Abstract
Objectives
The underlying mechanisms behind the therapeutic and side effects of deep brain stimulation (DBS) need further investigation. The utilization of transgenic mouse lines is a suitable approach to better understand the cellular and network effects of DBS. However, not many bilateral DBS studies have been conducted in mice. This might be due to a lack of commercially available bilateral DBS constructs.
Materials and Methods
We developed an approach to perform repetitive long‐term DBS in freely moving mice. In this study, we implanted an in‐house custom‐made DBS construct containing two bipolar concentric electrodes to target the subthalamic nucleus (STN) bilaterally. Subsequently, we stimulated half of the animals with clinically relevant parameters three to five times a week with a duration of 20 min for ten weeks. Several behavioral tests were conducted of which the open field test (OFT) is shown to validate the reliability of this electrode construct and implantation method. Furthermore, we performed fiber photometry measurements to show the acute effect of STN‐DBS on serotonin network activity in the dorsal raphe nucleus.
Results
Repetitive DBS and long‐term behavioral testing were performed without complications. STN‐DBS resulted in an increase of the distance traveled in the OFT and a reduction of calcium levels in serotonergic neurons of the dorsal raphe nucleus. None of the mice had lost their electrodes and postmortem evaluation of the tissue showed accurate targeting of the STN without excessive gliosis.
Conclusion
The DBS electrode construct and implantation method described can be used for long‐term DBS studies to further investigate the mechanisms underlying DBS.
Keywords: DBS, deep brain stimulation, electrodes, implantation, method, mice
INTRODUCTION
Deep brain stimulation (DBS) is a dynamic area of translational neuroscience and used to treat motor symptoms in patients with Parkinson's disease (PD) (1), other movement disorders (2, 3), and nonmotor symptoms for instance in patients with obsessive‐compulsive disorder (4, 5). Recently, DBS is being explored as a potential therapy in other neurological and psychiatric disorders, including Alzheimer's disease (6), depression (7, 8), and addiction (9). Despite the beneficial effects of DBS in movement disorders, these studies show variable clinical outcomes and side‐effect profiles. Therefore, it is important to gain more knowledge about its underlying mechanisms of action. DBS has been used in different neural targets using a variety of stimulation parameters, but its effect on a molecular, biochemical, and neural network level remains poorly understood.
A good way to study these underlying mechanisms would be by using transgenic animal models. Most transgenic animal models are created by using mice embryos, because mice are technically the easiest species for generation of transgenic lines (10). By using transgenic mice, the effects of DBS can be studied in different disease models. Furthermore, Cre transgenic mouse lines in combination with state of the art techniques, such as Optogenetics and Designer Receptors Exclusively Activated by Designer Drugs, give the opportunity to manipulate genes or cell types during DBS. However, not many bilateral DBS studies have been performed in freely moving mice. In the few bilateral DBS studies that have been done before, either a monopolar electrode or a bipolar twisted electrode was implanted per hemisphere (11, 12, 13, 14). Both types of electrodes are not ideal since the current spread is too big in monopolar stimulation and twisted electrodes might bend, untwist, and distort brain tissue leading to reduced accuracy of the implantation and more brain damage. So far, a bilateral DBS construct with high‐quality concentric bipolar electrodes is not commercially available.
Therefore, we developed a custom‐made electrode construct and implantation method to be able to perform long‐term bilateral DBS experiments. Using this technique, we were able to perform DBS and behavioral testing on a daily basis over the course of three months without losing any electrodes. In the present study, we show a systematic approach to perform DBS experiments in mice to stimulate further research into the mechanisms underlying the therapeutic and side effects of DBS.
MATERIALS AND METHODS
Overall Study Design
A proportion of patients with PD will develop adverse neuropsychiatric symptoms following subthalamic nucleus (STN)‐DBS treatment, such as depression and anxiety (15, 16). The overall experimental approach of this study was to investigate the therapeutic and side effects of STN‐DBS in ePet‐cre mice. Therefore, a behavioral test battery was performed in all mice to identify changes in locomotion, mood, and anxiety (timeline is shown in Fig. 1). To investigate possible changes in serotonin network activity that might be related to mood problems during STN‐DBS, fiber photometry was used. To specifically record from serotonergic neurons in the dorsal raphe nucleus, ePet‐cre mice (JAX stock #012712) were used in combination with a cre‐dependent GCaMP6s virus. To perform such a long‐term DBS experiment, it was necessary to establish a good method for implanting a high‐quality DBS construct in mice. Therefore, this paper focusses on the developed DBS‐construct and implantation method used for this study. Only data of the open field test (OFT) and fiber photometry measurements of one example animal are shown to validate the reliability of the DBS‐construct.
Figure 1.
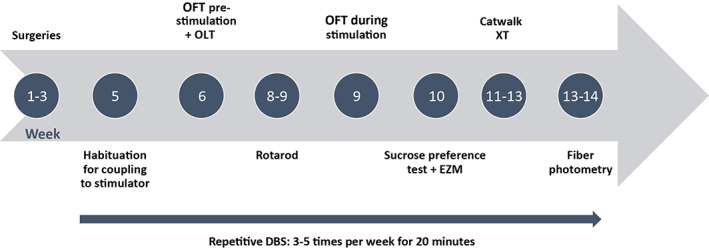
Experimental timeline. Timeline showing the sequence of events and behavioral testing. The arrow shows the application of DBS (and sham stimulation in the control group) for three to five times a week for a duration of 20 min over the course of ten weeks. DBS was given during behavioral testing or in the homecage. [Color figure can be viewed at wileyonlinelibrary.com]
Animals
Experiments were performed on ePet‐cre male mice (JAX stock #012712). Of the 53 mice that were implanted, 49 completed the whole study of three months. Mice were socially housed under controlled conditions (21 ± 2°C and 40‐60% humidity) in a reversed 12 hours day/night cycle (lights on, 7 pm) until they had received surgery. Mice were given ad libitum access to food and water. At the time of surgery, mice were six months of age. All tests and DBS took place under red light conditions during the active phase of the animals. Experiments were conducted according to the directive 2010/63/EU for animal experiments and in agreement with the Animal Experiments and Ethics Committee of the Maastricht University, Maastricht, The Netherlands.
