Abstract
Topical and systemic retinoids have long been used in the treatment of ichthyoses and other disorders of cornification. Due to the need for long‐term use of retinoids for these disorders, often beginning in childhood, numerous clinical concerns must be considered. Systemic retinoids have known side effects involving bone and eye. Additionally, potential psychiatric and cardiovascular effects need to be considered. Contraceptive concerns, as well as the additive cardiovascular and bone effects of systemic retinoid use with hormonal contraception must also be deliberated for patients of childbearing potential. The Pediatric Dermatology Research Alliance (PeDRA) Use of Retinoids in Ichthyosis Work Group was formed to address these issues and to establish best practices regarding the use of retinoids in ichthyoses based on available evidence and expert opinion.
Keywords: retinoid, ichthyosis, disorder of cornification, systemic therapy, topical therapy, adverse drug effects, drug monitoring, safety monitoring, bone health, hyperlipidemia, ectropion, contraception, iPLEDGE, depression, quality of life
1. INTRODUCTION
Disorders of cornification (DOC), including the inherited ichthyoses, represent a heterogeneous group of genetic disorders resulting from mutations in more than 50 genes that affect proteins of varied function including structural proteins, DNA repair proteins, and cholesterol biosynthesis enzymes among many others. All result in compromised cutaneous barrier function and are marked by increased scale and variable erythema and skin thickening. Ectropion, alopecia, nail thickening, and increased cutaneous infection are frequently associated. Pruritus, often severe, and skin fragility are commonly reported. Functional compromise, such as joint restriction and overheating, may result. The syndromic forms of ichthyosis include systemic manifestations, ranging from atopy to severe neurologic disease. The cutaneous and extracutaneous manifestations often have a profound psychosocial impact on patients, as well as their parents and siblings. 1 The financial burden of ichthyosis is also significant (mean cost $3,192 per year in 2010), with systemic retinoids comprising the greatest cost to insurers, while costs of emollients were incurred by patients. 2
Ichthyosis management requires a combination of therapeutic modalities, with frequent bathing, environmental humidification, daily topical emollients, and keratolytic agents as the foundation. 3 Retinoids, both topical and systemic, are a key component of ichthyosis management and have been used for decades with varying results. Tretinoin, adapalene, and tazarotene are applied topically, while isotretinoin and acitretin are used systemically; historically, etretinate, a potent metabolite of acitretin, was used but is no longer manufactured. In 2018, the European guidelines on management of congenital ichthyosis were published, detailing a comprehensive approach to the care of patients with ichthyosis from skin care to systemic treatment, including the use of retinoids. 4 , 5 However, the numerous clinical concerns regarding the use of retinoids were beyond the scope of that document.
In response to this identified need, a group of clinicians with experience in the management of disorders of cornification or tissues impacted by these medications, as well as a representative from the Foundation for Ichthyosis and Related Skin Types (FIRST), attended a meeting in October 2018, to review available literature and relate individual experience relative to the use of topical and systemic retinoids for ichthyosis. A modified Delphi process followed to reach consensus on new recommendations. These recommendations herein will provide guidance on when and how to use retinoids in the management of ichthyoses, as well as establish basic side‐effect monitoring standards. Through the process, numerous gaps in knowledge and practice were identified to serve as the foundation for further investigations.
1.1. Scope
These consensus recommendations specifically address the use of topical and systemic retinoids in the management of ichthyosis and other disorders of cornification (DOC) in children and adolescents. Issues regarding the impact of retinoid use on bone, eye, and cardiovascular health, as well as psychiatric effects, were reviewed with the goal to create recommendations for monitoring. Additionally, the teratogenic effects of retinoids prompted examination of gynecologic considerations and the role of the iPLEDGE program in the United States on patient access to isotretinoin.
1.2. Methods
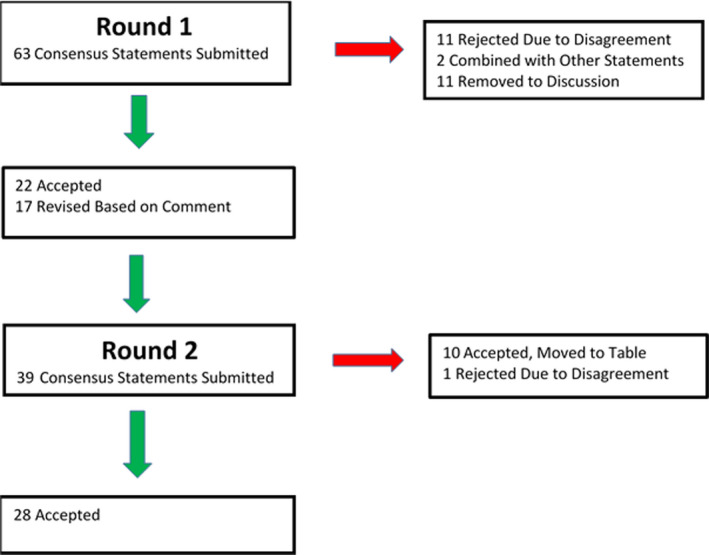
The Pediatric Dermatology Research Alliance (PeDRA) Use of Retinoids in Ichthyosis Work Group was selected by the co‐chairs based on expertise and was comprised of thirteen pediatric dermatologists, five general dermatologists, one pediatric cardiologist, one obstetrician‐gynecologist with expertise in family planning, a PhD researcher with expertise in bone development, and an ophthalmologist. The scope of the guidelines was determined and clinical questions identified by Work Group members (Table 1). These are listed at the beginning of each section. Work Group members were divided into subgroups and assigned specific clinical questions based on areas of expertise and interest. Each subgroup was responsible for performing an extensive literature search on the topics assigned, and the articles were centrally stored for group access and review. Searches were prospectively limited to publications in the English language. A total of 670 articles were identified as relevant and reviewed by Work Group members. The Delphi process was then used to attain consensus on the clinical question statements (Appendix I). An initial in‐person meeting was held in October 2018. Each subgroup presented its researched clinical questions, followed by discussion and comment by the entire Work Group. Following the meeting, the clinical questions were adapted into clinical statements and subsequently sent out for an initial round of blinded assessment of agreement based on a 0‐9 Likert scale and comment. Statements with greater than 80% agreement were carried forward as described or modified based on comment. Those with less than 80% agreement were modified by the co‐chairs based on comments or discarded. Several statements were combined with similar statements or moved to discussion. Recommendations for bone monitoring were approved by the group but presented in Table 8. The statement review process was performed two times and final statements approved. (Flowchart listed in Appendix II) Each subgroup then submitted a written summation of their assigned topics for publication. The quality of the available data methodology and the strength of recommendation were evaluated using Strength of Recommendation Taxonomy (SORT).
Good‐quality patient‐oriented evidence (ie, evidence measuring outcomes that matter to patients: morbidity, mortality, symptom improvement, cost reduction, and quality of life).
Limited quality patient‐oriented evidence.
Other evidence including consensus guidelines, opinion, case studies, or disease‐oriented evidence (ie, evidence measuring intermediate, physiologic, or surrogate end points that may or may not reflect improvements in patient outcomes).
Clinical recommendations were developed on the best available evidence tabled in the guideline. The strength of recommendation was ranked as follows:
Recommendation based on consistent and good‐quality patient‐oriented evidence.
Recommendation based on inconsistent or limited quality patient‐oriented evidence.
Recommendation based on consensus, opinion, case studies, or disease‐oriented evidence.
In those situations where documented evidence‐based data were not available, or showing inconsistent or limited conclusions, expert opinion and medical consensus was utilized to generate clinical recommendations
2. RETINOID EFFECTS ON THE SKIN
Retinoids are compounds structurally and functionally related to vitamin A (all‐trans retinol). Retinoids selectively bind, based on chemical structure, to specific protein isotopes (α, β,γ) of nuclear retinoic acid receptors (RAR) and retinoid X receptors (RXR). They have numerous and varied functions in the skin including modulating epidermal maturation, keratinocyte differentiation, apoptosis, immune function, and carcinogenesis. 6 , 7 The effects in the skin vary by route of administration (topical versus systemic), medication vehicle, and vary by the individual binding properties of the specific medication. The result is decreased thickening and scaling of the skin and decreased erythema. 8
Topical retinoids, primarily tretinoin and tazarotene, have been used to reduce scaling and hyperkeratotic thickening in several of the DOC, including X‐linked recessive ichthyosis, non‐erythrodermic autosomal recessive lamellar ichthyosis, autosomal dominant ichthyosis vulgaris, and ichthyosis bullosa of Siemens (IBS), as well as to improve ectropion, primarily in individuals with lamellar ichthyosis. 9 , 10 Clinical subtypes shown to respond favorably to systemic retinoids include: autosomal recessive congenital ichthyosis (ARCI) including harlequin ichthyosis, 11 , 12 , 13 , 14 , 15 , 16 lamellar ichthyosis, 9 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 and congenital ichthyosiform erythroderma (select genotypes). 25 , 26 , 27 In addition, recessive X‐linked ichthyosis, 28 IFAP (ichthyosis follicularis, atrichia and photophobia) syndrome, 29 Sjögren‐Larsson syndrome, 30 , 31 KID (keratitis, ichthyosis and deafness) syndrome, 32 , 33 , 34 neutral lipid storage disease with ichthyosis, 35 loricrin keratoderma, 36 erythrokeratodermia variabilis, 37 , 38 , 39 , 40 , 41 ichthyosis with confetti, 42 and KLICK (keratosis linearis with ichthyosis congenita and sclerosing keratoderma) syndrome 43 have all shown positive clinical responses to systemic retinoids based on published reports.
Table 1.
Clinical questions identified by work group members
| Retinoid Effects on Skin |
|
| Systemic Retinoid Dosing |
|
| Topical Retinoid Use |
|
| Retinoid Effects on Bone |
|
| Systemic Retinoid Effects on the Eye |
|
| Systemic Retinoid Effects on Lipids and Liver |
|
| Psychiatric Effects of Systemic Retinoid Therapy |
|
| Contraception Considerations of Long‐Term Systemic Retinoid Use |
|
| The Impact of iPLEDGE |
|
There are several subtypes of ichthyosis for which there are either no data for the use of retinoids or there have been data showing no improvement. Examples of such conditions include: Conradi‐Hunermann‐Happle syndrome, CHILD (congenital hemidysplasia with ichthyosiform erythroderma and limb defect) syndrome, ichthyosis‐hypotrichosis syndrome, ichthyosis‐hypotrichosis‐sclerosing cholangitis, trichothiodystrophy, Refsum syndrome, CHIME (colobomas, congenital heart defects, migratory ichthyosiform dermatosis, mental retardation, and ear anomalies) syndrome, MEDNIK (mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma) syndrome, peeling skin disease, and ichthyosis prematurity syndrome. Further, in some disorders with increased skin fragility, peeling skin, atopic diathesis, or excessive desquamation, such as Netherton syndrome, 44 , 45 retinoid use might, in fact, exacerbate disease manifestations and should be used with caution. 46 , 47 Consensus recommendations are listed in Table 2.
Table 2.
