Abstract
Objectives
The effectiveness of trimethoprim/sulfamethoxazole (TMP/SMZ) for pneumocystis pneumonia (PCP) is limited with adverse events. Caspofungin, by inhibiting the cyst form of Pneumocystis jirovecii, may be an alternative therapy for PCP. However, the availability of clinical data about caspofungin combined with TMP/SMZ in the treatment of PCP in HIV‐infected patients is limited. Thus, we aimed to examine the clinical effectiveness and safety of caspofungin combined with TMP/SMZ as a first‐line therapy for moderate‐to‐severe PCP in HIV‐infected patients.
Methods
From January 2017 to December 2019, data of HIV‐infected patients with moderate‐to‐severe PCP who received either TMP/SMZ alone or caspofungin combined with TMP/SMZ as first‐line therapy were retrospectively reviewed to assess the effectiveness and safety of each regimen. The Kaplan–Meier curve and log‐rank test were used for survival analysis.
Results
A total of 278 patients met the criteria. The overall positive response rate of PCP treatment was 48.92%, and the overall all‐cause in‐hospital mortality rate was 33.09%. Patients who received combination therapy consisting of caspofungin and TMP/SMZ had a better positive response rate (59.44% vs. 37.78%, P < 0.001) and lower all‐cause in‐hospital mortality rate (24.48% vs. 42.22%, P = 0.003). Also, patients who received combination therapy had higher survival rate during a hospital stay (75.52% vs. 57.78%, P = 0.004), and those who received longer combination therapy were more likely to have higher survival rate (P = 0.042). We found that age (P = 0.019), CD4 cell count (P = 0.001) and therapeutic regimen (P = 0.002) were significant risk factors for all‐cause in‐hospital mortality rate in univariate analysis. In multivariate analysis, only CD4 cell count and therapeutic regimen were statistically significant factors associated with all‐cause in‐hospital mortality rate. Patients with a CD4 count of > 30 cells/µL and patients who received combination therapy consisting of caspofungin and TMP/SMZ were more likely to survive from PCP (P = 0.011 and P = 0.002, respectively). There were no additional severe adverse events caused by adding caspofungin.
Conclusions
For HIV‐infected patients with moderate‐to‐severe PCP, combination therapy with caspofungin and TMP/SMZ is an effective and promising first‐line therapy with no greater number of adverse events compared with TMP/SMZ monotherapy. Patients who received caspofungin had better positive response rates and lower all‐cause in‐hospital mortality rates. Also, we recommend early initiation of caspofungin.
Keywords: caspofungin, HIV, pneumocystis pneumonia
Introduction
Pneumocystis pneumonia (PCP) is an acute and potentially life‐threatening pulmonary infection that occurs among HIV‐infected patients with a low CD4 cell count [1]. Owing to the widespread use of antiretroviral therapy (ART), the incidence of PCP has decreased. However, it is still a major HIV‐related opportunistic infection, particularly in patients who are not aware of their HIV infection and present with late‐stage disease [2].
Trimethoprim/sulfamethoxazole (TMP/SMZ) has been recommended as the first‐line treatment for all forms of PCP and this has remained unchanged for many years [3]. Despite this therapy, the mortality rate in HIV‐infected patients diagnosed with PCP remains high. The overall mortality rate of patients with PCP is c. 50%, and it is even higher in patients with severe PCP [4, 5]. Therefore, treatment of HIV‐infected patients with severe PCP remains challenging and alternative treatments that can improve the outcomes of PCP infection are eagerly required.
Caspofungin is an antifungal agent that inhibits the synthesis of β‐1,3‐glucan in the cell wall [6]. It is likely to be effective at treating PCP, because β‐1,3‐glucan is an important component of the cyst cell wall in Pneumocystis jirovecii [7]. In a mouse model, caspofungin treatment was effective in Pneumocystis jirovecii clearance, and reduced the cyst burden in lung tissue, which led to better survival in HIV‐infected mice [8, 9, 10]. Previous studies reported that caspofungin combined with TMP/SMZ could possibly have an additive treatment effect on PCP, and this combination regimen was found to be favourable as a first‐line or salvage therapy for PCP [11]. However, the availability of clinical data on caspofungin combined with TMP/SMZ in the treatment of PCP in HIV‐infected patients is limited. It remains unclear whether combination treatment of caspofungin and TMP/SMZ is more effective than TMP/SMZ alone [12, 13].