DBS Electrode Construct
Several stimulation paradigms and electrode types are possible with experimental DBS. Stimulation with bipolar electrodes is the most appropriate for targeting the small brain structures in rodents. The concentrated current around the tip of bipolar electrodes will influence a smaller tissue area compared to monopolar stimulation in which electrons will diffuse from the negative pole through the way of least resistance. For this reason, we used a custom‐made DBS construct with a bipolar concentric electrode for each hemisphere, which was manufactured by the engineering department (IDEE) of Maastricht University (Fig. 2).
Figure 2.
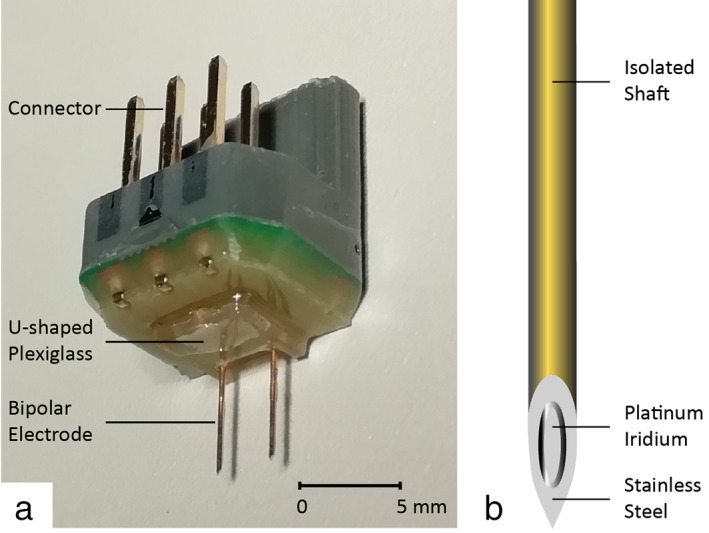
a. DBS construct. The DBS construct contains two bipolar concentric electrodes. The interelectrode distance is 3 mm and the length of the electrodes are 5.5 mm to target the STN. The little U‐shaped Plexiglas attaches the construct to the skull. b. Schematic drawing of a concentric bipolar electrode. Each bipolar electrode is gold‐coated. The stainless steel outer part functions as a positive contact and the platinum‐iridium inner part functions as the negative contact. The outer diameter of the concentric needle is 300 μm, the electrode surface is 0.021 mm2, and the distance between anode and cathode is 50 μm. [Color figure can be viewed at wileyonlinelibrary.com]
The bipolar electrodes used for this DBS construct are commercially available and clinically used for electromyography recordings in human subjects (TE/D125‐101, Technomed, Beek, The Netherlands). The bipolar electrode has an outer diameter of 300 μm and a tip diameter of approximately 50 μm. Apart from the 75 μm exposed tip, the whole electrode is insulated. The gold‐coated electrode has a stainless steel outer part, which functions as a positive contact, and a platinum‐iridium inner part, which functions as the negative contact. The distance between the positive and negative contact is 50 μm. The desired length of the bipolar electrodes was fixed in a custom‐designed print plate with an adjusted interelectrode distance. The inner wire and outer surface of the needles were soldered to the print plate. Thereafter, connecting pins were soldered on the print plate. Melted plastic was used to cover and insulate the connections. The edges of the construct were trimmed, making it as small as possible. A little Plexiglas u shape was added onto the construct for a perfect fit with the skull of the mouse. A dental adhesive and light‐cure flowable composite was used to create a stable and strong attachment between the Plexiglas u shape and the small skull of the mouse. A matching DBS construct holder was fabricated for easy and accurate stereotaxic implantation (shown in Fig. 3C).
Figure 3.