Consensus statements: retinoid effects on skin
| Both topical and systemic retinoids can improve scaling in patients with select forms of ichthyosis. | II, B |
|
In generalized disorders that feature prominent scale, there is evidence for the utilization of retinoids. Subtypes that have shown improvement with use of oral or topical retinoids include: congenital ichthyosiform erythroderma (select genotypes), epidermolytic ichthyosis, erythrokeratodermia variabilis, harlequin ichthyosis, ichthyosis with confetti, IFAP syndrome, KIDsyndrome, KLICKsyndrome, lamellar ichthyosis, loricrin keratoderma, neutral lipid storage disease with ichthyosis, recessive X‐linked ichthyosis, and Sjogren‐Larsson syndrome. |
II, B |
|
Subtypes for which there are either no data for the use of retinoids, or there has been data showing no improvement, include: CHILD syndrome, CHIME syndrome, Conradi‐Hunermann‐Happle syndrome, ichthyosis‐hypotrichosis syndrome, ichthyosis‐hypotrichosis‐sclerosing cholangitis, ichthyosis prematurity syndrome, MEDNIK syndrome, peeling skin disease, Refsum syndrome, and trichothiodystrophy. |
III, C |
| Utilization of retinoids in some disorders with skin fragility, peeling skin, atopic diathesis, or excessive desquamation (eg, Netherton syndrome) may exacerbate disease and should be used with caution. | II, B |
| Both adults and children with moderate to severe disorders of keratinization with significant functional or psychological impairment should be offered the opportunity to make a benefit/risk assessment of treatment with a systemic. | III, C |
3. TOPICAL RETINOID USE
Topical retinoids (tazarotene, tretinoin, and adapalene) have shown benefit in reducing scale, improving digital contractures in neonates, and reversing ectropion in congenital ichthyosis. 9 , 51 Most case reports have focused on lamellar ichthyosis, although improvement also has been reported for other forms of ichthyosis, including other autosomal recessive congenital ichthyoses, recessive X‐linked ichthyosis, 52 epidermolytic ichthyosis, 53 , 54 and erythrokeratodermia variabilis. 55 There are more reports on the effective use of tazarotene than other topical retinoids, but no direct comparisons have been performed.
Topical retinoids have a lower risk for adverse side effects than systemic retinoids and, therefore, should be= considered for use in milder disease and when the risks of systemic therapy outweigh the benefits. Cutaneous adverse effects include erythema, pruritus, irritation, and photosensitivity. 49 There is likely an increased risk for these adverse effects in ichthyosis vulgaris, patients with associated atopic dermatitis, and others with skin fragility. 49 No systemic adverse effects have been reported with widespread use. 56 With widespread use of tazarotene, some patients have had measurable but low plasma drug levels; thus, it is designated pregnancy category X and pregnancy should be avoided with tazarotene use. 56 , 57 , 58 There is no evidence to suggest systemic laboratory monitoring, or that monthly pregnancy tests in patients of childbearing potential, is needed; however, data are limited to single case reports and case series. 50 , 56 , 57 , 58 Consensus recommendations are listed in Table 3.
Table 3.
Consensus statements: topical retinoid use
| SORT | |
|---|---|
|
Topical retinoids are useful in treating scale, digital contractures, and ectropion in patients with ichthyosis. |
IIIC |
|
Systemic laboratory monitoring is not recommended with use of topical retinoids for ichthyosis. |
IIB |
4. SYSTEMIC RETINOID DOSING
The families of children and adolescents with moderate to severe disorders of cornification and significant functional or psychological impairment should be offered the opportunity to make a risk‐benefit assessment on the use of a systemic retinoid. While specific clinical features can help predict retinoid responsiveness, genetic testing has not added to our ability to predict retinoid benefit. In considering retinoid therapy, it is important to thoroughly discuss the expected outcome and the potential adverse effects with both the child and caregivers. Considerations include: age, severity, other comorbidities, grooming time, psychosocial effects, out‐of‐pocket costs, the ability to understand and participate in the risk: benefit analysis as well as to comply with contraception precautions. The expected benefit must outweigh the risks. A systemic medication should be considered and added to topical therapies if topical therapies provide insufficient improvement, the burden of disease is high, and there is a significant impact on the quality of life. General reduction in the thickness of scale can be expected. Retinoids can have a significant beneficial effect on ectropion or pseudoainhum. For those who fatigue easily because of overheating secondary to absent sweating, systemic retinoids may increase the ability to sweat, but not all patients note improved sweating with scale reduction.
The beneficial effects of retinoids in ichthyosis are recognized to be dose‐dependent. However, the dose‐escalation studies, conducted early in the use of isotretinoin, used a wide dosage range from 1 mg/kg/day to as much as 7 mg/kg/day. 59 While signs and symptoms of ichthyosis (scaling, induration, and crusting) improved with increasing dose, cutaneous toxicity was dose‐limiting at higher doses, particularly skin irritation, fragility, and dryness. 59 Based on these studies, investigators chose maintenance dosing for various forms of ichthyosis (ichthyosis vulgaris, X‐linked ichthyosis, lamellar ichthyosis, epidermolytic ichthyosis, Darier disease, and palmoplantar keratoderma) intended to produce maximal clearing with minimal side effects; the mean dosing from these studies was 1.8 to 2.1 mg/kg/day. 59 , 60 , 61 , 62 Given the cutaneous and extracutaneous toxicities of oral retinoids, lower doses were found to achieve the most acceptable risk‐benefit result. Few individuals now receive more than 1 mg/kg/day of isotretinoin or 0.5 mg/kg/day of acitretin (see bone section for relative therapeutic index for synthetic retinoids in animals). Many patients do well with lower doses, especially for epidermolytic ichthyoses (where blistering is common) and in erythrokeratodermia variabilis. A summary of dosing recommendations for topical and systemic retinoids in specific disorders is provided in Table 4. Tips for administering retinoid capsules to infants and children are provided in Table 5.
Table 4.
Retinoid dosing in ichthyosis and other disorders of cornification
| Formulation | Dosage | Considerations |
|---|---|---|
| Systemic | ||
|
Acitretin 10 mg, 25 mg capsule |
Child: 0.5 ‐ 1 mg/kg/day Adult: 0.5 ‐ 1 mg/kg/day Typical dose: 10‐25 mg/day Maximum dose: 75 mg/day |
|
|
Isotretinoin 10 mg, 20 mg, 30 mg, 40 mg capsule (contains soy) |
Child: 0.5 ‐ 1 mg/kg/day Adult: 0.5 ‐ 1 mg/kg/day |
|
| Topical | ||
|
Tazarotene 0.05%, 0.1% gel 0.05%, 0.1% cream |
Daily |
|
|
Tretinoin 0.025%, 0.05%, 0.1% cream |
Daily |
|
|
Adapalene 0.1%, 0.3% gel |
Daily |
|
Table 5.
Tips for administering retinoid capsule to child*
|
Keep out of direct sunlight, use capsule immediately after removing from packaging |
| Capsule can be swallowed whole or chewed |
| Poke a hole in capsule with sharp knife or nail clippers and add to food (note: capsule is very tough) |
| The capsule may be softened by placing in a small cup with warm water or milk for 2‐3 minutes |
| Add to limited amount (1 oz) of breast milk or formula for infants |
| Give with high fat food (such as whole milk, ice cream, peanut, or other nut butter) for best absorption |
| Freeze capsule in bite sized, soft centered chocolate and chew chocolate and capsule |
| *Adapted from https://www.stjude.org/treatment/patient‐resources/caregiver‐resources/medicines/a‐z‐list‐of‐medicines/isotretinoin.html. Accessed September 5, 2020. |
The initial short‐term studies suggested the therapeutic window was similar for the major clinical types of ichthyosis (except Netherton syndrome for which cutaneous toxicity usually exceeds benefit). However, individuals with ichthyosis have varying severity of disease, and thus tolerance of retinoids, which affects dosing. In addition, the clinical features of various forms of ichthyosis dictate response and tolerance to retinoids. For example, individuals with lamellar ichthyosis may use retinoids daily, adjusting dosing if cutaneous irritation occurs, while an individual with epidermolytic ichthyosis may require dose modifications or periods of discontinuation owing to skin fragility. Season of year, environmental change, and other poorly understood factors may affect the frequency of dosing, for example, in Darier disease. Topical modalities should be continued if tolerated during oral retinoid therapy because they may add to therapeutic benefit, as well as allow lower oral retinoid dosing or retinoid holidays. 3
Optimal dosing should be based on shared decision‐making between patient, caregiver and physician based on benefit, including appearance and comfort of skin, time and effort for skin care, heat or cold tolerance, and risk of both cutaneous and non‐cutaneous adverse effects. Ultimately, the optimal dose of a systemic retinoid is the lowest dose that will achieve and maintain the desired therapeutic effect with acceptable mucocutaneous and systemic toxicities. Again, any retinoid treatment should be used in conjunction with an optimized skin care routine with bathing and emollient usage.
Ichthyosis is a life‐long disorder, and therefore, systemic retinoid treatment of DOC is often long term. The skin reverts to its pretreatment condition upon cessation of retinoid treatment. Therefore, clinicians must counsel patients about potential long‐term toxicities. Although many patients have received systemic retinoids for decades, there have been no long‐term prospective studies to clarify maintenance dosing. Other than the adverse bone effects, no toxicity appears to depend on duration of use. 25 , 53 , 63 , 64 Additionally, some toxicities vary by age at exposure. For example, growing children exposed to high doses are at rare risk for premature epiphysial closure 65 , 66 but do not seem to develop the hyperostotic changes (enthesopathy) that commonly occurs in adults (see below, Retinoid Effects on Bone).
Tachyphylaxis does not seem to occur, and there is also no evidence that benefits change with age. Overall, use of the lowest effective dose of retinoid is recommended (typically 1 mg/kg/day or less).
While a week on‐week off schedule in the original isotretinoin studies was used, intermittent therapy has not been studied. Anecdotally, patients who have interrupted and then resumed therapy for family planning or other reasons experience neither a “flare” in their disease nor change in therapeutic window. Therefore, there may be a benefit to “retinoid holidays,” or less frequent dosing, such as twice weekly. 67
Neonates with harlequin ichthyosis whose constrictive scales significantly restrict breathing or threaten viability of distal extremities may benefit from treatment with systemic retinoids. 11 , 12 , 68 Those with a milder phenotype may not require a systemic retinoid. 69 Responses and risks of isotretinoin and acitretin as treatments for ichthyosis are generally similar. Some patients think results with one are better than the other, but no consistent preference is clear. Acitretin, similar to its predecessor, the aromatic retinoid etretinate, has a greater capacity to cause peeling of the palms and soles compared to isotretinoin (suggesting preferential use for palmoplantar involvement), but also a greater risk of persistent hair loss. 70 In contrast, isotretinoin has been found to lead to more facial desquamation. 70 A major advantage to long‐term use of isotretinoin is its short half‐life, simplifying dose changes, “drug holidays,” and family planning. Broadly, because of a 2‐3 year post‐treatment teratogenic risk, exposure to acitretin to patients of childbearing potential should be of particular concern (see below, Contraception Considerations of Long‐Term Systemic Retinoid Use). Consensus recommendations are listed in Table 6.
Table 6.
