Abstract
BACKGROUND:
Cognitive impairment in survivors of blood or bone marrow transplantation (BMT) is well documented. However, to the authors’ knowledge, the clinical relevance of self-endorsed cognitive problems and their relation to objectively assessed cognitive impairment is not known.
METHODS:
The authors assessed cognitive impairment in 378 BMT recipients (median age, 52.2 years, 40% of whom were female and 68% of whom were non-Hispanic white) and 98 healthy controls at 5 predetermined time points: at baseline (before BMT) and at 6 months, 1 year, 2 years, and 3 years after BMT. Self-endorsed cognitive problems were evaluated using the Neuropsychological Impairment Scale (NIS) and correlated with a standardized 2-hour battery of objective cognitive testing at each time point. The authors examined the magnitude of difference in self-endorsed cognitive problems between BMT recipients and healthy controls, and the rate of change in scores over time. Multivariable analyses were used to identify clinical and/or demographic variables associated with self-endorsed cognitive problems. The authors also examined the association between cognitive impairment and returning to work after BMT.
RESULTS:
Compared with healthy controls, BMT recipients endorsed more cognitive problems (P < .001) at all time points, and the rate of change in NIS scores was found to be significantly greater in BMT recipients. Fatigue was associated with greater endorsement of cognitive problems at 1 year after BMT (odds ratio, 4.23; 95% CI, 2.1-8.3 [P < .001]). Overall, there was a statistically significant, modest correlation noted between self-endorsed cognitive problems and objective cognitive impairment (range, 0.401-0.445 [P ≤ .01]). Higher self-endorsed cognitive problems were associated with a 3.7-fold (P = .02) higher odds of not returning to work at 3 years after BMT.
CONCLUSIONS:
The results of the current study demonstrated that self-endorsed cognitive problems can help to identify vulnerable patient subpopulations for detailed cognitive assessment and possible cognitive remediation.
Keywords: blood or bone marrow transplantation (BMT), cancer survivors, cognitive impairment, longitudinal, return to work
INTRODUCTION
Blood or bone marrow transplantation (BMT) is a well-established therapeutic option for patients with hematologic malignancies. Cognitive impairment in survivors of BMT is being increasingly recognized, and may include deficits in memory, attention, executive functioning, and processing speed.1 These deficits persist in the long term in approximately 40% of survivors,1–3 and have the potential to affect social and vocational outcomes, such as difficulty returning to work.3 These cognitive impairments are likely due to a combination of clinical (eg, total body irradiation or high-dose chemotherapy), demographic (eg, age and sex), and premorbid cognitive factors (eg, cognitive reserve) or comorbidities (eg, fatigue).4–6 These findings suggest a need to screen BMT recipients for cognitive impairment, such that targeted interventions can be implemented in a timely fashion.
Objective cognitive assessment using validated neuropsychological measures is the currently accepted modality. However, such assessments are time-intensive and require administration by trained specialists. Subjective assessment of cognitive impairment using validated self-report questionnaires is an alternative that potentially could be used to identify patients for further assessment. Self-report measures can be administered in the clinic or online in the patient’s home. This is especially important for BMT recipients, among whom the high burden of morbidity places logistical challenges in administering objective neuropsychological measures to all individuals.
We sought to examine the relation between self-endorsed cognitive problems and objectively determined cognitive impairment in BMT recipients. We also sought to determine the change in self-endorsed cognitive problems over time when compared with healthy controls and whether there were specific subgroups among BMT recipients who were at a higher risk of self-endorsed cognitive problems. Finally, we aimed to assess the clinical relevance of self-endorsed cognitive problems using the ability to return to work as the primary measure of relevance.
MATERIALS AND METHODS
Participants
BMT recipients were recruited from the City of Hope National Medical Center (City of Hope) between 2005 and 2011. Healthy controls were recruited from the community and included family or friends of the study participants. Study participants were aged ≥18 years and proficient in English. A diagnosis of a neurologic or psychiatric disorder, significant sensory and/or motor impairments, and/or participation in neuropsychological interventions within the prior 6 months precluded participation. The Neuropsychological Impairment Scale (NIS)7 and standardized neuropsychological tests (the results of which were reported in detail elsewhere3) were administered at 5 predetermined time points: baseline (before BMT) and at 6 months, 1 year, 2 years, and 3 years after BMT. Healthy controls were administered the same measures at corresponding time points. To be eligible for this study, participants needed to have completed the standardized neuropsychological and the corresponding NIS tool prior to undergoing BMT. We have provided detailed participation at each subsequent time point in Figure 1. At each time point, BMT recipients also rated their level of fatigue using the Profile of Mood States–Fatigue and Vigor subscales8 or the Fatigue Symptom Inventory9. The median score was used to identify those participants with high versus low fatigue. The Wechsler Abbreviated Scale of Intelligence10 or the Word Reading subtest of the Wide Range Achievement Test–Fourth Edition were administered at the pre-BMT time point as an estimation of cognitive reserve.11 The median score was used to identify those participants with high versus low cognitive reserve. Employment status and returning to work were tracked at each time point.
Figure 1.
