference data, and iso‐BMI 25 was used to categorise individuals as normal‐ or overweight.
The R software package, version 3.5.3, (The R Project for Statistical Computing) was used for the statistical analysis. A P‐value of <.05 was considered significant.
3. RESULTS
Demographic data are visualised in Table 1. Most of the study population were normal weight. Overweight was observed in 19.5% of females and 14% of males and obesity in 3.1% of females and 3.6% of males according to age‐adjusted BMI.
Table 1.
Demographic information of the study population by age, sex and BMI ± standard deviation in each subgroup
| Age (yo) | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | Total |
|---|---|---|---|---|---|---|---|---|---|
| Male (N) | 60 | 61 | 60 | 63 | 58 | 60 | 60 | 61 | 477 |
| BMI | 19.8 ± 2.4 | 20.7 ± 2.6 | 21.1 ± 3.2 | 21.4 ± 2.7 | 22.4 ± 4.0 | 22.6 ± 3.4 | 23.5 ± 4.0 | 23.1 ± 4.6 | 21.8 ± 3.6 |
| Female (N) | 62 | 62 | 60 | 60 | 61 | 59 | 57 | 60 | 481 |
| BMI | 21.4 ± 3.5 | 22.1 ± 3.0 | 22.0 ± 2,8 | 22.7 ± 3.7 | 23.5 ± 4.4 | 22.0 ± 3.6 | 22.5 ± 3.6 | 22.8 ± 2.7 | 22.4 ± 3.5 |
| Total | 122 | 123 | 120 | 123 | 119 | 119 | 117 | 121 | 958 |
Abbreviations: BMI, body mass index; N, number of subjects; yo, years old.
The chronological age of complete growth plate fusion followed a distinct pattern whereby the first site of complete growth plate closure was the calcaneus, followed by distal tibia, proximal tibia and distal femur, and then the distal radius, in ascending order. The age at which at least 50% and 90% of females had fused their growth plates occurred between 14‐17 and 15‐18 years, respectively (Table 2). In boys the age at which at least 50% and 90% had fused their growth plates occurred between 15‐18 and 17‐20 years, respectively. All females aged 19 years and older had closed growth plates at all sites while all males were closed at all sites at the age of 21 years.
Table 2.
Ratio among subjects with completely closed growth plates (stage 5), by growth plate for males and females in each age group
| Gender | Age | Radius (%) | Femur (%) | Proximal Tibia (%) | Distal Tibia (%) | Calcaneus (%) |
|---|---|---|---|---|---|---|
| Male | 14 | 0/59 (0) | 0/60 (0) | 0/60 (0) | 3/60 (5.0) | 11/60 (18.3) |
| 15 | 1/59 (1.7) | 0/60 (0) | 2/60 (3.3) | 11/60 (18.3) | 31/59 (52.5) | |
| 16 | 5/60 (8.5) | 10/60 (16.7) | 12/60 (20.0) | 35/60 (58.3) | 46/60 (76.7) | |
| 17 | 15/61 (24.6) | 26/61 (42.6) | 34/61 (55.7) | 54/61 (88.5) | 60/61 (98.4) | |
| 18 | 34/57 (59.6) | 48/58 (82.7) | 50/57 (87.7) | 57/58 (98.3) | 58/58 (100) | |
| 19 | 52/58 (89.7) | 58/60 (96.7) | 57/60 (95.0) | 58/60 (96.7) | 59/60 (98.3) | |
| 20 | 53/54 (98.1) | 54/54 (100) | 54/54 (100) | 52/54 (96.3) | 54/54 (100) | |
| 21 | 59/59 (100) | 60/60 (100) | 60/60 (100) | 60/60 (100) | 60/60 (100) | |
| Female | 14 | 3/62 (4.8) | 6/62 (9.7) | 12/62 (19.4) | 31/62 (50) | 44/61 (72.1) |
| 15 | 8/60 (13.3) | 25/62 (40.3) | 33/62 (53.2) | 52/62 (83.9) | 56/62 (90.3) | |
| 16 | 26/60 (43.3) | 42/60 (70.0) | 53/60 (88.3) | 55/59 (93.2) | 56/58 (96.6) | |
| 17 | 45/60 (75.0) | 51/60 (85.0) | 58/60 (96.7) | 59/60 (98.3) | 59/60 (98.3) | |
| 18 | 55/61 (90.2) | 59/60 (98.39) | 60/60 (100) | 61/61 (100) | 61/61 (100) | |
| 19 | 57/57 (100) | 58/58 (100) | 58/58 (100) | 58/58 (100) | 58/58 (100) | |
| 20 | 57/57 (100) | 57/57 (100) | 57/57 (100) | 57/57 (100) | 57/57 (100) | |
| 21 | 60/60 (100) | 60/60 (100) | 60/60 (100) | 60/60 (100) | 60/60 (100) |
Spearman's rank correlation coefficient for the different variables was calculated separately for each sex and can be seen in Tables 3 and 4. Spearman's intercorrelation matrix showed a moderate correlation (ρ = 0.514‐0.598; P < .001) between the Tanner score and the maturity of the growth plates for males and moderate to low correlation (ρ = 0.287 ‐0.513; P < .001) for females.
