Abstract
Tillandsia (Bromeliaceae) species have high endemism, and due to their strong ornamental potential, predatory extraction is threatening the extinction or drastic population reduction of many of them. In light of this scenario, it is necessary to find strategies for the conservation of these endangered species. The objective of this study was to evaluate two seed preservation strategies (freezing at − 5 °C and cryopreservation at − 196 °C) for 20 Tillandsia species occurring in the state of Bahia. We initially evaluated the morphometry, thousand-seed weight, and water content, followed by tests of germination and desiccation. After selecting the best result of the germination test (Germitest paper and incubation at 30 °C) and desiccation (3 h on silica gel), we established conservation tests utilizing two temperatures (freezing at − 5 °C and liquid nitrogen at − 196 °C), with storage times of 1, 7, 30, 180 and 450 days. Analysis of variance indicated that the 20 species had different behaviors when submitted to the two temperatures and different storage times. After 450 days there was a reduction in the germination percentage and germination speed index (GSI) of all the species studied when the seeds were preserved in the freezer. The storage in liquid nitrogen was efficient for the preservation of Tillandsia seeds when dried to a moisture content of approximately 7%. Our results support the establishment of a cryobank for Tillandsia to conserve these endemic species.
Keywords: Bromeliads, Dehydration, Endangered species, Endemic species, Liquid nitrogen, Tillandsioideae
Introduction
The genus Tillandsia L. is the most representative among the family Bromeliaceae Juss., with 755 recorded species (Gouda et al. 2021, continuous updated), distributed in seven subgenera (Barfuss et al. 2016), variously with high ornamental potential, ecological value and/or possible uses in the chemical and pharmaceutical industries (Estrella-Parra et al. 2019). In the Brazilian state of Bahia, there are 25 recorded species in four subgenera, Anoplophytum (Beer) Baker, Diaphoranthema (Beer) Baker, Phytarrhiza (Vis.) Baker and Tillandsia (G.Nicholson). These species are found in the Caatinga, Mata Atlântica (Atlantic Forest) and Cerrado (savanna) biomes (Flora do Brasil 2020).
Tillandsia species are in high demand as ornamental plants, both in the domestic and international markets (Flores-Palacios and Valencia-Díaz 2007; Castillo et al. 2013; Toledo-Aceves et al. 2014), mainly in the United States, Europe, and Asia (Dematê 2005; Negrelle et al. 2012). In addition, Tillandsia species are often used in traditional rituals and ceremonies in Latin America (Haeckel 2008; Hornung-Leoni 2011), but they are rarely grown commercially in a sustainable form, instead of being obtained by extractivism (Negrelle et al. 2012). The high degree of endemism, predatory extractivism for illegal trade, and destruction of natural habitats, are threatening the survival of many of these species (Hietz et al. 2002; Flores-Palacios and Valencia-Díaz 2007; Forzza et al. 2013; Toledo-Aceves et al. 2014; NOM-059-Semarnat 2019), and causing a need for methods for their conservation.
The conservation of germplasm allows safeguarding the genetic variability of species with ecological and economic importance and/or that are endangered (Machado et al. 2016; Silva et al. 2016; Malhotra et al. 2019; Rao and Parthasarathy 2019; Dar et al. 2020). Among the conservation strategies, cryopreservation enables long-term conservation of genetic material with small space requirements. This can be achieved by storage in liquid nitrogen (− 196 °C) or cold nitrogen vapor (− 150 °C) of vegetative parts, such as calli, stem tips, buds, pollen grains, and seeds (Souza et al. 2016; Jenderek and Reed 2017; Dinato et al. 2020; Wang et al. 2020). The ultra-low temperatures reduce the metabolism of cells until a level that halts the tissue development but allows regeneration with restoration of ambient temperature (Panis and Lambardi 2006; Jenderek and Reed 2017). In the case of preserving seeds, the most important aspect to be considered is the ideal moisture content, which can be a decisive factor to maintain the viability of seeds after freezing and thawing, and thus influence the final percentage of seed germination (Generoso et al. 2019; Vettorazzi et al. 2019; Silva et al. 2021).
Seeds are ideal reproductive structures to store the genetic variability of a species, assuring the conservation of the gene pool (Gosling 2003; Zotz 2013; Generoso et al. 2019; Silva et al. 2021). Therefore, studying the different methods of preserving seeds contributes to the conservation of species, and at the extreme can rescue a species from the threat of extinction. It is common knowledge that bromeliad seeds lose their viability very quickly (Zotz 2013). Several studies involving the conservation of seeds of Bromeliaceae species at low (Vasak 1969; Fernandez et al. 1989; Wester and Zotz 2011; Zotz 2013) and ultra-low temperatures have been published, such as Tarré et al. (2007), Rodrigues et al. (2014), Ferrari et al. (2016) and Silva et al. (2021).
Zotz (2013), studying seven bromeliad species of the genera Guzmania Ruiz & Pav., Tillandsia and Vriesea Lindl., observed that conservation of seeds at room temperature (20 °C) drastically reduced their viability after 12 months, while when conserved at − 20 °C, they remained viable for 26 months.
Tarré et al. (2007) and Ferrari et al. (2016) investigated cryopreservation of the species Encholirium Mart. ex Schult. and Schult.f. and Dyckia Schult. and Schult.f. and observed germination results similar to or better than the control treatment after one year, thus considering this strategy to be efficient for the two species in question. Rodrigues et al. (2014) mainly studied the optimal moisture content of the seeds of 10 endemic species of bromeliads threatened with extinction of the genera Alcantarea (E.Morren ex Mez) Harms, Nidularium Lem, Pitcairnia L’Hér., Vriesea and Wittrockia Lindm. and concluded that the species had orthodox behavior, so it was necessary to dehydrate the seeds before the conservation procedures. The authors also reported that water content lower than 7% affected the viability of the seeds and that the ideal content for cryopreservation was around 6–7% (dry mass basis, DMB). To the best of our knowledge, there are no studies of cryopreservation of species of the Tillandsia despite all importance of this genus in the Bromeliaceae family. Also, based on our experience with a seed bank maintained at Embrapa Mandioca e Fruticultura in a freezer at − 5 °C, we observed a reduction in viability of the stored seeds of some species of Bromeliaceae, mainly of the subfamily Tillandsioideae. Based on the data in the literature and our observations, we decided to study two storage temperatures: (i) − 5 °C, in a freezer, a technique used for many plant species, with easy repeatability and low cost, and (ii) cryopreservation in liquid nitrogen at − 196 °C, with a greater cost for laboratory structure, but with better results observed for other species.
Therefore, our objective was to evaluate the conservation of seeds in a freezer at − 5 °C and by cryopreservation at − 196 °C in liquid nitrogen of 20 Tillandsia species with ornamental potential occurring in the state of Bahia, Brazil, to develop a protocol that can safeguard the genetic variability of these species.
Materials and methods
Plant material
We studied 20 Tillandsia species occurring in Bahia, Brazil (Figs. 1, 2 and Table 1). The seeds were collected randomly from ripe fruits of plants growing naturally, to maintain the variability among the populations. Voucher specimens of each species were deposited in the Recôncavo da Bahia Herbarium (HURB) (Table 1) and the Bromeliad Germplasm Bank of the Embrapa Cassava and Fruits research unit (Embrapa Mandioca e Fruticultura), located in the municipality of Cruz das Almas, Bahia, Brazil. The seed collection was carried out in line with all Brazilin regulations on the protection of biodiversity.
Fig. 1.