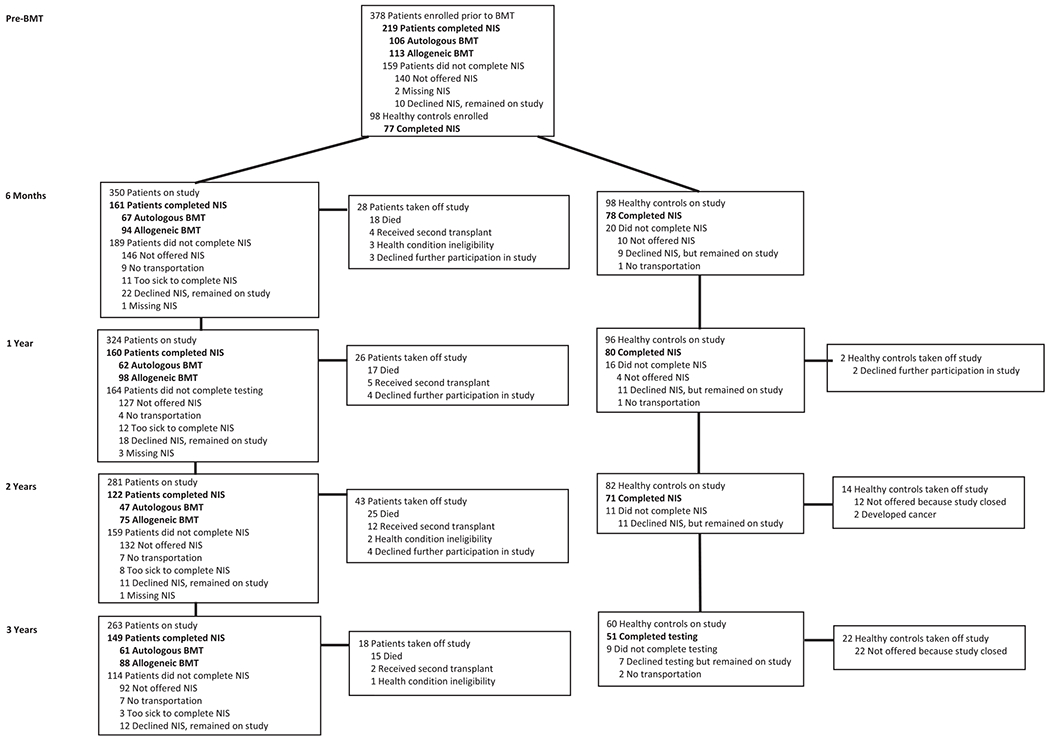
Flow diagram of participation in the study at each time point with completion of the Neuropsychological Impairment Scale (NIS). BMT indicates blood or bone marrow transplantation.
For the BMT recipients, information regarding the primary cancer diagnosis, conditioning regimens, risk of disease recurrence at BMT, type of BMT (autologous vs allogeneic), stem cell source (bone marrow, peripheral blood, or cord blood), post-BMT disease recurrence, and the development of chronic graft-versus-host disease were abstracted from the medical records. Additional demographic data were obtained through a self-report questionnaire. The study was approved by the institutional review board at the City of Hope. Informed consent was provided according to the Declaration of Helsinki.
Neuropsychological Impairment Scale
The NIS is a self-administered, 95-item questionnaire rated on a 5-point scale ranging from 0 (not at all) to 4 (extremely). Participants were asked to determine to what degree the statement applied to them during the past 30 days. The responses were used to obtain a Global Measure of Impairment (GMI) and 7 subscales related to specific areas of cognitive impairment: Critical Items (CRIT), Cognitive Efficiency (COG), Attention (ATT), Memory (MEM), Frustration Tolerance (FRU), Learning-Verbal (L-V), and Academic Skills (ACD). In addition, a subtest, the Subjective Distortion Index (SDI), provided a discrepancy score between the participant’s objective neuropsychological testing performance on the Wechsler Adult Intelligence Scale–Third Edition (WAIS-III) Digit Symbol Coding and Digit Span subscales and his or her self-endorsed cognitive performance (GMI score). For the GMI and each subscale, summary raw scores were transformed to population-based normed T scores. Higher T scores are indicative of greater self-endorsed cognitive problems, with T scores of 50 to 60 indicating mild impairment, T scores of 60 to 70 indicating moderate impairment, and T scores ≥70 indicating severe impairment.7 The NIS has adequate validity and reliability in both neurologic and psychiatric populations.12,13
Neuropsychological Measures
Objective neuropsychological tests administered as part of a larger study (the results of which have been reported else-where3) assessed the following cognitive domains: executive functioning, verbal fluency and speed, processing speed, working memory, auditory memory, and visual memory.
Statistical Analysis
Data were analyzed using SPSS statistical software (version 25.0; IBM Corporation, Armonk, New York). Analyses were conducted for the entire BMT cohort as well as by type of BMT (autologous vs allogeneic).
Relation between self-endorsed cognitive problems and objectively determined cognitive impairment
NIS subscale T scores were correlated at each time point with domain-specific neuropsychological measure T scores that most closely assessed the respective item content (see Supporting Table 1) using within-group first-order partial correlation analyses, controlling for age, sex, educational level, and cognitive reserve. A significance threshold of P ≤ 0.01 was used. GMI, an overall estimate of self-endorsed cognitive problems, was correlated with the Global Deficit Score (GDS)14 summarized from the scores for objective neuropsychological measures.
