Abstract
Background
Stratum corneum hydration (SCH) and transepidermal water loss (TEWL) provide useful information about skin barrier function. This study aimed to determine the value of GPSkin Pro, a new handheld device determining both SCH and TEWL, to measure skin barrier impairment and to monitor barrier function in rosacea in daily practice.
Materials and Methods
Two pilots were performed. Pilot 1: in 27 healthy participants, GPSkin SCH and TEWL were compared to Aquaflux® and Epsilon® values at the forearm before and after skin barrier perturbation via tapestripping. Moreover, GPSkin values were measured at both cheeks without intervention. Pilot 2: in 16 rosacea patients, GPSkin measurements were performed at the forearm, and at both cheeks before and during anti‐inflammatory treatment. They were compared to clinical symptoms and to GPSkin values from pilot 1.
Results
Pilot 1: after merging data from before and after tapestripping, a strong correlation was observed between GPSkin TEWL and Aquaflux® (Rs = 0.9256), and GPSkin SCH and Epsilon® (Rs = 0.8798). Pilot 2: SCH was significantly lower at the cheeks of rosacea patients compared to controls, with a normalizing trend during successful treatment. TEWL was comparable among patients and controls and did not change during treatment at all locations.
Conclusion
The GPSkin determines TEWL and SCH accurately in healthy and impaired skin barrier state and can monitor skin barrier function in rosacea during treatment. The GPSkin device is much more practical compared to previous skin barrier tools when used in clinical practice. Its further validation in other inflammatory skin diseases is recommended.
Keywords: capacitance, GPSkin, skin barrier function, skin hydration, stratum corneum, transepidermal water loss
1. INTRODUCTION
Measurement of stratum corneum hydration (SCH) and transepidermal water loss (TEWL) provide important information about the function of the skin barrier. 1 , 2 , 3 Impaired skin barrier function due to stratum corneum (SC) abnormalities is a hallmark of chronic inflammatory skin diseases, such as atopic dermatitis, psoriasis, and possibly also rosacea. 3 , 4 , 5 SCH and TEWL are also promising markers to distinguish healthy from inflamed skin and to monitor treatment. 6 , 7 , 8
A variety of skin barrier device methodologies is available to measure SCH and TEWL. 3 , 9 , 10 , 11 Unfortunately, these conventional devices have various disadvantages; they are expensive, bulky, not wireless, require repeated calibration, and intra‐ and inter‐instrument variation is large, making comparison of study outcomes challenging. 3 For all these reasons, assessment of skin barrier function is currently limited to research facilities with the available financial and logistic resources.
Recently, a new, noninvasive handheld device measuring SCH and TEWL simultaneously was introduced; the GPSkin. It is low‐cost, light‐weight, pocked‐sized, rapid, wireless, and data are directly transmitted to a smartphone application via Bluetooth. Earlier studies showed that the GPSkin provides precise and reliable SCH and TEWL values when compared to conventional devices in healthy skin. 12 , 13 , 14 Moreover, it is able to show skin barrier differences after application of topical agents. 14 However, to our knowledge its validity in case of a damaged skin barrier and its ability to monitor skin barrier function in patients with inflamed skin is not examined yet. As papules and pustules in rosacea often improve during anti‐inflammatory treatment, 15 we will use this facial dermatosis as a model to monitor inflamed skin state.
The aim of this study was to determine the value of GPSkin to measure accurate SCH and TEWL values after barrier function impairment, by comparing these values with conventional devices. Moreover, the value of the GPSkin to monitor skin barrier function in daily practice in rosacea patients during anti‐inflammatory treatment was determined. To do so, GPSkin values were compared to clinical scores.
2. MATERIALS AND METHODS
This explorative pilot study, approved by the local medical ethics committee, consisted of two sub‐pilots (Figure 1). In pilot 1, SCH and TEWL were determined with the GPSkin Pro (GPOWER Inc) at the volar forearm in healthy volunteers before and directly after skin barrier perturbation. As a validation for GPSkin values, parallel measurements with conventional devices were conducted; SCH with the Epsilon (E100, Biox), and TEWL with the Aquaflux (AF200, Biox). In pilot 2, GPSkin values were measured at both cheeks in rosacea patients before and during treatment. These values were linked to GPSkin values of healthy controls from pilot 1, and to their clinical symptoms.
FIGURE 1.
