Abstract
It is well recognized that human periodontal ligament cells (PDL cells) may represent local immune cells of the periodontal tissues. However, it is unclear whether they represent “true” immune cells, since they can produce pro‐inflammatory cytokines not only after stimulation with bacterial lipopolysaccharides but also in response to other stimuli such as mechanical stress. Stimulation with bacterial lipopolysaccharides strongly enhances PDL cell production of pro‐inflammatory cytokines through activation of toll‐like receptors and NF‐κB signaling. Less information is available regarding putative modulators of cytokine production and their mechanisms of action in PDL cells. The anti‐inflammatory glucocorticoid dexamethasone reduces lipopolysaccharide‐induced PDL cell production of cytokines. Recent observations show that vitamin D and the antimicrobial peptide LL‐37 antagonize lipopolysaccharide‐stimulated PDL cell production of pro‐inflammatory cytokines. Secretory leukocyte protease inhibitor is endogenously expressed by PDL cells, and this protein negatively regulates PDL cell‐evoked cytokine production. More information and knowledge about the regulation of PDL cell production of cytokines may clarify the role of PDL cells in oral innate immunity and their importance in periodontitis.
Keywords: inflammation, innate immunity, NF‐κB, periodontitis
1. INTRODUCTION
The human periodontal ligament cell (PDL cell) has been described as a mesenchymal fibroblast‐like cell with multifunctional properties. The PDL cell can produce collagen, a functional quality typical for fibroblasts, but it can also be transformed into an osteoblast‐like cell with capacity to form bone‐like mineralized tissue expressing bone marker proteins, suggesting that PDL cells can influence periodontal regeneration and tissue repair. 1 , 2 Human PDL cells have successfully been reprogrammed into induced pluripotent stem (iPS) cells, and thus, PDL cells may serve as an important source for iPS cells. 3 Interestingly, the PDL cell has also the capacity to produce pro‐inflammatory cytokines in response to stimulation with bacterial endotoxins such as lipopolysaccharide (LPS), indicating that PDL cells are key players in oral innate immunity. 4 The PDL cell produces important pro‐inflammatory cytokines and chemokines which mediate recruitment of white blood cells to the periodontal inflammation/infection, suggesting that this is indeed an essential cell type in the periodontal innate immune reaction. Blood neutrophils and monocytes/macrophages can also contribute to increased cytokine levels in periodontal inflammation, but one may speculate that the PDL cells are mainly responsible for the local cytokine/chemokine production in the acute early phase of periodontal inflammation/infection, since the vascular endothelium first has to become permeable to allow for trans‐endothelial transport of immune cells from blood to the periodontal tissues, a procedure which probably takes time.
Although it is well recognized that human PDL cells are able to produce pro‐inflammatory cytokines upon stimulation with bacteria and bacterial endotoxins, the modulation of this process and its underlying cellular and molecular mechanisms is poorly understood. LPS produced by various types of gram‐negative bacteria causes a rapid and profound stimulation of pro‐inflammatory cytokine gene expression and protein production. 4 , 5 Vitamin D reduces PDL expression of pro‐inflammatory cytokines probably via activation of the intracellular vitamin D receptor (VDR) expressed by human PDL cells. 6 , 7 , 8 Thus, also an agent like vitamin D which is produced by skin keratinocytes and/or taken up from the diet can modulate PDL cell pro‐inflammatory properties. Under inflammatory/infectious conditions, neutrophils are recruited to the inflamed tissue and they release the antimicrobial peptide LL‐37. High expression of the peptide has been detected in gingival tissues and gingival crevicular fluid from periodontitis patients, indicating that LL‐37 may have impact on PDL cell functional properties in periodontitis. 9 , 10 , 11 , 12 Interestingly, LL‐37 shows activity against many oral pathogens, suggesting that the peptide may play an important role in the defense against invading oral bacteria. 13 However, LL‐37 not only kills bacteria but it can also modulate host defense and innate immunity in multiple ways. 14 , 15 Recently, Aidoukovitch et al observed that LL‐37 is internalized by human PDL cells and that it reduces PDL cell production of the chemokine monocyte chemoattractant protein‐1 (MCP‐1). 16 Hence, LL‐37 seems to act anti‐inflammatory through an intracellular mechanism of action in PDL cells. Moreover, the protein secretory leukocyte protease inhibitor (SLPI), endogenously expressed by PDL cells, has recently been shown to act as a negative regulator of PDL cell pro‐inflammatory cytokine production, indicating that proteins expressed by PDL cells themselves also can modulate their production of cytokines. 17
In this mini review, I discuss both non‐PDL cell‐dependent (bacterial endotoxins, vitamin D, and LL‐37) and PDL cell‐dependent (the SLPI protein expressed by PDL cells) modulators and mechanisms involved in the regulation of PDL cell‐evoked cytokine/chemokine production. Information source for this mini review is published articles appearing in PubMed®. More knowledge about the mechanisms involved in the modulation of PDL cell production of pro‐inflammatory cytokines is necessary in order to understand the immune cell‐like properties of PDL cells and their importance in periodontitis.
