Abstract
BACKGROUND/OBJECTIVES
Frail participants are often under‐represented in randomized trials, raising questions about outcomes of interventions in real‐world settings. Frailty is strongly associated with vulnerability to illness and adverse health outcomes. We studied the impact of frailty on recombinant zoster vaccine (RZV) clinical outcomes.
DESIGN/SETTING
Data from two previously conducted phase III randomized trials of RZV were pooled. These two parent trials were conducted concurrently at the same study sites using the same methods.
PARTICIPANTS/INTERVENTION
In the two parent studies, participants aged ≥50 years (ZOE‐50 study) and ≥70 years (ZOE‐70 study), respectively, were randomized 1:1 to receive two doses of RZV or placebo.
MEASUREMENTS
In the current ZOE‐Frailty study (NCT03563183), a frailty index was created using previously validated methods. Clinical outcomes assessed by frailty status included vaccine efficacy, immunogenicity, reactogenicity, and safety.
RESULTS
Of 29,305 participants from the pooled ZOE‐50 and ZOE‐70 total vaccinated cohort, 92% were included in this study. Mean age was 68.8 years; 58.1% were women; 45.6% were pre‐frail and 11.3% frail. The percentage of frail participants increased with age from 5.7% aged 50–59 years to 22.7% aged ≥80 years. RZV vaccine efficacy against herpes zoster was >90% for all frailty subgroups (non‐frail: 95.8% (95% confidence interval = 91.6–98.2), pre‐frail: 90.4% (84.4–94.4), frail: 90.2% (75.4–97.0)). The RZV group demonstrated robust anti‐gE antibody and gE‐specific CD42+ responses, with mean concentrations remaining above pre‐vaccination levels at least 3 years post‐dose two, in all frailty subgroups. In the RZV group, the percentage of participants reporting solicited adverse events tended to decrease with increasing frailty.
CONCLUSION
The relatively nonrestrictive inclusion/exclusion criteria in the parent ZOE studies resulted in a range of participants that included frail and pre‐frail older adults. RZV significantly reduced the risk of herpes zoster across all frailty subgroups.
Keywords: older adults, frail, herpes zoster, quality of life, subunit vaccine
1. INTRODUCTION
Herpes zoster (HZ), or shingles, which results from the reactivation of latent varicella zoster virus (VZV), usually presents as a painful vesicular dermatomal rash. 1 VZV cell‐mediated immune (CMI) response declines with age, and this decline correlates with an increase in incidence and severity of HZ. 2 , 3 Older adults are at an increased risk of having severe pain during the acute phase, and of developing complications such as postherpetic neuralgia (PHN), 4 which can have a devastating impact on quality of life (QoL). 5 , 6 Particularly in frail individuals, HZ can lead to an inability to recover the lifestyle, interests, and level of functional activity that existed before HZ, and may also be associated with depression. 6 , 7
Antiviral therapy can reduce both rash extent and duration and acute pain severity, if administered within 72 hours of rash onset, but has not been shown to decrease the incidence of PHN. 8 Nonsteroidal anti‐inflammatory drugs or acetaminophen or opioids are commonly used for the treatment of acute pain and PHN associated with HZ. 1 However, the application of drugs to manage HZ in frail, co‐morbid, and often poly‐medicated patients must be carefully considered, as frail individuals could be affected more by treatment‐related side effects than non‐frail individuals. 9
Vaccines against other infections, such as influenza and pneumococcal disease, are less effective in older or frail individuals, resulting in less benefit. 10 , 11 , 12 , 13 , 14 Consequently, there is a paradox, that is, the people in most need of protection may benefit less from those vaccines. In contrast, a two‐dose adjuvanted recombinant zoster vaccine (RZV; Shingrix, GSK) demonstrated vaccine efficacy (VE) in preventing HZ of 97.2% in the pivotal phase III ZOE‐50 study that enrolled adults ≥50 years, and 91.3% in the pooled ZOE‐50 and ZOE‐70 analysis, that enrolled adults ≥70 years. 15 , 16 This vaccine consists of the VZV glycoprotein E (gE) antigen and an Adjuvant System (AS01B).