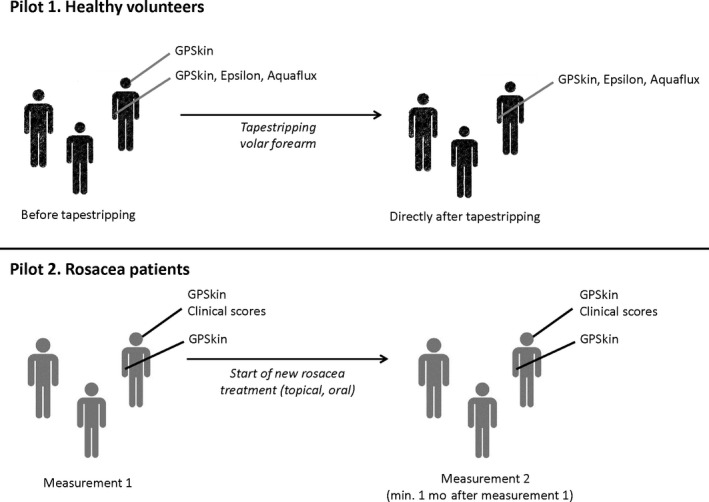
Overview of the study design, consisting of two sub‐pilots. Pilot 1, healthy volunteers. GPSkin, Epsilon, and Aquaflux measurements were performed at the right volar forearm before and directly after tapestripping. Moreover, GPSkin measurements were performed one at the left and right cheek without intervention. Pilot 2, rosacea patients. GPSkin values and clinical scores were determined at the left and right cheek before and min. 1 mo after start of new topical and/or oral anti‐inflammatory rosacea treatment. Additionally, GPSkin values were determined at both time points at the right volar forearm without intervention
2.1. Devices
2.1.1. GPSkin
The GPSkin measures SCH and TEWL simultaneously by placing its probe onto the skin during 5‐10 seconds. For SCH, two electronic sensors at the edge of the probe measure SC capacitance (ie dielectric constant). For TEWL, the probe opening (11 × 14 mm) contains a pseudo‐closed chamber system with temperature and humidity sensors; this system is similar to a closed chamber system, but provides chamber ventilation to decrease its humidity and pressure. All measurement results are directly sent by Bluetooth to a smartphone application for data access. 12 , 13 , 16 The device weights 40 g and is wireless.
2.1.2. Epsilon
Stratum corneum hydration can be measured with the Epsilon, a new generation corneometer, calculating the electrical capacitance of the SC by placing the probe of the device (1.3 × 1.5 cm) onto the skin for 30 seconds. Compared to conventional single sensor corneometers, the Epsilon contains 76 800 sensors, arranged in a 256 × 300 array with a spacial resolution of 50 µm and a sensing depth of 20 µm. Moreover, analysis software is integrated into the device, and linear water‐content‐based images can be obtained. This allows mapping of SCH and exclusion of regions with poor physical contact between sensor and skin. 17 , 18 , 19 The device is transported in a 2 kg case and measurements require connection to a laptop.
2.1.3. Aquaflux
This device measures TEWL by placing its probe onto the skin for a maximum of 180 s. The probe opening (7 × 7 mm) holds a closed chamber equipped with a condenser (−7.65°C). The condenser acts as a sink for incoming water vapor, crystallizing incoming moisture into ice. Water vapor flux due to diffusion is calculated using the humidity sensor with inbuilt calibration. No recovery time is necessary before starting the next measurement, as the chamber microclimate is controlled, independently of ambient humidity. 10 , 18 , 20 , 21 , 22 The device in total weights 1020 g and requires connection to a laptop.
2.2. Pilot 1 healthy skin
2.2.1. Participants
For pilot 1, healthy Caucasian volunteers were included. Informed consent was obtained from each participant. Measurements were performed in August 2018 at the department of Dermatology, Radboud University Medical Center, Nijmegen, the Netherlands. Exclusion criteria were: age <18 years, diagnosis of inflammatory/acneiform skin diseases, signs of inflammatory/acneiform skin diseases at the measurement sites, and use of immunosuppressive medication. Subjects did not use cream, body lotion, or foundation on the day of measurements and refrained from physical activity and showering within 3 hours before the measurements.
2.2.2. Study procedures
The measurements took place at the right volar forearm, because this location is easy to access, mainly refrained from UV‐light damage, hair, and sebaceous glands, and often used as a standard anatomical site for skin barrier studies. 3 , 22 , 23 , 24 All procedures were performed by one investigator (JGML). A circular area of approx. 3 × 3 cm was demarked with a pen at this location. The demarked skin was acclimatized to ambient air (room temperature: 22‐26°C; air humidity: 40%‐65%) for at least 10 minutes before start of measurements. Volunteers were placed in upright, sitting position during all study procedures.
First, SCH and TEWL were measured with the GPSkin once at the air‐exposed forearm. Then, SCH was determined by performing one measurement with the Epsilon. The Snapshot mode was used with a 5 seconds delay after first skin contact, and the average of three frames was calculated automatically. For both devices, moderate pressure was applied to keep contact with the skin surface. Thirdly, TEWL was measured with the Aquaflux. After calibration of this device, two measurements were performed with standard settings and a maximum measurement time of 180 seconds. The average of the two measurements was calculated. The Aquaflux was kept steady and perpendicular to the skin surface with very light skin pressure during measurements.
