Abstract
Background
Pharmacokinetics and pharmacodynamics of drugs in elderly individuals differ from those in younger adults; thus, adverse drug events (ADEs) are common in older patients with polypharmacy because co-existing comorbidities elevate the risk of ADEs occurring. However, ADEs have not yet been characterised based on the elderly patients of Japanese origin and polypharmacy.
Objective
The 100 most commonly reported ADEs were grouped into four classes (Class 1–Class 4) based on elderly patients with polypharmacy.
Patients and Methods
In this study, logistic regression analysis was performed using cases recorded in the Japanese Adverse Drug Event Report (JADER) database.
Results
ADEs in elderly patients treated with polypharmacy—in whom the risk of electrolyte abnormalities, renal and respiratory disorders, and coagulopathy was high—were categorised as ‘Class 1 [E(+), P(+)]’, while ADEs in elderly patients not treated with polypharmacy—in whom the risk of delirium and fall was high—were categorised as ‘Class 2 [E(+), P(−)]’. When there was no association with being elderly, ADEs associated with polypharmacy that carried a high risk of myelosuppression and infection were categorised as ‘Class 3 [E(−), P(+)]’, and allergic ADEs that were not affected by being elderly or polypharmacy, were categorised as ‘Class 4 [E(−), P(−)]’. Class 1 events as well as Class 3 ADEs occurred more frequently in females than in males, whereas Class 3 ADEs (deep vein thrombosis and pulmonary embolism) occurred more frequently in males.
Conclusions
Class 1 and Class 2 ADEs should be investigated in analyses that focus on individual drugs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40801-020-00221-8.
Key Points
| Using the Japanese Adverse Drug Event Report database, adverse drug events were categorized into four classes (Class 1–Class 4) based on age (elderly—≥ 70 years) and polypharmacy (six or more agents). |
| Among the adverse drug events associated with elderly patients, the risks of electrolyte abnormality, renal disorder, respiratory disorder, and coagulopathy were associated with polypharmacy (Class 1). |
| Among the adverse drug events associated with elderly patients, the risks of delirium and fall were not associated with polypharmacy (Class 2). |
Introduction
As a result of changes in physiological function that occur with aging, pharmacokinetics and organic drug responses (i.e. pharmacodynamics) differ between the elderly and the non-elderly [1–5]. Because of this, the risk of adverse drug events (ADEs) is higher in the elderly than in the non-elderly [1–6]. Since co-existing comorbidities tend to increase in frequency with age, there is a tendency for polypharmacy prescribing for the elderly [2, 3, 7, 8]. Polypharmacy, the simultaneous use of multiple medications by patients, has been linked to reduced adherence to medication, prolongation of hospitalisation, readmission to hospital immediately after discharge, mortality, ADE incidence, and elevated risks of drug interaction [3, 4]. Moreover, follow-up prescriptions (‘prescription cascades’) of concomitant drugs for occurring ADEs also give rise to polypharmacy [4]. This type of polypharmacy treatment in the elderly, which is linked to adverse outcomes, has been reported as a global public health issue [9]. ADEs induced by polypharmacy increase dramatically in patients prescribed five to six drugs [2].
In this way, aging and polypharmacy increase the risk of ADEs. It is said that the geriatric syndromes (such as delirium, falling, dizziness, and urinary incontinence) are non-specific and do not fit into a single deficit diagnosis [10]. In other words, it can be considered that the risk of certain ADEs in elderly patients receiving polypharmacy increases not because of specific drugs but because of an increase in the number of drugs administered. Therefore, we hypothesized that the elderly may be vulnerable to certain ADEs, given that they have higher sensitivity to drugs. Thus, the identification of which ADEs are likely to be caused by aging and polypharmacy would make it easier to observe these ADEs more closely for managing drug treatment effectively in the elderly.
Focusing on cases recorded in the Japanese Adverse Drug Event Report (JADER) database from the perspectives of polypharmacy and aging, we grouped the 100 most commonly reported ADEs into four classes (Class 1–Class 4) and investigated the influence of these two factors on the risk of ADEs.
Methods
Database Information
The JADER was downloaded from the Pharmaceuticals and Medical Devices Agency website (http://www.info.pmda.go.jp/fukusayoudb/CsvDownload.jsp). JADER is the database of collected and published cases of ADEs that were reported by pharmaceutical companies and medical staff, and is used for pharmacovigilance activities. All cases recorded from April 2004 to July 2018 were used. The JADER is composed of four tables: a ‘demo’ table, a ‘drug’ table, a ‘reac’ table, and a ‘hist’ table, across which each case is linked by the assignment of a common identification number. Data on patients such as age and sex are presented in the ‘demo’ table, data on drugs used in cases are presented in the ‘drug’ table, data on ADEs that have occurred in cases are presented in the ‘reac’ table, and primary disease data are presented in the ‘hist’ table. For this study, we used the ‘demo’, ‘drug’ and ‘reac’ tables. The ‘drug involvement’ from the drug table in JADER mentions ‘Suspected drug’, ‘Co-administered drug’ or ‘Interactions’ for each drug. However, this study did not use the ‘drug involvement’ information as the study did not focus on individual drugs. The study instead examined all the registered drugs. The ADEs used in this study were entered in the database using the preferred terms (PTs) from the Medical Dictionary for Regulatory Activities/J (MedDRA/J), version 21.0. All processing was performed at the PT level.
Adverse Drug Events (ADEs)
All ADEs recorded in JADER were extracted from the reac table, and the total number of reports was calculated for each. We then ranked the top 100 ADE cases according to their frequency based on the information that we referred to. This study analysed these 100 ADEs. Cases in which none of the 100 ADEs were observed were also included as a control group.
Data Cleaning
The data-cleaning procedure is shown in Fig. 1. First, cases clearly denoted by ‘sex’ and ‘age’ were extracted from the JADER demo table. Then, we used the reac table to prepare combinations of ‘identification number-ADEs-date of ADEs,’ applying each combination to a single case. ADEs reported with the same identification number were counted as different cases. Then, using the common identification number for each case, we extracted ‘sex’ and ‘age’ from the demo table, ‘drug name (generic name),’ ‘date of start of use’ and ‘date of termination of use’ from the drug table, and ‘ADEs’ and ‘date of ADEs occurrence’ from the reac table. Year, month and day were used to denote the start of drug use and the termination of drug use, and for the date of ADE occurrence. By excluding drugs that were administered before or after the ADE occurrence, the remaining drugs were considered to have been used at the time of occurrence. Drugs with no clear record of the start or termination dates of use and those that were used at the time of ADE occurrence were regarded as patient-use drugs. When different dosages of the same drug were included in multiple records for a single case, the drug was counted as a single agent. The JADER also includes clinical trial data, thus spontaneously reported cases were included in the analysis, while clinical trial data were excluded.
Fig. 1.

Data-cleaning procedure. ADE adverse drug event
Data Analysis
Cases were divided into men and women, and subsequent analyses were conducted separately for each sex. We counted the number of concomitant drugs for each case. Based on previous studies, polypharmacy was defined as cases where the patient was treated with six or more concomitant drugs and non-polypharmacy cases where the patient was treated with less than six drugs [11, 12]. Although ‘elderly’ generally refers to individuals aged 65 years and older, as age is grouped by decade in the JADER, ‘elderly’ was defined as patients aged 70 years or more and ‘non-elderly’ was defined as those aged less than 70 years [13].
