INTRODUCTION
COVID-19 is a multifaceted disease and respiratory failure is a common manifestation. Interestingly, several reports are suggesting that many patients with COVID-19 pneumonia seem to present less respiratory effort and experience less dyspnea when exposed to acute respiratory failure and hypoxemia, a phenomenon called “silent,” “happy,” or even “apathetic” hypoxemia. We present a personal view exploring potential pathways associated with this intriguing clinical feature. We also suggest that “nondyspnogenic acute hypoxemic respiratory failure” is a more accurate terminology to describe this clinical finding in COVID-19, as hypoxemia may not be the only trigger for dyspnea in patients with pneumonia associated with respiratory failure.
Acute hypoxemic respiratory failure is a common clinical manifestation in patients with pneumonia caused by the new severe acute respiratory coronavirus 2 (SARS-CoV-2) infection and is a marker of in-hospital mortality (1, 2). The autonomic nervous system orchestrates a complex respiratory and cardiovascular response during a hypoxemic stress. Besides the lung and cardiovascular manifestations documented in patients with the new coronavirus disease 2019 (COVID-19) (3), there is growing evidence suggesting that SARS-CoV-2 is a neurotropic virus and may invade the peripheral and central nervous system (CNS) by several routes, with widespread brain injury, including the respiratory and cardiovascular autonomic centers (4, 5).
In clinical practice, acute hypoxemic respiratory syndromes, such as those caused by bacterial or viral pneumonia, are generally accompanied by dyspnea sensation (6–8). In a cohort of 395 patients with community-acquired pneumonia, 94% of those with severe pneumonia presented with dyspnea (7). Interestingly, several anecdotal reports are suggesting that patients with COVID-19 pneumonia have an atypical nondyspnogenic response to acute hypoxemic respiratory failure (9–11). Some argue that these patients present with less respiratory effort and experience less dyspnea even when exposed to critical hypoxemia, a phenomenon called “silent,” “happy,” or even “apathetic” hypoxemia (11–13).
The term “silent hypoxemia” has been criticized because dyspnea is a complex and multifactorial symptom not only dependent on systemic hypoxemia (14). Previous studies in healthy humans suggest that hypoxemia per se is generally well tolerated and hypoxemia associated with acute respiratory failure may be only a marker of a more complex respiratory pathophysiology and not the determinant cause of dyspnea (15–17). Moreover, in controlled human experiments, respiratory effort is not necessarily associated with dyspnea sensation (18). Therefore, in this review, we prefer to use the term nondyspnogenic acute hypoxemic respiratory failure in COVID-19 pneumonia to describe the apparently comfortable status of patients with COVID-19, despite marked acute hypoxemic respiratory failure (9, 10).
Nondyspnogenic acute hypoxemic respiratory failure is not an expected feature in clinical practice when assisting patients with pneumonia. Conversely, nondyspnogenic respiratory failure is more common in chronic conditions, such as chronic pulmonary obstructive disease and pulmonary fibrosis, and can also be seen in high altitude sickness (19). The limited understanding of the complex mechanisms involved in dyspnea sensation and of the neuropathology associated with SARS-CoV-2 infection is a barrier to explain why patients with severe hypoxemia may present to the emergency department without any physical discomfort and almost asymptomatic. Two initial hypotheses have been speculated to explain the installation of nondyspnogenic acute hypoxemic respiratory failure in these circumstances: the association of iso/hypocapnia, preventing the uncomfortable feelings of air hunger (19); and the maintenance of pulmonary mechanics (total dead space, lung compliance, and resistance) in near-normal range in the first stages of respiratory failure, thus not increasing significantly the respiratory effort (19, 20).
Although both mechanisms are biologically plausible, they fail to address why acute respiratory failure in COVID-19 pneumonia seems to be peculiarly undersensed by these patients. To integrate the current knowledge regarding SARS-CoV-2 infection with the peculiar nondyspnogenic acute hypoxemic respiratory failure in COVID-19 pneumonia, we hypothesize a different mechanism related to an early and complex interaction between SARS-CoV-2 infection and the peripheral and central nervous system disturbing the activation of brain regions responsible for dyspnea sensation during acute hypoxemic respiratory failure.