Next, the skin barrier of the demarked forearm location was disturbed using tapestripping, a noninvasive, painless, widely applied procedure to analyze SC barrier function without interfering with deeper, living epidermal keratinocytes. 9 , 25 , 26 , 27 , 28 , 29 Repetitive adhesive tapes were applicated to the skin for 10 seconds with a standardized pressure pen (150 g/cm2; D'Squame) and sequentially removed until the skin became partly to homogeneously refulgent, corresponding to partial to almost complete removal of the SC; 13‐33 tapes per volunteer were needed. In this way, a wide range of SCH and TEWL values was obtained.
Directly after the tapestripping procedure, GPSkin, Epsilon, and Aquaflux measurements were repeated at the demarked location as described above. Lastly, one GPSkin measurement per cheek site was performed in each volunteer for later comparison to rosacea patients in pilot 2 (Figure 1).
2.3. Pilot 2 Rosacea
2.3.1. Participants
Patients with a clinical diagnosis of facial rosacea were included in pilot 2 after signing informed consent. They were recruited between July and December 2019 at the department of Dermatology, Radboud University Medical Center, Nijmegen, Netherlands. Patients needed to start with new topical or oral anti‐inflammatory rosacea treatment according to clinical daily practice via their physician. 15 Excluded were patients aged <18 years, using immunosuppressive medication, or having other facial dermatological conditions or underlying diseases able to interfere with rosacea diagnosis or assessment.
2.3.2. Study procedures
Forearm and facial skin were acclimatized to ambient air for at least 10 minutes before the start of measurements. Then, one GPSkin measurement per cheek site and at the right volar forearm was performed. Additionally, facial clinical assessment was performed including lesion count, investigator's global assessment (IGA), papules and pustules scale, erythema scale, and telangiectasia scale (Table S1). Directly after these measurements, anti‐inflammatory rosacea treatment was started; topical ivermectin (n = 13), topical metronidazole (n = 1), doxycycline (n = 1), or topical ivermectin combined with doxycycline (n = 1). Minimally 1 month later (median follow‐up time: 63 days; range: 35‐94 days), GPSkin measurements and clinical assessment at both cheeks and the right forearm were repeated.
2.4. Statistical analysis
Due to the nonparametric character of data from pilot 1, Spearman correlation analysis (R s) was used to calculate the relationship between GPSkin values and results obtained from the Aquaflux and Epsilon. Next, a simple linear regression analysis (R 2) was performed to test for a possible linear relationship between the measurements from the GPSkin and conventional devices. For both analyses, values from before and after tapestripping were merged. For pilot 2, differences between baseline GPSkin values of rosacea patients and healthy controls were analyzed using the Mann‐Whitney U test. A Friedman test with Dunn‐Bonferroni post hoc method was performed to demonstrate possible differences between GPSkin results among the three body sites. GPSkin and lesion count differences of rosacea patients at baseline and during therapy were explored with the Wilcoxon signed‐rank test. For all statistical tests, P‐values <.05 were considered significant. Statistical analysis was performed using GraphPad Prism 5.03 (GraphPad Software) and SPSS (SPSS statistics 25, IBM Corporation).
3. RESULTS
3.1. Pilot 1 healthy skin
Twenty‐seven volunteers (18 females and nine males; median age 37 years, range 23‐67 years; skin type I‐III) participated in pilot 1. Correlation of GPSkin TEWL and SCH with Aquaflux and Epsilon was very strong (Rs > 0.80; Figure 2), and also highly linearly related (R2 > 0.80). Interestingly, the range of Epsilon values was large with GPSkin values ≥ 60. Before tapestripping, median TEWL was 5.1 g/m2/h (range: 0.4‐19.6) for GPSkin and 12.2 g/m2/h (range: 8.1‐17.6) for the Aquaflux. Median SCH was 21 arbitrary units (a.u.; range: 9‐38) for the GPSkin and 9.6 a.u. (range: 5.3‐16.1) for the Epsilon. After tapestripping, median TEWL was 42.7 (range: 19.6‐80.0) for the GPSkin and 70.8 (range: 25.1‐88.3) for the Aquaflux. Median SCH was 73 (range: 27‐74) for the GPSkin and 45.7 (range: 10.6‐63.5) for the Epsilon.
FIGURE 2.
