Abstract
Introduction
Exosomes are an emerging candidate for biomarkers of Alzheimer's disease (AD). This study investigated whether exosomal synaptic proteins can predict AD at the asymptomatic stage.
Methods
We conducted a two‐stage‐sectional study (discovery stage: AD, 28; amnestic mild cognitive impairment [aMCI], 25; controls, 29; validation stage: AD, 73; aMCI, 71; controls, 72), a study including preclinical AD (160) and controls (160), and a confirmation study in familial AD (mutation carriers: 59; non‐mutation carriers: 62).
Results
The concentrations of growth associated protein 43 (GAP43), neurogranin, synaptosome associated protein 25 (SNAP25), and synaptotagmin 1 were lower in AD than in controls (P < .001). Exosomal biomarker levels were correlated with those in cerebrospinal fluid (R2 = 0.54–0.70). The combination of exosomal biomarkers detected AD 5 to 7 years before cognitive impairment (area under the curve = 0.87–0.89).
Discussion
This study revealed that exosomal GAP43, neurogranin, SNAP25, and synaptotagmin 1 act as effective biomarkers for prediction of AD 5 to 7 years before cognitive impairment.
Keywords: Alzheimer's disease, biomarker, diagnosis, exosome, prediction, synaptic protein
1. INTRODUCTION
Alzheimer's disease (AD) is the most common type of dementia and is the leading cause of disability in people older than 65 years worldwide. 1 Effective treatments are therefore urgently needed for AD. However, clinical trials treating AD after the onset of cognitive impairment face huge challenges. 2 Substantial evidence has shown that the pathophysiological process of AD commences in clinically normal older individuals long before the onset of dementia. 3 The pathology of AD has a broad clinical spectrum: cognitively normal, mild cognitive impairment (MCI), and dementia; this suggests that if the treatment begins at an earlier stage, such as during MCI or even the cognitively normal stage, the onset of clinical symptoms can be delayed. 4 These strategies require supportive approaches to detect AD at the asymptomatic stage.
Synaptic dysfunction has been implicated in AD. 5 Mounting evidence suggests that amyloid beta (Aβ) and P‐tau, the pathological hallmarks of AD, cause the synaptic pathology, which occurs even in the asymptomatic stage. 6 , 7 For example, the brain tissues of patients with AD exhibit decreased levels of synaptic proteins, such as growth associated protein 43 (GAP43), neurogranin, synaptotagmins, Rab3A, synaptosome associated protein 25 (SNAP25), and neurogranin. 8 , 9 Moreover, the increased expressions of GAP43, 10 , 11 , 12 neurogranin, 13 SNAP25, 14 , 15 and synaptotagmin 1 16 have also been observed in the cerebrospinal fluid (CSF) of such patients, indicating their potential as biomarkers for AD. As synaptic damages occur in the asymptomatic stage of AD, the current study investigated whether synaptic proteins can serve as predictive factors for AD at the asymptomatic stage.
Exosomes are transport microparticles (30—100 nm) secreted by numerous cell types, including neurons. The small size of exosomes and their structural similarity with cells allows them to easily cross the blood‐brain barrier 17 and remove pathological proteins from the central nervous system. 18 , 19 Changes in exosomal Aβ and tau are reportedly characteristic to AD, 20 , 21 and we and others have demonstrated that neuronal‐derived exosomes are ideal candidates for AD biomarkers. 20 , 22 , 23 , 24 , 25 , 26 Although several synaptic proteins were reported to be altered in the CSF of patients with MCI, 10 , 11 , 12 , 13 , 14 , 15 , 16 it has not been fully understood whether changes of synaptic proteins in the blood can be detected in the preclinical stages of AD. Studies have reported that neuronal‐derived exosomes in patients with AD or preclinical AD exhibit a decreased level of several synaptic proteins, 27 suggesting that exosomal synaptic proteins may be promising biomarkers to predict AD even in the non‐symptomatic stage. However, the data presented by these studies included relatively few patients (nine preclinical AD patients), and were not validated by CSF analyses, limiting their translation to clinical application.
This study aimed to (1) explore the capacity of exosomal GAP43, neurogranin, SNAP25, and synaptotagmin 1 to aid in the diagnosis of AD and amnestic mild cognitive impairment (aMCI), (2) verify the exosomal biomarker results with data obtained from CSF samples and validate the results from an initial discovery experiment with those acquired from a subsequent validation stage with more samples, and (3) investigate the capacity of exosomal biomarkers to detect preclinical AD before cognitive impairment.
RESEARCH IN CONTEXT
Systematic review: We searched PubMed using the terms “Alzheimer's disease,” “synaptic protein,” “exosomes,” and “CSF” since January 1, 1990. However, whether exosomal synaptic proteins can be used to predict preclinical AD at the asymptomatic stage has not yet been fully addressed.
Interpretation: This study is the first to validate Alzheimer's disease (AD)‐induced changes in exosomal synaptic proteins in blood with concomitant cerebrospinal fluid (CSF) findings; it shows that the combination of exosomal growth associated protein 43 (GAP43), neurogranin, synaptosome associated protein 25 (SNAP25), and synaptotagmin 1 can detect preclinical AD 5 to 7 years before cognitive impairment. This study may provide opportunities to the early diagnosis of AD at the non‐symptomatic stage, and make early treatment of AD possible.
Future directions: The clinical application of exosomal GAP43, neurogranin, SNAP25, and synaptotagmin 1 for screening AD will be strengthened by prospective longitudinal studies. In addition, more samples in multiple international centers will provide powerful evidence before extensive clinic use.
2. METHODS
2.1. Subjects
Four datasets were acquired in this study (Figure 1A). Participants assessed in the discovery experiment were enrolled from a Beijing center (n = 82: 28 patients with AD, 25 patients with aMCI, and 29 healthy controls); those involved in the validation phase, conducted from September 2016 to July 2018, were recruited from other centers in the provinces of Shandong, Guizhou, Henan, Hebei, Jilin, Guangxi, and the Inner Mongolia Autonomous Region (n = 216: 73 patients with AD, 71 patients with aMCI, and 72 healthy controls). Other participants, who were confirmed as cognitively normal at baseline 5 to 7 years (year 2012 to 2014; Figure 1B) before the current study, were recruited from a longitudinal study. The blood of all participants was drawn at baseline and stored at ‐80°C so that exosomes could be collected from blood in the current study. As exosomal proteins are stable over a long period of storage (> 5 years), 28 the exosomal samples are believed to reflect the participants’ situation 5 to 7 years ago. The levels of Aβ42, P‐tau, and T‐tau proteins in CSF, and the cognitive function of all participants were measured by follow‐up in the current study. The normal controls and preclinical AD were included retrospectively using the following criteria. For controls: (1) normal cognitive function at baseline (5–7 years prior) and (2) normal cognitive function at the time of the current study initiation; for preclinical AD: (1) normal cognitive function at baseline (5–7 years prior) and (2) AD at the time of the current study initiation (meeting the AD diagnosis criteria).