Magnitude of difference in self-endorsed cognitive problems between BMT recipients and healthy controls
Using the Student t test for continuous variables and the chi-square test for categorical variables, each BMT group (autologous vs allogeneic) was compared with healthy controls with regard to demographic variables. The results revealed differences in age and intelligence quotient (IQ); these were included in the model as covariates, in addition to other variables known to influence cognitive functioning (eg, sex, educational level, and fatigue). At each time point, a multivariate analysis of covariance was performed to examine the relation between groups (healthy controls vs autologous BMT recipients and healthy controls vs allogeneic BMT recipients) for each subscale of the NIS. Significant group interactions were followed by univariate analyses using the Tukey honestly significant difference post hoc test to determine group differences by NIS subscale. To conservatively account for multiple comparisons in addition to the Tukey honestly significant difference, a significance threshold of P ≤ 0.003 was used for comparison between groups.
Change in self-endorsed cognitive problems among BMT recipients over time when compared with healthy controls
Multilevel modeling was used to examine participants’ rates of change in GMI across time. A series of 2-level models were constructed using hierarchical linear modeling (HLM 7). Group membership (autologous and allogeneic BMT vs healthy controls) was examined as a predictor of variability in GMI scores, while including age, sex, educational level, IQ, and fatigue as covariates. These variables, group membership and covariates, were added to the intercept and slope functions to assess rates of change in GMI by group.
Clinical relevance of self-endorsed cognitive problems: association with returning to work
Multivariable Cox regression analysis, adjusted for relevant sociodemographic variables, was used to examine the association between self-endorsed cognitive problems and returning to work to examine the clinical relevance of the NIS.
RESULTS
Participant Characteristics
The demographic and clinical characteristics of the study participants at baseline are described in Table 1. The current study included 378 BMT recipients (178 autologous and 200 allogeneic recipients) and 98 healthy controls at the time of enrollment (Fig. 1). Sex, race and/or ethnicity, educational level, and annual household income did not appear to differ between BMT recipients and healthy controls. BMT recipients had lower cognitive reserve compared with healthy controls (IQ ≥50th percentile: 64.3% for healthy controls vs 47.8% for BMT recipients [P = .006]). The median age at the time of study enrollment for BMT recipients was 50.1 years (range, 18-73 years); 38.9% were female and 67.5% were non-Hispanic white.
TABLE 1.
Demographic and Clinical Characteristics of Study Participants at Baselinea
| All BMT Recipients N = 378 |
Autologous BMT Recipients N = 178 |
Allogeneic BMT Recipients N = 200 |
Heathy Controls N = 98 |
||||
|---|---|---|---|---|---|---|---|
| Characteristic | No (%) | Pb | No. (%) | P | No. (%) | P | No. (%) |
| Mean age (range), y | 50.1 (18-73) | .51 | 52.0 (18-73) | .94 | 48.3 (19-71) | .22 | 51.1 (19-72) |
| Sex | |||||||
| Female | 151 (39.9) | .28 | 71 (39.9) | .31 | 80 (40.0) | .33 | 45 (45.9) |
| Ethnicity | |||||||
| Non-Hispanic white | 256 (67.7) | .67 | 128 (71.9) | .29 | 128 (64.0) | .83 | 64 (65.3) |
| Educational level | |||||||
| <College degree | 183 (48.4) | .06 | 85 (47.8) | .12 | 98 (49.0) | .07 | 37 (37.8) |
| Annual household income | |||||||
| <$50,000 | 102 (27.0) | 43 (24.2) | 59 (29.5) | 35 (35.7) | |||
| $50,000 to <$100,000 | 150 (39.7) | 75 (42.1) | 75 (37.5) | 30 (30.6) | |||
| ≥$100,000 | 126 (33.3) | .15 | 60 (33.7) | .07 | 66 (33.0) | .43 | 33 (33.7) |
| IQc | |||||||
| ≥50th percentile | 181 (47.9) | .006 | 93 (52.2) | .05 | 88 (44.0) | .003 | 63 (64.3) |
| Primary diagnosis | |||||||
| ALL | 35 (9.2) | 0 (0) | 35 (17.5) | ||||
| AML | 115 (30.4) | 8 (4.5) | 107 (53.5) | ||||
| HL/NHL | 138 (36.5) | 104 (58.4) | 34 (17.0) | ||||
| MM | 71 (18.8) | 66 (37.1) | 5 (2.5) | ||||
| Other | 19 (5.0) | 0 (0) | 19 (9.5) | ||||
| Conditioning irradiation | |||||||
| 111In/90Y | 28 (7.4) | 23 (12.9) | 5 (2.5) | ||||
| Myeloablative TBI | 86 (22.7) | 13 (7.3) | 73 (36.5) | ||||
| Reduced intensity TBI/TMI | 11 (2.9) | 8 (4.5) | 3 (1.5) | ||||
| Risk of disease recurrence at BMTd | |||||||
| High | 233 (61.6) | 123 (69.1) | 110 (53.4) | ||||
Abbreviations: 111In, indium-111; 90Y, yttrium-90; ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; BMT, blood or bone marrow transplantation; HL, Hodgkin lymphoma; IQ, intelligence quotient; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; TBI, total body irradiation; TMI, total bone marrow irradiation.
Bolded values indicate a statistical significance level of P < .05.
P values were calculated by comparing each BMT group with healthy controls using the Student t test for continuous variables and the chi-square test for categorical variables.