DYSPNEA SENSATION DURING HYPOXEMIC RESPIRATORY FAILURE
The conscious sensation of dyspnea during hypoxemia is a complex defense response and has not been completely elucidated. It may be triggered not only by hypoxemia but also by different other stimuli such as hypercapnia, increased sense of effort, stimulation of irritant receptors in airways, and dynamic airway compression (6).
In typical cases, the hypoxemic stimulus triggers peripheral chemoreceptors located at the carotid and aortic bodies. This information advances through the glossopharyngeal and vagus nerves, reaching the nucleus tractus solitarius (NTS) in the brain stem (6). In the NTS, it activates second-order neurons, which receives the chemoreceptor inputs to trigger an increase in breathing activity. In this process, the increase in breathing output caused by peripheral chemoreceptor stimulation is seen as a resultant of excitatory drives to the ventral respiratory column neurons (21). Secondarily, the hyperventilatory response activates mechanoreceptors (localized primarily in the respiratory muscles) and lung receptors (localized in the larynx, trachea, and lungs) via vagal C-fibers in the lung (“J” receptors), which also convey this information to the NTS. Systemic CO2 levels influence all these afferents. Likewise, the increased levels of CO2/H+ directly activate central chemoreceptors located at the lateral aspect of the ventral medulla. The primary excitatory input to VRC neurons is seen as originating from the chemosensitive neurons located in the retrotrapezoid nucleus. Signals from the retrotrapezoid nucleus will activate glutamatergic receptors in the ventral respiratory column triggering breathing activity (21, 22). It is also important to consider that the chemoreceptor information ascends by a thalamocortical pathway to the viscerosensory insular cortex and is translated into efferent information perceived as dyspnea (23). In summary, the neural basis of dyspnea involves activation of peripheral and central chemosensors, activation of mechanoreceptors and lung receptors, integration of all these signals at the brain stem, and a final activation of forebrain and cortical areas, such as the limbic system and the somatosensory cortex (Fig. 1).
Figure 1.
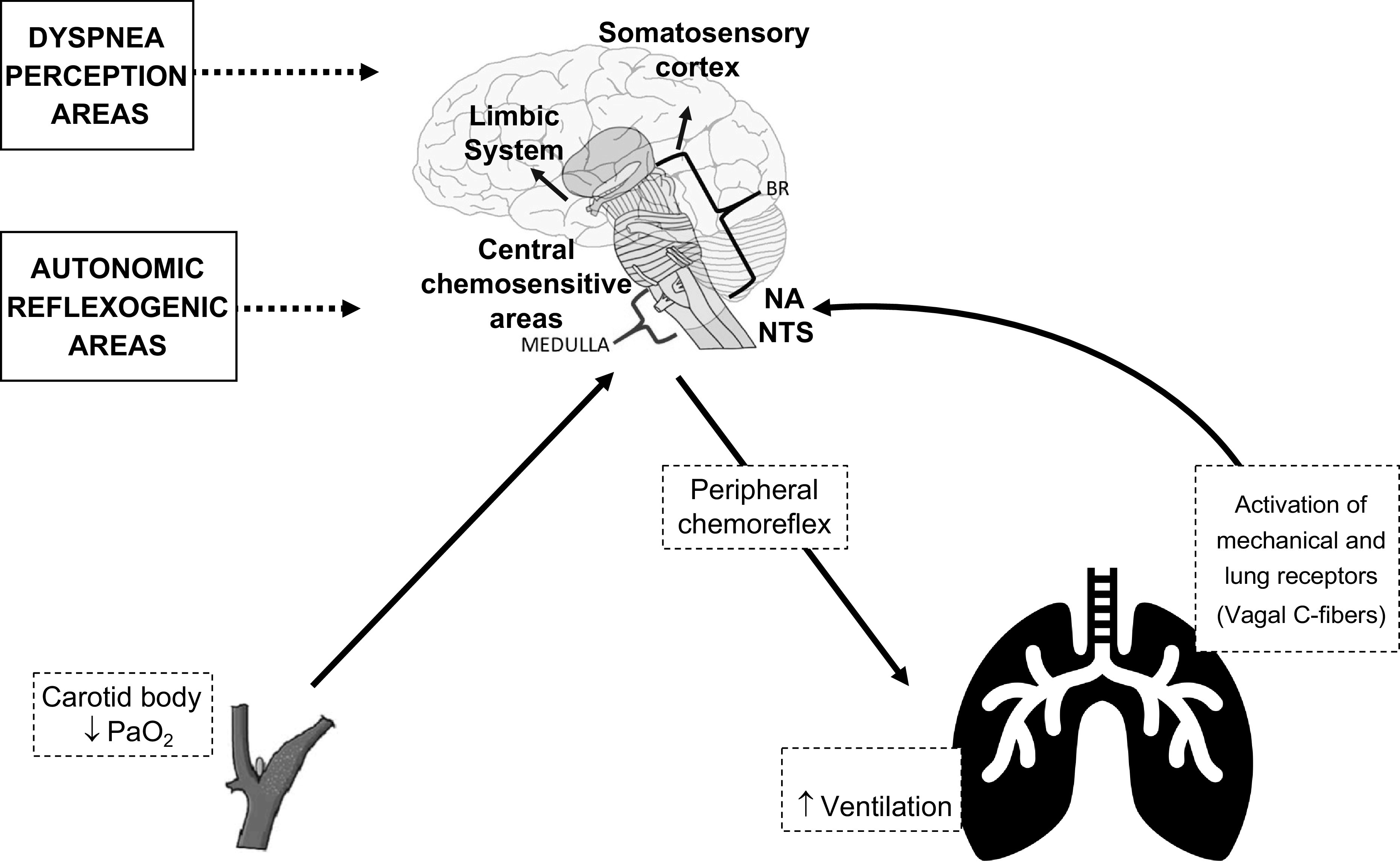