2. RECEPTORS AND SIGNALING IN LPS‐INDUCED STIMULATION OF PDL CELL PRODUCTION OF PRO‐INFLAMMATORY CYTOKINES
The bacterial cell wall component LPS is produced by gram‐negative bacteria such as the periodontitis pathogens P gingivalis and A actinomycetemcomitans. Notably, stimulation with LPS from different types of bacteria shows on the whole powerful stimulation of PDL cell production of pro‐inflammatory cytokines with similar time course and concentration‐response patterns, although differences in sensitivity to, for example, P gingivalis and E coli LPS have been reported. 5 , 18 LPS from E coli is often used to assess cellular cytokine production in experimental systems. Although LPS from P gingivalis has a different structure compared to E coli LPS, both types of LPS induce cytokine mRNA expression in human PDL cells in a similar pattern. 5 LPS from A actinomycetemcomitans has also been shown to enhance PDL cell cytokine expression, and thus, different types of bacterial LPS trigger PDL cell cytokine production. 19 E coli LPS is used in many experimental in vitro studies to stimulate oral cells, which may represent a weakness when it comes to in vivo relevance. Lipoteichoic acid (LTA) is a bacterial endotoxin produced by gram‐positive bacteria. Although there are reports showing that LTA also may enhance PDL cell production of pro‐inflammatory cytokines, much less information is available regarding LTA‐induced production of cytokines by PDL cells as compared to the well‐documented stimulatory effects of LPS on PDL cell cytokine production. 20 , 21 Im et al 19 show that LTA has no effect on PDL cell expression of IL‐8 and that LTA, in fact, antagonizes A actinomycetemcomitans LPS‐induced PDL cell IL‐8 expression. LPS acts as an agonist for toll‐like receptor 4 (TLR4), whereas LTA is a TLR2 agonist. Importantly, human PDL cells express both TLR2 and TLR4, and upon activation, these receptors promote cytokine expression mainly through NF‐κB activation. 18 , 22 , 23 Hence, it can be concluded that agonists for both TLR2 and TLR4 have an impact on PDL cell‐evoked cytokine production. Notably, there are findings indicating that human PDL cells may express higher transcript levels of TLR2 compared to TLR4. 18
High expression of IL‐6 is observed in diseased periodontal tissues, and polymorphism of the IL‐6 gene has been reported to be associated with periodontitis, strongly suggesting that IL‐6 is involved in the initiation/progression of the disease. 24 , 25 P gingivalis LPS seems to be a weak stimulator of PDL cell IL‐6 production compared to E coli LPS. 18 , 26 , 27 Interestingly, dental follicle cells, which are considered to represent periodontal progenitor cells, show enhanced IL‐6 gene expression in response to E coli LPS stimulation, whereas stimulation with P gingivalis LPS lacks effect on IL‐6 transcript expression in these cells. 28 These data suggest that also dental follicle cells are more sensitivity to E coli LPS than P gingivalis LPS, when it comes to stimulation of IL‐6 expression. However, it should be noted that there are several studies which clearly show that stimulation with both P gingivalis bacteria and P gingivalis LPS enhances PDL cell expression of IL‐6. 6 , 7 , 29 P gingivalis LPS has been reported to act as an agonist for TLR2 and as a potential antagonist for TLR4, whereas E coli LPS preferentially activates TLR4. 30 , 31 Taking into consideration that P gingivalis LPS is an agonist for TLR2 and that PDL cells have been reported to express high levels of transcript for TLR2 suggests that the low sensitivity of PDL cells to P gingivalis LPS is due to another mechanism than low PDL cell expression of TLR2. 18 Hence, it is more likely that differences in sensitivity of PDL cells to P gingivalis and E coli LPS are associated with LPS subtype‐specific signaling downstream of the TLRs. Importantly, we have also to consider that differences in sensitivity of PDL cells to LPS from P gingivalis and E coli may reflect LPS subtype‐specific structural properties and variations in purity between batches of LPS.
Both LPS‐induced production of IL‐6 and chemokine (C‐X‐C motif) ligand 1 (CXCL1, also named GROα) by PDL cells are antagonized by the glucocorticoid dexamethasone, suggesting that PDL cell production of pro‐inflammatory cytokines in periodontitis can be counteracted by treatment with glucocorticoids. 18 , 32 Notably, glucocorticoids are thought to abolish inflammation via inhibition of NF‐κB signaling leading to reduced production of pro‐inflammatory cytokines, but their exact mechanism of action is still not completely understood. 33
The concentration‐response relationship for E coli LPS‐stimulated PDL IL‐6 production shows that stimulation with 0.5 and 10 μg/mL LPS for 24 hours enhances IL‐6 protein production 3 and 9 times, whereas a lower concentration (0.1 μg/mL) of LPS has no effect. 34 Notably, researchers do not normally use higher concentrations of LPS than 10 μg/mL, since they may produce unspecific effects. Stimulation with 10 μg/mL LPS for a longer time (72 hours) increases IL‐6 protein by about 10 times, indicating that LPS‐stimulated IL‐6 production reaches a plateau within this time frame. Hence, it seems that E coli LPS causes a long‐term and powerful activation of IL‐6 production by PDL cells.
