The pathogenic role of γδ T cells in experimental cerebral malaria (ECM) is dependent on the liver stage of infection. In the presence of IFN‐γ‐producing γδ T cells, parasites that egress the liver are more virulent and lead to ECM. In humans, Vγ9Vδ2 T cells recognize soluble Plasmodium phosphoantigens and become activated, producing IFN‐γ and inducing CM. After repeated parasite exposure, Vγ9Vδ2 T cells decrease production of pro‐inflammatory cytokines, which associates with clinical tolerance.
Keywords: cerebral malaria, clinical immunity, experimental cerebral malaria, gamma‐delta T cells, interferon‐gamma, liver stage, Plasmodium, sporozoites, tolerance
Abstract
Malaria remains a devastating global health problem, resulting in many annual deaths due to the complications of severe malaria. However, in endemic regions, individuals can acquire ‘clinical immunity’ to malaria, characterized by a decrease in severe malaria episodes and an increase of asymptomatic Plasmodium falciparum infections. Recently, it has been reported that tolerance to ‘clinical malaria’ and reduced disease severity correlates with a decrease in the numbers of circulating Vγ9Vδ2 T cells, the major subset of γδ T cells in the human peripheral blood. This is particularly interesting as this population typically undergoes dramatic expansions during acute Plasmodium infections and was previously shown to play antiparasitic functions. Thus, regulated γδ T‐cell responses may be critical to balance immune protection with severe pathology, particularly as both seem to rely on the same pro‐inflammatory cytokines, most notably TNF and IFN‐γ. This has been clearly demonstrated in mouse models of experimental cerebral malaria (ECM) based on Plasmodium berghei ANKA infection. Furthermore, our recent studies suggest that the natural course of Plasmodium infection, mimicked in mice through mosquito bite or sporozoite inoculation, includes a major pathogenic component in ECM that depends on γδ T cells and IFN‐γ production in the asymptomatic liver stage, where parasite virulence is seemingly set and determines pathology in the subsequent blood stage. Here, we discuss these and other recent advances in our understanding of the complex—protective versus pathogenic—functions of γδ T cells in malaria.
Abbreviations
- CM
cerebral malaria
- ECM
experimental cerebral malaria
- IFN‐γ
interferon‐γ
- IL‐
interleukin
- MIP
macrophage inflammatory protein
- MSP1
merozoite surface protein 1
- P.
Plasmodium
- pRBCs
parasitized red blood cells
- RAMA
Rhoptry‐associated membrane antigen
- Spz
sporozoites
- TCR
T‐cell receptor
- TNF
tumor necrosis factor
- WT
wild‐type.
Introduction
Malaria remains a devastating global health problem, responsible for more than 228 million cases per year worldwide, leading to more than 405 000 annual deaths due to severe malaria, such as cerebral malaria (CM), mostly caused by Plasmodium (P.) falciparum [1]. The most vulnerable groups affected by malaria are children under 5 years old, which accounted for 67% of all malaria deaths worldwide, and pregnant women [1].
In endemic regions, adults and children older than 5 years acquire considerably rapid ‘clinical immunity’ to malaria, characterized by a decrease in severe malaria episodes and an increase of asymptomatic P. falciparum infections [2]. Our understanding of ‘clinical immunity’ is made difficult by the complex life cycle of Plasmodium in the host, comprising two stages in two different tissues, liver and blood, together with other factors, such as high genetic variation of the parasite, age of the host and frequency of infection [3].
In natural infections, malaria is transmitted through the bite of infected Anopheles mosquitoes, in which Plasmodium sporozoites (Spz) are delivered into the skin and from there find their way to the liver [4]. After invading a hepatocyte, the Spz develops and replicates producing a schizont containing thousands of merozoites. Merozoites then egress from hepatocytes and are released into the bloodstream where they invade red blood cells and initiate the blood‐stage infection. The clinically ‘silent’ liver stage is thus an essential step in the Plasmodium life cycle that always precedes the cyclic intraerythrocytic infection where the clinical symptoms of malaria, such as CM, appear [4].
Due to this complexity, stemming from both the malaria parasite and the human immune system, interactions between the parasite and the host during infection result in outcomes ranging from protective immunity to ‘clinical immunity’ or to highly deleterious immune responses, particularly in severe malaria [5, 6] One of the immune populations gathering increasing interest in this context are γδ T cells. In this viewpoint, we discuss and integrate recent advances from human and mouse studies toward a better understanding of the multifaceted functions of γδ T during malaria infection, with a particular focus on CM.
γδ T‐cell responses to Plasmodium infection
γδ T cells are one of the immune populations that respond most dramatically to Plasmodium infection, given that it induces very marked γδ T‐cell expansions both in mice [7, 8, 9] and in humans [10, 11, 12, 13].
Murine γδ T cells consist of various subsets with diverse properties regarding thymic ontogeny, homing to anatomical locations and functional potential [14]. The T‐cell receptor (TCR) Vγ chain usage can vary substantially across tissues, and for example, in the liver, γδ T cells can express Vγ1+, Vγ4+, or Vγ6+ TCRs [14]. Like in mice, γδ T cells are also a minor population (1–5% of leukocytes) in the human peripheral blood, but are more abundant in tissues, in particular epithelial layers, such as intestine and skin [15]. Human γδ cells are typically characterized according to the variable regions of TCRδ (instead of TCRγ) chain [16]. While Vδ1+ T cells are the major γδ T‐cell population at epithelial sites, Vδ2+ T cells, which most often contain a Vγ9 chain, are the main subset in peripheral blood [17]. Vγ9Vδ2 T cells are able to recognize low molecular weight non‐peptidic phosphoantigens, enabling them to respond to a diverse range of pathogens, including P. falciparum [18]. In fact, this subset can reach more that 40% of blood leukocytes after primary Plasmodium infections, while producing key pro‐inflammatory cytokines, especially type 1 effector cytokines like interferon γ (IFN‐γ) and tumor necrosis factor (TNF), in response to parasite antigen stimulation [12, 13, 19, 20].
A considerable number of studies with humans and murine γδ T cells suggest they may paradoxically contribute for both protection and pathology during Plasmodium infection. Some studies have shown that Vγ9Vδ2 T cells are able to control/ inhibit parasite replication by targeting and killing extracellular merozoites though a granulysin‐mediated process [21, 22, 23], as well as killing intracellular late‐stage parasites during the intraerythrocytic stage, also through granulysin‐mediated release of cytotoxic granzymes [24] (Fig. 1), and act as antigen‐presenting cells for αβ T cells in response to intraerythrocytic stage parasites [25]. However, other reports suggested that Vγ9Vδ2 T cells may be linked to pathological outcomes, since a decrease in their numbers (in the blood) is associated with tolerance to ‘clinical malaria’ and reduced disease severity [5, 26].
Fig. 1.