FIGURE 1.
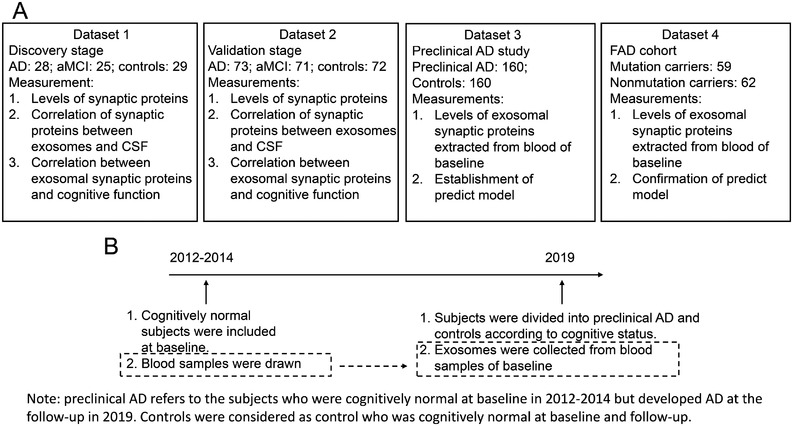
Four datasets were included in this study. A, Numbers of subjects, and measurements in four datasets. B, Cognitively normal subjects that were included from a longitudinal study between 2012 and 2014. The subjects were grouped into preclinical Alzheimer's disease and controls and followed up in the present study by measuring their cognitive function, and amyloid beta 42, P‐tau, and T‐tau in cerebrospinal fluid. The exosomes were extracted from the blood collected at baseline
In total, 320 subjects were included, of whom 160 subjects were cognitively normal controls, and 160 subjects were preclinical AD (Table 1). We aimed to assess whether exosomal synaptic proteins are different in the asymptomatic stage between preclinical AD and controls. If so, we determined that exosomal synaptic proteins can predict preclinical AD. In addition, participants from the familial Alzheimer's disease (FAD) cohort were also included, who are consanguineous members from families carrying mutations in the genes encoding amyloid beta precursor protein (APP), presenilin 1 (PSEN1), or presenilin 2 (PSEN2). The non‐mutation carriers of the family served as controls. It is believed that the onset age tends to be accordant for a given mutation; therefore, we can calculate an estimated years to symptom onset (EYO) for mutation carriers by the known onset age of individuals from the same family. 29 All FAD participants were included from The Chinese Familial Alzheimer's Disease Network, which has been partially published previously. 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 In this study, 62 controls and 59 mutation carriers, whose EYO were 5 to 7 years, were recruited from the FAD cohort (Table 1). Diagnoses of probable AD were performed according to the 2011 criteria of the National Institute on Aging and Alzheimer's Association; 39 and those of aMCI, according to the published criteria. 40 In addition, cutoff values of P‐tau/Aβ42 (0.14) and T‐tau/Aβ42 (0.67) were used to determine AD or normal controls, which were calculated from our published data, 21 and consistent with others. 41 Age‐matched individuals with normal psychological function and no comorbidities that could affect cognition were chosen as normal controls. All participants or their legal guardians were fully informed and signed written consent before their enrollment. The Institutional Review Board of Xuanwu Hospital, Capital Medical University, approved this study.
TABLE 1.
Characteristics of participants
| Characteristics of longitudinal dataset | |||||
|---|---|---|---|---|---|
| Baseline | Follow‐up | ||||
| Characteristic | Total Sample (n = 320) | Preclinical AD (n = 160) | Controls (n = 160) | Preclinical AD (n = 160) | Controls (n = 160) |
| Age, mean (SD), year | 65 (6) | 60 (4) | 60 (4) | 66 (5) | 65 (6) |
| Education, mean (SD), year | 10.6 (1.2) | 10.8 (1.2) | 10.4 (1.0) | 10.8 (1.2) | 10.4 (1.0) |
| Women, No. (%) | 176 (55) | 90 (51.1) | 86 (48.9) | 90 (51.1) | 86 (48.9) |
| APOE ε4 positive, No. (%) | 101 (31.6) | 72 (45) * | 29 (18.1) | 72 (45) * | 29 (18.1) |
| MMSE score, mean (SD) | 25.2 (1.6) (Follow‐up) | 29.2 (1.2) | 29.1 (1.2) | 20.7 (2.9) * | 29.1 (1.1) |
| CDR score of 0/0.5/1.0/2.0, No. | 153/51/85/31 (Follow‐up) | 157/3/0/0 | 157/3/0/0 | 0/44/85/31 * | 153/7/0/0 |
| CSF biomarkers (pg/ml) | (Follow‐up) | ||||
| Aβ42 | 532 (119) | 452 (122) * | 695 (151) | 376 (88) * | 687 (149) |
| total Tau | 474 (141) | 536 (162) * | 328 (90) | 612 (191) * | 336 (93) |
| P‐181‐Tau | 70 (19) | 71 (17) * | 46 (12) | 91 (25) * | 48 (12) |
| Characteristics of FAD dataset | |||||
|---|---|---|---|---|---|
| Characteristic | Non‐mutation (n = 62) | Mutation‐carriers (n = 59) | P values | ||
| Age, year | 48.5 (9.7) | 48.9 (9.5) | .819 | ||
| Women, No. (%) | 32 (51.6) | 30 (50.8) | .896 | ||
| Estimated year prior to onset | / | 6.2 (7.8) | / | ||
| MMSE | 29.9 (1.0) | 29.9 (0.8) | > .99 | ||
| CDR score of 0/0.5/1.0/2.0, No. | 61/1/0/0 | 58/1/0/0 | > .99 | ||
| Family mutation, n/total (%) | |||||
| PSEN1 | / | 43 (72.8) | / | ||
| PSEN2 | / | 4 (6.8) | / | ||
| APP | / | 12 (20.3) | / | ||
| CSF biomarkers (pg/ml) | |||||
| Aβ42 | 710 (160) | 389 (99) | < .001 | ||
| total Tau | 312 (91) | 602 (183) | < .001 | ||
| P‐181‐Tau | 48 (12) | 85 (23) | < .001 | ||
Abbreviations: AD, Alzheimer's disease; aMCI, amnestic mild cognitive impairment; APOE ε4, apolipoprotein ε4; APP, amyloid precursor protein; CDR, Clinical Dementia Rating; CSF, cerebrospinal fluid; FAD, familial Alzheimer's disease; MMSE, Mini‐Mental State Examination; PSEN, presenilin; SD, standard deviation.
