Abstract
Introduction
Reliable estimates of time from diagnosis until institutionalization and death in people with dementia from routine nationally representative databases are lacking.
Methods
We selected 9230 people with dementia and 24,624 matched controls from family physicians’ electronic records linked with national administrative databases to analyze time until institutionalization and death and associated factors.
Results
Median time from recorded diagnosis until institutionalization and until death for people with dementia was 3.9 and 5.0 years, respectively, which was considerably shorter than for controls. Once institutionalized, median time to death was longer for persons with dementia (2.5 years) than for controls (1.2 years). Older age and receiving home care were the strongest predictors of shorter time until institutionalization and death in people with dementia. Gender, cohabitation, migration status, frailty, polypharmacy, and dementia medication were other significant factors.
Discussion
The estimates could help to inform patients, their families, and policymakers about probable trajectories.
Keywords: care trajectories, data linkage, primary care, registry data, risk factors, survival, time to institutionalization
1. BACKGROUND
Dementia currently affects ≈50 million people worldwide. With the aging of the population, this number is expected to triple by 2050. 1 , 2 Dementia has huge consequences for affected persons, their relatives, and society. With the progression of the disease, persons with dementia become more dependent on support of others and at some point in time, admission to a long‐term care facility is often considered necessary. Once dementia is diagnosed, frequently asked questions are how long people will be able to live at home and how long it will last until death. Accurate estimates may help to inform people with dementia and their relatives about the prognosis. In addition, caring for people with dementia is associated with substantial (in)formal care use. Reliable estimates are relevant to support policymakers in planning appropriate services.
Previous studies have attempted to estimate time until institutionalization and associated factors in people with dementia. 3 Studies varied considerably in design, sample selection, sample size, follow‐up time, and consequently, in estimations with median time ranging from 2.5 to 7.3 years. There was a lack of methodological strength in a majority of studies, such as use of small and selected samples, relatively short follow‐up time, and selection of prevalent dementia cases with unclear disease onset. 4 , 5 , 6 , 7 , 8 , 9 , 10
With regard to the prognosis of survival, the average duration from diagnosis until death varied substantially across studies, ranging from 1.1 to 8.5 years. 11 , 12 Again, most studies used prevalent cases from study entry and included small or selected samples. Large population‐based studies of incident cases with extensive follow‐up length without missing outcomes are scarce. Only one identified study included a large, unscreened sample of (22,529) incident dementia cases and estimated a median survival time of 6 years. 13
The present study aims to advance current knowledge about time to institutionalization and death in people with dementia and their associated factors, and to address important methodological limitations of previous studies by analyzing these outcomes in nationwide registries from the first diagnosis of dementia by the family physician. Electronic health record data from family physicians linked with national administrative health databases and the population registry provide an opportunity to estimate time to institutionalization and death in a large sample without loss to follow‐up due to, for example, change or transfers out of practice. Insight into the time until these events occur from the point of recognition of dementia by physicians may be of high importance to patients, their family caregivers, and health services planning and policy‐making because such estimates are less biased than estimates from screened populations.
This research aimed to (1) estimate time from recorded dementia diagnosis to institutionalization and death compared with matched control persons without dementia, and (2) examine whether estimates were dependent on sociodemographic and clinical characteristics.
2. METHODS
We analyzed electronic health record data from family physicians (FPs) linked with national administrative databases to examine trajectories of people with dementia from the time the diagnosis was first recorded by their FP.
2.1. Data sources
In The Netherlands, FPs act as the “gatekeepers” to specialist care and are usually the first healthcare professional to be contacted with health problems. All citizens are registered in a family practice. About 90% of the older people visit their FP at least once a year, and on average nine times a year. 14 , 15 The dementia diagnosis is usually made by secondary care specialists and copied into the electronic health records system of FPs.
HIGHLIGHTS
Median time from recorded dementia diagnosis until institutionalization was 3.9 years
Median survival time from recorded dementia diagnosis to death was 5.0 years
Older age and receiving home care strongly predict earlier admission and death
People with dementia had much higher hazards to be admitted and die than controls
Once admitted, survival time was longer for persons with dementia than for controls
RESEARCH IN CONTEXT
Systematic review: In a PubMed literature review, we identified only one study that analyzed survival in a large, unscreened sample of people with incident dementia from routine care data.
Interpretation: In people with dementia, median time until institutionalization was 3.9 years, and 5.0 years until death. This was considerably shorter than in controls. But once persons with dementia were institutionalized, median time to death was longer for persons with dementia (2.5 years) than for controls (1.2 years). Older age and receiving home care were the strongest predictors of shorter time until institutionalization and death in people with dementia. Gender, cohabitation, migration status, frailty, polypharmacy, and prescribed dementia medication were other significant factors.
Future directions: Use of linked real‐world data provides tremendous possibilities for answering important questions for clinical practice and policy about care trajectories in people with dementia and for overcoming important methodological limitations of current studies.
We selected patients with a first recorded dementia diagnosis between 2008 and 2014 from the routine electronic health recordings from Dutch FPs participating in the NIVEL Primary Care Database (NIVEL‐PCD). 16 , 17 This database provides pseudonymized data from 451 family practices. It covers ≈10% of the Dutch population and is representative for Dutch family practices in terms of patients’ age and sex, practice size, and geographical distribution. Contact diagnoses are coded with ICPC‐1 (International Classification of Primary Care) 18 and grouped into disease episodes. FPs receive feedback on the quality of recording and are supported in coding. 19 In addition, a portion of FPs’ reimbursement is based on the quality of recording. 20
Institutionalization (between 2008 and 2015) and death (between 2008 and 2018) statistics were derived from administrative data sources made available for research by Statistics Netherlands (Centraal Bureau voor de Statistiek), the governmental institution responsible for processing statistical data in The Netherlands. Date of death originated from the Municipal Personal Records Database, including all persons residing in The Netherlands. Start and end dates of long‐term care admissions were derived from administrative data for the Dutch national long‐term care insurance scheme covering all institutionalizations (nursing, residential, or psychiatric home) of all Dutch adults.