Species of Tillandsia with ornamental potential occurring in Bahia, Brazil. a T. bulbosa; b T. juncea; c T. paraensis; d T. polystachia; e T. pruinosa; f T. loliaceae; g T. recurvata; h T. tricholepis; i T. streptocarpa 1; j T. streptocarpa 2; and k T. chapeuensis; l T. gardneri
Fig. 2.
Species of Tillandsia with ornamental potential occurring in Bahia, Brazil. a T. geminiflora; b T. globosa; c T. pohliana; d T. stricta 1; e T. stricta 2; f T. stricta subsp. piniformis; g T. tenuifolia; and h T. tenuifolia var. vaginata
Table 1.
Species of Tillandsia that occur in Bahia, Brazil, including subgenus, collection municipality, biome and voucher number of the specimen deposited in the HURB Herbarium
| Species | Subgenus | Collection municipality | Biome | HURB |
|---|---|---|---|---|
| T. bulbosa Hook.f. | Tillandsia | Ilhéus | Atlantic Forest/Restinga | 25395 |
| T. juncea (Ruiz and Pav.) Poir. | Tillandsia | Santa Terezinha | Caatinga | 22220 |
| T. paraensis Mez | Tillandsia | Entre Rios | Atlantic Forest | 25754 |
| T. polystachia (L.) L. | Tillandsia | Milagres | Caatinga | 22131 |
| T. pruinosa Sw. | Tillandsia | Brejões | Atlantic Forest | 22152 |
| T. loliacea Mart. ex Schult. and Schult.f. | Diaphoranthema | Milagres | Caatinga | 22130 |
| T. recurvata (L.) L. | Diaphoranthema | Milagres | Caatinga | 22149 |
| T. tricholepis Baker | Diaphoranthema | Ibitiara | Caatinga | 25753 |
| T. streptocarpa Baker 1 | Phyrarrhiza | Milagres | Caatinga | 18591 |
| T. streptocarpa Baker 2 | Phyrarrhiza | Milagres | Caatinga | 25006 |
| T. chapeuensis Rauh | Anoplophytum | Morro do Chapéu | Caatinga/Rupestrian grasslands | 23983 |
| T. gardneri Lindl. | Anoplophytum | Ilhéus | Atlantic Forest | 25393 |
| T. geminiflora Brongn. | Anoplophytum | Brejões | Atlantic Forest | 22222 |
| T. globosa Wawra | Anoplophytum | Muritiba | Atlantic Forest | 25569 |
| T. pohliana Mez | Anoplophytum | Morro do Chapéu | Caatinga/Rupestrian grasslands | 23978 |
| T. stricta Sol. 1 | Anoplophytum | Ilhéus | Atlantic Forest | 25384 |
| T. stricta Sol. 2 | Anoplophytum | Milagres | Caatinga | 22180 |
| T. stricta subsp. piniformis Rauh ex. Ehers and Heidt | Anoplophytum | Morro do Chapéu | Caatinga/Rupestrian grasslands | 23977 |
| T. tenuifolia L. | Anoplophytum | Milagres | Caatinga | 15626 |
| T. tenuifolia var. vaginata (Wawra) L.B.Sm. | Anoplophytum | Cruz das Almas | Atlantic Forest | 24541 |
Morphometry and thousand-seed weight
The plumose appendages were removed with scissors to prevent the proliferation of microorganisms. Then the number of seeds per capsule was counted, using three capsules per species from different plants. The length and width of 25 seeds were measured from images obtained with a stereomicroscope (Leica EZ4 D, Wetzlar, Germany) using the ImageJ 2 program (Rueden et al. 2017). The thousand-seed weight was determined with a precision scale, according to the Seed Analysis Rules (Brasil 2009).
Water content of the seeds
The seed moisture content was determined by examining three samples of 100 recently collected seeds of each species, applying the fresh weight method by heating to 105 °C for 24 h, also according to the Seed Analysis Rules (Brasil 2009).
Germination and desiccation testing
For the germination test, we used seeds of four species, each one representing one of the subgenera of plants occurring in Bahia: T. streptocarpa (subg. Phytarrhiza), T. recurvata (subg. Diaphoranthema), T. stricta 1 (subg. Anophophytum) and T. polystachia (subg. Tillandsia), with two substrates (Germitest® paper and MS culture medium), at three incubation temperatures (20, 25 and 30 °C).
One lot of seeds was placed to germinate in Petri dishes containing two sheets of autoclaved Germitest® paper moistened with 2.5 times their weight (in grams) of autoclaved distilled water. Another lot of seeds was disinfected in a laminar flow cabinet with 70% ethanol for 5 min and a 2% sodium hypochlorite solution for 20 min, followed by rinsing three times with autoclaved distilled water. The seeds were placed in Petri dishes containing MS medium (Murashige and Skoog 1962) containing half the normal salt concentration, supplemented with 3% sucrose, and solidified with 2 g L−1 of Phytagel®. The pH was adjusted to 5.8 before autoclaving.
Next, the dishes containing the seed samples were incubated at three temperatures (20, 25 and 30 °C) with an 8 h photoperiod under four cool daylight fluorescent lamps of 20 W (total flux rate 120 μmol m−2 s−1). The emergence of the embryonic axis (1 mm) was the criterion used for the start of germination. The germination percentage (G %) and germination speed index (GSI) were calculated according to: , where N = number of germinated seeds and A = total number of seeds; , where Gi = number of germinated seeds and ni = counting day.
For the desiccation tests, each seed batch was placed in a chamber with activated silica gel for 3, 6, 9 and 12 h. One lot was evaluated for water content according to the method described above and the seeds of the other lot were placed in Petri dishes to germinate on Germitest® paper at a temperature of 30 °C. The evaluation of germination was performed as described above.
The experimental design was completely randomized in a 4 × 2 × 3 factorial scheme (species × environments × temperature) with four repetitions, where each repetition was composed of 25 seeds, for a total of 100 seeds per treatment. The seeds were observed daily for 20 days. The data on germination percentage and GSI were submitted to analysis of variance and the means were compared by the Tukey test (p < 0.01), with the germination data first being transformed by arcsine . The analyses were carried out with the R program (R Development Core Team 2019).
Conservation of seeds
The seeds’ appendages were removed and then they were separated into lots of 100 (Fig. 3a, b), each of which was placed in a cryogenic tube (3 mL) and desiccated for 3 h on activated silica gel (best desiccation treatment). After selecting the best result of the germination tests, we established the conservation test, utilizing the two treatment methods (freezer at − 5 °C and liquid nitrogen at − 196 °C). For cryopreserved seeds, the tubes containing the seeds were rapidly immersed in liquid nitrogen.
Fig. 3.
a Tillandsia gardneri seeds with appendages. b Tillandsia gardneri seeds with appendage removed and separated into lots of 100. c Tillandsia gardneri seeds germinated after cryopreservation for 450 days. ap appendage, se seed
We evaluated the germination and GSI after 1, 7, 30, 180, and 450 days (Fig. 3c). The seeds were slowly thawed at room temperature for 30 min and placed to germinate in a B.O.D. (Biological Oxygen Demand) incubator growth chamber at 30 °C, with 16: 8 h photoperiod (light: dark). The evaluations were performed daily until the 20th day. The control treatment consisted of seeds removed from the desiccator and placed directly in Petri dishes on Germitest® paper in the same conditions as the conservation test.
The experimental design was completely randomized in a 20 × 2 × 5 factorial scheme (species x environments x evaluation times). Each treatment used 4 repetitions, each composed of 25 seeds, for a total of 100 seeds per treatment. The data were submitted to analysis of variance and the means were compared by the Scott-Knott test (p < 0.01) for species, the F test (p < 0.01) for conservation environments and the Tukey test (p < 0.01) for evaluation times. The germination data were first transformed to arcsine . All the analyses were carried out with the R program (R Development Core Team 2019).