P < .05 compared to controls
2.2. Collection of neuronal‐derived exosomes from the blood
Twenty milliliter blood samples were collected from all enrolled individuals in the morning after a 12‐hour fast and were kept in polypropylene tubes containing ethylene diamine tetraacetic acid. To obtain neuronal‐derived exosomes, the whole‐blood samples were immediately processed at the Beijing center (Xuanwu Hospital). At the other centers, the collected samples were immediately centrifuged at 4200 × g for 10 minutes at room temperature to obtain the plasma, which was then kept at 4°C and shipped in dry ice to the Beijing central laboratory within 12 hours. For the discovery (dataset 1) and validation (dataset 2) study, once the blood samples arrived at the Beijing center, they were immediately processed, and specific neuronal‐derived exosomes were isolated according to our published protocol. 21 For the preclinical AD (dataset 3) and FAD (dataset 4) study, blood samples were stored at –80°C until the collection of exosomes. In brief, using the ExoQuick exosome precipitation solution (EXOQ; System Biosciences, USA), total exosomes were collected from serum. Neuronal‐derived exosomes were then isolated by co‐immunoprecipitation using a mouse anti‐human neural cell adhesion molecule (NCAM) antibody, labeling with biotin by the EZ‐Link sulfo‐NHS‐biotin system (Thermo Fisher Scientific, USA).
2.3. Confirmation of neuronal‐derived exosomal collection
Transmission electron microscopy (TEM) and western blot were performed to confirm the success of exosomal collection, and L1 cell adhesion molecule (L1CAM) levels were measured to confirm the neuronal‐derived enrichment according to our previous protocols. 21
2.4. Collection of CSF
After the blood was drawn, CSF was immediately collected according to international guidelines. 42 Briefly, subjects were placed in the left lateral position when the lumbar puncture was performed. The L3‐L5 intervertebral disc spaces were chosen as the site of puncture. Fifteen‐milliliter samples of CSF were collected with 20‐gauge atraumatic needles and centrifugated at 2000 × g for 10 minutes at room temperature. The CSF samples were then stored in polypropylene tubes at –80°C. All the subjects were monitored for at least 12 hours after lumbar puncture.
2.5. Protein measurements
A pilot study was performed to determine the data ranges on an enzyme‐linked immunosorbent assay (ELISA). The concentrations of synaptotagmin 1 were found to be above the assay range, and the measured samples were therefore diluted accordingly in the following experiments. The mean value of the CD81 levels in each group was set to 1.00, and the relative values for each sample were used to normalize their recovery. 20 All the final data were within the assay ranges of ELISA kits (Table S1 in supporting information). All measurements were performed in a blinded manner.
2.6. Statistical analysis
Statistical analyses were performed using SPSS v.22 and Stata 13.0. The data from the discovery and validation stages were calculated independently. Group differences in categorical data, such as sex, clinical subgroups, and apolipoprotein ε4 (APOE ε4) carrier distributions, were analyzed using the χ2 test. Group differences in numerical data, such as the concentrations of biomarkers, were analyzed with Welch's t‐test or analyses of variance. The correlative analysis was performed using a linear regression model. In the discovery and validation stage, after the generation of an adjusted receiver operating characteristic (ROC) curve, the predicted values were calculated using a binary logistic regression model with age, sex, and APOE ε4 status as covariates. 43 For the preclinical AD and FAD dataset, the tolerance, variance inflation factor, eigenvalue, and condition index were calculated to examine the multicollinearity in the linear regression models. To avoid multicollinearity when establishing the predict models of synaptic proteins, ridge regression was performed in Stata 13.0 with elastic regress module. The age, sex, and APOE ε4 status was adjusted in the ridge regression. The dataset was randomly split into training dataset (0.67 of total) and test dataset (0.33 of total) using SPSS v.22. All tests were two‐tailed, and the level of significance was set to P < .05.
3. RESULTS
3.1. Participant characteristics
Recruited as part of a multicenter study, the participant characteristics in the discovery and validation stages have been described in a previous study (Table S2 in supporting information). 21 The participant characteristics in the preclinical AD dataset and the FAD dataset are shown in Table 1. The participants in each group were matched in terms of age, gender, APOE ε4 status, and education level.
3.2. Levels of GAP43, neurogranin, SNAP25, and synaptotagmin 1 in exosomes and CSF
The neuronal‐derived exosomes were confirmed by TEM, western blot, and L1CAM (Figure S1 in supporting information). A representative TEM image of the exosomes of a patient with AD depicts the exosomes (Figure S1A). Western blot analysis showed that Alix was expressed in the exosomal samples but not in the supernatants or negative controls (Figure S1B). The L1CAM content in the immunoprecipitated exosomes increased by approximately 10‐fold to that of non‐immunoprecipitated exosomes (Figure S1C). These data confirmed that neuronal‐derived exosomes were successfully collected.
We first measured the levels of CD9, CD63, and CD81 in all samples (Figure S2 in supporting information). No differences in CD9, CD63, and CD81 among AD, aMCI, and controls were found (all P > .05). The CD81 levels of each sample were used to normalize the subsequent exosomal measurements. We then measured the biomarkers in blood neuronal‐derived exosomes and the CSF in the discovery stage (Figure 2). The exosomal concentrations of GAP43, neurogranin, SNAP25, and synaptotagmin 1 in the AD group (1996 ± 515, 250 ± 67, 493 ± 144, and 302 ± 80 pg/ml, respectively) were significantly lower than those in the control group (2738 ± 724, 2010 ± 530, 634 ± 166, and 597 ± 151 pg/ml, respectively, P < .001); their concentrations in the aMCI group (2372 ± 450, 1567 ± 445, 575 ± 144, and 448 ± 117 pg/ml, respectively) were significantly higher than those in the AD group (P < .05 or 0.001) and significantly lower than those in the control group (P < .05; Figure 2A‐D).
FIGURE 2.