Overview of the physiological autonomic response to systemic hypoxemia. The neural basis of dyspnea perception involves activation of peripheral (carotid body and aortic bodies) and central chemosensors, activation of mechanical and lung receptors (vagal C-fibers), increase in ventilation, integration of all these signals at the brain stem, and a final activation of forebrain and cortical areas, such as the limbic system and the somatosensory cortex.
Although the sensation of dyspnea requires multiple integrated elements, it seems that the primary center in the brain stem receives viscerosensory information (i.e., the NTS) and plays a key role in the control of cortical areas. In human studies, the bilateral blockage of the vagus and glossopharyngeal nerves blunts the ventilatory response as well as the breathing discomfort elicited by hypercapnic stimulus (24, 25). Vagal C-fibers are known to mediate dyspnea associated with pneumonia and inflammatory airway disease through afferent mechanoreceptors and chemosensitive dyspnogenic sensors, further reinforcing the potential role of the vagal nerve in symptom decoding (6, 23, 26). Similarly, it is postulated that damage of both vagal and glossopharyngeal nerves and the NTS induced by Trypanosoma cruzi may explain the reduced central chemosensitivity to hyperoxic hypercapnia observed in patients with Chagas disease (27). This fact points out to the possibility of an infectious disease dysregulating the chemosensory control of breathing.
A NEURAL MECHANISM FOR NONDYSPNOGENIC ACUTE HYPOXEMIC RESPIRATORY FAILURE IN COVID-19 PNEUMONIA
Although COVID-19 was first defined as an acute respiratory disease (1), there was soon robust evidence of an early insult to the peripheral and the central nervous system. This was demonstrated by the sudden onset of olfactory and gustatory dysfunctions in these patients or by other neurological conditions that appeared even before the respiratory symptoms (28–31). Moreover, viral particles were also found in neural and capillary endothelial cells from frontal lobe tissue obtained by autopsy analyses in older adults (32).
SARS-CoV-2 may access the central nervous system by direct and indirect pathways (33). One of the direct mechanisms involves a retrograde neuronal invasion through the olfactory nerve and olfactory bulb neurons. This entry route is supported by early-onset anosmia in several patients (30). The virus can affect peripheral nerves, such as the trigeminal nerve, or sensory fibers of the vagus nerve, accessing the CNS via transsynaptic connections, and spreading to the brain stem and cortex (34, 35).