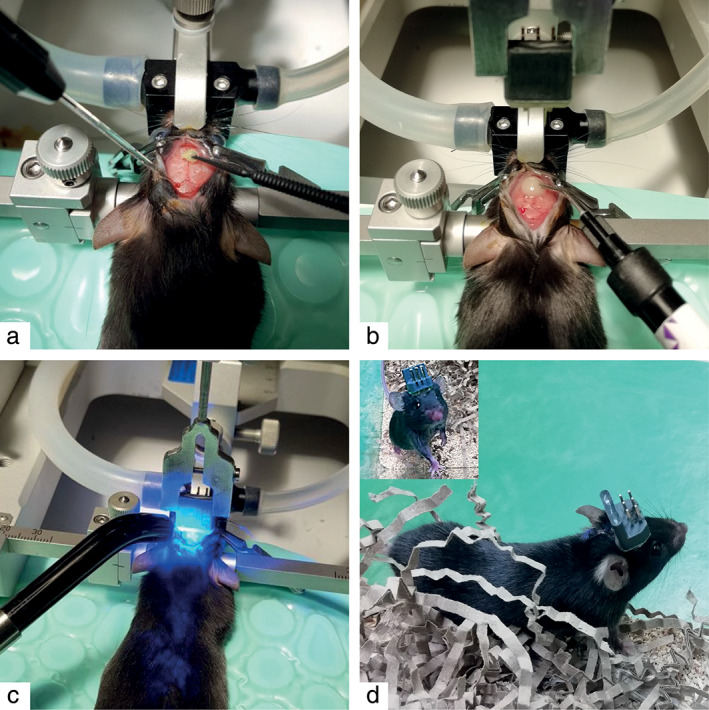
Implantation of the DBS electrode. a. After drilling the holes for the electrodes at the region of interest, self‐etch dental adhesive was applied and cured with UV light. b. Flowable composite was applied at the center of where the construct will be placed. c. The DBS construct was lowered until it reaches the desired DV coordinate. Thereafter, the composite was cured with UV light and small layers were added on each side until there was a thick encasement. d. Recovered mouse. [Color figure can be viewed at wileyonlinelibrary.com]
This electrode construct can be modified according to different targets and experimental designs. Both unilateral and bilateral stimulation are possible using this construct. The electrode length can be adapted to target any brain region of interest. For bilateral stimulation, the interelectrode distance can be modified as well. Furthermore, the addition of a recording electrode for electroencephalography (EEG) purposes is also possible (17). Another important advantage of this DBS construct is that only one connector is needed for connecting the mouse to the external device(s), regardless of number of implanted electrodes in the brain or on the skull, which makes connecting the animal easy and therefore less stressful. The total weight of this construct is 2 gm.
In this study, the STN was targeted bilaterally. Therefore, a construct was made with two bipolar electrodes having an interelectrode distance of 3 mm and a length of 5.5 mm.
Stereotaxic Surgery
Buprenorphine 0.1 mg/kg was subcutaneously injected half an hour prior to surgery as an analgesic. Inhalational anesthesia was induced and maintained with isoflurane (Abbot Laboratories, Maidenhead, UK) at 4% and 1.5‐3%, respectively. After adequate induction of the anesthesia, the mouse was placed in a small animal stereotaxic frame (Stoelting, Dublin, Ireland) and fixed by ear bars with zygoma ear cups (Kopf, Los Angeles, CA, USA) and a mouse gas anesthesia head holder (Stoelting). To maintain body temperature at 37°C throughout the whole procedure, the mouse was placed on a thermoregulator pad. An ocular lubricant was applied to prevent drying of the eyes. A subcutaneous injection of Lidocaine 1% (Streuli Pharma, Uznach, Switzerland) at the incision side was given for local anesthesia. After careful removal of hair and disinfection of the skin with betadine followed by 70% ethanol, a midline incision was made to identify the cranial sutures (e.g., bregma, lamda).
After leveling the skull, burr holes were made at the location of the brain region of interest by using a dental drill. The bipolar electrodes were targeted in the STN (AP −2.00 mm, ML ±1.50 mm, DV −4.55 mm) and the GCaMP6s virus and fiber (400 μm) were targeted in the DRN for fiber photometry measurements (AP −4.7, ML −0.00, DV −2.9 at a 32° angle from the left) (18). For fiber photometry, 500 nL of AAV5.Syn.Flex.GCaMP6s.WPRE.SV40 was injected in the DRN using the Nanoject II (Drummond Scientific). Subsequently, the fiber and DBS construct were placed in their holders. The fiber and one of the bipolar electrodes were calibrated with bregma and positioned to the correct AP and ML coordinates. Before lowering them to their right DV coordinates, the skull was completely dried and OptiBond All‐In‐One (Kerr, Los Angeles, CA, USA) was used (Fig. 3a). This dental adhesive has etching, priming, and bonding properties, which prepare the skull for proper attachment to the flow able composite (Diadent, Almere, The Netherlands). The dental adhesive is air dried and light cured with UV light. Subsequently, composite was added in the middle of the area where the DBS construct would be placed on the skull before, lowering it to the correct DV coordinate (Fig. 3b). The composite under the construct is light cured from all sides (Fig. 3c). Multiple small layers of composite (2‐3 mm) were added around the construct and light cured until there was a thick encasement. Next, the fiber was lowered until it reached the DV coordinate of the DRN and attached to the skull with the same composite. Afterward, the construct and fiber holder were removed and the skin of the mice sutured if necessary. After surgery, all mice were given a one‐week recovery period prior to behavioral testing. During this period, their welfare was closely monitored and analgesic and anti‐inflammatory agents were administered.
The electrode construct and dental composite add extra weight on top of the animal's head. Besides, attaching and detaching the wires of the external stimulator may induce stress and alter behavior. Therefore, sham stimulation was performed as well. These animals were implanted with the same electrode construct.
Stimulation Protocol
To apply DBS, the electrode construct was connected to the stimulator by external wires and a swivel. The swivel allows the animal to move freely without the risk of cables being tangled up. A 3800 MultiStim 8‐channel connected to Model 3820 Stimulus Isolation Units (A‐M Systems, Carlsborg, WA, USA) was used for DBS. Animals of the DBS group were stimulated three to five times a week for 20 min over the course of ten weeks, either during behavioral tests or in their home cage. The sham animals were coupled to the external stimulator, but did not receive DBS.