Therefore, in this retrospective study, we aimed to examine the clinical effectiveness and safety of caspofungin combined with TMP/SMZ as a first‐line therapy for moderate‐to‐severe PCP in HIV‐infected patients.
Methods
Patient population
We retrospectively reviewed the medical records of newly diagnosed adult patients with both HIV infection and moderate‐to‐severe PCP in our institute between January 2017 and December 2019. Patients were excluded if: (1) patients received prophylactic treatment for PCP; (2) patients had CD4 T lymphocyte count > 200 cells/μL; (3) patients were allergic or intolerant to related agents for PCP treatment; or (4) patients had a history of drug abuse. Our primary endpoint was all‐cause in‐hospital mortality rate, secondary endpoint was overall positive response rate. The study was approved by the ethics committee (2019‐003‐02‐KY).
Diagnosis and treatment of PCP
The definite diagnosis of PCP was based on aetiology diagnosis, finding cyst or trophozoite of Pneumocystis jirovecii in respiratory specimens or lung tissue. Also, HIV‐infected patients with typical clinical symptoms such as fever, cough and dyspnoea, accompanied by chest imaging consistent with interstitial pneumonitis were diagnosed with PCP clinically. The severity of PCP was classified into mild and moderate‐to‐severe by partial arterial oxygen pressure (PaO2) while breathing room air or the alveolar–arterial oxygen difference (AaDO2) [3]. In this study, we only included HIV‐infected patients with PaO2 ≤ 70 mmHg or AaDO2 ≥ 35 mmHg as having moderate‐to‐severe PCP.
Patients were divided into four groups according to their therapeutic regimens (Fig. 1). In group 1, patients received standard first‐line therapy of PCP, which consisted of TMP/SMZ at a daily dose of 15–20 mg/kg of TMP and 75–100 mg/kg of SMZ for 3 weeks. Patients in group 2 received caspofungin intravenously after 2 weeks standard therapy at a dose of 50 mg caspofungin daily with a 70 mg loading dose on day 1 for 1 week. In group 3, patients received caspofungin intravenously after 1 week standard therapy for 2 weeks, and in group 4, patients started caspofungin at the same time as TMP/SMZ for the whole 3 weeks. All patient received caspofungin reached a dose of 50 mg/day with a 70 mg loading dose on day 1. Adjunctive corticosteroids and ART were provided according to the current guidelines. The decisions on when to initiate caspofungin were at the discretion of at least two physicians and were discussed with patients. The primary outcome of the study was all‐cause in‐hospital mortality rate. Positive response was defined as fewer clinical symptoms, better PaO2 and resolution of pneumonitis on chest imaging after treatment.
Fig. 1.
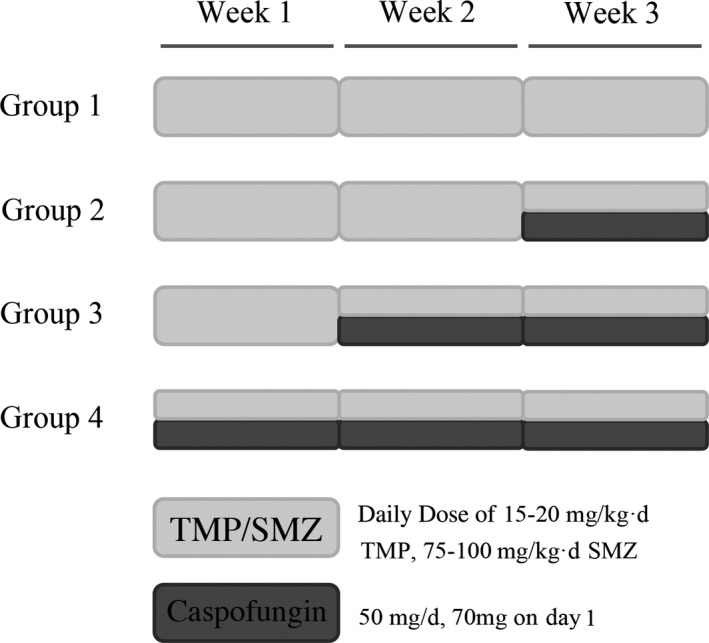
Therapeutic regimens of HIV‐infected patients diagnosed with moderate‐to‐severe pneumocystis pneumonia (PCP). TMP/SMZ, trimethoprim/sulfamethoxazole.