Evidence from the first SARS-CoV analyses reports that these neurotropic viruses may cause inflammation and demyelination in the NTS and nucleus ambiguous and can disseminate to the cortex up to 7 days from first nasal cavity infection (33). These lesions can be mediated by direct virus injury or by a systemic inflammatory response (the “cytokines storm”), which breaches the blood-brain barrier and facilitates neuroinvasion (34, 36). In normal activation states, the NTS receives sensory information from the mechanoreceptors and chemoreceptors in the lung and respiratory tracts, whereas the efferent fibers from the nucleus ambiguous and the NTS itself provide innervation of airway smooth muscle. However, the blunted afferent signals could modify the dyspnea sensation, leading patients with COVID-19 to present with normal breathing effort despite the hypoxemia caused by diffuse interstitial pulmonary damage (37). Moreover, we speculate that interleukin-6 (IL-6) and serotonergic system abnormalities at the medulla elicited by the “cytokines storm” may further blunt chemosensitivity and dyspnea perception (38).
SARS-CoV-2 could also reach the brain via general circulation and cross the blood-brain barrier, which is responsible for protecting the brain against infections. Indeed, the presence of viral particles has been reported individually and in small vesicles of endothelial cells of the blood-brain barrier in postmortem brain analysis of patients with COVID-19 (32). However, one cannot exclude the possibility of the virus reaching the brain stem via systemic circulation without necessarily crossing the blood-brain barrier. This direct access would occur at the level of the area postrema and median eminence (forebrain), which are circumventricular organs characterized by a lack of blood-brain barrier (39).
A potential contributor for the rapid neuroinvasion and CNS injury caused by SARS-CoV-2 is the angiotensin-converting enzyme 2 (ACE2) protein, which is commonly expressed in many human surfaces, such as heart, vessels, kidney, brain, and skeletal muscles (40). ACE2 proteins were already found in CNS under physiological conditions (41). Interestingly, recent studies demonstrate that they might also be receptors for SARS-CoV-2 infection (42). SARS-CoV-2 spike protein could link to the ACE2 receptor expressed in the capillary endothelium, allowing the virus to cross the blood-brain barrier and damage the central nervous system (43, 44). Unfortunately, the functional role of ACE2 proteins on carotid bodies in peripheral chemosensitivity remains elusive, precluding a pathophysiological inference regarding SARS-CoV-2, ACE2 receptors, and peripheral chemosensitivity.
Therefore, regardless of the proposed mechanism for central nervous system injury, it is reasonable to assume that the virus invasion can play a role in this injury and the subsequent dysregulation of the autonomic system, peripheral nerves, and brain stem, leading to a disruption of the respiratory autonomic control. These early and prominent neural lesions, even before the infection reaches the lower respiratory tract, might explain why nondyspnogenic acute hypoxemic respiratory failure prevails in these patients once the pulmonary damage occurs.
A synthesis of the proposed working hypothesis is demonstrated in Fig. 2. We believe SARS-CoV-2 may reach and injure the CNS by several routes (via transsynaptic transfer by the olfactory nerve or via the blood-brain barrier). The olfactory nerve pathway would occur early in disease natural history, probably a few days after nasal cavity invasion, and even in the absence of respiratory symptoms or systemic hypoxemia (“prehypoxemic” stage), spreading to the brain stem and viscerosensory cortex. Once SARS-CoV-2 reaches the systemic circulation, it could also enter the CNS by blood dissemination. At this point, the respiratory center would already be damaged and incapable of providing an effective response once the O2 levels decrease. With COVID-19 progression (“hypoxemic” stage), structural lung injury and the systemic action of cytokines, particularly IL-6, would further disturb peripheral chemoreceptors. In addition, considering the neurotropism of SARS-CoV-2, the severe alveolar inflammation associated with COVID-19 pneumonia may further damage pulmonary vagal C-fibers compromising dyspnogenic afferent sensors and contributing to the dysregulation of the autonomic respiratory control. Therefore, in our point of view, the underperception of dyspnea-associated acute hypoxemic respiratory failure would represent the ultimate clinical construct of these prominent neurological lesions in patients with COVID-19.
Figure 2.