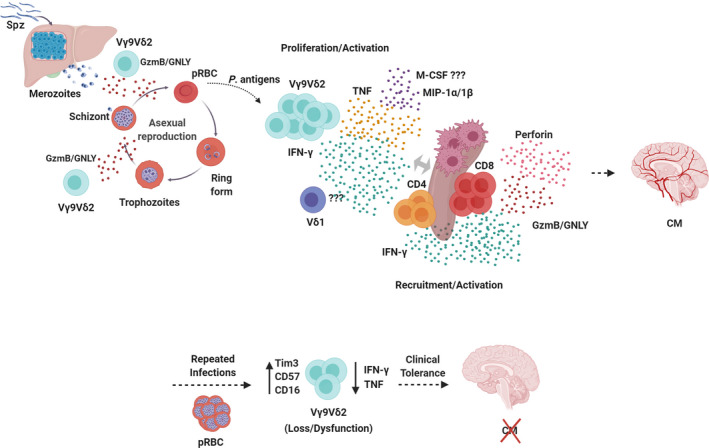
Functional activities of human γδ T cells in malaria. Infected Anopheles mosquitoes inject Plasmodium Spz into the host skin from where they migrate to the liver and invade hepatocytes to develop into schizonts containing thousands of merozoites. Merozoites then egress from hepatocytes and are released into the bloodstream where they invade red blood cells and initiate the blood‐stage infection, when clinical symptoms of malaria, such as CM, appear. Vγ9Vδ2 T cells are able to control/inhibit parasite replication by targeting and killing extracellular merozoites and intracellular late‐stage parasites though granulysin (GNLY)‐mediated release of cytotoxic granzymes (GzmB) during the intraerythrocytic stage. Vγ9Vδ2 T cells recognize soluble phosphoantigens released from schizont stage parasites and, potentially, other pRBC stages, and become activated, producing pro‐inflammatory cytokines, like IFN‐γ and TNF, and chemokines, like MIP‐1α and MIP‐1β. This promotes splenic activation and differentiation of CD4+ and CD8+ T cells into Th1, IFN‐γ‐producing, and cytotoxic cells, and subsequent migration to the brain, where they cause neuroinflammation and, ultimately, CM. However, after repeated parasite exposure, Vγ9Vδ2 T cells may increase expression of immunoregulatory molecules, such as Tim‐3, and decrease production of pro‐inflammatory cytokines, which associates with clinical tolerance.
In mice, most studies have been performed with parasitized red blood cells (pRBCs), which bypass the liver stage to directly induce blood‐stage infection. A recent study using a Plasmodium chabaudi infection model revealed a macrophage colony‐stimulating factor (M‐CSF)‐producing γδ T‐cell subset that provided protection at late stage of infection [27]. In this model, two different types of γδ T‐cell responses were observed: During the acute stage, these cells produced mainly IFN‐γ, while during the postacute stage, M‐CSF was the main cytokine produced and was essential to prevent parasite recrudescence [27]. Other studies have suggested that γδ T cells may exert an immunoregulatory role by controlling alpha‐beta (αβ) T‐cell function in Plasmodium yoelii 17X nonlethal (17XNL) and P. chabaudi infections [28, 29], whereas in Plasmodium berghei XAT (a nonlethal strain) infection model, γδ T cells expressing CD40L promoted dendritic cell activation and induced clearance of the parasite [30].
In the context of Spz immunization studies, several reports have shown that γδ T cells play an important protective role in malaria infection in humans and in P. yoelii 17XNL and P. berghei infection mouse models [31, 32, 33]. However, it is still not clear how γδ T cells exert their protective role in the context of immunization studies, namely if they function as effector cells independently of αβ T cells, in particular CD8+ T cells, or instead act as accessory cells, alongside CD8α+ dendritic cells (DC), to induce protective CD8+ T‐cell responses [31, 32, 33]. In any case, all studies have suggested an important protective role of γδ T cells during Spz vaccination studies.
Cerebral malaria
Severe malaria is a general term that includes various and overlapping lethal syndromes, such as CM and respiratory distress, that may coexist during the malaria infection [34]. The development of severe malaria, and ultimately death, may depend on several factors, such as the species of the parasite, the innate and acquired immunity of the host, as well as the efficacy of antimalarial treatment [34].
Cerebral malaria is one of the most common forms of severe malaria, responsible for the majority of child mortality, presenting between 15% and 25% fatality rate, and for which there is no effective therapy [35]. Although the nature of the cellular and molecular mechanisms leading to CM remains poorly understood two nonexclusive hypotheses, the mechanical (sequestration) obstruction and the immune‐driven inflammation, try to explain the complex interactions between the malaria parasite and the host that lead to this pathology [36, 37]. However, these two phenomena may not fully explain the genesis of CM [38]. More recently, a new hypothesis has been proposed stating that the involvement of acute liver failure, together with blood–brain barrier breakdown, may be sufficient and necessary for CM development [38]. This hypothesis is further supported by two phenomena that occur during experimental CM (ECM): liver damage due to parasite sequestration/accumulation [39], and activation of CD8+ T cells, a process that requires a metabolic shift from oxidative processes to aerobic glycolysis and glutaminolysis, thus requiring high levels of glutamine [40]. Indeed, several reports have linked high glutamine levels, and consequently high ammonia levels, to encephalopathy associated with acute fulminant liver failure [41]. More recently, a study showed the therapeutic potential of blocking glutamine metabolism to rescue mice from ECM development [42]. Overall these studies strengthen the importance of the liver in ECM pathogenesis.
Both the sequestration and immunopathology hypotheses have been widely tested in the mouse model for CM, P. berghei ANKA‐induced ECM in C57BL/6 mice [43, 44, 45]. The ECM model recapitulates many of the features of CM observed in children [46, 47], such as the accumulation of pRBCs and CD8+ T cells in the brain vasculature [45, 46, 48], and blood–brain barrier (BBB) dysfunction and edema [46]. On the other hand, ECM is also an immune‐mediated disease where CD8+T cells and the pro‐inflammatory cytokine IFN‐γ play central pathogenic roles [6, 49, 50]. Recently, a study showed definitively the presence of CD8+T cells in close contact with the microvasculature in brains of children that died with CM, as well as the presence of pRBC along the cerebrovasculature, which may promote endothelial antigen acquisition and cross‐presentation to CD8+T cells [47]. These findings corroborate the results obtained with the ECM model and reinforce the relevance of this experimental system to elucidate CM associated‐pathogenic processes in humans and to assess new therapeutic targets for CM adjunctive therapy.
The vast majority of the studies using the ECM model have challenged the mice with P. berghei‐pRBC, a route of infection that bypasses the liver stage of Plasmodium infection, thus neglecting the potential impact of the liver stage in the subsequent (erythrocytic and symptomatic phase) of Plasmodium infection and in CM pathogenesis. In fact, very few studies have shown that pre‐erythrocytic or early immune responses may modulate downstream immune responses and thereby impact ECM development or clinical symptoms, respectively, in mice and in humans [26, 51, 52, 53, 54, 55, 56]. Some of these studies used chemical or genetically modified P. berghei ANKA parasites that after Spz infection showed impaired development during liver and intraerythrocytic stages, thus impacting on subsequent systemic immune responses and, ultimately, on ECM development [53, 54]. By contrast, another study with a transgenic P. berghei ANKA parasite that moderately overexpress profilin, an immunomodulatory protein, and that after Spz infection did not show evident developmental impairments, induced an early production of the regulatory cytokine interleukin (IL)‐10 and pro‐inflammatory cytokines, such as IL‐12p70, IL‐6, and TNF [56]. This early immune response seemed to dampen the subsequent pro‐inflammatory responses during blood stage and prevented the development of ECM [56]. Notably, this transgenic parasite induced lower sterile immunity in the context of immunization studies when compared with wild‐type (WT) parasites, suggesting reduced hepatic immune responses [56]. It would be interesting to assess the functional interaction of γδ T cells with this transgenic parasite in the context of whole‐Spz vaccination strategies.