Consensus statements: retinoid dosing
| SORT | |
|---|---|
|
When choosing a systemic retinoid for treatment of disorders of cornification, isotretinoin should be considered first line for patients of reproductive potential. |
IA |
|
Because of the prolonged half‐life of acitretin (up to 3 years), clinicians should consider transitioning patients of childbearing potential from acitretin to isotretinoin before puberty if at risk of pregnancy. |
IIIC |
| The optimal dose of a systemic retinoid is the lowest dose that will achieve and maintain the desired therapeutic effect with acceptable mucocutaneous and systemic toxicities. | IA |
| Systemic retinoid treatment of the disorders of cornification is often long term. Therefore, the clinician must counsel patients about potential long‐term toxicities. | IA |
| Neonates with harlequin ichthyosis whose constrictive scales significantly restrict breathing or threaten viability of distal extremities may benefit from treatment with systemic retinoids. | IIIC |
|
Neonates with milder phenotypes of harlequin ichthyosis may not require a systemic retinoid. |
IIIC |
5. RETINOID EFFECTS ON BONE
Naturally occurring and synthetic retinoids have a wide spectrum of effects on bone, which have been observed in animal studies, in humans during clinical studies, and in real‐world treatment observations. Animal studies have shown a dose‐dependent impact on bones, which has been mirrored in humans. The toxicities of retinoid drugs emulate those previously observed with naturally occurring retinoids, particularly hypervitaminosis A. However, there are differences in the spectrum of abnormalities and effect of dose. Both naturally occurring and synthetic retinoids are metabolized in the body, and the relative effects of these different compounds are poorly understood. Many factors affect the type and severity of toxicity including retinoid chemical structure, age at exposure, dose and duration of exposure, genetic predisposition, and effects of other drugs.
Skeletal toxicities observed in children include premature epiphyseal closure, calcifications of tendons and ligaments, osteophytes or “bone spurs,” diffuse idiopathic skeletal hyperostosis (DISH), and potential alterations in bone density and growth. Monitoring for these toxicities should include a family and personal medical history considering risk factors for skeletal toxicity, annual growth assessment, regular inquiry about musculoskeletal symptoms, inquiry about diet, and periodic imaging studies. Retinoid effects on children with growing bones are likely to differ from effects on adults. 71 , 72 While retinoids are critical for bone growth, development, turnover, and mineralization, overdoses can cause fractures and calcification in tendons and ligaments. Pediatric oncology data, where high doses of systemic retinoids are used, are confounded by the use of multiple drugs, suggesting those data may not reflect the use of lower doses for dermatologic conditions.
Premature closure of the epiphyses is an uncommon but serious adverse effect reported with systemic retinoid therapy (above 1mg/kg/day) over prolonged periods of time (4‐6 years). While there are several case reports documenting this side effect in both long‐term use of etretinate and isotretinoin for DOC, 67 , 73 larger case‐based and prospective series are lacking. Additional data regarding growth plate disruption are reported from high‐dose isotretinoin use for childhood tumors 74 , 75 and fibrodysplasia ossificans progressiva 76 as well as from animal models. 65
Many observational studies during treatment, as well as prospective studies, have substantiated the role of systemic retinoids in accelerating the development of DISH‐like hyperostotic changes involving the tendon and ligaments of the spine and peripheral joints (osteophytes, tendon and ligament calcification, and hyperostosis). These changes appear to be related to both dose and duration of retinoid exposure. 63 DISH is thought to be an enthesopathy, impacting the attachment of tendons and ligaments to bone.
There may be a difference between retinoids in frequency of hyperostotic changes of the spine versus peripheral joints. 77 , 78 It is not known whether the variation observed between different retinoids and location of bone toxicity are due to pharmacological differences, constitutional differences between populations studied (age of exposure, genetics, etc), or possibly confounded by the diseases treated (eg, psoriasis patients are prone to a similar enthesopathy). Hyperostotic bony changes are extremely common in the general population and increase with age even without exposure to retinoid treatment. In the general population, one study found that 100% of 400 spine specimens of individuals older than 40 years of age showed some degree of anterior osteophyte formation, which increased in size with age. 79 In another study, up to 65% of individuals younger than 20 years of age had similar changes. 80
Available evidence does not support a threshold level below which skeletal toxicity would not occur. In a 3‐year, very‐low‐dose (0.14 mg/kg/day) study for basal cell carcinoma chemoprevention, more patients on isotretinoin had progression of existing hyperostotic abnormalities and new hyperostotic involvement compared with placebo, without preventing new basal cell carcinomas. 81 A follow‐up radiographic survey found no progression of hyperostotic vertebral abnormalities in post‐treatment films taken approximately 16‐17 months later, suggesting tha retinoid contribution to this toxicity does not extend after discontinuation of retinoid treatment. 82
For patients taking long‐term retinoid therapy for DOC, radiographic surveys of areas frequently involved with hyperostosis, as well as areas that are symptomatic, may be useful to characterize the extent of involvement and progression. 3 This includes lateral view of the cervical, thoracic, and sometimes lumbar spine, and may include the hips and lateral view of the calcanei of the feet, common areas of involvement with hyperostosis. Because unexpected body locations can be involved, symptomatic locations should also be imaged. 83 , 84 , 85 Once retinoid therapy is determined to be well tolerated and the patient is expected to continue long term, baseline imaging can be obtained and repeated after 3‐5 years or sooner for symptomatic areas. After long‐term exposure, some patients develop clinically significant stiffness, arthralgia, limitation of joint range of motion, or bone spurs that can impinge on nerves. However, many patients tolerate long‐term treatment. Non‐steroidal anti‐inflammatory drugs, physical therapy including range of motion exercises, and surgical removal of bone spurs may be helpful in some cases, 86 but controlled studies have not been done. Patients with bone symptoms may be co‐managed with an appropriate specialist.
While high‐dose retinoid exposure in animal studies have identified changes in bone mineral density, human clinical studies have observed variable results in different populations, with different retinoid drugs, and measurement techniques. Decreased bone mineral density associated with systemic retinoid treatment has been reported in some studies and not in others. 87 , 88 The most relevant clinically important endpoint of decreased bone mineral density is bone fracture. A large scale, population‐based Danish study including children and adults found none of the common retinoids (isotretinoin, acitretin, and topical retinoids) were associated with any change in fracture risk at any skeletal site, and there was no trend with either increasing dose or duration of treatment. 89 For patients on long‐term therapy, a baseline bone density evaluation can be considered, noting extra‐osseous calcification is associated with retinoid therapy and bone density measurements should be interpreted with caution. Based on the literature and expert opinion, the timing of radiographic evaluation for hyperostosis and bone mineral density is variable and should be individualized based on patient history and physical findings. Consensus statements are listed in Table 7, and bone monitoring recommendations are presented in Table 8.
Table 7.
Consensus statements: retinoid effects on bone
| SORT | |
|---|---|
| Long‐term use of systemic retinoids in ichthyosis/ DOC is associated with skeletal concerns. | I, A |
| The toxic effects of systemic retinoid use on bone are strongly dependent on dose and duration. | I, A |
| Potential bone toxicity should not preclude long‐term systemic retinoid use in patients with ichthyosis if there is a clear clinical benefit. | III, C |
| Genetic risk and modifiable factors that affect bone health, such as diet and physical activity, may impact susceptibility to systemic retinoid bone toxicity and should be discussed with the patient. | II, C |
Table 8.
Recommended bone monitoring for patients on long‐term systemic retinoid therapy
|
The following evaluation and testing should be considered for children (less than 18 years of age): Comprehensive family and personal medical history for risk factors for skeletal toxicity Annual growth assessment (height, weight, BMI, and growth curve) Inquire regularly about musculoskeletal symptoms; follow‐up with appropriate imaging Inquire about diet (including sufficient vitamin D intake and no additional vitamin A sources) At full growth (16‐18 years), a baseline skeletal radiographic survey, which may include imaging of the lateral cervical and thoracic spine, lateral view of the calcanei to include Achilles tendon, hips and symptomatic areas; and bone density evaluation (eg, DEXA scan) |
|
The following evaluation and testing should be considered for adults: Comprehensive family and personal medical history for risk factors for skeletal toxicity Inquire regularly about musculoskeletal symptoms; follow‐up with appropriate imaging Inquire about diet (including sufficient vitamin D intake and additional vitamin A sources) If therapy is likely to continue long‐term, a baseline skeletal radiographic survey which may include imaging of the lateral cervical and thoracic spine, lateral view of the calcanei to include Achilles tendon, hips and symptomatic areas; and bone density evaluation (eg, DEXA scan) Repeat radiographic bone evaluation every 3‐5 years or if symptomatic |
6. SYSTEMIC RETINOID EFFECTS ON THE EYE
To date, there is limited literature about the ophthalmologic effects of retinoids in patients with ichthyosis to provide evidence‐based surveillance and management guidelines. Both topical and systemic retinoids have been shown to reduce the severity of ectropion in children and adults. 9 , 17 , 90 However, the potential side effects of systemic retinoids on eye health are compounded by any ocular manifestations of the underlying ichthyosis. The acute and long‐term effects of retinoid use on ocular health in children and adolescents are unknown. Most of the current evidence is based on retrospective reviews of short‐term, episodic systemic isotretinoin use in adolescents and young adults with acne vulgaris.
Approximately 13.8% of adolescents on isotretinoin for acne experience ocular side effects. 62 , 91 , 92 The most common complaints include pruritus, photophobia, eye pain, burning, and gritty feeling or visual disturbance that may occur within days up to a few months following initiation of isotretinoin. These symptoms are dose dependent and can be attributed to ocular surface diseases, as well as refractive changes and retinal dysfunction. 93 Ocular surface diseases including conjunctivitis, hordeolum, chalazion, blepharitis, and dry eyes are likely due to decreased secretion from Meibomian glands that results in poor tear‐film quality. 91 , 94 , 95 , 96 Refractive changes are likely secondary to corneal steepening and edema that cause myopic shift. 97 , 98 Corneal opacity resulting from systemic therapy is rare, but can be permanent. 99 , 100 Ocular toxicity from acitretin use in psoriasis was not detected after one month of treatment. 101 Nonetheless, the lowest daily retinoid dose able to manage cutaneous symptoms is recommended to minimize risks of ocular toxicity.
Early ocular surface diseases and refractive changes are considered to be reversible side effects that can be improved with isotretinoin dose adjustment or discontinuation. 101 , 102 Although there is no significant change of the retinal nerve fiber layer measured by optical coherence tomography (OCT), 103 some cases of refractive change and most cases of retinal dysfunction with decreased color or night vision are considered permanent. If retinal damage occurs, termination of systemic therapy is recommended. Use of topical retinoids to periocular skin is considered a safe option, as ocular toxicity has not been noted among those using topical treatments long term for acne. 101 , 104
Since patients with ichthyosis often have underlying eye abnormalities, including dry eyes, ectropion, exposure keratopathy, and corneal defects, additional caution should be taken when choosing systemic retinoid therapy. Baseline comprehensive eye examination by an experienced ophthalmologist is recommended.. 94 , 101 Recommendations on ocular lubricants, as well as preservative‐free artificial tears, should be provided to patients during retinoid therapy if they are not already using them. Subsequent follow‐up with an ophthalmologist is recommended after 4‐6 months of systemic retinoid therapy to rule out progressive ocular surface diseases and increased retinal dysfunction. Interval clinical evaluation should be considered, especially when patients experience worsening symptoms. Therapeutic holidays or discontinuation should be considered if worsening retinal function is noted. Regardless, lowering dosage of systemic retinoids or transition to topical retinoids should be considered when cutaneous therapeutic goals are achieved. For patients who are on long‐term systemic retinoid therapy, routine eye examination (every 6‐12 months) should be considered depending on the severity of their ocular symptoms. Consensus recommendations are listed in Table 9.
Table 9.
