ABSTRACT
Importance
The use of factor VIII (FVIII) concentrates under pharmacokinetic (PK) guidance has become the main approach for treatment of hemophilia. However, limited PK research has been conducted in Chinese pediatric patients.
Objective
To investigate the PK parameters of various FVIII concentrates in Chinese pediatric patients.
Methods
Seventy‐nine patients were enrolled (28 treated with Kogenate FS®, 23 treated with Advate ®, and 28 treated with GreenMono™). All enrolled patients participated in single‐dose PK analysis after at least a 3‐day washout period. Blood samples were collected predose, as well as at 1 h, 9 h, 24 h, and 48 h after infusion; FVIII levels were measured using a one‐stage clotting assay. von Willebrand Factor Antigen (VWF:Ag) levels and blood types were also determined. PK parameters were evaluated by WAPPS‐Hemo.
Results
Mean values of terminal elimination half‐life time (t1/2) for the Kogenate FS®, Advate®, and GreenMono™ FVIII groups were 12.24 h, 10.18 h, and 9.62 h; median clearance values were 4.16, 6.23, and 5.11 mL·kg−1·h−1; and median in vivo recovery values were 1.97, 1.55, and 1.61 IU/dL per IU/kg. Longer t1/2, higher in vivo recovery, and lower clearance were observed in patients with higher VWF:Ag level who were treated with recombinant concentrates.
Interpretation
Chinese pediatric patients with hemophilia had FVIII PK characteristics similar to those previously observed in non‐Chinese children, including large variation among individuals. VWF:Ag level and FVIII brand were associated with differences in FVIII PK. Thus, PK‐guided dosing should be used to optimize individualized therapy in Chinese children.
Keywords: Hemophilia A, Pharmacokinetics, Pediatric patients, FVIII concentrates
1. INTRODUCTION
Hemophilia A is an X‐linked, inherited bleeding disorder caused by a deficiency in coagulation factor VIII (FVIII), which affects approximately 1 in 5000 live male births. As recommended by the International Society of Thrombosis and Hemostasis, regular replacement therapy (prophylaxis) has become the main treatment worldwide. 1 In 1965, Ahlberg noticed that patients with moderate and mild hemophilia A had fewer joint bleeds, compared with patients with severe hemophilia A; thus, he established the target trough level of 1 IU/dL as the initial goal of prophylaxis. 2 Compared with on‐demand therapy, prophylaxis has been shown to reduce both clinical and subclinical bleeding, which are presumed to cause joint issues like pain and disfunction. 3 , 4 , 5 Based on the current understanding of factor concentrates, the Malmo protocol suggests the infusion of 20–40 IU/kg body weight at a rate of at least three times per week, and up to every other day. 6 However, this therapeutic regimen is resource‐intensive and places a heavy venipuncture burden on patients, which may reduce treatment adherence (especially among pediatric patients). 7 Thus, some comparable treatments with less frequent infusion and lower doses have been adopted as alternatives to the standard prophylaxis regimen. 8 , 9 , 10 , 11 By using a combination of pharmacokinetic (PK) tests, the therapy can be adapted, such that it is more precise and individualized.
Dose calculation for most prophylactic regimens has been performed with the assumption that all patients exhibit similar PK characteristics. However, there is great inter‐individual variability in various PK parameters, 12 such as half‐life time (t1/2), clearance (CL), and in vivo recovery (IVR). Bjorkman et al 13 performed PK analyses with a large number of patients, which revealed considerable differences among individuals. In their study, the t1/2 of FVIII varied from 6 h to 25 h in patients with hemophilia A. Similar findings were more recently reported for a variety of concentrates; substantial variability was encountered regarding IVR and CL. 14 Furthermore, determinants of inter‐individual variability (e.g., body mass composition) were also identified; 15 these findings indicated that dosing based on body weight may lead to under or over‐dosing because of clearance variability among patients. 16 Thus, the use of individual PK parameters to optimize FVIII replacement therapy was adopted; this approach has been shown to improve important patient outcomes. 17 Knowledge of their own PK has helped patients to achieve better quality of life by either reducing unnecessary administration of FVIII or adding appropriate doses of FVIII, where necessary, to maintain the FVIII concentration above the target level. 18 Furthermore, patients may be able to choose a highly cost‐effective product from among the range of available concentrates. With the aid of individual PK profiles, patients with different bleeding phenotypes can precisely determine their target FVIII levels, thus reducing bleeding during routine prophylaxis.
PK‐guided prophylaxis is increasingly used and several affordable FVIII concentrates are available in China; however, there is very limited PK information concerning these FVIII concentrates in Chinese pediatric patients. A prior PK study conducted by Chen et al 19 involved performance of PK tests in 36 boys with hemophilia A, using different FVIII concentrates; it produced similar results of PK parameters. Based on the findings of the study by Chen et al, we performed this study involving 79 patients, grouped according to the FVIII concentrate brand used by each patient, instead of simply evaluating whether the patients used plasma‐derived or recombinant FVIII concentrates. The aim of our study was to investigate PK variability in Chinese pediatric patients with hemophilia A by analysis of PK profiles from patients who were treated with different FVIII concentrates.