Human γδ T cells in severe malaria
Several studies have suggested different roles for the two main human γδ T‐cell subsets, expressing either Vδ1+ or Vδ2+ TCRs, in response to P. falciparum in distinct experimental or clinical settings [3, 57]. In fact, the response of γδ T‐cell subsets seems to depend on several factors such as the age of the host (children or adults), ethnicity, that is, Caucasians or Africans, and malaria endemicity, that is, high or low endemic areas. Although Vγ9Vδ2 T cells seem to be the main γδ T‐cell subset in healthy Caucasians, this is not observed in healthy individuals living in malaria‐endemic areas [58]. Notably, it has been reported that both Vγ9+ and Vδ1+ subsets seem to increase proportionally following P. falciparum infection in patients from malaria‐endemic areas [58, 59]. Thus, the sustained Vγ9Vδ2 T cell‐dominated responses in studies using γδ T cells from peripheral blood of nonexposed individuals have not been corroborated by some African studies [60, 61]. Actually, it has been reported that in the context of endemic malaria, where populations are exposed to consecutive malaria infections and/or chronic infection, Vδ1+ T cells seem to be the main subset in circulation [58]. While there is no clear explanation for this observation, it has been suggested that the retention of active Vγ9Vδ2 T cells in the spleen and/or the reemergence of tissue‐resident Vδ1+ T cells, such as hepatic Vδ1+ T cells, into the circulation after antimalarial chemotherapy, may change the proportions of both subsets in the peripheral blood [60].
An emerging topic is the role of human γδ T cells in ‘clinical malaria’. Although multiple studies have been performed with malaria‐naïve and infected adults [12, 20, 62, 63], considerably fewer have been done in children from endemic countries that develop severe malaria, in particular CM, and are subjected to recurrent Plasmodium infections [5, 26, 55, 61, 63, 64, 65]. Of note, studies performed in children and adults from African endemic countries showed that percentage and activation markers of γδ T cells do not seem to discriminate ‘clinical malaria’ cases from asymptomatic infections [61, 62, 64]. Indeed, it has been reported that age, level of previous exposure, and antimalarial chemotherapy seem to be crucial determinants of malaria‐induced γδ T‐cell responses and in the observed proportions of Vγ9Vδ2 T cells and Vδ1+ T cells in peripheral blood [3, 64]. A study using convalescent samples from children with severe malaria and living in high endemic areas showed that CD14+ monocytes and γδ T cells were the predominant cellular sources of TNF, macrophage inflammatory protein (MIP)‐1β, and MIP‐1α after in vitro stimulation with pRBC [26]. Interestingly, recent studies have shown a decrease in Vγ9Vδ2 T‐cell numbers associated with tolerance to clinical malaria and reduced disease severity [5]. Thus, in malaria‐endemic areas, the loss and dysfunction of Vδ2+ T cells may represent a mechanism of disease tolerance that seems to contribute to the development of ‘clinical immunity’ in children that are subjected to successive malaria episodes [5, 65]. The production of pro‐inflammatory cytokines, such as TNF and IFN‐γ, by Vδ2+ T cells may have two opposing effects during malaria infection, on the one hand an antiparasitic effect that limits parasite burden, but on the other hand, it can promote the development of clinical symptoms [26]. Therefore, the acquisition of ‘clinical immunity’ may depend on the ability of the host to down‐modulate pro‐inflammatory responses by Vδ2+ T cells, which will favor the presence of asymptomatic infections and perpetuate P. falciparum transmission in endemic countries, as suggested by several studies [5, 19, 66] (Fig. 1). Nonetheless, it is still not very clear how Vγ9Vδ2 T cells contribute to both ‘clinical immunity’ and susceptibility to severe disease in the course of P. falciparum infection as well as the role of Vδ1+ T cells during infection [57].
Murine γδ T cells in experimental cerebral malaria
Effector lymphocytes, especially CD4+ and CD8+ T cells, as well as pro‐inflammatory cytokines, like IFN‐γ, TNF, and lymphotoxin alpha, have long been shown to play crucial roles in ECM pathogenesis [6]. In fact, mice (in the C57Bl/6 genetic background) deficient for all T cells, or just αβ T cells, or only CD8+ T cells, all fail to develop ECM upon P. berghei ANKA infection [8]. Although an early pro‐inflammatory immune response has been associated with protection against infection, this needs to be followed by a rapid resolution of inflammation in order to prevent immunopathology [67]. It is therefore critical to dissect the early, innate‐like immune responses that drive the induction of inflammation and subsequent pathological processes in ECM.
In fact, γδ T cells are endowed with an innate capacity to produce high amounts of IFN‐γ and IL‐17, which is preprogrammed during thymic development [9, 68, 69]. However, the pioneering study addressing the role of γδ T cells in ECM development, which used P. berghei ANKA pRBCs, showed that mice deficient for γδ T cells (TCRδ−/−) developed ECM similarly to control mice, while mice depleted of γδ T cells by monoclonal antibody were partially protected from CM [70]. This prompted us to recently readdress the role of γδ T cells in ECM in a setting that is closer to the natural infection, namely by using mosquito bite or Spzs to initiate the infection. Importantly, these routes, unlike pRBCs inoculation, lead to infection of the liver and development of the parasite inside hepatocytes before they egress to the blood. Importantly, until very recently nothing was known about the properties and contributions of γδ T cells during a primary Spz‐induced Plasmodium infection on the course to ECM development.
Pathogenic role for γδ T cells in ECM upon liver‐stage infection
The liver is a central organ for several crucial metabolic processes in addition to its nutrient storage and detoxifying capacities [71]. Besides these functions, its critical position between the gastrointestinal system and the systemic circulation system makes this organ crucial for innate and adaptive immunity against pathogens as well as for induction of tolerance to nonpathogens, such as dietary antigens [71, 72]. The liver is composed of parenchyma cells, among which hepatocytes comprises 60–80% of the cells, and nonparenchyma cells, with the lymphocyte population comprising ~ 25% of the total cells [71, 72]. In healthy conditions, the liver is an anti‐inflammatory or tolerogenic organ but under specific conditions is able to mount robust immune responses against infectious or noninfectious stimuli [72]. In fact, in a P. berghei Spzs infection model a robust innate type I IFN response was observed during the liver stage [73]. Despite this, the mechanisms regulating the balance between an efficient immune response and tolerance are essential for liver function, even if they remain poorly understood [71, 72].
The liver is highly enriched in innate immune cells, such as macrophages (Kupffer cells), natural killer (NK), natural killer T (NKT) cells, and also γδ T cells, in addition to more adaptive lymphocytes, namely αβT cells and B cells [74]. γδ T cells constitute 15–25% of the total number of hepatic T cells and have been suggested to be important inducers of hepatic inflammation. Hepatic γδ T cells can produce high levels of pro‐inflammatory cytokines, such as IL‐17, TNF, and IFN‐γ [71], and comprise various Vγ TCR chains, that is, Vγ1, Vγ4, and Vγ6 in mice and Vδ1 and Vδ3 in humans [16].
