Abstract
Aim
To determine the prevalence of inflammatory bowel disease (IBD) in patients with type 1 diabetes (T1D) and to characterise patients with both diseases.
Methods
Data of 65.147 patients with T1D ≤18 years of 379 centres in Germany and Austria participating in the DPV initiative were analysed. A total of 63 children had comorbid IBD; IBD prevalence was 0.1%. Regression models were used to analyse differences in metabolic control, acute complications and steroid intake.
Results
Mean BMI‐SDS in patients with T1D and IBD was lower (−0.15 ± 0.11) compared to patients with T1D only (0.27 ± 0.00, p < .001). Patients with T1D and IBD had a significantly higher use of steroids (22% ± 0.05% vs. 1% ± 0.00, p < .001) and a significantly higher rate of severe hypoglycaemic events per patient year (0.33 ± 0.07 vs. 0.16 ± 0.00, p = .001). No differences were found in HbA1c levels, insulin dose and occurrence of DKA.
Conclusion
Although children and adolescents with T1D and IBD take steroids more often, they suffer from severe hypoglycaemia more frequently and have a lower BMI‐SDS. These findings might be explained by chronic intestinal inflammation leading to malabsorption, malnutrition and increased severe hypoglycaemia.
Keywords: children, hypoglycaemia, inflammatory bowel disease, prevalence, type 1 diabetes
Abbreviations
- BMD
bone mineral density
- BMI
body mass index
- BMI‐SDS
body mass index standard deviation score
- CD
Crohn's disease
- CG
control group
- DKA
diabetic ketoacidosis
- DPV
diabetes patient follow‐up
- GFD
gluten‐free diet
- I. U.
international units
- IBD
inflammatory bowel disease
- IC
indeterminate colitis
- pos.
positive
- RR
relative risk
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- tTGA
tissue transglutaminase
- UC
ulcerative colitis
- vs.
versus
Key Notes.
Data on associations between type 1 diabetes (T1D) and inflammatory bowel disease (IBD) are conflicting.
We investigated correlations between T1D and IBD with prevalence data of the German and Austrian population, using the DPV database.
The risk for children and adolescents with T1D to develop IBD is elevated, and patients suffering from both diseases have a higher risk of severe hypoglycaemia.
1. INTRODUCTION
In the last two decades, an increase in the incidence of T1D in children and adolescents 1 as well as a progression in the incidence of IBD in children 2 , 3 has been observed in both developed and developing countries. The incidence of T1D in Austria for children below 15 years of age increased from 12.0 to 18.4 per 100.000 children between 1999 and 2007. 4 A similar rise of incidence rates has been observed in Germany for children with T1D younger than 15 years (in the 1990s 13.7/100.000/year; since 2000 19.4/100.000/year). 5 The prevalence of T1D in Germany below the age of 15 years was 126/100.000 in 2006; the prevalence in Germany at the end of 2026 is predicted to be 265/100.000. 5
In concordance with worldwide reports, 3 a rise in IBD has been observed in Austria and Germany. 6 , 7
In the general population, the average annual incidence rate in the province of Styria, Austria, from 1997 to 2007 was 4.8 (95% CI 4.5–5.2) per 100.000 for ulcerative colitis and 6.7 (95% CI 6.2–7.1) per 100.000 for Crohn's disease and rose significantly during the 11 year study period. 7 The percentage of patients who were under 18 years of age at initial diagnosis was 5% (34/674) for ulcerative colitis and 11% for Crohn's disease (89/813). Incidence rates for patients from 0 to 19 years were 2.2 (95% CI 1.7–2.8) for ulcerative colitis and 4.8 per 100.000 (95% CI 4.0–5.7) for Crohn's disease, respectively. 7
In Leipzig, province of Saxony, Germany, incidence rates for IBD in children and adolescents < 18 years of age, including not only data from the Paediatric IBD Registry of Saxony but additional data of patients who have been exclusively cared for by internists, were as follows:
11/100.000 (95% 7.3–14.6) for IBD; 4.4 (95% CI 2.1–6.7) for ulcerative colitis; 5.7 (95% CI 3.0–8.3) for Crohn's disease; and 0.9 (95% 0.0–2.0) for indeterminate colitis. 8
Average prevalence for children and adolescents <18 years of age with IBD in Saxony between 2000 and 2009 is quoted as 35.8/100.000 (95% CI 34.3–37.3). 9
Autoimmune comorbidities have been described in both T1D and IBD 10 , 11 ; based on this evidence, correlations between both diseases could be traced down. 12 , 13 , 14 Whereas correlations between T1D and other autoimmune diseases such as coeliac disease are very well investigated, 15 data on correlations between T1D and IBD remain conflicting. While associations between autoimmune disorders are generally presumed, a clear relation between T1D and IBD could not be confirmed by all study groups. 16
The aim of our study was to investigate whether the risk for children with T1D to develop IBD is elevated, and to compare clinical characteristics and complications of T1D patients with and without IBD, including anthropometrics and metabolic control.