2. METHODS
2.1. Ethical approval
The study was approved by the Ethics Committee of Beijing Children’s Hospital (2018‐60). Written informed consent was obtained from each enrolled patient or their legally authorized guardian(s).
2.2. Patients
The patients were enrolled from January 2018 to February 2019 at Beijing Children’s Hospital. Inclusion criteria were age <18 years; moderate or severe (FVIII activity < 2 IU/dL) hemophilia A; receipt of prophylactic treatment with a plasma‐derived or recombinant FVIII concentrate for more than 50 exposure days; and current use of Kogenate FS®, Advate®, or GreenMono™ for treatment. Exclusion criteria were the presence of a current FVIII inhibitor (anti‐FVIII antibody titer > 0.6 Bethesda units), fever, active bleeding, or a concurrent coagulation disorder.
2.3. Blood samples
After a washout period of at least 72 hours, each patient received a daily‐use FVIII infusion of 40–50 IU/kg. Peripheral venous blood samples were drawn using vacutainer tubes (3.2% trisodium citrate, 2 mL). In accordance with the method of Blanchette et al, 20 a reduced blood sampling strategy was used (predose, as well as at 1 h, 9 h, 24 h, and 48 h after concentrate infusion). Blood samples were immediately centrifuged at 2500 g for 15 min at 20–25°C. Plasma was divided into three EP tubes (600 μL per tube) and stored in a −80°C freezer for further analysis. For each patient, FVIII level, FVIII inhibitor, blood type (ABO), and von Willebrand factor antigen (VWF:Ag) level were tested.
2.4. Laboratory assay
The one‐stage‐based activated partial thromboplastin time assay (Instrumentation Laboratory, Bedford, MA, USA) was used to measure FVIII activity in a multi‐dilution mode, with FVIII‐deficient plasma. The Nijmegen modification of the Bethesda Assay was used for the determination of FVIII inhibitors. An ACL TOP‐700 analyzer was used to perform all measurements (Instrumentation Laboratory, Bedford, MA, USA). A latex particle‐enhanced immunoturbidimetric assay with HemosIL von Willebrand factor antigen kit (Instrumentation Laboratory, Bedford, MA, USA) was utilized to determine VWF:Ag levels.
2.5. PK evaluation
Individual PK profiles were estimated using WAPPS‐Hemo, 21 a PK‐guided dosing tool based on Bayesian methods and a population PK model that has been specifically designed for patients with hemophilia. In this study, the following information was entered into WAPPS: patient ID, height, body weight, date of birth, blood type, VWF:Ag level, endogenous baseline level, administered dose, FVIII level of all samples, FVIII measurement assay, and FVIII concentrate brand. Estimates for CL are not included in the standard WAPPS‐Hemo report and were generously provided by Dr. Alfonso Iorio upon request; details regarding the population PK model used for estimation of individual profiles (i.e., the generic factor VIII described by McEneny‐King et al 21 ) were also provided by Dr. Alfonso Iorio. In brief, the model was derived by using 174 PK studies on recombinant concentrates and 49 PK studies on plasma‐derived concentrates, all involving patients with severe hemophilia A. Baseline demographics were as follows: mean ages were 21 years for patients receiving recombinant concentrates and 21 years for patients receiving plasma‐derived concentrates; the proportions of patients < 18 years of age were 14.3% (recombinant) and 12.2% (plasma‐derived). Median bodyweights (min–max) were 68.1 kg (10.6–132.5 kg) for patients receiving recombinant concentrates and 58.2 kg (18.5–93.0 kg) for patients receiving plasma‐derived concentrates. Median body mass index (min–max) values were 23.1 kg/m2 (11.7–38.6 kg/m2) for patients receiving recombinant concentrates and 20.9 kg/m2 (14.0–30.5 kg/m2) for patients receiving plasma‐derived concentrates. Median numbers of measurements taken (min–max) were nine (4–11) for patients receiving recombinant concentrates and 10 (4–12) for patients receiving plasma‐derived concentrates. FVIII as measured with a one‐stage clotting test. The structural model constituted a two‐compartment model, with a covariate term for the specific concentrate (Kogenate FS®, Advate®, or plasma‐derived) on CL and Volume 1; for age on CL; and for fat‐free mass on CL, Volume 1, and Volume 2. A term for between‐participant variability was used on CL and Volume 1; no term was used for within‐participant variability, and the residual unexplained variability term was structured as a combined error. The median relative error for t1/2 estimates for the models was ≤ 1.5%, while the corresponding upper fifth percentile of the relative error was < 4.0%. IVR was calculated as follows: IVR (IU/dL per IU/kg) = (Cmax − FVIIIpreinfusion [IU/dL])/(FVIIIadministered [IU/dL]/bodyweight [kg]). 19
2.6. Statistical analysis
The statistical analysis was performed by Graphpad Prism 8.0. Normally distributed data were reported as mean ± standard deviation and non‐normally distributed data were reported as median (lower quartile, upper quartile). One way ANOVA and t‐tests were employed to evaluate the difference among normally distributed data, and for non‐normal data, Kruskal–Wallis and Mann–Whitney test were used to evaluate the difference. Categorical variables were compared by the Chi‐squared test to detect statistical differences. Pearson (normal distributed data) or Spearman (non‐normal distributed data) correlation coefficients were used to analyze the potential relations between PK parameters and the characteristics of patients. P values < 0.05 were considered statistically significant.