2.2. Study population
Dementia cases—Patients born in 1965 or before with a first recorded dementia diagnosis (ICPC code: P70) between 2008 and 2014 and living at home at the date of diagnosis were included. Persons with Down syndrome and dementia (ICPC code A90.01) were excluded, as these persons usually have different care trajectories.
Control group—For all people with dementia, an independent researcher matched a maximum of four patients without a dementia diagnosis or memory disturbances (ICPC code: P20) from the same practice in year of diagnosis. Matching occurred on age (5‐year intervals), gender, and cohabitation with a spouse. If more than four controls were available, random selection was performed. The entry date of control patients was set as the date on which the dementia diagnosis of the person they were matched with was first recorded in the FP's record. Controls with a dementia drug prescription in the linked data set were excluded. Similar to the dementia cases, controls who were institutionalized at the entry date were excluded.
2.3. Outcomes
Outcome measures included time from the first recorded dementia diagnosis in primary care to institutionalization and death, and the institutionalization rate and mortality rate per 1000 person‐years (ie, number of cases admitted/died during the study period divided by the person‐years at risk). Institutionalization was defined as permanent entry into an institution for long‐term care at any time after dementia diagnosis (ie, respite admissions were not included). Second, we examined time from institutionalization to death in persons who were admitted, and the place of death.
2.4. Sociodemographics and clinical characteristics
Age, gender, living alone (vs cohabitation), and migrant status at the time of dementia diagnosis were derived from the national population registry managed by Statistics Netherlands. Migrant status was categorized into non‐Western migration background (Surinamese, Antillean, Aruban, Moroccan, Turkish, or other non‐Western migration background) and Western background (a native Dutch background or Western migration background).
The use of care at home (domestic assistance, personal care for activities of daily living, and home nursing support) at any point during 2008 to 2014 was derived from a national registry from the Dutch Central Administration Office (CAK) made available for research by Statistics Netherlands. From 2011, use of day care (individual or in a group) was also recorded.
Polypharmacy was defined as the dispensing of 5 or more drugs and hyperpolypharmacy as the dispensing of 10 or more drugs from different chemical subgroups (Anatomical Therapeutic Chemical [ATC] level 4) in the year of dementia diagnosis. Data were derived from a nationwide database from the National Healthcare Institute and made available for research by Statistics Netherlands and contained all medicines dispensed by pharmacies for which the costs are reimbursed under the statutory basic medical insurance. This covers medicines for community‐dwelling people and for people living in residential care homes, but not medicines dispensed in hospitals and nursing homes. The database does not include over‐the‐counter medicines.
Prescribed dementia medication included the presence of prescriptions starting with ATC "N06D" during available data collections from NIVEL‐PCD (2008 to 2015) and the National Healthcare Institute (2008 to 2016). This variable was categorized into prescribed dementia medication in the year of diagnosis or before, after the year of diagnosis, or no prescribed dementia drugs.
A frailty index was created by screening the FP electronic health records for 35 predefined clinically relevant “health deficits” including ICPC codes of diseases and symptoms, and one deficit “polypharmacy.” 21 The proportion of deficits present in an individual resulted in the Frailty Index score (range 0 to 1). People were classified into three categories in accordance with prior studies: non‐frail (three or fewer deficits; Frailty Index ≤0.08), pre‐frail (four to eight deficits; 0.08< score <0.25), and frail (nine or more deficits; score ≥0.25). 22 , 23 , 24
2.5. Data linkage
FP data were pseudonymized at the source and transferred to Statistics Netherlands, which performed the linkage. Pseudonyms were based on the citizen service number, or combination of birth date, gender, and zip code.
2.6. Statistical analyses
Descriptive statistics were used to describe the sample characteristics. The institutionalization and mortality rate per 1000 person‐years adjusted for age and gender were calculated using Poisson regression analysis. Time until institutionalization was calculated in years between date of dementia diagnosis and permanent institutionalization. Calculations were also conducted for males and female patients of different age groups. Because death alters the probability of institutionalization (people who die before institutionalization cannot be institutionalized any longer), it was considered as a competing risk in this analysis. In the presence of a competing risk, the standard Cox proportional hazard regression yields biased results. 25 Therefore, the cumulative incidence function, as part of the competing risk approach, was used to estimate the time to institutionalization. If an individual had not died or entered an institution at the end of data collection (31 December, 2014), his or her time to institutionalization was censored at this date. Time until death was estimated in years between date of dementia diagnosis and date of death using Kaplan‐Meier curves. Persons who were still alive at the end of the observed period (31 December, 2017) were censored at this date.
Time from institutionalization to death was estimated with Kaplan‐Meier curves. Mortality data until 2015 were used, as institutionalization data were not available yet after this year. The association between sociodemographic and clinical characteristics and time until institutionalization and death was analyzed separately in dementia patients and controls using competing risk regression and Cox proportional hazards models, respectively. For each variable, Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated, adjusted for age and gender. The significance level for all analyses was set at 0.05. Analyses were performed with SPSS (version 22) and R studio.
2.7. Ethics
The ethics committee of the VU Medical Center approved the study. Because pseudonymized data collected for routine administrative registration purposes were used, informed consent of patients was not obtained. Patients were informed by their FP about the use of their pseudonymized record data and could object.