In this study, we used logistic regression analysis to analyse the factors ‘elderly’ and ‘polypharmacy’, because it can be used to simultaneously analyse multiple factors [14]. Next, to investigate the influence of elderly and polypharmacy, with xe as the presence or absence of elderly, and xp as the presence or absence of polypharmacy with respect to the reporting rate for each ADE in the JADER database, p, we performed logistic regression analysis according to Eq. 1:
| 1 |
The natural exponential function of the partial regression coefficient (ae or ap) derived from logistic regression analysis with Eqs. (2) and (3) being the adjusted odds ratio (adjusted OR), elderly risk (ORe) and polypharmacy risk (ORp) were calculated for each ADE. Non-association between ‘elderly’ or ‘polypharmacy’ and ADE onset is represented by OR 1, while it is represented by OR > 1 where there is risk of ADE onset. In this study, a significant risk was defined as a 95% confidence interval of OR.
| 2 |
| 3 |
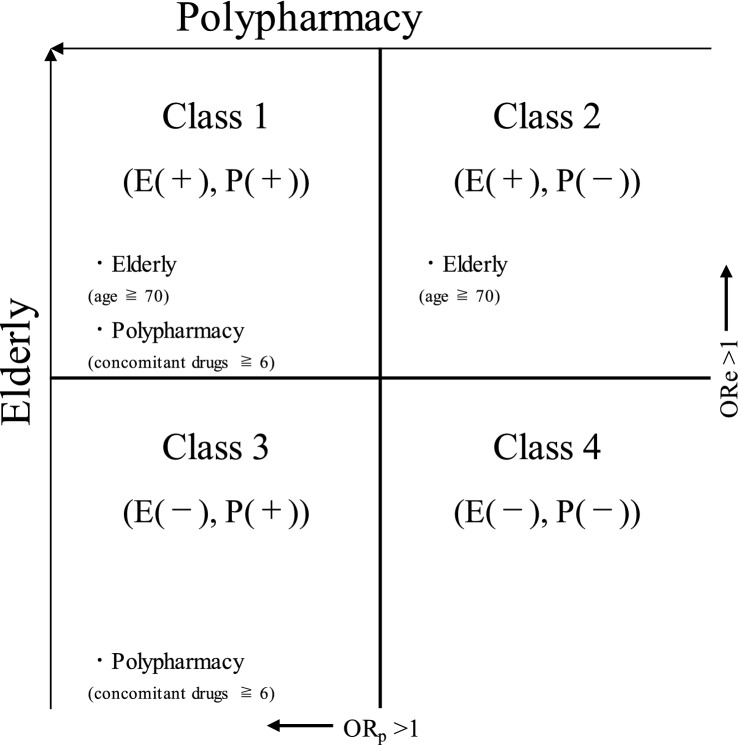
ADE Categorisation
Using ORe and ORp derived from logistic regression analysis, 100 types of ADEs were grouped into four classes (Fig. 2). First, ADEs for which ORe significantly exceeded 1 were grouped into Classes 1 and 2, and all other ADEs were grouped into Classes 3 and 4. Next, of the ADEs categorised as Class 1 and Class 2, those in which ORp significantly exceeded 1 were grouped into Class 1 [E(+), P(+)] and the remainder into Class 2 [E(+), P(−)]. Again, of the ADEs categorised as Class 3 and Class 4, those in which ORp significantly exceeded 1 were grouped into Class 3 [E(−), P(+)] and the remainder into Class 4 [E(−), P(−)].
Fig. 2.
Categorisation of adverse drug events (ADEs) considering elderly and polypharmacy. ORe adjusted odds ratio with elderly as a factor for the occurrence of ADEs, ORp adjusted odds ratio with polypharmacy as a factor for the occurrence of ADEs. E(+), P(+): ADEs where ORe and ORp significantly exceeded 1; E(+), P(−): ADEs where only ORe significantly exceeded 1; E(−), P(+): ADEs where only ORp significantly exceeded 1; E(−), P(−): ADEs where neither ORe nor ORp significantly exceeded 1
Results
ADEs in Identified Cases
Total cases registered in JADER (combinations of identification number-ADE-date of ADE) comprised 355,855 men and 349,460 women. The average number of drugs that the patients used simultaneously was 4.25 ± 4.56, and the median age of the study group was 70 years. The 100 most common types of ADEs are collated by report number in Table 1; these were subsequently targeted for analysis.
Table 1.
Top 100 adverse drug event (ADE) types considered for analysis
| No. | ADEs | No. | ADEs |
|---|---|---|---|
| 1 | Interstitial lung disease | 51 | Stomatitis |
| 2 | Platelet count decreased | 52 | Pneumocystis jirovecii pneumonia |
| 3 | Hepatic function abnormal | 53 | Alanine aminotransferase increased |
| 4 | Anaphylactic shock | 54 | Urticaria |
| 5 | Neutrophil count decreased | 55 | Hyponatraemia |
| 6 | White blood cell count decreased | 56 | Blood creatine phosphokinase increased |
| 7 | Pyrexia | 57 | Dizziness |
| 8 | Anaemia | 58 | Aspartate aminotransferase increased |
| 9 | Pneumonia | 59 | Hyperkalaemia |
| 10 | Liver disorder | 60 | Renal disorder |
| 11 | Neutropenia | 61 | Hypertension |
| 12 | Drug eruption | 62 | Bradycardia |
| 13 | Diarrhoea | 63 | Hypokalaemia |
| 14 | Renal impairment | 64 | Anaphylactoid reaction |
| 15 | Blood pressure decreased | 65 | Rash generalised |
| 16 | Decreased appetite | 66 | Delirium |
| 17 | Febrile neutropenia | 67 | Erythema |
| 18 | Rash | 68 | Renal failure |
| 19 | Acute kidney injury | 69 | Pleural effusion |
| 20 | Rhabdomyolysis | 70 | Depressed level of consciousness |
| 21 | Thrombocytopenia | 71 | Urinary retention |
| 22 | Pancytopenia | 72 | Diabetes mellitus |
| 23 | Hypoglycaemia | 73 | Electrocardiogram QT prolonged |
| 24 | Cerebral infarction | 74 | Deep vein thrombosis |
| 25 | Nausea | 75 | Pulmonary embolism |
| 26 | Erythema multiforme | 76 | Dehydration |
| 27 | Anaphylactic reaction | 77 | Melaena |
| 28 | Cardiac failure | 78 | Fall |
| 29 | Stevens-Johnson syndrome | 79 | Drug interaction |
| 30 | Haemoglobin decreased | 80 | Jaundice |
| 31 | Altered state of consciousness | 81 | Herpes zoster |
| 32 | Vomiting | 82 | Blood creatinine increased |
| 33 | Shock | 83 | Lymphoproliferative disorder |
| 34 | Seizure | 84 | Palmar-plantar erythrodysaesthesia syndrome |
| 35 | Leukopenia | 85 | Headache |
| 36 | Sepsis | 86 | Transfusion-related acute lung injury |
| 37 | Cerebral haemorrhage | 87 | Cytomegalovirus infection |
| 38 | Dyspnoea | 88 | Pancreatitis acute |
| 39 | Loss of consciousness | 89 | Lung disorder |
| 40 | Drug-induced liver injury | 90 | Respiratory failure |
| 41 | Drug reaction with eosinophilia and systemic symptoms | 91 | Hyperglycaemia |
| 42 | Malaise | 92 | Infection |
| 43 | Bone marrow failure | 93 | Ileus |
| 44 | Agranulocytosis | 94 | Pneumonia aspiration |
| 45 | Disseminated intravascular coagulation | 95 | Toxicity to various agents |
| 46 | Gastrointestinal haemorrhage | 96 | Septic shock |
| 47 | Death | 97 | Tubulointerstitial nephritis |
| 48 | Toxic epidermal necrolysis | 98 | Cellulitis |
| 49 | Neuroleptic malignant syndrome | 99 | Toxic skin eruption |
| 50 | Osteonecrosis of jaw | 100 | Peritonitis |
ADEs That are Associated with Both Elderly and Polypharmacy: Class 1 [E(+), P(+)]
ADEs categorised as Class 1 are presented by sex in Table 2 ((a) Males, (b) Females). The ADEs in Table 2 were ranked in descending order of ORe and ORp volume. A total of 19 and 26 male and female ADE types, respectively, were categorised as Class 1. Of these, 16 ADE types were categorised as common to both males and females.