3. RESULTS
3.1. Patient characteristics
This study included 79 children with hemophilia A, aged 2–13 years; five patients exhibited moderate hemophilia A and 74 patients exhibited severe hemophilia A. Table 1 shows the patient characteristics according to treatment products (28 patients were treated with Kogenate FS® [age range, 2–15 years], 23 patients were treated with Advate® [age range, 2–10 years], and 28 patients were treated with GreenMono™ [age range, 4–9 years]). Age, bodyweight, body mass index, and blood‐type distribution did not significantly differ among the three groups.
TABLE 1.
Baseline characteristics of patients with hemophilia A in the three concentrate brand groups
| Variables | Advate® (n = 23) | Kogenate FS® (n = 28) | GreenMono™ (n = 28) | Statistics | P |
|---|---|---|---|---|---|
| Age* (year) | 6.25 (4.25, 8.33) | 6.18 (4.52, 7.75) | 6.50 (5.22, 8.48) | 1.153# | 0.47 |
| Bodyweight* (kg) | 22.0 (20.0, 28.0) | 22.5 (18.0, 30.3) | 23.5 (20.1, 28.8) | 0.743# | 0.80 |
| BMI* (kg/m2) | 16.53 (14.42, 18.90) | 16.09 (14.80, 17.47) | 15.55 (14.51, 17.36) | 0.434# | 0.81 |
| VWF:Ag* (IU/dL) | 84.0 (74.2, 105.8) | 83.9 (69.4, 107.8) | 92.6 (80.0, 106.6) | 1.955# | 0.38 |
| HA type (moderate/severe) | 1/22 | 2/26 | 2/26 | 0.21† | 0.90† |
| Blood type | |||||
| O type (n) | 11 | 17 | 12 | 1.89† | 0.39 |
| Non‐O type (n) | 12 | 11 | 16 |
*median (lower quartile, upper quartile); #Kruskal–Wallis test; † Chi–squared test. BMI, body mass index; HA, hemophilia A.
3.2. PK parameters of FVIII concentrates
The relationship between post‐infusion FVIII levels and time after dosing is shown in Figure 1. Among all patients, the respective median values of t1/2, IVR, and CL were 10.25 h, 1.66 IU/kg per IU/dL, and 4.89 mL·kg−1·h−1. Patients in the Kogenate FS® group had a longer mean t1/2, compared with patients in the Advate® (12.24 vs 10.18 h, P = 0.01) and GreenMono™ (12.24 vs 9.62 h, P < 0.01) groups. Patients in the Kogenate FS® group had a higher median IVR, compared with patients in the Advate® (1.97 vs 1.55 IU/dL per IU/kg, P < 0.01) and GreenMono™ (1.97 vs 1.61 IU/dL per IU/kg, P < 0.01) groups. Lower CL was observed among patients in the Kogenate FS® group, compared with patients in the Advate® (4.16 vs 6.23 mL·kg−1·h−1, P < 0.01) and GreenMono™ (4.16 vs 5.11 mL·kg−1·h−1; P = 0.02) groups. There were no statistically significant differences between the GreenMono™ and Advate® groups in terms of t1/2 (10.18 vs 9.62 h, P = 0.71), IVR (1.55 vs 1.61 IU/dL per IU/kg, P > 0.99), or CL (6.23 vs 5.11 mL·kg−1·h−1, P > 0.99) (Table 2, Figure 2).
FIGURE 1.
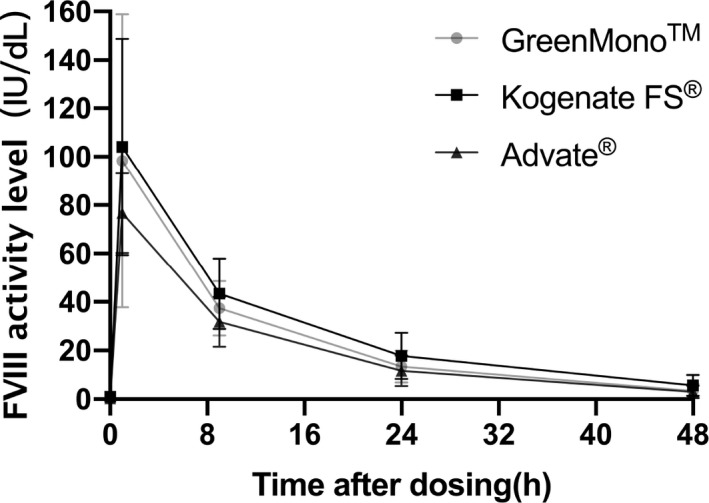
Plasma FVIII concentration (mean ± standard deviation) vs time curve of three FVIII concentrates after single infusion of 40–50 IU/kg FVIII. One‐stage assay was used to determine FVIII activity.
TABLE 2.