3. RESULTS
3.1. Study sample
We identified 11,534 patients with a recorded diagnosis of dementia in the FP electronic records and 28,750 matched controls who could be linked to the population registry of Statistics Netherlands. In total, 2304 people with dementia and 4126 controls were excluded, resulting in 9230 people with dementia and 24,624 included in the analyses (Figure 1). Table 1 presents the characteristics of the samples. About 60% were female and the average age was 80 years. Respectively, 56% and 52% of the people with dementia and controls were cohabiting with others. Fifty‐three percent of the people with dementia and 37% of the controls received home care at the time of diagnosis. The majority of people in both groups were "pre‐frail" and had polypharmacy.
Figure 1.
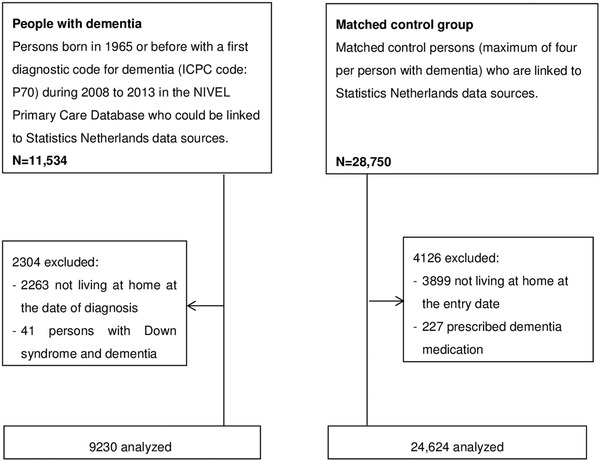
Flow diagram of the study sample
Table 1.
Sociodemographic and clinical characteristics of people with dementia and matched controls
| Persons with dementia (n = 9230) | Matched controls (n = 24,624) | |||
|---|---|---|---|---|
| N | % | N | % | |
| Female gender | 5553 | 60.3 | 15,010 | 61.0 |
| Age, mean (SD) | 79.7 | (7.9) | 79.5 | (7.7) |
| Under 65 | 465 | 5.0 | 1266 | 5.1 |
| 65‐74 | 1750 | 19.0 | 4758 | 19.3 |
| 75‐84 | 4596 | 49.8 | 12,687 | 51.5 |
| 85 and above | 2419 | 26.2 | 5913 | 24.0 |
| Cohabiting | 5149 | 55.8 | 12,772 | 51.9 |
| Migrant status | ||||
| Native Dutch | 8055 | 87.3 | 21,655 | 87.9 |
| Western migration background | 874 | 9.5 | 2304 | 9.4 |
| Surinamese/Antillean/Aruban | 124 | 1.3 | 233 | 0.9 |
| Moroccan/Turkish | 122 | 1.3 | 261 | 1.1 |
| Other non‐Western | 55 | 0.6 | 171 | 0.7 |
| Care at home during trajectory | 7681 | 83.2 | 12,367 | 50.2 |
| Care started before diagnosis | 4935 | 64.2 | 9050 | 73.2 |
| Care started after diagnosis | 2746 | 35.7 | 3317 | 26.8 |
|
Frailty Index (FI, 0‐1), median (range) |
0.11 | (0.47) | 0.11 | (0.47) |
| mean (SD) | 0.13 | (0.07) | 0.11 | (0.07) |
| Non‐frail (FI ≤ 0.08) | 1720 | 18.6 | 7871 | 32.0 |
| Pre‐frail (0.08 < FI < 0.25) | 6723 | 72.8 | 15,264 | 62.0 |
| Frail (FI ≥0.25) | 787 | 8.5 | 1489 | 6.0 |
| Polypharmacy | ||||
| Mean number of drugs (SD) | 7.43 | (4.71) | 6.79 | (4.75) |
| 0 to 4 drugs | 2701 | 29.3 | 8666 | 35.2 |
| 5 or more | 3750 | 40.6 | 9572 | 38.9 |
| 10 or more | 2779 | 30.1 | 6386 | 25.9 |
| Dementia medication prescribed | 2867 | 31.1 | – | – |
| Medication was prescribed in year of diagnosis or before a | 1609 | 17.4 | – | – |
| Medication was prescribed after the year of diagnosis | 1258 | 13.6 | – | – |
For 401 persons, dementia medication was prescribed before the year in which the diagnosis was recorded in the family physician's record, and for 1208 persons, dementia medication was prescribed in the year in which the diagnosis was recorded.
3.2. Institutionalization
When adjusting for age and gender, we found that 199 persons with dementia (95% CI 193, 205) and 17 controls (95% CI 16, 19) per 1000 person‐years were institutionalized during a median follow‐up time of, respectively 2.0 (IQR 2.2) and 2.3 (IQR 2.0) years. Median time to institutionalization for people with dementia was 3.9 years, and could not be estimated for controls, as the cumulative incidence curve remained <50% at the end of the study period, meaning that <50% of the controls were admitted at the end of the study period. Appendix 1 (Table A1), shows the median time to institutionalization for men and women from different age groups. For people with dementia, this ranged between 2.3 years for women of 85 or older to 6.2 years for men between 65‐ and 75‐years‐old. Table 2 presents the probability of being institutionalized within 6 months up to 6 years after diagnosis. People with dementia had a seven times higher hazard to be institutionalized than controls (HR 7.3, 95% CI 6.9, 7.7; P < 0.001) (Figure 2).
Table 2.