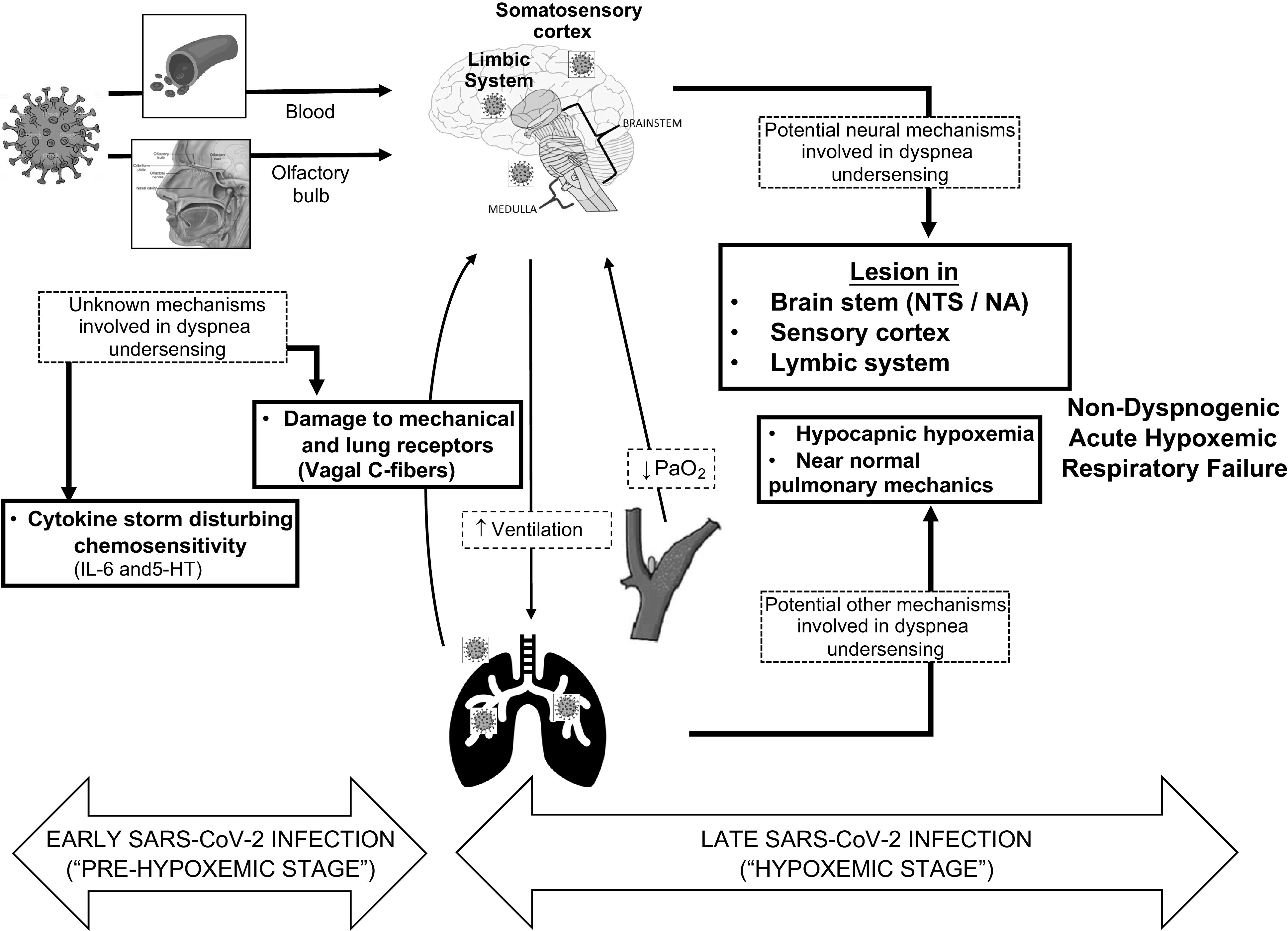
Proposed mechanisms by which SARS-CoV-2 neuroinvasion can play a role in the dysregulation of the autonomic system, peripheral nerves, and brain stem, leading to disruption of the respiratory autonomic control and explaining nondyspnogenic acute hypoxemic respiratory failure observed in clinical practice. IL-6, interleukin-6; NA, nucleus ambiguous; NTS, nucleus tractus solitarius; PaO2, partial pressure of oxygen; 5-HT, 5-hydroxytryptamine (serotonin).
POTENTIAL CLINICAL IMPLICATIONS
Dyspnea is a protective symptom against abnormalities in gas exchange. In COVID-19, dyspnea is the main symptom responsible for seeking emergency medical assistance (42). Hence, the association of hypoxemic respiratory failure with a blunted sensorial alert may have clinical implications.
