Abstract
The primary cilium is an antennae‐like structure extent outside the cell surface. It has an important role in regulating cell‐signaling transduction to affect proliferation, differentiation and migration. Evidence is accumulating that ciliary defects lead to ciliopathies and ciliary deregulation also play crucial roles in cancer formation and progression. Interestingly, restoring the cilia can suppress proliferation in some cancer cell. However, t he role of primary cilia in cancer still be debated. In this article, we review the role of the primary cilium in cancer through architecture, signaling pathways, cilia assembly and disassembly regulators, and summarized the new findings of the primary cilium in tumor microenvironments and different cancers, highlighting novel possibilities for therapeutic target in cancer.
Keywords: cancer, cilium assembly, cilium disassembly, primary cilium, signaling pathway, tumor microenvironment
The role of the primary cilium in cancer.
1. INTRODUCTION
Since researchers found that many tumor‐associated signaling pathways, such as receptor tyrosine kinases (RTK) signaling, Hedgehog signaling, Notch signaling, and Wnt signaling pathways, were regulated by the primary cilium, the relationship between the primary cilium and tumor development attracted more and more researchers' attention. About a decade years ago Michaud and Yoder (Michaud & Yoder, 2006) anticipated that the emerging discoveries being made about the function of the primary cilium in signaling pathways will also provide novel insights into the molecular mechanisms of carcinogenesis. After that, many researchers also proposed that the list of cancers associated with altered ciliary signaling would grow exponentially (Christensen et al., 2007; Mans et al., 2008; Wong & Reiter, 2008). Indeed, a number of research has been made to investigate the relationship between primary cilia and the cancer, and a number of cancer has been found to have some relationship with primary cilia defect. However, little is known about the specific molecular mechanism of primary cilium modulating oncogenesis in context of different cancers. A detailed understanding of its biological function is consequently crucial to the treatment of cancer. Herein we review architecture of primary cilium, primary cilium associated signaling pathways, cilia assembly and disassembly regulator's role in cancer and summarize current findings of the primary cilium in tumor microenvironments and different types of cancer.
2. THE ARCHITECTURE OF PRIMARY CILIUM
The cilium is a kind of projection at the apical surface of epithelial cells (CECs). It consists of a basal body which under the cell membrane and an axoneme that attaches to the basal body and extended outside the cell. The basal body is transformed from the mother centrosome, and the axoneme was supported by microtubules. Depend on the structure and the motility of the axoneme microtubules, cilia can be divided into motile cilia and primary cilia. The motile cilium has a 9 + 2 microtubule pattern in which nine peripheral doublets of microtubules surround two single centrally localized ones. While the microtubules in primary cilia were arranged in a 9 + 0 pattern: nine outer doublets were found at the periphery but with no central microtubules (Figure 1).
Figure 1.
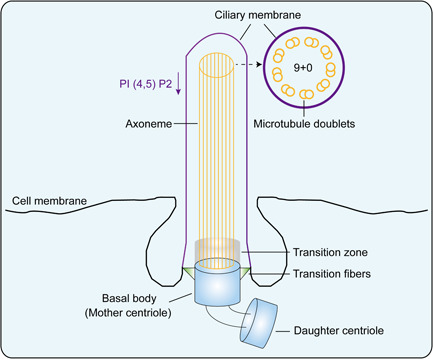
Basic architecture of primary cilium. The overall architecture of primary cilium is shown with the key structural elements. PI (4,5) P2, phosphatidylinositol 4,5 biphosphate
The microtubule outside the cell surface is coated with the ciliary membrane, which extended from and continued with the cell membrane. Nevertheless, the ciliary membrane has completely different characteristics from the cell membrane. This difference may be related to a significant decrease in the content of phosphatidylinositol 4,5 biphosphate (PI (4,5) P2) in the ciliary membrane (Chavez et al., 2015; Garcia‐Gonzalo et al., 2015), which were found to be determined by the enrichment of phosphoinositide 5 phosphatase (Inpp5e) at the basal of cilia (Chavez et al., 2015; Garcia‐Gonzalo et al., 2015). The section between the ciliary membrane and the cell membrane is called the transition zone (TZ), and the TZ connects to the underlying basal body by the transition fibers (TFs). The TFs together with the upper region of the basal body form a special permeable barrier called the cilium gate (Wu et al., 2012), this cilium gate could filter proteins that enter or leave the cilium (Figure 1).
3. CILIA ASSOCIATED SIGNALING PATHWAYS AND CANCER
Cell signaling pathways are crucial regulators of multicellular organisms in a wide variety of functions. Altered signaling pathways are often found in all stages of oncogenesis. More and more research has confirmed that the receptors and effectors are also found localized at the primary cilium, and primary cilium is widely considered the cellular sensorial antenna. Therefore, it is likely that primary cilia may contribute to the regulation of cell signaling pathways. The emerging role of primary cilia in cancer signaling pathways has been reported in recent research. Below, we will review the signaling pathways are regulated by primary cilia in cancer.
3.1. Hedgehog signaling pathway
The Hedgehog (Hh) signaling pathway is the most well‐known cilia‐related signaling pathway, which is essential for vertebrate embryonic development and also has roles in the adult. Hh signaling is activated by initiated by three secreted ligands including Sonic Hedgehog (Shh), Indian Hedgehog (Ihh), and Desert Hedgehog (Dhh). The activate Hh binds to a transmembrane (TM) protein called Patched (Ptch) leading to this interaction complex degraded. In the absence of Hh, Ptch constitutively inhibits the ciliary localization of the seven TM protein Smoothened (Smo). When Ptch is degraded by proteasome, activate Smo migrates membrane and leads to glioma‐associated oncogene family zinc finger family (Gli) moving into nucleus. Gli is a major effector molecule for transcription of target genes (Ng & Curran, 2011; Rohatgi et al., 2007). Aberrant activation of this pathway has evidently been related to cancer development. The first report demonstrates the relationship between the Hh signaling pathway and primary cilium in a screen for embryonic patterning mutations induced by ethylnitrosourea. The mutations in the cilia‐related genes such as Ift172, Ift88, Kif3a in mouse embryos have been found to result in blockade of the Shh signaling pathway and resulting in abnormalities in neural tube and brain development (Huangfu et al., 2003). The important Shh signaling Smo has been found localized to the cilium (Corbit et al., 2005), and both Smo and Ptch1 aggregated at the cilium under the stimulation of Shh signaling (Rohatgi et al., 2007). And the effector of Hh signaling Gli has also been found localized at cilium. These major players of Hh signaling gathering around cilium suggest that primary cilium is involved directly in regulating Hh signaling pathway.
The primary cilium mediated Hh signaling has dual and opposing roles in tumorigenesis. In mouse models Han et al. (2009), found that in the medulloblastoma models are driven by a constitutively active Smo, genetic ablation of primary cilia by Kif3a or Ift88 knockout could inhibit the tumor formation. However, in the medulloblastoma models driven by a constitutively active GLI2, removal of primary cilia could promote the growth of the tumor. Meanwhile, Wong et al. (2009) also found that ciliary ablation inhibited the growth of human basal cell carcinomas (BCCs)‐like tumors induced by an activated form of Smo, however, accelerated tumors induced by activated Gli2, a transcriptional effector of Hh signaling. Some other studies found that prostate cancer cell lines that lack demonstrable autocrine Hh signaling capacity, did not exhibit primary cilia even under proliferation‐limiting growth conditions (Zhang et al., 2009). Also, in ovarian cancer, the low frequency of primary cilia in cancer cells could lead to aberrant Hh signaling and ovarian tumorigenesis (Egeberg et al., 2012). Notably, a recent study found that EMT programs promote basal mammary stem cell and tumor‐initiating cell stemness through inducing primary ciliogenesis and Hh signaling (Guen et al., 2017). Therefore, modulation of tumorigenesis by primary cilium could be dependent on the states of Hh signaling pathway.
3.2. Wntsignaling pathway
Another important signaling pathway that recently been found could be regulated by cilia is the Wnt signaling pathway. The transduction of extracellular Wnt signaling is achieved through the binding of Wnt with the receptor complex Frizzled (Fz) and the low‐density lipoprotein receptor LRP5 or LRP6. The transduction of Wnt signaling depends on the localization of Dishevelled (Dvl) under different signal conditions. Nuclear localization of Dv1 is necessary for the activation of the β‐catenin dependent canonical Wnt signaling pathway (Itoh et al., 2005). β‐catenin is the major molecule for this pathway, which regulates crucial aspects of cell polarity, cell migration, and cell fate determination. Moreover, the current study found that Inversin (Inv), a protein localized at the primary cilium, could interact with Dvl and affect Dvl degradation, thereby mediating the exchange between classical Wnt signaling and noncanonical Wnt signaling (Simons et al., 2005). It has also been found that the protein Jouberin (Jbn), localized at the primary cilium, can bind to β‐catenin to mediate its nuclear localization. The absence of the primary cilium could lead to an increase of free Jbn in the cytoplasm, which promotes β‐catenin entry into the nucleus. However, extra cilia could lead to the reduction of cytoplasmic Jbn and thus inhibit the nucleus location of β‐catenin (Lancaster et al., 2011). The ciliated basal specific protein BBS protein family has also been found to be closely related to the Wnt signaling pathway in zebrafish (Gerdes et al., 2007). Other studies have also found that the deletion or mutation of cilium proteins such as Kif3a, Ift88, and Ofd1, could result in the enhancement of β‐catenin dependent transcriptional and become more sensitive to Wnt3a stimulation in mouse embryos (Corbit et al., 2008; Gerdes et al., 2007). Further, it has been identified that the protein Jade‐1 colocalized with NPHP1 at the TZ of primary cilia and interacts with NPHP4. While NPHP4 could stabilize the protein level of Jade‐1 and promotes its translocation to the nucleus. The stabilization and nuclear translocation of Jade‐1 by NPHP4 enhances the ability of Jade‐1 to negatively regulate canonical Wnt signaling. Loss of this repressor function in nephronophthisis might be an important factor in promoting Wnt activation (Borgal et al., 2012). These studies show that primary cilium has an inhibitory effect on the canonical Wnt pathway, and the absence of cilium could lead to an increased sensitivity of cells to the canonical Wnt stimulus.