Statistical analysis
We used descriptive statistics to present patients’ baseline characteristics in each group. Categorical variables were compared using χ 2 test and continuous variables using t‐test. Multivariate logistic regression analyses were performed. The Kaplan–Meier curve and log‐rank test were used for survival analysis. Two‐tailed P‐values were adopted, and P < 0.05 was considered significant. All statistical analyses were performed using SPSS statistical software v.20.0 (IBM, Chicago, IL, USA).
Results
Baseline characteristics
A total of 278 patients met the criteria. Overall baseline characteristics stratified by therapeutic regimens are presented in Table 1. The median age of these patients was 34 years old (range 19–65 years), and 83.45% of them (n = 232) were male. At the diagnosis of moderate‐to‐severe PCP, the median [interquartile range (IQR)] CD4 count was 30 (19–84) cells/µL, which was far below the normal level. Most patients showed bilateral ground‐glass opacity on computed tomography scan (n = 236, 84.89%), and 24.82% of patients (n = 69) developed pleural effusion. Concurrent pulmonary infections when diagnosed with PCP were assessed. The total concurrent pulmonary infection rate was 50.36% (n = 140), among whom 15.83% (n = 44) had tuberculosis infection, 7.91% (n = 22) had fungal infection and 32.01% (n = 89) had another kind of infection. Moreover, 19 patients had multiple infections. The median (IWR) time from symptom onset to PCP treatment was 7 (3–14) days.
Table 1.
Overall baseline characteristics in the cohort stratified by therapeutic regimen
| Variables | All (n = 278) | Group 1 (n = 135) | Group 2 (n = 39) | Group 3 (n = 79) | Group 4 (n = 25) | P‐value |
|---|---|---|---|---|---|---|
| Male [n (%)] | 232 (83.45) | 115 (85.19) | 31 (79.49) | 64 (81.01) | 22 (88.00) | 0.693 |
| Age (years) [median (IQR)] | 34 (31–38) | 34 (31–38) | 34 (32–37) | 35 (30–38) | 33 (30–36) | 0.873 |
| CD4 count (cells/µL) [median (IQR)] | 30 (19–84) | 32 (19–84) | 26 (15–98) | 32 (17–66) | 30 (25–46) | 0.826 |
| Radiographic findings | ||||||
| Bilateral GGOs [n (%)] | 236 (84.89) | 117 (86.67) | 33 (84.62) | 65 (82.28) | 21 (84.00) | 0.857 |
| Pleural effusion [n (%)] | 69 (24.82) | 35 (25.93) | 9 (23.08) | 18 (22.78) | 7 (28.00) | 0.927 |
| Use of corticosteroid [n (%)] | 269 (96.76) | 130 (96.30) | 38 (97.44) | 77 (97.47) | 24 (96.00) | 0.956 |
| Concurrent pulmonary infections [n (%)] | ||||||
| Tuberculosis infection [n (%)] | 44 (15.83) | 23 (17.04) | 6 (15.38) | 11 (13.92) | 4 (16.00) | 0.946 |
| Fungal infection [n (%)] | 22 (7.91) | 10 (7.41) | 3 (7.69) | 7 (8.86) | 2 (8.00) | 0.986 |
| Other infection [n (%)] | 89 (32.01) | 45 (33.33) | 13 (33.33) | 23 (29.11) | 8 (32.00) | 0.931 |
| Multiple infection [n (%)] | 19 (6.83) | 10 (7.41) | 3 (7.69) | 4 (5.06) | 2 (8.00) | 0.906 |
| Symptom onset to PCP treatment (days) [median (IQR)] | 7 (3–14) | 7 (3–14) | 6 (3–13) | 7 (3–15) | 8 (4–13) | 0.912 |
IQR, interquartile range; GGO, ground‐glass opacity; PCP, pneumocystis pneumonia.