Probability of being institutionalized within 6 months up to 6 years after diagnosis and to survive at least 6 months up to 9 years after diagnosis
| Probability of being institutionalized | Probability of surviving | |||||||
|---|---|---|---|---|---|---|---|---|
| Persons with dementia | Control group | Persons with dementia | Control group | |||||
| Years | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
| 0.5 | 0.10 | (0.093; 0.105) | 0.016 | (0.015; 0.018) | 0.97 | (0.96; 0.97) | 0.97 | (0.968; 0.972) |
| 1 | 0.18 | (0.17; 0.19) | 0.031 | (0.029; 0.034) | 0.92 | (0.92; 0.93) | 0.94 | (0.937; 0.943) |
| 2 | 0.31 | (0.30; 0.32) | 0.055 | (0.052; 0.058) | 0.83 | (0.83; 0.84) | 0.88 | (0.88; 0.89) |
| 3 | 0.42 | (0.41; 0.43) | 0.073 | (0.070; 0.077) | 0.73 | (0.72; 0.74) | 0.83 | (0.83; 0.84) |
| 4 | 0.51 | (0.49; 0.52) | 0.090 | (0.086; 0.095) | 0.61 | (0.60; 0.62) | 0.78 | (0.77; 0.78) |
| 5 | 0.58 | (0.56; 0.59) | 0.11 | (0.11; 0.12) | 0.50 | (0.49; 0.51) | 0.73 | (0.72; 0.73) |
| 6 | 0.63 | (0.62; 0.65) | 0.12 | (0.12; 0.13) | 0.40 | (0.39; 0.41) | 0.68 | (0.67; 0.68) |
| 7 | 0.32 | (0.31; 0.33) | 0.63 | (0.62; 0.63) | ||||
| 8 | 0.25 | (0.23; 0.26) | 0.58 | (0.57; 0.59) | ||||
| 9 | 0.18 | (0.17; 0.20) | 0.53 | (0.51; 0.54) | ||||
Figure 2.

Cumulative incidence of institutionalization within people with dementia and matched controls
3.3. Survival
When adjusting for age and gender, we found that 142 persons with dementia (95% CI 138, 146) and 53 controls (95% CI 52, 55) per 1000 person‐years died, irrespective of being institutionalized, during a median follow‐up time of, respectively, 4.5 years (IQR 3.3) and 5.0 years (IQR 2.2). People with dementia died mostly in an institution, whereas the majority of controls died at home (X2 = 2286, 3 df, P < 0.0001, Appendix 2 Table B1).
Median time to death for people with dementia was 5.0 years and 9.6 years for controls. Appendix 1 (Table A2) shows the median time to death for women and men from different age groups. This ranged from 2.7 years for men of 85 or older to 9.4 years for women younger than 65 years. Table 2 presents the probability to survive 6 months up to 9 years after diagnosis. People with dementia had more than a twice higher hazard of death than controls (HR 2.2, 95% CI 2.2, 2.3; P < 0.001) (Figure 3). Once institutionalized, people with dementia had a median time to death of 2.5 years, which was shorter for controls with 1.2 years (HR 0.6, 95% CI 0.5, 0.6; P < 0.001).
Figure 3.

Cox survival curves of time until death within people with dementia and matched controls
3.4. Factors of institutionalization and death
Older age, being female, living alone, having a native Dutch background, receiving home care, and having a dementia medication prescription in or before the year of diagnosis were significantly associated with shorter time to institutionalization, whereas being (pre)frail was associated with a longer time to institutionalization in people with dementia (Table 3). Older age, being male, having a native Dutch background, receiving home care, hyperpolypharmacy, and being (pre)frail were associated with a shorter time to death in people with dementia. People who were prescribed dementia medication after the year in which the dementia diagnosis was recorded had a prolonged time to death (Table 3).
Table 3.
Results of competing risk regression examining permanent institutionalization, and results of Cox proportional hazard regression examining death, associated with baseline factors in patients with dementia and age‐ and gender‐matched controls
| Time to institutionalization | Time to death | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| People with dementia (n = 9230) | Controls (n = 24,624) | People with dementia (n = 9230) | Matched controls (n = 24,624) | |||||||||
| Outcome | HR a | 95% CI | P‐value | HR a | 95% CI | P‐value | HR a | 95% CI | P‐value | HR a | 95% CI | P‐value |
| Older age (65+) | 2.5 | 2.07; 2.99 | <0.001 | 32.7 | 10.56; 101.44 | <0.001 | 2.8 | 2.39; 3.26 | <0.001 | 9.1 | 7.07; 11.71 | <0.001 |
|
Age category (ref under 65) |
Ref | Ref | Ref | Ref | ||||||||
| 65‐74 | 1.6 | 1.30; 1.94 | <0.001 | 5.5 | 1.73; 17.54 | 0.004 | 1.5 | 1.30; 1.81 | <0.001 | 3.0 | 2.28; 3.86 | <0.001 |
| 75‐84 | 2.5 | 2.04; 2.96 | <0.001 | 25.5 | 8.20; 79.10 | <0.001 | 2.8 | 2.35; 3.22 | <0.001 | 8.5 | 6.57; 10.90 | <0.001 |
| 85 and above | 3.5 | 2.91; 4.26 | <0.001 | 78.4 | 25.26; 243.47 | <0.001 | 5.3 | 4.49; 6.17 | <0.001 | 21.2 | 16.41; 27.26 | <0.001 |
| Female gender | 1.2 | 1.13; 1.28 | <0.001 | 1.2 | 1.09; 1.34 | <0.001 | 0.63 | 0.60; 0.66 | <0.001 | 0.63 | 0.61; 0.66 | <0.001 |
| Living alone (vs cohabiting) | 1.3 | 1.23; 1.40 | <0.001 | 1.5 | 1.36; 1.73 | <0.001 | 0.96 | 0.91; 1.02 | 0.20 | 0.91 | 0.87; 0.96 | <0.001 |
| Native Dutch or Western migration background (vs non‐Western) | 1.9 | 1.52; 2.48 | <0.001 | 1.6 | 0.99; 2.53 | 0.057 | 1.2 | 1.03: 1.44 | 0.025 | 1.2 | 1.03; 1.47 | 0.025 |
| Migrant status (ref Native Dutch) | Ref | Ref | Ref | Ref | ||||||||
| Surinamese/Antillean/Aruban | 0.69 | 0.50; 0.95 | 0.024 | 1.2 | 0.71; 1.96 | 0.520 | 0.67 | 0.51; 0.88 | 0.004 | 0.92 | 0.70; 1.21 | 0.55 |
| Western migration background | 0.86 | 0.77; 0.96 | 0.006 | 0.99 | 0.83; 1.18 | 0.910 | 0.92 | 0.84; 1.00 | 0.051 | 1.0 | 0.95; 1.11 | 0.53 |