LPS/TLR4 signaling involves different adaptor proteins such as myeloid differentiation factor 88 (MyD88). LPS‐induced activation of TLR4 may cause either MyD88‐dependent or MyD88‐independent downstream signaling. 30 For the MyD88‐dependent pathway, the LPS/TLR4/MyD88 complex binds and activates downstream adaptor proteins leading to activation of PI3K/Akt and NF‐κB. Besides activation of PI3K/Akt and NF‐κB, MyD88‐dependent signaling also involves activation of MAPKs such as ERK, JNK, and p38. The functional effects of NF‐κB are associated with transcriptional activation of multiple pro‐inflammatory genes such as IL‐1, IL‐6, and TNFα, whereas PI3K/Akt, ERK, JNK, and p38 activation is coupled to inflammatory pathways but also cell proliferation/cell survival and apoptotic signaling. Activation of the MyD88‐independent pathway causes activation and nuclear translocation of NF‐κB and IRF3 transcription factors leading to transcription of genes belonging to the interferon family of cytokines. MyD88 plays a critical role also for TLR2‐induced activation of NF‐κB and production of cytokines. In human PDL cells, knockdown of MyD88 prevents both P gingivalis LPS‐induced and synthetic TLR2 agonist‐induced IL‐6 and IL‐8 transcript expression, indicating that MyD88 is necessary for TLR2 agonist‐evoked PDL cell cytokine production. 35
3. NOT ONLY LPS BUT ALSO OTHER STIMULI TRIGGER PDL CELL PRODUCTION OF PRO‐INFLAMMATORY CYTOKINES
It is well recognized that mechanical loading can induce expression of cytokines associated with inflammation in cultured human PDL cells. 36 , 37 PDL cells subjected to mechanical force show increased IL‐6 gene expression and also enhanced IL‐6 protein production compared to untreated control cells, and these effects seem to be reduced by heat shock protein 70. 37 Up‐regulation of PDL cell pro‐inflammatory cytokine expression by mechanical/orthodontic stress may also involve TLR4 signaling, suggesting that mechanical forces induce PDL cell cytokine expression through multiple pathways. 38 Thermal stress has also been reported to induce IL‐6 and IL‐8 gene expression in human PDL cells, indicating that PDL cells may respond to many different types of stimuli with elevated cytokine expression. 39 Hence, classical infection/inflammation mediators, such as LPS, stimulate PDL cell production of pro‐inflammatory cytokines, but also other forms of stimuli, which are not primarily related to infections and infectious agents, can enhance production of cytokines in PDL cells.
What about the relative importance of PDL cell production of cytokines vs. that of classical immune cells in periodontitis? This is a complicated and challenging question to answer. To my knowledge, there are no reports published addressing this interesting issue. It is definitely a big challenge to establish an experimental system where both the total amounts and concentrations of all relevant cytokines/chemokines produced by PDL cells in response to different inflammation promoters are compared to those of neutrophils, monocytes, and macrophages.
In this context, it should be mentioned that bacterial endotoxins such as P gingivalis LPS and E coli LPS trigger pro‐inflammatory cytokine expression also in human gingival fibroblasts. 40 , 41 Thus, different types of periodontal tissue fibroblast‐like cells may contribute to cytokine production in periodontitis. It is difficult to assess the pathophysiological importance of cytokines produced by PDL cells compared to that of cytokines produced by gingival fibroblasts, since these cell types show different anatomical location and total cell number. Importantly, PDL cells are present in the interface between the root cementum and the alveolar bone tissue and thus very close to the osteoblasts of the bone tissue, arguing that PDL‐evoked cytokine production is indeed important.
4. VITAMIN D ATTENUATES PDL CELL PRODUCTION OF PRO‐INFLAMMATORY CYTOKINES
Pro‐vitamin D is produced by skin epithelial cells via a UV light‐dependent process. The keratinocytes release the pro‐hormone vitamin D3 which then undergoes metabolic transformation to biologically active 1α,25‐dihydroxyvitamin D3 in the liver and kidneys. 42 , 43 Besides the endogenous synthesis of pro‐hormone vitamin D3 by keratinocytes, humans can get the pro‐hormones vitamin D2 and D3 from the diet. Dietary vitamin D is taken up by intestinal epithelial cells and transported to the liver and kidneys where it is processed to 1α,25‐dihydroxyvitamin D3. In this review, vitamin D refers to biologically active 1α,25‐dihydroxyvitamin D3. The main functional responses of vitamin D involve regulation of calcium and phosphorus uptake from the gut, and hereby the hormone plays an important role in bone formation and bone metabolism, but vitamin D has also been shown to modulate the immune system. 43 , 44 Vitamin D is lipophilic and thus can diffuse over the plasma membrane and bind to its intracellular vitamin D receptor (VDR). The vitamin D/VDR forms a complex with retinoid X receptors, and this complex then activates or inactivates target genes. 43 Importantly, all transcriptional effects of vitamin D are regarded to be mediated via VDR. 43
Treatment with vitamin D, at a concentration which corresponds to a high plasma concentration of the vitamin, reduces E coli LPS‐induced IL‐6 and CXCL1 but not IL‐1β and MCP‐1 transcript expression in human PDL cells, suggesting that vitamin D differentially affects LPS‐stimulated pro‐inflammatory cytokine/chemokine expression in PDL cells. 8 Andrukhov et al have also reported that vitamin D affects LPS‐induced pro‐inflammatory cytokine production differentially in human PDL cells. 7 These authors show that vitamin D attenuates P gingivalis LPS‐stimulated IL‐8 and MCP‐1 production but has no effect on IL‐6. Thus, vitamin D can reduce LPS‐stimulated PDL cell production of pro‐inflammatory cytokines, but this effect seems to be dependent on experimental conditions such as the type of LPS used for stimulation of cytokine production. For E coli LPS‐stimulated IL‐6 expression in PDL cells, also protein production is down‐regulated by vitamin D (3‐300 ng/mL), indicating that vitamin D3‐induced anti‐inflammatory effects involve local attenuation of IL‐6 production in the periodontal tissues. 8 Human PDL cells express VDR protein and transcript, indicating that vitamin D‐induced attenuation of cytokine expression is mediated via activation of VDR. 7 , 8 Moreover, Andrukhov et al have demonstrated that knockdown of VDR with siRNA abolishes vitamin D‐induced down‐regulation of PDL cell cytokine production, providing strong evidence that VDR indeed mediates the anti‐inflammatory effects of vitamin D. 7 Presumably, the vitamin D/VDR‐induced attenuation of pro‐inflammatory cytokine production in PDL cells involves direct transcriptional mechanisms, but vitamin D/VDR‐evoked modulation of post‐transcriptional events cannot be completely excluded (Figure 1).