Outcomes in all patients
In the study, 48.20% of patients (n = 134) received mechanical ventilation. The median (IQR) time of hospital stay was 30 (21–34) days. The overall positive response rate of PCP treatment was 48.92% (n = 136), and the overall all‐cause in‐hospital mortality rate was 33.09% (n = 92). Patients who received caspofungin (patients in groups 2, 3 and 4) had a better positive response rate (59.44%, n = 143 vs. 37.78%, n = 135; P < 0.001) and lower all‐cause in‐hospital mortality rate (24.48%, n = 143 vs. 42.22%, n = 135; P = 0.003), differences that were all statistically significant. Clinical outcomes of the cohort at the end of PCP treatment are shown in Table 2.
Table 2.
Clinical outcomes of the cohort at the end of pneumocystis pneumonia (PCP) treatment
| Variables | All (n = 278) | Group 1 (n = 135) | Group 2 (n = 39) | Group 3 (n = 79) | Group 4 (n = 25) | P value |
|---|---|---|---|---|---|---|
| Mechanical ventilation [n (%)] | 134 (48.20) | 68 (50.37) | 19 (48.72) | 36 (45.57) | 11 (44.00) | 0.884 |
| Length of hospital stay (days) [median (IQR)] | 30 (21–34) | 28 (20–32) | 29 (19–33) | 30 (21–34) | 30 (21–34) | 0.833 |
| Positive response rate [n (%)] | 136 (48.92) | 51 (37.78) | 18 (46.15) | 48 (60.76) | 19 (76.00) | < 0.001 |
| All‐cause in‐hospital mortality [n (%)] | 92 (33.09) | 57 (42.22) | 14 (35.90) | 19 (24.05) | 2 (8.00%) | 0.001 |
The Kaplan–Meier curve indicated that patients who received caspofungin (patients in groups 2, 3 and 4) had higher survival rates during hospital stay (75.52%, n = 143 vs. 57.78%, n = 135; P = 0.004) (Fig. 2), and patients who received longer combination therapy with caspofungin and TMP/SMZ were more likely to have higher survival rates (P = 0.042) (Fig. 3). The survival rates of patients in each group were 57.78%, 64.10%, 75.95% and 92.00% respectively.
Fig. 2.
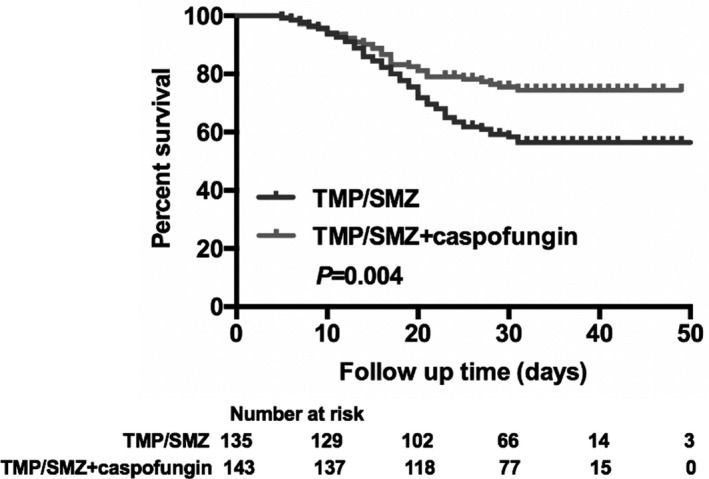
Kaplan–Meier curve of all‐cause in‐hospital mortality according to treatment with trimethoprim/sulfamethoxazole (TMP/SMZ) monotherapy and combination therapy consisting of caspofungin and TMP/SMZ during hospital stay.
Fig. 3.
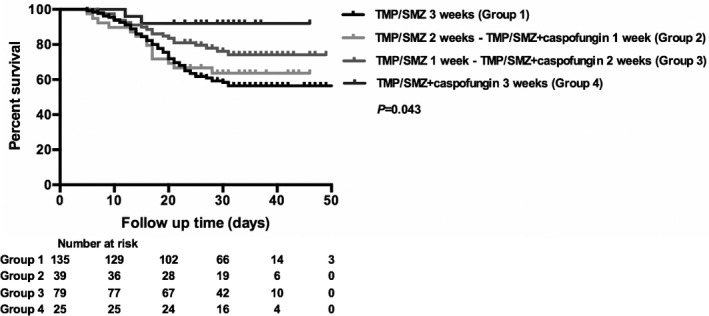
Kaplan–Meier curve of all‐cause in‐hospital mortality according to treatment with a different kind of combination therapy consisting of caspofungin and trimethoprim/sulfamethoxazole (TMP/SMZ) during hospital stay.