2. RESEARCH DESIGN AND METHODS
Diabetes patient follow‐up (diabetes patient follow‐up) is a prospective, observational, multicentre diabetes survey with continuous data acquisition. Twice a year, anonymous longitudinal data from patients are transmitted for central validation from 419 diabetes centres in Germany (n = 390) and Austria (n = 29). Inconsistent data are reported back to the centres for correction and then re‐entered into the database. 17
According to the guidelines of the German Diabetes Association, all centres are advised to document age at diabetes manifestation, sex, weight, height, BMI, blood pressure, migration background, insulin regimen, concomitant diseases, additional medications, laboratory markers and HbA1c levels.
2.1. Demographic data of study patients
From January 1995 to March 2015, data from 405.559 patients with diabetes were collected. 65.147 children and adolescents of these patients had T1D and were at/under the age of 18 years. Among this age group, we differentiated between patients with T1D and IBD (n = 63) and patients with T1D only (n = 65.084) (Figure 1). Patients with IBD were searched for and identified either by ICD‐10 code for IBD or written diagnosis. Only patients with confirmed diagnosis of IBD were included. Demographic data of study patients are displayed in Table 1.
FIGURE 1.
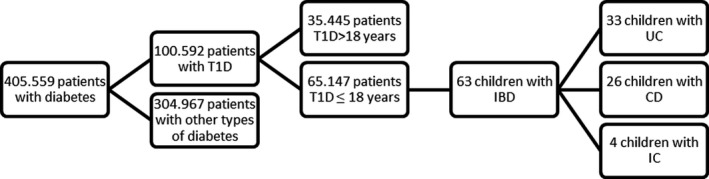
Selection of patients
TABLE 1.
Demographic data of study patients (unadjusted comparisons, presented as mean ± SD)
| Patients with T1D and IBD (n = 63) | Patients with T1D only (n = 65.084) | p‐values | |
|---|---|---|---|
| Males/females (%) | 50.8/49.2 | 52.7/47.3 | 0.7610 |
| Age (years) | 15.68 ± 3.08 | 14.0 ± 3.86 | <0.0001 |
| Age at T1D onset (years) | 10.77 ± 4.32 | 8.59 ± 4.36 | <0.0001 |
| Diabetes duration (years) | 4.91 ± 3.92 | 5.41 ± 4.20 | 0.3390 |
| BMI SDS | −0.16 ± 1.13 | 0.27 ± 0.90 | 0.0006 |
Bold letters are used when p‐values are signifikant (≤ 0.05).
For analysis, data on age, age at diabetes onset, diabetes duration, insulin dose (iu/kg/day), BMI, glycaemic control (HbA1c), percentage of patients with autoimmune thyroiditis, positive tissue transglutaminase (tTGA) or endomysial antibodies, dyslipidaemia, hypertension, insulin regimen, and steroid intake were collected (table 2).