Table 3.
Spearman correlation matrix for males for all five growth plates as well as Tanner, BMI and physical activity
| Stage Radius | Stage Femur | Stage Prox. Tibia | Stage Dist. Tibia | Stage Calcaneus | Tanner | BMI | Physical Activity | |
|---|---|---|---|---|---|---|---|---|
| Stage Radius | 1.000 |
0.855 <0.001 |
0.838 <0.001 |
0.703 <0.001 |
0.607 <0.001 |
0.596 <0.001 |
0.384 <0.001 |
−0.069 0.135 |
| Stage Femur | 1.000 |
0.908 <0.001 |
0.739 <0.001 |
0.573 <0.001 |
0.0592 <0.001 |
0.368 <0.001 |
−0.049 0.282 |
|
| Stage Prox. Tibia | 1.000 |
0.777 <0.001 |
0.667 <0.001 |
0.597 <0.001 |
0.365 <0.001 |
−0.056 0.219 |
||
| Stage Dist. Tibia | 1.000 |
0.788 <0.001 |
0.598 <0.001 |
0.346 <0.001 |
−0.037 0.414 |
|||
| Stage Calcaneus | 1.000 |
0.514 <0.001 |
0.308 <0.001 |
−0.058 0.203 |
||||
| Tanner | 1.000 |
0.319 <0.001 |
−0.036 0.436 |
|||||
| BMI | 1.000 |
0.041 0.366 |
||||||
| Physical activity | 1.000 |
The table shows whether there is a correlation between two variables and if it is significant.
Table 4.
Spearman correlation matrix for females for all five growth plates as well as Tanner, BMI and physical activity
| Stage Radius | Stage Femur | Stage Prox. Tibia | Stage Dist. Tibia | Stage Calcaneus | Tanner | BMI | Physical Activity | |
|---|---|---|---|---|---|---|---|---|
| Stage Radius | 1.000 |
0.828 <0.001 |
0.759 <0.001 |
0.551 <0.001 |
0.377 <0.001 |
0.513 <0.001 |
0.222 <0.001 |
0.095 <0.05 (0.043) |
| Stage Femur | 1.000 |
0.846 <0.001 |
0.615 <0.001 |
0.431 <0.001 |
0.500 <0.001 |
0.210 <0.001 |
0.043 0.343 |
|
| Stage Prox. Tibia | 1.000 |
0.717 <0.001 |
0.496 <0.001 |
0.445 <0.001 |
0.216 <0.001 |
0.019 0.686 |
||
| Stage Dist. Tibia | 1.000 |
0.516 <0.001 |
0.378 <0.001 |
0.219 <0.001 |
0.028 0.542 |
|||
| Stage Calcaneus | 1.000 |
0.287 <0.001 |
0.186 <0.001 |
−0.049 0.285 |
||||
| Tanner | 1.000 |
0.261 <0.001 |
−0.026 0.560 |
|||||
| BMI | 1.000 |
0.059 0.197 |
||||||
| Physical activity | 1.000 |
The table shows whether there is a correlation between two variables and if it is significant.