Frequency, pulse width, current intensity, and pulse type are parameters that can be altered in DBS. Most animal studies use DBS parameters that are also used for treatment purposes in the clinic, which is a high‐frequency stimulation (above ~100 Hz) and a pulse width between 60 and 90 μs (19, 20, 21). However, the current intensities used in animal studies are more variable. A very high current intensity can induce tissue damage and/or adverse behavioral side effects (22). Therefore, the maximum current intensity that can be used safely in neural tissue should be determined for the electrode used by charge density calculations (charge per phase divided by surface area of the electrode). The recommended charge density limit for DBS in clinical practice, which is also used in animal models, is 30 μC/cm2 based on the Shannon model of neuronal damage (23, 24, 25). In addition, dose response tests were performed with variable current intensities to determine which intensity elicits the desired behavioral response, but does not induce adverse behavioral side effects. In this study, monophasic stimulation at a frequency of 130 Hz, pulse width 60 μs, and a current intensity of 80 μA was used. The surface of the electrode is 0.021 mm2, so the chosen parameters result in a charge density of 22.9 μC/cm2, which is well below the limit of 30 μC/cm2. Moreover, these parameters did not induce adverse behavioral effects.
Open Field Test
To test the effect of STN‐DBS on locomotion, several behavioral tests were performed (Fig.1). In this manuscript, only the OFT is presented to show the validity and reliability of the electrode construct and implantation method. We performed the OFT two times in the same cohort of animals. The first time STN‐DBS was only given prior to the OFT for a duration of 10 minutes and the second time STN‐DBS was given during the OFT. The OFT arena consisted of a gray Plexiglas base (50 × 50 cm) and was divided into four equal arenas (25 × 25 cm each) separated by 25‐cm‐high black Plexiglas walls. A camera was placed above the center of the open field. Four mice were tracked simultaneously with a computerized system (Ethovision Color Pro, Noldus, The Netherlands) for 20 min. The floor of the open field was cleaned with ethanol after each session to prevent transmission of olfactory cues. Total distance moved was recorded for each animal.
Fiber Photometry
To investigate possible changes in serotonin network activity during STN‐DBS, fiber photometry was used. Mice were connected to a Doric Lenses Inc. photometry setup by a 400 μm 0.48 NA patchcord. A 465 nm LED was coupled to a fluorescence mini‐cube and a 1 × 1 fiber optic rotary joint to deliver light through the patchcord into the implanted optical fiber in the DRN. Light was delivered at an intensity of 40 microwatt at the tip of the patchcord. The emitted light that was collected from the brain was passed through the mini‐cube and measured with a Newport 2151 Femtowatt Photoreceiver Module. To link the calcium‐dependent fluorescent signal to DBS on or off epochs, the 3800 MultiStim 8‐channel was directly connected to an analog input channel of the photometry console.
For the photometry experiment, mice could move freely in their home cage while they were coupled to both the patchcord and DBS cable. During the photometry measurements, mice were allowed to habituate for 5 min before DBS was given intermittently (2 min on – 3 min off) for ten trials. This means that one photometry measurement lasted for 55 min.
Electrode Localization
After the completion of experiments, verification of the electrode position was verified. At the end of the experiment, mice were deeply anesthetized with pentobarbital and transcardially perfused with tyrode buffer, followed by ice‐cold 4% paraformaldehyde fixative in 0.1 M phosphate buffer. The electrode constructs were removed and the brains extracted from the crania. Brains were postfixed in 4% paraformaldehyde overnight and submerged in sucrose for cryoprotection (24 hours in 10% sucrose, thereafter 24 hours in 20% sucrose at 5°C). Coronal brain sections (20 μm) were cut on a cryostat, stained by hematoxylin and eosin (HE), and examined by an independent researcher. Animals with one or both electrodes misplaced were excluded from data analysis.
Data Analysis and Statistical Testing
Statistical analysis was performed using IBM SPSS Statistics for Windows (version 25.0, released 2017, IBM Corp., Armonk, NY, USA). Normality of OFT data was tested using the Kolmogorov‐Smirnov test and homogeneity of variance was tested using the Levene's test. The parametric data of the OFT were analyzed with the Student's t‐test and provided as mean ± standard error of the mean (SEM). A p‐value ≤0.05 was considered significant.
Fiber photometry data were extracted, processed, and analyzed using custom MATLAB (Mathworks) scripts. The first two and a half minute of the data during the habituation period was discarded to remove the initial fast bleaching of the fluorescent signal. Next, the original sample rate of a 100 Hz was downsampled to 1 Hz and low‐pass filtered using decimation. A two‐term exponential model was fitted and subtracted from the decimated data to account for slow bleaching artifacts. Then, a single baseline fluorescence value (F 0) was calculated by averaging the fluorescent signals during the 60‐sec time period pre‐DBS. Subsequently, the normalized change in fluorescence (dF/F) was calculated as F − F 0/F 0. Data are presented as a heatmap of all single trials and an average plot with SEM.
A permutation test was used to analyze the statistical significance of the DBS‐related fluorescent change. To compare the values of dF/F at each time point with the DBS‐related fluorescent change, 10,000 permutations were used. An α‐level of ≤0.05 was considered significant.
RESULTS
Histology
The surgical procedure was well tolerated by the operated animals. No complications such as infection, head/neck muscle injury, or intracranial ischemia were observed during or after surgery. Moreover, no behavioral abnormalities that could have been linked to internal brain damage were observed. At autopsy, macroscopic inspection of the scalp/skull did not indicate any sign of internal damage. Besides, there was no weight loss in both groups over the course of the study (data were presented during the reviewing process, but not shown here). After surgery, mice recovered fast. Initially, mice lost 10% of their weight during the first three days postsurgery (Fig. 4a). From day four onward, mice started gaining weight again and showed no signs of reduced welfare anymore. No electrodes were lost during this study. The location of the implanted electrodes was checked by histology. In 44 out of 49 mice, both electrodes were targeted correctly resulting in a targeting accuracy of approximately 90%. No excessive gliosis was found around the tip of the electrode (Fig. 4b).