Frail participants and those with multiple comorbidities are often under‐represented in randomized controlled trials, raising questions about vaccine efficacy in a real‐world setting. 17 Given these questions and the correlation of frailty with low immune responses and poor clinical outcomes with other vaccines, we undertook an additional analysis of data from the ZOE studies, describing the baseline frailty status of ZOE study participants and the impact of frailty on RZV efficacy, immunogenicity, reactogenicity, and safety.
2. METHODS
2.1. Study Design and Participants
This ZOE‐Frailty study (NCT03563183), was an international, observational, retrospective study designed to assess the baseline frailty status of participants in the ZOE‐50 and the ZOE‐70 studies. 15 , 16 These two parent phase III randomized, observer‐blinded, placebo‐controlled clinical trials were conducted concurrently at the same study sites using the same methods with participants aged ≥70 years randomly assigned to the ZOE‐50 or ZOE‐70 study. ZOE study participants belonging to sites willing to take part in the current study were included in the ZOE‐Frailty study. In the ZOE studies, while patient reported outcomes (PRO) data were collected from all participants, encoding of PRO questionnaires (Short Form Survey‐36 (SF‐36), EuroQol‐5 Dimension (EQ‐5D)) was only performed for participants who developed a suspected HZ episode during the study. In the ZOE‐Frailty study, we encoded the remaining PRO questionnaires. We linked this data with the data from the ZOE studies, which allowed us to assess baseline frailty status and perform the analysis of clinical outcomes as a function of frailty. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
In the parent studies, vaccine or placebo (0.9% saline solution) was administered (0.5 mL) intramuscularly at month 0 and month 2 with 1:1 randomization. 15 , 16
2.2. Objectives
The primary objective of the ZOE‐Frailty study was to evaluate the baseline frailty status of participants in the parent ZOE‐50 and ZOE‐70 phase III trials. The secondary objectives included the evaluation of VE against HZ, VE against HZ burden of illness (BOI), humoral and cellular immunogenicity, vaccine reactogenicity, and safety by frailty status.
2.3. Assessments
Frailty status was measured using the accumulation of deficits approach. 18 , 19 , 20 The different aspects of frailty composing the frailty index (FI) were assessed through the medical history and components of the SF‐36 and EQ‐5D questionnaires recorded before dose one, as previously validated. 21 The SF‐36 is a multi‐purpose health survey comprising 36 questions, including scales for physical functioning, role physical, bodily pain, general health, vitality, social function, role emotional, and mental health. 22 EQ‐5D is a generic measure of health status that defines health in terms of mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. 23 Further details on the deficits assessed and the scoring of the FI components are provided in the supplemental material (Supplementary File S1 and Supplementary Tables S1 and S2). Deficits were coded as 0 = absent to 1 = present. Each individual's deficits were summed to generate a total deficit score. The FI was then calculated by dividing by the number of possible deficits as follows: FI = (accumulation of deficits)/(41‐nmissQoL), where nmissQoL was the number of missing components of the 29 items from the SF‐36 and EQ‐5D questionnaires. Each study participant was assigned to one of three subgroups based on the FI as follows: FI ≤0.08 is classified as non‐frail; FI >0.08 to ≤0.25 is classified as pre‐frail; FI >0.25 is classified as frail. 20 Participants with a missing FI were classified as unknown.