| Care at home during trajectory | 2.1 | 1.86; 2.38 | <0.001 | 3.7 | 3.16; 4.34 | <0.001 | 1.6 | 1.48; 1.75 | <0.001 | 2.6 | 2.43: 2.72 | <0.001 |
| Care at home (ref none) | ||||||||||||
| Care started after diagnosis | 1.8 | 1.56; 2.01 | <0.001 | 2.6 | 2.14; 3.08 | <0.001 | 1.4 | 1.23; 1.47 | <0.001 | 2.4 | 2.27; 2.60 | <0.001 |
| Care started before diagnosis | 2.5 | 2.18; 2.83 | <0.001 | 4.3 | 3.66; 5.09 | <0.001 | 1.9 | 1.73; 2.07 | <0.001 | 2.7 | 2.50; 2.82 | <0.001 |
| Frailty index (ref non‐frail) | <0.001 | |||||||||||
| Pre‐frail | 0.77 | 0.71; 0.83 | <0.001 | 0.97 | 0.87; 1.09 | 0.580 | 1.2 | 1.08; 1.23 | <0.001 | 1.2 | 1.18; 1.31 | <0.001 |
| Frail | 0.66 | 0.57; 0.75 | <0.001 | 1.2 | 1.02; 1.47 | 0.030 | 1.5 | 1.32; 1.63 | <0.001 | 1.8 | 1.60; 1.90 | <0.001 |
| Number of drugs (ref 0‐4 drugs) | ||||||||||||
| Polypharmacy (≥5 drugs) | 0.97 | 0.90; 1.04 | 0.380 | 1.3 | 1.15; 1.50 | <0.001 | 1.1 | 1.02; 1.16 | 0.11 | 1.4 | 1.30; 1.46 | <0.001 |
| Hyperpolypharmacy (≥10 drugs) | 0.96 | 0.89; 1.04 | 0.280 | 2.1 | 1.86; 2.40 | <0.001 | 1.4 | 1.29; 1.47 | <0.001 | 2.5 | 2.32; 2.61 | <0.001 |
| Dementia medication (ref none) | n/a | n/a | n/a | n/a | n/a | n/a | ||||||
| Started before or in year of diagnosis | 1.2 | 1.07; 1.25 | <0.001 | n/a | n/a | n/a | 1.0 | 0.96; 1.10 | 0.43 | n/a | n/a | n/a |
| Started after year of diagnosis | 0.73 | 0.67; 0.80 | <0.001 | n/a | n/a | n/a | 0.72 | 0.66; 0.78 | <0.001 | n/a | n/a | n/a |
Hazard ratios are adjusted for age and gender.
4. DISCUSSION
4.1. Main findings and interpretation
This study is one of the largest that examined the trajectories of persons with dementia in linked routine nationally representative administrative databases. Until now, reliable estimates from high‐quality studies are scarce, but are important to help to inform patients and their families about probable care trajectories and policymakers to optimize the allocation of resources.
4.2. Comparison with literature
4.2.1. Institutionalization
A previous systematic review found that the mean time from estimated onset of dementia to admission ranged considerably between studies, from 2.5 to 7.3 years. 3 Compared to this, our estimated median time until admission of 3.9 years seems rather short. Differences could be due to multiple factors, such as specific sample characteristics, study designs, and differences between health care systems, such as capacity of and access to long‐term care services. For example, we identified people by a dementia diagnosis recorded in FP electronic records. Because FPs tend to be reluctant to assess for the dementia diagnosis at an early stage, or to refer, 26 we may have missed milder dementia cases, which might explain the shorter time to institutionalization.
Predictors of (time to) institutionalization in people with dementia have been studied widely, and summarized in some systematic reviews. 7 , 27 , 28 Older age, white race, being unmarried, living alone, and dementia severity significantly increased the risk of institutionalization in previous studies. 27 In line with this, we identified older age and living alone as significantly associated with shorter time to institutionalization. Consistent with two German studies, 10 , 29 we found that women were at higher hazard to be admitted than men. Likely, at older age, males often still have a spouse who can care for them, which may delay institutionalization, whereas women more often live alone at older ages. Having a native Dutch background, receiving home care and having a dementia medication prescription were other significant factors of shorter time to institutionalization. We speculate that native Dutch people are better able to access long‐term care, and admissions are more acceptable for their families. Home care is a probable indicator of peoples’ dependency and therefore associated with increased risk of institutionalization. Unexpectedly, being (pre) frail was associated with longer time to institutionalization. Frail people may have died before institutionalization, although we used a competing risk approach that accounts for this. We hypothesize that frail people will be seen on a more regular basis and the care offered may be better reviewed by their FP. This may help in the planning and delivery of services and enable them to live longer in the community. Previous studies with similar findings also hypothesized that highly dependent people are perhaps well supported by informal caregivers and community‐based services, which may delay institutionalization. 4 , 10
Finally, we found that people who were prescribed dementia medication in or before the year of diagnosis recording had an increased risk of being institutionalized earlier, whereas people who received a prescription after the year of diagnosis had a decreased risk of institutionalization, compared to people without prescribed dementia medication. An explanation could be that dementia medication is usually prescribed to people in early stages of dementia, whereas people who had already started with dementia medication at the first time their diagnosis was recorded by the FP were later in the disease trajectory. It may also indicate a registration artifact, indicating that people with prescribed dementia medication in or before the year of diagnosis recording had already been diagnosed with dementia for a longer time, but their FP had not yet documented this in the record.