Table 2.
Adverse drug events (ADEs) categorised as Class 1 (ORe and ORp volumes in descending order)
| (a) Male cases | ||||||
|---|---|---|---|---|---|---|
| No. | ADEs | ORe | (95% CI) | ORp | (95% CI) | Female class |
| 1 | Hyperkalaemia | 1.76 | (1.54–2.02) | 1.89 | (1.65–2.16) | 1 |
| 2 | Bradycardia | 2.03 | (1.77–2.32) | 1.49 | (1.30–1.70) | 1 |
| 3 | Hypoglycaemia | 2.03 | (1.86–2.22) | 1.29 | (1.18–1.41) | 1 |
| 4 | Transfusion-related acute lung injury | 1.43 | (1.25–1.64) | 1.71 | (1.49–1.97) | 1 |
| 5 | Hyponatraemia | 1.73 | (1.50–1.99) | 1.29 | (1.11–1.49) | 1 |
| 6 | Interstitial lung disease | 1.74 | (1.67–1.82) | 1.17 | (1.12–1.23) | 1 |
| 7 | Dehydration | 1.18 | (1.00–1.40) | 1.71 | (1.44–2.02) | 1 |
| 8 | Hypokalaemia | 1.48 | (1.26–1.74) | 1.36 | (1.15–1.60) | 1 |
| 9 | Lung disorder | 1.48 | (1.28–1.72) | 1.31 | (1.12–1.53) | 2 |
| 10 | Respiratory failure | 1.33 | (1.14–1.56) | 1.43 | (1.21–1.68) | 1 |
| 11 | Renal failure | 1.41 | (1.23–1.63) | 1.33 | (1.15–1.55) | 1 |
| 12 | Altered state of consciousness | 1.29 | (1.17–1.42) | 1.41 | (1.28–1.56) | 1 |
| 13 | Anaemia | 1.45 | (1.31–1.59) | 1.20 | (1.08–1.33) | 1 |
| 14 | Thrombocytopenia | 1.50 | (1.35–1.66) | 1.13 | (1.02–1.26) | 1 |
| 15 | Blood creatinine increased | 1.36 | (1.15–1.62) | 1.24 | (1.03–1.49) | 2 |
| 16 | Acute kidney injury | 1.20 | (1.10–1.30) | 1.36 | (1.25–1.47) | 1 |
| 17 | Dizziness | 1.30 | (1.12–1.50) | 1.22 | (1.05–1.43) | 2 |
| 18 | Pancytopenia | 1.24 | (1.13–1.37) | 1.12 | (1.00–1.24) | 1 |
| 19 | Blood pressure decreased | 1.10 | (1.02–1.18) | 1.16 | (1.07–1.26) | 1 |
| (b) Female cases | ||||||
|---|---|---|---|---|---|---|
| No. | ADEs | ORe | (95% CI) | ORp | (95% CI) | Male class |
| 1 | Hyperkalaemia | 3.68 | (3.12–4.37) | 1.90 | (1.64–2.21) | 1 |
| 2 | Hyponatraemia | 3.18 | (2.79–3.64) | 1.65 | (1.45–1.86) | 1 |
| 3 | Hypoglycaemia | 3.42 | (3.10–3.78) | 1.47 | (1.34–1.61) | 1 |
| 4 | Bradycardia | 2.82 | (2.46–3.24) | 1.43 | (1.25–1.64) | 1 |
| 5 | Renal failure | 2.49 | (2.08–2.98) | 1.61 | (1.35–1.91) | 1 |
| 6 | Dehydration | 2.48 | (2.05–3.01) | 1.60 | (1.32–1.93) | 1 |
| 7 | Acute kidney injury | 2.35 | (2.15–2.58) | 1.54 | (1.41–1.69) | 1 |
| 8 | Hypokalaemia | 2.16 | (1.89–2.48) | 1.66 | (1.45–1.90) | 1 |
| 9 | Drug interaction | 1.43 | (1.24–1.65) | 2.26 | (1.96–2.61) | 3 |
| 10 | Transfusion-related acute lung injury | 1.61 | (1.38–1.88) | 1.89 | (1.62–2.21) | 1 |
| 11 | Urinary retention | 2.32 | (1.96–2.76) | 1.26 | (1.06–1.50) | 2 |
| 12 | Pneumonia aspiration | 2.08 | (1.66–2.61) | 1.38 | (1.10–1.74) | 2 |
| 13 | Renal disorder | 2.04 | (1.74–2.39) | 1.25 | (1.05–1.47) | 4 |
| 14 | Renal impairment | 2.04 | (1.86–2.23) | 1.21 | (1.10–1.33) | 2 |
| 15 | Electrocardiogram QT prolonged | 1.72 | (1.48–1.99) | 1.42 | (1.22–1.66) | 3 |
| 16 | Altered state of consciousness | 1.71 | (1.56–1.87) | 1.40 | (1.27–1.53) | 1 |
| 17 | Decreased appetite | 1.84 | (1.65–2.06) | 1.21 | (1.07–1.35) | 2 |
| 18 | Rhabdomyolysis | 1.18 | (1.07–1.30) | 1.65 | (1.49–1.83) | 3 |
| 19 | Cardiac failure | 1.70 | (1.53–1.89) | 1.12 | (1.00–1.25) | 2 |
| 20 | Anaemia | 1.48 | (1.34–1.62) | 1.29 | (1.17–1.43) | 1 |
| 21 | Pancytopenia | 1.58 | (1.45–1.73) | 1.19 | (1.08–1.31) | 1 |
| 22 | Respiratory failure | 1.37 | (1.14–1.64) | 1.31 | (1.08–1.59) | 1 |
| 23 | Blood pressure decreased | 1.28 | (1.18–1.38) | 1.22 | (1.12–1.33) | 1 |
| 24 | Interstitial lung disease | 1.36 | (1.29–1.43) | 1.10 | (1.04–1.16) | 1 |
| 25 | Thrombocytopenia | 1.13 | (1.00–1.26) | 1.29 | (1.14–1.45) | 1 |
| 26 | Pneumonia | 1.30 | (1.19–1.41) | 1.11 | (1.01–1.22) | 2 |
Underlined ADEs are categorised in a separate Class in the other sex
ORe adjusted odds ratio with elderly as a factor for the occurrence of ADEs, ORp adjusted odds ratio with polypharmacy as a factor for the occurrence of ADEs, 95% CI 95% confidence interval of ORe and ORp, Female Class class in which ADEs common in females are categorised, Male Class class in which ADEs common in males are categorised
ORe for males included hypoglycaemia (OR 2.03), bradycardia (OR 2.03), hyperkalaemia (OR 1.76), interstitial lung disease (OR 1.74), and hyponatraemia (OR 1.73). For females, ADEs included hyperkalaemia (OR 3.68), hypoglycaemia (OR 3.42), hyponatraemia (OR 3.18), bradycardia (OR 2.82), and renal failure (OR 2.49).
ORp for males included hyperkalaemia (OR 1.89), transfusion-related acute lung injury (OR 1.71), dehydration (OR, 1.71), bradycardia (OR 1.49), and respiratory failure (OR 1.43). For females, the ADEs included disseminated drug interaction (OR 2.26), hyperkalaemia (OR 1.90), transfusion-related acute lung injury (OR 1.89), hypokalaemia (OR 1.66), and rhabdomyolysis (OR 1.65).