The Zoster Brief Pain Inventory (ZBPI) questionnaire severity of illness score was calculated as the area under the curve of the ZBPI worst pain score from day 0 until day 182. 24 The BOI was then estimated by aggregating the severity of illness scores over all the participants in a group and dividing by the total number of years of participant follow‐up. Consequently, this composite measure took into account the incidence of HZ as well as the severity and duration of pain. 25 Details are presented elsewhere. 26
The total vaccinated cohort (TVC) included all participants who received at least one dose of RZV or placebo. The primary cohort for efficacy analyses was the modified vaccinated cohort (mTVC), which excluded participants who did not receive the second dose or who had a confirmed HZ episode before 1 month post‐dose two. VE against HZ was defined as 1 minus the ratio of HZ incidence of confirmed cases in the RZV group to that in the placebo group, multiplied by 100. The VE in reducing the BOI was similarly defined and calculated. All statistical tests were performed two tailed using a .05 significance level. All statistical analyses were performed with SAS software, version 4.7 (SAS Institute).
The analysis of humoral immunogenicity was performed based on the according‐to‐protocol cohort for immunogenicity, at each time point, including all participants who received both doses, met all the eligibility criteria, complied with the protocol, and had immunogenicity data available. 27 Serum anti‐gE antibody concentrations were measured using a GSK in‐house enzyme‐linked immunosorbent assay (ELISA). gE‐specific CMI responses were measured by flow cytometry to assess the frequency of CD4+ T cells expressing two or more of the following activation markers (hereafter termed CD42+): interferon‐gamma (IFN‐γ), interleukin‐2 (IL‐2), tumor necrosis factor‐α (TNF‐α), and CD40 ligand, following ex vivo stimulation with gE peptides. gE‐specific CMI analysis was limited to a small subset of participants from the Czech Republic, Japan, and the United States (i.e., CMI subset, Supplementary Table S4). Details of this selection and of the immunologic assays are presented elsewhere. 27
The humoral response threshold for the calculation of vaccine response rates (VRR) was defined as a fourfold or more increase in the anti‐gE antibody concentration as compared to the pre‐vaccination concentration (for initially seropositive participants) or as compared to the anti‐gE antibody cut‐off value for seropositivity (97 milli‐International Units (mIU)/mL, for initially seronegative participants). The CMI‐response threshold was defined as a twofold or more increase in the frequency of CD42+ T cells, as compared to pre‐vaccination frequencies (for participants with pre‐vaccination CD42+ T‐cell frequencies above the cut‐off of 320 positive cells per 106 CD4 T cells counted) or a twofold or more increase above the cut‐off (for participants with pre‐vaccination frequencies below the cut‐off). Exact 95% CIs were computed at each time point for the percentage of humoral and CMI responders. Medians with interquartile ranges were calculated for CD42+ T‐cell frequencies. The 95% CI for GMCs was computed by anti‐log transformation of the 95% CI for the mean of log‐transformed concentrations (which were calculated assuming that log‐transformed values were normally distributed with unknown variance).
In the parent ZOE‐50 and ZOE‐70 studies, a randomly selected subgroup of age‐stratified participants (i.e., TVC diary card subset) recorded injection‐site reactions (pain, redness, and swelling) and systemic reactions (fatigue, fever, gastrointestinal symptoms, headache, myalgia, and shivering) on diary cards for 7 days after each injection. Depending on the severity, solicited adverse events (AEs) were graded from 0 to 3. 15 , 16 Unsolicited reports of AEs were recorded for 30 days after each dose for all participants. Serious AEs (SAEs) were recorded for all participants for 12 months after the second dose. Fatal AEs, vaccination‐related SAEs and potential immune‐mediated diseases (pIMDs) were recorded in all participants throughout the trial.
3. RESULTS
Of the 29,305 participants from the pooled ZOE‐50 and ZOE‐70 TVC, 26,976 participants (92%) were included in the TVC of ZOE‐Frailty (Figure 1, Supplementary Table S4). Participants mean age at baseline was 68.8 years, 58.1% were female and 74.6% were Caucasian (Supplementary Table S5). In the TVC, 42.7% of participants were classified as non‐frail, 45.6% as pre‐frail and 11.3% as frail (Table 1). The percentage of frail participants increased with age from 5.7% of participants aged 50–59 years to 22.7% of participants aged ≥80 years. A higher proportion of females were frail (12.5%) compared with males (9.5%; P < .001).