The correlation between BMI and growth plate maturity was low for males (ρ = 0.308‐0.384; P < .001) and low to very low for females (ρ = 0.186‐0.222; P < .001). There was no significant correlation between physical activity and growth plate maturity in either sex.
Logistic regression of the five growth plates showed that females in the study population were more likely than males to be stage 5, by an odds ratio of 8.57‐28.81 (Table 5). The regression model also showed that individuals were more likely to be stage 5 for each additional year of age, with an odds ratio of 4.18‐5.62. In the stratified version of the conditional logistic regression, it was more likely for an overweight or obese individual to be stage 5 than an individual of ordinary weight, with odds ratios for females between 2.65 and 8.71 and for males between 1.71 and 4.03 (Table 6). Odds ratios for females were overall significant (P < .05) for all growth plates with the exception of the proximal tibia. Odds ratios for males were significant only for the radius (P < .01) and the distal tibia (P < .05). Odds ratios for males were not significant for the distal femur (P < .1), the proximal tibia (P < .1), or the calcaneus (P < .3).
Table 5.
Odds ratio after logistic regression model
| Radius | Femur | Proximal Tibia | Distal Tibia | Calcaneus | |
|---|---|---|---|---|---|
| Age |
4.71 P < .01 |
5.06 P < .01 |
5.62 P < .01 |
4.28 P < .01 |
4.18 P < .01 |
| Gender |
8.57 P < .01 |
13.60 P < .01 |
28.81 P < .01 |
14.64 P < .01 |
9.20 P < .01 |
| BMI |
3.22 P < .01 |
2.75 P < .01 |
2.80 P < .01 |
3.13 P < .01 |
2.60 P < .02 |
The model evaluates how much age, gender or BMI affects the odds of an individual to be stage 5 or not.
Table 6.
Conditional logistic regression stratified for age and gender to calculate odds ratios for overweight individuals to be stage 5 in comparison with their peers
| Radius | Femur | Proximal Tibia | Distal Tibia | Calcaneus | |
|---|---|---|---|---|---|
| Male |
4.03 P < .01 |
2.45 P < .1 (P = .087) |
2.50 P < .1 (P = .073) |
2.97 P < .05 (P = .026) |
1.71 P < .3 (P = .258) |
| Female |
2.65 P < .01 |
2.73 P < .01 |
3.02 P = .11 |
4.23 P < .02 |
8.71 P < .05 |
4. DISCUSSION
In this study, we used MRI to assess growth plate fusion in 958 healthy subjects aged 14.0‐21.5 years old at five different growth plate locations and found that the closure of the different growth plates follows a distinct ascending pattern whereby the calcaneus is the first location to fuse, followed by the distal tibia, proximal tibia, and distal femur, with the distal radius being the last growth plate to fuse. These results are unique since no other MRI studies have examined five growth plates in the same study of such a large population.
As expected, female growth plates close approximately 2 years before male (Table 2). GP has considered 17‐year‐old females and 19‐year‐old males to have complete fusion of the radial growth plate. 1 In our study, complete fusion of the distal radius is seen in 75% of 17‐year‐old females and 90% of 19‐year‐old males.
Our inter‐observer agreement is excellent, in line with previous studies. 8 , 11 , 24 These results support our theory that our MRI staging scale is a reliable and functional tool for paediatric radiologists, but further evaluation of these results is needed.
The self‐assessed Tanner scores in comparison with the growth plate maturity showed moderate correlation among males and moderate to low correlation among females (Tables 3 and 4). Our study used a similar questionnaire to Chavarro et al 25 for self‐assessment, with drawings depicting the five Tanner stages 12 , 13 , and a description of each stage. Chavarro et al did not record a difference among either females or males between self‐assessment and clinical assessment of pubic hair staging. Males especially tended to overrate their genitalia staging, and therefore, their assessment was considered inferior to assessment by a clinician. Despite these results, there is no doubt that self‐assessment is subjective, and it is not considered a reference standard, while clinical assessment by a paediatrician is considered the gold standard for assessment of pubertal development.