Figure 4.
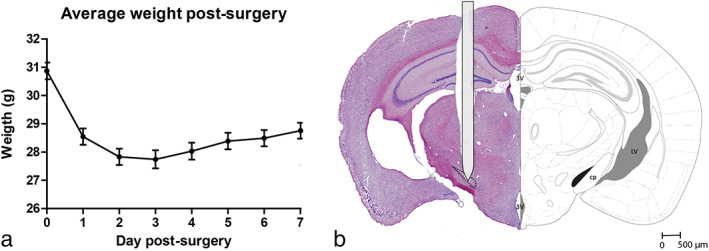
a. Weight postsurgery. After an initial loss of 10% of their bodyweight, mice started to gain weight again from day four postsurgery onward. b. Electrode localization STN. The tip of the electrode is localized in the STN (black). The electrode shaft is schematically drawn to scale. 3 V, third ventricle; LV, lateral ventricle; cp, cerebral peduncle. [Color figure can be viewed at wileyonlinelibrary.com]
Effect of STN‐DBS on Locomotion
DBS settings for mice were selected based on our previous rat studies, but started off with a reduced current intensity of 100 μA (26, 27, 28). However, this current intensity was still too high and induced behavioral side effects. Therefore, stimulation was applied at a frequency of 130 Hz, pulse width 60 μs, and a current intensity of 80 μA. These settings did not induce abnormal behaviors during and in the absence of DBS such as seizures or turning. STN‐DBS resulted in an increase of the distance traveled when given during the OFT (p < 0.01), but not when only given shortly before the test (Fig. 5).
Figure 5.
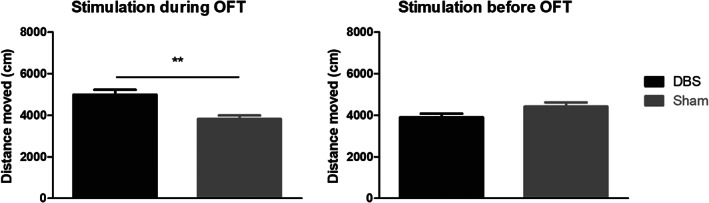
Open field test (OFT). Results of the OFT of the DBS group (n = 13) and sham control group (n = 12) are shown when DBS is given during the test (left) or shortly before the test (right). A significant increase in distance moved was seen when DBS is given during the test, while no differences between groups were found when DBS was given only shortly prior to the OFT in the same cohort of animals (**p < 0.01, independent samples t‐test).
Effect of STN‐DBS on In Vivo Activity of 5‐HT Neurons in the DRN Using the Genetically Coded Calcium Sensor GCaMP6s
Fiber photometry was used to find out if GCaMP6s fluorescence in 5‐HT neurons of the DRN changes in response to STN‐DBS. We found that upon the start of STN‐DBS, the GCaMP6s signal decreases over time. As soon as STN‐DBS stops, the fluorescent signal slowly goes back to baseline (Fig. 6). A permutation test revealed that this calcium related decrease in fluorescence was significant (p < 0.05). When STN‐DBS was stopped, it took approximately 90 sec before the fluorescent signal recovered back to baseline.
Figure 6.
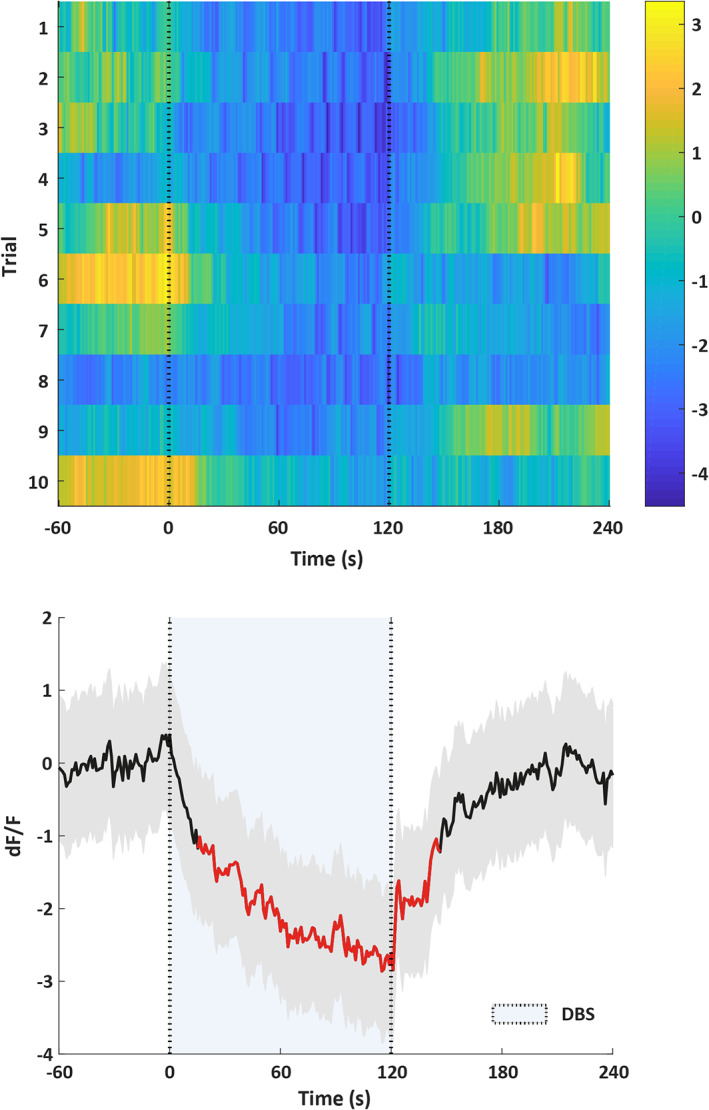
Fiber photometry data (n = 1). The top figure shows a heatmap of the change in fluorescence (dF/F) before, during (between the dotted lines), and after DBS. Each row plots one DBS trial (total of 10). Color scale at the right indicates dF/F (yellow = high and dark blue = low dF/F). The bottom figure shows the change in fluorescence averaged over the ten trials of the same animal. Thick black line indicates mean, shaded areas indicate SEM, and red segments indicate statistically significant decrease from baseline (p < 0.05; permutation test). [Color figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
By using a custom‐made electrode construct containing two small bipolar concentric electrodes, we were able to target the STN of mice with high accuracy and perform DBS experiments over the course of three months. The combination of a dental adhesive and light‐cured flowable composite ensures a strong bond between the electrode construct and the skull of mice, making it possible to perform DBS experiments for a long time. We performed these long‐term DBS experiments without any problems regarding infections, loss of electrodes, or abnormal behavior. High‐frequency DBS of the STN resulted in an increased distance traveled in the OFT, showing that DBS affects the basal ganglia network resulting in hyperactivity. Furthermore, STN‐DBS also affected the serotonin network of the DRN by lowering the activity of 5‐HT neurons, which might explain the occurrence or worsening of neuropsychiatric symptoms experienced by a proportion of PD patients following STN‐DBS treatment (15, 16).