Figure 1.
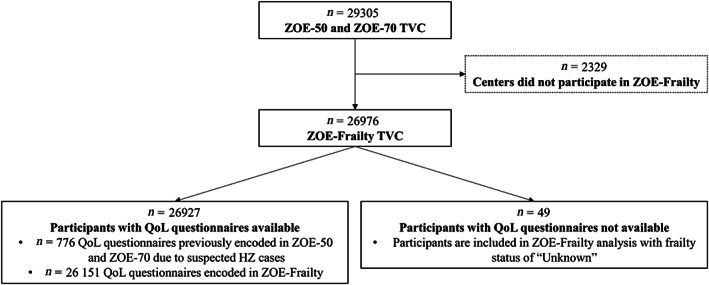
Study population. Abbreviations: HZ, herpes zoster; N, number of participants; QoL, quality of life; TVC, total vaccinated cohort; YOA, years of age.
Table 1.
Frailty Status by Age Group and Overall (Total Vaccinated Cohort)
| Frailty status | n (%) | ||||
|---|---|---|---|---|---|
| 50–59 YOA, N = 6,542 | 60–69 YOA, N = 4,000 | 70–79 YOA, N = 12,819 | ≥80 YOA, N = 3,615 | Total, N = 26,976 | |
| Non‐frail | 4,305 (65.8) | 2,102 (52.6) | 4,457 (34.8) | 654 (18.1) | 11,518 (42.7) |
| Pre‐frail | 1843 (28.2) | 1,614 (40.4) | 6,719 (52.4) | 2,114 (58.5) | 12,290 (45.6) |
| Frail | 375 (5.7) | 268 (6.7) | 1,575 (12.3) | 819 (22.7) | 3,037 (11.3) |
| Unknown | 19 (0.3) | 16 (0.4) | 68 (0.5) | 28 (0.8) | 131 (0.5) |
Notes: “Unknown” refers to participants who could not be assigned a frailty subgroup due to missing data. Percentages are based on the total number of participants in each age subgroup of the total vaccinated cohort.
Abbreviations: N, total number of participants in each age group and overall in the total vaccinated cohort; n (%), number (percentage) of participants in each subgroup by age and overall; YOA, years of age.
3.1. Vaccine Efficacy
A total of 430 and 31 subjects developed HZ in the Placebo and RZV groups, respectively. VE against HZ was 95.8% in non‐frail participants, 90.4% in pre‐frail participants and 90.2% in frail participants (Supplementary Table S3). The VE against ZBPI BOI score was 98.6% in non‐frail participants, 92.6% in pre‐frail participants and 85.2% in frail participants. The absolute reduction in BOI score between the Placebo and RZV groups was higher in frail (2.330–0.345 = 1.985) compared with non‐frail (1.173–0.017 = 1.156) participants (Figure 2).
Figure 2.
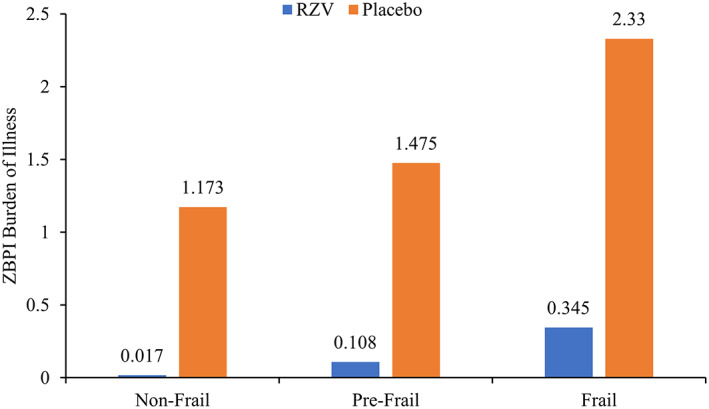
ZBPI burden of illness by vaccine group and frailty status (modified total vaccinated cohort). Abbreviations: RZV, adjuvanted recombinant zoster vaccine; ZBPI, Zoster Brief PainInventory.