Results and discussion
Morphometry, thousand-seed weight and water content
The analysis of variance revealed significant differences among the species for a number of seeds per capsule and length diameter and water content of the recently collected seeds. Table 2 reports the values of each of these traits along with the coefficient of variation.
Table 2.
Morphometry, water content, and thousand-seed weight of seeds of Tillandsia species (Bromeliaceae) occurring in Bahia, Brazil
| Species | NSC | LEM | DIA | WC | TSW |
|---|---|---|---|---|---|
| T. bulbosa | 63.00 ± 8.19 e | 3.13 ± 0.17 h | 0.47 ± 0.06 c | 11.98 ± 0.12 d | 0.35 |
| T. juncea | 117.00 ± 26.21 c | 3.10 ± 0.32 h | 0.46 ± 0.05 c | 13.56 ± 0.10 b | 0.41 |
| T. paraensis | 118.33 ± 17.95 c | 3.63 ± 0.29 g | 0.51 ± 0.09 b | 14.16 ± 0.08 a | 0.51 |
| T. polystachia | 155.33 ± 11.02 b | 3.93 ± 0.25 f | 0.39 ± 0.05 d | 13.58 ± 0.12 b | 0.42 |
| T. pruinosa | 60.00 ± 10.58 e | 2.94 ± 0.27 i | 0.35 ± 0.04 e | 11.31 ± 0.21 e | 0.21 |
| T. loliacea | 44.33 ± 14.47 f | 4.79 ± 0.56 d | 0.32 ± 0.07 e | 10.35 ± 0.10 f | 0.21 |
| T. recurvata | 52.33 ± 8.39 e | 3.51 ± 0.32 g | 0.31 ± 0.06 e | 11.18 ± 0.12 e | 0.21 |
| T. tricholepis | 65.00 ± 10.82 e | 4.35 ± 0.34 e | 0.32 ± 0.07 e | 10.16 ± 0.16 f | 0.09 |
| T. streptocarpa 1 | 82.67 ± 15.01 d | 5.63 ± 0.51 a | 0.33 ± 0.06 e | 13.33 ± 0.10 b | 0.42 |
| T. streptocarpa 2 | 34.33 ± 4.16 f | 5.68 ± 0.88 a | 0.52 ± 0.07 b | 14.58 ± 0.16 a | 0.51 |
| T. chapeuensis | 24.30 ± 9.00 f | 5.36 ± 0.48 b | 0.56 ± 0.07 a | 12.16 ± 0.21 c | 0.12 |
| T. gardneri | 71.33 ± 9.02 e | 4.36 ± 0.54 e | 0.47 ± 0.07 c | 14.31 ± 0.18 a | 0.54 |
| T. geminiflora | 57.33 ± 5.03 e | 5.11 ± 0.36 c | 0.41 ± 0.06 d | 11.86 ± 0.05 d | 0.25 |
| T. globosa | 53.33 ± 4.16 e | 4.70 ± 0.74 d | 0.33 ± 0.06 e | 12.86 ± 0.08 c | 0.35 |
| T. pohliana | 202.33 ± 14.64 a | 3.87 ± 0.25 f | 0.50 ± 0.06 b | 13.87 ± 0.11 b | 0.45 |
| T. stricta 1 | 66.67 ± 6.11 e | 3.75 ± 0.26 g | 0.31 ± 0.06 e | 12.13 ± 0.12 c | 0.35 |
| T. stricta 2 | 59.00 ± 10.82 e | 3.16 ± 0.36 h | 0.30 ± 0.06 e | 10.48 ± 0.14 f | 0.15 |
| T. stricta subsp. piniformis | 30.33 ± 2.52 f | 3.63 ± 0.18 g | 0.38 ± 0.05 d | 12.56 ± 0.09 c | 0.37 |
| T. tenuifolia | 97.33 ± 13.28 d | 3.94 ± 0.28 f | 0.41 ± 0.05 d | 11.67 ± 0.07 d | 0.32 |
| T. tenuifolia var. vaginata | 35.67 ± 5.51 f | 2.74 ± 0.35 i | 0.33 ± 0.07 e | 11.26 ± 0.11 e | 0.23 |
| CV (%) | 10.43 | 15.63 | 15.70 | 11.32 |
NSC number of seeds per capsule, LEN length of 25 seeds (mm), DIA diameter of 25 seeds (mm), WC water content in recently collected seeds, TSW thousand-seed weight
The greatest number of seeds per capsule was observed for T. pohliana, with a mean of 202.33, while the lowest quantities were observed for T. loliacea (44.33), T. streptocarpa 2 (34.33), T. chapeuensis (24.30), T. stricta subsp. piniformis (30.33), and T. tenuifolia var. vaginata (35.67), without significant pairwise differences among them (Table 2). According to Rodrigues et al. (1999) and Souza et al. (2015), the number of seeds per fruit is directly related to the number of ovules, so that species that produce more ovules have better chances of producing seeds, favoring their perpetuation in the environment. Other important factors related to the number of seeds are the efficiency of the pollination system (Scrok and Varassin 2011; Bastos et al. 2017) and the absence or presence of reproductive barriers (Souza et al. 2017).
With respect to seed length, T. streptocarpa 1 and T. streptocarpa 2 had the highest averages, with 5.63 and 5.68 mm, respectively, and T. pruinosa (2.94 mm) and T. tenuifolia var. vaginata (2.74 mm) had the lowest averages (Table 2). In relation to seed diameter, T. chapeuensis had the greatest mean, with 0.56 mm while T. stricta 2 (0.30 mm) had the smallest (Table 2). In general, all the species produced positive photoblastic seeds having the same morphology, with elongated, narrow, and filiform shape and plumose appendage in the basal region, despite small variations in the length and thickness of each species. These traits permit good seed dispersal by wind of the Tillandsioideae subfamily (Smith and Downs 1977; Magalhães and Mariath 2012; Barfuss et al. 2016). The need for light exposure (positive photoblasticity) for germination of seeds of Bromeliaceae species has been reported by Benzing (2000), Pereira et al. (2008) and Rodrigues et al. (2014). These researchers mainly correlated the size of seeds and the environment where the species normally occur.
In relation to the thousand-seed weight, there was a variation from 0.09 g for T. tricholepis to 0.54 g for T. gardneri (Table 2). Some authors have reported high positive correlations between the germination percentage and the germination speed (Martins et al. 2000; Silva et al. 2010; Socolowski et al. 2011). The results presented here corroborate those findings, because the lighter seeds (T. tricholepis, T. chapeuensis and T. stricta 2) presented the lowest germination percentages and GSI values.
Regarding the water content of the recently collected seeds, the highest values were observed for T. streptocarpa 2 (14.58%), T. gardneri (14.31%) and T. paraensis (14.16%), while the lowest values were for T. loliacea (10.35%), T. tricholepis (10.16%) and T. stricta 2 (10.48%). Naturally, orthodox seeds already have low moisture levels (Ferrari et al. 2016), and according to Stanwood (1985), orthodox or semi-recalcitrant seeds should be stored with moisture levels below 10%, similar to the results found in this study.
Desiccation and germination tests
After obtaining the morphometric, water content, and thousand-seed weight data, we performed tests of desiccation and germination with four Tillandsia species, one of each subgenus, to help support the other conservation studies (Fig. 4, Table 3).
Fig. 4.
Germination percentage and water content of seeds of Tillandsia species (Bromeliaceae) occurring in Bahia, Brazil after different desiccation times on activated silica gel
Table 3.