Various factors were associated with all‐cause in‐hospital mortality rate. We found that age (P = 0.019), CD4 cell count (P = 0.001) and therapeutic regimen (P = 0.002) were significant risk factors for all‐cause in‐hospital mortality rate in univariate analysis (Table 3), whereas in multivariate analysis, only CD4 cell count and therapeutic regimen were statistically significant factors associated with all‐cause in‐hospital mortality rate (Table 3). Patients with CD4 count > 30 cells/µL and those who received combination therapy with caspofungin and TMP/SMZ were more likely to survive PCP during hospital stay (P = 0.011 and P = 0.002, repectively).
Table 3.
Univariate and multivariable analysis of all‐cause in‐hospital mortality
| Variables |
All (n = 278) |
Survival (n = 186) |
% |
Death (n = 92) |
% |
Univariate P‐value |
Multivariate OR (95% CI), P‐value |
|---|---|---|---|---|---|---|---|
| Age | 0.019 | ||||||
| ≤ 35 years | 172 | 124 | 66.67 | 48 | 52.17 | Ref. | |
| > 35 years | 106 | 62 | 33.33 | 44 | 47.83 | 2.324 (0.978–5.652), 0.090 | |
| CD4 count | 0.001 | ||||||
| ≤ 30 cells/µL | 140 | 81 | 43.55 | 59 | 64.13 | Ref. | |
| > 30 cells/µL | 138 | 105 | 56.45 | 33 | 35.87 | 0.472 (0.266–0.840), 0.011 | |
| Concurrent pulmonary infections | 0.613 | ||||||
| No | 148 | 101 | 54.30 | 47 | 51.09 | ||
| Yes | 130 | 85 | 45.70 | 45 | 48.91 | ||
| Therapeutic regimens | 0.002 | ||||||
| TMP/SMZ monotherapy (group 1) | 135 | 78 | 41.94 | 57 | 61.96 | Ref. | |
| TMP/SMZ + caspofungin (groups 2, 3 and 4) | 143 | 108 | 58.06 | 35 | 38.04 | 0.402 (0.225–0.715), 0.002 |
OR, odds ratio; CI, confidence interval; TMP/SMZ, trimethoprim/sulfamethoxazole.
Adverse events
All reported adverse events were recorded. In our study, common adverse events of TMP/SMZ were gastrointestinal upset (n = 175, 62.95%), hepatic and renal toxicity (n = 94, 33.81%), rash (n = 28, 10.07%) and marrow suppression (n = 14, 5.04%). There were no serious events caused by caspofungin alone, and no patients discontinued caspofungin due to clinical or laboratory adverse events.
Discussion
To the best of our knowledge, this is currently the largest study that has investigated the clinical effectiveness of caspofungin as one of the first‐line regimens for confirmed moderate‐to‐severe PCP in HIV‐infected patients. Our results demonstrated that the positive response rate of patients who received caspofungin was 59.44%, which was significantly higher than patients who received only TMP/SMZ, and the all‐cause in‐hospital mortality rate was 24.48% in patients who received combination therapy, which was significantly lower than patients who only received TMP/SMZ. Also, caspofungin had fewer adverse events than TMP/SMZ.
Previous studies and case reports showed that caspofungin was an alternative treatment or one of the second‐line regimens for HIV‐infected patients with PCP [14, 15, 16, 17]. Huang et al. [14] found that echinocandin therapy, such as caspofungin, was an alternative treatment option in HIV‐infected patients with PCP who were unable to tolerate TMP/SMZ. Several case reports showed that after clinical failure or allergy of first‐line TMP/SMZ, most patients had their prognosis improved with second‐line therapy, which was combined with caspofungin and TMP/SMZ or other agents [15, 16, 17]. Moreover, studies focused on HIV‐negative patients with severe PCP showed that caspofungin combined with TMP/SMZ was a promising and effective first‐line therapy [18, 19]. Zhang et al. compared combined treatment with caspofungin and TMP/SMZ as first‐line therapy with the same regimens as second‐line therapy, and found that all‐cause hospital mortality was 42.86% (6/14), with mortality rates of 33.33% (3/9) and 60.00% (3/5) in the first‐line and second‐line therapy groups, respectively (P = 0.580). After combining with previously reported cases, the positive response rate was significantly greater in the first‐line therapy group (11/12, 91.67%) than in the second‐line therapy group (8/15, 53.33%, P = 0.043). Thus, we were interested in the effectiveness and safety of caspofungin as one of the first‐line regimens for HIV‐infected patients with moderate‐to‐severe PCP, which has rarely been investigated. Similarly, we found that patients who received combination therapy consisting of caspofungin and TMP/SMZ had better positive response rate and lower all‐cause in‐hospital mortality rate, which were comparable to findings of previous studies.