TABLE 2.
Comparison of patients after adjustment for age, sex and diabetes duration (estimated means ± SE)
| Patients with T1D and IBD | Patients with T1D only | p‐value | |
|---|---|---|---|
| HbA1c (%) | 8.16 ± 0.23 | 8.26 ± 0.01 | 0.656 |
| HbA1c (mmol/mol) | 65.80 ± 2.49 | 66.92 ± 0.08 | 0.656 |
| Insulin dose (iu/kg/day) | 0.90 ± 0.04 | 0.86 ± 0.00 | 0.300 |
| BMI‐SDS (kg/m2) | −0.15 ± 0.11 | 0.27 ± 0.00 | < 0.001 |
| Hypertension (%) | 27 ± 0.06 | 31 ± 0.00 | 0.480 |
| Dyslipidaemia (%) | 30 ± 0.07 | 40 ± 0.00 | 0.207 |
| Pump therapy (%) | 32 ± 0.07 | 28 ± 0.00 | 0.481 |
| Thyroid antibodies pos. (%) | 13 ± 0.04 | 17 ± 0.00 | 0.505 |
| tTGA positive (%) | 25 ± 0.06 | 17 ± 0.00 | 0.140 |
| Steroids (%) | 22 ± 0.05 | 1 ± 0.00 | <0.001 |
Bold letters are used when p‐values are signifikant (≤ 0.05).
2.2. BMI
Body mass index was derived from weight in kilograms divided by squared height in metres. Age‐ and sex‐specific BMI‐reference values of 17.641 children and adolescents from the population‐based German KIGGS study were used to calculate BMI‐SDS. 18
2.3. Glycaemic control
Glycated haemoglobin A1c (HbA1c) values were measured locally. To correct for different laboratory methods, they were mathematically standardised to the Diabetes Control and Complication Trial (DCCT) reference range of 4.05–6.05% (21‐43 mmol/mol). 19
Autoimmune thyroiditis was diagnosed by positive thyroid antibodies (thyroid peroxidase and/or thyroglobulin antibodies).
Dyslipidaemia was defined as either taking lipid modifying drugs, or having decreased high‐density lipoprotein (HDL) cholesterol values (< 35 mg/dl), or as increased values of total cholesterol (>200 mg/dl), low density lipoprotein (LDL) cholesterol (>130 mg/dl) or triglycerides (>150 mg/dl).
Hypertension was defined as the use of antihypertensive medication, or as increased systolic and/or diastolic arterial blood pressure, according to the KIGGS study. 18
Insulin regimen was categorised as conventional insulin therapy (CT, two injection time‐points per day), intensified insulin treatment (ICT, ≥2 injection time‐points per day) or continuous subcutaneous insulin infusion (CSII).
2.4. Acute complications
Data on acute complications such as severe hypoglycaemia, hypoglycaemic coma and DKA were documented and summarised as events per patient year. Severe hypoglycaemia was defined as hypoglycaemia with the need for external help.
2.5. IBD Prevalence
Published data on German IBD prevalence were used for comparison. 8 , 9 Prevalence for IBD among children and adolescents <18 years of age between 2000 and 2009 in Saxonia is 35.6/100.000 (95% CI 34.3–37.3), consisting of 11.7/100.000 with UC (95% CI 10.8–12.5), 21.3/100.00 with CD (95% CI 20.2–22.5) and 2.6/100.000 with IC (95% CI 2.2–3.0). Median age of Saxonian patients under 18 years with IBD was 13.9 years. 10
2.6. Statistical methods
The relative risk (RR) was calculated by MedCal, an open‐access calculation program. The standardised prevalence ratio (ie the ratio of observed to expected numbers of patients with IBD in the diabetic cohort) served as a measure of RR. The expected numbers of patients with IBD were calculated as the sum of age‐specific persons at risk in the type 1 diabetic cohort multiplied by corresponding national age‐specific IBD prevalence rates. National data were taken from a Saxonian register. 9 Ninety‐five per cent CIs for the RRs were estimated from the Wald test, assuming a Poisson distribution of the observed cases.