Germination percentage and germination speed index (GSI) of four Tillandsia species (Bromeliaceae) occurring in Bahia, Brazil in function of culture conditions
| T. streptocarpa 1 | T. recurvata | T. stricta 1 | T. polystachia | |||||
|---|---|---|---|---|---|---|---|---|
| MS | GP | MS | GP | MS | GP | MS | GP | |
| Germination (%) | ||||||||
| 20 °C | 80 aB | 76 aB | 96 aA | 95 aA | 86 aA | 88 aA | 82 aB | 79 aB |
| 25 °C | 96 aA | 92 aA | 92 aA | 91 aA | 88 aA | 81 aA | 91 aA | 90 aA |
| 30 °C | 92 aA | 89 aA | 98 aA | 94 aA | 82 aA | 83 aA | 94 aA | 92 aA |
| CV (%) | 22.38 | |||||||
| Germination speed index | ||||||||
| 20 °C | 1.40 aB | 1.15 aB | 3.08 aB | 2.96 aB | 0.44 aB | 0.35 aB | 2.30 aB | 2.60 aB |
| 25 °C | 3.25 aA | 3.02 aA | 4.34 aA | 4.56 aA | 2.23 aA | 2.33 aA | 4.21 aA | 4.21 aA |
| 30 °C | 3.06 aA | 3.86 aA | 4.77 aA | 4.80 aA | 2.21 aA | 2.01 aA | 4.11 aA | 3.83 aA |
| CV (%) | 16.22 | |||||||
Means followed by the same lowercase letters in the row and uppercase in the column do not differ at 1% probability by the Tukey test
MS culture medium containing half of the normal concentration of salts (Murashige and Skoog, 1962), GP Germitest® paper
The desiccation of the seeds on activated silica gel for 3 h significantly reduced the water content, from approximately 12–13% to 7–8%, after which the weights stabilized (Fig. 4). This reduction of water content did not influence the germination percentages of the seeds of the four species studied, indicating it is a good treatment for seed conservation.
The data obtained indicated that the seeds of the Tillandsia species had orthodox behavior, remaining viable with low moisture levels. This tolerance to desiccation of Bromeliaceae seeds was previously described by Rodrigues et al. (2014) and Silva et al. (2021), studying different species of various subfamilies, including the Tillandsioideae subfamily, the focus of this study. Those authors managed to reduce the seed water content to very low levels (around 2.2%) without affecting the viability.
To conserve the seeds of any plant species, it is necessary to reduce and then stabilize the water content (Figueiredo et al. 2017), to prevent the formation of ice crystals in the cells during cryopreservation, or tissue degradation when seeds are stored at higher temperatures. In both cases, this can impair the seed's integrity and viability (Pegg 2010; Figueiredo et al. 2017). Besides this, the tolerance to desiccation is an important characteristic for conservation, since a very low water content within the cells can cause the death of the embryo and compromise the viability of the seeds (Mello et al. 2010; Villela and Peres 2004; Masetto and Faria 2019).
With respect to the germination percentage and germination speed index (GSI), there was no significant interaction between the factors MS culture medium and Germitest® paper for the four species studied (Table 3). Regarding temperature, T. streptocarpa 1 and T. polystachia seeds had low germination percentages, irrespective of the substrate used, when cultured at 20 °C.
For the GSI, the temperature of 20 °C promoted slower germination of the four species. In turn, the germination percentages varied from 76% for T. streptocarpa 1 at 20 °C to 98% for T. recurvata at 30 °C (Table 3).
Temperature is a key factor for seed germination of many plant species, including overcoming dormancy and embryo growth, by affecting the water absorption speed and biochemical and physiological reactions (Silva et al. 2002; Castro et al. 2004; Vivian et al. 2008). We did not observe dormancy in any of the species studied, but the higher temperatures promoted higher germination and GSI. The absence of seed dormancy of Bromeliaceae species has been reported for various species of different genera (Pereira 1988; Pereira et al. 2008; Rodrigues et al. 2014). In contrast, Coelho et al. (2011) reported tegumental dormancy in Bromelia balansae Mez., which was broken by immersion in sulfuric acid PA (98%) for 1 min.
In the Bromeliaceae, the ideal temperature for seed germination appears to be strongly related to the species, although a range exists (15–35 °C) where the great majority of species’ seeds germinate. However, to maximize the germinative potential of a determined species, it is often necessary to optimize the temperature (Mercier and Guerreiro Filho 1990; Nara and Webber 2002; Pinheiro and Borghetti 2003; Vieira et al. 2007).
Pereira et al. (2010) reported no difference in the germinative potential of the bromeliad Nidularium innocentii Lem. when submitted to temperatures of 20 °C, 25 °C and an alternating regime of 20 °C/30 °C. Tarré et al. (2007) also found no significant differences in the germination percentages for species of the genera Encholirium Mart. ex Schult. & Schult.f. and Dyckia Schult. & Schult.f. when exposed to temperatures of 20, 25, and 30 °C. In turn, for Dyckia tuberosa (Vell.) Beer, Vieira et al. (2007) analyzed a variation from 10 to 45 °C and observed that these temperatures did not lead to germination and the ideal temperatures were 30–35 °C to maximize the germination and GSI.
Therefore, for a question of practicality and in light of the GSI of the four species studied, we decided to continue the conservation tests on Germitest® paper at a temperature of 30 °C.
Seed conservation
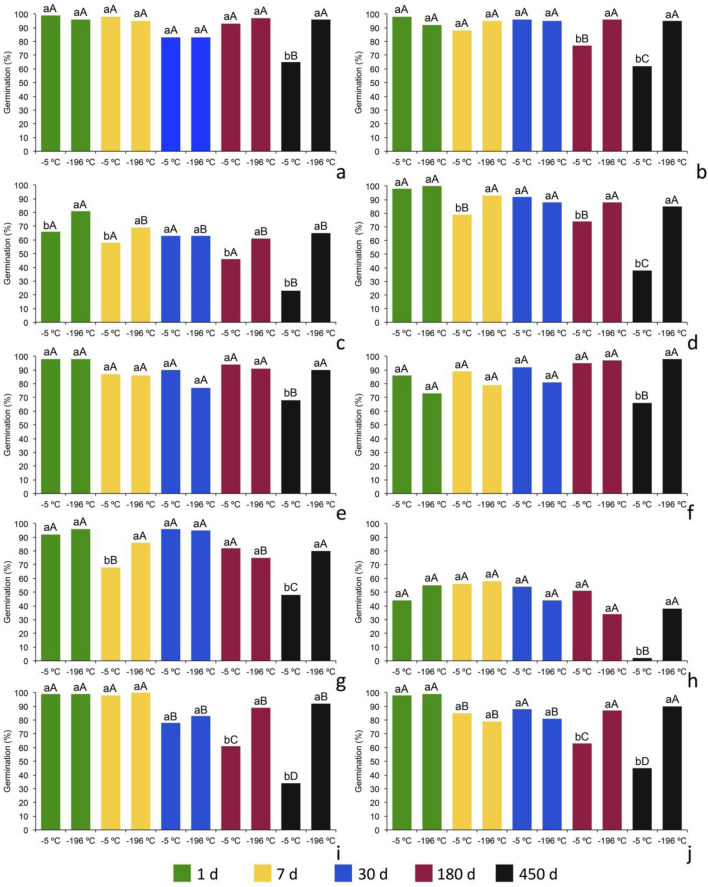
The analysis of variance revealed different behavior among the 20 species regarding the two conservation conditions (freezer at − 5 °C and liquid nitrogen at − 196 °C) and five evaluation times (1, 7, 30, 180 and 450 days). The results of the germination percentage after the different observation periods and conservation conditions are presented in Figs. 5 and 6 and the germination speed index values (GSI) are shown in Figs. 7 and 8.
Fig. 5.
