Abstract
Background
Abaloparatide is a parathyroid hormone receptor agonist that increases bone formation and reduces vertebral and nonvertebral fracture risk in women with postmenopausal osteoporosis. Animal studies indicate abaloparatide stimulates vertebral bone formation and enhances bony bridging and biomechanical stability of fracture calluses.
Aims
The current study is evaluating the potential utility for abaloparatide as an adjunct therapy for spinal fusions.
Material and Methods
The effects of 14 or 28 days of daily subcutaneous injections of abaloparatide (20 μg/kg/d) or vehicle were evaluated in 32 male Sprague‐Dawley rats starting 1 day after noninstrumented posterolateral fusion (PLF) with bone autograft. Fusion mass microarchitecture was analyzed by micro‐computed tomography (micro‐CT) and serum markers of bone formation and bone resorption were evaluated. Motion segments were scored in a blinded manner as fused or unfused by postmortem radiography and manual palpation.
Results
Abaloparatide‐treated rats showed higher bone formation (serum osteocalcin) at day 14 and 28 compared with vehicle controls, without increases in the bone resorption marker serum TRACP‐5b. Micro‐CT showed greater trabecular number in fusion masses from the abaloparatide group vs vehicle controls at day 14. Manual palpation and radiography indicated no fusions in either group at day 14, whereas 25% of vehicle‐treated rats and 50% of abaloparatide‐treated rats had bilateral fusion at day 28.
Discussion and Conclusion
In summary, this rat PLF model showed that abaloparatide treatment was associated with higher levels of the bone formation marker osteocalcin, improved fusion mass architecture, and a non‐ significant 2‐fold higher fusion rate compared with vehicle.
Keywords: arthrodesis, bone formation, bone‐forming agent, fusion mass, spine surgery
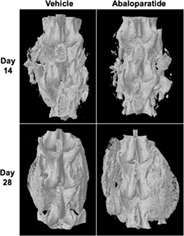
3D micro‐computed tomography images of motion segments showing lack of spinal fusion at day 14, and successful spinal fusion at day 28.
1. INTRODUCTION
Spinal fusion is a common procedure for patients with degenerative disc disease, spinal stenosis, spondylolisthesis, spondylosis, spinal fractures, scoliosis, and kyphosis. 1 Successful fusion surgery creates an environment that allows endochondral and intramembranous ossification processes to form a solid stabilizing bony bridge across decompressed segments, 2 , 3 , 4 , 5 , 6 but several biological and biomechanical challenges can hinder formation of this bridge. 7 Iliac crest bone grafting (ICBG) is the gold standard adjuvant biomaterial for spinal fusion, but fusion failure leading to nonunion or pseudoarthrosis is not uncommon even with ICBG. 5 Fusion failure can lead to poor clinical outcomes and may require costly and morbid revision surgery. 8 Risk factors for fusion failure include multilevel fusions, older age, smoking, diabetes, and osteoporosis. 5 Osteoporosis is relatively common in individuals undergoing spinal fusion, especially postmenopausal women, 9 which can contribute to complications, such as pseudoarthrosis, hardware failure, graft or interbody cage subsidence, adjacent‐level disc degeneration, and vertebral fractures. 10 BMP‐2 delivered in a collagen sponge (Infuse) within certain interbody devices is approved as an alternative to ICBG for anterior lumbar interbody fusions. 11 However, BMP‐2 does not improve vertebral bone mass and is sometimes associated with vertebral osteolysis, endplate erosion, ectopic bone formation, graft and cage subsidence, and other complications. 12 , 13
Systemically administered osteoporosis therapies, including antiresorptive bisphosphonates and the bone‐forming parathyroid hormone receptor (PTHR) agonist teriparatide, have been investigated as adjuvant therapies for spinal fusion. 14 Bisphosphonates and teriparatide increase spine bone mineral density (BMD) in patients with osteoporosis 15 , 16 and have been tested in patients undergoing spinal fusion for their effects on arthrodesis, vertebral bone density, adjacent vertebral fractures, instrumentation failure, fusion mass catabolism, and graft or cage subsidence. 10 A study of individuals undergoing interbody lumbar fusion showed favorable effects of bisphosphonates on bridging bone formation, cage subsidence, and vertebral fracture risk, though clinical assessments showed no improvements. 17 Bisphosphonates generally show neutral effects on fusion rates in animals. 14 Bone‐forming agents may offer advantages over antiresorptives for spinal fusion. 14 , 18 High‐dose teriparatide promotes fusion mass osteogenesis and chondrogenesis in animals, leading in some cases to enhanced arthrodesis. 4 , 19 , 20 A retrospective clinical study of patients undergoing instrumented fusion for osteoporotic vertebral fracture showed a lower rate of mechanical complications in those receiving postoperative teriparatide therapy vs those receiving bisphosphonate therapy. 21 Prospective clinical trials in women with postmenopausal osteoporosis (PMO) undergoing instrumented posterolateral lumbar fusion (PLF) show some beneficial effects of teriparatide vs bisphosphonates when treatments began 2 months preoperatively and continued postoperatively. 22 , 23 However, a placebo‐controlled trial in patients with PMO undergoing noninstrumented PLF showed no radiographic or clinical improvements with teriparatide initiated immediately postoperatively. 24 Teriparatide stimulates bone resorption in patients with osteoporosis, 15 an effect also observed in animals undergoing spinal fusion, 25 , 26 and this response may offset some of the benefits its bone‐forming effect would otherwise have on vertebral BMD, the fusion mass, and arthrodesis.