Many of the ADEs categorised as Class 1 were related to electrolyte abnormalities, renal and respiratory disorders, and coagulopathy. Comparison of male and female ADEs identified as Class 1 revealed that lung disorders, increased blood creatinine, and dizziness were significant for males only, and that ten ADE types (drug interaction, urinary retention, pneumonia aspiration, renal disorder, renal impairment, electrocardiogram QT prolonged, decreased appetite, rhabdomyolysis, cardiac failure, pneumonia) were significant for females only.
ADEs That are Associated with Elderly and Not With Polypharmacy: Class 2 [E(+), P(−)]
ADEs categorised as Class 2 are presented by sex in Table 3 ((a) Males, (b) Females). The ADEs in Table 3 were ranked in descending ORe order. A total of 19 male and female ADE types, respectively, were categorised as Class 2. Of these, 12 were categorised as being common to both males and females. ORe for males presented high values for urinary retention (OR 3.34), fall (OR 2.29), cardiac failure (OR 2.17), osteonecrosis of the jaw (OR 2.14), and gastrointestinal haemorrhage (OR 2.14). For females, high values were found for gastrointestinal haemorrhage (OR 3.69), melaena (OR 3.22), fall (OR 3.05), delirium (OR 2.89), and cerebral haemorrhage (OR 2.61). Except for mental and nervous disorders, various ADEs were categorised in Class 2. Hypertension was common to males, while decreased haemoglobin, infection, shock and stomatitis were common to females.
Table 3.
Adverse drug events (ADEs) categorised as Class 2 (in ORe descending order)
| (a) Male cases | ||||||
|---|---|---|---|---|---|---|
| No. | ADEs | ORe | (95% CI) | ORp | (95% CI) | Female class |
| 1 | Urinary retention | 3.34 | (2.93–3.82) | 0.79 | (0.69–0.91) | 1 |
| 2 | Fall | 2.29 | (1.93–2.73) | 1.04 | (0.87–1.25) | 2 |
| 3 | Cardiac failure | 2.17 | (1.94–2.43) | 1.07 | (0.95–1.20) | 1 |
| 4 | Osteonecrosis of jaw | 2.14 | (1.83–2.52) | 0.60 | (0.50–0.73) | 2 |
| 5 | Gastrointestinal haemorrhage | 2.14 | (1.89–2.43) | 0.77 | (0.67–0.89) | 2 |
| 6 | Pneumonia aspiration | 2.14 | (1.81–2.52) | 1.14 | (0.96–1.35) | 1 |
| 7 | Melaena | 2.08 | (1.78–2.43) | 0.91 | (0.77–1.07) | 2 |
| 8 | Cerebral haemorrhage | 1.88 | (1.70–2.07) | 0.89 | (0.80–0.99) | 2 |
| 9 | Pleural effusion | 1.81 | (1.54–2.12) | 1.00 | (0.84–1.18) | 2 |
| 10 | Cerebral infarction | 1.79 | (1.62–1.97) | 0.73 | (0.65–0.82) | 2 |
| 11 | Delirium | 1.70 | (1.49–1.94) | 1.01 | (0.87–1.16) | 2 |
| 12 | Decreased appetite | 1.55 | (1.40–1.72) | 1.07 | (0.96–1.20) | 1 |
| 13 | Platelet count decreased | 1.46 | (1.37–1.56) | 0.97 | (0.90–1.04) | 2 |
| 14 | Depressed level of consciousness | 1.40 | (1.21–1.63) | 1.06 | (0.90–1.24) | 2 |
| 15 | Pneumonia | 1.40 | (1.30–1.51) | 1.02 | (0.94–1.11) | 1 |
| 16 | Lymphoproliferative disorder | 1.40 | (1.16–1.68) | 0.11 | (0.06–0.16) | 2 |
| 17 | Death | 1.26 | (1.10–1.43) | 0.62 | (0.53–0.73) | 2 |
| 18 | Hypertension | 1.25 | (1.05–1.50) | 0.69 | (0.56–0.85) | 4 |
| 19 | Renal impairment | 1.17 | (1.08–1.26) | 1.02 | (0.94–1.11) | 1 |
| (b) Female cases | ||||||
|---|---|---|---|---|---|---|
| No. | ADEs | ORe | (95% CI) | ORp | (95% CI) | Male class |
| 1 | Gastrointestinal haemorrhage | 3.69 | (3.15–4.33) | 1.05 | (0.90–1.22) | 2 |
| 2 | Melaena | 3.22 | (2.69–3.86) | 0.93 | (0.77–1.11) | 2 |
| 3 | Fall | 3.05 | (2.63–3.54) | 0.88 | (0.75–1.03) | 2 |
| 4 | Delirium | 2.89 | (2.47–3.38) | 1.11 | (0.94–1.29) | 2 |
| 5 | Cerebral haemorrhage | 2.61 | (2.31–2.97) | 0.83 | (0.72–0.95) | 2 |
| 6 | Blood creatinine increased | 2.16 | (1.71–2.73) | 1.15 | (0.90–1.47) | 1 |
| 7 | Osteonecrosis of jaw | 2.16 | (1.95–2.39) | 0.71 | (0.63–0.80) | 2 |
| 8 | Death | 1.99 | (1.69–2.34) | 0.67 | (0.55–0.81) | 2 |
| 9 | Cerebral infarction | 1.91 | (1.71–2.13) | 0.63 | (0.55–0.72) | 2 |
| 10 | Haemoglobin decreased | 1.46 | (1.24–1.72) | 0.88 | (0.73–1.07) | 4 |
| 11 | Lymphoproliferative disorder | 1.42 | (1.26–1.60) | 0.10 | (0.07–0.13) | 2 |
| 12 | Infection | 1.26 | (1.04–1.54) | 0.50 | (0.38–0.64) | 4 |
| 13 | Platelet count decreased | 1.23 | (1.15–1.32) | 0.97 | (0.90–1.06) | 2 |
| 14 | Lung disorder | 1.21 | (1.01–1.45) | 1.06 | (0.87–1.30) | 1 |
| 15 | Depressed level of consciousness | 1.21 | (1.07–1.37) | 1.05 | (0.91–1.20) | 2 |
| 16 | Pleural effusion | 1.20 | (1.02–1.43) | 1.12 | (0.93–1.35) | 2 |
| 17 | Shock | 1.20 | (1.09–1.32) | 0.89 | (0.80–1.00) | 4 |
| 18 | Dizziness | 1.20 | (1.06–1.35) | 0.94 | (0.82–1.07) | 1 |
| 19 | Stomatitis | 1.19 | (1.02–1.38) | 1.01 | (0.85–1.19) | 4 |
Underlined ADEs are categorised in a separate Class in the other sex
ORe adjusted odds ratio with elderly as a factor for the occurrence of ADEs, ORp adjusted odds ratio with polypharmacy as a factor for the occurrence of ADEs, 95% CI 95% confidence interval of ORe and ORp, Female Class class in which ADEs common in females are categorised, Male Class class in which ADEs common in males are categorised
ADEs That are Associated with Polypharmacy and Not With Elderly: Class 3 [E(−), P(+)]
Class 3 ADEs are presented by sex in Table 4 ((a) Males, (b) Females). ADEs in Table 4 and ORp are ranked in descending order. In total, 14 and 20 ADE types common to both males and females, respectively, were categorised as Class 3. Of these, nine were common to both males and females.
Table 4.