Consensus statements: systemic retinoid effects on the eye
| SORT | |
|---|---|
|
Systemic retinoid therapy can cause retinal dysfunction, night blindness and dry eyes. Use may exacerbate existing eye symptoms related to underlying ichthyosis. |
I, A |
| For patients who are on long‐term systemic retinoid therapy, evaluation by ophthalmology is recommended 4‐6 months after initiation of systemic retinoid therapy, with routine follow‐up every 6‐12 months. | III, C |
7. SYSTEMIC RETINOID EFFECTS ON LIPIDS AND LIVER
Both isotretinoin and acitretin can cause elevations in serum lipids and have the rare potential to adversely affect liver function. Most of the literature on the long‐term use of oral retinoids is from patients with psoriasis, as isotretinoin is typically used for limited durations in the management of acne. Data on long‐term use in children and adolescents are lacking. The effects of acitretin on the liver were studied in a case series of 128 adults treated with 25‐75 mg/day. 105 At the end of the 2‐year period, liver biopsy was performed and compared to pretreatment biopsy. In this study, 59% exhibited no change, 24% improved, and 17% worsened. All changes were mild to moderate. Only four patients had liver function test (LFT) elevations, and two had increased triglyceride levels. No correlations between elevated LFTs and liver biopsy status were found. In a study of 104 patients with psoriasis treated with acitretin for a mean duration of 3.2 years, no patients developed hepatotoxicity or uncontrolled hyperlipidemia. 106 In another retrospective study of 18 children treated with acitretin for psoriasis, no significant alterations in liver function and lipid profiles were identified. 107 Children with psoriasis may be at increased risk for metabolic syndrome. 108 Whether or not patients with ichthyosis, in which some forms are also associated with chronic systemic inflammation, 109 are at similar risk has not been examined; therefore, the additive risk of medication‐induced hyperlipidemia is not known.
There are a limited number of studies specifically focusing on the use of oral retinoids for the treatment of DOC. One study evaluated the long‐term use of oral retinoid therapy for DOC in 23 adults and children. In this cohort, 8 patients had elevated lipid profiles, one required lipid‐lowering treatment and all were safely continued on the retinoid. 110 None of the adults in this study had elevated liver function tests, except one patient with continued alcohol dependence while on etretinate who experienced progressive end‐stage alcoholic liver cirrhosis. In a pediatric study of 46 children with DOC treated with acitretin for a cumulative follow‐up time of 472 months, only minor abnormalities in liver function tests were noted in four patients. 111 One patient developed transient elevated triglycerides, but therapy was not altered. No irreversible side effects developed in this cohort.
Severe lipid abnormalities and hepatotoxicity with the use of systemic retinoids are very rare. A serum lipid panel and liver function testing should be performed at baseline and, if in an acceptable range, repeated 1‐2 months after starting treatment. If the levels remain within the acceptable range, subsequent testing can be done every 6‐12 months. Prescribers should routinely document concomitant medications as well. Combined oral contraceptives, commonly used to adhere to contraceptive requirements in patients of childbearing potential, can also increase serum lipids. Patients on a long‐term systemic retinoid with persistent, abnormal, clinically significant elevations in serum lipids may continue retinoid treatment with effective lipid management if the risk: benefit assessment is deemed acceptable. Recommendations on the management of hyperlipidemia are provided by the American Academy of Pediatrics and cardiovascular risk reduction in high‐risk pediatric patients (which includes chronic inflammatory disorders but does not specify retinoid use) is detailed by the American Heart Association. 112 , 113 Long‐term effects on overall cardiovascular health are unknown. Consensus recommendations are listed in Table 10.
Table 10.
Consensus statements: systemic retinoid effects on lipids and liver
| SORT | |
|---|---|
| Severe lipid abnormalities and hepatotoxicity with the use of systemic retinoids are very rare. | II, B |
|
Serum lipid panels and liver function tests should be performed at baseline and, if in an acceptable range, repeated in 1‐2 months after starting treatment. If the levels remain within the acceptable range, subsequent testing can be done every 6‐12 months. |
II, B |
|
Patients on long‐term systemic retinoids with persistent, abnormal, clinically significant elevations in serum lipids may continue retinoid treatment with effective lipid management. |
II, C |
8. PSYCHIATRIC EFFECTS OF SYSTEMIC RETINOID THERAPY
The relationship between use of systemic retinoids and development of psychiatric symptoms is controversial. Often the systemic retinoid is used for a chronic disorder, which in itself can contribute to psychiatric symptoms. Additionally, the incidence of major depressive disorder in adolescents in the United States is quite high. In 2017, 13.3% of adolescents 12‐17 years old experienced major depressive disorder, with females affected more frequently than males (20% vs 6.6%). 114 The psychiatric effects of retinoid use in children and adolescents with ichthyosis, specifically, have not been examined.
Most studies exploring these relationships evaluate patients with acne. One large retrospective review of over 21,000 patients aged 10‐29 years found no increased relative risk of psychiatric symptoms for those taking antibiotics vs. systemic retinoids to treat acne. 115 A questionnaire of 3,775 teens with acne suggested the increase of mental health issues in candidates for systemic isotretinoin was, in part, due to the acne itself. 116 Multiple case reports have detailed worsening psychiatric symptoms in patients taking systemic retinoids, particularly isotretinoin. A systematic review of these reports found an average onset of psychiatric symptoms at 5.4 weeks into the course, but factors predisposing patients were unclear. 117 In a case‐crossover study exploring the timing of hospitalization for depression and use of systemic isotretinoin, authors reported an adjusted relative risk of 2.68 to develop depression when exposed to isotretinoin and a threefold increased risk for patients with a history of depression. 118 A review of the French National Pharmacovigilance Database for reports of adverse events to any systemic retinoid treating any disorder also suggested an increased risk of development of psychiatric symptoms in patients with a personal or family history of psychiatric disease. 119
These reports support monitoring patients for the development of psychiatric symptoms while taking systemic retinoids and co‐management with a mental health provider in patients with psychiatric symptoms. That said, ichthyosis itself can have a profound impact on a patient's quality of life. 120 In a cross‐sectional study of children and adults with ichthyosis, 30.2% of children screened positive for depression and 37.7% for anxiety. 121 In adults, 34.4% screened positive for depression and 27.3% were positive for anxiety. A mental health inquiry should be performed on every patient with a DOC at baseline and follow‐up regardless of therapy. Consensus recommendations are listed in Table 11.
Table 11.
Consensus statements: psychiatric effects of systemic retinoid therapy
| SORT | |
|---|---|
|
All patients, regardless of history, should be monitored for the development of psychiatric symptoms when they are taking systemic retinoids. |
I, A |
|
Patients with a personal history of depression, anxiety, and other affective disorders prior to initiation of systemic retinoid treatment should be monitored carefully for exacerbation of symptoms. Co‐management with a mental health provider should be considered. |
II, B |
9. CONTRACEPTION CONSIDERATIONS OF LONG‐TERM SYSTEMIC RETINOID USE
Systemic retinoids are known teratogens, with a “black box” warning of extreme risk to the exposed fetus for severe birth defects. Adolescents of childbearing potential taking systemic retinoids should be counseled about their options for contraception, inclusive of highly effective methods such as intrauterine devices (levonorgestrel‐releasing intrauterine system, intrauterine copper contraceptive) and subcutaneous injection (depot medroxyprogesterone acetate) and implants (etonogestrel‐releasing contraceptive implants). The failure rates of these long‐acting reversible contraceptives are less than 1%. 122 In the United States, all patients of childbearing potential on isotretinoin must adhere to the iPLEDGE program contraceptive regulations; specifically, those who are sexually active must use two forms of contraception, including one highly effective form, while on isotretinoin. Acitretin does not have a mandated risk management program, but contraceptive counseling should be as rigorous as for isotretinoin and include long‐term contraceptive planning, given the prolonged half‐life of the drug. The acitretin package insert also states that two forms of contraception are required. Teratogenicity resulting from male retinoid use has not been reported. Table 12 summarizes the pharmacokinetics of systemic retinoids.
Table 12.
Pharmacokinetics of systemic retinoids
| Systemic Retinoid | Metabolites | Pharmacokinetics |
|---|---|---|
|
Isotretinoin 126 |
|
Half‐life:
|
|
Acitretin 127 |
With alcohol:
|
Half‐life:
Greater than 98% of acitretin is eliminated within 2 months (assuming a mean elimination half‐life of 49 hours) Greater than 98% of etretinate formed is eliminated in 2 years (assuming a mean half‐life of 120 days) Greater than 98% of etretinate formed is eliminated in 3 years (based on half‐life of 168 days) |
While isotretinoin is cleared within several months, acitretin has a much longer half‐life. In addition, acitretin taken with ethanol converts to etretinate, which is stored in fat and has a prolonged elimination and release into circulation. 123 Therefore, pregnancy should be avoided for 3 years after cessation of acitretin therapy. When choosing a systemic retinoid for treatment of DOC, isotretinoin should be considered preferentially for patients of reproductive potential. In addition, because of the prolonged half‐life of acitretin, clinicians should consider transitioning patients of childbearing potential from acitretin to isotretinoin at the onset of puberty or at least consider long‐acting, reversible forms of contraception.
In adolescent patients continuing on a systemic retinoid from childhood, or those just starting a systemic retinoid with abstinence as the chosen form of contraception, discussions regarding contraception should be undertaken early and reviewed each visit. Providers, in shared decision‐making with patients, should have a plan to start contraception either at a specific age (eg, 16 years of age) or before sexual debut, employing the assistance of a gynecologist or other provider with expertise in contraception as needed.
Potential sequelae resulting from the long‐term use of combined oral contraception in conjunction with systemic retinoids, including cardiovascular and bone health, have not been explored. Both medications can increase serum lipids. The additive metabolic effects of chronic inflammation occurring in some forms of ichthyosis (such as CIE), along with the lipid elevations caused by retinoid and contraceptive use, have not been explored. Additionally, low‐estrogen and progestin‐only contraceptive formulations can increase osteoporosis risk. Subcutaneous medroxyprogesterone injection carries a “black box” warning regarding risk of osteoporosis. 124 Consensus recommendations are listed in Table 13.
Table 13.
Consensus statements: contraception considerations of long‐term systemic retinoid use
| SORT | |
|---|---|
|
Highly effective forms of contraception, such as intrauterine devices (IUD) or progestin implants, should be considered in shared decision‐making with sexually active adolescent patients of childbearing potential on systemic retinoid therapy. |
I,A |
| Patients of childbearing potential who are sexually active should use two forms of contraception, including one highly effective form, while on any systemic retinoid therapy. | I,A |
10. THE IMPACT OF IPLEDGE
In the United States, the iPLEDGE program serves as the Risk Evaluation and Mitigation program for isotretinoin. Implemented in 2006, iPLEDGE requires initial consent, monthly office visits and online verification by prescribers with patient verification and a 30‐day supply limit of medication each month for all patients. For patients of childbearing potential, additional requirements include two effective forms of contraception, monthly verification of pregnancy tests and patient understanding, and a seven‐day pickup window for prescriptions. 125 Isotretinoin is traditionally used for acne vulgaris, for which the typical duration of therapy is five to six months; when used for ichthyosis treatment, isotretinoin is commonly prescribed continuously for several years. Aside from the iPLEDGE requirements, monthly visits for long‐term isotretinoin use are generally not clinically indicated once a patient is on a stable dose. Patients with ichthyosis have increased financial burden due to medical costs and out‐of‐pocket expenses. 120 The monthly iPLEDGE requirements are burdensome for ichthyosis patients as they increase direct and indirect medical costs for patients and their families, including cost of visits, CLIA laboratory‐certified pregnancy testing and work and school absence. Presently, there are no exceptions to the iPLEDGE requirements for patients on long‐term therapy with isotretinoin for ichthyosis. Consensus recommendations are listed in Table 14.