Abaloparatide is selective PTHR agonist approved in the US for the treatment of PMO. Abaloparatide increases bone formation in women with PMO, leading to greater increases in spine BMD vs teriparatide during the first year of therapy and an overall 86% reduction in vertebral fracture risk vs placebo. 27 These results indicate a substantial effect of abaloparatide on vertebral bone mass that may be relevant to patients undergoing spinal fusion. Abaloparatide‐treated women with PMO show lesser increases in systemic bone resorption markers vs teriparatide treatment, 27 , 28 and several animal studies show that abaloparatide increases vertebral bone formation, density, and strength without increasing bone resorption parameters. 29 , 30 , 31 , 32 Abaloparatide also improved the early bridging and biomechanical stability of long bone fractures in rats, with bridging showing significant relationships with the extent of callus chondrogenesis and osteogenesis. 33 Spinal fusion is associated with chondrogenesis and osteogenesis, 2 , 3 , 6 and chondrogenic responses have been demonstrated with high‐dose teriparatide therapy in spinal fusion models 3 , 4 and with abaloparatide in mesenchymal cell cultures. 34 The current study is the first to examine the effects of abaloparatide as a postoperative adjuvant therapy in animals undergoing noninstrumented PLF.
2. MATERIALS AND METHODS
2.1. Animal care and surgery
All animal procedures and activities were approved by the University of Iowa's Institutional Animal Care and Use Committee (IACUC) and performed in an AAALAC‐accredited vivarium at the Bone Healing Research Lab and Iowa Spine Research Lab (Iowa City, Iowa, USA). Thirty‐two 8‐week‐old male Sprague‐Dawley rats were obtained from Covance and pair‐housed in standard Thorne racks. Animals were ear‐tagged and microchipped and underwent physical examinations and daily cage‐side observation over a 7 day acclimation period. Animals were weighed before surgery and weekly thereafter. Antibiotic (Enroflaxacin 5 mg/kg) was administered intramuscularly prior to surgery. Serum was prepared from saphenous vein blood obtained prior to surgery (day 0) and 14 and 28 days postoperatively.
All surgeries were performed by the same surgeon (Douglas C. Fredericks) who was blinded to postoperative treatment group assignments. Preoperatively, animals were anesthetized with isoflurane and fur was removed over the dorsal surgical region. Exposed skin was scrubbed with chlorhexidine soap and wiped with isopropyl alcohol, and chlorhexidine was applied prior to creating a dorsal ~5 cm long mid‐line incision from L1 to the sacrum. Fascia and muscles were incised over the L1‐L6 transverse processes, and the medial third‐to‐half of L4 and L5 transverse processes were decorticated with a high‐speed burr. To obtain bone autograft, bupivacaine (1 mg, 0.25%, 0.1 mL per site) was applied to the fascia above the iliac crest followed by incision and harvesting of ~0.4 cc of corticocancellous bone graft from each iliac crest. This graft was combined with bone harvested from L4‐L5 spinous processes for a total of ~1.0 cc of autograft per animal. Autograft was morselized with a rongeur and ~ 0.5 cc was applied to each of the left and right L4‐L5 paraspinal beds between their transverse processes. Autograft was placed along the medial third‐to‐half of the transverse processes, primarily toward the midline, with no direct contact with vertebral bodies. Fascia and skin were closed with 4‐0 Vicryl sutures and the skin stapled closed. Postoperative analgesics were administered per IACUC protocol, and animals were allowed free cage movement. All animals were closely observed twice per day over the subsequent 4 weeks and given additional pain medication as indicated by mobility, diet, disposition, and general activity.
Starting 1 day postoperatively, rats received daily subcutaneous injections of sterile saline (vehicle, n = 16) or abaloparatide at 20 μg/kg/d (n = 16), with abaloparatide doses adjusted weekly based on body weight. Assignments to treatment groups were determined by a random number generator. Eight animals from each treatment group were euthanized 14 days after surgery and the remaining animals were euthanized 28 days after surgery with Euthasol (120 mg/kg iv). Necropsies included examination of all external surfaces, orifices, cranial, thoracic, abdominal, and pelvic cavities including contents. The entire lumbar column was removed en bloc and soft tissues were removed from the surgically treated spinal unit. The grafted site was examined for graft migration, infection, and soft tissue abnormalities.
2.2. Bone turnover markers
Serum concentrations of the bone formation marker osteocalcin were determined by the Immutopics Rat Osteocalcin ELISA Kit (Quidel). Serum concentrations of the bone resorption marker tartrate‐resistant acid phosphatase isoform 5b (TRACP‐5b) were measured with the RatTRAP ELISA (IDS).
2.3. Micro‐computed tomography analyses
The excised lumbar spine was scanned with a SkyScan 1176 Micro‐CT unit. Thresholding was performed manually based on the visualization of host cancellous bone. Micro‐computed tomography (micro‐CT) thresholding, analyses, and data collection were performed by a staff engineer who was not involved in the in vivo phase of the study. A region of interest comprising the entire fusion mass including transverse processes was used to calculate total bone volume (BV), trabecular number (Tb.N), and trabecular thickness (Tb.Th) for the left and right sides of each grafted site, results of which were averaged.
2.4. Radiography
Anesthetized rats were radiographed dorsoventrally 2 and 4 weeks postoperatively using a Quantum DR Digital X‐ray unit and Carestream Image software (Crestream Health, Inc, Rochester, NY, USA). Three blinded reviewers evaluated fusion qualitatively (fused or unfused) on each of the left and right sides of the motion segment, with fusion defined as the presence of continuous bridging bone spanning the L4 and L5 transverse processes. To assure proper blinding, none of the three reviewers were involved in the administration of abaloparatide or vehicle. The plain films were also assessed qualitatively for graft migration, osteolysis, fracture, and other adverse events.