4.2.2. Survival
Our median estimated survival time of 5.0 years falls within the estimated ranges reported by two earlier reviews 11 , 12 (between 1.1 to 8.5 years and 3.2 to 6.6 years). Previously, Rait et al. (2010) performed the only study with a large number of incident dementia cases in a primary care record database. 13 Their estimated survival for age groups that differed from the age categories used in our study and without making distinction between men and women, makes it difficult to compare these with our results. Similar to this and other studies 12 we found that people with dementia were at higher hazard to live for shorter duration than people without dementia. Once people with dementia were institutionalized, their survival was longer compared to controls. This could indicate that, at the time of institutionalization, people with dementia are physically relatively healthy compared to people without dementia and might therefore live longer once admitted.
Older age, being male, having a native Dutch background, receiving home care, hyperpolypharmacy, and frailty were associated with shorter survival time in people with dementia. Although previous studies have differed greatly in the examination of predictors of mortality in dementia, increased age and male gender have been associated consistently with reduced survival, and our results contribute to this evidence. The association with migrant status is less conclusive, but studies from the United States found that African Americans and Latinos with dementia had significantly lower risks of mortality than Caucasians did (eg 30 ). We also concluded that people with dementia with a Surinamese, Antillean, or Aruban migration background survived longer than native Dutch people with dementia. The underlying reason may be that before immigrating for reasons such as study or employment they already enjoyed good health (the so‐called "healthy immigrant effect"). 31 Frailty and hyperpolypharmacy increased the mortality risk, which indicates that it is important to give particular attention to these vulnerable subgroups of patients. Home care utilization was associated with decreased survival and is probably a strong indicator of disability. In addition, people who were prescribed dementia medication after the year the dementia diagnosis was first recorded in primary care had a prolonged time to death. We hypothesize that this is because these medications are mainly prescribed in earlier disease stages and in younger patients.
4.3. Strengths and limitations
We state that this study enhances understandings of care trajectories, prognosis of people with dementia, and the factors associated with these, by using real world data and thereby overcoming important methodological limitations of previous studies. We were able to analyze a follow‐up duration of up to 6 years after diagnosis for institutionalization and up to 9 years for mortality. In contrast with earlier studies, we could use an unbiased population derived from routinely recorded data from large numbers of family practices. We followed trajectories from the time the diagnosis was first recorded by their FP, who is generally the first contact of care. In addition, we compared data of people with dementia with data of a matched control group without a dementia diagnosis or cognitive impairments. Finally, there were no missing outcomes, as we used continuous data from national registries.
Nevertheless, there were also limitations. Our sample consisted of people identified as having dementia from recordings in the medical records of FPs. Dementia remains poorly recognized in primary care, and FPs are reluctant to record the diagnosis. 26 , 32 , 33 Underreporting of dementia diagnoses in family practice records concerns especially early stages. Although, this identification is likely to have a low sensitivity, the recorded dementia diagnoses are likely to be accurate (ie, high specificity). 33 , 34 The diagnosis is usually made by secondary care specialists and communicated with the FP, which may lead to a registration delay of several weeks or months. An earlier Dutch study estimated a time lag of 21 months between first symptoms and recorded dementia diagnosis by FPs. 35 We therefore emphasize that our data reflect the time from dementia diagnosis as recorded in FP records until institutionalization and death.
With regard to risk factors of institutionalization and death, no information was available on type or severity of dementia, rate of decline, behavioral problems and caregiver distress, whereas these have found to be important factors in predicting these outcomes. 3 , 12 , 27 , 28 , 36 In The Netherlands, FPs do not (yet) structurally record this information in a standardized way. If present in the electronic health records systems at all, this information will be hidden in free text fields and letters from other health care professionals, which makes it difficult or even impossible to retrieve for research purposes. We expect that including this type of information would improve our understanding of care trajectories. At the same time, however, this would also increase the administrative burden for family physicians. Linking FP electronic health records with other relevant data sources such as, for example, records from memory clinics might be an alternative source of information, but only for patients visiting those clinics. Despite these limitations, our study shows that the linkage of electronic health records and administrative data can be a useful alternative for costly and time‐consuming prospective studies with primary data collection, provided these data sets are available for research purposes and of sufficient quality. In many countries this is not the case, and our study could be seen as an incentive to provide better opportunities for the re‐use of health and administrative data for research purposes.
4.4. Conclusion and implications
This study is the first to estimate the time that people with dementia live at home and survive from the moment a diagnosis is first recorded in primary care based on population‐based routine care data. The availability of registries in The Netherlands, their linkability, and the fact that Statistics Netherlands brings them together and serves as a trusted third party, provides tremendous possibilities to answer important questions for clinical practice and policy about time to institutionalization and time to death in people with dementia. FPs can use the gender‐ and age‐specific estimations from this study to better inform patients and family caregivers about a probable prognosis when recording the dementia diagnosis in their record. These estimations are also highly relevant for care planning by policymakers. The predictive effect of the risk factors in our study can be used to more precisely assess the risk of institutionalization and potentially link those at higher risk to appropriate services. Results can also be used as a basis for monitoring effects of health care policies on transitions. For this, structural use of population‐based routine care data is needed. If policy efforts or interventions are able to increase the time people with dementia can spend with their families in their own environment, this may improve their quality of life.