Thus, from our perspective, caspofungin can be used as an alternative treatment for HIV‐infected patients with PCP when other second‐line agents are unavailable, while data remain too limited to support caspofungin alone as first‐line therapy for these patients. An advantage of TMP/SMZ over caspofungin is its activity against bacteria. Physicians should be alert to any concurrent bacterial infections while treating patients with caspofungin alone.
According to previous data, about one‐quarter of patients cannot complete the full course of TMP/SMZ because of treatment failure or severe adverse events such as marrow suppression, hepatic and renal toxicity, gastrointestinal upset and so on [2, 20]. In our study, we did not include patients who were allergic or intolerant to TMP/SMZ. The decisions on when to initiate caspofungin were at the discretion of at least two physicians and were discussed with patients themselves. The primary factor associated with different choices was economic condition, because caspofungin was expensive.
Moreover, we found that age, CD4 cell count and therapeutic regimen were significant risk factors for all‐cause in‐hospital mortality rate. In multivariate analysis, CD4 cell count and therapeutic regimen remained statistically significant. Jin et al. [19] showed that low lymphocyte counts, high serum lactate dehydrogenase levels at the time of diagnosis of PCP and progression to shock were significant risk factors for death.
There are several limitations to our study. First, this was a retrospective study. However, this was a relatively large dataset with uniform inclusive and exclusive criteria. Second, there was selection bias in patients with TMP/SMZ failure after adding caspofungin. For these patients, the effectiveness of caspofungin could cover up the evidence of TMP/SMZ failure. Caspofungin was no longer one of the first‐line therapies; in fact, it was a second‐line therapy after treatment failure with TMP/SMZ. Third, we did not examine the drug resistance of PCP. Fourth, there was no fixed schedule of adjunctive corticosteroids, such as dose and duration. Thus, it was difficult to eliminate the effect of corticosteroids.
In conclusion, for HIV‐infected patients with moderate‐to‐severe PCP, combination therapy with caspofungin and TMP/SMZ is an effective and promising first‐line treatment, with no greater number of adverse events than are seen with TMP/SMZ monotherapy. Patients who received caspofungin had a better positive response rate and a lower all‐cause in‐hospital mortality rate. Moreover, we recommend early initiation of caspofungin. Further assessment is needed to confirm the results of our study.
Author contributions
QT and JS wrote the article. RX, BW, BH, QL and ZJ collected and analysed the data. FJ provided administrative support.
Acknowledgements
Financial disclosure: This work was supported by grants from the National Science and Technology Major Project of China during the 13th Five‐year plan period (project no. 2018ZX10302104).
Conflicts of interest: The authors declare there are no conflicts of interest.
References
- 1. Shibata S, Kikuchi T. Pneumocystis pneumonia in HIV‐1‐infected patients. Respir Investig 2019; 57: 213–219. [DOI] [PubMed] [Google Scholar]
- 2. Huang YS, Yang JJ, Lee NY et al. Treatment of Pneumocystis jirovecii pneumonia in HIV‐infected patients: a review. Expert Rev Anti Infect Ther 2017; 15: 873–892. [DOI] [PubMed] [Google Scholar]
- 3. Panel on Opportunistic Infections in HIV‐Infected Adults and Adolescents . Guidelines for the prevention and treatment of opportunistic infections in HIV‐infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at: https://aidsinfo.nih.gov/guidelines [Accessed Date 10 January 2020].