Because Wnt signaling is an important signaling that could control cell differentiation and promote tumorigenesis, the primary cilium may also play a role in tumorigenesis. Indeed, it has been reported that in mesenchymal stem cell (MSC) increased primary cilia assembly was associated with decreased levels of nuclear active β‐catenin, axin‐2 induction, and proliferation, in response to wnt3a (McMurray et al., 2013). And also it has been found that loss of liver kinase B1 (LKB1) resulted in disassembly of the primary cilium and induced aberrant signaling through the Wnt pathway, which could be countered by restoring primary cilia through inhibiting histone deacetylase 6 (HDAC6; Jacob et al., 2011). Moreover, tumor suppressor PTEN could regulate cilia through Dishevelled (Shnitsar et al., 2015). To conclude, it seems that primary cilium disassembly induces aberrant Wnt signaling pathway contributing to tumor development.
3.3. Notchsignaling pathway
Notch signaling pathway is an evolutionarily conserved pathway, which regulates cell proliferation, cell fate, cell survival, and cell communication. Notch receptor is a single‐pass TM protein and has four homologous proteins (Notch1, Notch2, Notch3, and Notch4), which bind to their ligands including Delta‐like (Dll‐1, ‐3, and ‐4) and Jagged (Jagged 1 and Jagged 2). The structural domains of these receptors are the Notch extracellular domain, TM, and Notch intracellular domain (NICD). Notch receptor is processed through cleavage to release NICD into the nucleus in the signal‐receiving cell, when the receptor bonds to ligand. NICD interaction with the transcriptional co‐activator induces the expression of target genes (Kovall et al., 2017; Meurette & Mehlen, 2018). Some studies also demonstrated that the Notch signaling pathway was influenced by ciliary mutants. Notch receptors and Notch‐processing enzymes colocalized with cilia in wild‐type epidermal cells and ciliary mutants could lead to defects in Notch signaling and commitment of progenitors to differentiate in skin development. While Shh signaling defects in ciliary mutants occurred later than Notch signaling (Ezratty et al., 2011). Other studies found that primary cilium has functions in maintaining homeostasis of the corneal epithelium by balancing proliferation and vertical migration of basal corneal CECs through modulation of Notch signaling (Grisanti et al., 2016). And also in choroid plexus (CP) tumours the distinct ciliogenesis caused by aberrant Notch signaling may lead to Shh signaling activity and promote tumor cell proliferation (Li et al., 2016). Therefore, Notch signaling pathway is regulated by primary cilium, and aberrant Notch signaling affects ciliogenesis and the formation of the primary cilium.
3.4. mTORsignaling pathway
Mammalian target of rapamycin (mTOR) signaling pathway is the major regulator of amino acid metabolism and protein synthesis in cells. The mTOR is a serine‐threonine kinase, and forms mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). The important components of mTORC1 are the regulatory‐associated protein of mTOR (Raptor) and roline‐rich AKT substrate 40 kDa (PRAS40), wherein Raptor could regulate the assembly of mTORC1 to activate this complex, and PRAS40 negatively regulates activity through inhibiting the interaction of substrate and kinase. Other significant components are the rapamycin‐insensitive companion of mTOR (Rictor) and rapamycin‐insensitive companion of mTOR (Rictor) in mTORC2. They could stabilize each other to regulate the activity of mTORC2. Both intracellular and extracellular could regulate mTOR activity (Guertin & Sabatini, 2007; Guri & Hall, 2016). Recent studies have demonstrated that the primary cilium also interact with mTOR signaling. Tuberous sclerosis complex (TSC) 1 (TSC1) and TSC2 proteins function as a heterodimer to form TSC. TSC act as the upstream inhibitor of the mTOR1 that integrates environmental signals to regulate cell growth, proliferation, and survival. In mouse, TSC1 localizes to the basal body of the primary cilium, and TSC deletion could promote the cilia formation through protein synthesis (Hartman et al., 2009; Yuan et al., 2012). Also, recent studies found that mTORC1 inactivation could induce ciliogenesis through p27KIP1 upregulation (Takahashi et al., 2017). On the other hand, it is reported that in zebrafish the mTOR pathway also becomes aberrantly activated when ciliary mutants (DiBella et al., 2009). In mammalian, studies also demonstrated that primary cilia could control the mTOR pathway through LKB1 (Aznar & Billaud, 2010; Boehlke et al., 2010). Further, FLCN could recruit LKB1 to the primary cilium for activation of AMPK resided at the basal body, which causes mTORC1 downregulation (Zhong et al., 2016). Thus, these findings suggest that the signaling network regulated by cilia can feedback into the mTOR pathway and that TSC regulates the formation of the cilium itself.
3.5. RTKs signaling pathway
RTKs signaling pathways play key roles in the regulation of cellular proliferation, differentiation, migration, survival, and metabolism. RTKs constitute a family of receptors, and change the conformation by binding ligands to activate the downstream signaling, which includes mitogen‐activated protein kinase, phosphoinositide 3‐kinase/Akt, and Janus kinase/signal transducer and activator of transcription (Butti et al., 2018; Lemmon & Schlessinger, 2010). Many studies have found that receptors in RTK signaling such as platelet‐derived growth factor receptor α, insulin‐like growth factor receptor 1, epidermal growth factor receptor, Tie1/2, and their downstream molecules such as Mek1/2, AKT, PKD2, IRS‐1 were colocated with cilia (Anderson & Stearns, 2009; Awan et al., 2010; Jacoby et al., 2009; Ma et al., 2005; Schneider et al., 2005; Schneider et al., 2010; Wu et al., 2009; Zhu et al., 2009). Christensen et al. (2012) have already summarized the role of primary cilia in coordinating RTK signaling pathways in an excellent review. As a result, the activation of RTKs decreases primary cilium. Although the direct connection between RTK signaling regulated by cilia and cancer should continue to investigate, it has emphasized that the crosstalk between mTOR through LKB1 in the primary cilium assume an important role of the primary cilium in tumor development (Aznar & Billaud, 2010; Boehlke et al., 2010; Christensen et al., 2012).
3.6. Hippo signaling pathway
Hippo signaling pathway controls the size of the organ by regulating cell proliferation and apoptosis. In the Hippo signaling cascade, large tumor suppressor kinases control the phosphorylation of nuclear effector molecules, which are Yes‐associated protein (YAP) and transcriptional coactivator with PDZ‐binding motif (TAZ). YAP/TAZ can be phosphorylated by Last1/2 and degraded by proteasome at cytoplasmic when Hippo signaling active. Moreover, the nonphosphorylated state makes them bind transcriptional coactivators TEAD for target gene transcription (Misra & Irvine, 2018; Zheng & Pan, 2019). A recent study has found that Hippo signaling pathway may also have connections with the primary cilium. The primary cilium TZ protein NPHP4, which is mutated in ciliopathies, could modulate the Hippo signaling pathway by both inhibiting Lats1‐mediated phosphorylation of YAP/TAZ and inducing YAP/TAZ release from 14‐3‐3 binding (Habbig et al., 2011). Recently, Nagai and Mizuno (2017) also found that jasplakinolide (Jasp), a potent inducer of actin polymerization on ciliogenesis, treatment could induce ciliogenesis and the phosphorylation and cytoplasmic localization of YAP and suppressed cell proliferation in low density‐cultured cells. In addition, overexpression of an active form of YAP could suppresse Jasp‐induced ciliogenesis. They also found that the downregulation of Src activity may be involved in Jasp‐induced YAP inactivation and ciliogenesis. Consequently, the Hippo signaling may feedback to control the primary cilium formation.
4. PRIMARY CILIUM ASSEMBLY REGULATORS AND CANCER
The primary cilium is enriched in numerous important signaling molecules, and regulates oncogenesis as a mediator of signaling pathways. Therefore, it is interesting and important to understand the mechanisms of ciliary regulation, which is cilium assembly and cilium disassembly. The defective cilia have been observed in all common cancers, which suggests the proteins that regulate primary cilium assembly and disassembly could take part in the tumor development. Below, we will review the processes of ciliary assembly and molecular mechanisms of ciliary assembly and disassembly regulators in cancer (Table 1).
Table 1.