Concerning the relationship between weight and closure of the growth plate, odds ratios for stage 5 closure are significantly higher for overweight or obese subjects vs normal‐weight subjects in the female population (for all growth plates except the proximal tibia). Among obese and overweight males, the same pattern is observed for the growth plates of the radius and distal tibia. These results are in line with results by Sopher et al, 17 which found that obese adolescent boys had more advanced bone age than their peers. The bone age assessment should be possible even in plain radiographs, although we used MRI to evaluate bone maturation. To investigate further, we compared the height of overweight and normal‐weight individuals with completely closed growth plates at all five locations. We did not observe a significant difference in height between these groups, either among females (normal weight N = 234, mean height = 166.4 ± SD 6.4 cm; overweight N = 77, mean height = 166.2 ± SD 7.0 cm) or males (normal weight N = 166, mean height = 180.7 ± SD 8.8 cm; overweight N = 53, mean height = 179.3 ± SD 7.4 cm). These results are similar to Brener et al, 26 and as such, we find their conclusion that obese adolescents' “risk … impairment of their potential genetic adult height” to be a tendentious assertion. Our study also contradicts conclusions of Pinhas‐Hamel et al 27 that obese children have more advanced BA associated with lower mature height. Our population (Table 2) is more mature than the population reported by McKern et al, 28 whose autopsy study found that 29% of 17‐ to 18‐year‐olds (16 out of 55) and 40% of 19‐year‐olds (21 out of 52) had completely closed growth plates of the distal radius. Our results support our hypothesis that the age of complete maturity decreased during the second half of the past century.
The prevalence of overweight and obese adolescents seems to have increased among females but remained stable among males in our study, in comparison with a questionnaire study from 2008. 29 That study showed that a population of 15‐year‐olds in Western Sweden contained 9.9% overweight and 1.9% obese females and 15.1% overweight and 3.0% obese males. Globally, there has been an increase of childhood and adolescent obesity since Winquist's study was conducted. 17 Adipose tissue is a complex organ with an endocrine component that synthetises leptin and aromatase. Aromatase converts androgens to oestrogen which may be a factor in thelarche in females. Therefore, obesity may itself affect the onset of puberty. An increase of adipose tissue also raises insulin resistance, hyperinsulinism, increased insulin levels of factor 1 (IGF‐1), and a decrease of the sex‐binding hormone protein (SHBG). 15 , 30 , 31 IGF‐1 is linked to growth hormone (GH), which together with sex hormones is responsible for the growth spurt during puberty. Most studies have been performed on pre‐pubescent populations and may not be relevant for comparison with our population which is pubescent and/or post‐pubescent. Sopher et al 17 assert that a sole focus on BMI does not account for other potentially significant factors such as muscle‐fat quota or percentage of body fat. Ergo, BMI may well be higher in very active subjects due to greater muscle mass in comparison with their peers, as opposed to adipose tissue.
Our results show no significant correlation between regular physical activity and the maturation of the growth plates except for a very low correlation in the female distal radius (Tables 3 and 4), indicating regular physical activity. It has been difficult to prove a correlation between growth plate fusion and physical activity/mechanical load in the general population. 19 , 20 In contrast, a review of the literature 18 found that elite male soccer players often have a more mature bone age while elite female gymnasts often have a lower bone age than chronological age. Obvious limitations of these studies are the lack of objectively established chronological age, i.e. birth certificates, and the lack of control groups. It is likely that selection bias, at least in part, explains these findings. It is plausible that underdeveloped male soccer players do not have the same physical capacity and therefore cannot perform as well on an elite level than their more mature counterparts. In gymnastics, smaller and thinner girls have an advantage that may result in a selection bias against more physically mature female gymnasts. Therefore, the extent to which various physical activities and mechanical loads may affect maturation of the growth plate is still unclear. In addition, the extreme loading associated with elite gymnastics and weight lifting is limited to a statistically insignificant percentage of the larger population and will thus have little impact on the average tempo of skeletal maturation and growth plate fusion.
4.1. Limitations
Firstly, pubertal development was self‐assessed and not assessed by a paediatrician. Therefore, it is uncertain if the stages of puberty were accurately reported. One must also consider that our study population was either in puberty or post‐puberty. (Puberty starts at the age of 8‐13 years for females and 9‐14 for males.) In addition, measurements of sex hormone levels (IGF‐1 or GH) were not taken to evaluate if hormonal levels correlated with pubertal stages. Assessment of sexual characteristics by a paediatrician and blood sample analysis would have been costly, invasive, and stressful, thus complicating the recruitment process.