- 4. Monnet X, Vidal‐Petiot E, Osman D et al. Critical care management and outcome of severe Pneumocystis pneumonia in patients with and without HIV infection. Crit Care 2008; 12: R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roembke F, Heinzow HS, Gosseling T et al. Clinical outcome and predictors of survival in patients with Pneumocystis jirovecii pneumonia–results of a tertiary referral centre. Clin Respir J 2014; 8: 86–92. [DOI] [PubMed] [Google Scholar]
- 6. Espinel‐Ingroff A. Novel antifungal agents, targets or therapeutic strategies for the treatment of invasive fungal diseases: a review of the literature (2005–2009). Rev Iberoam Micol 2009; 26: 15–22. [DOI] [PubMed] [Google Scholar]
- 7. Kutty G, Davis AS, Ma L, Taubenberger JK, Kovacs JA. Pneumocystis encodes a functional endo‐beta‐1,3‐glucanase that is expressed exclusively in cysts. J Infect Dis 2015; 211: 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cushion MT, Linke MJ, Ashbaugh A et al. Echinocandin treatment of pneumocystis pneumonia in rodent models depletes cysts leaving trophic burdens that cannot transmit the infection. PLoS One 2010; 5: e8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cushion MT, Collins MS. Susceptibility of Pneumocystis to echinocandins in suspension and biofilm cultures. Antimicrob Agents Chemother 2011; 55: 4513–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lobo ML, Esteves F, de Sousa B et al. Therapeutic potential of caspofungin combined with trimethoprim‐sulfamethoxazole for pneumocystis pneumonia: a pilot study in mice. PLoS One 2013; 8 (8): e70619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mu XD, Que CL, He B, Wang GF, Li HC. Caspofungin in salvage treatment of severe pneumocystis pneumonia: case report and literature review. Chin Med J 2009; 122: 996–999. [PubMed] [Google Scholar]
- 12. Kamboj M, Weinstock D, Sepkowitz KA. Progression of Pneumocystis jiroveci pneumonia in patients receiving echinocandin therapy. Clin Infect Dis 2006; 43: e92–94. [DOI] [PubMed] [Google Scholar]
- 13. Li H, Huang H, He H. Successful treatment of severe Pneumocystis pneumonia in an immunosuppressed patient using caspofungin combined with clindamycin: a case report and literature review. BMC Pulm Med 2016; 16: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang YS, Liu CE, Lin SP et al. Echinocandins as alternative treatment for HIV‐infected patients with Pneumocystis pneumonia. AIDS 2019; 33: 1345–1351. [DOI] [PubMed] [Google Scholar]
- 15. Lee WS, Hsueh PR, Hsieh TC, Chen FL, Ou TY, Jean SS. Caspofungin salvage therapy in Pneumocystis jirovecii pneumonia. J Microbiol Immunol Infect 2017; 50: 547–548. [DOI] [PubMed] [Google Scholar]
- 16. Ceballos ME, Ortega M, Andresen M, Wozniak A, Garcia P, Balcells ME. Successful treatment with echinocandin in an HIV‐infected individual failing first‐line therapy for Pneumocystis jirovecii pneumonia. AIDS 2011; 25: 2192–2193. [DOI] [PubMed] [Google Scholar]
- 17. Armstrong‐James D, Stebbing J, John L et al. A trial of caspofungin salvage treatment in PCP pneumonia. Thorax 2011; 66: 537–538. [DOI] [PubMed] [Google Scholar]
- 18. Zhang G, Chen M, Zhang S et al. Efficacy of caspofungin combined with trimethoprim/sulfamethoxazole as first‐line therapy to treat non‐HIV patients with severe pneumocystis pneumonia. Exp Ther Med 2018; 15: 1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jin F, Liu XH, Chen WC, Fan ZL, Wang HL. High initial (1, 3) Beta‐d‐Glucan concentration may be a predictor of satisfactory response of c aspofungin combined with TMP/SMZ for HIV‐negative patients with moderate to severe Pneumocystis jirovecii pneumonia. Int J Infect Dis 2019; 88: 141–148. [DOI] [PubMed] [Google Scholar]
- 20. Benfield T, Atzori C, Miller RF, Helweg‐Larsen J. Second‐line salvage treatment of AIDS‐associated Pneumocystis jirovecii pneumonia: a case series and systematic review. J Acquir Immune Defic Syndr 2008; 48: 63–67. [DOI] [PubMed] [Google Scholar]