Figure 1.
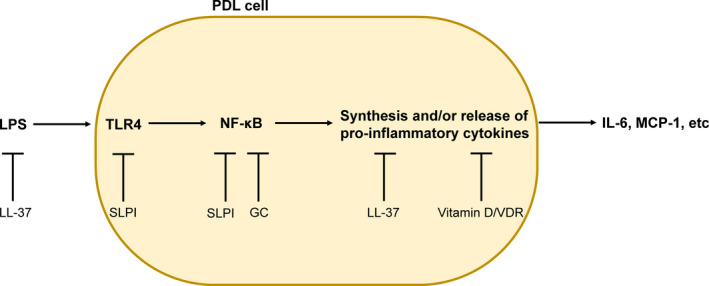
Modulation of LPS‐induced pro‐inflammatory cytokine production in human PDL cells. Vitamin D/VDR presumably attenuates cytokine production at the transcriptional level. The antimicrobial peptide LL‐37 can bind and neutralize LPS extracellularly, but it may also interact with the synthesis of cytokines downstream of NF‐κB, leading to reduced cellular cytokine production. Secretory leukocyte protease inhibitor (SLPI) is a protein expressed in PDL cells, and it seems to act as a negative regulator of cytokine production via interaction with TLR4 expression and NF‐κB activity. Glucocorticoids (GC) reduce PDL cell production of pro‐inflammatory cytokines probably through inhibition of NF‐κB activity. Please, note that TLR4 is a plasma membrane receptor
Circulating levels of 25‐hydroxyvitamin D have been reported to be lower in chronic periodontitis patients compared to healthy controls, suggesting that low plasma levels of vitamin D are associated with the disease. 45 However, other systematic review studies cannot unambiguously show that low plasma concentrations of vitamin D are associated with periodontitis. 46 In patients with severe periodontal disease, a higher proportion of individuals has been reported to be vitamin D deficient compared to healthy controls. 47 Interestingly, no correlation between serum vitamin D concentrations and the clinical parameters bleeding on probing, clinical attachment level, and periodontal probing depth is observed in the group of patients with periodontal disease, indicating that low concentrations of vitamin D in serum are associated with the initiation of the disease. 47 Thus, supplementation with vitamin D may has beneficial anti‐inflammatory effects in periodontitis through down‐regulation of PDL cell production of pro‐inflammatory cytokines. However, it has to be considered that positive effects of vitamin D on periodontal health may also be coupled to the well‐documented vitamin D‐induced stimulation of bone formation.
5. THE HUMAN ANTIMICROBIAL PEPTIDE LL‐37 REGULATES PDL CELL PRODUCTION OF MCP‐1
LL‐37 is the only human member of the cathelicidin family of antimicrobial peptides. Neutrophils and epithelial cells synthesize and secrete the protein hCAP18 which is a pro‐form of LL‐37. 15 , 48 hCAP18 is processed to LL‐37 in an extracellular reaction catalyzed by either serine protease 3 or kallikrein 5, for neutrophils and epithelial cells, respectively. 49 , 50 LL‐37 has been detected in whole saliva of both adults and children. 51 , 52 , 53 , 54 Notably, LL‐37 is detected in both isolated parotid and submandibular/sublingual saliva, indicating that the peptide is produced by cells of the major salivary glands. 55 Hence, salivary LL‐37 may originate from the major salivary glands, but it can also be derived from oral epithelial cells and/or from neutrophils of the gingival tissues and gingival crevicular fluid. Indeed, high concentrations of LL‐37 are reported in gingival tissues and gingival crevicular fluid of periodontitis patients. 9 , 10 , 11 , 12 Thus, it is reasonable to suggest that PDL cells in periodontitis patients probably face high concentrations of LL‐37.