Several studies have shown that hepatic γδ T cells may play different functional roles, that is, pathogenic or protective, depending on the experimental models studied [71]. For example, during Listeria monocytogenes infection, Vγ4+ T cells, which are the major IL‐17 producing cell type in the liver, are crucial for protective immunity during early infection [75]. In contrast, during Schistosoma japonicum infection, IL‐17 production by γδ T cells, also the major IL‐17‐producing cell type in this infection model apparently, plays a pathogenic role since the neutralization of IL‐17 reduced liver inflammation and pathology [76]. Moreover, it was recently shown that hepatic γδ T cells predominantly producing high levels of IL‐17A exhibited a Vγ chain repertoire distinct from γδ T cells of other organs [77].
Besides their potential role in immunization studies [31, 32, 33], the function of γδ T cells in primary pre‐erythrocytic Plasmodium infection remains understudied and is of utmost importance to understand if the innate immune responses that occur in the liver may impact ECM pathogenesis. In addition, the crosstalk between liver and blood stages of Plasmodium infection has been poorly studied and remains incompletely understood but is crucial for inducing effective adaptive immune responses against the infection [78, 79].
We have addressed the impact of γδ T cells and liver‐stage infection on ECM development using a Spz‐induced infection model [51]. We showed that TCRδ−/−mice are resistant to ECM when infected with P. berghei ANKA Spzs, the liver‐infective form of the parasite and the natural route of infection, in contrast to the susceptible phenotype when challenged with P. berghei ANKA‐pRBC [51]. The observed pathogenic role of γδ T cells in ECM development was strictly dependent on the liver stage without affecting the intrahepatic development of the parasite or inhibiting parasite replication during the intraerythrocytic stage of infection [51]. In fact, a decreased pro‐inflammatory microenvironment was observed in TCRδ−/− livers, suggesting a mechanism of disease tolerance since the lack of immunopathology did not involve reduced parasite growth rate or load [80, 81, 82]. These findings raise some issues in the context of immunization studies and, consequently, in the balance between sterile immunity and inflammation‐induced immunopathology.
Interestingly, during Spz‐induced liver infection, hepatic γδ T cells were the main IL‐17A‐producing cells, as seen in other infections [83, 84], while IFN‐γ+ γδ T cells were only a fraction of the total hepatic IFN‐γ+ cells; however, IFN‐γ+ γδ T cells seem to be required for optimal IFN‐γ production by other hepatic lymphocytes, such as CD4 + and CD8 + T and NK cells (unpublished data). Along these lines, a new specific M‐CSF‐producing γδ T‐cell subset was recently identified in the liver (as well as spleen and lung) of mice infected with P. chabaudi, suggesting that these cells might shape the myeloid compartment in postacute stage of the infection [27]. In fact, the crosstalk between γδ T cells and myeloid cells has already been observed in other infections and cancer models [85, 86]. Therefore, it would be interesting to assess the role of these M‐CSF‐producing γδ T cells in the liver, their crosstalk with other immune cells and the potential impact in malaria pathogenesis after P. berghei Spz infection.
Importantly, in our study, liver infection impacted on the subsequent intraerythrocytic stage of the parasite by promoting an early IFN‐γ response by γδ T cells that conditioned IFN‐γ production by splenic CD4+ and CD8+ T cells (Fig. 2) [51]. Indeed, previous studies have shown the importance of innate IFN‐γ production by γδ T cells from malaria naïve human donors, as well as the impact of IFN‐γ on the differentiation of effector CD4+ Th1 cells that promote CD8+ T‐cell accumulation in the brain, leading to ECM development [87, 88]. Consistent with these studies, our Spz infection study showed that γδ T cells promoted the accumulation of inflammatory IFN‐γ‐producing and cytotoxic T cells in the brain, key features of ECM development (Fig. 2) [51]. It would be interesting to address the potential interaction between γδ T cells and CD8+ T cells in/with the cerebrovasculature in the ECM model and in human samples.
Fig. 2.
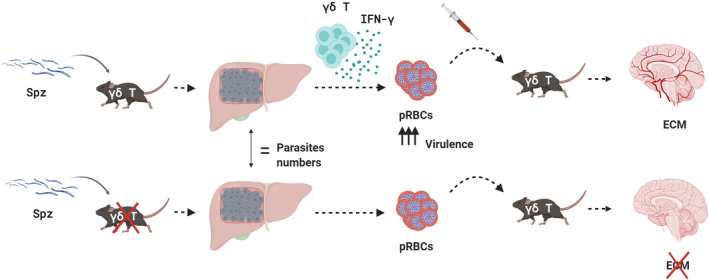
γδ T cells and IFN‐γ modulate the pathogenicity of liver‐derived parasites in ECM development. Graphical summary of adoptive transfer experiments showing that pathogenic role of γδ T cells in ECM is dependent on the liver stage of infection. In the presence of IFN‐γ producing γδ T cells, the parasite that egresses the liver is more virulent and induces the inflammatory cascade that leads to ECM development. By contrast, pRBCs collected from TCRδ−/− mice are substantially less pathogenic than those from WT mice.
Surprisingly, during liver stage, the relative quantity of parasites developing in the liver and the prepatency period of the infection was not significantly different between TCRδ−/− and WT mice (Fig. 2). Therefore, we hypothesized that parasites derived from the liver of both mouse strains were qualitatively different, resulting in different degrees of virulence [51]. In fact, it has been known for some time that parasite virulence and disease severity increases with serial blood passage of Plasmodium through mice, primates, or humans and that mosquito transmission resets Plasmodium virulence [89, 90, 91]. In addition, recent studies have corroborated these findings showing differences in gene expression between blood and mosquito passage parasites and their impact in parasite virulence and host immune responses [91, 92, 93].
Our transcriptional analyses of parasites derived from TCRδ−/− and WT mice following Spz infection revealed differential expression of various surface and rhoptry glycosylphosphatidy inositol‐anchored merozoite proteins, such as MSP1 and RAMA [51]. Notably, several of these proteins are potential targets for host immune cells during the intraerythrocytic stage, since it was shown that they induce pro‐inflammatory responses and contribute to malaria pathogenesis [94, 95, 96, 97]. Of note, these parasite proteins have been considered as potential components of a multivalent subunit vaccine against malaria [98, 99, 100]. Importantly, the transcriptional changes (relative to WT controls) observed in liver stage‐derived parasites from TCRδ−/− mice or from IFN‐γ−/− mice were very similar, suggesting a key role for IFN‐γ in the γδ T cell‐dependent transcriptional modulation of Plasmodium parasites. To functionally demonstrate the impact of this modulation in ECM pathogenesis, we performed adoptive transfer experiments, in which we found pRBCs collected from TCRδ−/− mice to be substantially less pathogenic than those from WT mice, as indicated by higher survival rates, independently of the recipient host genotype (Fig. 2) [51].
Overall, these observations firmly established the role of γδ T cells in promoting an IFN‐γ‐rich inflammatory microenvironment and impacting the expression of Plasmodium immunogenic proteins, thus increasing parasite virulence and promoting immunopathology in ECM (Fig. 2).