2.5. Manual palpation for fusion status
Stiffness of the motion segment was assessed by manual palpation according to accepted practice, as defined in prior investigations. 35 Two independent observers who were blinded to treatment allocation graded motion segments as fused if there was no detectable motion at the disc space in flexion or extension, and unfused if motion was detected. Neither observer was involved in the administration of abaloparatide or vehicle.
2.6. Statistical analyses
Results are expressed as group means and SE. Bone turnover marker and micro‐CT results were analyzed using multiple two‐tailed t‐tests assuming unequal variance. Holm‐Sidak method was used to correct for multiple comparisons, with an adjusted P value of <.05 indicating statistical significance. All statistical analyses including linear regressions were performed using GraphPad Prism V8.1.1 (GraphPad Software Inc., San Diego, CA, USA).
3. RESULTS
All animals in the day 28 groups survived in good health until scheduled necropsy. Two animals from the day 14 vehicle group and one from the day 14 abaloparatide group were euthanized 1‐2 days after surgery due to graft harvesting complications, leaving an n of 6 and 7 animals in the day 14 vehicle and abaloparatide groups, respectively. Rats were alert and eating within 3 hours of surgery, and there were no complications related to abaloparatide administration, including no effect on body weight. All necropsies were unremarkable, with no adverse changes such as inflamed, necrotic, or devascularized tissue surrounding the grafted levels.
The bone formation marker serum osteocalcin was significantly higher in the abaloparatide vs vehicle groups at days 14 and 28 (Table 1). The bone resorption marker serum TRACP‐5b was similar in abaloparatide and vehicle groups at days 14 and 28 (Table 1).
TABLE 1.
Biochemical markers of bone formation (serum osteocalcin) and bone resorption (serum TRACP‐5b)
| Vehicle | Abaloparatide | P value | |
|---|---|---|---|
| Serum osteocalcin (ng/mL) | |||
| Day 0 | 44.2 ± 1.9 | 44.9 ± 2.2 | .826 |
| Day 14 | 34.3 ± 1.5 | 45.4 ± 1.4* | <.0001 |
| Day 28 | 24.7 ± 1.0 | 35.7 ± 2.1* | <.001 |
| Serum TRACP‐5b (U/L) | |||
| Day 0 | 5.91 ± 0.31 | 5.90 ± 0.32 | .982 |
| Day 14 | 6.64 ± 0.54 | 5.47 ± 0.38 | .230 |
| Day 28 | 5.25 ± 0.29 | 5.62 ± 0.39 | .711 |
Note: Data represent means ± SE, n = 14 to 15 per group for days 0 and 14, and 8 per group for day 28. *P < .05 vs vehicle control.
3D micro‐CT assessment of fusion masses indicated similar bone volume (BV) in the two treatment groups at day 14. At day 28, the abaloparatide group showed a trend toward greater BV vs vehicle (multiplicity‐adjusted P = .109; Table 2). Trabecular number was significantly higher in the abaloparatide group vs vehicle controls at day 14 (adjusted P = .002), with a trend to higher Tb.N at day 28 (adjusted P = .081). Trabecular thickness also showed a trend to higher values in the abaloparatide group vs vehicle controls at day 28 (adjusted P = .095, Table 2).
TABLE 2.
Micro‐CT data for fusion masses
| Vehicle | Abaloparatide | P value | |
|---|---|---|---|
| Bone volume (mm3) | |||
| Day 14 | 58.3 ± 2.3 | 59.5 ± 3.0 | .765 |
| Day 28 | 51.7 ± 2.5 | 61.4 ± 3.9 | .109 |
| Trabecular number (1/mm) | |||
| Day 14 | 7.78 ± 0.21 | 11.09 ± 0.68* | .002 |
| Day 28 | 8.38 ± 0.27 | 9.87 ± 0.74 | .081 |
| Trabecular thickness (μm) | |||
| Day 14 | 50.3 ± 0.8 | 50.3 ± 1.7 | .997 |
| Day 28 | 50.2 ± 1.6 | 55.0 ± 1.6 | .095 |
Note: Data are based on average values for left and right sides. Data represent means ± SE; n = 6 to 7/group for Veh and 7 to 8/group for abaloparatide. *P < .05 vs vehicle control.
Abbreviation: micro‐CT, micro‐computed tomography.
Parasagittal 2D micro‐CT reconstructions of fusion masses were also generated across the transverse processes spanning the motion segment for unblinded visual evaluation of fusion masses. Figure 1 shows reconstructions for the right side of all day 28 animals. Successful fusion is visually evident in 2 of 8 vehicle controls based on the continuous presence of mineralized bone between the grafted transverse processes. Most of the vehicle control samples show residual autograft particles in the form of dense white bone fragments that are largely unincorporated into the fusion mass. Fusion is evident by micro‐CT in 4 of the 8 animals in the abaloparatide group, with several cases showing residual graft particles (Figure 1). Figure 2 provides 3D reconstructed micro‐CT images from representative unfused motion segments at day 14, and successfully fused segments at day 28. The larger fusion mass in the abaloparatide vs vehicle sample at day 28 is consistent with the 19% greater average fusion mass bone volume in the abaloparatide vs vehicle group at that time point.
FIGURE 1.