Whether anosmia is closely associated with nondyspnogenic acute hypoxemic respiratory failure remains to be established. Unlike other viral syndromes, olfactory dysfunction is an early feature of COVID-19 and not often associated with nasal symptoms, which supports the hypothesis of sensory neural loss as the mainstay of anosmia pathophysiology rather than a conductive mechanism (44, 45). Nevertheless, this potential association would need further endorsement by experimental and clinical studies.
Like silent myocardial infarction, nondyspnogenic acute hypoxemic respiratory failure in COVID-19 pneumonia may also be associated with delayed presentation to the hospital and unfavorable outcomes. It remains uncertain whether patients with COVID-19 and a nondyspnogenic response would have worse prognosis than those without this peculiar clinical feature. In one study including non-COVID-19 patients, the blunted perception of dyspnea by older adults was associated with higher mortality and medical care costs (45).
Considering the hypothesis that nondyspnogenic acute hypoxemic respiratory failure is a marker of cerebral and/or central autonomic dysfunction, it is tempting to speculate that this manifestation may be a clinical marker of worse outcomes. Despite the biological plausibility on which we built the grounds for our hypothesis, we acknowledge its speculative nature as a potential field for future research. However, it is safe to conclude from our data that clinicians should be aware of nondyspnogenic acute hypoxemic respiratory failure in COVID-19 and possibly increase surveillance mechanisms (e.g., pulse oximetry monitoring), particularly in high-risk populations (46). Assuming that further studies would confirm the hypothesis that SARS-CoV-2 infection is associated with marked respiratory autonomic dysfunction, we could also expect cardiovascular autonomic dysfunction to participate in the pathophysiology of cardiovascular manifestations, including heart failure and cardiac arrhythmias, and related clinical outcomes. In the long term, it could be reasonable to follow-up patients with history of COVID-19 and monitor autonomic clinical manifestations secondary to CNS injury.
CONCLUSIONS
Nondyspnogenic acute hypoxemic respiratory failure is emerging as a clinical manifestation of COVID-19. Although we still need robust empirical data to confirm that nondyspnogenic acute hypoxemic respiratory failure is a clinical entity and it is more frequent in COVID-19 than in bacterial or other types of viral pneumonia, several clinical observations have suggested that patients with COVID-19 seem to be more comfortable with hypoxemia than patients with other pulmonary syndromes. Further experimental and clinical studies may clarify which is the best nomenclature to describe this condition and its underlying pathophysiology. Nevertheless, based on clinical experience and emerging reports, we believe that patients with acute hypoxemic respiratory failure in COVID-19 pneumonia seem to present with a peculiar blunted dyspnea perception (9, 10, 13, 46). Herein, we presented a speculative personal view reviewing some recent evidence that link SARS-CoV-2 to neuropathological findings that may explain the dysregulation in dyspnea awareness during hypoxemic respiratory failure. Furthermore, in confirming that nondyspnogenic acute hypoxemic respiratory failure is a peculiar finding of COVID-19, we anticipate potential adverse implications of these findings to clinical outcomes in patients with COVID-19. Future investigations will be paramount to clarify the epidemiology, pathophysiology, and clinical aspects of this intriguing clinical feature that emerges from the COVID-19 pandemic.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A.B-F., J.D.S-G., F.B.G., and L.F.D. conceived and designed research; J.A.B-F., J.D.S-G., F.B.G., T.S.M., and L.F.D. analyzed data; J.A.B-F., J.D.S-G., F.B.G., T.S.M., and L.F.D. prepared figures; J.A.B-F., J.D.S-G., F.B.G., T.S.M., and L.F.D. drafted manuscript; J.A.B-F., J.D.S-G., F.B.G., T.S.M., and L.F.D. edited and revised manuscript; J.A.B-F., J.D.S-G., F.B.G., T.S.M., and L.F.D. approved final version of manuscript.