Pharmacokinetic parameters of patients with hemophilia A in the three concentrate brand groups
| PK parameters | Total (n = 79) | Advate® (n = 23) | Kogenate FS® (n = 28) | GreenMono™ (n = 28) | Statistics | P |
|---|---|---|---|---|---|---|
| t1/2 (h) | 10.25 (8.75,12.75) | 10.18 ± 2.55 | 12.24 ± 2.60 | 9.62 ± 2.44 | 8.24* | < 0.01 |
| IVR (IU/kg per IU/dL) | 1.66 (1.53, 1.98) | 1.55 (1.48, 1.74) | 1.97 (1.66, 2.23) | 1.61 (1.45, 1.85) | 3.54# | < 0.01 |
| CL (mL·kg−1·h−1) | 4.89 (3.93, 6.34) | 6.23 (4.55, 6.86) | 4.16 (2.93, 5.72) | 5.11 (4.39, 6.67) | 2.77# | < 0.01 |
*t‐test; #Kruskal–Wallis test. t1/2 for each of the three divided groups is shown as mean ± standard deviation, t1/2 of all patients is shown as median (lower quartile, upper quartile); IVR and CL are both shown as median (lower quartile, upper quartile). PK, pharmacokinetic; t1/2, half‐life time; IVR, in vivo recovery; CL, clearance.
FIGURE 2.
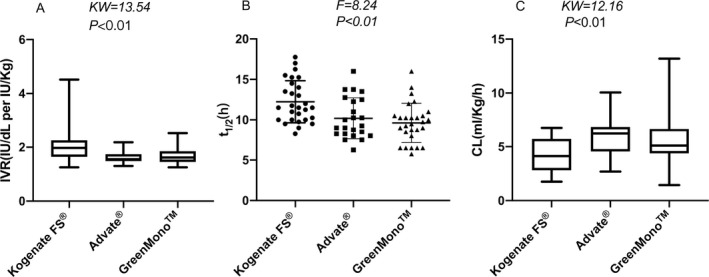
Comparison of IVR, t1/2, and CL among the three concentrate brand groups. IVR and CL data are shown as median (range), while t1/2 data are shown as mean ± standard deviation. t1/2, half‐life time; IVR, in vivo recovery; CL, clearance.
The correlation between VWF:Ag level and individual PK parameters was evaluated further in each of the three FVIII concentrates. t1/2 increased with increasing VWF:Ag level in all groups (Kogenate FS®: r = 0.57, P < 0.01; Advate®: r = 0.78, P < 0.01; GreenMono™: r = 0.73, P < 0.01). CL decreased with increasing VWF:Ag level in all groups (Kogenate FS®: r = −0.53, P < 0.01; Advate®: r = −0.78, P < 0.01; GreenMono™: r = −0.54, P < 0.01). IVR increased with increasing VWF:Ag level in two groups (Kogenate FS®: r = 0.44, P = 0.02; Advate®: r = 0.78, P < 0.01; Figure 3). The relationships between blood type and these PK parameters were also analyzed. t1/2 tended to be shorter, whereas CL tended to be higher in patients with blood type O, compared with patients who exhibited non‐O blood type, in all FVIII groups in our study; however, no statistical difference was found between IVR and blood type in our study (all P > 0.05; Table 3). Furthermore, CL decreased with age in the Kogenate FS® (r = −0.41, P = 0.03) and GreenMono™ (r = −0.40, P = 0.04) groups; IVR increased with age in the GreenMono™ group (r = 0.38, P = 0.04).
FIGURE 3.
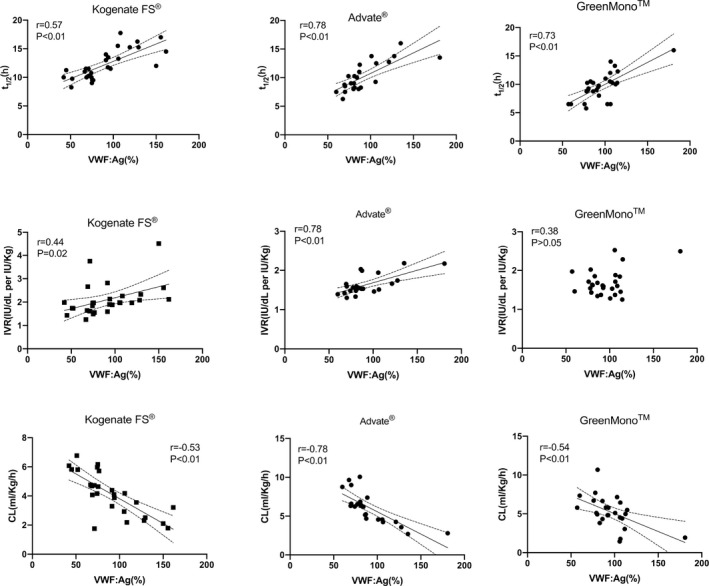
Correlations of VWF:Ag levels (%) with each of three PK parameters (t1/2, IVR, and CL) among the three FVIII concentrate brands. t1/2, half‐life time; IVR, in vivo recovery; CL, clearance; PK, pharmacokinetic.
TABLE 3.