Adverse drug events (ADEs) categorised as Class 3 (in ORp descending order)
| (a) Male cases | ||||||
|---|---|---|---|---|---|---|
| No. | ADEs | ORe | (95% CI) | ORp | (95% CI) | Female class |
| 1 | Drug interaction | 1.05 | (0.91–1.21) | 2.16 | (1.87–2.49) | 1 |
| 2 | Agranulocytosis | 0.69 | (0.60–0.79) | 1.62 | (1.42–1.84) | 3 |
| 3 | Rhabdomyolysis | 0.86 | (0.80–0.93) | 1.60 | (1.48–1.73) | 1 |
| 4 | Erythema | 0.91 | (0.78–1.06) | 1.41 | (1.20–1.66) | 3 |
| 5 | Toxic skin eruption | 0.69 | (0.57–0.84) | 1.31 | (1.07–1.60) | 3 |
| 6 | Septic shock | 0.80 | (0.66–0.97) | 1.28 | (1.05–1.55) | 4 |
| 7 | Electrocardiogram qt prolonged | 1.17 | (0.98–1.39) | 1.25 | (1.04–1.49) | 1 |
| 8 | Anaphylactic shock | 0.70 | (0.67–0.74) | 1.22 | (1.15–1.28) | 3 |
| 9 | Neuroleptic malignant syndrome | 0.31 | (0.26–0.35) | 1.19 | (1.05–1.34) | 3 |
| 10 | Hepatic function abnormal | 0.88 | (0.83–0.93) | 1.17 | (1.11–1.25) | 3 |
| 11 | Drug eruption | 0.71 | (0.65–0.78) | 1.16 | (1.05–1.27) | 3 |
| 12 | Disseminated intravascular coagulation | 1.11 | (0.98–1.25) | 1.16 | (1.01–1.32) | 3 |
| 13 | Pyrexia | 0.87 | (0.82–0.94) | 1.13 | (1.05–1.22) | 3 |
| 14 | White blood cell count decreased | 0.88 | (0.80–0.97) | 1.11 | (1.01–1.23) | 4 |
| (b) Female cases | ||||||
|---|---|---|---|---|---|---|
| No. | ADEs | ORe | (95% CI) | ORp | (95% CI) | Male class |
| 1 | Sepsis | 0.91 | (0.79–1.05) | 1.60 | (1.38–1.84) | 4 |
| 2 | Cellulitis | 1.13 | (0.93–1.38) | 1.48 | (1.20–1.81) | 4 |
| 3 | Hyperglycaemia | 0.90 | (0.75–1.08) | 1.47 | (1.22–1.77) | 4 |
| 4 | Neuroleptic malignant syndrome | 0.49 | (0.42–0.57) | 1.45 | (1.26–1.67) | 3 |
| 5 | Erythema | 0.62 | (0.54–0.72) | 1.39 | (1.20–1.59) | 3 |
| 6 | Pancreatitis acute | 0.65 | (0.54–0.78) | 1.38 | (1.14–1.66) | 4 |
| 7 | Disseminated intravascular coagulation | 1.06 | (0.92–1.22) | 1.38 | (1.19–1.59) | 3 |
| 8 | Blood creatine phosphokinase increased | 0.92 | (0.79–1.07) | 1.34 | (1.14–1.57) | 4 |
| 9 | Toxic skin eruption | 0.75 | (0.63–0.89) | 1.31 | (1.09–1.56) | 3 |
| 10 | Stevens-Johnson syndrome | 0.70 | (0.63–0.77) | 1.29 | (1.16–1.42) | 4 |
| 11 | Jaundice | 1.04 | (0.88–1.24) | 1.26 | (1.05–1.51) | 4 |
| 12 | Aspartate aminotransferase increased | 0.87 | (0.74–1.03) | 1.24 | (1.04–1.47) | 4 |
| 13 | Toxic epidermal necrolysis | 0.81 | (0.70–0.93) | 1.23 | (1.06–1.42) | 4 |
| 14 | Anaphylactic shock | 0.68 | (0.64–0.72) | 1.19 | (1.12–1.27) | 3 |
| 15 | Vomiting | 1.11 | (1.00–1.22) | 1.18 | (1.05–1.31) | 4 |
| 16 | Drug-induced liver injury | 0.57 | (0.50–0.65) | 1.15 | (1.01–1.31) | 4 |
| 17 | Pyrexia | 0.74 | (0.69–0.80) | 1.14 | (1.06–1.23) | 3 |
| 18 | Agranulocytosis | 0.62 | (0.55–0.69) | 1.13 | (1.01–1.26) | 3 |
| 19 | Drug eruption | 0.61 | (0.56–0.67) | 1.12 | (1.03–1.22) | 3 |
| 20 | Hepatic function abnormal | 0.79 | (0.75–0.84) | 1.09 | (1.02–1.16) | 3 |
Underlined ADEs are categorised in a separate Class in the other sex
ORe adjusted odds ratio with elderly as a factor for the occurrence of ADEs, ORp adjusted odds ratio with polypharmacy as a factor for the occurrence of ADEs, 95% CI 95% confidence interval of ORe and ORp, Female Class class in which ADEs common in females are categorised, Male Class class in which ADEs common in males are categorised
ORp for males included drug interaction (OR 2.16), agranulocytosis (OR 1.62), rhabdomyolysis (OR 1.60), erythema (OR 1.41), and toxic skin eruption (OR 1.31). For females, the ranking was sepsis (OR 1.60), cellulitis (OR 1.48), hyperglycaemia (OR 1.47), neuroleptic malignant syndrome (OR 1.45), and erythema (OR 1.39).
In Class 3, the PTs infectious diseases and decreased white blood cell count predominated. Septic shock and decreased white blood cell count were categorised as common to males, while sepsis, cellulitis, hyperglycaemia, acute pancreatitis, increased blood creatine phosphokinase, Stevens–Johnson syndrome, jaundice, increased aspartate aminotransferase, toxic epidermal necrolysis, vomiting, and drug-induced liver injury were categorised as common to females.
ADEs That are Not Associated with Either Polypharmacy or Elderly: Class 4 [E(−), P(−)]
Class 4 ADEs are presented by sex in Table 5 ((a) Males, (b) Females). ORe and ORp volumes are ranked in ascending order to clarify that ADEs were unaffected by elderly or polypharmacy. A total of 48 and 35 ADE types were categorised as male and female, respectively. Of these, 32 were common to both sexes. Class 4 ADEs were dominated by allergic symptoms, including anaphylactic reaction.
Table 5.