IQ scores were missing for 7 allogeneic BMT recipients.
Patients with the following diagnoses were considered to be at standard risk of disease recurrence at BMT: ALL, AML (except secondary AML), HL/NHL in first or second complete remission, MM in complete remission, first chronic phase of chronic myeloid leukemia, myelodysplastic syndrome (refractory anemia), myelofibrosis, or myeloproliferative disorder. All other patients were categorized as being at high risk.
Relationship Between Self-Endorsed Cognitive Problems and Objectively Determined Cognitive Impairment
All BMT recipients
The GMI scores were positively correlated with GDS scores at 1 year after BMT (correlation coefficient [r], 0.28; P = .001), indicating higher overall self-endorsed cognitive problems (GMI) correlated with greater global deficits in neuropsychological measures (GDS). There were no significant correlations noted between any of the NIS subscales and corresponding neuropsychological measures at baseline or at 6 months after BMT. At 1 year after BMT, the Delis-Kaplan Executive Function System Color-Word Interference Test was negatively correlated with the COG subscale of the NIS (r, −0.236; P = .004) and WAIS-III Arithmetic was negatively correlated with ACD (r, −0.262; P = .01). At 2 years after BMT, WAIS-III Arithmetic was negatively correlated with ACD (r, −0.251; P = .007). At 3 years after BMT, WAIS-III Arithmetic was negatively correlated with ACD (r, −0.308; P < .001) and Wechsler Memory Scale–Third Edition Family Pictures was negatively correlated with MEM (r, −0.218; P = .01). Significant negative correlations indicated that poorer performance on neuropsychological measures correlated with greater self-endorsed cognitive problems.
Autologous BMT recipients
The GMI was not correlated with GDS scores at any time point, nor were there significant correlations noted between NIS subscales and neuropsychological measures.
Allogeneic BMT recipients
A significant positive correlation between GMI and GDS was found at 1 year after BMT (r, 0.35; P = .001). There was no significant correlation noted between NIS subscales and neuropsychological measures for 6 months, 1 year, and 2 years after BMT. WAIS-III Arithmetic was negatively correlated with ACD at baseline (r, −0.298; P = .004) and 3 years after BMT (r −0.305; P = .006).
Magnitude of Difference in Self-Endorsed Cognitive Problems Between BMT Recipients and Healthy Controls
The overall multivariate analysis of covariance results (Wilks’ λ) demonstrated a significant difference between BMT recipients and healthy controls with regard to GMI scores at all time points (see Supporting Table 2). Although no statistically significant differences were observed between autologous and allogeneic BMT recipients, significant differences were found between each BMT group and healthy controls at each time point (Table 2).
TABLE 2.
Post Hoc Pairwise Comparisons (Tukey HSD) of Scores at Each Time Point Between Recipients of Autologous and Allogeneic BMT and Healthy Controlsa
| Autologous BMT Versus Healthy Control |
Allogeneic BMT Versus Healthy Control |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time Point | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 |
| NIS subscale | ||||||||||
| Critical Items | 9.34 | 8.47 | 7.51 | 7.01 | 5.60 | 8.26 | 7.62 | 6.23 | 9.87 | 10.83 |
| 95% CI | 12.5/6.1 | 12.1/4.8 | 11.3/3.8 | 10.9/3.1 | 9.7/1.5 | 11.4/5.1 | 11.0/4.3 | 9.6/2.9 | 13.3/6.5 | 14.6/7.0 |
| P | <.001 | <.001 | <.001 | <.001 | .004 | <.001 | <.001 | <.001 | <.001 | <.001 |
| Cognitive Efficiency | 10.2 | 10.1 | 7.36 | 6.20 | 7.63 | 9.06 | 8.33 | 8.17 | 8.79 | 10.08 |
| 95% CI | 13.9/6.5 | 14.6/5.1 | 11.6/3.2 | 10.9/1.5 | 12.3/2.9 | 12.8/5.4 | 12.4/4.3 | 11.9/4.4 | 12.9/4.7 | 14.5/5.7 |
| P | <.001 | <.001 | <.001 | .006 | .001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| Attention | 6.03 | 6.82 | 5.91 | 4.47 | 5.87 | 5.46 | 3.14 | 4.85 | 6.47 | 7.51 |
| 95% CI | 10.1/2.0 | 11.5/2.1 | 10.6/1.2 | 9.6/−0.60 | 11.2/0.57 | 9.5/1.5 | 7.5/−1.2 | 9.0/0.67 | 10.9/2.0 | 12.4/2.6 |