LL‐37 shows activity against both gram‐positive and gram‐negative bacteria including many oral pathogens. 13 The peptide exerts its antimicrobial activity via different mechanisms. LL‐37 has high affinity for bacteria, and it can form pores in their cell walls leading to bacterial cell lysis. 56 , 57 , 58 Besides LL‐37‐induced permeabilization of bacteria, LL‐37 may also bind and neutralize bacterial LPS and LTA. 59 , 60 Importantly, LL‐37 does not only show antibacterial activity, but it also modulates the host defense and innate immune system. 14 , 15
Human PDL cells show no endogenous expression of LL‐37, but they rapidly internalize extracellular LL‐37, suggesting that the peptide can have intracellular actions. 16 Thus, LL‐37 produced by salivary gland cells, oral epithelial cells, and/or neutrophils may be imported by PDL cells and thereby affect PDL cell function through a paracrine mechanism. In PDL cells incubated with exogenous LL‐37, immunoreactivity for LL‐37 is mainly observed in the cytoplasm. 16 Treatment with LL‐37 at a concentration (1 μmol/L) similar to that observed in the gingival crevicular fluid of periodontitis patients abolishes E coli LPS‐induced MCP‐1 production in PDL cells. 12 , 16 The LL‐37‐evoked reversal of LPS‐stimulated MCP‐1 production is probably associated with intracellular actions of LL‐37, since pre‐incubation with LL‐37 for 60 minutes followed by stimulation with LPS for 24 hours in the absence of LL‐37 prevents LPS‐evoked up‐regulation of MCP‐1 production. 16 The mechanism behind LL‐37‐induced down‐regulation of PDL cell MCP‐1 production seems not dependent on interactions with NF‐κB activation but may instead involve effects downstream of NF‐κB such as LL‐37‐induced transcriptional and/or post‐transcriptional effects (Figure 1). However, although LL‐37 presumably reduces PDL cell production of cytokines via intracellular actions, the peptide has probably also anti‐inflammatory effects in PDL cells through extracellular binding and neutralization of LPS. Hence, LL‐37 produced by neutrophils in the inflamed gingival tissues may have a local anti‐inflammatory effect in PDL cells via these different mechanisms. Interestingly, vitamin D is a very strong inducer of LL‐37 (CAMP) gene activity in different types of human cells, and thus, vitamin D may exert both antibacterial and anti‐inflammatory effects through stimulation of LL‐37 production. 61 , 62
6. HUMAN PDL CELLS EXPRESS SLPI AND CELLULAR LEVELS OF SLPI NEGATIVELY CORRELATE TO IL‐6 AND MCP‐1 PRODUCTION
Secretory leukocyte protease inhibitor is produced by human oral and bronchial epithelial cells and also by human skin HaCaT keratinocytes, and the protein is detected in human saliva and nasal secretion. 63 , 64 , 65 , 66 , 67 The SLPI protein may thus show both intracellular and extracellular distribution. Intracellular SLPI has been shown to translocate from cytoplasm to nucleus and interact with NF‐κB signaling in monocytes. 68 We have shown by immunocytochemistry and Western blot that PDL cells express SLPI, although PDL cells show much lower expression of the protein compared to HaCaT cells. 17 E coli LPS increases both IL‐6 and MCP‐1 mRNA in PDL cells, whereas it has no effect in HaCaT cells, and thus, expression levels of SLPI negatively correlate with pro‐inflammatory cytokine expression. 17 Knockdown of PDL cell SLPI gene activity by siRNA enhances both basal and LPS‐stimulated IL‐6 and MCP‐1 expression, providing further evidence that SLPI indeed is a negative regulator of PDL cell production of pro‐inflammatory cytokines. 17 Treatment with SLPI siRNA increases expression of mRNA for both TLR2 and TLR4, and moreover, it also enhances phosphorylated NF‐κB p105 levels in PDL cells, suggesting that SLPI is a negative regulator of TLRs expression and NF‐κB activity. 17 Hence, the mechanisms behind the regulation of PDL cell cytokine production by SLPI probably involve SLPI‐induced regulation of both TLRs expression and NF‐κB activity (Figure 1). Strong evidence thus suggests that cellular SLPI is a negative regulator of PDL cell production of pro‐inflammatory IL‐6 and MCP‐1. Reduced PDL cell expression of SLPI would probably make the cells more sensitive to bacterial endotoxins. However, to the best of my knowledge, there is no information available regarding alterations in PDL cell SLPI expression in response to bacteria and/or in periodontitis vs. healthy conditions.
7. CONCLUSIONS
Much data and many reports show that human PDL cells can produce pro‐inflammatory cytokines in response to stimulation with bacterial endotoxins and thus act as local immune cells. However, little information is available regarding the regulation of PDL cell production of pro‐inflammatory cytokines. The well‐known anti‐inflammatory glucocorticoid dexamethasone reduces LPS‐induced cytokine production in PDL cells. Vitamin D and LL‐37 antagonize LPS‐stimulated PDL cell production of cytokines and thus represent two modulators of PDL cell‐evoked cytokine production. Further, SLPI, which is a protein endogenously expressed by PDL cells, negatively regulates PDL cell production of cytokines. More information and knowledge about modulators and regulatory mechanisms involved in PDL cell production of pro‐inflammatory cytokines is warranted, since it may clarify the role of PDL cells in periodontitis.
CONFLICTS OF INTEREST
The author reports no conflicts of interest.
ACKNOWLEDGEMENTS
The author is supported by grants from The Alfred Österlunds Foundation and The Research Funds for Oral Health Related Research by Region Skåne and by the Medical Faculty, Lund University.