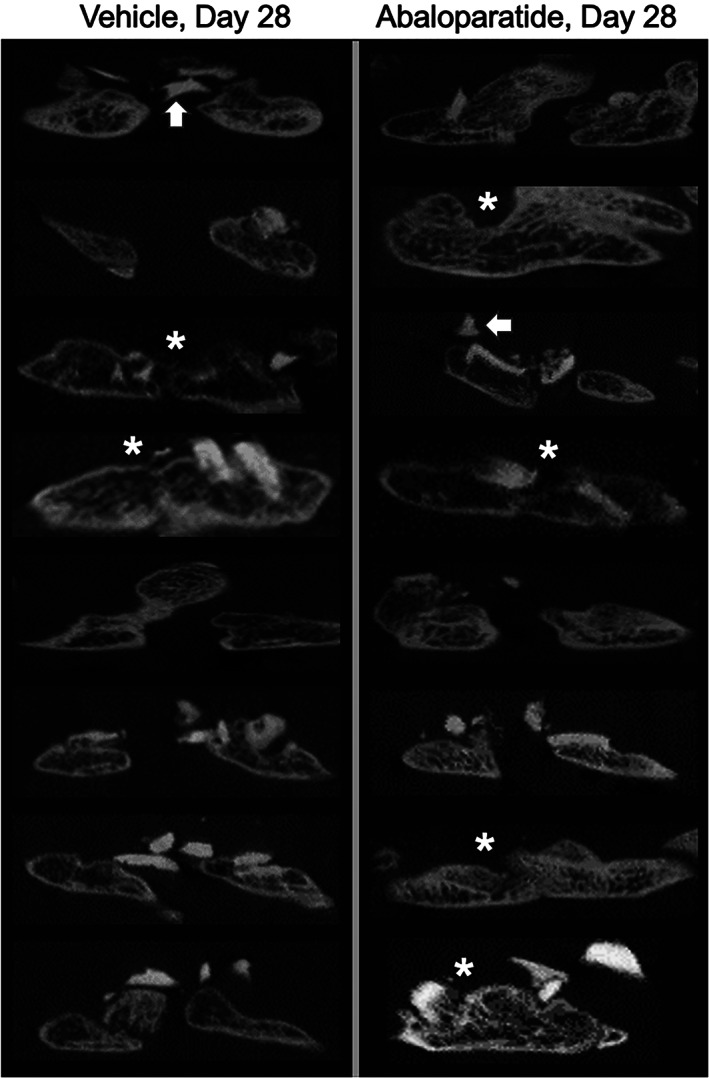
2D micro‐computed tomography (micro‐CT) images of L4‐L5 transverse processes with interposed fusion masses comprising newly formed bone and bone autograft remnants. Images on the left and right are from animals that received daily injections of vehicle or abaloparatide 20 μg/kg/d, respectively, starting the day after surgery and continuing for 28 days. Asterisks indicate motion segments judged to be fused by radiographic and manual palpation assessments, which is corroborated visually in these micro‐CT images. White arrows highlight residual bone autograft fragments, many of which are not incorporated into the established fusion masses
FIGURE 2.

3D micro‐computed tomography (micro‐CT) images of motion segments showing lack of fusion in representative animals from both treatment groups at day 14 and successful fusion in each treatment group at day 28. The day 14 examples were selected based on fusion mass bone volume that was closest to their group mean, whereas fusion mass bone volumes for the day 28 examples were closest to their group mean among the subset of animals deemed fused by radiographic and manual palpation asessments
Observations of higher trabecular number and higher serum osteocalcin with abaloparatide vs vehicle led to explorations of serum osteocalcin as a determinant of microarchitectural parameters. Linear regressions revealed that day 14 serum osteocalcin was significantly and positively correlated with day 14 trabecular number, with an overall Pearson r value of 0.67 (P < .05; Figure 3). Day 28 osteocalcin correlated positively with day 28 trabecular number (r = 0.55, P < .05; data not shown), as did osteocalcin vs trabecular number for both time points combined (r = 0.59, P < .001; data not shown). Day 28 serum osteocalcin also correlated positively with day 28 bone volume (r = 0.52, P < .05; Figure 3), and day 14 osteocalcin correlated with day 28 trabecular thickness (r = 0.54, P < .05; data not shown). Serum TRACP‐5b was not associated with bone volume, trabecular number, or trabecular thickness.
FIGURE 3.

Linear regressions of serum osteocalcin vs fusion mass bone microarchitecture. A, Day 14 serum osteocalcin vs day 14 trabecular number (Tb.N) by micro‐CT. B, Day 28 serum osteocalcin vs day 28 trabecular bone volume per tissue volume (BV/TV). R values represent both groups combined
Radiographs at 2 and 4 weeks showed qualitatively normal healing responses over time. Loss of graft distinction at the host bone margins indicated progression of host site integration, new bone formation, and bone remodeling over time. Blinded scoring of radiographic fusion by three independent reviewers indicated that none of the vehicle or abaloparatide‐treated animals achieved unilateral or bilateral fusion at day 14 (Figure 4A). At day 28, 2 of 8 rats in the vehicle group and 4 of 8 rats in the abaloparatide group achieved radiographic fusion bilaterally, with no other animals achieving unilateral or bilateral fusion (Figure 4A). There was 100% concordance among reviewers on fusion status at the individual animal level. Figure 4C depicts radiographs of representative unfused and fused examples at day 14 and 28, respectively.
FIGURE 4.