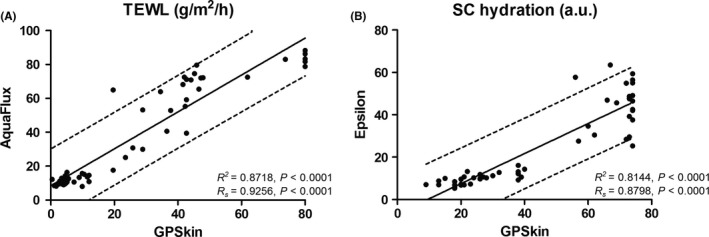
Linear regression with R2‐values and 95% confidence intervals (dotted lines) for GPSkin vs conventional devices. Spearman correlation coefficient (Rs) is also displayed. GPSkin was tested against the Aquaflux to measure TEWL (A) and the Epsilon to measure hydration (B)
3.2. Pilot 2 Rosacea
3.2.1. Comparison to healthy controls
Sixteen rosacea patients (11 females and five males; median age 51 years, range 21‐84 years) participated in pilot 2. No significant differences were found in TEWL GPSkin readings between rosacea patients and controls (Figure 3A). Post hoc analysis revealed that TEWL at the forearm was significantly lower compared to the left and right cheek, both in rosacea patients as well as controls (P < .05). SCH was significantly lower in rosacea patients compared to controls at the left and right cheek (Table 1, Figure 3B). SCH values showed no significant anatomical differences.
FIGURE 3.
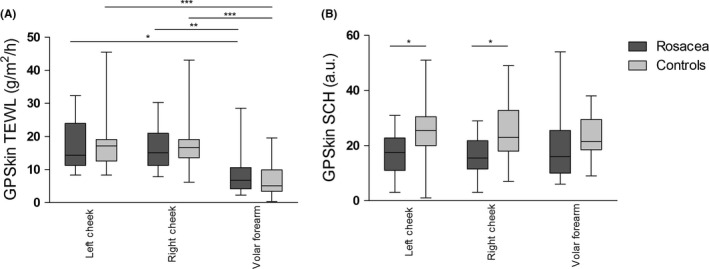
GPSkin results at the left cheek, right cheek, and volar forearm of rosacea patients at baseline and healthy controls. (A), TEWL, transepidermal water loss. (B), SCH, stratum corneum hydration. The boxes indicate the median value with 75th percentile and range. *0.01 ≥ P<.05, **0.001 ≥ P<.01, ***P < .001
TABLE 1.
GPSkin values of rosacea patients at baseline compared to healthy controls
| Left cheek | Right cheek | Volar forearm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rosacea (n = 16) | Controls (n = 27) | P‐value | Rosacea (n = 16) | Controls (n = 27) | P‐value | Rosacea (n = 16) | Controls (n = 27) | P‐value | |
| TEWL, g/m2/h | 14.4 (8.3‐32.4) a | 17.2 (8.3‐45.5) | .77 | 15.1 (7.8‐30.3) | 16.7 (6.2‐43.1) | .91 | 6.8 (2.3‐28.5) | 5.1 (0.4‐19.6) | .24 |
| SCH, a.u. | 19 (3‐31) | 25 (1‐51) | .010 | 16 (3‐29) | 23 (7‐49) | .013 | 16 (6‐54) | 21 (9‐38) | .062 |
Abbreviations: a.u., arbitrary unit; SCH, stratum corneum hydration; TEWL, transepidermal water loss.
Values are expressed as median (range).
Bold values indicate statistically significant P < .05.
3.2.2. Effect of treatment
All 16 rosacea patients attended the follow‐up visit. The GPSkin follow‐up data from the volar forearm of one patient were excluded, because TEWL and SCH were very low (4 g/m2/h and 1 a.u. respectively), probably due to a low battery. Median number of facial inflammatory lesions significantly decreased from 19 (range 0‐45) at baseline to 3 (range 0‐21; P = .001) during treatment. Improvement in IGA and erythema was noticed; telangiectasias remained unaffected (Figure 4). Compared to baseline, TEWL at both cheeks and the forearm did not change at follow‐up (Table 2, Figure 5). Although not statistically significant, a clear trend toward increased SCH at the left and right cheek was seen during treatment; this increase in SCH was not seen at the forearm. Figure 6 showed that SCH and TEWL were significantly and negatively correlated (Rs=−0.3970, P = .024). No correlations were found between GPSkin values and clinical scores (R2 all < 0.25; data not shown).
FIGURE 4.
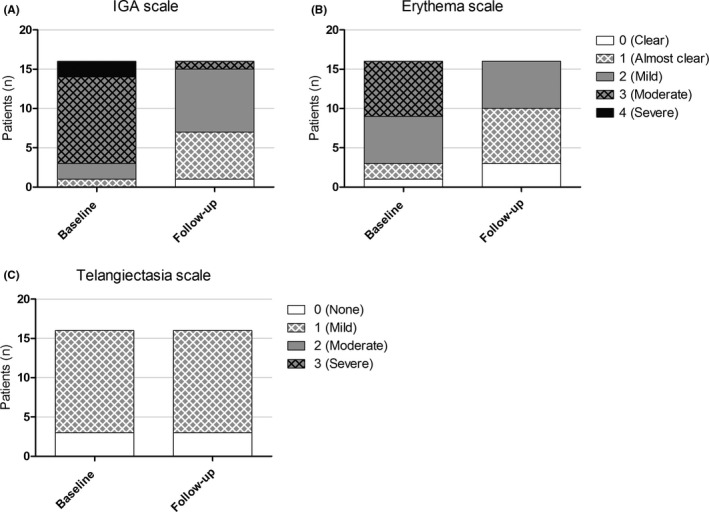
Clinical scores of all rosacea patients (n = 16) at baseline and during treatment (= follow‐up). (A‐C), IGA scale, erythema scale, and telangiectasia scale. IGA, investigator's global assessment
TABLE 2.