Pharmacokinetic parameters of patients with hemophilia A who exhibit different blood types
| PK parameters | FVIII | Blood group O | Blood group non‐O | t | P |
|---|---|---|---|---|---|
| t1/2 (h) | Kogenate FS® | 10.29 ± 1.32 | 15.05 ± 1.76 | −7.253 | < 0.01 |
| Advate® | 8.35 ± 1.29 | 11.60 ± 2.40 | −3.641 | < 0.01 | |
| GreenMono™ | 8.67 ± 1.64 | 10.50 ± 2.51 | −2.099 | 0.03 | |
| IVR (IU/kg per IU/dL) | Kogenate FS® | 2.04 ± 0.62 | 2.43 ± 0.81 | −1.288 | 0.21 |
| Advate® | 1.58 ± 0.23 | 1.67 ± 0.27 | −0.850 | 0.41 | |
| GreenMono™ | 1.70 ± 0.34 | 1.69 ± 0.33 | 0.034 | 0.97 | |
| CL (mL·kg−1·h−1) | Kogenate FS® | 6.36 ± 2.01 | 4.51 ± 1.21 | 2.576 | 0.02 |
| Advate® | 10.94 ± 2.69 | 6.63 ± 2.01 | 4.171 | < 0.01 | |
| GreenMono™ | 9.66 ± 4.01 | 6.38 ± 3.62 | 2.580 | 0.02 |
PK, pharmacokinetic; t1/2, half‐life time; IVR, in vivo recovery; CL, clearance.
4. DISCUSSION
Because of the existence of inter‐individual variability, individual PK testing has been a useful tool to guide individualized prophylaxis for patients with hemophilia. We have shown that the inter‐individual differences of PK parameters in the Chinese pediatric population are similar to those observed in non‐Chinese children. For each PK parameter, the maximum and minimum values differed greatly within the patient population. Furthermore, within FVIII concentrate groups, variability among patients was obvious. The respective ranges of t1/2 in Kogenate FS®, Advate®, and GreenMono™ groups were 8.25–17.75 h, 6.25–16.00 h, and 5.75–16.00 h, fully overlapping with the ranges observed in non‐Chinese populations. In each group, the longest t1/2 was more than twofold greater, compared with the shortest t1/2; comparable findings were also made concerning IVR and CL, which could lead to great discrepancies in FVIII dosing in traditional dosing regimens.
Inter‐individual differences have been widely discussed in the literature. 14 , 16 , 22 , 23 PK studies of non‐Chinese populations have also been conducted. 24 , 25 A PK study of Kogenate FS® in Canada revealed that the mean t1/2, CL, and IVR were 10.7 h, 4.1 mL·kg−1·h−1, and 1.87 U/mL per IU/kg, respectively. 24 Our study revealed similar CL and IVR results, along with longer t1/2 (12.24 h). When adjusted for their higher median age, Chinese pediatric patients seemed to have a longer t1/2 while using Kogenate FS®. This may be due to ethnic differences, as well as variability in patient characteristics (e.g., age). Another study was conducted in Spain, involving 13 pediatric patients aged 7–15 years who were using Advate®; in that study, the reported mean t1/2 and CL were 10.2 h and 4.3 mL·kg−1·h−1, respectively. 25 The t1/2 was nearly identical to ours (10.18 h), whereas the CL was lower than in the present study; this discrepancy may be related to inter‐individual variability, age difference, and ethnicity‐related differences. 25 , 26 Further research is needed to compare PK among populations.
Kogenate FS®, Advate®, and GreenMono™ are three widely used concentrates in our center. Both Kogenate FS® and Advate® are full‐length recombinant FVIII concentrates, while GreenMono™ is a plasma‐derived FVIII product. Considerable variability was found among the FVIII concentrate groups. In the Advate® and GreenMono™ groups, the three PK parameters (t1/2, IVR, and CL) showed no significant differences. Although Morfini et al 27 found no significant difference in t1/2 between plasma‐derived and recombinant FVIII concentrates, a statistical difference in t1/2 was observed between the Kogenate FS® and GreenMono™ groups in our study. This difference may be attributable to the population difference between groups, as well as the difference between plasma‐derived and recombinant FVIII products in our study. Morfini et al 27 concluded that complete bioequivalence was not achieved in their headto‐head study because a higher IVR was observed for recombinant FVIII concentrates, presumably because of activated molecules in the formulation of recombinant FVIII concentrates. Another study compared Advate® with a recombinant VIII single‐chain product; latter had a more favorable PK profile, which permitted a longer dosing interval. 28 This may be partly related to different modifications in molecular structure. Some previous studies also demonstrated variability in PK parameters (e.g., t1/2 and CL) among FVIII concentrates. 29 , 30 , 31 In our study, the Kogenate FS® group exhibited longer t1/2, higher IVR, and lower CL, compared with the Advate® and GreenMono™ group; in contrast, PK parameters were very similar between the Advate® and GreenMono™ groups. Because improved glycosylation has been detected in patients using Kogenate FS® and extra VWF:Ag was only observed in patients using GreenMono™, differences in PK parameters may be related to distinct manufacturing technologies and other factors (e.g., extra VWF:Ag in GreenMono™), separate from inter‐individual variability. 32
Some factors that influence PK parameters were also analyzed in our study. Key characteristics that influenced t1/2 and CL were patient age and the VWF:Ag level in plasma. In the Kogenate FS® and GreenMono™ groups, CL decreased with increasing age; IVR increased with age in the GreenMono™ group. According to Björkman et al and Kepa et al, t1/2 is associated with age. 16 , 33 In studies by Björkman et al and Chen et al, respective enhancements of 0.4 h and 0.6 h per year in t1/2 were observed in children with severe hemophilia A. 19 , 34 However, a study of patients using Advate®, in which patients were divided into three groups according to their age (<6 years, 6–12 years, and >12 years) revealed that the respective mean t1/2 values of the three groups were 8.78 h, 9.44 h, and 8.52 h.28 Thus, no obvious correlation between age and t1/2 was identified. Furthermore, no significant correlation between t1/2 and age was found in our study. This might be related to the narrow age distribution of our patients and considerable individual PK variability among children in the study.