Second, as stated above, BMI is but a blunt tool to evaluate obesity. Additional assessment of muscle‐fat ratio along with measurements to evaluate lean muscle mass or the amount of visceral/subcutaneous fat could have improved the accuracy of classification as overweight or normal weight. We included self‐assessment of physical activity as a proxy to determine whether a higher BMI was caused by greater muscle mass or adipose tissue.
5. CONCLUSION
Implementation of MRI to determine the degree of growth plate fusion is feasible. In agreement with clinical observation, female growth plates close significantly earlier than male growth plates. Interestingly, regular or ordinary physical activity may not have significant effect on the maturation process among normal populations of healthy subjects. However, overweight seems to have a modest but significant effect on growth plate fusion. To date, no large, prospective, population‐based study has assessed the appearance of the growth plate in multiple regions in healthy adolescents and young adults. A prospective study with a large population of younger subjects to re‐examine growth plate maturation at different ages (pre‐/post‐puberty) would be of value.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the National Board of Health and Welfare and all the people who participated in this study such as radiographers, physicists, recruitment staff (Karolinska Trial Alliance) and staff at BTH Health Technology Research Lab, as well as all participants.
Kvist OFT, Luiza Dallora A, Nilsson O, et al. A cross‐sectional magnetic resonance imaging study of factors influencing growth plate closure in adolescents and young adults. Acta Paediatr. 2021;110:1249–1256. 10.1111/apa.15617
Funding information
The National Board of Health and Welfare funded this study.
REFERENCES
- 1. Greulich WW, Pyle SI. Radiographic Atlas of Skeletal Development of the Hand and the Wrist. Stanford, CA: Stanford U.P; 1959. [Google Scholar]
- 2. Tanner J, Whitehouse RH, Cameron N, Marshall WA, Healy MJR, Goldstein H. Assessment of Skeletal Maturity and Prediction of Adult Height (TW2 method). London and New York, Academic Press; 1983. [Google Scholar]
- 3. Abad V, Uyeda JA, Temple HT, De Luca F, Baron J. Determinants of spatial polarity in the growth plate. Endocrinology. 1999;140:958‐962. [DOI] [PubMed] [Google Scholar]
- 4. Dvorak J, George J, Junge A, Hodler J. Application of MRI of the wrist for age determination in international U‐17 soccer competitions. Br J Sports Med. 2007;41(8):497‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tscholl PM, Junge A, Dvorak J, Zubler V. MRI of the wrist is not recommended for age determination in female football players of U‐16/U‐17 competitions. Scand J Med Sci Sports. 2016;26(3):324‐328. [DOI] [PubMed] [Google Scholar]
- 6. Schmeling A, Schulz R, Reisinger W, Mühler M, Wernecke KD, Geserick G. Studies on the time frame for ossification of the medial clavicular epiphyseal cartilage in conventional radiography. Int J Legal Med. 2004;118(1):5‐8. [DOI] [PubMed] [Google Scholar]
- 7. Kellinghaus M, Schulz R, Vieth V, Schmidt S, Pfeiffer H, Schmeling A. Enhanced possibilities to make statements on the ossification status of the medial clavicular epiphysis using an amplified staging scheme in evaluating thin‐slice CT scans. Int J Legal Med. 2010;124(4):321‐325. [DOI] [PubMed] [Google Scholar]
- 8. Dedouit F, Auriol J, Rousseau H, Rougé D, Crubézy E, Telmon N. Age assessment by magnetic resonance imaging of the knee: a preliminary study. Forensic Sci Int. 2012;217(1–3):232.e1‐7. [DOI] [PubMed] [Google Scholar]
- 9. Saint‐Martin P, Rérolle C, Pucheux J, Dedouit F, Telmon N. Contribution of distal femur MRI to the determination of the 18‐year limit in forensic age estimation. Int J Legal Med. 2015;129(3):619‐620. [DOI] [PubMed] [Google Scholar]
- 10. Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332‐336. [DOI] [PubMed] [Google Scholar]