REFERENCES
- 1.Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, Li P, Zhou Y, Lin Y, Duan Q, Luo G, Fan S, Lu Y, Feng A, Zhan Y, Liang B, Cai W, Zhang L, Du X, Li L, Shu Y, Zou H. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect 80: 656–665, 2020. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie J, Covassin N, Fan Z, Singh P, Gao W, Li G, Kara T, Somers VK. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc 95: 1138–1147, 2020. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061–1069, 2020. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 92: 552–555, 2020. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease. JAMA Neurol 77: 1018–1027, 2019. [32469387] doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burki NK, Lee LY. Mechanisms of dyspnea. Chest 138: 1196–1201, 2010. doi: 10.1378/chest.10-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewig S, Ruiz M, Mensa J, Marcos MA, Martinez JA, Arancibia F, Niederman MS, Torres A. Severe community-acquired pneumonia. Am J Respir Crit Care Med 158: 1102–1108, 1998. doi: 10.1164/ajrccm.158.4.9803114. [DOI] [PubMed] [Google Scholar]
- 8.Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, Calverley PM, Gift AG, Harver A, Lareau SC, Mahler DA, Meek PM, O’Donnell DE; American Thoracic Society Committee on Dyspnea. An official American thoracic society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 185: 435–452, 2012. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuglebjerg NJU, Jensen TO, Hoyer N, Ryrsø CK, Lindegaard B, Barrella Harboe Z. Silent hypoxia in patients with SARS CoV-2 infection before hospital discharge. Int J Infect Dis 99: 100–101, 2020. doi: 10.1016/j.ijid.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jouffroy R, Jost D, Prunet B. Prehospital pulse oximetry: a red flag for early detection of silent hypoxemia in COVID-19 patients. Crit Care 24: 4–5, 2020. doi: 10.1186/s13054-019-2709-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ottestad W, Seim M, Mæhlen JO. COVID-19 with silent hypoxemia. Tidsskr Nor Laegeforen 140 (7), 2020. [First published April 11, 2020. Print 2020 May 5.] doi: 10.4045/tidsskr.20.0299. [DOI] [PubMed] [Google Scholar]
- 12.Couzin-Frankel J. The mystery of the pandemic’s ‘happy hypoxia’. Science 368: 455–456, 2020. doi: 10.1126/science.368.6490.455. [DOI] [PubMed] [Google Scholar]
- 13.Wilkerson RG, Adler JD, Shah NG, Brown R. Silent hypoxia: a harbinger of clinical deterioration in patients with COVID-19. Am J Emerg Med 38: 2243.e5–2243.e6, 2020. doi: 10.1016/j.ajem.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med 202: 356–360, 2020. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fatemian M, Robbins PA. Human ventilatory response to CO2 after 8 h of isocapnic or poikilocapnic hypoxia. J Appl Physiol 85: 1922–1928, 1998. doi: 10.1152/jappl.1998.85.5.1922. [DOI] [PubMed] [Google Scholar]
- 16.Rapanos T, Duffin J. The ventilatory response to hypoxia below the carbon dioxide threshold. Can J Appl Physiol 22: 23–36, 1997. doi: 10.1139/h97-003. [DOI] [PubMed] [Google Scholar]
- 17.Steinback CD, Poulin MJ. Cardiovascular and cerebrovascular responses to acute isocapnic and poikilocapnic hypoxia in humans. J Appl Physiol 104: 482–489, 2008. doi: 10.1152/japplphysiol.00553.2007. [DOI] [PubMed] [Google Scholar]
- 18.Demediuk BH, Manning H, Lilly J, Fencl V, Weinberger SE, Weiss JW, Schwartzstein RM. Dissociation between dyspnea and respiratory effort. Am Rev Respir Dis 146: 1222–1225, 1992. doi: 10.1164/ajrccm/146.5_Pt_1.1222. [DOI] [PubMed] [Google Scholar]
- 19.Ottestad W, Søvik S. COVID-19 patients with respiratory failure: what can we learn from aviation medicine? Br J Anaesth 125: e280–e281, 2020. doi: 10.1016/j.bja.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care 24: 198, 2020. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4: 1151–1162, 2014. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron 87: 946–961, 2015. doi: 10.1016/j.neuron.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med 333: 1547–1553, 1995. doi: 10.1056/NEJM199512073332307. [DOI] [PubMed] [Google Scholar]
- 24.Davies SF, McQuaid KR, Iber C, McArthur C, Path M, Beebe DS, Helseth HK. Extreme dyspnea from unilateral pulmonary venous obstruction: demonstration of a vagal mechanism and relief by right vagotomy. Am Rev Respir Dis 136: 184–188, 1987. doi: 10.1164/ajrccm/136.1.184. [DOI] [PubMed] [Google Scholar]