3.2. Vaccine Immunogenicity
VRRs for anti‐gE antibody were highest at 1 month post‐dose two in the RZV group for all frailty subgroups: that is, 98.0%, 97.9%, and 95.7% for non‐frail, pre‐frail, and frail, respectively (Figure 3). At 36 months post‐dose two, the VRR for anti‐gE antibody in the RZV group remained high for all frailty subgroups: 81.7%, 74.6%, and 62.0% for non‐frail, pre‐frail, and frail, respectively. In frail participants, geometric mean anti‐gE antibody concentrations increased by approximately 31‐fold at 1 month post‐dose two and remained 6‐fold over baseline in RZV recipients 36 months post‐dose two. The corresponding estimates for the non‐frail subgroup were 42‐fold and 10‐fold increases over baseline in RZV recipients at 1 month post‐dose two and 36 months post‐dose two. In the CMI subset (Supplementary Table S4), VRRs for gE‐specific CD42+ T‐cells were highest at 1 month post‐dose two in the RZV group for all frailty subgroups: 91.7%, 94.2%, and 92.9% in non‐frail, pre‐frail, and frail participants, respectively. At 36 months post‐dose two, the CMI VRR in the RZV group was 58.6%, 48.9%, and 33.3% for non‐frail, pre‐frail, and frail subgroups, respectively. In frail participants, median CD42+ T‐cell frequencies increased by approximately 21‐fold (1 month post‐dose two) and remained sevenfold (36 months post‐dose two) over baseline in RZV recipients. The corresponding estimates for the non‐frail subgroup were 22‐fold and sevenfold increases over baseline in RZV recipients at 1 month post‐dose two and 36 months post‐dose two.
Figure 3.
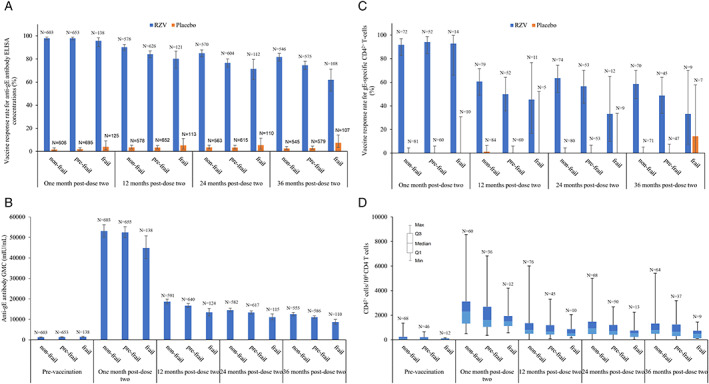
RZV‐induced anti‐glycoprotein E antibody responses: percentage of responders by frailty status (A), GMCs by frailty status (B); and RZV‐induced glycoprotein E‐specific cell‐mediated immunity: percentage of responders by frailty status (C), CD42+frequencies by frailty status (D). Abbreviations: ELISA, enzyme‐linked immunosorbent assay; gE, glycoprotein E; GMC, geometric mean concentration; IU, international units; N, number of participants with available results in each group; Q1/Q3, first and third quartiles; RZV, adjuvanted recombinant zoster vaccine.
3.3. Reactogenicity
The percentage of participants reporting any solicited AE was higher in the RZV group than in the placebo group for all frailty subgroups during the 7‐day (days 0–6) post‐vaccination periods following each dose (Supplementary Table S6). The percentage of participants reporting any solicited AEs decreased with increasing frailty in the RZV group (87.3%, 83.4%, and 73.5% for non‐frail, pre‐frail, and frail, respectively) and were similar across frailty subgroups in the placebo group (32.2%, 33.7%, and 36.1% for non‐frail, pre‐frail, and frail, respectively). The percentage of participants reporting any grade 3 solicited AEs were similar across frailty subgroups in the RZV group (17.4%, 14.1%, and 15.3% for non‐frail, pre‐frail, and frail, respectively) and were higher in the frail than the pre‐frail and non‐frail subgroups in the placebo group (1.8%, 2.2%, and 5.1% for non‐frail, pre‐frail, and frail, respectively).