| P | .002 | .002 | .009 | .097 | .026 | .004 | .20 | .018 | .002 | .001 |
| Memory | 4.54 | 6.45 | 6.06 | 3.86 | 6.05 | 5.05 | 2.79 | 4.51 | 6.56 | 8.71 |
| 95% CI | 8.4/0.71 | 10.7/2.1 | 10.3/1.8 | 8.8/−1.0 | 10.9/1.2 | 8.8/1.3 | 6.7/−1.1 | 8.3/0.71 | 10.9/2.3 | 13.2/4.2 |
| P | .015 | .001 | .003 | .15 | .01 | .005 | .22 | .015 | .001 | <.001 |
| Frustration Tolerance | 7.10 | 5.95 | 3.83 | 4.10 | 3.90 | 6.65 | 4.52 | 3.68 | 7.95 | 7.09 |
| 95% CI | 11.1/3.2 | 9.8/2.1 | 7.7/−0.04 | 8.5/−0.28 | 8.3/−0.48 | 10.2/3.1 | 8.1/0.95 | 7.2/0.22 | 11.8/4.1 | 11.2/3.0 |
| P | <.001 | .001 | .05 | .072 | .127 | <.001 | .009 | .034 | <.001 | <.001 |
| Learning-Verbal | 6.36 | 7.9 | 7.23 | 6.54 | 4.66 | 6.62 | 4.49 | 6.04 | 7.40 | 8.65 |
| 95% CI | 9.9/2.8 | 12.1/3.6 | 11.2/3.2 | 11.3/1.8 | 8.1/0.01 | 10.2/3.1 | 8.4/0.60 | 9.6/2.5 | 11.6/3.2 | 13.0/4.3 |
| P | <.001 | <.001 | <.001 | .004 | .049 | <.001 | .019 | <.001 | <.001 | <.001 |
| Academic Skills | 6.54 | 4.79 | 4.70 | 3.46 | 3.62 | 5.70 | 3.08 | 4.42 | 5.95 | 7.23 |
| 95% CI | 10.5/2.6 | 9.2/0.41 | 9.0/0.40 | 8.4/−1.5 | 8.5/−1.2 | 9.6/1.8 | 7.1/−0.95 | 8.3/0.57 | 10.3/1.6 | 11.7/2.7 |
| P | <.001 | .028 | .028 | .225 | .183 | .002 | .17 | .02 | .004 | .001 |
| GMI | 9.51 | 9.09 | 7.73 | 6.61 | 7.36 | 8.60 | 6.43 | 7.54 | 10.26 | 11.72 |
| 95% CI | 13.5/5.5 | 13.8/4.4 | 12.4/3.1 | 11.6/1.6 | 12.4/2.3 | 12.5/4.7 | 10.7/2.1 | 11.7/3.4 | 14.7/5.8 | 16.4/7.0 |
| P | <.001 | <.001 | <.001 | .006 | .002 | <.001 | .001 | <.001 | <.001 | <.001 |
Abbreviations: 0, before blood or bone marrow transplantation; 1, 6 months after blood or bone marrow transplantation; 2, 1 year after blood or bone marrow transplantation; 3, 2 years after blood or bone marrow transplantation; 4, 3 years after blood or bone marrow transplantation; BMT, blood or bone marrow transplantation; GMI, Global Measure of Impairment; HSD, honestly significant difference; NIS, Neuropsychological Impairment Scale.
Data are the estimate of the mean score difference. 95% CIs indicate upper bound/lower bound values. Bolded values indicate a P ≤ .003 significance level, adjusted for multiple comparisons. A positive value indicates greater self-endorsed cognitive problems on the NIS subscale in BMT recipients when compared with healthy controls.
Autologous BMT recipients versus healthy controls
GMI was significantly higher in autologous BMT recipients when compared with healthy controls before BMT at baseline and at 6 months, 1 year, and 3 years after BMT. CRIT scores were higher in the BMT group compared with the healthy controls at all time points from baseline through 2 years after BMT. The COG and L-V subscales were higher in the BMT group compared with healthy controls at baseline and at 6 months and 1 year after BMT. The FRU and ATT subscale scores were higher in the BMT group compared with healthy controls at baseline and at 6 months after BMT. ACD scores were significantly higher in the BMT group compared with healthy controls at the pre-BMT baseline time point (Table 2).
Allogeneic BMT recipients versus healthy controls
GMI scores and CRIT and COG subscale scores were found to be significantly higher in allogeneic BMT recipients compared with healthy controls at all time points. Higher scores were observed for several subscales among the allogeneic BMT recipients compared with healthy controls at later time points: 2 years after BMT (CRIT, COG, ATT, MEM, FRU, and L-V) and 3 years after BMT (all subscales) (Table 2).
Trajectory of NIS Subscales Over Time
Results from the HLM analyses demonstrated a significant difference in the trajectory of GMI across autologous and allogeneic BMT recipients and healthy controls (Table 3) (Fig. 2). The rate of change in GMI scores (slope) was significantly different among groups (P = .035), with both BMT groups demonstrating an increase in GMI scores over time whereas healthy controls demonstrated a slight decline in GMI scores. Group assignment and fatigue were the only 2 predictors to reach statistical significance, with sex, age, educational level, and IQ not found to be related to GMI scores at time point 0.
TABLE 3.