Concluding remarks
Several studies have significantly enhanced our knowledge on the diverse roles played by γδ T cells in malaria infection. This notwithstanding, additional mechanistic and functional studies are still required to answer several open questions, such as how to integrate the evidence that on one hand γδ T cells are required, either as effector or accessory cells, while on the other hand, they seem to contribute to severe malaria pathogenesis. In fact, γδ T cells seem to play a dual role in malaria infection, that is, a protective function in whole‐Spz sterile immunity and a pathogenic role in severe malaria. How to balance this tradeoff when developing γδ T cell‐based therapeutic strategies will be challenging, since on the one hand sterile immunity presupposes the presence of hepatic γδ T cells and on the other hand these cells seem to be drivers of immunopathology under the natural route of infection.
Although mouse models have been an irreplaceable tool to study the function of γδ T cells [101, 102], it is essential to translate and apply such findings in human clinical settings. However, this is complicated by distinct developmental programs and tissue locations of γδ T cells between human and mice and because there are no mouse orthologues to the human Vγ9+ and Vδ1+ subsets. Importantly, it is crucial to understand the complexity of γδ T cells in terms of their different tissue‐specific homing, functional plasticity, activation mode, antigen recognition, recall functions, and crosstalk with other immune cells, in order to elucidate their role in malaria infection and, in particular, CM.
Though sterile immunity to Plasmodium may be the ultimate goal of vaccination strategies, therapies inducing clinical tolerance to malaria seem to be a more achievable goal in the short term. Importantly, a more comprehensive knowledge of the interaction between the host immune responses and the virulence mechanisms of the parasite in severe malaria will be fundamental for the development of effective immunological therapies. Furthermore, a better understanding of the basic biology and functions of liver‐resident γδ T cells will be most valuable for the development of more efficacious Spz‐based vaccines to induce sterile immunity and/or improved γδ T cell‐based prophylactic or therapeutic strategies to induce ‘clinical immunity’ and overcome susceptibility to severe disease.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
AP and BSS conceived and wrote the manuscript.
Acknowledgements
This viewpoint was supported by the European Research Council (CoG_646701 to BSS). AP currently holds a research position financially supported by Fundação para a Ciência e a Tecnologia (under the Decree‐law no. 57/2016 of July 19, as amended by the Law no. 57/2017).
Contributor Information
Ana Pamplona, Email: anapamplona@medicina.ulisboa.pt.
Bruno Silva‐Santos, Email: bssantos@medicina.ulisboa.pt.
References
- 1. WHO (2019) https://www.who.int/publications‐detail/world‐malaria‐report‐2019
- 2. Marsh K (1992) Malaria–a neglected disease? Parasitology 104 (Suppl), S53–S69. [DOI] [PubMed] [Google Scholar]
- 3. Dantzler KW & Jagannathan P (2018) gammadelta T cells in antimalarial immunity: new insights into their diverse functions in protection and tolerance. Front Immunol 9, 2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, Krueger A, Pollok JM, Menard R & Heussler VT (2006) Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313, 1287–1290. [DOI] [PubMed] [Google Scholar]
- 5. Jagannathan P, Kim CC, Greenhouse B, Nankya F, Bowen K, Eccles‐James I, Muhindo MK, Arinaitwe E, Tappero JW, Kamya MR et al. (2014) Loss and dysfunction of Vdelta2(+) gammadelta T cells are associated with clinical tolerance to malaria. Sci Transl Med 6, 251ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schofield L & Grau GE (2005) Immunological processes in malaria pathogenesis. Nat Rev Immunol 5, 722–735. [DOI] [PubMed] [Google Scholar]
- 7. Pied S, Roland J, Louise A, Voegtle D, Soulard V, Mazier D & Cazenave PA (2000) Liver CD4‐CD8‐ NK1.1+ TCR alpha beta intermediate cells increase during experimental malaria infection and are able to exhibit inhibitory activity against the parasite liver stage in vitro . J Immunol 164, 1463–1469. [DOI] [PubMed] [Google Scholar]
- 8. Yanez DM, Manning DD, Cooley AJ, Weidanz WP & van der Heyde HC (1996) Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J Immunol 157, 1620–1624. [PubMed] [Google Scholar]
- 9. Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ et al. (2009) CD27 is a thymic determinant of the balance between interferon‐gamma‐ and interleukin 17‐producing gammadelta T cell subsets. Nat Immunol 10, 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ho M, Tongtawe P, Kriangkum J, Wimonwattrawatee T, Pattanapanyasat K, Bryant L, Shafiq J, Suntharsamai P, Looareesuwan S, Webster HK et al. (1994) Polyclonal expansion of peripheral gamma delta T cells in human Plasmodium falciparum malaria. Infect Immun 62, 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perera MK, Carter R, Goonewardene R & Mendis KN (1994) Transient increase in circulating gamma/delta T cells during Plasmodium vivax malarial paroxysms. J Exp Med 179, 311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roussilhon C, Agrapart M, Guglielmi P, Bensussan A, Brasseur P & Ballet JJ (1994) Human TcR gamma delta+ lymphocyte response on primary exposure to Plasmodium falciparum . Clin Exp Immunol 95, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roussilhon C, Agrapart M, Ballet JJ & Bensussan A (1990) T lymphocytes bearing the gamma delta T cell receptor in patients with acute Plasmodium falciparum malaria. J Infect Dis 162, 283–285. [DOI] [PubMed] [Google Scholar]
- 14. Miguel Muñoz‐Ruiz NS, Pennington DJ & Silva‐Santos B (2017) Thymic determinants of γδ T cell differentiation. Trends Immunol 38, 336–344. [DOI] [PubMed] [Google Scholar]
- 15. Hayday AC (2009) Gammadelta T cells and the lymphoid stress‐surveillance response. Immunity 31, 184–196. [DOI] [PubMed] [Google Scholar]
- 16. Pang DJ, Neves JF, Sumaria N & Pennington DJ (2012) Understanding the complexity of gammadelta T‐cell subsets in mouse and human. Immunology 136, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pang DJ, Neves JF, Sumaria N & Pennington DJ (2012) Understanding the complexity of γδ T‐cell subsets in mouse and human. Immunology 136, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Behr C, Poupot R, Peyrat MA, Poquet Y, Constant P, Dubois P, Bonneville M & Fournie JJ (1996) Plasmodium falciparum stimuli for human gammadelta T cells are related to phosphorylated antigens of mycobacteria. Infect Immun 64, 2892–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tran TM, Jones MB, Ongoiba A, Bijker EM, Schats R, Venepally P, Skinner J, Doumbo S, Quinten E, Visser LG et al. (2016) Transcriptomic evidence for modulation of host inflammatory responses during febrile Plasmodium falciparum malaria. Sci Rep 6, 31291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ho M, Webster HK, Tongtawe P, Pattanapanyasat K & Weidanz WP (1990) Increased gamma delta T cells in acute Plasmodium falciparum malaria. Immunol Lett 25, 139–141. [DOI] [PubMed] [Google Scholar]