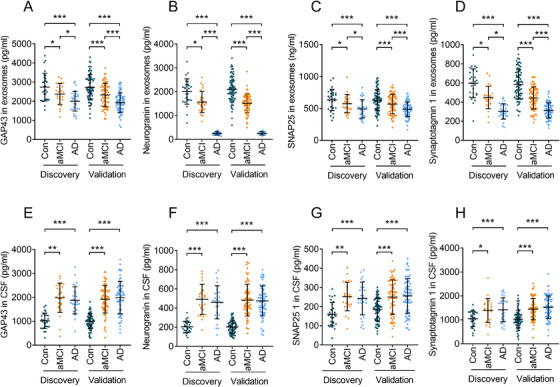
Biomarkers were measured in the discovery and validation datasets. Panels A—D show levels of neuronal‐derived exosomal growth associated protein 43 (GAP43; A), neurogranin (B), synaptosome associated protein 25 (SNAP25; C), and synaptotagmin 1 (D). Panels E–H show levels of cerebrospinal fluid (CSF) GAP43 (E), neurogranin (F), SNAP25 (G), and synaptotagmin 1 (H). In the discovery stage, n = 28 (Alzheimer's disease [AD]), 25 (amnestic mild cognitive impairment [aMCI]), and 29 (controls). In the validation stage, n = 73 (AD), 71 (aMCI), and 72 (controls). Abbreviation: Con, controls *** P < 0.001, ** P < 0.01, * P < 0.05
We further assessed these same measurements in the validation stage. The exosomal concentrations of GAP43, neurogranin, SNAP25, and synaptotagmin 1 in the AD group (1926 ± 509, 254 ± 69, 489 ± 114, and 312 ± 81 pg/ml, respectively) were significantly lower than those in the control group (2722 ± 664, 2099 ± 540, 628 ± 166, and 586 ± 153 pg/ml, respectively, P < .001); their concentrations in the aMCI group (2325 ± 606, 1511 ± 390, 569 ± 152, and 442 ± 115 pg/ml, respectively) were significantly higher than those in the AD group (P < .001) and significantly lower than those in the control group (P < .001; Figure 2A‐D). In addition, the non‐normalized levels of GAP43, neurogranin, SNAP25, and synaptotagmin 1 showed similar data as CD81‐normalized biomarkers (Figure S3 in supporting information). These data indicated that the biomarkers in the blood exosomes differentiated patients with AD, controls, and patients with aMCI in both the discovery and validation datasets, with no significant difference between the two. Assessments of the levels of GAP43, neurogranin, SNAP25, and synaptotagmin 1 in the CSF (Figure 2E‐H) revealed that the AD or aMCI group had significantly different levels of all biomarkers relative to controls (P < .05 and P < .01 or .001, respectively). Furthermore, the levels of exosomal and CSF biomarkers showed no difference between the discovery and validation datasets (P > .05), indicating that the biomarkers exhibited the same performance in the two stages.
3.3. Biomarker correlation analysis between blood exosomes and CSF
To validate GAP43, neurogranin, SNAP25, and synaptotagmin 1 in neuronal‐derived exosomes as candidates for the diagnosis of AD, we performed a correlation analysis between exosomal and CSF biomarkers. In the discovery stage, we found that the blood exosomal levels of GAP43, neurogranin, SNAP25, and synaptotagmin 1 were inversely correlated with their levels in the CSF (Figure 3): GAP43 in the AD group, R2 = 0.65, P < .0001, Figure 3A; GAP43 in aMCI group, R2 = 0.70, P < .0001, Figure 3B; GAP43 in controls, R2 = 0.67, P < .0001, Figure 3C; neurogranin in AD group, R2 = 0.58, P < .0001, Figure 3D; neurogranin in aMCI group, R2 = 0.54, P < .0001, Figure 3E; neurogranin in controls, R2 = 0.57, P < .0001, Figure 3F; SNAP25 in AD group, R2 = 0.63, P < .0001, Figure 3G; SNAP25 in aMCI group R2 = 0.64, P < .0001, Figure 3H; SNAP25 in controls, R2 = 0.64, P < .0001, Figure 3I; synaptotagmin 1 in AD group, R2 = 0.61, P < .0001, Figure 3J; synaptotagmin 1 in aMCI group, R2 = 0.62, P < .0001, Figure 3K; and synaptotagmin 1 in controls R2 = 0.61, P < .0001, Figure 3L. We then confirmed the correlation analysis in the validation data set and found the same associations between exosomal and CSF biomarkers (Figure 3). We found that levels of GAP43, neurogranin, SNAP25, and synaptotagmin 1 expressed by exosomes and in the CSF were highly correlated, indicating that exosomal biomarkers may reflect pathological changes in the brain and can be used to help inform the diagnosis of AD.
FIGURE 3.
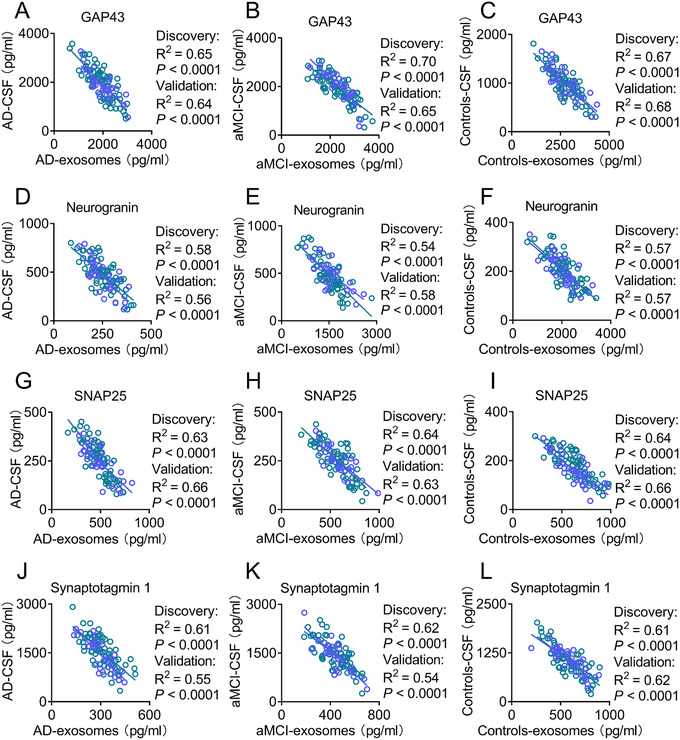
The concentrations of exosomal biomarkers are highly correlated with those in the cerebrospinal fluid (CSF). Panels A–C show that levels of growth associated protein 43 in exosomes and the CSF were closely correlated in patients with Alzheimer's disease (AD, A), patients with amnestic mild cognitive impairment (aMCI, B), and controls (C). Panels D–F show robust correlations between neurogranin levels in exosomes and those in the CSF of patients with AD (D), patients with aMCI (E), and controls (F). Panels G–I show significant correlations between synaptosome associated protein 25 (SNAP25) expression in exosomes and those in the CSF in patients with AD (G), patients with aMCI (H), and controls (I). Panels J–L show high correlations between synaptotagmin 1 levels in exosomes and the CSF of patients with AD (J), patients with aMCI (K), and controls (L). The blue circles and lines correspond to the discovery dataset, while the green circles and lines correspond to the validation dataset. In the discovery stage, n = 28 (AD), 25 (aMCI), and 29 (controls). In the validation stage, n = 73 (AD), 71 (aMCI), and 72 (controls)
3.4. Diagnostic power of each biomarker in blood exosomes and CSF