Table 14.
Consensus statements: the impact of iPLEDGE
| SORT | |
|---|---|
|
All patients and prescribers of isotretinoin in the United States must comply with current iPLEDGE guidelines. |
I, A |
|
The iPLEDGE program was not designed for long‐term use of isotretinoin in patients with ichthyosis and imposes a significant burden on this patient population. |
III, C |
11. CONCLUSION
The use of systemic retinoids in children and adolescents with ichthyosis and other disorders of cornification can be highly effective in reducing scaling and improving both function and appearance. However, the long‐term use of these medications results in several important health considerations, particularly regarding bone and cardiovascular health, as well as contraceptive concerns. This publication provides recommendations regarding the use of systemic and topical retinoids based on the best evidence available. However, numerous knowledge and practice gaps have been identified (Table 15). Providers must be aware of what is known and unknown regarding the safety and efficacy of long‐term retinoid use. These risks should be discussed candidly with patients and their families in shared decision‐making and recognize long‐term safety data are lacking. It is the hope of this work group these efforts and the numerous knowledge gaps identified will serve as the framework for future study.
Table 15.
Practice gaps/ unmet needs
|
Retinoid Effects on the Skin: 1. The exact mechanism of action of retinoids on the skin and their effects in DOC has not been adequately examined. |
|
Retinoid Dosing: 2. The optimal formulation of retinoid, as well as dosing amount and frequency, based on ichthyosis subtype and individual features is not known. 3. The adverse effects of long‐term retinoid treatment and the optimal means to monitor for them need more thorough examination. 4. Whether there is a benefit to intermittent therapy with respect to risk of toxicity and maintenance of efficacy is unknown. 5. Evaluation of optimal timing of initiation of retinoid therapy regarding safety and efficacy is needed. |
|
Topical Effects of Retinoids: 6. The potential for systemic absorption of topical retinoids is not known. 7. Best practices to minimize the local adverse effects of topical retinoids needs examination. |
|
Retinoid Effects on Bone: 8. A “bone‐safe” duration of retinoid therapy (if it exists) requires further study. 9. Which individuals are at greater risk for bone toxicity from retinoid treatment is not known. 10. The best formulation and timing of initiation of a systemic retinoid on potential bone toxicity needs to be determined. 11. How to minimize symptoms and progression of DISH‐like changes, if feasible, is not known. |
|
Systemic Retinoid Effects on the Eye: 12. Further study of the long‐term effects of systemic retinoid therapy on patients with ichthyosis is needed. 13. A better understanding of the ophthalmologic concerns from the use of topical retinoids on the eyelids is required. |
|
Systemic Retinoid Effects on Lipids and Liver 14. The chronic effects of systemic retinoids on lipids and liver function are unknown. 15. Optimal monitoring frequency of lipids and liver function with long‐term use of systemic retinoids should be examined. 16. The potential effects of systemic retinoid therapy on patients’ long‐term cardiovascular health are not known. |
|
Psychiatric Effects of Retinoids: 17. Data on the prevalence of psychiatric disorders in ichthyosis patients are lacking. 18. Studies exploring the psychiatric effects of retinoids on patients taking long‐term systemic retinoids have not been performed. |
|
Contraception Considerations of Long‐Term Systemic Retinoid Use 19. The combined effects of using hormonal contraception and systemic retinoids on cardiovascular and bone health in females are unknown. |
|
The Impact of iPLEDGE: 20. The financial burden of iPLEDGE program on patients and their families has not been determined. 21. The iPLEDGE program should be modified to accommodate the use of isotretinoin in the management of ichthyoses and disorders of cornification to reduce undue burden. |
CONFLICTS OF INTEREST
Andrea L. Zaenglein, MD was an investigator for Abbvie and Incyte, was on an advisory board for Cassiopea, was an investigator and consultant for Pfizer and Sun Pharmaceuticals, was on an advisory board for Verrica, is an editor‐in‐chief for Pediatric Dermatology, and was an author for UpToDate. Moise L. Levy, MD was on an advisory board and consultant for Cassiopea, Regeneron, and UCB, was an investigator for Fibrocell, Galderma, Janssen, and Pfizer, was on a DSMB for Novan, and was a section editor and author for UpToDate. Latanya T. Benjamin, MD was a consultant for Dove, was on a speakers bureau for Regeneron and Sanofi Genzyme, was on an advisory board and consultant for Mustela, CeraVe, and La Roche‐Posay, was on an advisory board for Bioderma, Cutanea, Biofrontera, Menlo Therapeutics, and Pfizer. Anna L. Bruckner, MD was a investigator for Mayne Pharma. Keith Choate, MD, PhD was on a advisory board and speaker for Abbvie and Janssen, was a consultant and investigator for Mayne Pharma, was a consultant for Timber Pharmaceuticals, and was an author for UpToDate. Brittany G. Craiglow, MD was on a advisory board and consultant for Aclaris, was on an advisory board for Arena Pharmaceuticals, was a consultant for Concert Pharmaceuticals, was on an advisory board, consultant, and speaker for Pfizer, and was a speaker for Regeneron and Sanofi‐Genzyme. Lawrence F. Eichenfield, MD was an investigator and consultant for Abbvie, was on an advisory board for Cassiopea, was an investigator and consultant for Galderma, Incyte, Pfizer, Regeneron, Sanofi‐Genzyme, and Verrica, and was an author for UpToDate. Nicole Stefanko, MD, John DiGiovanna, MD, Peter Elias, MD, Philip Fleckman, MD, Richard Alan Lewis, MD, Anne W. Lucky, MD, Leonard M. Milstone, MD, Sonali S. Patel, MD, Joyce Teng, MD, PhD, Lauren Thaxton MD, MBA, MSBS, and Mary L. Williams, MD: None. Leslie Potter Lawley, MD was an author for UpToDate. Erin F. Mathes, MD was a consultant for Aldeyra Therapeutics and Rodan + Fields, was an author for UpToDate, and was a consultant for Pierre Fabre Amy S. Paller, MD was an investigator in AbbVie, Anaptysbio, Celgene, Eli Lilly, Incyte, and Janssen, was a consultant for Almirall, Amgen, Asana, Boehringer‐Ingelheim, Castle Creek, Dermavant, Dermira, Exicure, Lenus, MEDA Corp, Meiji Seika, Novan, Pfizer, Sanofi‐Genzyme, and Sol Gel, was an investigator and consultantfor Galderma, Leo, Novartis, and Regeneron. Dawn H. Siegel, MD was a consultant ifor Arqule, was an author for UpToDate, and was an investigator for SUN. Sherry A. Tanumihardjo, PhD was on an expert panel for the World Health Organization.
DISCLAIMER
The Work Group acknowledges that the ichthyoses and other disorders of cornification are a heterogeneous group of disorders with widely varying prevalence and pathogenetic mechanisms. Clinically, the severity of cutaneous expression, systemic associations, and comorbidities are also disparate. High quality, randomized clinical trials are not available. Thus, these recommendations are made using best available data and the collective clinical experience of the Work Group. These recommendations should not be applied to any patient without careful individualization of the therapeutic plan based on specific patient information and thoughtful provider‐patient discussion of potential risks and benefits.
ACKNOWLEDGMENTS
The Work Group would like to thank all of our patients with ichthyosis who inspire our desire to understand safe and effective therapies. We would also like to acknowledge Melissa Butt, MPh (Penn State Health) for research support and RedCap expertise and Katherine Devenport (PeDRA) for her administrative support.
Appendix I.
DELPHI PROCESS
The Delphi process is an iterative, structured methodology used to gain consensus from groups of experts on complex topics: 128
Round 1: Either the relevant individuals are invited to provide opinions on a specific matter, based on their knowledge and experience, or the team undertaking the Delphi expresses opinions on a specific matter and selects suitable experts to participate in subsequent questionnaire rounds; these opinions are grouped together under a limited number of headings and statements drafted for circulation to all participants on a questionnaire;
Round 2: Participants rank their agreement with each statement in the questionnaire; the rankings are summarized and included in a repeat version of the questionnaire;
Round 3: Participants re‐rank their agreement with each statement in the questionnaire, with the opportunity to change their score in view of the group's response; the re‐rankings are summarized and assessed for degree of consensus: if an acceptable degree of consensus is obtained the process may cease, with final results fed back to participants; if not, the third round is repeated.
Appendix II.
Workgroup Consensus Flowchart
Zaenglein AL, Levy ML, Stefanko NS, et al; PeDRA Use of Retinoids in Ichthyosis Work Group . Consensus recommendations for the use of retinoids in ichthyosis and other disorders of cornification in children and adolescents. Pediatr Dermatol 2021;38:164–180. 10.1111/pde.14408
Funding information
This project was funded by unrestricted educational grants from Sun Pharmaceuticals and the Foundation for Ichthyosis and Related Skin Types (FIRST). Administrative support was provided by the Pediatric Dermatology Research Alliance (PeDRA).