Molecular mechanisms of ciliary assembly and disassembly regulators in cancer
| Regulators | Expression in cancer | Mechanism of ciliary assembly and disassembly | References |
|---|---|---|---|
| Cep131 | Deregulated | Basal body component | Li et al. (2016) |
| Plk4 | Upregulated | Centrosome protein | Lu et al. (2015) |
| BUBR1 | Upregulated | Essential for the primary cilium formation | Kim et al. (2011) |
| EHD1 | Deregulated | Help proteins targeting to the mother centrosome |
Kowal et al. (2015); Mahjoub et al. (2012); Mans et al. (2019) |
| Cotraffic with Smo | McMurray et al. (2013) | ||
| Aurora A | Upregulated | Trichoplein‐Aur A pathway suppresses ciliary assembly | Menzl et al. (2014) |
| Ndel1 regulates Trichoplein‐Aur A pathway | Michaud et al. (2006) | ||
| Inactivation of HDAC2 decreases Aur A | Miyamoto et al. (2015) | ||
| HDAC6 | Upregulated | Deacetylating α‐tubulin and destabilizing microtubules |
Gradilone et al. (2013); Hubbert et al. (2002) |
| Hypomethylation and NRF2 regulate the expression of HDAC6 | Lam et al. (2013) | ||
| Plk1 | Upregulated | Dvl2‐Plk1‐mediated ciliary disassembly through stabilizing HEF1 and activating Aur A |
Moser et al. (2009); Nagai et al. (2017) |
| Phosphorylate Kif2a at T554 (microtubule‐depolymerizing activity) | Nobutani et al. (2014) | ||
| Brefeldin A suppresses interaction of CK1varepsilon‐Dvl2 | Phua et al. (2017) | ||
| Nek2 | Upregulated in breast cancer | Phosphorylate and enhance Kif24 (microtubule depolymerizing kinesin), and ablation of Nek2 and Kif24 restores ciliation in cancer cells |
Rohatgi et al. (2007); Sarkisian et al. (2014) |
| VHL | Mutaion/loss of function in renal cancer | Aur A ubiquitination |
Schmidt et al. (2012) |
| β‐catenin regulates Aur A | Schraml et al. (2009) |
The primary cilium assembly can be divided into several different stages. The beginning of the cilium formation is the transformation of the mother centrosome into the basal body. Living cell imaging has revealed that within 10–15 min after mitogens are removed, cytoplasmic vesicles from Golgi or recycling endosome begin to attach to the distal appendage of the mother centrioles, and these vesicles are called distal appendage vesicles (DAVs; Kobayashi et al., 2014; Lu et al., 2015; Schmidt et al., 2012). The gradual integration of these vesicles form a cap‐like membranous structure at the distal end of the mother centrioles, called primary ciliary vesicles (Sorokin, 1962). The microtubule structure below the cilium vesicles begins to extend, while the cap‐like structure also continues to fuse with its surrounding vesicles to expand and form ciliary sheath. Then the mother centrosome moves forward to the apical surface of the cell and finally the ciliated sheath fusion with the cell membrane to form the ciliary membrane (Figure 2).
Figure 2.
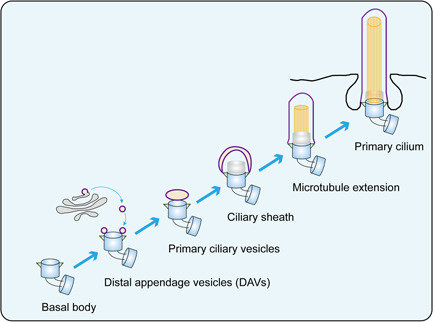
Processes of primary cilium assembly. The mother centrosome transforms into the basal body. The cytoplasmic vesicles attaches to mother centrioles to form distal appendage vesicles (DAVs). These vesicles gradually integrate into primary ciliary vesicles at the distal end of mother centrioles. They continue to expand and form ciliary sheath. The microtubule begin to extend from basal body. The mother centrosome moves forward to the apical surface of cell to form primary cilium
4.1. Centrosome proteins
During assembly of the primary cilium, numbers of basal body proteins or centriolar proteins were changed (Gupta et al., 2015). Many of these proteins have been shown to have some relationships with cancer. It has been found that depletion of the Cep131, a basal body component essential for cilium formation, could result in a reduction in centriole amplification, chromosomal instability, and an increase in postmitotic DNA damage (Staples et al., 2012). Another centrosome protein Plk4 which is required for centriolar satellite function and chromosomal stability has also been found to play a role in ciliogenesis (Hori et al., 2016). Also, studies found that cells with extra centrioles often formed more than one cilium, had reduced ciliary concentration of Smo in response to Shh stimulation, and reduced Shh pathway transcriptional activation (Mahjoub & Stearns, 2012). In addition, the mitotic spindle checkpoint regulator BUBR1 was also essential for the primary cilium formation (42). These results suggest that the primary cilium may have a function in regulating genomic instability through these centrosome proteins that play important role in both cilia assembly and mitosis.
4.2. Vesicular transport proteins
Since no protein synthesis was found at the basal body of the primary cilium, vesicular transport was the need to transfer the proteins that required for cilia formation to the basal body or the cilium tip. It has been demonstrated that Rab8a positive and Rab11 positive vesicles together with EHD1 could help proteins targeting the mother centrosome (Lu et al., 2015; Westlake et al., 2011; Yoshimura et al., 2007). And the intraflagellar transport complexes were responded to the transport of proteins in the primary cilium (Lechtreck, 2015). Some of these proteins have effects on cancer‐associated signaling pathways. It has been certified that the endocytic recycling regulator EHD1 protein colocalizes with the Shh receptor Smo in the primary cilia upon ligand stimulation. In addition, EHD1 was shown to cotraffic with Smo into the developing primary cilia and act as a direct binding partner of Smo (Bhattacharyya et al., 2016). Thus, the results illustrate the absence of vesicular transport proteins at the ciliary location could reflect the imbalance of cilia‐associated signaling pathways in cancer.
5. PRIMARY CILIUM DISASSEMBLY REGULATORS AND CANCER
Until now, the mechanisms underlying disassembly of the primary in normal or pathological conditions are largely unknown. Moreover, how cell cycle progression is linked to cilium disassembly is unclear. Current studies in cultured mammalian cells suggest that the disassemble of the primary cilium occurring in the G1 phase shortly after mitogen stimulation of quiescent cells. Some initial studies found that NEDD9 (also known as HEF1) and calcium–calmodulin activate Aurora A kinase, which in turn phosphorylates and active the histone deacetylase HDAC6, promoting the de‐acetylation and destabilization of tubulins within the cilium axoneme. Two kinesin‐13 families of depolymerizing kinesins Kif2a and Kif24 also implicated in the destabilization and depolymerization of axonemal microtubules. Some of those cilium disassembly regulators also play an important role in cancer.
5.1. Aurora A kinase
Aurora A (Aur A), as a regulator of the cell cycle, is widely deregulated in human cancer through overexpression or gene amplification. Recent studies found that its cell cycle control function might partly achieve through the primary cilium. It reported that knockdown Aur A or Trichoplein, which could activate Aur A and suppress primary cilia assembly, could induce G0/G1 arrest, but this phenotype was reversed by cilia formation suppress. The trichoplein‐Aur A pathway is required for G1 progression through a key role in the continuous suppression of primary cilia assembly (Inoko et al., 2012). Further, Ndel1, a well‐known modulator of dynein activity, have been found localize at the subdistal appendage of the mother centriole and acts as an upstream regulator of the trichoplein‐Aur A pathway to inhibit primary cilia assembly (Inaba et al., 2016). Another study found that inactivation of HDAC2 could result in decreased Aur A expression and then promote disassembly of primary cilia (Kobayashi et al., 2017). Therefore, the expression and activity of Aur A could regulate ciliary assembly and disassembly.
5.2. HDAC6
HDAC6 is a member of Class II of the histone deacetylase/acuc/apha family, which changes the chromosome structure to affect transcription by histone deacetylation. It is also located in the cytoplasm and regulates the deacetylation of nonhistone proteins (Peixoto et al., 2020). Because α‐tubulin is a target for deacetylation in the ciliary axoneme, HDAC6 has been shown to regulate primary cilia disassembly and shortening through microtubule destabilization (Gradilone et al., 2013; Hubbert et al., 2002). Cigarette smoke exposure could regulate autophagy‐mediated primary cilia shortening through upregulating HDAC6 by hypomethylation and NRF2 (Lam et al., 2013). In cholangiocarcinoma, nucleotides localized in the primary cilium inhibited tumor migration, invasion, and growth by LKB1/PTEN/AKT signaling pathway, and HDAC6‐dependent ciliophagy should regulate ciliary disassembly and tumor growth (Mansini et al., 2019; Peixoto et al., 2020). Hence HDAC6 could promote tumor migration, invasion, and proliferation by facilitating primary cilium disassembly.
5.3. Plk1
Another cell cycle protein polo‐like kinase 1 (Plk1), which highly expresses during mitosis and elevated levels are found in many different types of cancer, could interact with Dishevelled 2 (Dvl2) and then mediate primary cilia disassembly by stabilizing the HEF1 scaffold and activating Aurora A (Lee et al., 2012; Seeger‐Nukpezah et al., 2012). Plk1 could also phosphorylated Kif2a at T554 and active the microtubule‐depolymerizing activity of Kif2a (Miyamoto et al., 2015). Further, the recent study found that the application of a small molecular KY‐0120, identified as Brefeldin A, could disturbed Dvl2‐Plk1‐mediated ciliary disassembly through suppression of the interaction of CK1varepsilon‐Dvl2 and the expression of Plk1 messenger RNA (Lee et al., 2017). Thus, these results suggest that Plk1 could regulate ciliary disassembly via interacting Dvl2 and phosphorylating Kif2a.