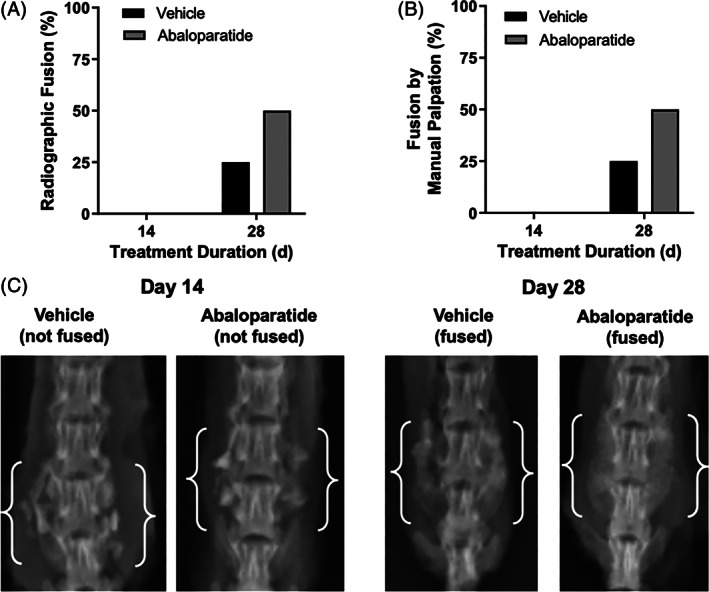
Fusion status assessed by radiography, A and manual palpation, B indicated no fusions in either group at day 14, whereas both assessments indicated successful fusion in 2 of 8 vehicle control group animals and 4 of 8 abaloparatide group animals at day 28. Lumbar spine radiographs, C, show representative unfused examples at day 14, and fused examples at day 28. Day 14 examples were selected based on fusion mass bone volume closest to their group mean, whereas fusion mass bone volumes for the day 28 examples were closest to their group mean among the subset of animals deemed fused by radiography and manual palpation. White brackets identify motion segments and associated fusion masses
Similar to radiographic fusion assessments, fusion evaluation by manual palpation showed no fusions in either treatment group at day 14, whereas 2 of 8 vehicle control animals and 4 of 8 abaloparatide animals were fused at day 28 (Figure 4B). There was 100% concordance among reviewers for manual palpation assessments, and all individual animals deemed fused or unfused by manual palpation were also deemed fused or unfused by blinded radiography and by unblinded review of micro‐CT reconstructions.
4. DISCUSSION
The effects of abaloparatide in rats undergoing spinal fusion were evaluated based on previous animal data indicating that this novel PTH receptor agonist increases vertebral bone formation, density, and strength, and also improves long bone fracture healing. 29 , 30 , 31 , 32 , 33 , 36 , 37 Abaloparatide has high homology to PTH‐related protein (PTHrP), an endogenous factor that plays important roles in osteogenesis, chondrogenesis, and fracture healing. 38 , 39 A previous rat PLF study showed that local administration of another PTHrP analog in a controlled release carrier increased fusion mass BMD and bone volume, with more animals achieving solid fusions compared with empty carrier controls. 40 The current rat PLF study shows that systemic abaloparatide therapy was associated with increased bone formation markers, improvements in fusion mass microarchitecture, and a 2‐fold higher incidence of fusion at day 28 compared with vehicle controls. Fusion status was assessed on days 14 and 28 based on data from a similar rat PLF model indicating that those time points reflect the transition from an unfused to a fused state. 2 , 25 Radiography and manual palpation showed no solid fusions in the vehicle or abaloparatide group at day 14, similar to findings from a rat PLF model evaluating teriparatide. 25 At day 28, 25% and 50% of the vehicle and abaloparatide animals (respectively) demonstrated fusion by both assessment approaches.
Abaloparatide increased serum levels of the bone formation marker osteocalcin compared with vehicle controls. Regression analyses indicated that serum osteocalcin was associated with fusion mass microarchitectural parameters, suggesting the osteoblast‐stimulating effects of abaloparatide may influence fusion processes. This hypothesis is tempered by the lack of fusion mass histomorphometry data, and it is possible that abaloparatide effects on fusion mass microarchitecture were related at least in part to increased chondrogenesis (endochondral ossification). The lack of increased serum TRACP‐5b in the abaloparatide group is consistent with the lack of increased systemic and tissue‐level parameters of bone resorption with abaloparatide in animal models of osteoporosis. 29 , 30 , 31 , 36 , 41 A study in male mice showed that abaloparatide and teriparatide both increased bone resorption markers at 4‐fold the current dose, whereas bone formation markers were significantly higher with high‐dose abaloparatide vs high‐dose teriparatide. 42
The pharmacodynamic profile of increased bone formation with minimal effects on bone resorption may be desirable in patients undergoing spinal fusion, as inadequate anabolism and premature catabolism of fusion masses are potential contributors to pseudoarthrosis. 18 Several observations indirectly support this hypothesis. For example, the confluence of low bone formation markers and high serum TRACP‐5b was the most significant risk factor for nonunion in patients undergoing spinal fusion surgery. 43 A recent prospective controlled clinical study showed an early increase in fusion rate in patients receiving a weekly teriparatide regimen that increases bone formation markers but not resorption markers, 44 , 45 whereas a more recent prospective study with placebo control showed that the standard daily teriparatide regimen, which increases bone formation and resorption markers, 15 had no effects on arthrodesis‐related endpoints or fusion mass. 24 Another spinal fusion study indicated that patients receiving daily teriparatide along with the potent osteoclast inhibitor denosumab had a higher fusion rate at month 6 compared with patients receiving teriparatide alone. 46 Animal data also indicate that BMP‐2 promotes better bony bridging when its osteoclast‐stimulating effects are inhibited. 47