- 21. Costa G, Loizon S, Guenot M, Mocan I, Halary F, de Saint‐Basile G, Pitard V, Dechanet‐Merville J, Moreau JF, Troye‐Blomberg M et al. (2011) Control of Plasmodium falciparum erythrocytic cycle: gammadelta T cells target the red blood cell‐invasive merozoites. Blood 118, 6952–6962. [DOI] [PubMed] [Google Scholar]
- 22. Elloso MM, van der Heyde HC, vande Waa JA, Manning DD & Weidanz WP(1994) Inhibition of Plasmodium falciparum in vitro by human gamma delta T cells. J Immunol 153, 1187–1194. [PubMed] [Google Scholar]
- 23. Farouk SE, Mincheva‐Nilsson L, Krensky AM, Dieli F & Troye‐Blomberg M (2004) Gamma delta T cells inhibit in vitro growth of the asexual blood stages of Plasmodium falciparum by a granule exocytosis‐dependent cytotoxic pathway that requires granulysin. Eur J Immunol 34, 2248–2256. [DOI] [PubMed] [Google Scholar]
- 24. Hernández‐Castañeda MA, Happ K, Cattalani F et al. (2020) γδ T cells kill Plasmodium falciparum in a granzyme‐ and granulysin‐dependent mechanism during the late blood stage. J Immunol 204, 1798–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Howard J, Loizon S, Tyler CJ, Duluc D, Moser B, Mechain M, Duvignaud A, Malvy D, Troye‐Blomberg M, Moreau JF et al. (2017) The antigen presenting potential of Vgamma9Vdelta2 T‐cells during Plasmodium falc iparum blood‐stage infection. J Infect Dis 215: 1569–1579. [DOI] [PubMed] [Google Scholar]
- 26. Stanisic DI, Cutts J, Eriksson E, Fowkes FJ, Rosanas‐Urgell A, Siba P, Laman M, Davis TM, Manning L, Mueller I et al. (2014) gammadelta T cells and CD14+ monocytes are predominant cellular sources of cytokines and chemokines associated with severe malaria. J Infect Dis 210, 295–305. [DOI] [PubMed] [Google Scholar]
- 27. Mamedov MR, Scholzen A, Nair RV, Cumnock K, Kenkel JA, Oliveira JHM, Trujillo DL, Saligrama N, Zhang Y, Rubelt F et al. (2018) A macrophage colony‐stimulating‐factor‐producing γδ T cell subset prevents malarial parasitemic recurrence. Immunity 48, 350–363.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kopacz J & Kumar N (1999) gamma delta T‐cells may interfere with a productive immune response in Plasmodium yoelii infections. Int J Parasitol 29, 737–742. [DOI] [PubMed] [Google Scholar]
- 29. Seixas E, Fonseca L & Langhorne J (2002) The influence of gammadelta T cells on the CD4+ T cell and antibody response during a primary Plasmodium chabaudi chabaudi infection in mice. Parasite Immunol 24, 131–140. [DOI] [PubMed] [Google Scholar]
- 30. Inoue S, Niikura M, Takeo S, Mineo S, Kawakami Y, Uchida A, Kamiya S & Kobayashi F (2012) Enhancement of dendritic cell activation via CD40 ligand‐expressing gammadelta T cells is responsible for protective immunity to Plasmodium parasites . Proc Natl Acad Sci USA 109, 12129–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsuji M, Mombaerts P, Lefrancois L, Nussenzweig RS, Zavala F & Tonegawa S (1994) Gamma delta T cells contribute to immunity against the liver stages of malaria in alpha beta T‐cell‐deficient mice. Proc Natl Acad Sci USA 91, 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A et al. (2013) Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341, 1359–1365. [DOI] [PubMed] [Google Scholar]
- 33. Zaidi I, Diallo H, Conteh S, Robbins Y, Kolasny J, Orr‐Gonzalez S, Carter D, Butler B, Lambert L, Brickley E et al. (2017) gammadelta T cells are required for the induction of sterile immunity during irradiated sporozoite vaccinations. J Immunol 199, 3781–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. WHO (2014) Severe malaria. Trop Med Int Health 19, 7–131. [DOI] [PubMed] [Google Scholar]
- 35. Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, Birbeck GL, Bradley WG, Fox LL, Glover SJ et al. (2015) Brain swelling and death in children with cerebral malaria. N Engl J Med 372, 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hunt NH, Ball HJ, Hansen AM, Khaw LT, Guo J, Bakmiwewa S, Mitchell AJ, Combes V & Grau GE (2014) Cerebral malaria: gamma‐interferon redux. Front Cell Infect Microbiol 4, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pais TF & Penha‐Goncalves C (2018) Brain endothelium: the "innate immunity response hypothesis" in cerebral malaria pathogenesis. Front Immunol 9, 3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martins YC & Daniel‐Ribeiro CT (2013) A new hypothesis on the manifestation of cerebral malaria: the secret is in the liver. Med Hypotheses 81, 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haque A, Best SE, Amante FH, Ammerdorffer A, de Labastida F, Pereira T, Ramm GA & Engwerda CR (2011) High parasite burdens cause liver damage in mice following Plasmodium berghei ANKA infection independently of CD8(+) T cell‐mediated immune pathology. Infect Immun 79, 1882–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A, Turay AM & Frauwirth KA (2010) Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol 185, 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ott P & Vilstrup H (2014) Cerebral effects of ammonia in liver disease: current hypotheses. Metab Brain Dis 29, 901–911. [DOI] [PubMed] [Google Scholar]
- 42. Gordon EB, Hart GT, Tran TM, Waisberg M, Akkaya M, Kim AS, Hamilton SE, Pena M, Yazew T, Qi CF et al. (2015) Targeting glutamine metabolism rescues mice from late‐stage cerebral malaria. Proc Natl Acad Sci USA 112, 13075–13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, Chora A, Rodrigues CD, Gregoire IP, Cunha‐Rodrigues M et al. (2007) Heme oxygenase‐1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med 13, 703–710. [DOI] [PubMed] [Google Scholar]
- 44. Pena AC, Penacho N, Mancio‐Silva L, Neres R, Seixas JD, Fernandes AC, Romao CC, Mota MM, Bernardes GJ & Pamplona A (2012) A novel carbon monoxide‐releasing molecule (CO‐RM) fully protects mice from severe malaria. Antimicrob Agents Chemother 56: 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baptista FG, Pamplona A, Pena AC, Mota MM, Pied S & Vigario AM (2010) Accumulation of Plasmodium berghei‐infected red blood cells in the brain is crucial for the development of cerebral malaria in mice. Infect Immun 78, 4033–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sierro F & Grau GER (2019) The ins and outs of cerebral malaria pathogenesis: immunopathology, extracellular vesicles, immunometabolism, and trained immunity. Front Immunol 10, 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Riggle BA, Manglani M, Maric D, Johnson KR, Lee MH, Lopes Abath Neto O, Taylor TE, Seydel KB, Nath A, Miller LH et al. (2019) CD8+ T cells target cerebrovasculature in children with cerebral malaria. J Clin Invest 130, 1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bagot S, Nogueira F, Collette A, do Rosario V, Lemonier F, Cazenave P‐A & Pied S (2004) Comparative study of brain CD8+ T cells induced by sporozoites and those induced by blood‐stage Plasmodium berghei ANKA involved in the development of cerebral malaria. Infect Immun 72, 2817–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Belnoue E, Kayibanda M, Vigario AM, Deschemin JC, van Rooijen N, Viguier M, Snounou G & Renia L (2002) On the pathogenic role of brain‐sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J Immunol 169, 6369–6375. [DOI] [PubMed] [Google Scholar]