To analyze the performance of biomarkers in exosomes and the CSF to distinguish patients with AD or aMCI from controls, we performed a ROC analysis. GAP43, neurogranin, SNAP25, and synaptotagmin 1 levels in exosomes and CSF featured significantly high areas under the curve (AUCs), which far exceeded random chance (AUC of 50%). In the validation stage, the levels of GAP43, neurogranin, SNAP25, and synaptotagmin 1 showed significant AUCs in the comparisons of AD/controls (GAP43 in exosomes, 0.83, P < .0001, Figure S4A; GAP43 in the CSF, 0.90, P < .0001, Figure S4E; neurogranin in exosomes, 1.00, P < .0001, Figure S4B; neurogranin in the CSF, 0.94, P < .0001, Figure S4F; SNAP25 in exosomes, 0.75, P < .0001, Figure S4C; SNAP25 in the CSF, 0.74, P < .0001, Figure S4G; synaptotagmin 1 in exosomes, 0.95, P < .0001, Figure S4D; and synaptotagmin 1 in the CSF, 0.80, P < .0001, Figure S4H) and in the comparisons of aMCI/controls (GAP43 in exosomes, 0.66, P < .001, Figure S4A; GAP43 in the CSF, 0.91, P < .001, Figure S4E; neurogranin in exosomes, 0.82, P < .0001, Figure S4B; neurogranin in the CSF, 0.95, P < .0001, Figure S4F; SNAP25 in exosomes, 0.59, P = .05, Figure S4C; SNAP25 in the CSF, 0.73, P < .0001, Figure S4G; synaptotagmin 1 in exosomes, 0.82, P < .0001, Figure S4D; and synaptotagmin 1 in the CSF, 0.77, P < .0001, Figure S4H); however, while the AUCs in the comparisons of aMCI/AD were significant in the exosomal biomarkers, these were not significant in the CSF samples. The comparisons of the AUCs of the aMCI and AD groups revealed significant differences in the levels of exosomal biomarkers and nonsignificant differences in CSF biomarker levels (GAP43 in exosomes, 0.68, P = .0001, Figure S4A; GAP43 in the CSF, 0.53, P = .55, Supplementary Figure S1E; neurogranin in exosomes, 1.00, P < .0001, Figure S1B; neurogranin in the CSF, 0.50, P = .99, Figure S1F; SNAP25 in exosomes, 0.66, P = .0011, Figure S1C; SNAP25 in the CSF, 0.51, P = .83, Figure S4G; synaptotagmin 1 in exosomes, 0.76, P < .0001, Figure S4D; synaptotagmin 1 in the CSF, 0.55, P = .29, Figure S4H). Analysis of the ROCs in the discovery dataset revealed similar results concerning the AUCs (data not shown). These data indicate that GAP43, neurogranin, SNAP25, and synaptotagmin 1 levels in blood exosomes have the potential to supplement the diagnosis of AD.
3.5. Correlations of Mini‐Mental State Examination (MMSE) values with exosomal synaptic protein levels
To further examine the relationships between synaptic protein levels and cognitive decline in AD, we performed a linear correlation analysis between MMSE scores and the levels of GAP43, neurogranin, SNAP25, and synaptotagmin 1 in patients with AD. Our results showed significant correlations between MMSE scores and exosomal GAP43 (discovery: R2 = 0.59, P < .0001; validation: R2 = 0.59, P < .0001, Figure S5A), neurogranin (discovery: R2 = 0.60, P < .0001; validation: R2 = 0.62, P < .0001, Figure S5B), SNAP25 (discovery: R2 = 0.58, P < .0001; validation: R2 = 0.59, P < .0001, Figure S5C), and synaptotagmin 1 (discovery: R2 = 0.60, P < .0001; validation: R2 = 0.58, P < .0001, Figure S5D). We also assessed the performance of CSF biomarkers, and found similar correlations between MMSE scores and biomarker levels (R2 = 0.31–0.50, P < .0001 or P = .0006, Figure S5E–H). Our data indicated that exosomal GAP43, neurogranin, SNAP25, and synaptotagmin 1 can be used to predict cognitive decline.
3.6. Prediction of exosomal synaptic proteins for preclinical AD
It has been demonstrated that synaptic dysfunction occurs in the brain before the emergence of AD symptoms. 6 We speculated that exosomal synaptic proteins may be changed in preclinical AD. Our data showed that GAP43, neurogranin, SNAP25, and synaptotagmin 1 were slightly reduced in preclinical AD groups (2573 ± 653, 1938 ± 510, 568 ± 135, and 546 ± 135 pg/ml, respectively) than controls (2726 ± 704, 2049 ± 491, 600 ± 145, and 597 ± 149 pg/ml, respectively, P = .045–.050; Figure 4A–D). The ROC analyses showed that the AUCs of each biomarker ranged from 0.56 to 0.60 (Figure 4E–H), indicating that a single biomarker was not effective to detect preclinical AD, probably due to minimal alteration of exosomal synaptic proteins in preclinical AD. These data suggested that GAP43, neurogranin, SNAP25, and synaptotagmin 1 cannot independently predict AD. However, when all the synaptic biomarkers and the APOE ε4 status, a well‐known risk factor for AD, were put into a model (composite) generated by ridge regression, they performed together effectively to predict AD (Figure 4I–K). The AUC was 0.88 for total dataset (Figure 4I). To avoid overfitting, we split the total dataset into training and test datasets. The prediction model was generated in the training dataset and then applied to the test dataset. The AUCs were 0.89 for training dataset (Figure 4J) and 0.89 for test dataset (Figure 4K). These results indicated that the prediction model was fitted well and can work in other datasets. In addition, the ROC of APOE ε4 status was analyzed independently and showed an AUC of 0.61 (Figure 4L), indicating that high performance of the prediction model was generated by combination of exosomal GAP43, neurogranin, SNAP25, and synaptotagmin 1.
FIGURE 4.