Nilsson B‐O. Mechanisms involved in regulation of periodontal ligament cell production of pro‐inflammatory cytokines: Implications in periodontitis. J Periodont Res. 2021;56:249–255. 10.1111/jre.12823
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Somerman MJ, Young MF, Foster RA, Moehring JM, Imm G, Sauk JJ. Characteristics of human periodontal ligament cells in vitro. Arch Oral Biol. 1990;35:241‐247. [DOI] [PubMed] [Google Scholar]
- 2. Arceo N, Sauk JJ, Moehring J, Foster RA, Somerman MJ. Human periodontal cells initiate mineral‐like nodules in vitro. J Periodontol. 1991;62:499‐503. [DOI] [PubMed] [Google Scholar]
- 3. Nomura Y, Ishikawa M, Yashiro Y, et al. Human periodontal ligament fibroblasts are the optimal cell source for induced pluripotent stem cells. Histochem Cell Biol. 2012;137:719‐732. [DOI] [PubMed] [Google Scholar]
- 4. Jönsson D, Nebel D, Bratthall G, Nilsson BO. The human periodontal ligament cell: a fibroblast‐like cell acting as an immune cell. J Periodontal Res. 2011;46:153‐157. [DOI] [PubMed] [Google Scholar]
- 5. Yamaji Y, Kubota T, Sasaguri K, et al. Inflammatory cytokine gene expression in human periodontal ligament fibroblasts stimulated with bacterial lipopolysaccharides. Infect Immun. 1995;63:3576‐3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang X, Pan Y, Zhao Y. Vitamin D inhibits the expression of interleukin‐8 in human periodontal ligament cells stimulated with Porphyromonas gingivalis. Arch Oral Biol. 2013;58:397‐407. [DOI] [PubMed] [Google Scholar]
- 7. Andrukhov O, Andrukhova O, Hulan U, Tang Y, Bantleon H‐P, Rausch‐Fan X. Both 25‐hydroxyvitamin‐D3 and 1,25‐dihydroxyvitamin‐D3 reduces inflammatory response in human periodontal ligament cells. PLoS One. 2014;9:e90301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nebel D, Svensson D, Arosenius K, Larsson E, Jönsson D, Nilsson BO. 1α,25‐dihydroxyvitamin D3 promotes osteogenic activity and downregulates proinflammatory cytokine expression in human periodontal ligament cells. J Periodontal Res. 2015;50:666‐673. [DOI] [PubMed] [Google Scholar]
- 9. Puklo M, Guentsch A, Hiemstra PS, Eick S, Potempa J. Analysis of neutrophil‐derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL‐37 in the innate immune response against periodontogenic bacteria. Oral Microbiol Immunol. 2008;23:328‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turkoglu O, Emingil G, Kutukculer N, Atilla G. Gingival crevicular fluid levels of cathelicidin LL‐37 and interleukin‐18 in patients with chronic periodontitis. J Periodontol. 2009;80:969‐976. [DOI] [PubMed] [Google Scholar]
- 11. Turkoglu O, Kandiloglu G, Berdeli A, Emingil G, Atilla G. Antimicrobial peptide hCAP‐18/LL‐37 protein and mRNA expressions in different periodontal diseases. Oral Dis. 2011;17:60‐67. [DOI] [PubMed] [Google Scholar]
- 12. Turkoglu O, Emingil G, Eren G, Atmaca H, Kutukculer N, Atilla G. Gingival crevicular fluid and serum hCAP18/LL‐37 levels in generalized aggressive periodontitis. Clin Oral Investig. 2017;21:763‐769. [DOI] [PubMed] [Google Scholar]
- 13. Bechinger B, Gorr SU. Antimicrobial peptides: mechanisms of action and resistance. J Dent Res. 2017;96:254‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cederlund A, Gudmundsson GH, Agerberth B. Antimicrobial peptides important in innate immunity. FEBS J. 2011;278:3942‐3951. [DOI] [PubMed] [Google Scholar]
- 15. Hancock RE, Haney EF, Gill EE. The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol. 2016;16:321‐334. [DOI] [PubMed] [Google Scholar]
- 16. Aidoukovitch A, Anders E, Dahl S, Nebel D, Svensson D, Nilsson BO. The host defense peptide LL‐37 is internalized by human periodontal ligament cells and prevents LPS‐induced MCP‐1 production. J Periodontal Res. 2019;54:662‐670. [DOI] [PubMed] [Google Scholar]
- 17. Svensson D, Aidoukovitch A, Anders E, Jönsson D, Nebel D, Nilsson BO. Secretory leukocyte protease inhibitor regulates human periodontal ligament cell production of pro‐inflammatory cytokines. Inflamm Res. 2017;66:823‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nebel D, Arvidsson J, Lillqvist J, Holm A, Nilsson BO. Differential effects of LPS from Escherichia coli and Porphyromonas gingivalis on IL‐6 production in human periodontal ligament cells. Acta Odontol Scand. 2013;71:892‐898. [DOI] [PubMed] [Google Scholar]
- 19. Im J, Baik JE, Kim KW, et al. Enterococcus faecalis lipoteichoic acid suppresses Aggregatibacter actinomycetemcomitans lipopolysaccharide‐induced IL‐8 expression in human periodontal ligament cells. Int Immunol. 2015;27:381‐391. [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Wang T, Lu Y, et al. Tumor necrosis factor receptor‐associated factor 6 plays a role in the inflammatory responses of human periodontal ligament fibroblasts to Enterococcus faecalis. J Endod. 2015;41:1997‐2001. [DOI] [PubMed] [Google Scholar]