VWF acts as a chaperone for FVIII and is known to protect FVIII from protease degradation. 35 In our study, PK parameters were affected by VWF:Ag levels in all three groups. Patients with a higher level of VWF:Ag tended to have longer t1/2, higher IVR, and lower CL. A study conducted in Vienna reached a similar conclusion. 33
Our result was also in accordance with studies by Vlot et al and Fisher et al concerning the relationships of VWF:Ag level with PK parameters. 35 , 36 However, no statistically significant correlation was found between VWF:Ag level and IVR in the GreenMono™ group; this might have been due to the existence of extra VWF:Ag in plasma‐derived FVIII concentrates, which was not present in recombinant FVIII products. The extra VWF:Ag in the GreenMono™ FVIII concentrate reduced the importance of endogenous levels of VWF:Ag and led to loss of the correlation between the endogenous VWF:Ag level and IVR. Investigation of the relationships of blood type with individual PK parameters revealed that patients with non‐O blood types tended to have longer t1/2 and lower CL, relative to patients with blood type O, which could help reduce FVIII consumption and venipuncture frequency. The effect of blood type maybe partly caused by differences in VWF:Ag levels among patients with different blood types. Notably, our results were consistent with those of Chen et al and Fischer et al. 19 , 36
Our study had some limitations. First, only the one‐stage assay was used to test FVIII level, whereas the chromogenic assay was not used due to its high cost. 37 Although the use of chromogenic testing has implications for regulators, the one‐stage clotting test is most commonly used in clinical practice. Second, although the demographic characteristics of patients were not significantly different among groups, the differences observed among concentrates may have reflected differences in the underlying population, rather than true differences among concentrates. Third, the WAPPS models used for individual PK estimation were derived from studies of non‐Chinese populations with relatively limited pediatric representation. Formal demonstration of the presence or absence of an ethnicity‐linked covariate effect would require derivation of a new set of models based on the pooled Caucasian and Chinese sample, thereby assessing the impact of ethnicity as a covariate.
Three considerations suggest that our results exhibit considerable accuracy. First, the observed variability demonstrated comparable magnitude with the derivation cohort. Second, the Chinese PK profiles were based on five time point curves, with a standard dose administration and a wash‐out period; these conditions made the individual PK estimation closer to the individual estimates than to the underlying population estimates. Third, the generic model ensured adoption of a common structural base and covariate parametrization for the estimation, which would minimize the likelihood of model‐associated differences in the observations.
Because China is a developing country, limited access to coagulation factor concentrates has been known to cause insufficient therapy for patients with hemophilia. However, several FVIII concentrate brands are now available; thus, patients can optimize their therapy with PK guidance and achieve better outcomes. The results of this study will be useful for adjustment of the participants’ dosing regimens and could potentially aid in additional studies. We presume that the present study and future investigations will facilitate the application of PK‐tailored dosing of FVIII concentrate in routine clinical practice. In addition, the submission of our data will enhance the accuracy of WAPPS‐Hemo evaluated PK parameters, especially in Asian pediatric patients.
In conclusion, we have confirmed the existence of interindividual variability among Chinese pediatric patients, irrespective of the specific FVIII concentrate they were receiving (limited to the Kogenate FS®, Advate®, and GreenMono™ concentrates). Both VWF:Ag and FVIII concentrate brand were independent influencing factors for FVIII PK.
5. Funding source
This work was supported in part by grants from research on the application of clinical characteristics of the Beijing Municipal Science and Technology Commission (code Z181100001718182), Capital Health Development Research Project (No. Capital Development 2018‐2‐2094), Beijing Natural Science Foundation of China (No. 7162059), Beijing Municipal Administration of Hospitals Clinical medicine Development of special funding (code ZY201404).
CONFLICT OF INTEREST
The authors confirmed that there were no interests that might cause conflict or bias.