This study has several limitations, including anatomical differences between the rat and human lumbar spine. 48 Rats are an appropriate initial test system for spinal fusion pharmacology studies, 49 but studies in larger species are necessary to corroborate the current findings and to assess abaloparatide effects in instrumented fusion models. The rats were not ovariectomized or osteopenic, a limitation that may be more relevant to instrumented spinal fusion models that rely on systemic BMD for screw fixation and construct stability. The lack of fusion mass histology is another limitation, though biochemical markers of bone formation and resorption provided some mechanistic insights into abaloparatide's pharmacodynamic effects. This study was not designed or powered to evaluate abaloparatide effects on fusion incidence rate. Sequential radiographic fusion assessments would have improved statistical power, but such in‐life assessments were avoided based on concerns that the additional handling could perturb fusion processes. 49 Greater animal numbers would also have improved statistical power, though a previous study in a similar rat PLF model that used binary (fused/not‐fused) scoring showed that even with 27 to 29 rats per group, a 40% higher fusion rate in one treatment group vs another did not achieve statistical significance. 3 Micro‐CT segmentation and microarchitectural analyses of fusion masses can be challenging compared with that of normal bones, a limitation that is likely to have affected both groups and may have increased the variability of those parameters. Another limitation is the lack of time points beyond 4 weeks, which should be addressed in future studies to evaluate longer‐term fusion outcomes. Preliminary data from a rabbit spinal fusion study showed that 6 weeks of systemic abaloparatide therapy significantly increased spinal fusion rate compared with vehicle controls. 50 A recent case report of a patient with cervical fusion nonunion and two failed revision surgeries showed successful fusion after 12 weeks of abaloparatide therapy as a postoperative adjuvant to corpectomy and fusion, though the specific contribution of abaloparatide to this result is unclear. 51 While not a part of this non‐instrumented fusion study, an important question for future studies is how abaloparatide affects vertebral bone volume and density at the fusion level and adjacent vertebrae. Studies using 2‐ to 4‐fold lower doses than the current study indicate that abaloparatide increases bone formation and BMD of rodent vertebral bodies within the four‐week duration of the current study. 31 , 52 Such effects may have favorable implications for the fixation of pedicle or interbody screws and for adjacent‐ and remote‐segment fracture risk. Future studies may also determine whether preoperative vertebral bone augmentation with abaloparatide increases the primary stability of spinal fusion implants.
Strengths of this study include excellent inter‐observer and inter‐modality concordance in fusion assessments. It is also notable that positive effects were achieved with a relatively modest abaloparatide dose, one that represents a substantially lower multiple of its approved clinical dose compared with doses of teriparatide used in most rat spinal fusion studies. 3 , 4 , 19 , 20 , 25 , 26 Abaloparatide increases vertebral BMD and promotes fracture bridging and callus strength in rats when dosed as low as 5 μg/kg/d, 31 , 33 and future studies may determine whether similarly low doses improve spinal fusion in animals.
In summary, systemic abaloparatide administration was associated with early positive effects on fusion mass architecture in rats undergoing non‐instrumented PLF, in association with elevated bone formation markers without increased bone resorption markers. These findings, which were accompanied by a 2‐fold higher fusion rate at day 28, warrant follow‐up in larger species to provide additional insights into abaloparatide's potential efficacy in spinal fusion settings.
CONFLICT OF INTEREST
Heike Arlt, Tatiana Besschetnova, Michael S. Ominsky, and Beate Lanske are current or former employees of Radius Health and may own shares or stock options in Radius Health. The University of Iowa received financial support from Radius Health for DCF to conduct the animal study.
AUTHOR CONTRIBUTIONS
Heike Arlt, Beate Lanske, and Douglas C. Fredericks designed the study, and in addition with Tatiana Besschetnova and Michael S. Ominsky analyzed the data and contributed to writing the manuscript. All authors read and approved the final submitted manuscript.
ACKNOWLEDGMENTS
Funding for this study was provided by Radius Health. Medical writing support was provided by Paul Kostenuik, PhD, of Phylon Pharma Services, and funded by Radius Health. Authors Beate Lanske, Heike Arlt, Tatiana Besschetnova, and Michael S. Ominsky are current employees of Radius Health and report being Radius Health shareholders.
Arlt H, Besschetnova T, Ominsky MS, Fredericks DC, Lanske B. Effects of systemically administered abaloparatide, an osteoanabolic PTHrP analog, as an adjuvant therapy for spinal fusion in rats. JOR Spine. 2021;4:e1132. 10.1002/jsp2.1132
Some of these results were presented in abstract form at the annual meeting of the American Society for Bone and Mineral Research, Sept. 20‐23, 2019, in Orlando, FL, USA.