Hierarchical Linear Modeling of Change Over Time for GMI
| Coefficient | Standard Error | t Ratio | pa | |
|---|---|---|---|---|
| Intercept (time point 0) | ||||
| Constant, γ00 | 44.376 | 2.604 | 17.039 | <.001 |
| Age, γ01 | −0.022 | 0.036 | −0.619 | .536 |
| Education, γ02 | −0.201 | 0.546 | −0.368 | .713 |
| Sex, γ03 | 1.415 | 1.012 | 1.399 | .163 |
| Groupb, γ04 | 2.124 | 0.685 | 3.102 | .002 |
| IQ, γ05 | −1.621 | 1.023 | −1.584 | .114 |
| Fatigue, γ06 | 9.735 | 1.027 | 9.483 | <.001 |
| Slope (linear growth rate in days) | ||||
| Constant, γ10 | 0.0013 | 0.0019 | 0.707 | .480 |
| Age, γ11 | 0.000013 | 0.000027 | 0.473 | .636 |
| Education, γ12 | −0.00042 | 0.00039 | −1.077 | .282 |
| Sex, γ13 | −0.00075 | 0.00075 | −0.999 | .318 |
| Group, γ14 | 0.00051 | 0.00035 | 1.46 | .035 |
| IQ, γ15 | −0.0010 | 0.00084 | −1.213 | .225 |
| Fatigue, γ16 | −0.00078 | 0.00078 | −0.986 | .225 |
Abbreviations: time point 0, before blood or bone marrow transplantation; GMI, Global Measure of Impairment; IQ, intelligence quotient.
Bolded values indicate a P < .05 significance level.
Group assignment was as follows: autologous blood or bone marrow transplantation (BMT) recipients, allogeneic BMT recipients, and healthy controls.
Figure 2.
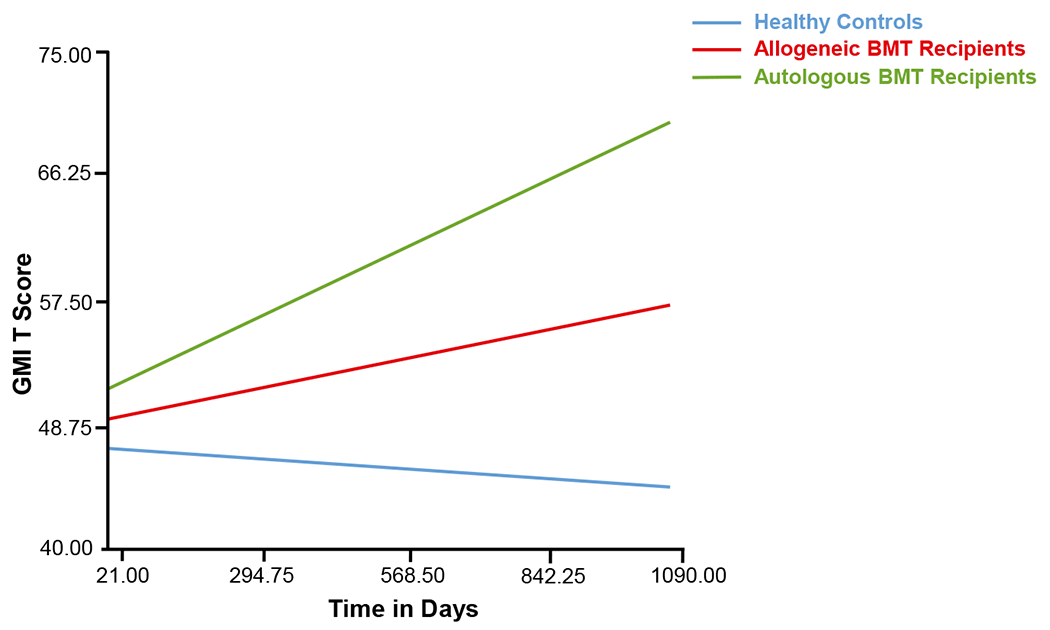
Change in Global Measure of Impairment (GMI) over time (in days) since baseline (designated as 0 days) for recipients of autologous and allogeneic blood or bone marrow transplantation (BMT) and healthy controls.
Predictors of Self-Endorsed Cognitive Problems
The 1-year post-BMT time point was selected to determine predictors of self-endorsed cognitive problems given the highest NIS scores (GMI and subscale) and greatest correlation of GMI with GDS observed at this time point. Fatigue was the only variable found to be associated with greater endorsement of cognitive problems at 1 year after BMT among all BMT recipients taken together (odds ratio, 4.23; 95% CI, 2.1-8.3 [P < .001]).
Cognitive Problems and Returning to Work
Among the BMT recipients who participated at the 3-year time point, approximately 22.8% had not returned to work. Two separate multivariable models were used to examine the association between GMI and returning to work and GDS and returning to work (adjusting for age, sex, educational level, fatigue, cognitive reserve, and type of BMT received). These analyses demonstrated that higher self-reported cognitive problems (GMI) and higher objectively assessed cognitive impairment (CDS) were associated with a 3.7-fold (P = .02) and 4.3-fold (P = .02) higher odds of not returning to work, respectively (see Supporting Table 3).
Subjective Distortion index
The SDI provides a discrepancy score between the participant’s objective neuropsychological testing performance and his or her self-endorsed cognitive problems on the NIS. Multivariable analyses identified no differences in the SDI between allogeneic and autologous BMT recipients, but did identify differences between healthy controls and BMT recipients only at the baseline time point. Specifically, significant differences were found between autologous BMT recipients and healthy controls (mean difference, 11.19; standard error, 2.58 [P < .001]) and allogeneic BMT recipients and healthy controls (mean difference, 9.36; standard error, 2.99 [P = .01]).