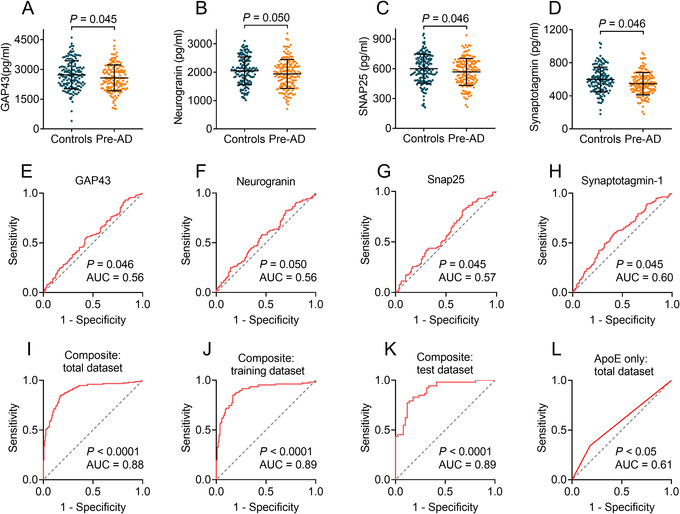
Prediction of exosomal growth associated protein 43 (GAP43), neurogranin, synaptosome associated protein 25 (SNAP25), and synaptotagmin 1 for preclinical Alzheimer's disease (AD). Panels A–D show levels of neuronal‐derived exosomal GAP43 (A), neurogranin (B), SNAP25 (C), and synaptotagmin 1 (D) in preclinical AD and controls. Panels E–H show receiver operating characteristic (ROC) analyses of GAP43 (E), neurogranin (F), SNAP25 (G), and synaptotagmin 1 (H). Panels I–K show ROC analyses by combining exosomal GAP43, neurogranin, SNAP25, synaptotagmin 1, and APOE status in total dataset (I), randomly selected training dataset (J), and test dataset (K). In addition, the ROC of APOE ε4 status was analyzed independently in total dataset (L). n = 160 (preclinical AD) and 160 (controls). Pre‐AD = preclinical AD
To further confirm the prediction model, we tested the model in subjects from FAD. To do so, we first measured the single level of exosomal biomarkers (Figure 5A–D). Our data showed that GAP43 (Figure 5A), neurogranin (Figure 5B), SNAP25 (Figure 5C), and synaptotagmin 1 (Figure 5D) were reduced in mutation carriers (2515 ± 692, 1890 ± 540, 555 ± 143, and 534 ± 143 pg/ml, respectively) compared to normal controls (2714 ± 673, 2029 ± 473, 600 ± 140, and 597 ± 150pg/ml, respectively, P = .046–.13). However, the ROC analyses showed poor AUCs of each biomarker, ranging from 0.56 to 0.60 (Figure 5E–H). These data were similar to those in preclinical AD dataset. We applied the model generated from the preclinical AD dataset to FAD subjects and found that the AUC was 0.87 (Figure 5I), indicating that the prediction model has a high performance in mutation carriers from FAD. Taken together, our model generated by exosomal GAP43, neurogranin, SNAP25, and synaptotagmin 1 may help in the prediction of AD 5 to 7 years before the onset of cognitive impairment.
FIGURE 5.
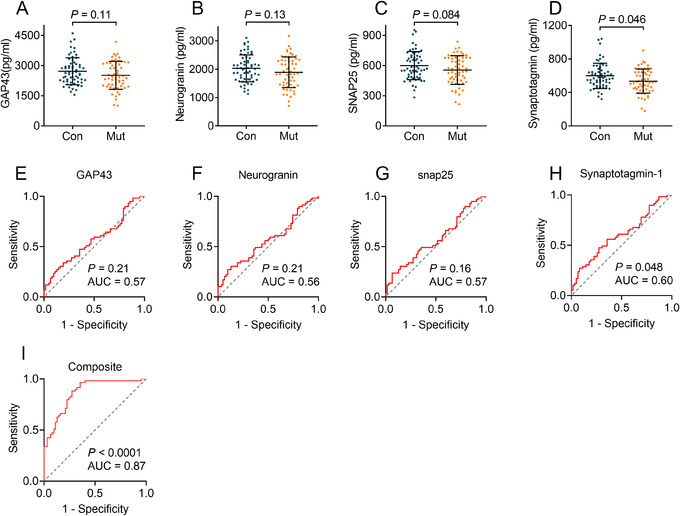
Prediction of exosomal growth associated protein 43 (GAP43), neurogranin, synaptosome associated protein 25 (SNAP25), and synaptotagmin 1 for mutation carriers in a familial Alzheimer's disease (FAD) cohort (dataset 4). Panels A–D show levels of neuronal‐derived exosomal GAP43 (A), neurogranin (B), SNAP25 (C), and synaptotagmin 1 (D) in mutation carriers and non‐mutation controls. Panels E–H show ROC analyses of GAP43 (E), neurogranin (F), SNAP25 (G), and synaptotagmin 1 (H). Panel I shows receiver operating characteristic (ROC) analyses by applying the predict model generated from preclinical AD dataset (dataset 3). n = 59 (mutation carriers) and 62 (non‐mutation carriers). Abbreviations: Con, controls, Mut, mutation carriers
4. DISCUSSION
While multiple studies have shown that the brain tissues and CSF of patients with AD exhibit changes in synaptic proteins, few studies have focused on changes in the serum or plasma. In the current multicenter study, we validated the diagnostic capacities of GAP43, neurogranin, SNAP25, and synaptotagmin 1 by assessing their levels in both neuronal‐derived exosomes and the CSF. Our data revealed that the concentrations of GAP43, neurogranin, SNAP25, and synaptotagmin 1 in exosomes were strongly correlated with those in the CSF in patients with AD or aMCI and controls, indicating that the levels of exosomal synaptic proteins may reflect synaptic changes in the brains. To further investigate the capacity of exosomal biomarkers to predict the preclinical AD, we conducted an investigation in preclinical AD and FAD subjects, and showed that the combination of exosomal GAP43, neurogranin, SNAP25, and synaptotagmin 1 can detect preclinical AD 5 to 7 years before cognitive impairment. To the best of our knowledge, this study is the first to validate AD‐induced changes in synaptic protein biomarkers in blood neuronal‐derived exosomes with concomitant CSF findings, therefore establishing a prediction model by using these exosomal synaptic biomarkers to detect preclinical AD. The current study may provide opportunities for the early diagnosis of AD at the asymptomatic stage, making the early treatment of AD possible.
In this study, exosomal GAP43, SNAP25, and synaptotagmin 1 achieved similar efficacy to CSF biomarkers in distinguishing patients with AD or aMCI from controls but performed slightly better in discriminating patients with AD from those with aMCI. Interestingly, exosomal neurogranin distinguished patients with AD from those with aMCI and controls with the highest accuracy; this finding agrees with previously published data. 23 In addition, we found lower levels of exosomal synaptic biomarkers in AD, which were negatively correlative to their counterparts in the CSF, and the same association was found between the levels of Aβ42 expressed in the CSF and exosomes. 21 The lower exosomal synaptic proteins were also found in other studies. 27 , 44 However, the mechanisms of this finding require further exploration. Moreover, we also investigated the association between changes in synaptic proteins and cognitive decline. Our results showed that, consistent with previous studies, 27 , 44 the levels of biomarkers in both exosomal and CSF can be used to predict cognitive decline.