REFERENCES
- 1. Rajaee SS, Bae HW, Kanim LE, et al. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila pa 1976). 2012;37:67‐76. [DOI] [PubMed] [Google Scholar]
- 2. Boden SD, Schimandle JH, Hutton WC. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine (Phila pa 1976). 1995;20:412‐420. [DOI] [PubMed] [Google Scholar]
- 3. Lawrence JP, Ennis F, White AP, et al. Effect of daily parathyroid hormone (1‐34) on lumbar fusion in a rat model. Spine J. 2006;6:385‐390. [DOI] [PubMed] [Google Scholar]
- 4. O'Loughlin PF, Cunningham ME, Bukata SV, et al. Parathyroid hormone (1‐34) augments spinal fusion, fusion mass volume, and fusion mass quality in a rabbit spinal fusion model. Spine (Phila pa 1976). 2009;34:121‐130. [DOI] [PubMed] [Google Scholar]
- 5. Kaiser MG, Groff MW, Watters WC 3rd, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 16: bone graft extenders and substitutes as an adjunct for lumbar fusion. J Neurosurg Spine. 2014;21:106‐132. [DOI] [PubMed] [Google Scholar]
- 6. Okamoto S, Ikeda T, Sawamura K, et al. Positive effect on bone fusion by the combination of platelet‐rich plasma and a gelatin beta‐tricalcium phosphate sponge: a study using a posterolateral fusion model of lumbar vertebrae in rats. Tissue Eng Part A. 2012;18:157‐166. [DOI] [PubMed] [Google Scholar]
- 7. Reid JJ, Johnson JS, Wang JC. Challenges to bone formation in spinal fusion. J Biomech. 2011;44:213‐220. [DOI] [PubMed] [Google Scholar]
- 8. Dagostino PR, Whitmore RG, Smith GA, Maltenfort MG, Ratliff JK. Impact of bone morphogenetic proteins on frequency of revision surgery, use of autograft bone, and total hospital charges in surgery for lumbar degenerative disease: review of the Nationwide inpatient sample from 2002 to 2008. Spine J. 2014;14:20‐30. [DOI] [PubMed] [Google Scholar]
- 9. Burch S, Feldstein M, Hoffmann PF, Keaveny TM. Prevalence of poor bone quality in women undergoing spinal fusion using biomechanical‐CT analysis. Spine (Phila pa 1976). 2016;41:246‐252. [DOI] [PubMed] [Google Scholar]
- 10. McCoy S, Tundo F, Chidambaram S, Baaj AA. Clinical considerations for spinal surgery in the osteoporotic patient: a comprehensive review. Clin Neurol Neurosurg. 2019;180:40‐47. [DOI] [PubMed] [Google Scholar]
- 11. Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP‐2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337‐349. [DOI] [PubMed] [Google Scholar]
- 12. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein‐2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471‐491. [DOI] [PubMed] [Google Scholar]
- 13. Helgeson MD, Lehman RA Jr, Patzkowski JC, et al. Adjacent vertebral body osteolysis with bone morphogenetic protein use in transforaminal lumbar interbody fusion. Spine J. 2011;11:507‐510. [DOI] [PubMed] [Google Scholar]
- 14. Hirsch BP, Unnanuntana A, Cunningham ME, Lane JM. The effect of therapies for osteoporosis on spine fusion: a systematic review. Spine J. 2013;13:190‐199. [DOI] [PubMed] [Google Scholar]
- 15. Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013;382:50‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207‐1215. [DOI] [PubMed] [Google Scholar]
- 17. Nagahama K, Kanayama M, Togawa D, Hashimoto T, Minami A. Does alendronate disturb the healing process of posterior lumbar interbody fusion? A prospective randomized trial. J Neurosurg Spine. 2011;14:500‐507. [DOI] [PubMed] [Google Scholar]
- 18. Bransford R, Goergens E, Briody J, Amanat N, Cree A, Little D. Effect of zoledronic acid in an L6‐L7 rabbit spine fusion model. Eur Spine J. 2007;16:557‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qiu Z, Wei L, Liu J, et al. Effect of intermittent PTH (1‐34) on posterolateral spinal fusion with iliac crest bone graft in an ovariectomized rat model. Osteoporos Int. 2013;24:2693‐2700. [DOI] [PubMed] [Google Scholar]
- 20. Lina IA, Puvanesarajah V, Liauw JA, et al. Quantitative study of parathyroid hormone (1‐34) and bone morphogenetic protein‐2 on spinal fusion outcomes in a rabbit model of lumbar dorsolateral intertransverse process arthrodesis. Spine (Phila pa 1976). 2014;39:347‐355. [DOI] [PubMed] [Google Scholar]
- 21. Kawabata A, Yoshii T, Hirai T, et al. Effect of bisphosphonates or teriparatide on mechanical complications after posterior instrumented fusion for osteoporotic vertebral fracture: a multi‐center retrospective study. BMC Musculoskelet Disord. 2020;21:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ohtori S, Inoue G, Orita S, et al. Teriparatide accelerates lumbar posterolateral fusion in women with postmenopausal osteoporosis: prospective study. Spine (Phila pa 1976). 2012;37:E1464‐E1468. [DOI] [PubMed] [Google Scholar]
- 23. Ohtori S, Inoue G, Orita S, et al. Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective. Spine (Phila pa 1976). 2013;38:E487‐E492. [DOI] [PubMed] [Google Scholar]
- 24. Jespersen AB, Andresen ADK, Jacobsen MK, Andersen MØ, Carreon LY. Does systemic administration of parathyroid hormone after noninstrumented spinal fusion surgery improve fusion rates and fusion mass in elderly patients compared to placebo in patients with degenerative lumbar spondylolisthesis? Spine (Phila pa 1976). 2019;44:157‐162. [DOI] [PubMed] [Google Scholar]
- 25. Abe Y, Takahata M, Ito M, Irie K, Abumi K, Minami A. Enhancement of graft bone healing by intermittent administration of human parathyroid hormone (1‐34) in a rat spinal arthrodesis model. Bone. 2007;41:775‐785. [DOI] [PubMed] [Google Scholar]
- 26. Sugiura T, Kashii M, Matsuo Y, et al. Intermittent administration of teriparatide enhances graft bone healing and accelerates spinal fusion in rats with glucocorticoid‐induced osteoporosis. Spine J. 2015;15:298‐306. [DOI] [PubMed] [Google Scholar]
- 27. Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316:722‐733. [DOI] [PubMed] [Google Scholar]
- 28. Leder BZ, O'Dea LS, Zanchetta JR, et al. Effects of abaloparatide, a human parathyroid hormone‐related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2015;100:697‐706. [DOI] [PubMed] [Google Scholar]