- 21. Behm C, Blufstein A, Gahn J, et al. Soluble CD14 enhances the response of periodontal ligament stem cells to toll‐like receptor 2 agonists. Mediators Inflamm. 2019;2019:8127301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Q, Verma IM. NF‐kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725‐734. [DOI] [PubMed] [Google Scholar]
- 23. Hayden MS, West AP, Ghosh S. NF‐kappaB and the immune response. Oncogene. 2006;25:6758‐6780. [DOI] [PubMed] [Google Scholar]
- 24. Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998;9:248‐266. [DOI] [PubMed] [Google Scholar]
- 25. Trevilatto PC, Scarel‐Caminaga RM, de Brito RB, Jr. , De Souza AP, Line SR. Polymorphism at position ‐174 of IL‐6 gene is associated with susceptibility to chronic periodontitis in a Caucasian Brazilian population. J Clin Periodontol. 2003;30:438‐442. [DOI] [PubMed] [Google Scholar]
- 26. Jones KJ, Ekhlassi S, Montufar‐Solis D, Klein JR, Schaefer JS. Differential cytokine patterns in mouse macrophages and gingival fibroblasts after stimulation with Porphyromonas gingivalis or Escherichia coli lipopolysaccharide. J Periodontol. 2010;81:1850‐1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morandini AC, Sipert CR, Gasparoto TH, et al. Differential production of macrophage inflammatory protein‐1α, stromal‐derived factor‐1, and IL‐6 by human cultured periodontal ligament and gingival fibroblasts challenged with lipopolysaccharide from P. gingivalis. J Periodontol. 2010;81:310‐317. [DOI] [PubMed] [Google Scholar]
- 28. Morsczeck CO, Drees J, Gosau M. Lipopolysaccharide from Escherichia coli but not from Porphyromonas gingivalis induce pro‐inflammatory cytokines and alkaline phosphatase in dental follicle cells. Arch Oral Biol. 2012;57:1595‐1601. [DOI] [PubMed] [Google Scholar]
- 29. Jian C, Li C, Ren Y, et al. Hypoxia augments lipopolysaccharide‐induced cytokine expression in periodontal ligament cells. Inflammation. 2014;37:1413‐1423. [DOI] [PubMed] [Google Scholar]
- 30. Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86:9‐22. [DOI] [PubMed] [Google Scholar]
- 31. Yoshimura A, Kaneko T, Kato Y, Golenbock DT, Hara Y. Lipopolysaccharides from periodontopathic bacteria Porphyromonas gingivalis and Capnocytophaga ochracea are antagonists for human toll‐like receptor 4. Infect Immun. 2002;70:218‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jönsson D, Amisten S, Bratthall G, Holm A, Nilsson BO. LPS induces GROα chemokine production via NF‐κB in oral fibroblasts. Inflamm Res. 2009;58:791‐796. [DOI] [PubMed] [Google Scholar]
- 33. Clark AR. Anti‐inflammatory functions of glucocorticoid‐induced genes. Mol Cell Endocrinol. 2007;275:79‐97. [DOI] [PubMed] [Google Scholar]
- 34. Jönsson D, Nebel D, Bratthall G, Nilsson BO. LPS‐induced MCP‐1 and IL‐6 production is not reversed by oestrogen in human periodontal ligament cells. Arch Oral Biol. 2008;53:896‐902. [DOI] [PubMed] [Google Scholar]
- 35. Morandini AC, Chaves Souza PP, Ramos‐Junior ES, Souza Costa CA, Santos CF. MyD88 or TRAM knockdown regulates interleukin (IL)‐6, IL‐8, and CXCL12 mRNA expression in human gingival and periodontal ligament fibroblasts. J Periodontol. 2013;84:1353‐1360. [DOI] [PubMed] [Google Scholar]
- 36. Long P, Liu F, Piesco NP, Kapur R, Agarwal S. Signaling by mechanical strain involves transcriptional regulation of proinflammatory genes in human periodontal ligament cells in vitro. Bone. 2002;30:547‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marciniak J, Lossdörfer S, Kirschneck C, Deschner J, Jäger A, Wolf M. Heat shock protein 70 dampens the inflammatory response of human PDL cells to mechanical loading in vitro. J Periodontal Res. 2019;54:481‐488. [DOI] [PubMed] [Google Scholar]
- 38. Marciniak J, Lossdörfer S, Knaup I, et al. Orthodontic cell stress modifies proinflammatory cytokine expression in human PDL cells and induces immunomodulatory effects via TLR‐4 signaling in vitro. Clin Oral Investig. 2020;24:1411‐1419. [DOI] [PubMed] [Google Scholar]
- 39. Son GY, Hong JH, Chang I, Shin DM. Induction of IL‐6 and IL‐8 by activation of thermosensitive TRP channels in human PDL cells. Arch Oral Biol. 2015;60:526‐532. [DOI] [PubMed] [Google Scholar]
- 40. Wang PL, Ohura K. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts‐CD14 and toll‐like receptors. Crit Rev Oral Biol Med. 2002;13:132. [DOI] [PubMed] [Google Scholar]
- 41. Andrukhov O, Ertlschweiger S, Moritz A, Bantleon H‐P, Rausch‐Fan X. Different effects of P. gingivalis LPS and E. coli LPS on the expression of interleukin‐6 in human gingival fibroblasts. Acta Odontol Scand. 2014;72:337‐345. [DOI] [PubMed] [Google Scholar]
- 42. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266‐281. [DOI] [PubMed] [Google Scholar]