5.4. Nek2
Kif24, another microtubule depolymerizing kinesin that suppress ciliary assembly during the cell cycle, could be phosphorylated and enhanced by Nek2, a serine/threonine‐protein kinase that is involved in mitotic regulation. Nek2 is localized to the centrosome and undetectable during the G1 phase, but expresses progressively throughout the S phase, reaching maximal levels in the late G2 phase. The expression of Nek2 during the S and G2 phase ensures Kif24 activity and prevents the outgrowth of cilia in cells that lack cilia. Further, Nek2 and Kif24 are overexpressed in breast cancer cells, and ablation of these proteins restores ciliation in these cells, thereby reducing proliferation (Kim et al., 2015). In addition, Nek2 is one of the hallmarks of oncoproteins, and the inhibitors of Nek2 are currently in clinical trials (Frett et al., 2014). Thus, overexpression of Nek2 in cancer could suppress ciliary assembly through phosphorylating and enhancing the activity and levels of Kif24.
5.5. VHL
Patients with mutations in the von Hippel–Lindau tumour suppressor gene (VHL) predispose to renal cysts and clear cell renal cell carcinoma (ccRCC). It has been reported that Aurora kinase A is a novel, hypoxia‐independent target for VHL ubiquitination (Hasanov et al., 2017). Moreover, the low ciliation status in ccRCC was associated with its high VHL mutation status. In addition, the primary cilia degeneration in sporadic ccRCC also depends on VHL inactivation (Schraml et al., 2009). Guinot et al. (2016) also found that primary cilium loss, in addition to VHL and P53 losses, promotes the transition towards malignancy in kidneys. Additionally, it has been explained that in renal cell carcinoma β‐catenin could regulate Aur A and primary cilia formation in the setting of VHL deficiency (Dere et al., 2015). In renal cancer, loss of VHL function could regulate and stabilize Aur A to suppress ciliary assembly.
6. PRIMARY CILIUM AND TUMOR MICROENVIRONMENT
The primary cilium is an important sensor of environmental signals. Despite its regulation of calcium signals and mechanical signals, many cell environment signals that associated with cancer such as inflammatory cytokine and metabolic signals also have some relationship with primary cilia. Furthermore, the primary cilium could also act as a media for cell‐cell signals transition. This section will provide a brief review on the tumor microenvironment, which has been associated with primary cilium.
6.1. Inflammation
It reported that in human fibroblasts, IL‐1 could induce cilia elongation and this elongation occurs via the protein kinase A‐dependent mechanism. These results indicated that ciliary assembly regulates the response to inflammatory cytokines (Wann & Knight, 2012). Studies also found that inflammatory cytokine tumor necrosis factor‐alpha could trigger a dose‐dependent loss of the primary cilia in MSC. These findings maintain a concept that the primary cilium may serve as a biomarker reflecting the tumor‐supporting potential of MSC and their capacity to adapt to hypoxic and proinflammatory environments. Therefore, inflammation seems to regulate primary cilium formation through cytokines, and the hypoxic and proinflammatory environments may counteract primary cilium, which leads to tumor development.
6.2. Metabolism
In hypothalamic neurons, primary cilia have been found to play a critical role in sensing metabolic signaling (Kang et al., 2015). Willemarck et al. (2010) also found that in prostate cancer cells the tumor‐associated lipogenesis could disturb cilia formation and contribute to impaired environmental sensing, aberrant cell signaling, and distortion of polarized tissue architecture. Other study found that cilia also have a role in obesity (Mariman et al., 2016). Notably, recent studies found that glucose deprivation (Takahashi et al., 2017) and autophagy (Shin et al., 2015; Tang et al., 2013) could also regulate the primary cilium formation in different ways, and therefore, the metabolic signals in the environment could influence the cilia formation and then feedback to the cell metabolism conditions.
6.3. Cilia excision
Recent studies found that the primary cilia tips can be excises by serum induction. The cilia excision is called cilia decapitation, and necessary to cilia disassembly. The ciliary disassembly directly determines the advancement of the cell cycle during the G1/S phase and at the transition to mitosis. The absence of cilia would thus lead to uncontrolled entry and passage of the cell cycle tumor cells (Rocha et al., 2014). Undergrowth stimulation, ciliary Inpp5e loss, and PI(4,5)P2 redistribution in primary cilia tips could induce actin polymerization leading to cilia excision, and this cilia excision induces mitogenic signaling and constitutes a molecular link between the cilia life cycle and cell‐division cycle (Phua et al., 2017). Another Rab GTPases, key regulators of membrane trafficking and endosomal biogenesis, were reported as an essential role in cilia excision. Rab7 could directly interact with F‐actin and not change the ciliary accumulation of Inpp5e and PI(4,5)P2, which regulates cilia excision through control of intraciliary actin polymerization (Wang et al., 2019). Furthermore, the vesicles formed by the cilia excision could content cell signals such as GPCRs and secreted outside the cell forming extracellular vesicles, which could transport the signaling from one cell to another. Thus, cilia excision could not only mediate the cell cycle but also transport extracellular signaling.
7. PRIMARY CILIUM ABNORMAL IN DIFFERENT CANCERS
Until now, abnormal primary cilium station has been found in numbers of tumors. Most studies found that primary cilium assembly is suppressed in cancer cells. The absence of primary cilia is found in cholangiocarcinoma, and inhibition of HDAC6 activity in cholangiocarcinoma cells can inhibit the degradation of the cilium to maintain the stability of cilium, thereby inhibiting the growth of cholangiocarcinoma [109]. Studies in gliomas also found that cell cycle‐related kinase in glioma cells may inhibit the ciliary assembly through its downstream substrate intestinal cell kinase, whereas the cilia are restored the tumor growth is also suppressed (Yang et al., 2013). Another study found that the absence of primary cilia is present in pancreatic cancer cell lines, pancreatic intraepithelial neoplasia (PanIN), and pancreatic ductal adenocarcinoma tissues (Bailey et al., 2009; Seeley et al., 2009). In fact, primary cilia loss has been found in a variety of other tumors including clear cell renal carcinoma (Schraml et al., 2009), epithelial ovarian cancer (Egeberg et al., 2012), prostatic cancer (Hassounah et al., 2013), melanoma carcinoma (Kim et al., 2011), glioblastoma (Moser et al., 2009), and breast cancer (Menzl et al., 2014; Nobutani et al., 2014; Yuan et al., 2010). Notably, Hassounah et al. (2017) found that in the polyoma middle T spontaneous breast cancer mouse model, inhibit cilium formation led to earlier tumorigenesis, faster tumor growth, and more lung metastases. However, the inhibition of the formation of the primary cilium in normal mice without other oncogene mutations did not induce tumors.
The role of the primary cilium in tumorigenesis could be dual and opposing. It has been noted that in medulloblastoma (Han et al., 2009) and BCCs (Wong et al., 2009) loss of primary cilia can be either positive or negative in tumor growth. Studies also found that in subpopulations of cells within human glioblastoma tumors are ciliated (Sarkisian et al., 2014) and human medulloblastoma cells critically rely on the establishment of primary cilia to drive Shh‐mediated cell division (Gate et al., 2015). The presence of primary cilia also been found in the spindle and epithelioid gastric gastrointestinal stromal tumors cells (Castiella et al., 2013; Dvorak et al., 2014), HeLa (human epithelial adenocarcinoma), and other cancer cell lines such as MG63 (human osteosarcoma; Kowal & Falk, 2015), though the significance of this persistent primary cilium in cancer cells still unclear.
Studies also found compounds and drugs that could affect primary cilia assembly. It has been illustrated that a selection of compounds such as Clofibrate, Gefitinib, Sirolimus, Imexon, and Dexamethasone, all have an ability to restore ciliogenesis in different cancer cell lines (pancreas, lung, kidney, breast; Khan et al., 2016). To conclude, primary cilia play the emerging roles in tumorigenesis, but their functions contribute to tumor promotion and suppression because of different cell types and organs in the context. In addition, it seems that some compounds could treat the cancer though affecting primary cilia.
8. CONCLUSIONS
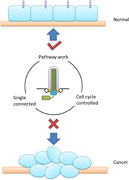
The primary cilium, as the sensor and regulator of the outside cell signaling, has been pay more and more attention to its role in tumorigenesis and cancer development. Numbers of the signaling pathways have been demonstrated under the regulation of the primary cilium, absence of cilia would thus lead to uncontrolled entry and passage of the cell cycle and the proteins that regulate cilia assembly and disassembly also have close relationship with cancer. These facts indicate that the primary cilium should have some effects in cancer developing process (Figure 3). However, the role of primary cilia in cancer is still unclear. Though lots of cancer exhibit primary cilia defects, the significances of these cilia changes and the mechanisms of how these changes were happened are all still unknown. Hence, further study in cilia characteristic and its assembly mechanism may help make the role of primary cilia in cancer more clear.
Figure 3.