- 11. Kramer JA, Schmidt S, Jürgens K‐U, Lentschig M, Schmeling A, Vieth V. Forensic age estimation in living individuals using 3.0 T MRI of the distal femur. Int J Legal Med. 2014;128(3):509‐514. [DOI] [PubMed] [Google Scholar]
- 12. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nilsson O, Baron J. Impact of growth plate senescence on catch‐up growth and epiphyseal fusion. Pediatr Nephrol. 2005;20(3):319‐322. [DOI] [PubMed] [Google Scholar]
- 15. Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140(3):399‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vandewalle S, Taes Y, Fiers T, et al. Sex steroids in relation to sexual and skeletal maturation in obese male adolescents. J Clin Endocrinol Metab. 2014;99(8):2977‐2985. [DOI] [PubMed] [Google Scholar]
- 17. Sopher AB, Jean AM, Zwany SK, et al. Bone age advancement in prepubertal children with obesity and premature adrenarche: possible potentiating factors. Obesity. 2011;19(6):1259‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malina RM. Skeletal age and age verification in youth sport. Sports Med. 2011;41(11):925‐947. [DOI] [PubMed] [Google Scholar]
- 19. Muller L, Müller E, Hildebrandt C, Kapelari K, Raschner C. The assessment of biological maturation for talent selection ‐ which method can be used? Sportverletz Sportschaden. 2015;29(1):56‐63. [DOI] [PubMed] [Google Scholar]
- 20. Malina RM, Coelho E Silva MJ, Figueiredo AJ, Carling C, Beunen GP. Interrelationships among invasive and non‐invasive indicators of biological maturation in adolescent male soccer players. J Sports Sci. 2012;30(15):1705‐1717. [DOI] [PubMed] [Google Scholar]
- 21. Kvist Ola, Dallora Ana Luiza, Nilsson Ola, Anderberg Peter, Berglund Johan Sanmartin, Flodmark Carl‐Erik, Diaz Sandra. Comparison of reliability of magnetic resonance imaging using cartilage and T1‐weighted sequences in the assessment of the closure of the growth plates at the knee. Acta Radiologica Open. 2020;9 (9):205846012096273. 10.1177/2058460120962732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70(4):213‐220. [DOI] [PubMed] [Google Scholar]
- 23. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22(3):276‐282. [PMC free article] [PubMed] [Google Scholar]
- 24. Ekizoglu O, Hocaoglu E, Inci E, Can IO, Aksoy S, Kazimoglu C. Forensic age estimation via 3‐T magnetic resonance imaging of ossification of the proximal tibial and distal femoral epiphyses: Use of a T2‐weighted fast spin‐echo technique. Forensic Sci Int. 2016;260:102e1‐102e7. [DOI] [PubMed] [Google Scholar]
- 25. Chavarro JE, Watkins DJ, Afeiche MC, et al. Validity of self‐assessed sexual maturation against physician assessments and hormone levels. J Pediatr. 2017;186:172‐178 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brener A, Bello R, Lebenthal Y, Yackobovitch‐Gavan M, Phillip M, Shalitin S. The impact of adolescent obesity on adult height. Horm Res Paediatr. 2017;88(3–4):237‐243. [DOI] [PubMed] [Google Scholar]
- 27. Pinhas‐Hamiel O, Benary D, Mazor‐Aronovich K, et al. Advanced bone age and hyperinsulinemia in overweight and obese children. Endocr Pract. 2014;20(1):62‐67. [DOI] [PubMed] [Google Scholar]
- 28. McKern TW, Stewart TD. Skeletal changes in young American males, in U S. Army Quartermaster Research and Development Command ; 1957.
- 29. Winkvist A, Hultén B, Kim J‐L, et al. Dietary intake, leisure time activities and obesity among adolescents in Western Sweden: a cross‐sectional study. Nutr J. 2016;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee HS, Shim YS, Jeong HR, Kwon EB, Hwang JS. The association between bone age advancement and insulin resistance in prepubertal obese children. Exp Clin Endocrinol Diabetes. 2015;123(10):604‐607. [DOI] [PubMed] [Google Scholar]
- 31. de Groot CJ, van den Berg A, Ballieux BEPB, et al. Determinants of advanced bone age in childhood obesity. Horm Res Paediatr. 2017;87(4):254‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