To investigate whether exosomal synaptic proteins can predict AD before the onset of symptoms, we recruited subjects from a longitudinal study that began 10 years ago. We followed up the population who were cognitively normal 5 to 7 years ago in the study, and selected subjects who developed cognitive impairments with abnormal cutoff values of P‐tau/Aβ42 and T‐tau/Aβ42 at present as preclinical AD. The time point of 5 to 7 years before cognitive impairments should be proper, which is not too far from the onset of symptoms, providing insights into the biomarkers’ ability to predict differences between preclinical AD and controls. In addition, the term of 5 to 7 years is enough to start early treatment for the disease. Biomarkers such as Pittsburgh compound B positron emission tomography (PiB PET) and CSF Aβ42 have been extensively used to group subjects to Aβ (+) or AD. 12 45 In the current study, we used the CSF ratios of P‐tau/Aβ42 and T‐tau/Aβ42 to group subjects into AD or controls. The measurement of single synaptic protein showed tendency to decline (P = .45–.05) in preclinical AD. However, ROCs of each biomarker showed poor performance (AUCs = 0.56–0.60), possibly because the changes in exosomal synaptic proteins, 5 to 7 years before onset of disease, were minimal. We then established a prediction model to detect the preclinical AD by combining APOE status, exosomal GAP43, neurogranin, SNAP25, and synaptotagmin 1. Logistic regression is commonly used in studies of prediction models. However, the coefficient estimates may change erratically in response to minor changes in the model when multicollinearity exists. To avoid multicollinearity between values of synaptic biomarkers, ridge regression was performed, which is a stricter statistical method than logistic regression. The prediction model was generated in the training dataset and confirmed in the test dataset, and then further validated in subjects from the FAD cohort. The AUC in each dataset was around 0.87 to 0.89, demonstrating that the prediction model was effective and can be used in other datasets. Our findings suggest that the combination of exosomal synaptic GAP43, neurogranin, SNAP25, and synaptotagmin 1 could predict AD 5 to 7 years before the onset of cognitive impairment, thus making early treatment of AD possible.
This study found that neuronal‐derived exosomal GAP43, neurogranin, SNAP25, and synaptotagmin 1 show promise as blood biomarkers for AD and aMCI. The verification of exosomal GAP43, neurogranin, SNAP25, and synaptotagmin 1 in the CSF confirmed that these biomarkers may reflect pathological changes in the AD brain and have the capacity to differentiate AD and aMCI. More importantly, the combination of exosomal synaptic GAP43, neurogranin, SNAP25, and synaptotagmin 1 can detect preclinical AD 5 to 7 years before the onset of cognitive impairment. However, these findings warrant further investigation by more multiple center studies.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
J.J. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. L.J. and J.J. participated in the study design. L.J., M.Z., C.K., Y.P., H.Z., C.W, Y.T., Q.W., Y.I.L., Y.A.L., and Y.W. contributed to the acquisition, analysis, and interpretation of data. L.J. and F.L. performed the statistical analysis. L.J. and J.J. drafted the manuscript. L.J., M.Z., Q.Q., and Y.P. provided the administrative, technical, and material support. J.J. supervised the whole study. All authors critically reviewed the article and approved the final manuscript.
Supporting information
Supplementary information
Supplementary information
Supplementary information
Supplementary information
Supplementary information
Supplementary information
Supplementary information
ACKNOWLEDGMENTS
This study was supported by the Key Project of the National Natural Science Foundation of China (81530036); the National Key Scientific Instrument and Equipment Development Project (31627803); Mission Program of Beijing Municipal Administration of Hospitals (SML20150801); Beijing Scholars Program; Beijing Brain Initiative from Beijing Municipal Science & Technology Commission (Z161100000216137); Innovation Base Training and Development Special Program (Z171100002217007); CHINA‐CANADA Joint Initiative on Alzheimer's Disease and Related Disorders (81261120571); Project for Outstanding Doctor with Combined Ability of Western and Chinese Medicine, National Natural Science Foundation of China (81870825); and Beijing Municipal Natural Science Foundation (7202061).
Jia L, Zhu M, Kong C, et al. Blood neuro‐exosomal synaptic proteins predict Alzheimer's disease at the asymptomatic stage. Alzheimer's Dement. 2021;17:49–60. 10.1002/alz.12166
REFERENCES
- 1. Jia L, Quan M, Fu Y, et al. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. 2020;19:81‐92. [DOI] [PubMed] [Google Scholar]
- 2. Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid‐beta‐targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15:73‐88. [DOI] [PubMed] [Google Scholar]
- 3. Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572‐580. [DOI] [PubMed] [Google Scholar]
- 6. Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789‐791. [DOI] [PubMed] [Google Scholar]
- 7. Spires‐Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer's disease. Neuron. 2014;82:756‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reddy PH, Mani G, Park BS, et al. Differential loss of synaptic proteins in Alzheimer's disease: implications for synaptic dysfunction. J Alzheimers Dis. 2005;7:103‐117. discussion 73‐80. [DOI] [PubMed] [Google Scholar]
- 9. Bereczki E, Francis PT, Howlett D, et al. Synaptic proteins predict cognitive decline in Alzheimer's disease and Lewy body dementia. Alzheimers Dement. 2016;12:1149‐1158. [DOI] [PubMed] [Google Scholar]
- 10. Sjogren M, Minthon L, Davidsson P, et al. CSF levels of tau, beta‐amyloid(1‐42) and GAP‐43 in frontotemporal dementia, other types of dementia and normal aging. J Neural Transm (Vienna). 2000;107:563‐579. [DOI] [PubMed] [Google Scholar]
- 11. Sjogren M, Davidsson P, Gottfries J, et al. The cerebrospinal fluid levels of tau, growth‐associated protein‐43 and soluble amyloid precursor protein correlate in Alzheimer's disease, reflecting a common pathophysiological process. Dement Geriatr Cogn Disord. 2001;12:257‐264. [DOI] [PubMed] [Google Scholar]