Adverse drug events (ADEs) categorised as Class 4 (ORe and ORp volumes in ascending order)
| (a) Male cases | ||||||
|---|---|---|---|---|---|---|
| No. | ADEs | ORe | (95% CI) | ORp | (95% CI) | Female class |
| 1 | Cytomegalovirus infection | 0.33 | (0.26–0.41) | 0.74 | (0.60–0.92) | 4 |
| 2 | Drug reaction with eosinophilia and systemic symptoms | 0.37 | (0.33–0.42) | 0.77 | (0.68–0.88) | 4 |
| 3 | Diabetes mellitus | 0.49 | (0.41–0.59) | 0.65 | (0.53–0.79) | 4 |
| 4 | Headache | 0.44 | (0.33–0.56) | 0.85 | (0.65–1.09) | 4 |
| 5 | Tubulointerstitial nephritis | 0.53 | (0.44–0.63) | 0.74 | (0.61–0.88) | 4 |
| 6 | Palmar-plantar erythrodysaesthesia syndrome | 0.95 | (0.75–1.19) | 0.44 | (0.31–0.59) | 4 |
| 7 | Anaphylactoid reaction | 0.43 | (0.37–0.51) | 1.15 | (0.99–1.34) | 4 |
| 8 | Anaphylactic reaction | 0.51 | (0.46–0.56) | 1.02 | (0.92–1.13) | 4 |
| 9 | Pancreatitis acute | 0.50 | (0.42–0.59) | 1.04 | (0.88–1.22) | 3 |
| 10 | Peritonitis | 0.69 | (0.57–0.84) | 0.79 | (0.63–0.97) | 4 |
| 11 | Urticaria | 0.52 | (0.45–0.60) | 1.09 | (0.94–1.26) | 4 |
| 12 | Neutrophil count decreased | 0.92 | (0.84–1.01) | 0.65 | (0.58–0.73) | 4 |
| 13 | Infection | 0.99 | (0.82–1.19) | 0.61 | (0.48–0.76) | 2 |
| 14 | Drug-induced liver injury | 0.74 | (0.65–0.84) | 0.84 | (0.72–0.96) | 3 |
| 15 | Pulmonary embolism | 0.58 | (0.47–0.72) | 1.11 | (0.89–1.37) | 4 |
| 16 | Pneumocystis jirovecii pneumonia | 0.90 | (0.78–1.04) | 0.72 | (0.61–0.85) | 4 |
| 17 | Stevens-Johnson syndrome | 0.62 | (0.56–0.69) | 1.06 | (0.95–1.18) | 3 |
| 18 | Deep vein thrombosis | 0.70 | (0.56–0.86) | 0.95 | (0.75–1.18) | 4 |
| 19 | Rash generalised | 0.78 | (0.66–0.92) | 0.85 | (0.71–1.02) | 4 |
| 20 | Erythema multiforme | 0.73 | (0.64–0.83) | 0.92 | (0.80–1.05) | 4 |
| 21 | Alanine aminotransferase increased | 0.75 | (0.64–0.87) | 0.90 | (0.76–1.06) | 4 |
| 22 | Bone marrow failure | 0.99 | (0.88–1.11) | 0.68 | (0.59–0.79) | 4 |
| 23 | Neutropenia | 0.84 | (0.75–0.92) | 0.81 | (0.72–0.91) | 4 |
| 24 | Rash | 0.67 | (0.60–0.74) | 1.02 | (0.92–1.14) | 4 |
| 25 | Leukopenia | 0.85 | (0.73–0.98) | 0.82 | (0.70–0.97) | 4 |
| 26 | Blood creatine phosphokinase increased | 0.66 | (0.59–0.74) | 1.06 | (0.94–1.20) | 3 |
| 27 | Stomatitis | 0.88 | (0.75–1.03) | 0.80 | (0.66–0.96) | 2 |
| 28 | Toxicity to various agents | 0.64 | (0.54–0.76) | 1.15 | (0.96–1.37) | 4 |
| 29 | Seizure | 0.77 | (0.69–0.85) | 0.98 | (0.87–1.09) | 4 |
| 30 | Liver disorder | 0.78 | (0.72–0.84) | 1.04 | (0.96–1.12) | 4 |
| 31 | Hyperglycaemia | 0.84 | (0.71–0.99) | 0.97 | (0.80–1.16) | 3 |
| 32 | Shock | 0.97 | (0.89–1.06) | 0.87 | (0.78–0.96) | 2 |
| 33 | Toxic epidermal necrolysis | 0.73 | (0.63–0.84) | 1.16 | (1.00–1.35) | 3 |
| 34 | Aspartate aminotransferase increased | 0.83 | (0.71–0.96) | 1.02 | (0.86–1.21) | 3 |
| 35 | Nausea | 0.87 | (0.76–0.98) | 1.00 | (0.87–1.14) | 4 |
| 36 | Diarrhoea | 0.97 | (0.88–1.07) | 0.94 | (0.84–1.04) | 4 |
| 37 | Herpes zoster | 0.81 | (0.63–1.04) | 1.13 | (0.87–1.47) | 4 |
| 38 | Febrile neutropenia | 1.04 | (0.93–1.15) | 0.89 | (0.79–1.00) | 4 |
| 39 | Sepsis | 0.86 | (0.76–0.98) | 1.08 | (0.94–1.23) | 3 |
| 40 | Malaise | 1.02 | (0.89–1.16) | 0.93 | (0.80–1.08) | 4 |
| 41 | Loss of consciousness | 1.10 | (0.99–1.22) | 0.90 | (0.80–1.01) | 4 |
| 42 | Haemoglobin decreased | 1.17 | (1.00–1.38) | 0.84 | (0.70–1.01) | 2 |
| 43 | Ileus | 1.05 | (0.88–1.25) | 0.96 | (0.79–1.16) | 4 |
| 44 | Renal disorder | 1.05 | (0.92–1.20) | 0.97 | (0.83–1.12) | 1 |
| 45 | Jaundice | 1.05 | (0.90–1.22) | 0.97 | (0.82–1.14) | 3 |
| 46 | Cellulitis | 0.90 | (0.71–1.14) | 1.14 | (0.89–1.46) | 3 |
| 47 | Vomiting | 1.00 | (0.88–1.14) | 1.13 | (0.98–1.29) | 3 |
| 48 | Dyspnoea | 1.05 | (0.94–1.16) | 1.11 | (0.99–1.23) | 4 |
| (a) Female cases | ||||||
|---|---|---|---|---|---|---|
| No. | ADEs | ORe | (95% CI) | ORp | (95% CI) | Male class |
| 1 | Headache | 0.31 | (0.25–0.37) | 0.59 | (0.49–0.71) | 4 |
| 2 | Anaphylactoid reaction | 0.36 | (0.30–0.42) | 1.03 | (0.88–1.21) | 4 |
| 3 | Cytomegalovirus infection | 0.46 | (0.36–0.57) | 0.84 | (0.66–1.06) | 4 |
| 4 | Anaphylactic reaction | 0.39 | (0.35–0.43) | 1.00 | (0.90–1.10) | 4 |
| 5 | Palmar-plantar erythrodysaesthesia syndrome | 0.79 | (0.61–1.02) | 0.51 | (0.36–0.71) | 4 |
| 6 | Pulmonary embolism | 0.55 | (0.47–0.64) | 0.81 | (0.69–0.95) | 4 |
| 7 | Drug reaction with eosinophilia and systemic symptoms | 0.47 | (0.41–0.53) | 0.98 | (0.86–1.11) | 4 |
| 8 | Neutrophil count decreased | 0.73 | (0.65–0.81) | 0.64 | (0.56–0.73) | 4 |
| 9 | Neutropenia | 0.61 | (0.54–0.68) | 0.82 | (0.73–0.93) | 4 |
| 10 | Deep vein thrombosis | 0.70 | (0.61–0.81) | 0.72 | (0.61–0.85) | 4 |
| 11 | Urticaria | 0.49 | (0.42–0.56) | 1.07 | (0.93–1.23) | 4 |
| 12 | Febrile neutropenia | 0.61 | (0.53–0.70) | 0.87 | (0.75–1.00) | 4 |
| 13 | Diabetes mellitus | 0.54 | (0.44–0.66) | 1.00 | (0.82–1.23) | 4 |
| 14 | Tubulointerstitial nephritis | 0.65 | (0.54–0.78) | 0.85 | (0.69–1.05) | 4 |
| 15 | Rash | 0.56 | (0.51–0.61) | 1.05 | (0.96–1.16) | 4 |
| 16 | Erythema multiforme | 0.71 | (0.64–0.80) | 0.84 | (0.74–0.94) | 4 |
| 17 | Seizure | 0.71 | (0.65–0.79) | 0.84 | (0.75–0.95) | 4 |
| 18 | Malaise | 0.70 | (0.61–0.79) | 0.94 | (0.81–1.08) | 4 |
| 19 | Peritonitis | 0.79 | (0.65–0.96) | 0.84 | (0.66–1.04) | 4 |