Diabetes patient follow‐up data were analysed using the SAS 9.4 software (version 9.4; SAS Institute). Descriptive statistics (mean, SD and percentage) were calculated. For group comparison, Wilcoxon testing for continuous data and chi‐square tests for categorical data were used. The Holm method was applied to adjust p‐values for multiple comparisons. Multiple linear regression analysis for continuous variables (age, diabetes duration, BMI, HbA1c and insulin dose) and multiple logistic regression models for dichotomous variables (hypertension, dyslipidaemia, insulin regimen, positivity of thyroid antibodies, positivity of tTGA and steroid use) were applied for adjustment. Analysis of frequency of severe hypoglycaemia, hypoglycaemic coma and DKA was performed assuming a Poisson distribution.
Two‐sided p‐values <0.05 were considered to be statistically significant.
3. RESULTS
3.1. Diabetes prevalence
The DPV pool (03/2015) contains 65.147 patients with T1D aged 18 years and below. We compared patients aged 18 years and younger than 18 years with T1D and IBD with those who had T1D only. The cohort of patients with both, T1D and IBD, consists of 63 children (33 children with ulcerative colitis, 26 children with Crohn's disease and four children with indetermined colitis, Figure 1). The percentage of patients with IBD among the patients with T1D ≤18 years is therefore 0.097%, the percentage of cases with ulcerative colitis 0.050%, Crohn's disease 0.040% and indetermined colitis 0.006%.
3.2. Relative risk for IBD
Comparing our data to IBD prevalence data from Saxony, the prevalence for IBD in T1D patients is higher than in the general population. The RR for a patient with T1D to develop any subtype of IBD until his/her 18th birthday was 3.25 (CI 95% 2.17–4.88, p < .001). The highest RR is related to ulcerative colitis. Therefore, a patient with T1D has a 4.67‐fold risk to develop ulcerative colitis as a comorbidity. Mean age of patients with T1D and IBD was 15.7 years; mean age of patients with IBD without T1D is 13.0 years (Table 3).
TABLE 3.
Relative risk for IBD among patients with T1D <18 years
| Patients | Relative risk | p‐values |
|---|---|---|
| All patients with IBD | 3.25 (CI 95% 2.17–4.88) | <0.0010 |
| UC | 4.67 (CI 95% 2.35–9.26) | <0.0001 |
| Crohn's disease | 2.06 (CI 95% 1.17–3.65) | <0.0127 |
| IC | 1.20 (CI 95% 0.57–2.54) | 0.6260 |
Bold letters are used when p‐values are signifikant (≤ 0.05).
3.3. Comparison of T1D patients with and without IBD
In a linear regression model, after adjustment for age, sex and diabetes duration, no differences between both groups (estimated means ± SE) were seen neither in glycaemic control (HbA1c 8.16% ± 0.23 vs. 8.26 ± 0.01; 65.80 mmol/mol ± 2.49 vs. 66.92 ± 0.08, p = .656) nor in insulin requirement (units per kg per day 0.90 ± 0.04 vs. 0.86 ± 0.00, p = .300). A significant difference between both groups, however, was found in BMI‐SDS (–0.15 ± 0.11 vs. 0.27 ± 0.00, p < .001) (Table 2).
In a logistic regression model, after adjustment for age, sex and diabetes duration, both groups were compared and no differences were found concerning hypertension, dyslipidaemia, insulin regimen (pump therapy), the coexistence of thyroiditis or the presence of tTGA (Table 2).
However, a significant difference was found in steroid intake. The proportion of patients who took steroids at least once was significantly higher in the group of patients suffering from both diseases (22% vs. 1%, p < .001) (Table 2).