- 43. Carlberg C, Campbell MJ. Vitamin D receptor signaling mechanisms: Integrated actions of a well‐defined transcription factor. Steroids. 2013;78:127‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482‐496. [DOI] [PubMed] [Google Scholar]
- 45. Machado V, Lobo S, Proenca L, Mendes JJ, Botelho J. Vitamin D and periodontitis: a systematic review and meta‐analysis. Nutrients. 2020;12:E2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peric M, Cavalier E, Toma S, Lasserre JF. Serum vitamin D levels and chronic periodontitis in adult, Caucasian population‐a systematic review. J Periodontal Res. 2018;53:645‐656. [DOI] [PubMed] [Google Scholar]
- 47. Laky M, Bertl K, Haririan H, et al. Serum levels of 25‐hydroxyvitamin D are associated with periodontal disease. Clin Oral Investig. 2017;21:1553‐1558. [DOI] [PubMed] [Google Scholar]
- 48. Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr Issues Mol Biol. 2005;7:179‐196. [PubMed] [Google Scholar]
- 49. Sorensen OE, Follin P, Johnsen AH, et al. Human cathelicidin hCAP‐18, is processed to the antimicrobial peptide LL‐37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951‐3959. [DOI] [PubMed] [Google Scholar]
- 50. Yamasaki K, Schauber J, Coda A, et al. Kallkrein‐mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068‐2080. [DOI] [PubMed] [Google Scholar]
- 51. Murakami M, Ohtake T, Dorschner RA, Gallo RL. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J Dent Res. 2002;81:845‐850. [DOI] [PubMed] [Google Scholar]
- 52. Tao R, Jurevic RJ, Coulton KK, et al. Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob Agents Chemother. 2005;49:3883‐3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Davidopoulou S, Diza E, Sakellari D, Menexes G, Kalfas S. Salivary concentration of free LL‐37 in edentulism, chronic periodontitis and healthy periodontium. Arch Oral Biol. 2013;58:930‐934. [DOI] [PubMed] [Google Scholar]
- 54. Colombo NH, Ribas LF, Pereira JA, et al. Antimicrobial peptides in saliva of children with severe early childhood caries. Arch Oral Biol. 2016;69:40‐46. [DOI] [PubMed] [Google Scholar]
- 55. Svensson D, Aidoukovitch A, Anders E, et al. The host defense peptide LL‐37 is detected in human parotid and submandibular/sublingual saliva and expressed in glandular neutrophils. Eur J Oral Sci. 2018;126:93‐100. [DOI] [PubMed] [Google Scholar]
- 56. Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI. Activities of LL‐37, a cathelicidin‐associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206‐2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454‐457. [DOI] [PubMed] [Google Scholar]
- 58. Burton MF, Steel PG. The chemistry and biology of LL‐37. Nat Prod Rep. 2009;26:1572‐1584. [DOI] [PubMed] [Google Scholar]
- 59. Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: a novel antimicrobial lipopolysaccharide‐binding protein. Infect Immun. 1995;63:1291‐1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL‐37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883‐3891. [DOI] [PubMed] [Google Scholar]
- 61. Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25‐dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909‐2912. [DOI] [PubMed] [Google Scholar]
- 62. Liu PT, Stenger S, Wenzel L, et al. Toll‐like receptor triggering of a vitamin D‐mediated human antimicrobial response. Science. 2006;311:1770‐1773. [DOI] [PubMed] [Google Scholar]
- 63. Sumi Y, Muramatsu H, Hata K, Ueda M, Muramatsu T. Secretory leukocyte protease inhibitor is a novel inhibitor of fibroblast‐mediated collagen gel contraction. Exp Cell Res. 2000;256:203‐212. [DOI] [PubMed] [Google Scholar]
- 64. Jaumann F, Elssner A, Mazur G, Dobmann S, Vogelmeier C. Transforming growth factor‐beta1 is a potent inhibitor of secretory leukocyte protease inhibitor expression in a bronchial epithelial cell line. Munich Lung Transplant Group. Eur Respir J. 2000;15:1052‐1057. [DOI] [PubMed] [Google Scholar]
- 65. Bando M, Hiroshima Y, Kataoka M, et al. Interleukin‐1α regulates antimicrobial peptide expression in human keratinocytes. Immunol Cell Biol. 2007;85:532‐537. [DOI] [PubMed] [Google Scholar]
- 66. McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti‐human immunodeficiency virus 1 activity in vitro. J Clin Invest. 1995;96:456‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Thijs W, Janssen K, van Schadewijk AM, et al. Nasal levels of antimicrobial peptides in allergic asthma patients and healthy controls: differences and effect of a short 1,25(OH)2 vitamin D3 treatment. PLoS One. 2015;10:e0140986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Taggart CC, Cryan SA, Weldon S, et al. Secretory leukoprotease inhibitor binds to NF‐kappaB binding sites in monocytes and inhibits p65 binding. J Exp Med. 2005;202:1659‐1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