DISCUSSION
The current study examined the clinical relevance of self-endorsed cognitive problems in adult BMT recipients during the first 3 years after undergoing transplantation. We found that both autologous and allogeneic BMT recipients endorsed greater cognitive problems compared with healthy controls from before BMT through 3 years after BMT. Furthermore, although the majority of self-endorsed cognitive problems (with the exception of COG) for autologous BMT recipients at 3 years after BMT were not different from those of healthy controls, these cognitive problems continued to be significantly higher among allogeneic BMT recipients compared with healthy controls at 3 years. We found that both allogeneic and autologous BMT recipients demonstrated an increase in self-endorsed cognitive problems over time whereas the healthy controls demonstrated a slight decline. Self-endorsed cognitive scores did not correlate with objective neuropsychological scores for autologous BMT recipients, but did demonstrate a modest correlation at baseline and at 1 year after BMT for allogeneic BMT recipients. Self-endorsed cognitive problems were found to be associated with not returning to work after BMT and the magnitude of that association was similar to that observed when using objectively measured cognitive function.
Similar to previous studies that had used objective assessment of cognition, 3,15 we did not find an association between clinical characteristics and self-endorsed cognitive problems. However, fatigue did influence self-endorsed cognitive problems in both autologous and allogeneic BMT recipients. The inverse association between fatigue and cognitive functioning in healthy16,17 and cancer populations18–20 has been described previously. Allogeneic BMT recipients also were more likely to endorse global subjective cognitive impairment at 3 years after BMT, whereas autologous BMT recipients had a self-perception of cognitive impairment starting at baseline. The differing relationship between time from BMT and self-endorsed cognitive problems by type of BMT needs additional investigation, but further identifies vulnerable subpopulations of patients who possibly could benefit from interventions at specific times in relation to BMT. Cognitive behavioral therapy is reported to be effective in reducing fatigue and improving self-endorsed cognitive problems in cancer populations,18 thereby suggesting its usefulness in BMT recipients as well. As such, those patients who endorse significant fatigue should be identified early as a vulnerable subpopulation who could benefit from behavioral intervention.
In the current longitudinal and concurrent assessment of subjective and objective cognitive impairment, the results demonstrated that self-endorsed cognitive problems demonstrated a modest correlation with objective cognitive assessment in allogeneic BMT recipients but not autologous BMT recipients. Using the NIS subscale, SDI, we were able to discern that BMT recipients endorsed more cognitive problems than were evident from cognitive testing at baseline; this was not the case for healthy controls. The autologous BMT recipient group in particular endorsed greater cognitive problems across all NIS subscales at baseline, although their baseline neuropsychological test scores did not differ from those of the healthy controls.3 Overall, the current study results demonstrated that although moderate correlations between the NIS and objective neuropsychological measures were observed in allogeneic BMT recipients, there were no significant correlations noted between any of the NIS subscales and corresponding neuropsychological measures for autologous BMT recipients at any time point. This may suggest that, among autologous BMT recipients, additional clinical factors, in combination with levels of fatigue, affect their subjective ratings of cognitive functioning compared with the allogeneic BMT recipients. In addition, our research has indicated that the NIS is most correlated with objective testing at the time point of 1 year after BMT. This is consistent with other studies assessing fatigue and quality-of-life metrics, both of which have an impact on both objective and subjective cognitive functioning, in which the most vulnerable time point appears to be 1 year after BMT.21,22 We have speculated that the trajectories of self-endorsed cognitive problems and of objective cognitive impairment differ after the first year after BMT, resulting in the weakening or absence of a correlation. Nonetheless, the major implication of our observations is that the 1-year time point is an optimum time at which to screen patients regarding their need for further cognitive assessment and/or intervention using a simple self-administered assessment.
Psychological research in other populations has consistently found that self-perception of health can significantly affect behavioral and functional outcomes.23–25 Self-endorsed cognitive problems were found to be influenced significantly by fatigue among BMT recipients. Furthermore, self-endorsed cognitive problems was comparable to objective testing as a predictor for not returning to work. Indeed, the results of the current study indicated that self-endorsed cognitive problems on the NIS was a significant risk factor for not returning to work among the allogeneic BMT recipients. Allogeneic BMT recipients also were more likely to endorse global subjective cognitive impairment at 3 years after BMT, whereas autologous BMT recipients had a self-perception of cognitive impairment starting at baseline. These are important subgroups to identify: 1) those who may need more comprehensive follow-up; and 2) those who may best benefit from cognitive behavioral therapy to improve functional outcomes. Additionally, specific subgroups may benefit from different timing of the implementation of these behavioral therapeutic interventions.
The current study needs to be placed within the context of its limitations. Attrition over the course of the study, largely due to illness and death, had the potential to influence the results presented herein. HLM was used to account for the attrition, in addition to the conventional general linear model multivariate analyses. In addition, the current study was subject to potential biases common to all self-report research such as the desire to underemphasize or overemphasize cognitive impairment or to respond to questions in a way that the participant believes the researchers wish them to respond. These biases were handled by using a validated and reliable self-report measure (NIS) and by following specific administration guidelines of the measure to reduce researcher-related biases. In addition, we were unable to examine the outcome in patients who were treated with reduced intensity conditioning. Last, additional measures that could influence either self-ratings or objective testing of cognitive abilities such as depression or quality of life were not collected longitudinally.