In a Poisson regression model, after adjustment for age, sex and diabetes duration, no differences between both groups were seen, neither in DKA nor in hypoglycaemic coma (Figure 2). However, a significant difference was found in severe hypoglycaemia. Patients with T1D and IBD reported 0.33 ± 0.22 events of severe hypoglycaemia per year, whereas patients with T1D only had 0.16 ± 0.01 events, p = .001 (Figure 2).
FIGURE 2.
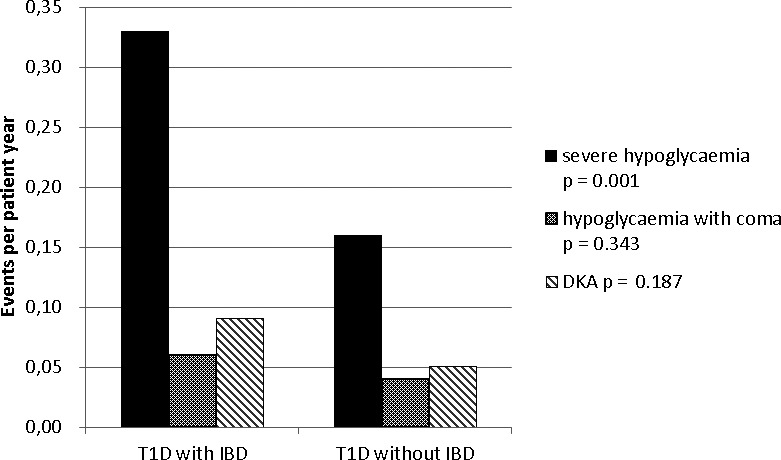
Acute complications (adjusted for age, sex and diabetes duration)
4. DISCUSSION
In this study, we show that IBD occurs more frequently in patients with T1D than in the general population of the same age group (≤18 years). We see that patients with both diseases, IBD and T1D, are older at the onset of T1D than patients with T1D only. We find differences in BMI‐SDS, the intake of steroids, and the frequency of severe hypoglycaemia between T1D patients with and without IBD. Patients with both diseases, T1D and IBD, have a lower BMI‐SDS, take steroids more often and experience more events of severe hypoglycaemia. Although the patients with T1D and IBD take steroids more often and although a rise of glucose is a side effect of steroids, they suffer from severe hypoglycaemia more often. Our interpretation is that these effects are caused by malabsorption, due to bowel inflammation.
Our data are in consistence with most epidemiological studies showing an overlap between T1D and IBD, 11 , 12 , 13 , 14 except for an US study using patient‐level medical and drug claims to trace back the diagnosis of IBD and T1D. 16 Kappelmann et al 13 found an elevated risk for paediatric patients with UC to develop T1D (OR 2.7, 95% CI 1.1–6.6) but not for patients with CD.
Recent observations suggest a rise in IBD cases by the use of dipeptidyl peptidase‐4 (DPP‐4) inhibitors in patients with type 2 diabetes (T2D), reaching a peak after three to 4 years of treatment. 20 DPP‐4 inhibitors increase insulin secretion by inhibiting the degradation of glucagon‐like peptide (GLP‐1). 21 A recent nationwide cohort study from Denmark shows an increased risk of T2D in IBD. 22 The risk was highest the first year after a diagnosis of IBD (95% CI 4.16–4.38) and remained increased for more than 20 years following the diagnosis (95% CI 1.16–1.38). 22 Enzymes in the insulin secretion pathway might play a role in the pathogenesis of IBD. 20
Our data indicate an increased prevalence of IBD among patients with T1D and therefore an increased risk among the patients with T1D to develop IBD. The same observation has been described for other autoimmune diseases such as juvenile arthritis 23 and multiple sclerosis. 24 Aetiological causes including genetics, epigenetics, immunological and/or environmental factors may be shared.