Pain was the most frequently reported solicited local AE for each frailty subgroup in both treatment groups (Table 2). Pain decreased with increasing frailty in the RZV group (82.5%, 75.6%, and 65.8% for non‐frail, pre‐frail, and frail, respectively) and was similar across frailty subgroups in the placebo group (10.3%, 10.7%, and 10.9% for non‐frail, pre‐frail, and frail, respectively). Pain was also the most frequently reported grade 3 solicited local AE in each frailty subgroup in both treatment groups. Reporting of grade 3 pain was similar across frailty subgroups in both the RZV and placebo groups.
Table 2.
Incidence of solicited local and general AEs reported during the 7‐day (days 0–6) post‐vaccination period overall per participant by frailty status (total vaccinated cohort diary card subset)
| Non‐frail, n (%) | Pre‐frail, n (%) | Frail, n (%) | Unknown, n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| RZV group | Placebo group | RZV group | Placebo group | RZV group | Placebo group | RZV group | Placebo group | |
| Solicited local AEs | N = 2,007 | N = 2,034 | N = 1,977 | N = 1,964 | N = 489 | N = 468 | N = 21 | N = 17 |
| Pain | ||||||||
| All | 1,655 (82.5) | 209 (10.3) | 1,494 (75.6) | 211 (10.7) | 322 (65.8) | 51 (10.9) | 17 (81.0) | 1 (5.9) |
| Grade 3 | 115 (5.7) | 4 (0.2) | 125 (6.3) | 6 (0.3) | 38 (7.8) | 5 (1.1) | 1 (4.8) | 0 (0.0) |
| Redness (mm) | ||||||||
| All | 805 (40.1) | 25 (1.2) | 734 (37.1) | 28 (1.4) | 160 (32.7) | 2 (0.4) | 11 (52.4) | 0 (0.0) |
| >100 | 75 (3.7) | 0 (0.0) | 46 (2.3) | 0 (0.0) | 12 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Swelling (mm) | ||||||||
| All | 545 (27.2) | 18 (0.9) | 497 (25.1) | 22 (1.1) | 107 (21.9) | 3 (0.6) | 6 (28.6) | 1 (5.9) |
| >100 | 24 (1.2) | 0 (0.0) | 19 (1.0) | 0 (0.0) | 2 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Solicited general AEs | N = 2,007 | N = 2,034 | N = 1,971 | N = 1,964 | N = 488 | N = 468 | N = 21 | N = 18 |
|---|---|---|---|---|---|---|---|---|
| Fatigue | ||||||||
| All | 951 (47.4) | 301 (14·8) | 848 (43.0) | 339 (17.3) | 164 (33.6) | 96 (20.5) | 5 (23.8) | 4 (22.2) |
| Grade 3 | 114 (5.7) | 15 (0.7) | 93 (4.7) | 17 (0.9) | 22 (4.5) | 8 (1.7) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal symptoms | ||||||||
| All | 369 (18.4) | 157 (7.7) | 325 (16.5) | 166 (8.5) | 65 (13.3) | 56 (12.0) | 2 (9.5) | 2 (11.1) |
| Grade 3 | 26 (1.3) | 11 (0.5) | 28 (1.4) | 8 (0.4) | 6 (1.2) | 5 (1.1) | 0 (0.0) | 0 (0.0) |
| Headache | ||||||||
| All | 838 (41.8) | 287 (14.1) | 676 (34.3) | 311 (15.8) | 147 (30.1) | 89 (19.0) | 6 (28.6) | 3 (16.7) |
| Grade 3 | 74 (3.7) | 8 (0.4) | 53 (2.7) | 12 (0.6) | 17 (3.5) | 10 (2.1) | 0 (0.0) | 0 (0.0) |
| Myalgia | ||||||||
| All | 952 (47.4) | 209 (10.3) | 828 (42.0) | 225 (11.5) | 173 (35.5) | 75 (16.0) | 5 (23.8) | 2 (11.1) |
| Grade 3 | 100 (5.0) | 8 (0.4) | 89 (4.5) | 8 (0.4) | 28 (5.7) | 12 (2.6) | 0 (0.0) | 0 (0.0) |