- 50. Rudin W, Favre N, Bordmann G & Ryffel B (1997) Interferon‐gamma is essential for the development of cerebral malaria. Eur J Immunol 27, 810–815. [DOI] [PubMed] [Google Scholar]
- 51. Ribot JC, Neres R, Zuzarte‐Luis V, Gomes AQ, Mancio‐Silva L, Mensurado S, Pinto‐Neves D, Santos MM, Carvalho T, Landry JJM et al. (2019) gammadelta‐T cells promote IFN‐gamma‐dependent Plasmodium pathogenesis upon liver‐stage infection. Proc Natl Acad Sci USA 116, 9979–9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fernandes P, Howland SW, Heiss K, Hoffmann A, Hernandez‐Castaneda MA, Obrova K, Frank R, Wiedemann P, Bendzus M, Renia L et al. (2018) A Plasmodium cross‐stage antigen contributes to the development of experimental cerebral malaria. Front Immunol 9, 1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haussig JM, Matuschewski K & Kooij TW (2011) Inactivation of a Plasmodium apicoplast protein attenuates formation of liver merozoites. Mol Microbiol 81, 1511–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lewis MD, Behrends J, Sa ECC, Mendes AM, Lasitschka F, Sattler JM, Heiss K, Kooij TW, Prudencio M, Bringmann G et al. (2015) Chemical attenuation of Plasmodium in the liver modulates severe malaria disease progression. J Immunol 194, 4860–4870. [DOI] [PubMed] [Google Scholar]
- 55. D'Ombrain MC, Robinson LJ, Stanisic DI, Taraika J, Bernard N, Michon P, Mueller I & Schofield L (2008) Association of early interferon‐gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin Infect Dis 47, 1380–1387. [DOI] [PubMed] [Google Scholar]
- 56. Sato Y, Ries S, Stenzel W, Fillatreau S & Matuschewski K (2019) The liver‐stage plasmodium infection is a critical checkpoint for development of experimental cerebral malaria. Front Immunol 10, 2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Howard J, Zaidi I, Loizon S, Mercereau‐Puijalon O, Dechanet‐Merville J & Mamani‐Matsuda M (2018) Human Vgamma9Vdelta2 T lymphocytes in the immune response to P. falciparum infection. Front Immunol 9, 2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hviid L, Akanmori BD, Loizon S, Kurtzhals JA, Ricke CH, Lim A, Koram KA, Nkrumah FK, Mercereau‐Puijalon O & Behr C (2000) High frequency of circulating gamma delta T cells with dominance of the v(delta)1 subset in a healthy population. Int Immunol 12, 797–805. [DOI] [PubMed] [Google Scholar]
- 59. Worku S, Bjorkman A, Troye‐Blomberg M, Jemaneh L, Farnert A & Christensson B (1997) Lymphocyte activation and subset redistribution in the peripheral blood in acute malaria illness: distinct gammadelta+ T cell patterns in Plasmodium falciparum and P. vivax infections. Clin Exp Immunol 108, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hviid L, Kurtzhals JA, Adabayeri V, Loizon S, Kemp K, Goka BQ, Lim A, Mercereau‐Puijalon O, Akanmori BD & Behr C (2001) Perturbation and proinflammatory type activation of V delta 1(+) gamma delta T cells in African children with Plasmodium falciparum malaria. Infect Immun 69, 3190–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Harawa V, Njie M, Keller T, Kim K, Jaworowski A, Seydel K, Rogerson SJ & Mandala W (2019) Malawian children with uncomplicated and cerebral malaria have decreased activated Vgamma9Vdelta2 gammadelta T cells which increase in convalescence. PLoS One 14, e0223410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Diallo H, Katile A, Kwan JL, Sissoko MS, Healy SA, Doumbo OK, Duffy PE & Zaidi I (2019) Longitudinal analysis of gamma delta T cell subsets during malaria infections in Malian adults. Malar J 18, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Goodier M, Krause‐Jauer M, Sanni A, Massougbodji A, Sadeler BC, Mitchell GH, Modolell M, Eichmann K & Langhorne J (1993) Gamma delta T cells in the peripheral blood of individuals from an area of holoendemic Plasmodium falciparum transmission. Trans R Soc Trop Med Hyg 87, 692–696. [DOI] [PubMed] [Google Scholar]
- 64. Hviid L, Kurtzhals JA, Dodoo D, Rodrigues O, Ronn A, Commey JO, Nkrumah FK & Theander TG (1996) The gamma/delta T‐cell response to Plasmodium falciparum malaria in a population in which malaria is endemic. Infect Immun 64, 4359–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jagannathan P, Lutwama F, Boyle MJ, Nankya F, Farrington LA, McIntyre TI, Bowen K, Naluwu K, Nalubega M, Musinguzi K et al. (2017) Vdelta2+ T cell response to malaria correlates with protection from infection but is attenuated with repeated exposure. Sci Rep 7, 11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Portugal S, Moebius J, Skinner J, Doumbo S, Doumtabe D, Kone Y, Dia S, Kanakabandi K, Sturdevant DE, Virtaneva K et al. (2014) Exposure‐dependent control of malaria‐induced inflammation in children. PLoS Pathog 10, e1004079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hirunpetcharat C, Finkelman F, Clark IA & Good MF (1999) Malaria parasite‐specific Th1‐like T cells simultaneously reduce parasitemia and promote disease. Parasite Immunol 21, 319–329. [DOI] [PubMed] [Google Scholar]
- 68. Goncalves‐Sousa N, Ribot JC, deBarros A, Correia DV, Caramalho I & Silva‐Santos B (2010) Inhibition of murine gammadelta lymphocyte expansion and effector function by regulatory alphabeta T cells is cell‐contact‐dependent and sensitive to GITR modulation. Eur J Immunol 40, 61–70. [DOI] [PubMed] [Google Scholar]
- 69. Ribot JC, Chaves‐Ferreira M, d'Orey F, Wencker M, Goncalves‐Sousa N, Decalf J, Simas JP, Hayday AC & Silva‐Santos B (2010) Cutting edge: adaptive versus innate receptor signals selectively control the pool sizes of murine IFN‐gamma‐ or IL‐17‐producing gammadelta T cells upon infection. J Immunol 185, 6421–6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yanez DM, Batchelder J, van der Heyde HC, Manning DD & Weidanz WP (1999) Gamma delta T‐cell function in pathogenesis of cerebral malaria in mice infected with Plasmodium berghei ANKA. Infect Immun 67, 446–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hammerich L & Tacke F (2014) Role of gamma‐delta T cells in liver inflammation and fibrosis. World J Gastrointest Pathophysiol 5, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kubes P & Jenne C (2018) Immune responses in the liver. Annu Rev Immunol 36, 247–277. [DOI] [PubMed] [Google Scholar]