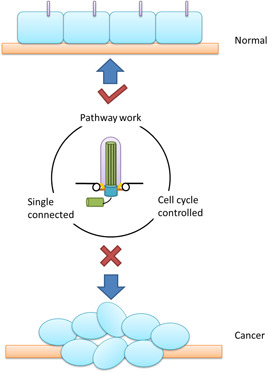
Primary cilium and cancer. In normal cells, the primary cilium acts as the sensor and regulator of the outside cell signaling. It ensures the completeness of several signal pathways. It determines the advancement of the cell cycle. In cancer cells, the primary cilium was absent. Absence of the primary block the signal from outside and neighbors, and disturb the signal pathways inside cells, thus lead to uncontrolled cell cycle process and tumorigenesis
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Peijun Liu conceptualized the idea for this manuscript; Bo Wang and Zheyong Liang wrote the manuscript; Bo Wang prepared the figures and table. All authors helped shape the manuscript.
ACKNOWLEDGMENTS
This work was supported by grants from National Natural Science Foundation of China (No. 81872272 and 81672876) and Key Research and Development Program of Shaanxi Province of China (No. 2019SF‐147 and 2018KW‐064).
Wang B, Liang Z, Liu P. Functional aspects of primary cilium in signaling, assembly and microenvironment in cancer. J Cell Physiol. 2021;236:3207–3219. 10.1002/jcp.30117
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Anderson, C. T. , & Stearns, T. (2009). Centriole age underlies asynchronous primary cilium growth in mammalian cells. Current Biology, 19(17), 1498–1502. 10.1016/j.cub.2009.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awan, A. , Oliveri, R. S. , Jensen, P. L. , Christensen, S. T. , & Andersen, C. Y. (2010). Immunoflourescence and mRNA analysis of human embryonic stem cells (hESCs) grown under feeder‐free conditions. Methods in Molecular Biology, 584, 195–210. 10.1007/978-1-60761-369-5_11 [DOI] [PubMed] [Google Scholar]
- Aznar, N. , & Billaud, M. (2010). Primary cilia bend LKB1 and mTOR to their will. Developmental Cell, 19(6), 792–794. 10.1016/j.devcel.2010.11.016 [DOI] [PubMed] [Google Scholar]
- Bailey, J. M. , Mohr, A. M. , & Hollingsworth, M. A. (2009). Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene, 28(40), 3513–3525. 10.1038/onc.2009.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya, S. , Rainey, M. A. , Arya, P. , Mohapatra, B. C. , Mushtaq, I. , Dutta, S. , George, M. , Storck, M. D. , McComb, R. D. , Muirhead, D. , Todd, G. L. , Gould, K. , Datta, K. , Waes, J. G. , Band, V. , & Band, H. (2016). Endocytic recycling protein EHD1 regulates primary cilia morphogenesis and SHH signaling during neural tube development. Scientific Reports, 6, 20727. 10.1038/srep20727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehlke, C. , Kotsis, F. , Patel, V. , Braeg, S. , Voelker, H. , Bredt, S. , Beyer, T. , Janusch, H. , Hamann, C. , Gödel, M. , Müller, K. , Herbst, M. , Hornung, M. , Doerken, M. , Köttgen, M. , Nitschke, R. , Igarashi, P. , Walz, G. , & Kuehn, E. W. (2010). Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nature Cell Biology, 12(11), 1115–1122. 10.1038/ncb2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgal, L. , Habbig, S. , Hatzold, J. , Liebau, M. C. , Dafinger, C. , Sacarea, I. , Hammerschmidt, M. , Benzing, T. , & Schermer, B. (2012). The ciliary protein nephrocystin‐4 translocates the canonical Wnt regulator Jade‐1 to the nucleus to negatively regulate beta‐catenin signaling. Journal of Biological Chemistry, 287(30), 25370–25380. 10.1074/jbc.M112.385658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butti, R. , Das, S. , Gunasekaran, V. P. , Yadav, A. S. , Kumar, D. , & Kundu, G. C. (2018). Receptor tyrosine kinases (RTKs) in breast cancer: Signaling, therapeutic implications and challenges. Molecular Cancer, 17(1), 34. 10.1186/s12943-018-0797-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiella, T. , Munoz, G. , Luesma, M. J. , Santander, S. , Soriano, M. , & Junquera, C. (2013). Primary cilia in gastric gastrointestinal stromal tumours (GISTs): An ultrastructural study. Journal of Cellular and Molecular Medicine, 17(7), 844–853. 10.1111/jcmm.12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez, M. , Ena, S. , Van Sande, J. , de Kerchove d'Exaerde, A. , Schurmans, S. , & Schiffmann, S. N. (2015). Modulation of ciliary phosphoinositide content regulates trafficking and sonic hedgehog signaling output. Developmental Cell, 34(3), 338–350. 10.1016/j.devcel.2015.06.016 [DOI] [PubMed] [Google Scholar]
- Christensen, S. T. , Clement, C. A. , Satir, P. , & Pedersen, L. B. (2012). Primary cilia and coordination of receptor tyrosine kinase (RTK) signalling. Journal of Pathology, 226(2), 172–184. 10.1002/path.3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, S. T. , Pedersen, L. B. , Schneider, L. , & Satir, P. (2007). Sensory cilia and integration of signal transduction in human health and disease. Traffic, 8(2), 97–109. 10.1111/j.1600-0854.2006.00516.x [DOI] [PubMed] [Google Scholar]
- Corbit, K. C. , Aanstad, P. , Singla, V. , Norman, A. R. , Stainier, D. Y. , & Reiter, J. F. (2005). Vertebrate smoothened functions at the primary cilium. Nature, 437(7061), 1018–1021. 10.1038/nature04117 [DOI] [PubMed] [Google Scholar]
- Corbit, K. C. , Shyer, A. E. , Dowdle, W. E. , Gaulden, J. , Singla, V. , & Reiter, J. F. (2008). Kif3a constrains beta‐catenin‐dependent Wnt signalling through dual ciliary and non‐ciliary mechanisms. Nature Cell Biology, 10(1), 70–76. 10.1038/ncb1670 [DOI] [PubMed] [Google Scholar]
- Dere, R. , Perkins, A. L. , Bawa‐Khalfe, T. , Jonasch, D. , & Walker, C. L. (2015). beta‐catenin links von Hippel‐Lindau to aurora kinase A and loss of primary cilia in renal cell carcinoma. Journal of the American Society of Nephrology, 26(3), 553–564. 10.1681/ASN.2013090984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBella, L. M. , Park, A. , & Sun, Z. (2009). Zebrafish Tsc1 reveals functional interactions between the cilium and the TOR pathway. Human Molecular Genetics, 18(4), 595–606. 10.1093/hmg/ddn384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak, J. , Sitorova, V. , Nikolov, D. H. , Filipova, A. , Ryska, A. , Melichar, B. , Richter, I. , Buka, D. , Mokry, J. , Filip, S. , & Petera, J. (2014). Primary cilia in gastrointestinal stromal tumors. Neoplasma, 61(3), 305–308. [DOI] [PubMed] [Google Scholar]
- Egeberg, D. L. , Lethan, M. , Manguso, R. , Schneider, L. , Awan, A. , Jørgensen, T. S. , Byskov, A. G. , Pedersen, L. B. , & Christensen, S. T. (2012). Primary cilia and aberrant cell signaling in epithelial ovarian cancer. Cilia, 1(1), 15. 10.1186/2046-2530-1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty, E. J. , Stokes, N. , Chai, S. , Shah, A. S. , Williams, S. E. , & Fuchs, E. (2011). A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell, 145(7), 1129–1141. 10.1016/j.cell.2011.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frett, B. , Brown, R. V. , Ma, M. , Hu, W. , Han, H. , & Li, H. Y. (2014). Therapeutic melting pot of never in mitosis gene a related kinase 2 (Nek2): A perspective on Nek2 as an oncology target and recent advancements in Nek2 small molecule inhibition. Journal of Medicinal Chemistry, 57(14), 5835–5844. 10.1021/jm401719n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Gonzalo, F. R. , Phua, S. C. , Roberson, E. C. , Garcia, G., 3rd , Abedin, M. , Schurmans, S. , Inoue, T. , & Reiter, J. F. (2015). Phosphoinositides regulate ciliary protein trafficking to modulate hedgehog signaling. Developmental Cell, 34(4), 400–409. 10.1016/j.devcel.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gate, D. , Danielpour, M. , Bannykh, S. , & Town, T. (2015). Characterization of cancer stem cells and primary cilia in medulloblastoma. CNS & Neurological Disorders Drug Targets, 14(5), 600–611. [DOI] [PubMed] [Google Scholar]
- Gerdes, J. M. , Liu, Y. , Zaghloul, N. A. , Leitch, C. C. , Lawson, S. S. , Kato, M. , Beachy, P. A. , Beales, P. L. , DeMartino, G. N. , Fisher, S. , Badano, J. L. , & Katsanis, N. (2007). Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nature Genetics, 39(11), 1350–1360. 10.1038/ng.2007.12 [DOI] [PubMed] [Google Scholar]