Germination percentage of seeds of Tillandsia species (Bromeliaceae) occurring in Bahia, Brazil stored at temperature of − 5 °C or cryopreserved at − 196 °C for 1–450 days. a T. bulbosa; b T. juncea; c T. paraensis; d T. polystachia; e T. pruinosa; f T. loliacea; g T. recurvata; h T. tricholepis; i T. streptocarpa 1; and j T. streptocarpa 2. Means followed by the same lowercase letters (temperature) and uppercase letters (time) do not differ by the F test and Tukey test (p < 0.01), respectively
Fig. 6.
Germination percentage of seeds of Tillandsia species (Bromeliaceae) occurring in Bahia, Brazil stored at a temperature of − 5 °C or cryopreserved at − 196 °C for 1–450 days. a T. chapeuensis; b T. gardneri; c T. geminiflora; d T. globosa; e T. pohliana; f T. stricta 1; g T. stricta 2; h T. stricta subsp. piniformis; i T. tenuifolia; and j T. tenuifolia var. vaginata. Means followed by the same lowercase letters (temperature) and uppercase letters (time) do not differ by the F test and Tukey test (p < 0.01), respectively
Fig. 7.
Germination speed index (GSI) of seeds of Tillandsia species (Bromeliaceae) occurring in Bahia, Brazil stored at temperature of − 5 °C or cryopreserved at − 196 °C for 1–450 days. a T. bulbosa; b T. juncea; c T. paraensis; d T. polystachia; e T. pruinosa; f T. loliacea; g T. recurvata; h T. tricholepis; i T. streptocarpa 1; and j T. streptocarpa 2. Means followed by the same lowercase letters (temperature) and uppercase letters (time) do not differ by the F test and Tukey test (p < 0.01), respectively
Fig. 8.
Germination speed index (GSI) of seeds of Tillandsia species (Bromeliaceae) occurring in Bahia, Brazil stored at temperature of − 5 °C or cryopreserved at − 196 °C for 1–450 days. a) T. chapeuensis; b T. gardneri; c T. geminiflora; d T. globose; e T. pohliana; f T. stricta 1; g T. stricta 2; h T. stricta subsp. piniformis; i T. tenuifolia; and j T. tenuifolia var. vaginata. Means followed by the same lowercase letters (temperature) and uppercase letters (time) do not differ by the F test and Tukey test (p < 0.01), respectively
According to the results found T. bulbosa (Figs. 5a, 7a), T. pruinosa (Figs. 5e, 7e), T. loliacea (Figs. 5f, 7f), T. tricholepis (Figs. 5h, 7h), T. gardneri (Figs. 6b, 8b), T. pohliana (Figs. 6e, 8e), T. stricta 1 (Figs. 6f, 8f), T. stricta subsp. piniformis (Figs. 6h, 8h) and T. tenuifolia (Figs. 6i, 8i) did not differ significantly regarding germination percentage (Figs. 5 and 6) and germination speed index (Figs. 7 and 8) between the two storage temperatures during 180 days of seed conservation. All the species presented germination higher than 75%, with average of 90%, except for T. tricholepis (Fig. 5h), with an average of 45%, and T. tenuifolia, with 60%. Regarding the GSI, the values varied from 3 to 6, with the smallest ones recorded for T. tricholepis and T. tenuifolia, with values of 3 to 3.5 (Figs. 7h, 8i) in this same evaluation period.
However, after 180 days there was an initial reduction of the germination and GSI when the seeds were stored in the freezer (− 5 °C) for the species T. juncea (Figs. 5b, 7b), T. paraensis (Figs. 5c, 7c), T. polystachia (Figs. 5d, 7d), T. streptocarpa (Figs. 5i, 7i), T. streptocarpa (Figs. 5j, 7j), T. chapeuensis (Figs. 6a, 8a), T. geminiflora (Figs. 6c, 8c), T. globosa (Figs. 6d, 8d) and T. tenuifolia var. vaginata (Figs. 6j, 8j), with an absence of germination and hence GSI of zero for T. stricta 2 (Figs. 6g, 8g). In contrast, there was no decrease in viability of the cryopreserved seeds in this same period for all the species.
Nevertheless, in the evaluations of the seeds stored for 450 days, all the species presented significant differences between the two storage temperatures, with drastic declines of the germination and GSI of the seeds kept in the freezer (− 5 °C). For T. paraensis (Fig. 5c), T. polystachia (Fig. 5d), T. tricholepis (Fig. 5h), T. streptocarpa 1 (Fig. 5i), T. streptocarpa 2 (Fig. 5j), T. chapeuensis (Fig. 6a), T. geminiflora (Fig. 6c), T. globosa (Fig. 6d), T. stricta 2 (Fig. 6g). T. tenuifolia (Fig. 6i) and T. tenuifolia var. vaginata (Fig. 6j), the reduction in the germination percentage was greater than 50%, and no T. chapeuensis seed germination of (Fig. 6a), T. globosa (Fig. 6d) and T. stricta 2 (Fig. 6g).
Of the 20 species evaluated, 13 germinated above 80% when preserved in liquid nitrogen for 450 days and five had percentages between 60 and 78%. The lowest germination percentages after cryopreservation were observed for T. stricta 2 (Fig. 6g), with an average of 33%, and T. tricholepis (Fig. 5h), with an average of 38%.
The highest germination speed index (GSI) values after conservation in liquid nitrogen (− 196 °C) for 450 days were observed for T. streptocarpa 2 (6.55) and T. globosa (6.01), and the lowest values were found for T. tricholepsis (2.50) and T. stricta 2 (2.99). For T. chapeuensis (Figs. 6a, 8a), there were increases in the germination and GSI after the seeds were stored in liquid nitrogen when compared to − 5 °C on days 1, 30, 180, and 450 (Figs. 5 and 6). Increases of 10% in germination percentage after cryopreservation were reported for Vriesea bahiana Leme (Silva et al. 2021) and for Encholirum magalhaestii L.B.Sm. and E. subsecundum Mez (Tarré et al. 2007). The increase in germination after cryopreservation of seeds has been reported for various other species (Pence 1991; Salomão 2002; Khanna et al. 2014; Araújo et al. 2019), besides species of Bromeliaceae (Silva et al. 2021). Salomão (2002), analyzing various Cerrado species, reported the influence of cryopreservation on the physical breaking of dormancy by inducing cracks in the endocarp or seed coat immediately surrounding the embryo. Likewise, Khanna et al. (2014), studying several species of Sarracenia, also reported the same observations. However, Araújo et al. (2019) and Silva et al. (2021) reported that the increase is related to the kinetic acceleration of germination of the seeds triggered by their sensitivity to desiccation, thus enabling them to withstand ultralow temperatures.
The longevity of orthodox seeds, when conserved at low temperatures, has been reported for various species, so cryopreservation can be of great importance for long-term storage of orthodox seeds with short lifetimes (Pritchard et al. 1999; Walters et al. 2004; Pritchard 2007; Pritchard and Nadarajan 2008), as we observed for some species of Tillandsia, which after 180 days already presented low viability when stored in a conventional freezer. The longevity of cryopreserved seeds was reported by Walters et al. (2004), who suggested increase of up to 175 times the normal interval when compared to the normal temperature of seed banks. This extended longevity of cryopreserved seeds was previously studied in lettuce by Pritchard (2007), who observed a 74-fold longer period in comparison with storage at temperature of − 18 °C.