The OFT is a widely used behavioral task to study general locomotor activity during DBS on and off conditions (29, 30). The “distance moved” is a well‐characterized readout parameter of the OFT that is affected in rodent models of PD which is (partly) restored by STN‐DBS (31, 32). Since STN‐DBS improves locomotion in rodent models of PD, it is not surprising that it increases distance moved in healthy mice as well. The increase of activity in the OFT might be explained by activation of the motor cortex because of STN‐DBS (31, 33).
Our fiber photometry experiments showed that the calcium level in the serotonin network of the DRN was significantly lower during STN‐DBS. This is in line with previous research in which STN‐DBS inhibits the firing rate of 5‐HT neurons of the DRN in anesthetized rats (28, 34). Interestingly, there is no direct connection between the STN and DRN. Therefore, polysynaptic connections between the STN and DRN are likely to be involved. Future experiments are necessary to unravel which brain regions might play a role. Possible candidates might be the substantia nigra pars reticulata or the globus pallidus externa, because both brain regions project to serotonergic neurons of the DRN and receive direct inputs from STN (35, 36).
Based on this study, it is possible to perform bilateral DBS experiments in mice for a long period of time. Therefore, mechanisms underlying DBS can be further investigated by using transgenic mouse lines. By using transgenic mouse models in combination with state of the art techniques (e.g., optogenetics, fiber photometry, Designer Receptors Exclusively Activated by Designer Drug), molecular, cellular, and network manipulations can be applied and recorded to better understand the impact of DBS on the brain (37, 38, 39). This would not be possible in other animal species. Furthermore, several genetic mouse models for a variety of diseases are available nowadays, which can be used for preclinical testing of DBS (40).
A few studies have already been performed where bilateral stimulation was applied in mice. Either a monopolar electrode or a bipolar twisted electrode was implanted per hemisphere (11, 12, 13, 14). The disadvantage of monopolar electrodes is that the current travels from the monopolar electrode through the brain to a ground electrode that is often attached to the skull or neck muscle (11, 13), which means that the current spread is relatively big. Therefore, bipolar electrodes are preferred when targeting the small brain structures in mice. Based on our own experience, we know that twisted bipolar electrodes carry the potential to untwist inside the brain. In addition, twisting the electrodes can damage the insulation layer creating a short circuit. Furthermore, the rough shape of these electrodes might distort brain tissue during implantation. Moreover, the relative location of the anode and cathode is not consistent as well as the distance between them, as these twisted electrodes are cut arbitrarily. Another disadvantage is that the electrodes used in the aforementioned studies are not available as a bilateral construct. Therefore, one electrode has to be implanted per hemisphere and two connectors need to be attached to the skull. This can be difficult in brain regions where you need a small interelectrode distance. For these reasons, we developed together with the engineering department (IDEE) of Maastricht University the described bilateral electrode construct that can be adapted to the researcher's wishes in terms of electrode length and interelectrode distance (Fig. 2). Furthermore, this construct provides a platform to incorporate additional stimulating, recording, and EEG electrodes, which can be connected to the other pins (17, 41). Therefore, only one connector is needed to connect the construct to external devices. This results in a shorter handling time and therefore less stress for the animal, which is of course beneficial for the animal's welfare and behavioral testing.
A limitation of our study is the use of monophasic stimulation. Monophasic stimulation can cause electrochemical damage and is therefore not often used in a clinical setting (42). In the majority of patients, charged balanced stimulation is used. Most external stimulators for rodents are not able to stimulate in a charged balanced manner. For this reason, most preclinical studies have been done using monophasic stimulation. Even though monophasic stimulation can induce tissue damage, in this study and previous studies of our group we have not seen this after histological examination (26, 28). Moreover, the primary aim of this article was to introduce a reliable and reproducible electrode implantation method in mice. The DBS construct that we have described here can be used for all types of stimulation waveforms.