- Gradilone, S. A. , Radtke, B. N. , Bogert, P. S. , Huang, B. Q. , Gajdos, G. B. , & LaRusso, N. F. (2013). HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Research, 73(7), 2259–2270. 10.1158/0008-5472.Can-12-2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti, L. , Revenkova, E. , Gordon, R. E. , & Iomini, C. (2016). Primary cilia maintain corneal epithelial homeostasis by regulation of the Notch signaling pathway. Development, 143(12), 2160–2171. 10.1242/dev.132704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guen, V. J. , Chavarria, T. E. , Kroger, C. , Ye, X. , Weinberg, R. A. , & Lees, J. A. (2017). EMT programs promote basal mammary stem cell and tumor‐initiating cell stemness by inducing primary ciliogenesis and Hedgehog signaling. Proceedings of the National Academy of Sciences of the United States of America, 114, E10532–E10539. 10.1073/pnas.1711534114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin, D. A. , & Sabatini, D. M. (2007). Defining the role of mTOR in cancer. Cancer Cell, 12(1), 9–22. 10.1016/j.ccr.2007.05.008 [DOI] [PubMed] [Google Scholar]
- Guinot, A. , Lehmann, H. , Wild, P. J. , & Frew, I. J. (2016). Combined deletion of Vhl, Trp53 and Kif3a causes cystic and neoplastic renal lesions. Journal of Pathology, 239(3), 365–373. 10.1002/path.4736 [DOI] [PubMed] [Google Scholar]
- Gupta, G. D. , Coyaud, É. , Gonçalves, J. , Mojarad, B. A. , Liu, Y. , Wu, Q. , Gheiratmand, L. , Comartin, D. , Tkach, J. M. , Cheung, S. W. T. , Bashkurov, M. , Hasegan, M. , Knight, J. D. , Lin, Z. Y. , Schueler, M. , Hildebrandt, F. , Moffat, J. , Gingras, A. C. , Raught, B. , & Pelletier, L. (2015). A dynamic protein interaction landscape of the human centrosome‐cilium interface. Cell, 163(6), 1484–1499. 10.1016/j.cell.2015.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guri, Y. , & Hall, M. N. (2016). mTOR signaling confers resistance to targeted cancer drugs. Trends in Cancer, 2(11), 688–697. 10.1016/j.trecan.2016.10.006 [DOI] [PubMed] [Google Scholar]
- Habbig, S. , Bartram, M. P. , Müller, R. U. , Schwarz, R. , Andriopoulos, N. , Chen, S. , Sägmüller, J. G. , Hoehne, M. , Burst, V. , Liebau, M. C. , Reinhardt, H. C. , Benzing, T. , & Schermer, B. (2011). NPHP4, a cilia‐associated protein, negatively regulates the Hippo pathway. Journal of Cell Biology, 193(4), 633–642. 10.1083/jcb.201009069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y. G. , Kim, H. J. , Dlugosz, A. A. , Ellison, D. W. , Gilbertson, R. J. , & Alvarez‐Buylla, A. (2009). Dual and opposing roles of primary cilia in medulloblastoma development. Nature Medicine (New York, NY, United States), 15(9), 1062–1065. 10.1038/nm.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman, T. R. , Liu, D. , Zilfou, J. T. , Robb, V. , Morrison, T. , Watnick, T. , & Henske, E. P. (2009). The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin‐insensitive and polycystin 1‐independent pathway. Human Molecular Genetics, 18(1), 151–163. 10.1093/hmg/ddn325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanov, E. , Chen, G. , Chowdhury, P. , Weldon, J. , Ding, Z. , Jonasch, E. , Sen, S. , Walker, C. L. , & Dere, R. (2017). Ubiquitination and regulation of AURKA identifies a hypoxia‐independent E3 ligase activity of VHL. Oncogene, 36(24), 3450–3463. 10.1038/onc.2016.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassounah, N. B. , Nagle, R. , Saboda, K. , Roe, D. J. , Dalkin, B. L. , & McDermott, K. M. (2013). Primary cilia are lost in preinvasive and invasive prostate cancer. PLoS One, 8(7), e68521. 10.1371/journal.pone.0068521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassounah, N. B. , Nunez, M. , Fordyce, C. , Roe, D. , Nagle, R. , Bunch, T. , & McDermott, K. M. (2017). Inhibition of ciliogenesis promotes hedgehog signaling, tumorigenesis, and metastasis in breast cancer. Molecular Cancer Research, 15, 1421–1430. 10.1158/1541-7786.MCR-17-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori, A. , Barnouin, K. , Snijders, A. P. , & Toda, T. (2016). A noncanonical function of Plk4 in centriolar satellite integrity and ciliogenesis through PCM1 phosphorylation. EMBO Reports, 17(3), 326–337. 10.15252/embr.201541432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu, D. , Liu, A. , Rakeman, A. S. , Murcia, N. S. , Niswander, L. , & Anderson, K. V. (2003). Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature, 426(6962), 83–87. 10.1038/nature02061 [DOI] [PubMed] [Google Scholar]
- Hubbert, C. , Guardiola, A. , Shao, R. , Kawaguchi, Y. , Ito, A. , Nixon, A. , Yoshida, M. , Wang, X. F. , & Yao, T. P. (2002). HDAC6 is a microtubule‐associated deacetylase. Nature, 417(6887), 455–458. 10.1038/417455a [DOI] [PubMed] [Google Scholar]
- Inaba, H. , Goto, H. , Kasahara, K. , Kumamoto, K. , Yonemura, S. , Inoko, A. , Yamano, S. , Wanibuchi, H. , He, D. , Goshima, N. , Kiyono, T. , Hirotsune, S. , & Inagaki, M. (2016). Ndel1 suppresses ciliogenesis in proliferating cells by regulating the trichoplein‐Aurora A pathway. Journal of Cell Biology, 212(4), 409–423. 10.1083/jcb.201507046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoko, A. , Matsuyama, M. , Goto, H. , Ohmuro‐Matsuyama, Y. , Hayashi, Y. , Enomoto, M. , Ibi, M. , Urano, T. , Yonemura, S. , Kiyono, T. , Izawa, I. , & Inagaki, M. (2012). Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. Journal of Cell Biology, 197(3), 391–405. 10.1083/jcb.201106101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, K. , Brott, B. K. , Bae, G. U. , Ratcliffe, M. J. , & Sokol, S. Y. (2005). Nuclear localization is required for Dishevelled function in Wnt/beta‐catenin signaling. Journal of Biology, 4(1), 3. 10.1186/jbiol20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob, L. S. , Wu, X. , Dodge, M. E. , Fan, C. W. , Kulak, O. , Chen, B. , Tang, W. , Wang, B. , Amatruda, J. F. , & Lum, L. (2011). Genome‐wide RNAi screen reveals disease‐associated genes that are common to Hedgehog and Wnt signaling. Science signaling, 4(157), ra4. 10.1126/scisignal.2001225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby, M. , Cox, J. J. , Gayral, S. , Hampshire, D. J. , Ayub, M. , Blockmans, M. , Pernot, E. , Kisseleva, M. V. , Compère, P. , Schiffmann, S. N. , Gergely, F. , Riley, J. H. , Pérez‐Morga, D. , Woods, C. G. , & Schurmans, S. (2009). INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nature Genetics, 41(9), 1027–1031. 10.1038/ng.427 [DOI] [PubMed] [Google Scholar]
- Kang, G. M. , Han, Y. M. , Ko, H. W. , Kim, J. , Oh, B. C. , Kwon, I. , & Kim, M. S. (2015). Leptin elongates hypothalamic neuronal cilia via transcriptional regulation and actin destabilization. Journal of Biological Chemistry, 290(29), 18146–18155. 10.1074/jbc.M115.639468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, N. A. , Willemarck, N. , Talebi, A. , Marchand, A. , Binda, M. M. , Dehairs, J. , Rueda‐Rincon, N. , Daniels, V. W. , Bagadi, M. , Raj, D. B. T. G. , Vanderhoydonc, F. , Munck, S. , Chaltin, P. , & Swinnen, J. V. (2016). Identification of drugs that restore primary cilium expression in cancer cells. Oncotarget, 7(9), 9975–9992. 10.18632/oncotarget.7198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Dabiri, S. , & Seeley, E. S. (2011). Primary cilium depletion typifies cutaneous melanoma in situ and malignant melanoma. PLoS One, 6(11), e27410. 10.1371/journal.pone.0027410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Lee, K. , Choi, J. H. , Ringstad, N. , & Dynlacht, B. D. (2015). Nek2 activation of Kif24 ensures cilium disassembly during the cell cycle. Nature Communications, 6, 8087. 10.1038/ncomms9087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, T. , Kim, S. , Lin, Y. C. , Inoue, T. , & Dynlacht, B. D. (2014). The CP110‐interacting proteins Talpid3 and Cep290 play overlapping and distinct roles in cilia assembly. Journal of Cell Biology, 204(2), 215–229. 10.1083/jcb.201304153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, T. , Nakazono, K. , Tokuda, M. , Mashima, Y. , Dynlacht, B. D. , & Itoh, H. (2017). HDAC2 promotes loss of primary cilia in pancreatic ductal adenocarcinoma. EMBO Reports, 18(2), 334–343. 10.15252/embr.201541922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovall, R. A. , Gebelein, B. , Sprinzak, D. , & Kopan, R. (2017). The canonical Notch signaling pathway: Structural and biochemical insights into shape, sugar, and force. Developmental Cell, 41(3), 228–241. 10.1016/j.devcel.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal, T. J. , & Falk, M. M. (2015). Primary cilia found on HeLa and other cancer cells. Cell Biology International, 39(11), 1341–1347. 10.1002/cbin.10500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, H. C. , Cloonan, S. M. , Bhashyam, A. R. , Haspel, J. A. , Singh, A. , Sathirapongsasuti, J. F. , Cervo, M. , Yao, H. , Chung, A. L. , Mizumura, K. , An, C. H. , Shan, B. , Franks, J. M. , Haley, K. J. , Owen, C. A. , Tesfaigzi, Y. , Washko, G. R. , Quackenbush, J. , Silverman, E. K. , … Choi, A. M. K. (2013). Histone deacetylase 6‐mediated selective autophagy regulates COPD‐associated cilia dysfunction. Journal of Clinical Investigation, 123(12), 5212–5230. 