- 25.Guz A, Noble MIM, Widdicombe JG, Trenchard D, Mushin WW. The effect of bilateral block of vagus and glossopharyngeal nerves on the ventilatory response to CO2 of conscious man. Respir Physiol 1: 206–210, 1966. doi: 10.1016/0034-5687(66)90017-X. [DOI] [PubMed] [Google Scholar]
- 26.Undem BJ, Nassenstein C. Airway nerves and dyspnea associated with inflammatory airway disease. Respir Physiol Neurobiol 167: 36–44, 2009. doi: 10.1016/j.resp.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Soares Barreto-Filho JA, Consolim-Colombo FM, Ferreira Lopes H, Martins Sobrinho CR, Guerra-Riccio GM, Krieger EM. Dysregulation of peripheral and central chemoreflex responses in Chagas’ heart disease patients without heart failure. Circulation 104: 1792–1798, 2001. doi: 10.1161/hc4001.097039. [DOI] [PubMed] [Google Scholar]
- 28.Butowt R, Bilinska K. SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem Neurosci 11: 1200–1203, 2020. doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- 29.Chigr F, Merzouki M, Najimi M. Autonomic brain centers and pathophysiology of COVID-19. ACS Chem Neurosci 11: 1520–1522, 2020. doi: 10.1021/acschemneuro.0c00265. [DOI] [PubMed] [Google Scholar]
- 30.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, Antinori S, Galli M. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis 71: 889–890, 2020. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77: 683–690, 2020. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paniz‐Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Sordillo EM, Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). J Med Virol 92: 699–702, 2020. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 87: 18–22, 2020. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, Talbot PJ. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 12: 14–28, 2019. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Weyhern CH, Kaufmann I, Neff F, Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet 395: e109, 2020. doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci 12: 1–15, 2018. doi: 10.3389/fncel.2018.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tassorelli C, Mojoli F, Baldanti F, Bruno R, Benazzo M. COVID‐19: what if the brain had a role in causing the deaths? Eur J Neurol 27: e41–e42, 2020. doi: 10.1111/ene.14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rognum IJ, Haynes RL, Vege Å, Yang M, Rognum TO, Kinney HC. Interleukin-6 and the serotonergic system of the medulla oblongata in the sudden infant death syndrome. Acta Neuropathol 118: 519–530, 2009. doi: 10.1007/s00401-009-0535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaur C, Ling E-A. The circumventricular organs. Histol Histopathol 32: 879–892, 2017. [DOI] [PubMed] [Google Scholar]
- 40.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11: 995–998, 2020. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 41.Li H, Xue Q, Xu X. Involvement of the nervous system in SARS-CoV-2 infection. Neurotox Res 38: 1–7, 2020. doi: 10.1007/s12640-020-00219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrejak C, Blanc F-X, Costes F, Crestani B, Debieuvre D, Perez T, Philippe B, Plantier L, Schlemmer F, Sesé L, Stach B, Uzunhan Y, Zanetti C, Zysman M, Raherison C, Maitre B. Guide for follow-up of patients with SARS-CoV-2 pneumonia management proposals developed by the French-language respiratory medicine society. Rev Mal Respir 37: 505–510, 2020. doi: 10.1016/j.rmr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebihara S, Niu K, Ebihara T, Kuriyama S, Hozawa A, Ohmori-Matsuda K, Nakaya N, Nagatomi R, Arai H, Kohzuki M, Tsuji I. Impact of blunted perception of dyspnea on medical care use and expenditure, and mortality in elderly people. Front Physiol 3: 1–8, 2012. doi: 10.3389/fphys.2012.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lechien JR, Chiesa-Estomba CM, Hans S, Barillari MR, Jouffe L, Saussez S. Loss of Smell and taste in 2013 European patients with mild to moderate COVID-19. Ann Intern Med 173: 672–675, 2020. doi: 10.7326/M20-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, , et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolarryngol 277: 2251–2261, 2020. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman J, Calderón-Villarreal A, Bojorquez I, Vera Hernández C, Schriger DL, Tovar Hirashima E. Excess out-of-hospital mortality and declining oxygen saturation: the sentinel role of emergency medical services data in the COVID-19 crisis in Tijuana, Mexico. Ann Emerg Med 76: 413–426, 2020. doi: 10.1016/j.annemergmed.2020.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]