CONCLUSION
The implantation procedure and electrode‐construct described here can be used to perform long‐lasting studies in mice to unravel the mechanisms underlying DBS. We showed that it is feasible to perform a long‐term bilateral DBS study in mice without complications. Combining this method of bilateral DBS in mice with genetic mouse models and state of the art techniques can contribute to gaining knowledge about the mechanisms of DBS and eventually clinical treatments of brain disorders.
Authorship Statement
Drs. Sylvana Pol, Dr. Ali Jahanshahi, and Professor Yasin Temel designed the study. Drs. Sylvana Pol conducted the study, including data collection, and data analysis. Drs. Sylvana Pol prepared the manuscript draft with important intellectual input from Dr. Ali Jahanshahi and Professor Yasin Temel. All authors approved the final manuscript.
Acknowledgement
We thank Instrument Development, Engineering and Evaluation (IDEE) department of MUMC+ for developing the deep brain stimulation electrode constructs.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: This work has been supported by the Dutch Research Council (NWO) through VENI 2015 project number 91616043.
Conflict of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Sylvana Pol, Email: sylvana.pol@maastrichtuniversity.nl.
Ali Jahanshahi, Email: a.jahanshahi@maastrichtuniversity.nl.
REFERENCES
- 1. Deuschl G, Schade‐Brittinger C, Krack P et al. A randomized trial of deep‐brain stimulation for Parkinson's disease. N Engl J Med 2006;355:896–908. [DOI] [PubMed] [Google Scholar]
- 2. Barbe MT, Reker P, Hamacher S et al. DBS of the PSA and the VIM in essential tremor: a randomized, double‐blind, crossover trial. Neurology 2018;91:e543–e550. [DOI] [PubMed] [Google Scholar]
- 3. Cury RG, Fraix V, Castrioto A et al. Thalamic deep brain stimulation for tremor in Parkinson disease, essential tremor, and dystonia. Neurology 2017;89:1416–1423. [DOI] [PubMed] [Google Scholar]
- 4. Mulders AEP, Leentjens AFG, Schruers K, Duits A, Ackermans L, Temel Y. Choreatic side effects of deep brain stimulation of the anteromedial subthalamic nucleus for treatment‐resistant obsessive‐compulsive disorder. World Neurosurg 2017;104:1048.e9–1048.e13. [DOI] [PubMed] [Google Scholar]
- 5. Mulders AEP, Plantinga BR, Schruers K et al. Deep brain stimulation of the subthalamic nucleus in obsessive‐compulsive disorder: neuroanatomical and pathophysiological considerations. Eur Neuropsychopharmacol 2016;26:1909–1919. [DOI] [PubMed] [Google Scholar]
- 6. Leoutsakos JS, Yan H, Anderson WS et al. Deep brain stimulation targeting the fornix for mild Alzheimer dementia (the ADvance trial): a two year follow‐up including results of delayed activation. J Alzheimers Dis 2018;64:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bergfeld IO, Mantione M, Hoogendoorn ML et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment‐resistant depression: a randomized clinical trial. JAMA Psychiat 2016;73:456–464. [DOI] [PubMed] [Google Scholar]
- 8. Dougherty DD, Rezai AR, Carpenter LL et al. A randomized sham‐controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment‐resistant depression. Biol Psychiatry 2015;78:240–248. [DOI] [PubMed] [Google Scholar]
- 9. Muller UJ, Sturm V, Voges J et al. Nucleus accumbens deep brain stimulation for alcohol addiction ‐ safety and clinical long‐term results of a pilot trial. Pharmacopsychiatry 2016;49:170–173. [DOI] [PubMed] [Google Scholar]
- 10. Pradhan BS, Majumdar SS. An efficient method for generation of transgenic rats avoiding embryo manipulation. Mol Ther Nucleic Acids 2016;5:e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang AD, Berges VA, Chung SJ, Fridman GY, Baraban JM, Reti IM. High‐frequency stimulation at the subthalamic nucleus suppresses excessive self‐grooming in autism‐like mouse models. Neuropsychopharmacology 2016;41:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Haas R, Struikmans R, van der Plasse G et al. Wireless implantable micro‐stimulation device for high frequency bilateral deep brain stimulation in freely moving mice. J Neurosci Methods 2012;209:113–119. [DOI] [PubMed] [Google Scholar]
- 13. Dournes C, Beeske S, Belzung C, Griebel G. Deep brain stimulation in treatment‐resistant depression in mice: Comparison with the CRF1 antagonist, SSR125543. Prog Neuropsychopharmacol Biol Psychiatry 2013;40:213–220. [DOI] [PubMed] [Google Scholar]
- 14. Pinhal CM, van den Boom BJG, Santana‐Kragelund F et al. Differential effects of deep brain stimulation of the internal capsule and the striatum on excessive grooming in Sapap3 mutant mice. Biol Psychiatry 2018;84:917–925. [DOI] [PubMed] [Google Scholar]
- 15. Mosley PE, Marsh R. The psychiatric and neuropsychiatric symptoms after subthalamic stimulation for Parkinson's disease. J Neuropsychiatry Clin Neurosci 2015;27:19–26. [DOI] [PubMed] [Google Scholar]
- 16. Cyron D. Mental side effects of deep brain stimulation (DBS) for movement disorders: The futility of denial. Front Integr Neurosci 2016;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smit JV, Jahanshahi A, Janssen MLF, Stokroos RJ, Temel Y. Hearing assessment during deep brain stimulation of the central nucleus of the inferior colliculus and dentate cerebellar nucleus in rat. PeerJ 2017;5:e3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fraklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates, third edition. New York: Academic Press, 2007. [Google Scholar]
- 19. Lozano AM, Lipsman N, Bergman H et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol 2019;15:148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roet M, Pol S, Schaper F, Hoogland G, Jahanshahi A, Temel Y. Severe seizures as a side effect of deep brain stimulation in the dorsal peduncular cortex in a rat model of depression. Epilepsy Behav 2019;92:269–275. [DOI] [PubMed] [Google Scholar]
- 21. Benazzouz A, Hallett M. Mechanism of action of deep brain stimulation. Neurology 2000;55:S13–S16. [PubMed] [Google Scholar]
- 22. Temel Y, Visser‐Vandewalle V, van der Wolf M et al. Monopolar versus bipolar high frequency stimulation in the rat subthalamic nucleus: differences in histological damage. Neurosci Lett 2004;367:92–96. [DOI] [PubMed] [Google Scholar]
- 23. Grill WM. Safety considerations for deep brain stimulation: review and analysis. Expert Rev Med Devices 2005;2:409–420. [DOI] [PubMed] [Google Scholar]
- 24. Medtronic . A610 Activa Clinician Programmer Application for Deep Brain Stimulation. Clinical Programming Manual. 2018.