- 29. Varela A, Chouinard L, Lesage E, Smith SY, Hattersley G. One year of abaloparatide, a selective activator of the PTH1 receptor, increased bone formation and bone mass in osteopenic ovariectomized rats without increasing bone resorption. J Bone Miner Res. 2017;32:24‐33. [DOI] [PubMed] [Google Scholar]
- 30. Doyle N, Varela A, Haile S, et al. Abaloparatide, a novel PTH receptor agonist, increased bone mass and strength in ovariectomized cynomolgus monkeys by increasing bone formation without increasing bone resorption. Osteoporos Int. 2018;29:685‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chandler H, Lanske B, Varela A, et al. Abaloparatide, a novel osteoanabolic PTHrP analog, increases cortical and trabecular bone mass and architecture in orchiectomized rats by increasing bone formation without increasing bone resorption. Bone. 2019;120:148‐155. [DOI] [PubMed] [Google Scholar]
- 32. Varela A, Chouinard L, Lesage E, et al. One year of abaloparatide, a selective peptide activator of the PTH1 receptor, increased bone mass and strength in ovariectomized rats. Bone. 2017;95:143‐150. [DOI] [PubMed] [Google Scholar]
- 33. Lanske B, Chandler H, Pierce A, et al. Abaloparatide, a PTH receptor agonist with homology to PTHrP, enhances callus bridging and biomechanical properties in rats with femoral fracture. J Orthop Res. 2019;37:812‐820. [DOI] [PubMed] [Google Scholar]
- 34. Yang Y, Lei H, Wang B. Effect of the PTHrP(1‐34) analog abaloparatide on inducing chondrogenesis involves inhibition of intracellular reactive oxygen species production. Biochem Biophys Res Commun. 2019;509:960‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fredericks D, Petersen EB, Watson N, Grosland N, Gibson‐Corley K, Smucker J. Comparison of two synthetic bone graft products in a rabbit posterolateral fusion model. Iowa Orthop J. 2016;36:167‐173. [PMC free article] [PubMed] [Google Scholar]
- 36. Bahar H, Gallacher K, Downall J, Nelson CA, Shomali M, Hattersley G. Six weeks of daily abaloparatide treatment increased vertebral and femoral bone mineral density, microarchitecture and strength in ovariectomized osteopenic rats. Calcif Tissue Int. 2016;99:489‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bernhardsson M, Aspenberg P. Abaloparatide versus teriparatide: a head to head comparison of effects on fracture healing in mouse models. Acta Orthop. 2018;89:674‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang M, Nasiri AR, Broadus AE, Tommasini SM. Periosteal PTHrP regulates cortical bone remodeling during fracture healing. Bone. 2015;81:104‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kostenuik P, Mirza FM. Fracture healing physiology and the quest for therapies for delayed healing and nonunion. J Orthop Res. 2017;35:213‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liang B, Huang J, Xu J, et al. Local delivery of a novel PTHrP via mesoporous bioactive glass scaffolds to improve bone regeneration in a rat posterolateral spinal fusion model. RCS Adv. 2018;8:12484‐12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Makino A, Takagi H, Takahashi Y, et al. Abaloparatide exerts bone anabolic effects with less stimulation of bone resorption‐related factors: a comparison with teriparatide. Calcif Tissue Int. 2018;103:289‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Le Henaff C, Ricarte F, Finnie B, et al. Abaloparatide at the same dose has the same effects on bone as PTH (1‐34) in mice. J Bone Miner Res. 2020;35(4):714‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Inose H, Yamada T, Mulati M, et al. Bone turnover markers as a new predicting factor for nonunion after spinal fusion surgery. Spine (Phila pa 1976). 2018;43:E29‐E34. [DOI] [PubMed] [Google Scholar]
- 44. Nakamura T, Sugimoto T, Nakano T, et al. Randomized Teriparatide [human parathyroid hormone (PTH) 1‐34] once‐weekly efficacy research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab. 2012;97:3097‐3106. [DOI] [PubMed] [Google Scholar]
- 45. Ebata S, Takahashi J, Hasegawa T, et al. Role of weekly teriparatide administration in osseous union enhancement within six months after posterior or transforaminal lumbar interbody fusion for osteoporosis‐associated lumbar degenerative disorders: a multicenter, prospective randomized study. J Bone Joint Surg Am. 2017;99:365‐372. [DOI] [PubMed] [Google Scholar]
- 46. Ide M, Yamada K, Kaneko K, et al. Combined teriparatide and denosumab therapy accelerates spinal fusion following posterior lumbar interbody fusion. Orthop Traumatol Surg Res. 2018;104:1043‐1048. [DOI] [PubMed] [Google Scholar]
- 47. Bougioukli S, Jain A, Sugiyama O, et al. Combination therapy with BMP‐2 and a systemic RANKL inhibitor enhances bone healing in a mouse critical‐sized femoral defect. Bone. 2016;84:93‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jaumard NV, Leung J, Gokhale AJ, Guarino BB, Welch WC, Winkelstein BA. Relevant anatomic and morphological measurements of the rat spine: considerations for rodent models of human spine trauma. Spine (Phila pa 1976). 2015;40:E1084‐E1092. [DOI] [PubMed] [Google Scholar]
- 49. Drespe IH, Polzhofer GK, Turner AS, Grauer JN. Animal models for spinal fusion. Spine J. 2005;5:209S‐216S. [DOI] [PubMed] [Google Scholar]
- 50. Hahn W, Kumagai H, Moore HG, et al. Abaloparatide enhances fusion and bone formation in a rabbit spinal arthrodesis model. J Orthop Res. 2020;44:962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parikh S, Lubitz SE, Sharma A. Novel use of abaloparatide to augment spinal fusion in patient undergoing cervicothoracic revision surgery. J Endocrine Soc. 2020;4(Suppl 1):MON‐365. [Google Scholar]
- 52. Arlt H, Mullarkey T, Hu D, et al. Effects of abaloparatide and teriparatide on bone resorption and bone formation in female mice. Bone Rep. 2020;13:100291. [DOI] [PMC free article] [PubMed] [Google Scholar]