| 20 | Hypertension | 0.88 | (0.73–1.06) | 0.76 | (0.60–0.94) | 2 |
| 21 | Bone marrow failure | 1.07 | (0.95–1.21) | 0.66 | (0.56–0.76) | 4 |
| 22 | Leukopenia | 0.71 | (0.61–0.82) | 1.00 | (0.85–1.17) | 4 |
| 23 | Liver disorder | 0.73 | (0.68–0.79) | 0.97 | (0.90–1.06) | 4 |
| 24 | Toxicity to various agents | 0.69 | (0.59–0.80) | 1.05 | (0.89–1.23) | 4 |
| 25 | Dyspnoea | 0.78 | (0.70–0.86) | 1.00 | (0.89–1.12) | 4 |
| 26 | Rash generalised | 0.82 | (0.72–0.95) | 1.05 | (0.90–1.22) | 4 |
| 27 | Nausea | 0.89 | (0.80–0.99) | 0.98 | (0.88–1.10) | 4 |
| 28 | Herpes zoster | 0.85 | (0.70–1.02) | 1.06 | (0.86–1.30) | 4 |
| 29 | Alanine aminotransferase increased | 0.81 | (0.69–0.95) | 1.12 | (0.94–1.32) | 4 |
| 30 | White blood cell count decreased | 0.87 | (0.80–0.95) | 1.05 | (0.95–1.16) | 3 |
| 31 | Loss of consciousness | 0.99 | (0.90–1.09) | 0.93 | (0.83–1.03) | 4 |
| 32 | Diarrhoea | 0.92 | (0.83–1.01) | 1.04 | (0.93–1.16) | 4 |
| 33 | Ileus | 1.16 | (0.95–1.42) | 0.86 | (0.68–1.07) | 4 |
| 34 | Septic shock | 0.86 | (0.71–1.05) | 1.22 | (0.99–1.49) | 3 |
| 35 | Pneumocystis jirovecii pneumonia | 1.07 | (0.95–1.20) | 1.04 | (0.91–1.18) | 4 |
ORe adjusted odds ratio with elderly as a factor for the occurrence of ADEs, ORp adjusted odds ratio with polypharmacy as a factor for the occurrence of ADEs, 95% CI 95% confidence interval of ORe and ORp, Female Class class in which ADEs common in females are categorised, Male Class class in which ADEs common in males are categorised
Summary of the Results
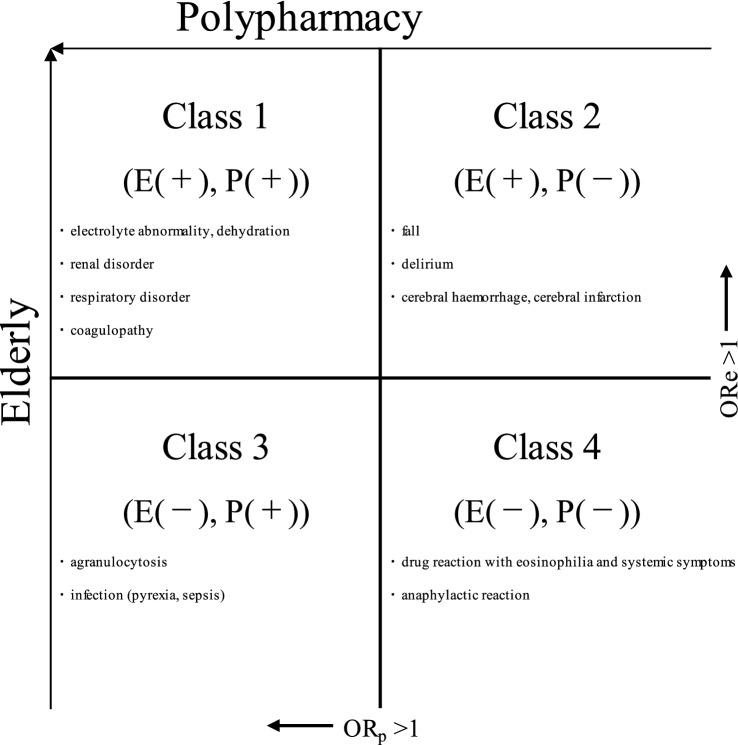
ADE classes are presented in Fig. 3.
Fig. 3.
Typical adverse drug events (ADEs) categorised into each class. ORe adjusted odds ratio with elderly as a factor for the occurrence of ADEs, ORp adjusted odds ratio with polypharmacy as a factor for the occurrence of ADEs. E(+), P(+): ADEs where ORe and ORp significantly exceeded 1; E(+), P(−): ADEs where only ORe significantly exceeded 1; E(−), P(+): ADEs where only ORp significantly exceeded 1; E(−), P(−): ADEs where neither ORe nor ORp significantly exceeded 1
Discussion
Class 2 ADEs [E(+), P(−)], including the PTs delirium and fall, have previously been identified as those with a high risk of occurrence in elderly patients treated with polypharmacy [10]. However, these ADEs were associated with the elderly but not with polypharmacy in this study. Moreover, it is difficult to distinguish between symptoms associated with drugs and those associated with aging, and symptoms may actually include symptoms of aging.
Conversely, the results of the present study support existing reports that the risk of Class 1 ADEs [E(+), P(+)], including electrolyte abnormalities [15–17], renal disorder [3] and pneumonia aspiration [18, 19], in elderly patients treated with polypharmacy is high. Dehydration and electrolyte imbalance are common in elderly persons, who have a high threshold value for throat dryness; moreover, polypharmacy including diuretics and selective serotonin receptor inhibitors has been reported [15–17] to increase these risks. In addition to the reduction in renal function associated with the elderly [3], multi-drug combinations of non-steroidal anti-inflammatory drugs, hypotensive drugs and diuretics have been reported to increase the risk of renal disorders. Similarly, reduced mobility in the elderly leads to decreased swallowing function, and multi-drug combinations including those with muscle-relaxing properties and diuretics carry a risk of pneumonia aspiration [18, 19].
The PTs decreased white blood cell count and infectious diseases predominate among the Class 3 ADEs [E(−), P(+)] that are common to both sexes. This indicates that there is an age-independent risk of myelosuppression with combinations of anti-cancer agents, which subsequently increase the risk of infections. In addition, given that many Class 4 ADEs [E(−), P(−)] were found to be allergic in nature, the high risk of allergy-type ADEs unrelated to polypharmacy or elderly should be considered.
In this study, sex differences in the occurrence of ADEs were investigated. ADEs of which the risk of onset is associated with polypharmacy occurred more frequently in women (Tables 2, 3), which may suggest that women are more sensitive to the effects of polypharmacy than are men [20, 21]. However, the type of drug used was not included in the analysis conditions. Thus, detailed investigations focusing on the types of drugs used are necessary for a detailed discussion on the reasons for these sex differences.
This study has the following limitations: since this study focused on the 100 most common ADE types reported in the JADER database, it is possible that ADEs categorised as Class 1 were not the only events that carry a high risk of occurrence in elderly patients treated with polypharmacy: other ADEs may carry a high risk of occurrence in polypharmacy-treated patients. In addition, because ADEs were categorised using only basic data (age, sex and number of concomitant drugs) in the JADER, questions as to whether Class 1-categorised ADEs were really caused by polypharmacy, or whether other factors (e.g. background diseases or individual drugs) were involved highlight the need for studies from both pharmacological and physiological perspectives.