REFERENCES
- 1. Dreyfus I, Bourrat E, Maruani A, et al. Factors associated with impaired quality of life in adult patients suffering from ichthyosis. Acta Derm Venereol. 2014;94(3):344‐346. 10.2340/00015555-1710 [DOI] [PubMed] [Google Scholar]
- 2. Styperek AR, Rice ZP, Kamalpour L, et al. Annual direct and indirect health costs of the congenital ichthyoses. Pediatr Dermatol. 2010;27(4):325‐336. 10.1111/j.1525-1470.2010.01160.x [DOI] [PubMed] [Google Scholar]
- 3. Digiovanna JJ, Mauro T, Milstone LM, Schmuth M, Toro JR. Systemic retinoids in the management of ichthyoses and related skin types. Dermatol Ther. 2013;26(1):26‐38. 10.1111/j.1529-8019.2012.01527.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mazereeuw‐Hautier J, Vahlquist A, Traupe H, et al. Management of congenital ichthyoses: European guidelines of care, part one. Br J Dermatol. 2019;180(2):272‐281. 10.1111/bjd.17203. [DOI] [PubMed] [Google Scholar]
- 5. Mazereeuw‐Hautier J, Hernández‐Martín A, O'Toole EA, et al. Management of congenital ichthyoses: European guidelines of care, part two. Br J Dermatol. 2019;180(3):484‐495. 10.1111/bjd.16882 [DOI] [PubMed] [Google Scholar]
- 6. DiGiovanna JJ. Systemic retinoid therapy. Dermatol Clin. 2001;19(1):161‐167. 10.1016/s0733-8635(05)70237-0 [DOI] [PubMed] [Google Scholar]
- 7. Eichner R, Kahn M, Capetola RJ, Gendimenico GJ, Mezick JA. Effects of topical retinoids on cytoskeletal proteins: implications for retinoid effects on epidermal differentiation. J Invest Dermatol. 1992;98(2):154‐161. 10.1111/1523-1747.ep12555767 [DOI] [PubMed] [Google Scholar]
- 8. Onnis G, Chiaverini C, Hickman G, et al. Alitretinoin reduces erythema in inherited ichthyosis. Orphanet J Rare Dis. 2018;13(1):46. 10.1186/s13023-018-0783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanson B, Becker L, Hook K, Polcari I, Areaux RG, Maguiness S. Ectropion improvement with topical tazarotene in children with lamellar ichthyosis. Pediatr Dermatol. 2017;34(5):584‐589. 10.1111/pde.13240 [DOI] [PubMed] [Google Scholar]
- 10. Hofmann B, Stege H, Ruzicka T, Lehmann P. Effect of topical tazarotene in the treatment of congenital ichthyoses. Br J Dermatol. 1999;141(4):642‐646. 10.1046/j.1365-2133.1999.03101.x [DOI] [PubMed] [Google Scholar]
- 11. Arjona‐Aguilera C, Albarrán‐Planelles C, Jiménez‐Gallo D. Treatment of harlequin ichthyosis with acitretin. Actas Dermosifiliogr. 2015;106(9):759. 10.1016/j.ad.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 12. Chang LM, Reyes M. A case of harlequin ichthyosis treated with isotretinoin. Dermatol Online J. 2014. ;20(2):doj_21540. [PubMed] [Google Scholar]
- 13. Giam YC. Etretinate treatment for harlequin baby. J Singapore Paediatr Soc. 1992;34(3–4):217‐219. [PubMed] [Google Scholar]
- 14. Singh S, Bhura M, Maheshwari A, Kumar A, Singh CP, Pandey SS. Successful treatment of harlequin ichthyosis with acitretin. Int J Dermatol. 2001;40(7):472‐473. 10.1046/j.1365-4362.2001.01173.x [DOI] [PubMed] [Google Scholar]
- 15. Pejaver RK, Prasad RS, Garg AK, Jelly A, Shawkat S. Etretinate in the management of harlequin siblings. Indian J Pediatr. 1998;65(2):320‐323. 10.1007/bf02752311. [DOI] [PubMed] [Google Scholar]
- 16. Damodaran K, Bhutada A, Rastogi S. A unique preparation and delivery method for acitretin for neonatal harlequin ichthyosis. J Pediatr Pharmacol Ther. 2018;23(2):164‐167. 10.5863/1551-6776-23.2.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh M, Kaur M, Kaur R, Singh S. Severe ectropion in lamellar ichthyosis managed medically with oral acitretin. Pediatr Dermatol. 2018;35(2):e117‐e120. 10.1111/pde.13410 [DOI] [PubMed] [Google Scholar]
- 18. Behera B, Chandrashekar L, Singh N, Thappa DM, Gochhait D. Lamellar ichthyosis associated bilateral pseudoainhum of fingers and toes successfully treated with tazarotene. Dermatol Ther. 2017;30(5): 10.1111/dth.12516.Epub [DOI] [PubMed] [Google Scholar]
- 19. Vahlquist A, Blockhuys S, Steijlen P, et al. Oral liarozole in the treatment of patients with moderate/severe lamellar ichthyosis: results of a randomized, double‐blind, multinational, placebo‐controlled phase II/III trial. Br J Dermatol. 2014;170(1):173‐181. 10.1111/bjd.12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kundu RV, Garg A, Worobec SM. Lamellar ichthyosis treated with tazarotene 0.1% gel. J Am Acad Dermatol. 2006;55(5 Suppl):S94‐95. 10.1016/j.jaad.2005.09.018 [DOI] [PubMed] [Google Scholar]
- 21. Marulli GC, Campione E, Chimenti MS, Terrinoni A, Melino G, Bianchi L. Type I lamellar ichthyosis improved by tazarotene 0.1% gel. Clin Exp Dermatol. 2003;28(4):391‐393. [DOI] [PubMed] [Google Scholar]
- 22. Hashimoto K, Gee S, Tanaka K. Lamellar ichthyosis: response to etretinate with transglutaminase 1 recovery. Am J Dermatopathol. 2000;22(3):277‐280. 10.1097/00000372-200006000-00014 [DOI] [PubMed] [Google Scholar]
- 23. Steijlen PM, Van Dooren‐Greebe RJ, Van de Kerkhof PC. Acitretin in the treatment of lamellar ichthyosis. Br J Dermatol. 1994;130(2):211‐214. 10.1111/j.1365-2133.1994.tb02902.x [DOI] [PubMed] [Google Scholar]
- 24. Mirrer E, McGuire J. Lamellar ichthyosis–response to retinoic acid (tretinoin). A case report. Arch Dermatol. 1970;102(5):548‐551. [PubMed] [Google Scholar]
- 25. Macbeth AE, Johnston GA. Twenty‐one years of oral retinoid therapy in siblings with nonbullous ichthyosiform erythroderma. Clin Exp Dermatol. 2008;33(2):190‐191. 10.1111/j.1365-2230.2007.02531.x [DOI] [PubMed] [Google Scholar]
- 26. Eriksen L, Cormane RH. Oral retinoic acid as therapy for congenital ichthyosiform erythroderma. Br J Dermatol. 1975;92(3):343‐345. 10.1111/j.1365-2133.1975.tb03086.x [DOI] [PubMed] [Google Scholar]
- 27. Thomson J, Milne JA. The use of retinoic acid in congenital ichthyosiform erythroderma. Br J Dermatol. 1969;81(6):452‐455. 10.1111/j.1365-2133.1969.tb14017.x [DOI] [PubMed] [Google Scholar]
- 28. Bruckner‐Tuderman L, Sigg C, Geiger JM, Gilardi S. Acitretin in the symptomatic therapy for severe recessive x‐linked ichthyosis. Arch Dermatol. 1988;124(4):529‐532. [PubMed] [Google Scholar]
- 29. Khandpur S, Bhat R, Ramam M. Ichthyosis follicularis, alopecia and photophobia (IFAP) syndrome treated with acitretin. J Eur Acad Dermatol Venereol. 2005;19(6):759‐762. 10.1111/j.1468-3083.2005.01318.x [DOI] [PubMed] [Google Scholar]
- 30. Jagell S, Lidén S. Treatment of the ichthyosis of the Sjögren‐Larsson syndrome with etretinate (Tigason). Acta Derm Venereol. 1983;63(1):89‐91. [PubMed] [Google Scholar]
- 31. Vural S, Vural A, Akçimen F, et al. Clinical and molecular characterization and response to acitretin in three families with Sjögren‐Larsson syndrome. Int J Dermatol. 2018;57(7):843‐848. 10.1111/ijd.14013 [DOI] [PubMed] [Google Scholar]
- 32. Patel V, Sun G, Dickman M, Khuu P, Teng JM. Treatment of keratitis‐ichthyosis‐ deafness (KID) syndrome in children: a case report and review of the literature. Dermatol Ther. 2015;28(2):89‐93. 10.1111/dth.12192 [DOI] [PubMed] [Google Scholar]
- 33. Sahoo B, Handa S, Kaur I, Radotra BD, Kumar B. KID syndrome: response to acitretin. J Dermatol. 2002;29(8):499‐502. 10.1111/j.1346-8138.2002.tb00315.x [DOI] [PubMed] [Google Scholar]
- 34. Werchau S, Toberer F, Enk A, Helmbold P. Keratitis‐ichthyosis‐deafness syndrome: response to alitretinoin and review of literature. Arch Dermatol. 2011;147(8):993‐995. 10.1001/archdermatol.2011.216 [DOI] [PubMed] [Google Scholar]
- 35. Israeli S, Pessach Y, Sarig O, Goldberg I, Sprecher E. Beneficial effect of acitretin in Chanarin‐Dorfman syndrome. Clin Exp Dermatol. 2012;37(1):31‐33. 10.1111/j.1365-2230.2011.04164.x [DOI] [PubMed] [Google Scholar]
- 36. Nico MMS, Fernandes JD. Low‐dose isotretinoin prevents digital amputation in loricrin keratoderma (Vohwinkel syndrome with ichthyosis). J Dtsch Dermatol Ges. 2017;15(6):665‐667. 10.1111/ddg.13254 [DOI] [PubMed] [Google Scholar]
- 37. Zhang L, Hong Y, Zheng S, et al. Both low‐dose arotinoid ethylester and acitretin are effective in the treatment of familial erythrokeratodermia variabilis. Dermatol Ther. 2014;27(4):240‐243. 10.1111/dth.12127 [DOI] [PubMed] [Google Scholar]
- 38. Singh N, Thappa DM. Erythrokeratoderma variabilis responding to low‐dose isotretinoin. Pediatr Dermatol. 2010;27(1):111‐113. 10.1111/j.1525-1470.2009.01044.x [DOI] [PubMed] [Google Scholar]
- 39. Rappaport IP, Goldes JA, Goltz RW. Erythrokeratodermia variabilis treated with isotretinoin. A clinical, histologic, and ultrastructural study. Arch Dermatol. 1986;122(4):441‐445. [PubMed] [Google Scholar]
- 40. Graham‐Brown RA, Chave TA. Acitretin for erythrokeratodermia variabilis in a 9‐year‐old girl. Pediatr Dermatol. 2002;19(6):510‐512. 10.1046/j.1525-1470.2002.00221.x [DOI] [PubMed] [Google Scholar]
- 41. Balci DD, Yaldiz M. Erythrokeratodermia variabilis: successful palliative treatment with acitretin. Indian J Dermatol Venereol Leprol. 2008;74(6):649‐650. 10.4103/0378-6323.45114 [DOI] [PubMed] [Google Scholar]
- 42. Guerra L, Diociaiuti A, El Hachem M, Castiglia D, Zambruno G. Ichthyosis with confetti: clinics, molecular genetics and management. Orphanet J Rare Dis. 2015;10:115. 10.1186/s13023-015-0336-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vahlquist A, Pontén F, Pettersson A. Keratosis linearis with ichthyosis congenita and sclerosing keratoderma (KLICK‐syndrome): a rare, autosomal recessive disorder of keratohyaline formation? Acta Derm Venereol. 1997;77(3):225‐227. 10.2340/0001555577225227 [DOI] [PubMed] [Google Scholar]