- 12. Sandelius A, Portelius E, Kallen A, et al. Elevated CSF GAP‐43 is Alzheimer's disease specific and associated with tau and amyloid pathology. Alzheimers Dement. 2019;15:55‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kester MI, Teunissen CE, Crimmins DL, et al. Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol. 2015;72:1275‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brinkmalm A, Brinkmalm G, Honer WG, et al. SNAP‐25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer's disease. Mol Neurodegener. 2014;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohrfelt A, Brinkmalm A, Dumurgier J, et al. A novel ELISA for the measurement of cerebrospinal fluid SNAP‐25 in patients with Alzheimer's disease. Neuroscience. 2019;420:136‐144. [DOI] [PubMed] [Google Scholar]
- 16. Ohrfelt A, Brinkmalm A, Dumurgier J, et al. The pre‐synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer's disease. Alzheimers Res Ther. 2016;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wood MJ, O'Loughlin AJ, Samira L. Exosomes and the blood‐brain barrier: implications for neurological diseases. Ther Deliv. 2011;2:1095‐1099. [DOI] [PubMed] [Google Scholar]
- 18. Rajendran L, Honsho M, Zahn TR, et al. Alzheimer's disease beta‐amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172‐11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vingtdeux V, Sergeant N, Buee L. Potential contribution of exosomes to the prion‐like propagation of lesions in Alzheimer's disease. Front Physiol. 2012;3:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fiandaca MS, Kapogiannis D, Mapstone M, et al. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case‐control study. Alzheimers Dement. 2015;11:600‐607. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jia L, Qiu Q, Zhang H, et al. Concordance between the assessment of Abeta42, T‐tau, and P‐T181‐tau in peripheral blood neuronal‐derived exosomes and cerebrospinal fluid. Alzheimers Dement. 2019;15:1071‐1080. [DOI] [PubMed] [Google Scholar]
- 22. Goetzl EJ, Schwartz JB, Abner EL, Jicha GA, Kapogiannis D. High complement levels in astrocyte‐derived exosomes of Alzheimer disease. Ann Neurol. 2018;83:544‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winston CN, Goetzl EJ, Akers JC, et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst). 2016;3:63‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kapogiannis D, Boxer A, Schwartz JB, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural‐derived blood exosomes of preclinical Alzheimer's disease. FASEB J. 2015;29:589‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goetzl EJ, Mustapic M, Kapogiannis D, et al. Cargo proteins of plasma astrocyte‐derived exosomes in Alzheimer's disease. FASEB J. 2016;30:3853‐3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goetzl EJ, Nogueras‐Ortiz C, Mustapic M, et al. Deficient neurotrophic factors of CSPG4‐type neural cell exosomes in Alzheimer disease. FASEB J. 2019;33:231‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goetzl EJ, Kapogiannis D, Schwartz JB, et al. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer's disease. FASEB J. 2016;30:4141‐4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen IH, Xue L, Hsu CC, et al. Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc Natl Acad Sci U S A. 2017;114:3175‐3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med. 2019;25:277‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dong J, Qin W, Wei C, Tang Y, Wang Q, Jia J. A novel PSEN1 K311R mutation discovered in Chinese families with late‐onset Alzheimer's disease affects amyloid‐beta production and tau phosphorylation. J Alzheimers Dis. 2017;57:613‐623. [DOI] [PubMed] [Google Scholar]
- 31. Wang Q, Jia J, Qin W, et al. A novel AbetaPP M722K mutation affects amyloid‐beta secretion and tau phosphorylation and may cause early‐onset familial Alzheimer's disease in Chinese individuals. J Alzheimers Dis. 2015;47:157‐165. [DOI] [PubMed] [Google Scholar]
- 32. Jia J, Xu E, Shao Y, Jia J, Sun Y, Li D. One novel presenilin‐1 gene mutation in a Chinese pedigree of familial Alzheimer's disease. J Alzheimers Dis. 2005;7:119‐124. discussion 73‐80. [DOI] [PubMed] [Google Scholar]
- 33. Qiu Q, Jia L, Wang Q, et al. Identification of a novel PSEN1 Gly111Val missense mutation in a Chinese pedigree with early‐onset Alzheimer's disease. Neurobiol Aging. 2020;85:155.e1‐155.e4. [DOI] [PubMed] [Google Scholar]
- 34. Qiu Q, Shen L, Jia L, et al. A novel PSEN1 M139L mutation found in a Chinese pedigree with early‐onset Alzheimer's disease increases Abeta42/Abeta40 ratio. J Alzheimers Dis. 2019;69:199‐212. [DOI] [PubMed] [Google Scholar]
- 35. Shen L, Qin W, Wu L, Zhou A, et al. Two novel presenilin‐1 mutations (I249L and P433S) in early onset Chinese Alzheimer's pedigrees and their functional characterization. Biochem Biophys Res Commun. 2019;516:264‐269. [DOI] [PubMed] [Google Scholar]
- 36. Zhang G, Xie Y, Wang W, Feng X, Jia J. Clinical characterization of an APP mutation (V717I) in five Han Chinese families with early‐onset Alzheimer's disease. J Neurol Sci. 2017;372:379‐386. [DOI] [PubMed] [Google Scholar]
- 37. Jia J, Jia L, Fu Y, et al. Genetic study of 404 pedigrees with familial Alzheimer's disease in China. Alzheimer's & Dementia. 2019;15:P921‐P2. [Google Scholar]
- 38. Jia L, Fu Y, Shen L, et al. PSEN1, PSEN2, and APP mutations in 404 Chinese pedigrees with familial Alzheimer's disease. Alzheimers Dement. 2020;16:178‐191. [DOI] [PubMed] [Google Scholar]
- 39. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367:1262‐1270. [DOI] [PubMed] [Google Scholar]
- 41. Seeburger JL, Holder DJ, Combrinck M, et al. Cerebrospinal fluid biomarkers distinguish postmortem‐confirmed Alzheimer's disease from other dementias and healthy controls in the OPTIMA cohort. J Alzheimers Dis. 2015;44:525‐539. [DOI] [PubMed] [Google Scholar]
- 42. Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73:1914‐1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid‐beta biomarkers for Alzheimer's disease. Nature. 2018;554:249‐254. [DOI] [PubMed] [Google Scholar]
- 44. Agliardi C, Guerini FR, Zanzottera M, Bianchi A, Nemni R, Clerici M. SNAP‐25 in serum is carried by exosomes of neuronal origin and is a potential biomarker of Alzheimer's disease. Mol Neurobiol. 2019;56:5792‐5798. [DOI] [PubMed] [Google Scholar]
- 45. Ashton NJ, Nevado‐Holgado AJ, Barber IS, et al. A plasma protein classifier for predicting amyloid burden for preclinical Alzheimer's disease. Sci Adv. 2019;5:eaau7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information
Supplementary information
Supplementary information
Supplementary information
Supplementary information
Supplementary information