Type 1 diabetes and IBD, moreover its co‐occurrence, have multiple life‐long effects. Adult IBD patients with concomitant diabetes have been shown to have a significantly higher morbidity and mortality over a 10‐year period compared to patients with IBD alone. 25 Some of the potential mechanisms to explain these adverse outcomes include additional alterations in the intestinal microbiome in the combined disease group, a chronic systemic inflammation and overlapping immune activity. 25 Inflammation of the bowel can lead to malabsorption, malnutrition, failure to thrive, a low BMI and an increased risk of hypoglycaemia, as shown in this study of children with T1D.
To summarise: As in all chronic diseases, it is important to diagnose and treat as early as possible. Although the prevalence is extremely low, we suggest to be aware of IBD in patients with T1D and screen for IBD in the presence of gastrointestinal symptoms. Faecal calprotectin represents an easy, non‐invasive biomarker for an early detection of IBD in patients with T1D. By early detection and consistent treatment, adverse factors such as inflammation, malabsorption and severe hypoglycaemia may be avoided.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
We thank the German Center for Diabetes Research (DZD, grant number FKZ 82DZD01402), German Diabetes Association (DDG) and Robert Koch Institute (RKI) for their support. I thank my team at the children's hospital Graz, especially Elke Fröhlich‐Reiterer and Kerstin Faninger, for their supervision and support.
We thank all centres participating in the DPV initiative and contributing their data to this study. For a full list of centres, please see the online supplementary file on our website.
Jasser‐Nitsche H, Bechtold‐Dalla Pozza S, Binder E, et al. Comorbidity of inflammatory bowel disease in children and adolescents with type 1 diabetes. Acta Paediatr. 2021;110:1353–1358. 10.1111/apa.15643
REFERENCES
- 1. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on a systematic review. Gastroenterology. 2012;142:46‐54.e42. [DOI] [PubMed] [Google Scholar]
- 3. Lopez RN, Evans HM, Appleton L, et al. Prospective incidence of paediatric inflammatory bowel disease in New Zealand in 2015: results from the paediatric inflammatory bowel disease in New Zealand (PINZ) study. J Pediatric Gastroenterol Nutr. 2018;66:e122‐e126. [DOI] [PubMed] [Google Scholar]
- 4. Schober E, Waldhoer T, Rami B, Hofer S, Austrian Diabetes Incidence Study Group . Incidence and time trend of type 1 and type 2 diabetes in Austrian children 1999–2007. J Pediatr. 2009;155:190‐193.e1. [DOI] [PubMed] [Google Scholar]
- 5. Ehehalt S, Dietz K, Willasch AM, Neu A. Prediction model for the incidence and prevalence of type 1 diabetes in childhood and adolescence: evidence for a cohort‐dependent increase within the next two decades in Germany. Pediatr Diabetes. 2012;13:15‐20. [DOI] [PubMed] [Google Scholar]
- 6. Hein R, Köster I, Bollschweiler E, Schubert I. Prevalence of inflammatory bowel disease: estimates for 2010 and trends in Germany from a large insurance‐based cohort. Scand J Gastroenterol. 2014;49:1325‐1335. [DOI] [PubMed] [Google Scholar]
- 7. Petritsch W, Fuchs S, Berghold A, et al. Incidence of inflammatory bowel disease in the province of Styria, Austria, from 1997 to 2007: a population‐based study. J Crohns Colitis. 2013;7:58‐69. [DOI] [PubMed] [Google Scholar]
- 8. Zurek M, Kern I, Manuwald U, et al. Epidemiology and care structures for children and adolescents and young adults up to the 26th year of life with inflammatory bowel diseases (IBD) in Leipzig/Saxony/Germany. J Public Health. 2018;26:437‐442. [Google Scholar]