Chen Z, Huang K, Li G, Zhen Y, Wu X, Ai D, et al. Pharmacokinetic variability of factor VIII concentrates in Chinese pediatric patients with moderate or severe hemophilia A. Pediatr Investig. 2021;5:38‐45. 10.1002/ped4.12252
REFERENCES
- 1. Ragni MV, Croteau SE, Morfini M, Cnossen MH, Iorio A. Subcommittee on Factor VIII, Factor IX, and Rare Bleeding Disorders. Pharmacokinetics and the transition to extended half‐life factor concentrates: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16:1437‐1441. [DOI] [PubMed] [Google Scholar]
- 2. Ahlberg A. Haemophilia in Sweden. VII. Incidence, treatment and prophylaxis of arthropathy and other musculo‐skeletal manifestations of haemophilia A and B. Acta Orthop Scand. 1965;Suppl 77:3‐132. [DOI] [PubMed] [Google Scholar]
- 3. Manco‐Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535‐544. [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez‐Merchan EC, Jimenez‐Yuste V, Aznar JA, Hedner U, Knobe K, Lee CA, et al. Joint protection in haemophilia. Haemophilia. 2011;17 Suppl 2:1‐23. [DOI] [PubMed] [Google Scholar]
- 5. Iorio A, Marchesini E, Marcucci M, Stobart K, Chan AK. Clotting factor concentrates given to prevent bleeding and bleeding‐related complications in people with hemophilia A or B. Cochrane Database Syst Rev. 2011;(9):CD003429. [DOI] [PubMed] [Google Scholar]
- 6. Nilsson IM, Berntorp E, Löfqvist T, Pettersson H. Twenty‐five years’ experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25‐32. [DOI] [PubMed] [Google Scholar]
- 7. Schrijvers LH, Uitslager N, Schuurmans MJ, Fischer K. Barriers and motivators of adherence to prophylactic treatment in haemophilia: a systematic review. Haemophilia. 2013;19:355‐361. [DOI] [PubMed] [Google Scholar]
- 8. Fischer K, Steen Carlsson K, Petrini P, Holmström M, Ljung R, van den Berg HM, et al. Intermediate‐dose versus high‐dose prophylaxis for severe hemophilia: comparing outcome and costs since the 1970s. Blood. 2013;122:1129‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pasca S, Milan M, Sarolo L, Zanon E. PK‐driven prophylaxis versus standard prophylaxis: When a tailored treatment may be a real and achievable cost‐saving approach in children with severe hemophilia A. Thromb Res. 2017;157:58‐63. [DOI] [PubMed] [Google Scholar]
- 10. Wu R, Luke KH, Poon MC, Wu X, Zhang N, Zhao L, et al. Low dose secondary prophylaxis reduces joint bleeding in severe and moderate haemophilic children: a pilot study in China. Haemophilia. 2011;17:70‐74. [DOI] [PubMed] [Google Scholar]
- 11. Wu R, Sun J, Xiao J, Liu Y, Xue F, Wang H, et al. A prospective study of health‐related quality of life of boys with severe haemophilia A in China: comparing on‐demand to prophylaxis treatment. Haemophilia. 2017;23:430‐436. [DOI] [PubMed] [Google Scholar]
- 12. Ar MC, Vaide I, Berntorp E, Björkman S. Methods for individualising factor VIII dosing in prophylaxis. Eur J Haematol Suppl. 2014;76:16‐20. [DOI] [PubMed] [Google Scholar]
- 13. Björkman S, Folkesson A, Jönsson S. Pharmacokinetics and dose requirements of factor VIII over the age range 3–74 years: a population analysis based on 50 patients with long‐term prophylactic treatment for haemophilia A. Eur J Clin Pharmacol. 2009;65:989‐998. [DOI] [PubMed] [Google Scholar]
- 14. McEneny‐King A, Iorio A, Foster G, Edginton AN. The use of pharmacokinetics in dose individualization of factor VIII in the treatment of hemophilia A. Expert Opin Drug Metab Toxicol. 2016;12:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 15. Henrard S, Speybroeck N, Hermans C. Body weight and fat mass index as strong predictors of factor VIII in vivo recovery in adults with hemophilia A. J Thromb Haemost. 2011;9:1784‐1790. [DOI] [PubMed] [Google Scholar]
- 16. Björkman S, Blanchette VS, Fischer K, Oh M, Spotts G, Schroth P, et al. Comparative pharmacokinetics of plasma‐ and albumin‐free recombinant factor VIII in children and adults: the influence of blood sampling schedule on observed age‐related differences and implications for dose tailoring. J Thromb Haemost. 2010;8:730‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagao A, Yeung CHT, Germini F, Suzuki T. Clinical outcomes in hemophilia A patients undergoing tailoring of prophylaxis based on population‐based pharmacokinetic dosing. Thromb Res. 2019;173:79‐84. [DOI] [PubMed] [Google Scholar]
- 18. Megías‐Vericat JE, Bonanad S, Haya S, Cid AR, Marqués MR, Monte E, et al. Bayesian pharmacokinetic‐guided prophylaxis with recombinant factor VIII in severe or moderate haemophilia A. Thromb Res. 2019;174:151‐162. [DOI] [PubMed] [Google Scholar]