GPSkin values of rosacea patients at baseline and during treatment
| Left cheek | Right cheek | Volar forearm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 16) | During treatment (n = 16) | P‐value | Baseline (n = 16) | During treatment (n = 16) | P‐value | Baseline (n = 16) | During treatment (n = 15) | P‐value | |
| TEWL, g/m2/h | 14.4 (8.3‐32.4) a | 13.6 (6.7‐25.3) | .23 | 15.1 (7.8‐30.3) | 14.9 (6.7‐34.1) | .59 | 6.8 (2.3‐28.5) | 7.8 (3.4‐28.9) | .36 |
| SCH, a.u. | 19 (3‐31) | 22 (13‐38) | .11 | 16 (3‐29) | 21 (7‐34) | .059 | 16 (6‐54) | 16 (9‐43) | .70 |
Abbreviations: a.u., arbitrary unit; SCH, stratum corneum hydration; TEWL, transepidermal water loss.
Values are expressed as median (range).
FIGURE 5.
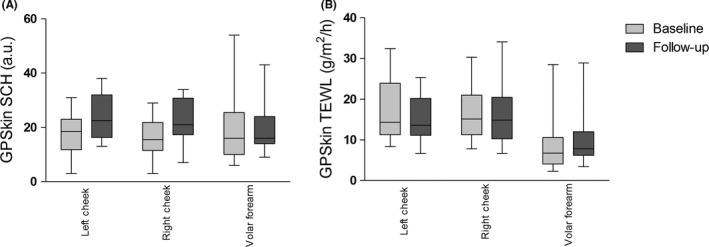
GPSkin results at the left cheek, right cheek, and volar forearm of rosacea patients at baseline and during follow‐up. (A), TEWL, transepidermal water loss. (B), SCH, stratum corneum hydration. The boxes indicate the median value with 75th percentile and range
FIGURE 6.
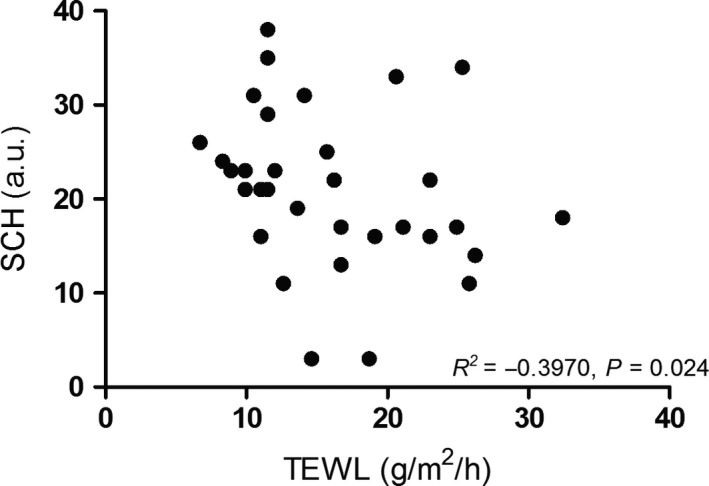
Weak inverse correlation found between stratum corneum hydration and transepidermal water loss in rosacea patients (baseline and follow‐up data combined), measured with the GPSkin
4. DISCUSSION
We showed that the GPSkin is able to provide reliable and accurate TEWL and SCH values compared to conventional devices, also after skin barrier perturbation. Moreover, we found that SCH, measured with the GPSkin, was significantly lower in rosacea patients compared to controls, with a recovering trend toward normal values after successful rosacea treatment. TEWL values in rosacea patients were comparable to healthy controls and did not change during treatment.
The GPSkin offers interesting advantages for application in clinical practice compared to conventional skin barrier devices. First, it measures TEWL and SCH simultaneously, preventing precise replacements of probes on the same skin site. 14 Second, data about skin temperature and humidity are displayed. Third, it is extremely portable, affordable, allows rapid, and simple measuring and has a long battery life (months, depending on use frequency), resulting in much higher ease of use compared to the non‐wireless, heavier‐weighted Epsilon and Aquaflux. Forth, data results are immediately visible at the smartphone screen, allowing immediate feedback to the patient. Based on our experiences within this study, this is very beneficial for improvement of therapy compliance.