- 9. Zurek M. Epidemiologie chronisch entzündlicher Darmerkrankungen bei Kindern und Jugendlichen in Sachsen sowie jungen Erwachsenen in Leipzig. Dissertation, University of Leipzig; 2013. https://nbn‐resolving.org/urn:nbn:de:bsz:15‐qucosa‐11574 [Google Scholar]
- 10. Benchimol EI, Manuel DG, To T, et al. Asthma, type 1 and type 2 diabetes mellitus, and inflammatory bowel disease amongst South Asian immigrants to Canada and their children: a population‐based cohort study. PLoS One. 2015;10:e0123599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fazeli Farsani S, Souverein PC, van der Vorst MMJ, Knibbe CA, de Boer A, Mantel‐Teeuwisse AK. Chronic comorbidities in children with type 1 diabetes: a population‐based cohort study. Arch Dis Child. 2015;100:763‐768. [DOI] [PubMed] [Google Scholar]
- 12. Bar Yehuda S, Axlerod R, Toker O, et al. The association of inflammatory bowel disease with autoimmune disorders: a report from the epi‐IIRN. J Crohns Colitis. 2019;13:324‐329. [DOI] [PubMed] [Google Scholar]
- 13. Kappelmann MD, Galanko JA, Porter CQ, Sandler RS. Association of paediatric inflammatory bowel disease with other immune‐mediated diseases. Arch Dis Child. 2011;96:1042‐1046. [DOI] [PubMed] [Google Scholar]
- 14. Halling ML, Kjeldsen J, Knudsen T, Nielsen J, Hansen LK. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017;23:6137‐6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kurppa K, Laitinen A, Agardh D. Coeliac disease in children with type 1 diabetes. Lancet Child Adolesc Health. 2018;2:133‐143. [DOI] [PubMed] [Google Scholar]
- 16. Cohen R, Robinson D, Paramore C, Fraeman K, Renahan K, Bala M. Autoimmune disease concomitance among inflammatory bowel disease patients in the United States, 2001–2002. Inflamm Bowel Dis. 2008;14:738‐743. [DOI] [PubMed] [Google Scholar]
- 17. Hofer SE, Schwandt A, Holl RW. Standardized documentation in pediatric diabetology: experience from Austria and Germany. J Diabetes Sci Technol. 2016;10:1042‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koch‐Institut R. Referenzperzentile für anthropometrische Maßzahlen und Blutdruck aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS). 2013;100‐111. 10.25646/3179 [DOI]
- 19. Fröhlich‐Reiterer EE, Rosenbauer J, Bechtold Dalla‐Pozza S, et al. Predictors of increasing BMI during the course of diabetes in children and adolescents with type 1 diabetes: data from the German/Austrian DPV multicentre survey. Arch Dis Child. 2014;99:738‐743. [DOI] [PubMed] [Google Scholar]
- 20. Abrahami D, Douros A, Yin H, et al. Dipeptidyl peptide‐4 inhibitors and incidence of inflammatory bowel disease among patients with type 2 diabetes: population based cohort study. BMJ. 2018;360:k872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thornberry NA, Gallwitz B. Mechanisms of action of inhibitors of dipeptidyl‐peptidase‐4 (DPP‐4). Best Pract Res Clin Endocrinol Metab. 2009;23:479‐486. [DOI] [PubMed] [Google Scholar]
- 22. Jess T, Jensen BW, Andersson M, Villumsen M, Allin KH. Inflammatory bowel disease increases risk of type 2 diabetes in a nationwide cohort study. Clin Gastroenterol Hepatol. 2020;18:881‐888.e1. [DOI] [PubMed] [Google Scholar]
- 23. Schenck S, Rosenbauer J, Niewerth M, et al. Comorbidity of type 1 diabetes mellitus in patients with juvenile idiopathic arthritis. J Pediatr. 2018;192:196‐203. [DOI] [PubMed] [Google Scholar]
- 24. Bechtold S, Blaschek A, Raile K, et al. Higher relative risk for multiple sclerosis in a pediatric diabetic population: analysis from the DPV database. Diabetes Care. 2014;37:96‐101. [DOI] [PubMed] [Google Scholar]
- 25. Teslova T, Kim M, Lukin D. Diabetes is associated with worse outcome in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(Suppl 1):19. [Google Scholar]