These limitations notwithstanding, the current study has several strengths. To our knowledge, this is the first report of a longitudinal and concurrent assessment of subjective and objective cognitive impairment in BMT recipients and a comparison group of healthy controls. An additional strength of the current study was the availability of baseline data before BMT. Furthermore, we used a valid and reliable measure (NIS) to evaluate self-endorsed cognitive problems.13 Using this approach, we found that both autologous and allogeneic BMT recipients self-endorsed cognitive problems up until 3 years after BMT. Fatigue is a strong contributor to the self-endorsement of these cognitive problems. Finally, there is a significant association between self-endorsement of cognitive problems and not returning to work. These findings suggest that a simple self-administered assessment could help to identify vulnerable subpopulations that could benefit from follow-up objective testing and referral to cognitive behavioral therapy programs.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
Supported in part by the Leukemia and Lymphoma Society (62771-11 to Smita Bhatia).
Footnotes
Additional supporting information may be found in the online version of this article.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Syrjala KL, Dikmen S, Langer SL, Roth-Roemer S, Abrams JR. Neuropsychologic changes from before transplantation to 1 year in patients receiving myeloablative allogeneic hematopoietic cell transplant. Blood. 2004;104:3386–3392. [DOI] [PubMed] [Google Scholar]
- 2.Syrjala KL, Artherholt SB, Kurland BF, et al. Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. J Clin Oncol. 2011;29:2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharafeldin N, Bosworth A, Patel SK, et al. Cognitive functioning after hematopoietic cell transplantation for hematologic malignancy: results from a prospective longitudinal study. J Clin Oncol. 2018;36:463–475. [DOI] [PubMed] [Google Scholar]
- 4.Ganz PA, Kwan L, Castellon SA, et al. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013;105:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65:123–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganz PA, Kwan L, Castellon SA, et al. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013;105:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Donnell WE, DeSoto C, DeSoto J, Reynolds D. The Neuropsychological Impairment Scale. Western Psychological Services; 2009. [Google Scholar]
- 8.McNair DM, Heuchert JWP. Profile of Mood States: Technical Update. Multi-Health Systems Inc; 2005. [Google Scholar]
- 9.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310. [DOI] [PubMed] [Google Scholar]
- 10.Stano JF. Wechsler Abbreviated Scale of Intelligence. Rehabil Couns Bull. 2004;48:56. [Google Scholar]
- 11.Wilkinson GS, Robertson GJ. WRAT 4: Wide Range Achievement Test. Psychological Assessment Resources; 2006. [Google Scholar]
- 12.O’Donnell WE, De Soto CB, De Soto JL. Validity and reliability of the revised Neuropsychological Impairment Scale (NIS). J Clin Psychol. 1993;49:372–382. [DOI] [PubMed] [Google Scholar]
- 13.O’Donnell WE, Reynolds DQ, de Soto CB. Neuropsychological Impairment Scale (NIS): initial validation study using trailmaking test (A & B) and WAIS digit symbol (scaled score) in a mixed grouping of psychiatric, neurological, and normal patients. J Clin Psychol. 1983;39:746–748. [DOI] [PubMed] [Google Scholar]
- 14.Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommenddations for future research. J Clin Oncol. 2007;25:2455–2463. [DOI] [PubMed] [Google Scholar]
- 15.Scherwath A, Schirmer L, Kruse M, et al. Cognitive functioning in allogeneic hematopoietic stem cell transplantation recipients and its medical correlates: a prospective multicenter study. Psychooncology. 2013;22:1509–1516. [DOI] [PubMed] [Google Scholar]
- 16.Barwick F, Arnett P, Slobounov S. EEG correlates of fatigue during administration of a neuropsychological test battery. Clin Neurophysiol. 2012;123:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rollinson DC, Rathlev NK, Moss M, et al. The effects of consecutive night shifts on neuropsychological performance of interns in the emergency department: a pilot study. Ann Emerg Med. 2003;41:400–406. [DOI] [PubMed] [Google Scholar]
- 18.Goedendorp MM, Knoop H, Gielissen MF, Verhagen CA, Bleijenberg G. The effects of cognitive behavioral therapy for postcancer fatigue on perceived cognitive disabilities and neuropsychological test performance. J Pain Symptom Manage. 2014;47:35–44. [DOI] [PubMed] [Google Scholar]
- 19.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen MZ, Rozmus CL, Mendoza TR, et al. Symptoms and quality of life in diverse patients undergoing hematopoietic stem cell transplantation. J Pain Symptom Manage. 2012;44:168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prieto JM, Blanch J, Atala J, et al. Clinical factors associated with fatigue in haematologic cancer patients receiving stem-cell transplantation. Eur J Cancer. 2006;42:1749–1755. [DOI] [PubMed] [Google Scholar]
- 22.Hendriks MG, Schouten HC. Quality of life after stem cell transplantation: a patient, partner and physician perspective. Eur J Intern Med. 2002;13:52–56. [DOI] [PubMed] [Google Scholar]
- 23.Levy BR, Myers LM. Preventive health behaviors influenced by self-perceptions of aging. Prev Med. 2004;39:625–629. [DOI] [PubMed] [Google Scholar]
- 24.Levy BR. Mind matters: cognitive and physical effects of aging self-stereotypes. J Gerontol B Psychol Sci Soc Sci. 2003;58:P203–P211. [DOI] [PubMed] [Google Scholar]
- 25.Von Ah D, Habermann B, Carpenter JS, Schneider BL. Impact of perceived cognitive impairment in breast cancer survivors. Eur J Oncol Nurs. 2013;17:236–241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