10.1172/jci69636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, M. A. , Schroth, J. , & Gleeson, J. G. (2011). Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nature Cell Biology, 13(6), 700–707. 10.1038/ncb2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck, K. F. (2015). IFT‐cargo interactions and protein transport in cilia. Trends in Biochemical Sciences, 40(12), 765–778. 10.1016/j.tibs.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. H. , Johmura, Y. , Yu, L. R. , Park, J. E. , Gao, Y. , Bang, J. K. , Zhou, M. , Veenstra, T. D. , Yeon Kim, B. , & Lee, K. S. (2012). Identification of a novel Wnt5a‐CK1varepsilon‐Dvl2‐Plk1‐mediated primary cilia disassembly pathway. EMBO Journal, 31(14), 3104–3117. 10.1038/emboj.2012.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, U. , Kim, S. O. , Hwang, J. A. , Jang, J. H. , Son, S. , Ryoo, I. J. , Ahn, J. S. , Kim, B. Y. , & Lee, K. H. (2017). The fungal metabolite brefeldin A inhibits Dvl2‐Plk1‐dependent primary cilium disassembly. Molecules and Cells, 40(6), 401–409. 10.14348/molcells.2017.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon, M. A. , & Schlessinger, J. (2010). Cell signaling by receptor tyrosine kinases. Cell, 141(7), 1117–1134. 10.1016/j.cell.2010.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Grausam, K. B. , Wang, J. , Lun, M. P. , Ohli, J. , Lidov, H. G. W. , Calicchio, M. L. , Zeng, E. , Salisbury, J. L. , Wechsler‐Reya, R. J. , Lehtinen, M. K. , Schüller, U. , & Zhao, H. (2016). Sonic Hedgehog promotes proliferation of Notch‐dependent monociliated choroid plexus tumour cells. Nature Cell Biology, 18(4), 418–430. 10.1038/ncb3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Q. , Insinna, C. , Ott, C. , Stauffer, J. , Pintado, P. A. , Rahajeng, J. , Baxa, U. , Walia, V. , Cuenca, A. , Hwang, Y. S. , Daar, I. O. , Lopes, S. , Lippincott‐Schwartz, J. , Jackson, P. K. , Caplan, S. , & Westlake, C. J. (2015). Early steps in primary cilium assembly require EHD1/EHD3‐dependent ciliary vesicle formation. Nature Cell Biology, 17(4), 531. 10.1038/ncb3155 [DOI] [PubMed] [Google Scholar]
- Ma, R. , Li, W. P. , Rundle, D. , Kong, J. , Akbarali, H. I. , & Tsiokas, L. (2005). PKD2 functions as an epidermal growth factor‐activated plasma membrane channel. Molecular and Cellular Biology, 25(18), 8285–8298. 10.1128/mcb.25.18.8285-8298.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjoub, M. R. , & Stearns, T. (2012). Supernumerary centrosomes nucleate extra cilia and compromise primary cilium signaling. Current Biology, 22(17), 1628–1634. 10.1016/j.cub.2012.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans, D. A. , Voest, E. E. , & Giles, R. H. (2008). All along the watchtower: Is the cilium a tumor suppressor organelle? Biochimica et Biophysica Acta/General Subjects, 1786(2), 114–125. 10.1016/j.bbcan.2008.02.002 [DOI] [PubMed] [Google Scholar]
- Mansini, A. P. , Peixoto, E. , Jin, S. , Richard, S. , & Gradilone, S. A. (2019). The chemosensory function of primary cilia regulates cholangiocyte migration, invasion, and tumor growth. Hepatology, 69(4), 1582–1598. 10.1002/hep.30308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariman, E. C. M. , Vink, R. G. , Roumans, N. J. T. , Bouwman, F. G. , Stumpel, C. T. R. M. , Aller, E. E. J. G. , van Baak, M. A. , & Wang, P. (2016). The cilium: A cellular antenna with an influence on obesity risk. British Journal of Nutrition, 116(4), 576–592. 10.1017/S0007114516002282 [DOI] [PubMed] [Google Scholar]
- McMurray, R. J. , Wann, A. K. , Thompson, C. L. , Connelly, J. T. , & Knight, M. M. (2013). Surface topography regulates wnt signaling through control of primary cilia structure in mesenchymal stem cells. Scientific Reports, 3, 3545. 10.1038/srep03545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzl, I. , Lebeau, L. , Pandey, R. , Hassounah, N. B. , Li, F. W. , Nagle, R. , Weihs, K. , & McDermott, K. M. (2014). Loss of primary cilia occurs early in breast cancer development. Cilia, 3, 7. 10.1186/2046-2530-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurette, O. , & Mehlen, P. (2018). Notch signaling in the tumor microenvironment. Cancer Cell, 34(4), 536–548. 10.1016/j.ccell.2018.07.009 [DOI] [PubMed] [Google Scholar]
- Michaud, E. J. , & Yoder, B. K. (2006). The primary cilium in cell signaling and cancer. Cancer Research, 66(13), 6463–6467. 10.1158/0008-5472.CAN-06-0462 [DOI] [PubMed] [Google Scholar]
- Misra, J. R. , & Irvine, K. D. (2018). The Hippo signaling network and its biological functions. Annual Review of Genetics, 52, 65–87. 10.1146/annurev-genet-120417-031621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, T. , Hosoba, K. , Ochiai, H. , Royba, E. , Izumi, H. , Sakuma, T. , Yamamoto, T. , Dynlacht, B. D. , & Matsuura, S. (2015). The microtubule‐depolymerizing activity of a mitotic kinesin protein KIF2A drives primary cilia disassembly coupled with cell proliferation. Cell Reports, 10, 664–673. 10.1016/j.celrep.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, J. J. , Fritzler, M. J. , & Rattner, J. B. (2009). Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer, 9, 448. 10.1186/1471-2407-9-448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, T. , & Mizuno, K. (2017). Jasplakinolide induces primary cilium formation through cell rounding and YAP inactivation. PLoS One, 12(8), e0183030. 10.1371/journal.pone.0183030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, J. M. , & Curran, T. (2011). The Hedgehog's tale: Developing strategies for targeting cancer. Nature Reviews Cancer, 11(7), 493–501. 10.1038/nrc3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobutani, K. , Shimono, Y. , Yoshida, M. , Mizutani, K. , Minami, A. , Kono, S. , Mukohara, T. , Yamasaki, T. , Itoh, T. , Takao, S. , Minami, H. , Azuma, T. , & Takai, Y. (2014). Absence of primary cilia in cell cycle‐arrested human breast cancer cells. Genes to Cells, 19(2), 141–152. 10.1111/gtc.12122 [DOI] [PubMed] [Google Scholar]
- Peixoto, E. , Jin, S. , Thelen, K. , Biswas, A. , Richard, S. , Morleo, M. , Mansini, A. , Holtorf, S. , Carbone, F. , Pastore, N. , Ballabio, A. , Franco, B. , & Gradilone, S. A. (2020). HDAC6‐dependent ciliophagy is involved in ciliary loss and cholangiocarcinoma growth in human cells and murine models. American Journal of Physiology, Gastrointestinal, and Liver Physiology, 318(6), G1022–G1033. 10.1152/ajpgi.00033.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phua, S. C. , Chiba, S. , Suzuki, M. , Su, E. , Roberson, E. C. , Pusapati, G. V. , Setou, M. , Rohatgi, R. , Reiter, J. F. , Ikegami, K. , & Inoue, T. (2017). Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell, 168(1‐2), 264–279. e215 10.1016/j.cell.2016.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha, C. , Papon, L. , Cacheux, W. , Marques Sousa, P. , Lascano, V. , Tort, O. , Giordano, T. , Vacher, S. , Lemmers, B. , Mariani, P. , Meseure, D. , Medema, J. P. , Bièche, I. , Hahne, M. , & Janke, C. (2014). Tubulin glycylases are required for primary cilia, control of cell proliferation and tumor development in colon. EMBO Journal, 33(19), 2247–2260. 10.15252/embj.201488466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi, R. , Milenkovic, L. , & Scott, M. P. (2007). Patched1 regulates hedgehog signaling at the primary cilium. Science, 317(5836), 372–376. 10.1126/science.1139740 [DOI] [PubMed] [Google Scholar]
- Sarkisian, M. R. , Siebzehnrubl, D. , Hoang‐Minh, L. , Deleyrolle, L. , Silver, D. J. , Siebzehnrubl, F. A. , Guadiana, S. M. , Srivinasan, G. , Semple‐Rowland, S. , Harrison, J. K. , Steindler, D. A. , & Reynolds, B. A. (2014). Detection of primary cilia in human glioblastoma. Journal of Neuro‐Oncology, 117(1), 15–24. 10.1007/s11060-013-1340-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, K. N. , Kuhns, S. , Neuner, A. , Hub, B. , Zentgraf, H. , & Pereira, G. (2012). Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. Journal of Cell Biology, 199(7), 1083–1101. 10.1083/jcb.201202126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, L. , Cammer, M. , Lehman, J. , Nielsen, S. , Guerra, C. , Veland, I. , Stock, C. , Hoffmann, E. , Yoder, B. , Schwab, A. , Satir, P. , & Christensen, S. (2010). Directional cell migration and chemotaxis in wound healing response to PDGF‐AA are coordinated by the primary cilium in fibroblasts. Cellular Physiology and Biochemistry, 25(2‐3), 279–292. 10.1159/000276562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, L. , Clement, C. A. , Teilmann, S. C. , Pazour, G. J. , Hoffmann, E. K. , Satir, P. , & Christensen, S. T. (2005). PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Current Biology, 15(20), 1861–1866. 10.1016/j.cub.2005.09.012 [DOI] [PubMed] [Google Scholar]