- 73. Liehl P, Zuzarte‐Luis V, Chan J, Zillinger T, Baptista F, Carapau D, Konert M, Hanson KK, Carret C, Lassnig C et al. (2014) Host‐cell sensors for Plasmodium activate innate immunity against liver‐stage infection. Nat Med 20, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Crispe IN (2011) Liver antigen‐presenting cells. J Hepatol 54, 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K et al. (2008) IL‐17A produced by gammadelta T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J Immunol 181, 3456–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen D, Luo X, Xie H, Gao Z, Fang H & Huang J (2013) Characteristics of IL‐17 induction by Schistosoma japonicum infection in C57BL/6 mouse liver. Immunology 139, 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li F, Hao X, Chen Y, Bai L, Gao X, Lian Z, Wei H, Sun R & Tian Z (2017) The microbiota maintain homeostasis of liver‐resident gammadeltaT‐17 cells in a lipid antigen/CD1d‐dependent manner. Nat Commun 7, 13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Portugal S, Carret C, Recker M, Armitage AE, Goncalves LA, Epiphanio S, Sullivan D, Roy C, Newbold CI, Drakesmith H et al. (2011) Host‐mediated regulation of superinfection in malaria. Nat Med 17, 732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ocana‐Morgner C, Mota MM & Rodriguez A (2003) Malaria blood stage suppression of liver stage immunity by dendritic cells. J Exp Med 197, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Melchor SJ & Ewald SE (2019) Disease tolerance in toxoplasma infection. Front Cell Infect Microbiol 9, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ayres JS & Schneider DS (2012) Tolerance of infections. Annu Rev Immunol 30, 271–294. [DOI] [PubMed] [Google Scholar]
- 82. Soares MP, Teixeira L & Moita LF (2017) Disease tolerance and immunity in host protection against infection. Nat Rev Immunol 17, 83–96. [DOI] [PubMed] [Google Scholar]
- 83. Hamada S, Umemura M, Shiono T, Hara H, Kishihara K, Tanaka K, Mayuzumi H, Ohta T & Matsuzaki G (2008) Importance of murine Vdelta1gammadelta T cells expressing interferon‐gamma and interleukin‐17A in innate protection against Listeria monocytogenes infection. Immunology 125, 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chen D, Xie H, Luo X, Yu X, Fu X, Gu H, Wu C, Tang X & Huang J (2013) Roles of Th17 cells in pulmonary granulomas induced by Schistosoma japonicum in C57BL/6 mice. Cell Immunol 285, 149–157. [DOI] [PubMed] [Google Scholar]
- 85. Shibata K, Yamada H, Hara H, Kishihara K & Yoshikai Y (2007) Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL‐17 production. J Immunol 178, 4466–4472. [DOI] [PubMed] [Google Scholar]
- 86. Mensurado S, Rei M, Lanca T, Ioannou M, Goncalves‐Sousa N, Kubo H, Malissen M, Papayannopoulos V, Serre K & Silva‐Santos B (2018) Tumor‐associated neutrophils suppress pro‐tumoral IL‐17+ gammadelta T cells through induction of oxidative stress. PLoS Biol 16, e2004990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Villegas‐Mendez A, Greig R, Shaw TN, de Souza JB, Gwyer Findlay E, Stumhofer JS, Hafalla JC, Blount DG, Hunter CA, Riley EM et al. (2012) IFN‐gamma‐producing CD4+ T cells promote experimental cerebral malaria by modulating CD8+ T cell accumulation within the brain. J Immunol 189, 968–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. D'Ombrain MC, Hansen DS, Simpson KM & Schofield L (2007) gammadelta‐T cells expressing NK receptors predominate over NK cells and conventional T cells in the innate IFN‐gamma response to Plasmodium falciparum malaria. Eur J Immunol 37, 1864–1873. [DOI] [PubMed] [Google Scholar]
- 89. Ebert D (1998) Infectivity, multiple infections, and the genetic correlation between within‐host growth and parasite virulence: a reply to Hochberg. Evolution 52, 1869–1871. [DOI] [PubMed] [Google Scholar]
- 90. Spence PJ, Jarra W, Levy P, Nahrendorf W & Langhorne J (2012) Mosquito transmission of the rodent malaria parasite Plasmodium chabaudi . Malar J 11, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Spence PJ, Jarra W, Levy P, Reid AJ, Chappell L, Brugat T, Sanders M, Berriman M & Langhorne J (2013) Vector transmission regulates immune control of Plasmodium virulence . Nature 498, 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Preiser PR, Khan S, Costa FT, Jarra W, Belnoue E, Ogun S, Holder AA, Voza T, Landau I, Snounou G et al. (2002) Stage‐specific transcription of distinct repertoires of a multigene family during Plasmodium life cycle. Science 295, 342–345. [DOI] [PubMed] [Google Scholar]
- 93. Bachmann A, Petter M, Krumkamp R, Esen M, Held J, Scholz JA, Li T, Sim BK, Hoffman SL, Kremsner PG et al. (2016) Mosquito passage dramatically changes var gene expression in controlled human plasmodium falciparum infections. PLoS Pathog 12, e1005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sanders PR, Gilson PR, Cantin GT, Greenbaum DC, Nebl T, Carucci DJ, McConville MJ, Schofield L, Hodder AN, Yates JR 3rd et al. (2005) Distinct protein classes including novel merozoite surface antigens in Raft‐like membranes of Plasmodium falciparum . J Biol Chem 280, 40169–40176. [DOI] [PubMed] [Google Scholar]
- 95. Gilson PR, Nebl T, Vukcevic D, Moritz RL, Sargeant T, Speed TP, Schofield L & Crabb BS (2006) Identification and stoichiometry of glycosylphosphatidylinositol‐anchored membrane proteins of the human malaria parasite Plasmodium falciparum . Mol Cell Proteomics 5, 1286–1299. [DOI] [PubMed] [Google Scholar]
- 96. Sanders PR, Kats LM, Drew DR, O'Donnell RA, O'Neill M, Maier AG, Coppel RL & Crabb BS (2006) A set of glycosylphosphatidyl inositol‐anchored membrane proteins of Plasmodium falciparum is refractory to genetic deletion. Infect Immun 74, 4330–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Deroost K, Pham TT, Opdenakker G & Van den Steen PE (2016) The immunological balance between host and parasite in malaria. FEMS Microbiol Rev 40, 208–257. [DOI] [PubMed] [Google Scholar]
- 98. Topolska AE, Richie TL, Nhan DH & Coppel RL (2004) Associations between responses to the rhoptry‐associated membrane antigen of Plasmodium falciparum and immunity to malaria infection. Infect Immun 72, 3325–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Naik RS, Branch OH, Woods AS, Vijaykumar M, Perkins DJ, Nahlen BL, Lal AA, Cotter RJ, Costello CE, Ockenhouse CF et al. (2000) Glycosylphosphatidylinositol anchors of Plasmodium falciparum: molecular characterization and naturally elicited antibody response that may provide immunity to malaria pathogenesis. J Exp Med 192, 1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Beeson JG, Drew DR, Boyle MJ, Feng G, Fowkes FJ & Richards JS (2016) Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev 40, 343–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Born WK, Yin Z, Hahn YS, Sun D & O'Brien RL (2010) Analysis of gamma delta T cell functions in the mouse. J Immunol 184, 4055–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Deroost K & Langhorne J (2018) Gamma/delta T cells and their role in protection against malaria. Front Immunol 9, 2973. [DOI] [PMC free article] [PubMed] [Google Scholar]