The results of storage in a freezer (− 5 °C) made it evident that the decrease in seed viability with longer conservation time can be related to the maintenance of the seeds’ metabolism, leading to the deterioration of the tissue. Voronkova et al. (2018) stated that storage of seeds at room temperature or in places with high humidity, such as freezers, can negatively affect the germination percentage, besides favoring the proliferation of microorganisms. Ultralow temperatures combined with reduced water content depress the metabolism, thus minimizing the deterioration reactions during storage. It is thus necessary to understand the involvement of the process of tolerance to desiccation with the behavior of seeds during storage. According to Li and Pritchard (2009), cryopreservation is particularly important to conserve orthodox seeds with low longevity, which quickly lose viability in conventional germplasm banks.
Conclusion
After conservation for 450 days, there was a reduction of the germination and the GSI of all the species studied when the seeds were preserved in a freezer, with total absence of germination (and thus GSI of zero) for T. chapeuensis, T. globosa and T. stricta 2. Cryopreservation is an efficient strategy to conserve seeds of the 20 Tillandsia species analyzed when desiccated to the water content of approximately 7% (dry mass basis, DMB). Our results support the establishment of a cryobank of Tillandsia that can be used to assure the preservation of endemic species that are vulnerable or in danger of extinction. Based on these results, obtained with a substantial number of species of the Tillandsia genus, including representatives of four subgenera, we believe it would be possible to apply this protocol to the other species of the genus, with emphasis on those that are vulnerable or threatened with extinction, thus preventing loss of important biodiversity.
Acknowledgments
This paper is part of RSO MSc dissertation and this study was financed in part by the Fundação de Amparo a Pesquisa do Estado da Bahia (BOL0177/2018) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. The authors would like to thank the support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (PROCAD—88887.124186/2014-00). EHS acknowledges the support of CAPES/PNPD/UFRB (88882.315208/2019-01), FVDS currently holds a productivity grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq—304269/2018-2).
Abbreviations
- HURB
Herbario do Recôncavo da Bahia
- GSI
Germination speed index
- MS
Murashige and Skoog (1962) culture medium
Author contributions
RSO, ILS and SOS: data curation, formal analysis, investigation, methodology, visualization, writing—original draft. FVDS and LYSA: conceptualization, funding acquisition, project administration, resources, supervision, writing—review and editing. EHS: conceptualization, formal analysis, funding acquisition, methodology, project administration, resources, supervision, writing—original draft, writing—review and editing.
Funding
There are no financial conflicts of interest to disclose.
Declarations
Conflict of interest
The authors declare that they have no con ict of interest in the publication.
References
- Araújo S, Pagano A, Dondi D, Lazzaroni S, Pinela E, Macovei A, Balestrazzi A. Metabolic signatures of germination triggered by kinetin in Medicago truncatula. Sci Rep. 2019;9:10466. doi: 10.1038/s41598-019-46866-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfuss MHJ, Till W, Leme EMC, Pinzón JP, Manzanares JM, Halbritter H, Samuel R, Brown JK. Taxonomic revision of Bromeliaceae subfam. Tillandsioideae based on a multi-locus DNA sequence phylogeny and morphology. Phytotaxa. 2016;279(1):1–97. doi: 10.11646/phytotaxa.279.1.1. [DOI] [Google Scholar]
- Bastos MJSM, Bastos LP, Souza EH, Soares TL, Morais DV, Souza FVD, Costa MAPC. Floral and reproductive biology of Alcantarea nahoumii (Bromeliaceae), a vulnerable endemic species of the Atlantic Forest. Acta Bot Bras. 2017;31(4):665–676. doi: 10.1590/0102-33062017abb0102. [DOI] [Google Scholar]
- Benzing DH. Bromeliaceae: profile of an adaptive radiation. Cambrigde: University Press; 2000. [Google Scholar]
- Brasil. Ministério da Agricultura, Pecuária E Abastecimento . Ministério da agricultura, pecuária e abastecimento. Brasília: Secretaria de Defesa Agropecuária Mapa/ACS; 2009. Regras para análise de sementes. [Google Scholar]
- Castillo RF, Trujillo‐Argueta S, Rivera‐García R, Gómez‐Ocampo Z, Mondragón‐Chaparro D (2013) Possible combined effects of climate change, deforestation, and harvesting on the epiphyte: a multidisciplinary approach. Ecol Evol 3(11):3935–3946. 10.1002/ece3.765 [DOI] [PMC free article] [PubMed]
- Castro RD, Bradford KJ, Hilhorst HWM. Desenvolvimento de sementes e conteúdo de água. In: Ferreira AG, Borghetti F, editors. Germinação: do básico ao aplicado. Porto Alegre: Artmed; 2004. pp. 51–67. [Google Scholar]
- Coelho MFB, Vieira SN, Chig LA, Santos LW, Albuquerque MCF. Superação da dormência em sementes de Bromelia balansae (Bromeliaceae) Hortic Bras. 2011;29(4):472–476. doi: 10.1590/S0102-05362011000400005. [DOI] [Google Scholar]
- Dar JA, Kareem M, Zargar SM, Wani AA, Rasool S, Bhat KA. Strategies for conservation of genetic resources. In: Salgotra R, Zargar S, editors. Rediscovery of genetic and genomic resources for future food security. Singapore: Springer; 2020. pp. 315–334. [Google Scholar]
- Dematê MESP. Information on Brazilian ornamental species of the genus Tillandsia L. (Bromeliaceae) Acta Hort. 2005;683:293–200. doi: 10.17660/ActaHortic.2005.683.35. [DOI] [Google Scholar]
- Dinato NB, Santos IRI, Vigna BBZ, Paula AF, Fávero AP. Pollen cryopreservation for plant breeding and genetic resources conservation. CryoLetters. 2020;41(3):115–127. [PubMed] [Google Scholar]
- Estrella-Parra E, Flores-Cruz M, Blancas-Flores G, Koch SD, Alarcón-Aguilar FJ. The Tillandsia genus: history, uses, chemistry, and biological activity. Bol Latinoam Caribe Plantas Med Aromat. 2019;18(3):239–264. [Google Scholar]
- Fernandez LV, Beltramo J, Caldiz DO. Germinacion y longevidad de semillas de Tillandsia recurvata. Revista de la Facultad de Agronomia. 1989;65(1–2):81–85. [Google Scholar]
- Ferrari EAP, Colombo RC, Faria RT, Takane RJ. Cryopreservation of seeds of Encholirium spectabile Martius ex Schultes f. by the vitrification method. Rev Ciênc Agron. 2016;47(1):127–177. doi: 10.5935/1806-6690.20160020. [DOI] [Google Scholar]
- Figueiredo JC, David AMSS, Silva CD, Amaro HTR, Alves DD. Maturação de sementes de pimenta em função de épocas de colheita dos frutos. Sci Agr. 2017;18(3):1–7. doi: 10.5380/rsa.v18i3.51324. [DOI] [Google Scholar]