- 25. Shannon RV. A model of safe levels for electrical stimulation. IEEE Trans Biomed Eng 1992;39:424–426. [DOI] [PubMed] [Google Scholar]
- 26. van Zwieten G, Jahanshahi A, van Erp ML et al. Alleviation of tinnitus with high‐frequency stimulation of the dorsal cochlear nucleus: a rodent study. Trends Hear 2019;23:2331216519835080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan SK, Hartung H, Visser‐Vandewalle V, Steinbusch HW, Temel Y, Sharp T. A combined in vivo neurochemical and electrophysiological analysis of the effect of high‐frequency stimulation of the subthalamic nucleus on 5‐HT transmission. Exp Neurol 2012;233:145–153. [DOI] [PubMed] [Google Scholar]
- 28. Temel Y, Boothman LJ, Blokland A et al. Inhibition of 5‐HT neuron activity and induction of depressive‐like behavior by high‐frequency stimulation of the subthalamic nucleus. Proc Natl Acad Sci U S A 2007;104:17087–17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Melse M, Temel Y, Tan SK, Jahanshahi A. Deep brain stimulation of the rostromedial tegmental nucleus: an unanticipated, selective effect on food intake. Brain Res Bull 2016;127:23–28. [DOI] [PubMed] [Google Scholar]
- 30. Thiele S, Furlanetti L, Pfeiffer LM, Coenen VA, Dobrossy MD. The effects of bilateral, continuous, and chronic deep brain stimulation of the medial forebrain bundle in a rodent model of depression. Exp Neurol 2018;303:153–161. [DOI] [PubMed] [Google Scholar]
- 31. Li Q, Ke Y, Chan DC et al. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron 2012;76:1030–1041. [DOI] [PubMed] [Google Scholar]
- 32. Brown AR, Antle MC, Hu B, Teskey GC. High frequency stimulation of the subthalamic nucleus acutely rescues motor deficits and neocortical movement representations following 6‐hydroxydopamine administration in rats. Exp Neurol 2011;231:82–90. [DOI] [PubMed] [Google Scholar]
- 33. Dejean C, Hyland B, Arbuthnott G. Cortical effects of subthalamic stimulation correlate with behavioral recovery from dopamine antagonist induced akinesia. Cereb Cortex 2009;19:1055–1063. [DOI] [PubMed] [Google Scholar]
- 34. Hartung H, Tan SK, Steinbusch HM, Temel Y, Sharp T. High‐frequency stimulation of the subthalamic nucleus inhibits the firing of juxtacellular labelled 5‐HT‐containing neurones. Neuroscience 2011;186:135–145. [DOI] [PubMed] [Google Scholar]
- 35. Pollak Dorocic I, Furth D, Xuan Y et al. A whole‐brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron 2014;83:663–678. [DOI] [PubMed] [Google Scholar]
- 36. Galvan A, Devergnas A, Wichmann T. Alterations in neuronal activity in basal ganglia‐thalamocortical circuits in the parkinsonian state. Front Neuroanat 2015;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deisseroth K. Form meets function in the brain: observing the activity and structure of specific neural connections. In: Kennedy H, Van Essen DC, Christen Y, eds. Micro‐, meso‐ and macro‐connectomics of the brain. Cham, Switzerland: Springer, 2016:19–29. [PubMed] [Google Scholar]
- 38. Roth BL. DREADDs for neuroscientists. Neuron 2016;89:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roet M, Hescham SA, Jahanshahi A, Rutten BPF, Anikeeva PO, Temel Y. Progress in neuromodulation of the brain; a role for magnetic nanoparticles? Prog Neurobiol 2019;177:1–14. [DOI] [PubMed] [Google Scholar]
- 40. Trancikova A, Ramonet D, Moore DJ. Genetic mouse models of neurodegenerative diseases. Prog Mol Biol Transl Sci 2011;100:419–482. [DOI] [PubMed] [Google Scholar]
- 41. Schipper S, Aalbers MW, Rijkers K et al. Accelerated cognitive decline in a rodent model for temporal lobe epilepsy. Epilepsy Behav 2016;65:33–41. [DOI] [PubMed] [Google Scholar]
- 42. Piallat B, Chabardes S, Devergnas A et al. Monophasic but not biphasic pulses induce brain tissue damage during monopolar high‐frequency deep brain stimulation. Neurosurgery 2009;64:156–162. discussion 162‐153. [DOI] [PubMed] [Google Scholar]