In pilot 1, the correlation of TEWL and SCH measured by the GPSkin and conventional tools was very high, both before and after tapestripping. The Aquaflux has a sophisticated chamber system to measure changes in TEWL after tapestripping, 10 , 20 and the Epsilon provides precise SCH values due to its multi‐sensor character. 17 This implicates that the GPSkin is able to provide very accurate skin barrier values as well. Aquaflux TEWL values were consistently higher compared to GPSkin with equal ranges, both before and after tapestripping. This can be explained by calibration differences between both devices. 12 Theoretically, unventilated closed chamber systems such as GPSkin could result in divergent values after prolonged measuring due to water vapor accumulation, 3 , 10 but this was not observed in this study. Regarding SCH, GPSkin values were higher than Epsilon values. As the Epsilon has multiple sensors, the sensing depth is more superficial compared to conventional devices (which have only one sensor), confining measurement to the ‘dry’ SC only before tapestripping. 17 After tapestripping, SCH measured by the Epsilon showed larger diversity than those measured by the GPSkin (Figure 2). This may be caused by tapestripping heterogeneity; this technique often results in skin areas with high damage surrounded by relatively undamaged SC. 10 Due to the relatively large probe surface and multi‐sensory character of the Epsilon, all these areas were integrated into the measurement, while GPSkin values were determined based on only one sensor with a smaller probe. Moreover, if the SC is mostly removed, capacitance measurements primarily reflect the hydration state of the ‘wet’ stratum granulosum rather than the SC 23 , 29 ; especially the Epsilon device seems less reliable in this situation.
The number of tape strips removed varied between the healthy volunteers; we tried to induce a partial to almost complete skin barrier removal, in order to obtain a wide range of SCH and TEWL values. A stronger SC cohesion results in less mass removal, requiring more strips to be applicated for the same barrier disruption effect. 9 Tape stripping procedure may be influenced by contact time, anatomical location, and applied pressure. 30 Therefore, a standardized protocol is needed, which we used.
Current literature is inconclusive regarding potential SCH and TEWL differences in rosacea skin compared to healthy skin, probably due to a large heterogeneity in studied rosacea subtypes, measurement locations, and biophysical devices. 31 We hypothesize that rosacea skin displays decreased SCH due to skin dryness, a frequently mentioned symptom in this skin disease. Application of topical treatment, such as ivermectin, reduces skin dryness and thereby increases SCH. SCH and TEWL were negatively correlated in the rosacea group, implying that an increase in skin hydration could slow down the TEWL. 14 Decreased TEWL after treatment was however not observed in this study. Possibly, skin barrier recovery measured by TEWL takes longer than the immediate moisturizing effect, especially in nonprotected areas such as the face. 14 , 32 , 33
Both in rosacea patients and in controls, TEWL of the forearm was lower than the cheeks. This is in line with previous work; anatomical differences in TEWL may be caused by intrinsic differences in eccrine sweat gland and sebaceous follicle activity, skin temperature, blood flow, SC thickness, lipid content, and corneocyte size and turnover time. 33 , 34 , 35 , 36 Most likely, also external physical effects in the face may cause differences in TEWL values. No chiral skin barrier differences seem to occur between the left and right forearm. 36 , 37 However, regional differences for both TEWL as well as SCH exist within short distances of the face, requiring measurements at exactly the same place during follow‐up. 33 , 36 , 37 , 38 This makes rosacea a challenging model for skin inflammation.
In pilot 2, we deliberately chose not to use a climate room during measurements, as this prevents translation of our results into the daily, clinical setting. We accepted normal fluctuations in weather, season, and daytime. Despite this, TEWL values were constant at baseline and at follow‐up, implying that external factors did not have a large impact on the results at both time points. This is a very interesting finding, making application in daily practice certainly feasible. However, it remains important to interpret skin barrier results in the light of potential influencing external factors such as temperature, humidity, occlusion, UV‐light, anatomical location, cream use, physical activity, and sweating. 5 , 35 , 39 , 40 Considering the inter‐individual variations in SCH and TEWL, a baseline value should always be registered in each patient, and lesional skin should be compared with non‐lesional skin. 9
5. CONCLUSION
The GPSkin allows accurate, simple, and rapid determination of TEWL and SCH, both in normal as well as in impaired skin barrier. Moreover, the GPSkin is able to measure improvement in skin barrier parameters in inflamed skin during successful treatment and could therefore possibly contribute to objectification of treatment effectiveness. Based on our results, influence of external factors on GPSkin values seems to be limited. Further validation of the GPSkin in other inflammatory skin diseases with impaired skin barrier, such as atopic dermatitis and psoriasis, is preferred. Ultimately, the GPSkin would replace the conventional, expensive, and relatively complex skin barrier tools, both in research and clinical setting. This paves the way for objective, home‐based skin barrier monitoring for patients with a variety of inflammatory skin diseases, further improving patient‐centered care and therapy compliance.