CONFLICT OF INTEREST
None declared.
FUNDING SOURCES
This work was supported by the Netherlands Organization for Health Research and Development (ZonMw) [grant number 733050403].
ACKNOWLEDGMENT
This study has been approved according to the governance code of Nivel Primary Care Database, under number NZR‐00315.063.
APPENDIX 1.
1.1.
Table A1.
Median time to institutionalization (in years) in people with dementia and matched control persons, for men and women of different age groups
| Persons with dementia (n = 9230) | Matched controls (n = 24,624) | |||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| Age | ||||
| Under 65 | n/a | n/a | n/a | n/a |
| 65‐74 | 5.1 | 6.2 | n/a | n/a |
| 75‐84 | 3.3 | 4.6 | n/a | n/a |
| 85 and above | 2.3 | 2.6 | n/a | n/a |
| Overall | 3.4 | 4.8 | n/a | n/a |
n/a: the median time could not be calculated, as the cumulative incidence curve remained below 50%, indicating that <50% of the persons were admitted at the end of the study period.
Table A2.
Median time to death (in years) in people with dementia and matched control persons, for men and women of different age groups
| Persons with dementia (n = 9230) | Matched controls (n = 24,624) | |||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| Age | ||||
| Under 65 | 9.4 | 8.3 | n/a | n/a |
| 65‐74 | 8.0 | 6.4 | n/a | n/a |
| 75‐84 | 5.7 | 4.3 | n/a | 7.9 |
| 85 and above | 3.8 | 2.7 | 5.3 | 4.1 |
| Overall | 5.4 | 4.4 | 9.7 | 9.1 |
n/a: the median time could not be calculated as the survival curve did not cross 50%, indicating that >50% of the persons had died at the end of the study period.
APPENDIX 2.
2.1.
Table B1.
Place of death of people with dementia and matched controls
| Persons with dementia (n = 6020) | Matched controls (n = 7929) | |||
|---|---|---|---|---|
| n | % | n | % | |
| At home | 886 | 14.7 | 2812 | 35.5 |
| Hospital | 698 | 11.6 | 2199 | 27.8 |
| Institution a | 4191 | 69.7 | 2290 | 28.9 |
| Other/unknown | 242 | 4.0 | 618 | 7.8 |
Nursing home, residential care facility, psychiatric hospital.
Joling KJ, Janssen O, Francke AL, et al. Time from diagnosis to institutionalization and death in people with dementia. Alzheimer's Dement. 2020;16:662–671. 10.1002/alz.12063
REFERENCES
- 1. WHO . Dementia . 2017. [cited 27 June 2018]; Available from: http://www.who.int/mediacentre/factsheets/fs362/en/.
- 2. WHO . Dementia: number of people affected to triple in next 30 years . 2017. [cited 27 June 2018]; Available from: http://www.who.int/mediacentre/news/releases/2017/dementia-triple-affected/en/.
- 3. Luppa M, Luck T, Brähler E, König HH, Riedel‐Heller SG. Prediction of institutionalisation in dementia. A systematic review. Dement Geriatr Cogn Disord. 2008;26(1):65‐78. [DOI] [PubMed] [Google Scholar]
- 4. Belger M, Haro JM, Reed C, et al. Determinants of time to institutionalisation and related healthcare and societal costs in a community‐based cohort of patients with Alzheimer's disease dementia. Eur J Health Econ. 2019;20:343‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brodaty H, Connors MH, Xu J, Woodward M, Ames D. PRIME study group. Predictors of institutionalization in dementia: a three year longitudinal study. J Alzheimers Dis. 2014;40(1):221‐226. [DOI] [PubMed] [Google Scholar]
- 6. Eska K, Graessel E, Donath C, Schwarzkopf L, Lauterberg J, Holle R. Predictors of institutionalization of dementia patients in mild and moderate stages: a 4‐year prospective analysis. Dement Geriatr Cogn Dis Extra. 2013;3(1):426‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luppa M, Luck T, Brähler E, König HH, Riedel‐Heller SG. Prediction of institutionalisation in dementia. A systematic review. Dement Geriatr Cogn Disord. 2008;26(1):65‐78. [DOI] [PubMed] [Google Scholar]
- 8. Luppa M, Riedel‐Heller SG, Stein J, et al. Predictors of institutionalisation in incident dementia–results of the German study on ageing, cognition and dementia in primary care patients (AgeCoDe study). Dement Geriatr Cogn Disord. 2012;33(4):282‐288. [DOI] [PubMed] [Google Scholar]
- 9. Rongve A, Vossius C, Nore S, Testad I, Aarsland D. Time until nursing home admission in people with mild dementia: comparison of dementia with Lewy bodies and Alzheimer's dementia. Int J Geriatr Psychiatry. 2014;29(4):392‐398. [DOI] [PubMed] [Google Scholar]
- 10. Runte R. Predictors of institutionalization in people with dementia: a survey linked with administrative data. Aging Clin Exp Res. 2018;30(1):35‐43. [DOI] [PubMed] [Google Scholar]
- 11. Brodaty H, Seeher K, Gibson L. Dementia time to death: a systematic literature review on survival time and years of life lost in people with dementia. Int Psychogeriatr. 2012;24(7):1034‐1045. [DOI] [PubMed] [Google Scholar]
- 12. Todd S, Barr S, Roberts M, Passmore AP. Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatry. 2013;28(11):1109‐1124. [DOI] [PubMed] [Google Scholar]
- 13. Rait G, Walters K, Bottomley C, Petersen I, Iliffe S, Nazareth I. Survival of people with clinical diagnosis of dementia in primary care: cohort study. Bmj. 2010;341:c3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boersma‐van Dam E, Weesie Y, Hek K, et al. Zorg door de huisarts. Zorg door de huisarts ‐ Nivel Zorgregistraties eerste lijn: Jaarcijfers 2017 en trendcijfers 2011‐2017 2019 03‐12‐2018 [cited 2019 21‐02‐2019]; Available from: www.nivel.nl/nl/zorgregistraties-eerste-lijn/aantal-patienten-met-minimaal-1-contact-met-de-huisartsenpraktijk.