- 44. Aksoy H, Cinar L, Acmaz G, et al. The effect of isotretinoin on ovarian reserve based on hormonal parameters, ovarian volume, and antral follicle count in women with acne. Gynecol Obstet Invest. 2015;79(2):78‐82. 10.1159/000371551 [DOI] [PubMed] [Google Scholar]
- 45. Ng E, Hale CS, Meehan SA, Cohen DE. Netherton syndrome with ichthyosis linearis circumflexa and trichorrhexis invaginatum. Dermatol Online J. 2014;20(12):13030/qt7m95t6v6. [PubMed] [Google Scholar]
- 46. Hartschuh W, Hausser I, Petzoldt D. Successful retinoid therapy of Netherton syndrome. Hautarzt. 1989;40(7):430‐433. [PubMed] [Google Scholar]
- 47. Lazaridou E, Apalla Z, Patsatsi A, Trigoni A, Ioannides D. Netherton's syndrome: successful treatment with isotretinoin. J Eur Acad Dermatol Venereol. 2009;23(2):210‐212. 10.1111/j.1468-3083.2008.02795.x [DOI] [PubMed] [Google Scholar]
- 48. Liu RH, Becker B, Gunkel J, Teng J. Rapid improvement in digital ischemia and acral contracture in a collodion baby treated with topical tazarotene. J Drugs Dermatol. 2010;9(6):713‐716. [PubMed] [Google Scholar]
- 49. Muller SA, Belcher RW, Esterly NB, et al. Keratinizing dermatoses. Combined data from four centers on short‐term topical treatment with tretinoin. Arch Dermatol. 1977;113(8):1052‐1054. 10.1001/archderm.113.8.1052 [DOI] [PubMed] [Google Scholar]
- 50. Thomas JR, Doyle JA. The therapeutic uses of topical vitamin A acid. J Am Acad Dermatol. 1981;4(5):505‐513. 10.1016/s0190-9622(81)70049-5 [DOI] [PubMed] [Google Scholar]
- 51. Ogawa M, Akiyama M. Successful topical adapalene treatment for the facial lesions of an adolescent case of epidermolytic ichthyosis. J Am Acad Dermatol. 2014;71(3):e103‐105. 10.1016/j.jaad.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 52. Cotellessa C, Cuevas‐Covarrubias SA, Valeri P, Fargnoli MC, Peris K. Topical tazarotene 0.05% versus glycolic acid 70% treatment in X‐linked ichthyosis due to extensive deletion of the STS gene. Acta Derm Venereol. 2005;85(4):346‐348. 10.1080/00015550510026613 [DOI] [PubMed] [Google Scholar]
- 53. Nassif PW, Nakandakari S, Fogagnolo L, Contin LA, Alves CJ. Epidermolytic hyperkeratosis: a follow‐up of 23 years of use of systemic retinoids. An Bras Dermatol. 2011;86(4 Suppl 1):S72‐75. 10.1590/s0365-05962011000700018 [DOI] [PubMed] [Google Scholar]
- 54. Liang J, Chen P, Chen H, et al. Long‐term safety and efficacy of continuous acitretin monotherapy for three children with different severe hyperkeratotic disorders in China. J Dermatol. 2018;45(8):1003‐1008. 10.1111/1346-8138.14462 [DOI] [PubMed] [Google Scholar]
- 55. Yoo S, Simzar S, Han K, Takahashi S, Cotliar R. Erythrokeratoderma variabilis successfully treated with topical tazarotene. Pediatr Dermatol. 2006;23(4):382‐385. 10.1111/j.1525-1470.2006.00252.x [DOI] [PubMed] [Google Scholar]
- 56. Nguyen V, Cunningham BB, Eichenfield LF, Alió AB, Buka RL. Treatment of ichthyosiform diseases with topically applied tazarotene: risk of systemic absorption. J Am Acad Dermatol. 2007;57(5 Suppl):S123‐125. 10.1016/j.jaad.2006.05.028 [DOI] [PubMed] [Google Scholar]
- 57. Buchan P, Eckhoff C, Caron D, Nau H, Shroot B, Schaefer H. Repeated topical administration of all‐trans‐retinoic acid and plasma levels of retinoic acids in humans. J Am Acad Dermatol. 1994;30(3):428‐434. [DOI] [PubMed] [Google Scholar]
- 58. Kaplan YC, Ozsarfati J, Etwel F, Nickel C, Nulman I, Koren G. Pregnancy outcomes following first‐trimester exposure to topical retinoids: a systematic review and meta‐analysis. Br J Dermatol. 2015;173(5):1132‐1141. 10.1111/bjd.14053 [DOI] [PubMed] [Google Scholar]
- 59. Baden HP, Buxman MM, Weinstein GD, Yoder FW. Treatment of ichthyosis with isotretinoin. J Am Acad Dermatol. 1982;6(4 Pt 2 Suppl):716‐720. 10.1016/s0190-9622(82)70062-3. [DOI] [PubMed] [Google Scholar]
- 60. Bergfeld WF, Derbes VJ, Elias PM, Frost P, Greer KE, Shupack JL. The treatment of keratosis palmaris et plantaris with isotretinoin. A multicenter study. J Am Acad Dermatol. 1982;6(4 Pt 2 Suppl):727‐731. 10.1016/s0190-9622(82)80053-4. [DOI] [PubMed] [Google Scholar]
- 61. Dicken CH, Bauer EA, Hazen PG, et al. Isotretinoin treatment of Darier's disease. J Am Acad Dermatol. 1982;6(4 Pt 2 Suppl):721‐726. 10.1016/s0190-9622(82)80052-2. [DOI] [PubMed] [Google Scholar]
- 62. Windhorst DB. The use of isotretinoin in disorders of keratinization. J Am Acad Dermatol. 1982;6(4 Pt 2 Suppl):708‐709. 10.1016/s0190-9622(82)80051-0. [DOI] [PubMed] [Google Scholar]
- 63. DiGiovanna JJ. Isotretinoin effects on bone. J Am Acad Dermatol. 2001;45(5):S176‐182. 10.1067/mjd.2001.113721 [DOI] [PubMed] [Google Scholar]
- 64. Lee E, Koo J. Single‐center retrospective study of long‐term use of low‐dose acitretin (Soriatane) for psoriasis. J Dermatolog Treat. 2004;15(1):8‐13. 10.1080/095466303100184473 [DOI] [PubMed] [Google Scholar]
- 65. Standeven AM, Davies PJ, Chandraratna RA, Mader DR, Johnson AT, Thomazy VA. Retinoid‐induced epiphyseal plate closure in guinea pigs. Fundam Appl Toxicol. 1996;34(1):91‐98. 10.1006/faat.1996.0179 [DOI] [PubMed] [Google Scholar]
- 66. Duvalyan A, Cha A, Goodarzian F, Arkader A, Villablanca JG, Marachelian A. Premature epiphyseal growth plate arrest after isotretinoin therapy for high‐risk neuroblastoma: a case series and review of the literature. Pediatr Blood Cancer. 2020;67(8):e28236. 10.1002/pbc.28236 [DOI] [PubMed] [Google Scholar]
- 67. Prendiville J, Bingham EA, Burrows D. Premature epiphyseal closure–a complication of etretinate therapy in children. J Am Acad Dermatol. 1986;15(6):1259‐1262. 10.1016/s0190-9622(86)70300-9 [DOI] [PubMed] [Google Scholar]
- 68. Rajpopat S, Moss C, Mellerio J, et al. Harlequin ichthyosis: a review of clinical and molecular findings in 45 cases. Arch Dermatol. 2011;147(6):681‐686. 10.1001/archdermatol.2011.9 [DOI] [PubMed] [Google Scholar]
- 69. Milstone LM, Choate KA. Improving outcomes for harlequin ichthyosis. J Am Acad Dermatol. 2013;69(5):808‐809. 10.1016/j.jaad.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 70. Windhorst DB, Nigra T. General clinical toxicology of oral retinoids. J Am Acad Dermatol. 1982;6(4 Pt 2 Suppl):675‐682. 10.1016/s0190-9622(82)70056-8. [DOI] [PubMed] [Google Scholar]
- 71. DiGiovanna JJ, Peck GL. Oral synthetic retinoid treatment in children. Pediatr Dermatol. 1983;1(1):77‐88. 10.1111/j.1525-1470.1983.tb01096.x [DOI] [PubMed] [Google Scholar]
- 72. Lawson JP, McGuire J. The spectrum of skeletal changes associated with long‐term administration of 13‐cis‐retinoic acid. Skeletal Radiol. 1987;16(2):91‐97. 10.1007/bf00367754 [DOI] [PubMed] [Google Scholar]
- 73. Milstone LM, McGuire J, Ablow RC. Premature epiphyseal closure in a child receiving oral 13‐cis‐retinoic acid. J Am Acad Dermatol. 1982;7(5):663‐666. 10.1016/s0190-9622(82)70148-3 [DOI] [PubMed] [Google Scholar]
- 74. Steineck A, MacKenzie JD, Twist CJ. Premature physeal closure following 13‐cis‐retinoic acid and prolonged fenretinide administration in neuroblastoma. Pediatr Blood Cancer. 2016;63(11):2050‐2053. 10.1002/pbc.26124 [DOI] [PubMed] [Google Scholar]
- 75. Nishimura G, Mugishima H, Hirao J, Yamato M. Generalized metaphyseal modification with cone‐shaped epiphyses following long‐term administration of 13‐cis‐retinoic acid. Eur J Pediatr. 1997;156(6):432‐435. 10.1007/s004310050631 [DOI] [PubMed] [Google Scholar]
- 76. Marini JC, Hill S, Zasloff MA. Dense metaphyseal bands and growth arrest associated with isotretinoin therapy. Am J Dis Child. 1988;142(3):316‐618. 10.1001/archpedi.1988.02150030090029 [DOI] [PubMed] [Google Scholar]
- 77. DiGiovanna JJ, Helfgott RK, Gerber LH, Peck GL. Extraspinal tendon and ligament calcification associated with long‐term therapy with etretinate. N Engl J Med. 1986;315(19):1177‐1182. 10.1056/NEJM198611063151901 [DOI] [PubMed] [Google Scholar]
- 78. Gerber LH, Helfgott RK, Gross EG, Hicks JE, Ellenberg SS, Peck GL. Vertebral abnormalities associated with synthetic retinoid use. J Am Acad Dermatol. 1984;10(5 Pt 1):817‐823. 10.1016/s0190-9622(84)70097-1 [DOI] [PubMed] [Google Scholar]
- 79. Nathan M, Pope MH, Grobler LJ. Osteophyte formation in the vertebral column: a review of the etiologic factors–Part II. Contemp Orthop. 1994;29(2):113‐119. [PubMed] [Google Scholar]
- 80. Jones MD, Pais MJ, Omiya B. Bony overgrowths and abnormal calcifications about the spine. Radiol Clin North Am. 1988;26(6):1213‐1234. [PubMed] [Google Scholar]
- 81. Tangrea JA, Kilcoyne RF, Taylor PR, et al. Skeletal hyperostosis in patients receiving chronic, very‐low‐dose isotretinoin. Arch Dermatol. 1992;128(7):921‐925. 10.1001/archderm.1992.01680170053004 [DOI] [PubMed] [Google Scholar]
- 82. Tangrea JA, Taylor PR, Hartman AM, et al. Isotretinoin and the axial skeleton. Lancet. 1992;340(8817):495‐496. 10.1016/0140-6736(92)91825-s [DOI] [PubMed] [Google Scholar]
- 83. Sillevis Smitt JH, de Mari F. A serious side‐effect of etretinate (Tigason). Clin Exp Dermatol. 1984;9(6):554‐556. 10.1111/j.1365-2230.1984.tb00858.x [DOI] [PubMed] [Google Scholar]
- 84. Tosti A, Albisinni U, Bettoli V, Merlini L, Lama L. Ossification of the posterior longitudinal ligament associated with etretinate therapy. Dermatologica. 1987;175(5):257‐258. 10.1159/000248915 [DOI] [PubMed] [Google Scholar]
- 85. Zabarino P, Krause E, Gary‐Bobo A, Dandurand M, Sany J, Guilhou JJ. Ossification of the antibrachial interosseous membrane caused by etretinate. Ann Dermatol Venereol. 1989;116(2):123‐126. [PubMed] [Google Scholar]