CONFLICTS OF INTEREST
JGML has received a research grant from Galderma. She carried out clinical trials for Abbvie, Novartis, Janssen, and LEO Pharma. She has received reimbursement for attending meetings from Abbvie. RJBD has received a research grant from Galderma. She carried out clinical trials for Cutanea Life Sciences, Galderma, Abbvie, Novartis, and Janssen. She has received reimbursement for attending meetings from Abbvie and Galderma. She has served as a consultant for Abbvie, Galderma, and Novartis. EMGJ has received research grants from AbbVie, Pfizer, Novartis, Janssen Pharmaceuticals, and Leo Pharma. All fees were paid directly to the institution. PEJE has no conflicts of interest to declare.
Supporting information
Tab S1
Logger JGM, Driessen RJB, de Jong EMGJ, van Erp PEJ. Value of GPSkin for the measurement of skin barrier impairment and for monitoring of rosacea treatment in daily practice. Skin Res Technol.2021;27:15–23. 10.1111/srt.12900
REFERENCES
- 1. Loden M. Effect of moisturizers on epidermal barrier function. Clin Dermatol. 2012;30:286‐296. [DOI] [PubMed] [Google Scholar]
- 2. Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771‐788. [DOI] [PubMed] [Google Scholar]
- 3. Berardesca E, Loden M, Serup J, et al. The revised EEMCO guidance for the in vivo measurement of water in the skin. Skin Res Technol. 2018;24:351‐358. [DOI] [PubMed] [Google Scholar]
- 4. Addor FA. Skin barrier in rosacea. An Bras Dermatol. 2016;91:59‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sahle FF, Gebre‐Mariam T, Dobner B, et al. Skin diseases associated with the depletion of stratum corneum lipids and stratum corneum lipid substitution therapy. Skin Pharmacol Physiol. 2015;28:42‐55. [DOI] [PubMed] [Google Scholar]
- 6. Ortiz A, Elkeeb L, Truitt A, et al. Topical PRK 124 (0.125%) lotion for improving the signs and symptoms of rosacea. J Drugs Dermatol. 2009;8:459‐462. [PubMed] [Google Scholar]
- 7. Xie HF, Huang YX, He L, et al. An observational descriptive survey of rosacea in the Chinese population: clinical features based on the affected locations. PeerJ. 2017;5:e3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhong S, Sun N, Liu H, et al. Topical tranexamic acid improves the permeability barrier in rosacea. Dermatologica Sinica. 2015;33:112‐117. [Google Scholar]
- 9. Darlenski R, Sassning S, Tsankov N, et al. Non‐invasive in vivo methods for investigation of the skin barrier physical properties. Eur J Pharm Biopharm. 2009;72:295‐303. [DOI] [PubMed] [Google Scholar]
- 10. Farahmand S, Tien L, Hui X, et al. Measuring transepidermal water loss: a comparative in vivo study of condenser‐chamber, unventilated‐chamber and open‐chamber systems. Skin Res Technol. 2009;15:392‐398. [DOI] [PubMed] [Google Scholar]
- 11. Rogiers V. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol Appl Skin Physiol. 2001;14:117‐128. [DOI] [PubMed] [Google Scholar]
- 12. Grinich EE, Shah AV, Simpson EL. Validation of a novel smartphone application‐enabled, patient‐operated skin barrier device. Skin Res Technol. 2019;25:612‐617. [DOI] [PubMed] [Google Scholar]
- 13. Ye L, Wang Z, Li Z, et al. Validation of GPSkin Barrier((R)) for assessing epidermal permeability barrier function and stratum corneum hydration in humans. Skin Res Technol. 2019;25:25‐29. [DOI] [PubMed] [Google Scholar]
- 14. Caberlotto E, Cornillon C, Njikeu S, et al. Synchronized in vivo measurements of skin hydration and trans‐epidermal water loss. Exploring their mutual influences. Int J Cosmet Sci. 2019;41:437‐442. [DOI] [PubMed] [Google Scholar]
- 15. van Zuuren EJ, Fedorowicz Z, Tan J, et al. Interventions for rosacea based on the phenotype approach: an updated systematic review including GRADE assessments. Br J Dermatol. 2019;181:65‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gpower . Meet the GPSkin Barrier 2018. https://mygpskin.com/device. Accessed 12‐08‐2019.