- 15. Cardol M, van Dijk L, de Jong JD, de Bakker D, Westert GP. Huisartsenzorg: wat doet de poortwachter? Tweede Nationale Studie naar ziekten en verrichtingen in de huisartspraktijk. (Second Dutch National Survey on General Practice: Care by the general practitioner: what does the gatekeeper do?). Utrecht/Bilthoven. 2004. [Google Scholar]
- 16. NIVEL . NIVEL Primary Care Database . [cited 2019 07‐06‐2019]; Available from: https://www.nivel.nl/en/dossier/nivel-primary-care-database.
- 17. Schweikardt C, Verheij RA, Donker GA, Coppieters Y. The historical development of the Dutch Sentinel General Practice Network from a paper based into a digital primary care monitoring system. J Public Health. 2016;24(6):18. [Google Scholar]
- 18. Lamberts H, Wood M. International classification of primary care (ICPC). Oxford: Oxford University Press; 1987. [Google Scholar]
- 19. Verheij RA, Curcin V, Delaney BC, McGilchrist MM. Possible sources of bias in primary care electronic health record data use and reuse. J Med Internet Res. 2018;20(5):e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van der Bij S, Verheij RA. Pay for performance scheme 2013 led to improvement in EHR data recording [Inzet variabiliseringsgelden 2013 leidt tot belangrijke verbetering EPD]. SynthesHIS. 2013;4(12):2. [Google Scholar]
- 21. Drubbel I, de Wit NJ, Bleijenberg N, Eijkemans RJ, Schuurmans MJ, Numans ME. Prediction of adverse health outcomes in older people using a frailty index based on routine primary care data. J Gerontol A Biol Sci Med Sci. 2013;68(3):301‐308. [DOI] [PubMed] [Google Scholar]
- 22. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738‐743. [DOI] [PubMed] [Google Scholar]
- 23. Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community‐dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004;59(12):1310‐1317. [DOI] [PubMed] [Google Scholar]
- 24. Song X, Mitnitski A, Rockwood K. Prevalence and 10‐year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681‐687. [DOI] [PubMed] [Google Scholar]
- 25. Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58(4):783‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009;23(4):306‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cepoiu‐Martin M, Tam‐Tham H, Patten S, Maxwell CJ, Hogan DB. Predictors of long‐term care placement in persons with dementia: a systematic review and meta‐analysis. Int J Geriatr Psychiatry. 2016;31(11):1151‐1171. [DOI] [PubMed] [Google Scholar]
- 28. Gaugler JE, Yu F, Krichbaum K, Wyman JF. Predictors of nursing home admission for persons with dementia. Med Care. 2009;47(2):191‐198. [DOI] [PubMed] [Google Scholar]
- 29. Schulze J, van den Bussche H, Kaduszkiewicz H, Koller D, Hoffmann F. Institutionalization in incident dementia cases in comparison to age‐ and sex‐ matched controls: a 5‐year follow‐up from Germany. Soc Psychiatry Psychiatr Epidemiol. 2015;50(1):143‐151. [DOI] [PubMed] [Google Scholar]
- 30. Mehta KM, Yaffe K, Pérez‐Stable EJ, et al. Race/ethnic differences in AD survival in US Alzheimer's disease centers. Neurology. 2008;70(14):1163‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kennedy S, KiddEmail MP, McDonald JT, Biddle N. The healthy immigrant effect: patterns and evidence from four countries. J Int Migr Integra. 2015;16(2):317‐332. [Google Scholar]
- 32. Mitchell AJ, Meader N, Pentzek M. Clinical recognition of dementia and cognitive impairment in primary care: a meta‐analysis of physician accuracy. Acta Psychiatr Scand. 2011;124(3):165‐183. [DOI] [PubMed] [Google Scholar]
- 33. van den Dungen P, van Marwijk HW, van der Horst HE. The accuracy of family physicians' dementia diagnoses at different stages of dementia: a systematic review. Int J Geriatr Psychiatry. 2012;27(4):342‐354. [DOI] [PubMed] [Google Scholar]
- 34. van Hout H, Vernooij‐Dassen M, Poels P, Hoefnagels W, Grol R. Are general practitioners able to accurately diagnose dementia and identify Alzheimer's disease? A comparison with an outpatient memory clinic. Br J Gen Pract. 2000;50(453):311‐312. [PMC free article] [PubMed] [Google Scholar]
- 35. van Hout HP, Vernooij‐Dassen MJ, Stalman WA. Diagnosing dementia with confidence by GPs. Fam Pract. 2007;24(6):616‐621. [DOI] [PubMed] [Google Scholar]
- 36. Garcia‐Ptacek S, Farahmand B, Kåreholt I, Religa D, Cuadrado ML, Eriksdotter M. Mortality risk after dementia diagnosis by dementia type and underlying factors: a cohort of 15,209 patients based on the Swedish Dementia Registry. J Alzheimers Dis. 2014;41(2):467‐477. [DOI] [PubMed] [Google Scholar]