- 19. Chen Z, Li P, Li G, Tang L, Zhen Y, Wu X, et al. Pharmacokinetic studies of factor VIII in Chinese boys with severe Hemophilia A: a single‐center study. Chin Med J (Engl). 2018;131:1780‐1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blanchette VS, Shapiro AD, Liesner RJ, Hernández Navarro F, Warrier I, Schroth PC, et al. Plasma and albumin‐free recombinant factor VIII: pharmacokinetics, efficacy and safety in previously treated pediatric patients. J Thromb Haemost. 2008;6:1319‐1326. [DOI] [PubMed] [Google Scholar]
- 21. McEneny‐King A, Foster G, Iorio A, Edginton AN. Data analysis protocol for the development and evaluation of population pharmacokinetic models for incorporation into the web‐accessible population pharmacokinetic service–hemophilia (WAPPS–Hemo). JMIR Res Protoc. 2016;5:e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collins PW, Björkman S, Fischer K, Blanchette V, Oh M, Schroth P, et al. Factor VIII requirement to maintain a target plasma level in the prophylactic treatment of severe hemophilia A: influences of variance in pharmacokinetics and treatment regimens. J Thromb Haemost. 2010;8:269‐275. [DOI] [PubMed] [Google Scholar]
- 23. Collins PW, Blanchette VS, Fischer K, Björkman S, Oh M, Fritsch S, et al. Break‐through bleeding in relation to predicted factor VIII levels in patients receiving prophylactic treatment for severe hemophilia A. J Thromb Haemost. 2009;7:413‐420. [DOI] [PubMed] [Google Scholar]
- 24. Barnes C, Lillicrap D, Pazmino‐Canizares J, Blanchette VS, Stain AM, Clark D, et al. Pharmacokinetics of recombinant factor VIII (Kogenate‐FS®) in children and causes of inter‐patient pharmacokinetic variability. Haemophilia. 2006;12:40‐49. [Google Scholar]
- 25. Mingot‐Castellano ME, Parra R, Núñez R, Martorell M. Improvement in clinical outcomes and replacement factor VIII use in patients with haemophilia A after factor VIII pharmacokinetic‐guided prophylaxis based on Bayesian models with myPKFiT® . Haemophilia. 2018;24:e338‐e343. [DOI] [PubMed] [Google Scholar]
- 26. Abrantes JA, Nielsen EI, Korth‐Bradley J, Harnisch L, Jönsson S. Elucidation of factor VIII activity pharmacokinetics: a pooled population analysis in patients with hemophilia A treated with moroctocog alfa. Clin Pharmacol Ther. 2017;102:977‐988. [DOI] [PubMed] [Google Scholar]
- 27. Morfini M, Marchesini E, Paladino E, Santoro C, Zanon E, Iorio A. Pharmacokinetics of plasma‐derived vs. recombinant FVIII concentrates: a comparative study. Haemophilia. 2015;21:204‐209. [DOI] [PubMed] [Google Scholar]
- 28. Klamroth R, Simpson M, von Depka‐Prondzinski M, Gill JC, Morfini M, Powell JS, et al. Comparative pharmacokinetics of rVIII‐SingleChain and octocogalfa (Advate(®)) in patients with severe haemophilia A. Haemophilia. 2016;22:730‐738. [DOI] [PubMed] [Google Scholar]
- 29. Schwartz RS, Abildgaard CF, Aledort LM, Arkin S, Bloom AL, Brackmann HH, et al. Human recombinant DNA‐derived antihemophilic factor (factor VIII) in the treatment of hemophilia A. recombinant Factor VIII Study Group. N Engl J Med. 1990;323:1800‐1805. [DOI] [PubMed] [Google Scholar]
- 30. Fijnvandraat K, Berntorp E, ten Cate JW, Johnsson H, Peters M, Savidge G, et al. Recombinant, B‐domain deleted factor VIII (r‐VIII SQ): pharmacokinetics and initial safety aspects in hemophilia A patients. Thromb Haemost. 1997;77:298‐302. [PubMed] [Google Scholar]
- 31. Lee CA, Owens D, Bray G, Giangrande P, Collins P, Hay C, et al. Pharmacokinetics of recombinant factor VIII (recombinate) using one‐stage clotting and chromogenic factor VIII assay. Thromb Haemost. 1999;82:1644‐1647. [PubMed] [Google Scholar]
- 32. Castaman G, Linari S. Pharmacokinetic drug evaluation of recombinant factor VIII for the treatment of hemophilia A. Expert Opin Drug Metab Toxicol. 2018;14:143‐151. [DOI] [PubMed] [Google Scholar]
- 33. Kepa S, Horvath B, Reitter‐Pfoertner S, Schemper M, Quehenberger P, Grundbichler M, et al. Parameters influencing FVIII pharmacokinetics in patients with severe and moderate haemophilia A. Haemophilia. 2015;21:343‐350. [DOI] [PubMed] [Google Scholar]
- 34. Björkman S, Oh MS, Spotts G, Schroth P, Fritsch S, Ewenstein BM, et al. Population pharmacokinetics of recombinant factor VIII: the relationships of pharmacokinetics to age and body weight. Blood. 2012;119:612‐618. [DOI] [PubMed] [Google Scholar]
- 35. Vlot AJ, Koppelman SJ, van den Berg MH, Bouma BN, Sixma JJ. The affinity and stoichiometry of binding of human factor VIII to von Willebrand factor. Blood. 1995;85:3150‐3157. [PubMed] [Google Scholar]
- 36. Fischer K, Pendu R, van Schooten CJ, van Dijk K, Denis CV, van den Berg HM, et al. Models for prediction of factor VIII half‐life in severe haemophiliacs: distinct approaches for blood group O and non‐O patients. PLoS One. 2009;4:e6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mazurkiewicz‐Pisarek A, Płucienniczak G, Ciach T, Płucienniczak A. The factor VIII protein and its function. Acta Biochim Pol. 2016;63:11‐16. [DOI] [PubMed] [Google Scholar]