- Schraml, P. , Frew, I. J. , Thoma, C. R. , Boysen, G. , Struckmann, K. , Krek, W. , & Moch, H. (2009). Sporadic clear cell renal cell carcinoma but not the papillary type is characterized by severely reduced frequency of primary cilia. Modern Pathology, 22(1), 31–36. 10.1038/modpathol.2008.132 [DOI] [PubMed] [Google Scholar]
- Seeger‐Nukpezah, T. , Liebau, M. C. , Hopker, K. , Lamkemeyer, T. , Benzing, T. , Golemis, E. A. , & Schermer, B. (2012). The centrosomal kinase Plk1 localizes to the transition zone of primary cilia and induces phosphorylation of nephrocystin‐1. PLoS One, 7(6), e38838. 10.1371/journal.pone.0038838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, E. S. , Carriere, C. , Goetze, T. , Longnecker, D. S. , & Korc, M. (2009). Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Research, 69(2), 422–430. 10.1158/0008-5472.CAN-08-1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, J. H. , Kim, P. S. , Kim, E. S. , Park, S. J. , Jo, Y. K. , Hwang, J. J. , Park, T. J. , Chang, J. W. , Seo, J. H. , & Cho, D. H. (2015). BIX‐01294‐induced autophagy regulates elongation of primary cilia. Biochemical and Biophysical Research Communications, 460(2), 428–433. 10.1016/j.bbrc.2015.03.050 [DOI] [PubMed] [Google Scholar]
- Shnitsar, I. , Bashkurov, M. , Masson, G. R. , Ogunjimi, A. A. , Mosessian, S. , Cabeza, E. A. , Hirsch, C. L. , Trcka, D. , Gish, G. , Jiao, J. , Wu, H. , Winklbauer, R. , Williams, R. L. , Pelletier, L. , Wrana, J. L. , & Barrios‐Rodiles, M. (2015). PTEN regulates cilia through Dishevelled. Nature Communications, 6, 8388. 10.1038/ncomms9388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, M. , Gloy, J. , Ganner, A. , Bullerkotte, A. , Bashkurov, M. , Krönig, C. , Schermer, B. , Benzing, T. , Cabello, O. A. , Jenny, A. , Mlodzik, M. , Polok, B. , Driever, W. , Obara, T. , & Walz, G. (2005). Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nature Genetics, 37(5), 537–543. 10.1038/ng1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin, S. (1962). Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. Journal of Cell Biology, 15, 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples, C. J. , Myers, K. N. , Beveridge, R. D. D. , Patil, A. A. , Lee, A. J. X. , Swanton, C. , Howell, M. , Boulton, S. J. , & Collis, S. J. (2012). The centriolar satellite protein Cep131 is important for genome stability. Journal of Cell Science, 125(Pt 20), 4770–4779. 10.1242/jcs.104059 [DOI] [PubMed] [Google Scholar]
- Takahashi, K. , Nagai, T. , Chiba, S. , Nakayama, K. , & Mizuno, K. (2017). Glucose deprivation induces primary cilium formation through mTORC1 inactivation. Journal of Cell Science, 131, jcs208769. 10.1242/jcs.208769 [DOI] [PubMed] [Google Scholar]
- Tang, Z. , Lin, M. G. , Stowe, T. R. , Chen, S. , Zhu, M. , Stearns, T. , Franco, B. , & Zhong, Q. (2013). Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature, 502(7470), 254–257. 10.1038/nature12606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G. , Hu, H. B. , Chang, Y. , Huang, Y. , Song, Z. Q. , Zhou, S. B. , Chen, L. , Zhang, Y. C. , Wu, M. , Tu, H. Q. , Yuan, J. F. , Wang, N. , Pan, X. , Li, A. L. , Zhou, T. , Zhang, X. M. , He, K. , & Li, H. Y. (2019). Rab7 regulates primary cilia disassembly through cilia excision. Journal of Cell Biology, 218(12), 4030–4041. 10.1083/jcb.201811136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wann, A. K. , & Knight, M. M. (2012). Primary cilia elongation in response to interleukin‐1 mediates the inflammatory response. Cellular and Molecular Life Science, 69(17), 2967–2977. 10.1007/s00018-012-0980-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake, C. J. , Baye, L. M. , Nachury, M. V. , Wright, K. J. , Ervin, K. E. , Phu, L. , Chalouni, C. , Beck, J. S. , Kirkpatrick, D. S. , Slusarski, D. C. , Sheffield, V. C. , Scheller, R. H. , & Jackson, P. K. (2011). Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex‐dependent trafficking of Rabin8 to the centrosome. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 2759–2764. 10.1073/pnas.1018823108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemarck, N. , Rysman, E. , Brusselmans, K. , Van Imschoot, G. , Vanderhoydonc, F. , Moerloose, K. , Lerut, E. , Verhoeven, G. , van Roy, F. , Vleminckx, K. , & Swinnen, J. V. (2010). Aberrant activation of fatty acid synthesis suppresses primary cilium formation and distorts tissue development. Cancer Research, 70(22), 9453–9462. 10.1158/0008-5472.CAN-10-2324 [DOI] [PubMed] [Google Scholar]
- Wong, S. Y. , & Reiter, J. F. (2008). The primary cilium at the crossroads of mammalian hedgehog signaling. Current Topics in Developmental Biology, 85, 225–260. 10.1016/S0070-2153(08)00809-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, S. Y. , Seol, A. D. , So, P. L. , Ermilov, A. N. , Bichakjian, C. K. , Epstein, E. H., Jr. , Dlugosz, A. A. , & Reiter, J. F. (2009). Primary cilia can both mediate and suppress Hedgehog pathway‐dependent tumorigenesis. Nature Medicine (New York, NY, United States), 15(9), 1055–1061. 10.1038/nm.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , Du, H. , Wang, X. , Mei, C. , Sieck, G. C. , & Qian, Q. (2009). Characterization of primary cilia in human airway smooth muscle cells. Chest, 136(2), 561–570. 10.1378/chest.08-1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, V. M. , Chen, S. C. , Arkin, M. R. , & Reiter, J. F. (2012). Small molecule inhibitors of Smoothened ciliary localization and ciliogenesis. Proceedings of the National Academy of Sciences of the United States of America, 109(34), 13644–13649. 10.1073/pnas.1207170109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Roine, N. , & Makela, T. P. (2013). CCRK depletion inhibits glioblastoma cell proliferation in a cilium‐dependent manner. EMBO Reports, 14(8), 741–747. 10.1038/embor.2013.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura, S. , Egerer, J. , Fuchs, E. , Haas, A. K. , & Barr, F. A. (2007). Functional dissection of Rab GTPases involved in primary cilium formation. Journal of Cell Biology, 178(3), 363–369. 10.1083/jcb.200703047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, K. , Frolova, N. , Xie, Y. , Wang, D. , Cook, L. , Kwon, Y. J. , Steg, A. D. , Serra, R. , & Frost, A. R. (2010). Primary cilia are decreased in breast cancer: Analysis of a collection of human breast cancer cell lines and tissues. Journal of Histochemistry and Cytochemistry, 58(10), 857–870. 10.1369/jhc.2010.955856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, S. , Li, J. , Diener, D. R. , Choma, M. A. , Rosenbaum, J. L. , & Sun, Z. (2012). Target‐of‐rapamycin complex 1 (Torc1) signaling modulates cilia size and function through protein synthesis regulation. Proceedings of the National Academy of Sciences of the United States of America, 109(6), 2021–2026. 10.1073/pnas.1112834109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Lipinski, R. J. , Gipp, J. J. , Shaw, A. K. , & Bushman, W. (2009). Hedgehog pathway responsiveness correlates with the presence of primary cilia on prostate stromal cells. BMC Developmental Biology, 9, 50. 10.1186/1471-213X-9-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y. , & Pan, D. (2019). The Hippo signaling pathway in development and disease. Developmental Cell, 50(3), 264–282. 10.1016/j.devcel.2019.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, M. , Zhao, X. , Li, J. , Yuan, W. , Yan, G. , Tong, M. , Guo, S. , Zhu, Y. , Jiang, Y. , Liu, Y. , & Jiang, Y. (2016). Tumor suppressor folliculin regulates mTORC1 through primary cilia. Journal of Biological Chemistry, 291(22), 11689–11697. 10.1074/jbc.M116.719997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, D. , Shi, S. , Wang, H. , & Liao, K. (2009). Growth arrest induces primary‐cilium formation and sensitizes IGF‐1‐receptor signaling during differentiation induction of 3T3‐L1 preadipocytes. Journal of Cell Science, 122(Pt 15), 2760–2768. 10.1242/jcs.046276 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