- Flora do Brasil 2020 (cont.updated) Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB6361. Accessed 12 june 2020
- Flores-Palacios A, Valencia-Díaz S. Local illegal trade reveals unknown diversity and involves a high species richness of wild vascular epiphytes. Biol Conserv. 2007;136(3):372–387. doi: 10.1016/j.biocon.2006.12.017. [DOI] [Google Scholar]
- Forzza RC, Costa AF, Leme EMC, Versieux LM, Wanderley MGL, Louzada RB, Monteiro RF, Judice DM, Fernandez EP, Borges RAX, Penedo TSA, Monteiro NP, Moraes MA. Bromeliaceae. In: Martinelli G, Moraes MA, editors. Livro vermelho da flora do Brasil. Rio de Janeiro: Andrea Jakobsson & amp; 2013. pp. 315–397. [Google Scholar]
- Generoso AL, Carvalho VS, Walter R, Campbell G, Araújo LS, Santana JGS, Cunha M. Mature-embryo culture in the cryopreservation of passion fruit (Passiflora edulis Sims) seeds. Sci Hortic. 2019;256:108638. doi: 10.1016/j.scienta.2019.108638. [DOI] [Google Scholar]
- Gosling PG. Viability Testing. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ, editors. Seed conservation: turning science into practice. Kew: Royal Botanic Gardens; 2003. pp. 445–481. [Google Scholar]
- Gouda EJ, Butcher D, Gouda K (2021) Encyclopaedia of bromeliads. http://bromeliad.nl/bromNames/. Accessed 17 March 2021
- Haeckel IB. The “Arco Floral”: ethnobotany of Tillandsia and Dasylirion spp. in a Mexican religious adornment. Econ Bot. 2008;62:90–95. doi: 10.1007/s12231-008-9009-8. [DOI] [Google Scholar]
- Hietz P, Ausserer J, Schindler G (2002) Growth, maturation and survival of epiphytic bromeliads in a Mexican humid montane forest. J Trop Ecol 18(2):177–191. 10.1017/S0266467402002122
- Hornung-Leoni CT. Avances sobre usos etnobotánicos de las Bromeliaceae en Latinoamérica. Bol Latinoam Caribe Plant Med Aromat. 2011;10:297–314. [Google Scholar]
- Jenderek MM, Reed BM. Cryopreserved storage of clonal germplasm in the USDA national plant germplasm system. Vitro Cell Dev Biol-Plant. 2017;53:299–308. doi: 10.1007/s11627-017-9828-3. [DOI] [Google Scholar]
- Khanna S, Jenkins H, Bucalo K, Determann RO, Cruse-Sanders JM, Pullman GS. Effects of seed cryopreservation, stratification and scarification on germination for five rare species of pitcher plants. CryoLetters. 2014;35(1):29–39. [PubMed] [Google Scholar]
- Li DZ, Pritchard HW. The science and economics of ex situ plant conservation. Trends Plant Sci. 2009;14(11):614–621. doi: 10.1016/j.tplants.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Machado LC, Oliveira VC, Paraventi MD, Cardoso RNR, Martins DS, Ambrósio CE. Maintenance of Brazilian biodiversity by germplasm bank. Pesq Vet Bras. 2016;36(1):62–66. doi: 10.1590/S0100-736X2016000100010. [DOI] [Google Scholar]
- Magalhães RI, Mariath JEA. Seed morphoanatomy and its systematic relevance to Tillandsioideae (Bromeliaceae) Plant Syst Evol. 2012;298:1881–1895. doi: 10.1007/s00606-012-0688-3. [DOI] [Google Scholar]
- Malhotra N, Panatu S, Singh B, Negi N, Singh D, Singh M, Chandora R. Genetic resources: collection, conservation, characterization and maintenance. In: Singh M, editor. Lentils: potential resources for enhancing genetic gains. Elsevier; 2019. pp. 21–41. [Google Scholar]
- Martins CC, Nakagawa J, Bovi MLA. Influência do peso das sementes de palmito-vermelho (Euterpe espiritosantensis Fernandes) na porcentagem e na velocidade de germinação. Rev Bras Sem. 2000;22(1):47–53. doi: 10.17801/0101-3122/rbs.v22n1p47-53. [DOI] [Google Scholar]
- Masetto TE, Faria JMR. In situ DNA fragmentation during the re-establishment of desiccation tolerance in germinated seeds of Cedrela fissilis Vell. J Seed Sci. 2019;41(2):244–249. doi: 10.1590/2317-1545v42n2207417. [DOI] [Google Scholar]
- Mello JIO, Barbedo CJ, Salatino A, Figueiredo-Ribeiro RCL. Reserve carbohydrates and lipids from the seeds of four tropical tree species with different sensitivity to desiccation. Braz Arch Biol Technol. 2010;53(4):889–899. doi: 10.1590/S1516-89132010000400019. [DOI] [Google Scholar]
- Mercier H, Guerreiro Filho O. Propagação sexuada de algumas bromélias nativas da Mata Atlântica: efeito da luz e da temperatura na germinação. Hoehnea. 1990;17(1):19–26. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nara AK, Webber AC. Biologia floral e polinização de Aechmea beeriana (Bromeliaceae) em vegetação de baixio na Amazônia Central. Acta Amaz. 2002;32(4):571–588. doi: 10.1590/1809-43922002324588. [DOI] [Google Scholar]
- Negrelle RRB, Mitchell D, Anacleto A. Bromeliad ornamental species: conservation issues and challenges related to commercialization. Acta Sci Biol Sci. 2012;34(1):91–100. doi: 10.4025/actascibiolsci.v34i1.731. [DOI] [Google Scholar]
- NOM-059-SEMARNAT (2019) Lista de especies en riesgo de la Norma Oficial Mexicana. Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Diario Oficial de la Federación 14/11/2019
- Panis B, Lambardi M. Status of cryopreservation technologies in plants (crops and forest trees) The Role of Biotechnol. 2006;5(7):43–54. [Google Scholar]
- Pegg DE. The relevance of ice crystal formation for the cryopreservation of tissues and organs. Cryobiol. 2010;60(3):36–44. doi: 10.1016/j.cryobiol.2020.01.005. [DOI] [PubMed] [Google Scholar]
- Pence VC. Cryopreservation of immature embryos of Theobroma cacao. Plant Cell Rep. 1991;10:144–147. doi: 10.1007/BF00232046. [DOI] [PubMed] [Google Scholar]
- Pereira TS. Bromelioideae (Bromeliaceae): morfologia do desenvolvimento pós-seminal de algumas espécies. Arq Jardim Bot Rio de Janeiro. 1988;29:115–154. [Google Scholar]
- Pereira AR, Pereira TS, Andrade ACS. Morfologia de sementes e do desenvolvimento pós-seminal de espécies de Bromeliaceae. Acta Bot Bras. 2008;22(4):1150–1162. doi: 10.1590/S0102-33062008000400026. [DOI] [Google Scholar]
- Pereira CC, Francine L, Panobianco M. Germinação e armazenamento de sementes de Nidularium innocentii (Lem.) Rev Bras Sem. 2010;32(2):36–41. doi: 10.1590/S0101-31222010000200004. [DOI] [Google Scholar]
- Pinheiro F, Borghetti F. Light and temperature requirements for germination of seeds of Aechmea nudicaulis (l.) Griesebach and Streptocalyx floribundus (Martius ex Shultes f.) Mez (Bromeliaceae) Acta Bot Bras. 2003;17(1):27–35. doi: 10.1590/S0102-33062003000100003. [DOI] [Google Scholar]
- Pritchard HW. Cryopreservation of desiccation-tolerant seeds. In: Day JG, Stacey GN, editors. Cryopreservation and freeze-drying protocols methods in molecular biologyTM. Humana Press; 2007. pp. 185–201. [DOI] [PubMed] [Google Scholar]
- Pritchard HW, Nadarajan J. Cryopreservation of orthodox (desiccation tolerant) seeds. In: Reed BM, editor. Plant cryopreservation: a practical guide. New York: Springer; 2008. pp. 485–501. [Google Scholar]