- 86. Urrutia J, Bono CM. Long‐term results of surgical treatment of dysphagia secondary to cervical diffuse idiopathic skeletal hyperostosis. Spine J. 2009;9(9):e13‐137. 10.1016/j.spinee.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 87. Leachman SA, Insogna KL, Katz L, Ellison A, Milstone LM. Bone densities in patients receiving isotretinoin for cystic acne. Arch Dermatol. 1999;135(8):961‐965. 10.1001/archderm.135.8.961 [DOI] [PubMed] [Google Scholar]
- 88. Hoover KB, Miller CG, Galante NC, Langman CB. A double‐blind, randomized, Phase III, multicenter study in 358 pediatric subjects receiving isotretinoin therapy demonstrates no effect on pediatric bone mineral density. Osteoporos Int. 2015;26(10):2441‐2447. 10.1007/s00198-015-3158-2 [DOI] [PubMed] [Google Scholar]
- 89. Vestergaard P, Rejnmark L, Mosekilde L. High‐dose treatment with vitamin A analogues and risk of fractures. Arch Dermatol. 2010;146(5):478‐482. 10.1001/archdermatol.2010.59 [DOI] [PubMed] [Google Scholar]
- 90. Craiglow BG, Choate KA, Milstone LM. Topical tazarotene for the treatment of ectropion in ichthyosis. JAMA Dermatol. 2013;149(5):598‐600. 10.1001/jamadermatol.2013.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fraunfelder FT, Fraunfelder FW, Edwards R. Ocular side effects possibly associated with isotretinoin usage. Am J Ophthalmol. 2001;132(3):299‐305. [DOI] [PubMed] [Google Scholar]
- 92. Neudorfer M, Goldshtein I, Shamai‐Lubovitz O, Chodick G, Dadon Y, Shalev V. Ocular adverse effects of systemic treatment with isotretinoin. Arch Dermatol. 2012;148(7):803‐808. 10.1001/archdermatol.2012.352 [DOI] [PubMed] [Google Scholar]
- 93. Cumurcu T, Sezer E, Kilic R, Bulut Y. Comparison of dose‐related ocular side effects during systemic isotretinoin administration. Eur J Ophthalmol. 2009;19(2):196‐200. 10.1177/112067210901900204 [DOI] [PubMed] [Google Scholar]
- 94. Aragona P, Cannavò SP, Borgia F, Guarneri F. Utility of studying the ocular surface in patients with acne vulgaris treated with oral isotretinoin: a randomized controlled trial. Br J Dermatol. 2005;152(3):576‐578. 10.1111/j.1365-2133.2005.06389.x [DOI] [PubMed] [Google Scholar]
- 95. Karalezli A, Borazan M, Altinors DD, Dursun R, Kiyici H, Akova YA. Conjunctival impression cytology, ocular surface, and tear‐film changes in patients treated with systemic isotretinoin. Cornea. 2009;28(1):46‐50. 10.1097/ICO.0b013e318183a396 [DOI] [PubMed] [Google Scholar]
- 96. Mathers WD, Shields WJ, Sachdev MS, Petroll WM, Jester JV. Meibomian gland morphology and tear osmolarity: changes with Accutane therapy. Cornea. 1991;10(4):286‐290. 10.1097/00003226-199107000-00002 [DOI] [PubMed] [Google Scholar]
- 97. Santodomingo‐Rubido J, Barrado‐Navascués E, Rubido‐Crespo MJ. Drug‐induced ocular side‐effects with isotretinoin. Ophthalmic Physiol Opt. 2008;28(5):497‐501. 10.1111/j.1475-1313.2008.00590.x [DOI] [PubMed] [Google Scholar]
- 98. Yuksel N, Ozer MD, Akcay E, Ozen U, Uzun S. Reduced central corneal thickness in patients with isotretinoin treatment. Cutan Ocul Toxicol. 2015;34(4):318‐321. 10.3109/15569527.2014.990155 [DOI] [PubMed] [Google Scholar]
- 99. Ellies P, Dighiero P, Legeais JM, Pouliquen YJ, Renard G. Persistent corneal opacity after oral isotretinoin therapy for acne. Cornea. 2000;19(2):238‐239. 10.1097/00003226-200003000-00020 [DOI] [PubMed] [Google Scholar]
- 100. Weiss J, Degnan M, Leupold R, Lumpkin LR. Bilateral corneal opacities. Occurrence in a patient treated with oral isotretinoin. Arch Dermatol. 1981;117(3):182‐183. 10.1001/archderm.117.3.182 [DOI] [PubMed] [Google Scholar]
- 101. Bergler‐Czop B, Bilewicz‐Stebel M, Stańkowska A, Bilewicz‐Wyrozumska T. Side effects of retinoid therapy on the quality of vision. Acta Pharm. 2016;66(4):471‐478. 10.1515/acph-2016-0039 [DOI] [PubMed] [Google Scholar]
- 102. Lerman S. Ocular side effects of accutane therapy. Lens Eye Toxic Res. 1992;9(3–4):429‐438. [PubMed] [Google Scholar]
- 103. Sekeryapan B, Dılek N, Oner V, Turkyılmaz K, Aslan MG. Retinal nerve fiber layer and ganglion cell layer thickness in patients receiving systemic isotretinoin therapy. Int Ophthalmol. 2013;33(5):481‐484. 10.1007/s10792-013-9726-6 [DOI] [PubMed] [Google Scholar]
- 104. Gold JA, Shupack JL, Nemec MA. Ocular side effects of the retinoids. Int J Dermatol. 1989;28(4):218‐225. 10.1111/j.1365-4362.1989.tb04806.x [DOI] [PubMed] [Google Scholar]
- 105. Roenigk HH, Callen JP, Guzzo CA, et al. Effects of acitretin on the liver. J Am Acad Dermatol. 1999;41(4):584‐588. [PubMed] [Google Scholar]
- 106. Chularojanamontri L, Silpa‐Archa N, Wongpraparut C, Limphoka P. Long‐term safety and drug survival of acitretin in psoriasis: a retrospective observational study. Int J Dermatol. 2019;58(5):593‐599. 10.1111/ijd.14349 [DOI] [PubMed] [Google Scholar]
- 107. Di Lernia V, Bonamonte D, Lasagni C, et al. Effectiveness and safety of acitretin in children with plaque psoriasis: a multicenter retrospective analysis. Pediatr Dermatol. 2016;33(5):530‐535. 10.1111/pde.12940 [DOI] [PubMed] [Google Scholar]
- 108. Tollefson MM, Van Houten HK, Asante D, Yao X, Maradit KH. Association of psoriasis with comorbidity development in children with psoriasis. JAMA Dermatol. 2018;154(3):286‐292. 10.1001/jamadermatol.2017.5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Malik K, He H, Huynh TN, et al. Ichthyosis molecular fingerprinting shows profound T(H)17 skewing and a unique barrier genomic signature. J Allergy Clin Immunol. 2019;143(2):604‐618. 10.1016/j.jaci.2018.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Katugampola RP, Finlay AY. Oral retinoid therapy for disorders of keratinization: single‐centre retrospective 25 years' experience on 23 patients. Br J Dermatol. 2006;154(2):267‐276. 10.1111/j.1365-2133.2005.06906.x [DOI] [PubMed] [Google Scholar]
- 111. Lacour M, Mehta‐Nikhar B, Atherton DJ, Harper JI. An appraisal of acitretin therapy in children with inherited disorders of keratinization. Br J Dermatol. 1996;134(6):1023‐1029. 10.1046/j.1365-2133.1996.d01-895.x [DOI] [PubMed] [Google Scholar]
- 112. Daniels SR, Greer FR, Committee on Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122(1):198‐208. 10.1542/peds.2008-1349 [DOI] [PubMed] [Google Scholar]
- 113. de Ferranti SD, Steinberger J, Ameduri R, et al. Cardiovascular risk reduction in high‐risk pediatric ratients: a scientific statement from the American Heart Association. Circulation. 2019;139(13):e603‐e634. 10.1161/CIR.0000000000000618 [DOI] [PubMed] [Google Scholar]
- 114. National Institute of Mental Health . Depression. February 2019. https://www.nimh.nih.gov/health/statistics/major‐depression.shtml. Accessed January 2, 2020.
- 115. Jick SS, Kremers HM, Vasilakis‐Scaramozza C. Isotretinoin use and risk of depression, psychotic symptoms, suicide, and attempted suicide. Arch Dermatol. 2000;136(10):1231‐1236. 10.1001/archderm.136.10.1231 [DOI] [PubMed] [Google Scholar]
- 116. Halvorsen JA, Stern RS, Dalgard F, Thoresen M, Bjertness E, Lien L. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population‐based study. J Invest Dermatol. 2011;131(2):363‐370. 10.1038/jid.2010.264 [DOI] [PubMed] [Google Scholar]
- 117. Le Moigne M, Fournier JP, Bulteau S, et al. Psychiatric disorders with systemic retinoids: a systematic review of case reports. Br J Dermatol. 2018;178(1):278‐280. 10.1111/bjd.15745 [DOI] [PubMed] [Google Scholar]
- 118. Azoulay L, Blais L, Koren G, LeLorier J, Berard A. Isotretinoin and the risk of depression in patients with acne vulgaris: a case‐crossover study. J Clin Psychiatry. 2008;69(4):526‐532. 10.4088/jcp.v69n0403 [DOI] [PubMed] [Google Scholar]
- 119. Le Moigne M, Bulteau S, Grall‐Bronnec M, et al. Psychiatric disorders, acne and systemic retinoids: comparison of risks. Expert Opin Drug Saf. 2017;1‐7: 10.1080/14740338.2017.1344641 [DOI] [PubMed] [Google Scholar]
- 120. Dreyfus I, Pauwels C, Bourrat E, et al. Burden of inherited ichthyosis: a French national survey. Acta Derm Venereol. 2015;95(3):326‐328. 10.2340/00015555-1955 [DOI] [PubMed] [Google Scholar]
- 121. Sun Q, Ren I, Zaki T, Maciejewski K, Choate K. Ichthyosis affects mental health in adults and children: a cross‐sectional study. J Am Acad Dermatol. 2020;83(3):951‐954. 10.1016/j.jaad.2020.01.052 [DOI] [PubMed] [Google Scholar]
- 122. Sundaram A, Vaughan B, Kost K, et al. Contraceptive failure in the United States: estimates from the 2006–2010 National Survey of Family Growth. Perspect Sex Reprod Health. 2017;49(1):7‐16. 10.1363/psrh.12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Brazzell RK, Colburn WA. Pharmacokinetics of the retinoids isotretinoin and etretinate. A comparative review. J Am Acad Dermatol. 1982;6(4 Pt 2 Suppl):643‐651. 10.1016/s0190-9622(82)70053-2. [DOI] [PubMed] [Google Scholar]
- 124. Depo‐provera‐medroxyprogesterone acetate injection, suspension. Pharmacia and Upjohn Company . 2017. https://www.pfizer.com/files/products/uspi_depo_provera_400mg.pdf. Accessed January 5, 2020.
- 125. iPLEDGE: Committed to Pregnancy Prevention . 2016. https://www.ipledgeprogram.com/iPledgeUI/aboutProgram. Accessed January 24, 2019.
- 126. Amnesteem‐ isotretinoin capsule . August 7, 2019. https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b2cb63c9‐f825‐4991‐9a2c‐6260f1bbcc2c. Accessed January 5, 2020.
- 127. Soriatane (acitretin) capsules . September 2017. https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Soriatane/pdf/SORIATANE‐PI‐MG.PDF. Accessed March 28, 2020.
- 128. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376‐380. 10.1136/bmj.311.7001.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient‐centered approach to grading evidence in the medical literature. J Am Board Fam Pract. 2004;17(1):59‐67. 10.3122/jabfm.17.1.59 [DOI] [PubMed] [Google Scholar]