- 17. Logger JGM, Munchhoff CU, Olydam JI, et al. Anatomical site variation of water content in human skin measured by the epsilon: a pilot study. Skin Res Technol. 2019;25:333‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Logger JGM, Olydam JI, Woliner‐van der Weg W, et al. Noninvasive skin barrier assessment: multiparametric approach and pilot study. Cosmetics. 2019;6:20. [Google Scholar]
- 19. Zhang X, Bontozoglou C, Chirikhina E, et al. Capacitive imaging for skin characterizations and solvent penetration measurements. Cosmetics. 2018;5:52. [Google Scholar]
- 20. Alexander H, Brown S, Danby S, et al. Research techniques made simple: transepidermal water loss measurement as a research tool. J Invest Dermatol. 2018;138(11):2295‐2300.e1. [DOI] [PubMed] [Google Scholar]
- 21. Imhof RE, De Jesus ME, Xiao P, et al. Closed‐chamber transepidermal water loss measurement: microclimate, calibration and performance. Int J Cosmet Sci. 2009;31:97‐118. [DOI] [PubMed] [Google Scholar]
- 22. Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. A report from the standardization group of the European society of contact dermatitis. Contact Dermatitis. 1990;22:164‐178. [DOI] [PubMed] [Google Scholar]
- 23. Jansen van Rensburg S, Franken A, Du Plessis JL. Measurement of transepidermal water loss, stratum corneum hydration and skin surface pH in occupational settings: a review. Skin Res Technol. 2019;25:595‐605. [DOI] [PubMed] [Google Scholar]
- 24. Bazin R, Fanchon C. Equivalence of face and volar forearm for the testing of moisturizing and firming effect of cosmetics in hydration and biomechanical studies. Int J Cosmet Sci. 2006;28:453‐460. [DOI] [PubMed] [Google Scholar]
- 25. Myer K, Maibach H. Stratum corneum evaluation methods: overview. Skin Res Technol. 2013;19:213‐219. [DOI] [PubMed] [Google Scholar]
- 26. Koppes SA, Kemperman P, Van Tilburg I, et al. Determination of natural moisturizing factors in the skin: Raman microspectroscopy versus HPLC. Biomarkers. 2017;22:502‐507. [DOI] [PubMed] [Google Scholar]
- 27. Janssens M, van Smeden J, Gooris G, et al. Increase in short‐chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res. 2012;53(12):2755‐2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Erp PEJ, Peppelman M, Falcone D. Noninvasive analysis and minimally invasive in vivo experimental challenges of the skin barrier. Exp Dermatol. 2018;27:867‐875. [DOI] [PubMed] [Google Scholar]
- 29. Fluhr JW, Dickel H, Kuss O, et al. Impact of anatomical location on barrier recovery, surface pH and stratum corneum hydration after acute barrier disruption. Br J Dermatol. 2002;146:770‐776. [DOI] [PubMed] [Google Scholar]
- 30. Breternitz M, Flach M, Prassler J, et al. Acute barrier disruption by adhesive tapes is influenced by pressure, time and anatomical location: integrity and cohesion assessed by sequential tape stripping. A randomized, controlled study. Br J Dermatol. 2007;156:231‐240. [DOI] [PubMed] [Google Scholar]
- 31. Logger JGM, de Vries FMC, van Erp PEJ, et al. Noninvasive objective skin measurement methods for rosacea assessment: a systematic review. Br J Dermatol. 2019;182(1):55‐66. [DOI] [PubMed] [Google Scholar]
- 32. Voegeli R, Gierschendorf J, Summers B, et al. Facial skin mapping: from single point bio‐instrumental evaluation to continuous visualization of skin hydration, barrier function, skin surface pH, and sebum in different ethnic skin types. Int J Cosmet Sci. 2019;41:411‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tagami H. Location‐related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int J Cosmet Sci. 2008;30:413‐434. [DOI] [PubMed] [Google Scholar]
- 34. Kleesz P, Darlenski R, Fluhr JW. Full‐body skin mapping for six biophysical parameters: baseline values at 16 anatomical sites in 125 human subjects. Skin Pharmacol Physiol. 2012;25:25‐33. [DOI] [PubMed] [Google Scholar]
- 35. Marrakchi S, Maibach HI. Biophysical parameters of skin: map of human face, regional, and age‐related differences. Contact Dermatitis. 2007;57:28‐34. [DOI] [PubMed] [Google Scholar]
- 36. Wa CV, Maibach HI. Mapping the human face: biophysical properties. Skin Res Technol. 2010;16:38‐54. [DOI] [PubMed] [Google Scholar]
- 37. Chilcott RP, Farrar R. Biophysical measurements of human forearm skin in vivo: effects of site, gender, chirality and time. Skin Res Technol. 2000;6:64‐69. [DOI] [PubMed] [Google Scholar]
- 38. Kobayashi H, Tagami H. Distinct locational differences observable in biophysical functions of the facial skin: with special emphasis on the poor functional properties of the stratum corneum of the perioral region. Int J Cosmet Sci. 2004;26:91‐101. [DOI] [PubMed] [Google Scholar]
- 39. Nam GW, Baek JH, Koh JS, et al. The seasonal variation in skin hydration, sebum, scaliness, brightness and elasticity in Korean females. Skin Res Technol. 2015;21:1‐8. [DOI] [PubMed] [Google Scholar]
- 40. Akdeniz M, Gabriel S, Lichterfeld‐Kottner A, et al. Transepidermal water loss in healthy adults: a systematic review and meta‐analysis update. Br J Dermatol. 2018;179:1049‐1055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1