- Pritchard HW, Poyner ALC, Seaton PT. Interspecific variation in orchid seed longevity in relation to ultra-dry storage and cryopreservation. Lindleyana. 1999;14:92–101. [Google Scholar]
- R Development Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2019. [Google Scholar]
- Rao VR, Parthasarathy V. Horticultural genetic resources conservation: priorities, challenges and way forward. In: Rajasekharan P, Rao V, editors. Conservation and utilization of horticultural genetic resource. Springer; 2019. pp. 27–47. [Google Scholar]
- Rodrigues LR, Dornelles ALC, Wittmann MTS. Poliembrionia e número de sementes por fruto de quatro cultivares de tangerineira. Cienc Rural. 1999;29(3):469–474. doi: 10.1590/S0103-84781999000300015. [DOI] [Google Scholar]
- Rodrigues ARP, Forzza RC, Andrade ACS. Physiological characteristics underpinning successful cryopreservation of endemic and endangered species of Bromeliaceae from the Brazilian Atlantic Forest. Bot J Linn Soc. 2014;176(4):567–578. doi: 10.1111/boj.12219. [DOI] [Google Scholar]
- Rueden CT, Schindelin J, Hiner MC, Dezonia BR, Walter AE, Arena ET, Eliceiri K. W. Image J2: imageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18(529):1–26. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomão AN. Tropical seed species’ responses to liquid nitrogen exposure. Braz J Plant Physiol. 2002;14(2):133–138. doi: 10.1590/S1677-04202002000200008. [DOI] [Google Scholar]
- Scrok GJ, Varassin IG. Reproductive biology and pollination of Aechmea distichantha Lem. (Bromeliaceae) Acta Bot Bras. 2011;25(3):571–576. doi: 10.1590/S0102-33062011000300009. [DOI] [Google Scholar]
- Silva LMM, Rodrigues TJD, Aguiar IB. Efeito da luz e da temperatura na germinação de sementes de aroeira (Myracrodruon urundeuva Allemão) Rev Árvore. 2002;26(6):691–697. doi: 10.1590/S0100-67622002000600006. [DOI] [Google Scholar]
- Silva KS, Mendonça V, Medeiros LF, Freitas PSC, Góis GB. Influência do tamanho da semente na germinação e vigor de mudas de jaqueira (Artocarpus heterophyllus Lam.) Rev Verde Agroecologia Desenvolv Sustent. 2010;5(4):217–221. [Google Scholar]
- Silva RL, Ferreira CF, Ledo CAS, Souza EH, Silva PH, Costa MAPC, Souza FVD. Viability and genetic stability of pineapple germplasm after ten years of in vitro conservation. Plant Cell Cult Microprop. 2016;127(1):123–133. doi: 10.1007/s11240-016-1035-0. [DOI] [Google Scholar]
- Silva SSS, Souza EH, Souza FVD, Max DAS, Rossi ML, Costa MAPC. Post-seminal development and cryopreservation of endemic or endangered bromeliads. An Acad Bras Ciênc; 2021. [DOI] [PubMed] [Google Scholar]
- Smith LB, Downs RJ. Tillandsioideae (Bromeliaceae) Fl Neotrop Monogr. 1977;14(2):663–1492. [Google Scholar]
- Socolowski F, Vieira DCM, Takaki M. Seed mass of Tecoma stans L. Juss. ex Kunth (Bignoniaceae): effects on emergence and seedling development under full sun and shade. Biota Neotrop. 2011;11(2):171–178. doi: 10.1590/S1676-06032011000200017. [DOI] [Google Scholar]
- Souza EH, Souza FVD, Rossi ML, Brancalleão N, Ledo CAS, Martinelli AP. Viability, storage and ultrastructure analysis of Aechmea bicolor (Bromeliaceae) pollen grains, an endemic species to the Atlantic forest. Euphytica. 2015;204:13–28. doi: 10.1007/s10681-014-1273-3. [DOI] [Google Scholar]
- Souza FVD, Kaya E, Vieira LJ, Souza EH, Amorim VBO, Skogerboe D, Matsumoto T, Alves AAC, Ledo CAS, Jenderek MM. Droplet-vitrification and morphohistological studies of cryopreserved shoot tips of cultivated and wild pineapple genotypes. Plant Cell Tissue Org Cult. 2016;126:351–360. doi: 10.1007/s11240-015-0899-8. [DOI] [Google Scholar]
- Souza EH, Versieux LM, Souza FVD, Rossi ML, Costa MAPC, Martinelli AP. Interspecific and intergeneric hybridization in Bromeliaceae and their relationships to breeding systems. Sci Hortic. 2017;221(1):53–61. doi: 10.1016/j.scienta.2017.04.027. [DOI] [Google Scholar]
- Stanwood PC. Cryopreservation of seed germplasm for genetic conservation. In: Kartha K, editor. Cryopreservation of plant cells and organs. Boca Raton: CRC Press; 1985. pp. 199–225. [Google Scholar]
- Tarré E, Pires BBM, Guimarães APM, Carneiro LA, Forzza RC, Mansur E. Germinability after desiccation, storage and cryopreservation of seeds from endemic Encholirium Mart. ex Schult. and Schult. f. and Dyckia Schult. and Schult. f. species (Bromeliaceae) Acta Bot Bras. 2007;21(4):777–783. doi: 10.1590/S0102-33062007000400003. [DOI] [Google Scholar]
- Toledo-Aceves T, García-Franco JG, López-Bezerra F. Bromeliad rain: An opportunity for cloud forest management. Forest Ecol Manag. 2014;329:129–136. doi: 10.1016/j.foreco.2014.06.022. [DOI] [Google Scholar]
- Vasak V. The viability of bromeliad seeds. Bromeliad Soc Bull. 1969;19:102–104. [Google Scholar]
- Vettorazzi RG, Carvalho VS, Teixeira MC, Campostrini E, Cunha M, Matos EM, Viccini LF. Cryopreservation of immature and mature seeds of Brazilian orchids of the genus Cattleya. Sci Hortic. 2019;256:108603. doi: 10.1016/j.scienta.2019.108603. [DOI] [Google Scholar]
- Vieira DCM, Socolowski F, Takaki M. Germinação de sementes de Dyckia tuberosa (Vell.) Beer (Bromeliaceae) sob diferentes temperaturas em luz e escuro. Braz J Bot. 2007;30(2):183–188. doi: 10.1590/S0100-84042007000200003. [DOI] [Google Scholar]
- Villela FA, Peres WB. Coleta, beneficiamento e armazenamento. In: Borghetti F, Ferreira AG, editors. Germinação: do básico ao aplicado. Porto Alegre: Artmed; 2004. pp. 265–281. [Google Scholar]
- Vivian R, Silva AA, Gimenes Junior M, Fagan EB, Ruiz ST, Labonia V. Dormência em sementes de plantas daninhas como mecanismo de sobrevivência: breve revisão. Planta Daninha. 2008;26(3):695–706. doi: 10.1590/S0100-83582008000300026. [DOI] [Google Scholar]
- Voronkova NM, Kholina AB, Koldaeva MN, Nakonechnaya OV, Nechaev VA. Morphophysiological dormancy, germination, and cryopreservation in Aristolochia contorta seeds. Plant Ecol Evol. 2018;151(1):77–86. doi: 10.5091/plecevo.2018.1351. [DOI] [Google Scholar]
- Walters C, Wheeler L, Stanwood PC. Longevity of cryogenically stored seeds. Cryobiology. 2004;48(3):229–244. doi: 10.1016/j.cryobiol.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Wang M, Lambardi M, Engelmann F, Pathirana R, Panis B, Volk GM, Wang QC. Advances in cryopreservation of in vitro-derived propagules: technologies and explant sources. Plant Cell Tiss Organ Cult. 2020 doi: 10.1007/s11240-020-01770-0. [DOI] [Google Scholar]
- Wester S, Zotz G. Seed comas of bromeliads promote germination and early seedling growth by wick-like water uptake. J Trop Ecol. 2011;27:115–119. doi: 10.1017/S0266467410000593. [DOI] [Google Scholar]
- Zotz G. A longer story than expected: seeds of several species (Tillandsioideae) remain viable for up to two years. J Bromeliad Soc. 2013;63(1):83–86. [Google Scholar]