In conclusion, analysis of ADEs considering polypharmacy and the elderly revealed that the risk of the 100 most common ADEs reported in the JADER database could be categorised as follows: electrolyte abnormalities, renal and respiratory disorders, and coagulopathy in Class 1 [E(+), P(+)]; delirium, falls in Class 2 [E(+), P(−)]; myelosuppression arising from anti-cancer multi-drug combinations in Class 3 [E(−), P(+)]; and allergy-type ADEs in Class 4 [E(−), P(−)]. These categories may provide potentially beneficial information for the future pharmaceutical management of elderly patients. In particular, the grouping of some ADEs into Class 2 that are considered classifiable as Class 1 in existing reports highlights the need for studies from pharmacological and physiological perspectives. In this study, we grouped ADEs by age (elderly—≥ 70 year) and polypharmacy (six or more agents) without specifying the drugs. Since the risk of ADEs grouped in Class 1 increased in the elderly using six or more drugs, monitoring is particularly important for elderly patients under polypharmacy. However, the risk of Class 2 ADEs increased in the elderly who were taking fewer than five drugs, and Class 2 ADEs should therefore always be monitored very cautiously in the elderly. Further investigations that focus on drug types are warranted.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Not applicable.
Conflict of interest
All authors declare that they have no conflicts of interest.
Data availability
The datasets supporting the conclusions of this article are available in the Pharmaceuticals and Medical Devices Agency repository, https://www.pmda.go.jp/.
Ethics approval
Not applicable.
Consent
Not applicable.
Author contributions
AN, NI and DK contributed to the conception and initial design of the manuscript. AN was the major contributor to the design and writing of the manuscript. MM and DK contributed to the final design and writing of the manuscript. SO contributed to the project administration. AN and NH contributed to the data curation, methodology, software and formal analysis. SN and SOh contributed to the review and editing. DK contributed to the conceptualization. All authors read and approved the final manuscript.
References
- 1.Sönnerstam E, Sjölander M, Lövheim H, Gustafsson M. Clinically relevant drug–drug interactions among elderly people with dementia. Eur J Clin Pharmacol. 2018;74:1351–1360. doi: 10.1007/s00228-018-2514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HA, Shin JY, Kim MH, Park BJ. Prevalence and predictors of polypharmacy among Korean elderly. PLoS ONE. 2014;9:e98043. doi: 10.1371/journal.pone.0098043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abe J, Umetsu R, Uranishi H, Suzuki H, Nishibata Y, Kato Y, Ueda N, Sasaoka S, Hatahira H, Motooka Y, Masuta M, Nakamura M. Analysis of polypharmacy effects in older patients using Japanese Adverse Drug Event Report database. PLoS ONE. 2017;12:e0190102. doi: 10.1371/journal.pone.0190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mortazavi SS, Shati M, Keshtkar A, Malakouti SK, Bazargan M, Assari S. Defining polypharmacy in the elderly: a systematic review protocol. BMJ Open. 2016;6:e010989. doi: 10.1136/bmjopen-2015-010989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Best O, Gnjidic D, Hilmer SN, Naganathan V, McLachlan AJ. Investigating polypharmacy and drug burden index in hospitalised older people. Intern Med J. 2013;43:912–918. doi: 10.1111/imj.12203. [DOI] [PubMed] [Google Scholar]
- 6.Pfister B, Jonsson J, Gustafsson M. Drug-related problems and medication reviews among old people with dementia. BMC Pharmacol Toxicol. 2017;18:52. doi: 10.1186/s40360-017-0157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leiss W, Méan M, Limacher A, Righini M, Jaeger K, Beer HJ, Osterwalder J, Frauchiger B, Matter CM, Kucher N, Angelillo-Scherrer A, Cornuz J, Banyai M, Lämmle B, Husmann M, Egloff M, Aschwanden M, Rodondi N, Aujesky D. Polypharmacy is associated with an increased risk of bleeding in elderly patients with venous thromboembolism. J Gen Intern Med. 2015;30:17–24. doi: 10.1007/s11606-014-2993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevenson JM, Davies JG, Martin FC. Medication-related harm: a geriatric syndrome. Age Aging. 2019 doi: 10.1093/ageing/afz121. [DOI] [PubMed] [Google Scholar]
- 10.Davies EA, O'Mahony MS. Adverse drug reactions in special populations—the elderly. Br J Clin Pharmacol. 2015;80:796–807. doi: 10.1111/bcp.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuzuya M, Masuda Y, Hirakawa Y, Iwata M, Enoki H, Hasegawa J, Cheng XW, Iguchi A. Underuse of medications for chronic diseases in the oldest of community-dwelling older frail Japanese. J Am Geriatr Soc. 2006;54:598–605. doi: 10.1111/j.1532-5415.2006.00659.x. [DOI] [PubMed] [Google Scholar]
- 12.Kojima T, Akishita M, Kameyama Y, Yamaguchi K, Yamamoto H, Eto M, Ouchi Y. High risk of adverse drug reactions in elderly patients taking six or more drugs: analysis of inpatient database. Geriatr Gerontol Int. 2012;12:761–762. doi: 10.1111/j.1447-0594.2012.00868.x. [DOI] [PubMed] [Google Scholar]
- 13.Chisaki Y, Aoji S, Yano Y. Analysis of adverse drug reaction risk in elderly patients using the Japanese Adverse Drug Event Report (JADER) database. Biol Pharm Bull. 2017;40:824–829. doi: 10.1248/bpb.b16-00930. [DOI] [PubMed] [Google Scholar]
- 14.Ueda N, Umetsu R, Abe J, Kato Y, Nakayama Y, Kato Z, Kinosada Y, Nakamura M. Analysis of neuropsychiatric adverse events in patients treated with oseltamivir in spontaneous adverse event reports. Biol Pharm Bull. 2015;38:1638–1644. doi: 10.1248/bpb.b15-00253. [DOI] [PubMed] [Google Scholar]
- 15.Mack GW, Weseman CA, Langhans GW, Scherzer H, Gillen CM, Nadel ER. Body fluid balance in dehydrated healthy older men: thirst and renal osmoregulation. J Appl Physiol. 1985;1994(76):1615–1623. doi: 10.1152/jappl.1994.76.4.1615. [DOI] [PubMed] [Google Scholar]
- 16.Koch CA, Fulop T. Clinical aspects of changes in water and sodium homeostasis in the elderly. Rev Endocr Metab Disord. 2017;18:49–66. doi: 10.1007/s11154-017-9420-5. [DOI] [PubMed] [Google Scholar]
- 17.Miller M, Moses AM. Drug-induced stated of impaired water excretion. Kidney Int. 1976;10:96–103. doi: 10.1038/ki.1976.81. [DOI] [PubMed] [Google Scholar]
- 18.Wirth R, Dziewas R, Beck AM, Clavé P, Hamdy S, Heppner HJ, Langmore S, Leischker AH, Martino R, Pluschinski P, Rösler A, Shaker R, Warnecke T, Sieber CC, Volkert D. Oropharyngeal dysphagia in older persons—from pathophysiology to adequate intervention: a review and summary of an international expert meeting. Clin Interv Aging. 2016;11:189–208. doi: 10.2147/CIA.S97481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tagliaferri S, Lauretani F, Pelá G, Meschi T, Maggio M. The risk of dysphagia is associated with malnutrition and poor functional outcomes in a large population of outpatient older individuals. Clin Nutr. 2018;38:2684–2689. doi: 10.1016/j.clnu.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Pereira KG, Peres MA, Iop D, Boing AC, Boing AF, Aziz M, d'Orsi E. Polypharmacy among the elderly: a population-based study. Rev Bras Epidemiol. 2017;20:335–344. doi: 10.1590/1980-5497201700020013. [DOI] [PubMed] [Google Scholar]
- 21.Marković-Peković V, Škrbić R, Petrović A, Vlahović-Palčevski V, Mrak J, Bennie M, Fadare J, Kwon HY, Schiffers K, Truter I, Godman B. Polypharmacy among the elderly in the Republic of Srpska: extent and implications for the future. Expert Rev Pharmacoecon Outcomes Res. 2016;16:609–618. doi: 10.1586/14737167.2016.1115347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are available in the Pharmaceuticals and Medical Devices Agency repository, https://www.pmda.go.jp/.