| Shivering | ||||||||
| All | 623 (31.0) | 111 (5.5) | 459 (23.3) | 107 (5.4) | 86 (17.6) | 41 (8.8) | 3 (14.3) | 3 (16.7) |
| Grade 3 | 103 (5.1) | 4 (0.2) | 57 (2.9) | 6 (0.3) | 18 (3.7) | 2 (0.4) | 0 (0.0) | 0 (0.0) |
| Temperature (°C) | ||||||||
| All | 465 (23.2) | 52 (2.6) | 368 (18.7) | 55 (2.8) | 70 (14.3) | 19 (4.1) | 1 (4.8) | 0 (0.0) |
| >39 | 6 (0.3) | 4 (0.2) | 5 (0.3) | 3 (0.2) | 2 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Abbreviations: AE, adverse event; All, Grade > 0; N, number of participants in each group; n (%), number (percentage) of participants reporting the adverse event at least once; RZV, adjuvanted recombinant zoster vaccine.
Fatigue and myalgia were the most frequently reported solicited general AEs for each frailty subgroup in both treatment groups. The percentage of participants reporting fatigue decreased with increasing frailty in the RZV group (47.4%, 43.0%, and 33.6% for non‐frail, pre‐frail, and frail, respectively) and tended to increase with increasing frailty in the placebo group (14.8%, 17.3%, and 20.5% for non‐frail, pre‐frail, and frail, respectively). Similarly, the percentage of participants reporting myalgia decreased with increasing frailty in the RZV group (47.4%, 42.0%, and 35.5% for non‐frail, pre‐frail, and frail, respectively) and tended to increase with increasing frailty in the placebo group (10.3%, 11.5%, and 16.0% for non‐frail, pre‐frail, and frail, respectively).
3.4. Safety
The percentage of participants reporting at least one unsolicited AE within the 30‐day post‐vaccination (days 0–29) was similar across frailty subgroups in the RZV group (51.6%, 49.7%, and 47.9% for non‐frail, pre‐frail, and frail, respectively), and tended to increase with increasing frailty in the placebo group (29.6%, 32.7%, and 35.5% for non‐frail, pre‐frail, and frail, respectively (Supplementary Figure S1). The percentage of participants experiencing at least one pIMD was 1.3%, 1.3%, and 1.0% in the non‐frail, pre‐frail, and frail subgroups of the RZV group, respectively. This was similar to the percentages observed in the placebo group (1.2%, 1.4%, and 1.8% in the non‐frail, pre‐frail, and frail subgroups, respectively). The percentage of participants experiencing at least one SAE was 6.2%, 11.5%, and 18.6% in the non‐frail, pre‐frail, and frail subgroups of the RZV group, respectively, and 5.7%, 12.1%, and 22.7% in the non‐frail, pre‐frail, and frail subgroups of the placebo group, respectively. The percentage of participants who died during the study follow‐up (and who were classified per protocol as experiencing a fatal SAE, independent of relationship to vaccination) was 2.1%, 4.9%, and 11.1% in the non‐frail, pre‐frail, and frail subgroups of the RZV group, respectively, and 1.9%, 5.5%, and 12.4% in the non‐frail, pre‐frail, and frail subgroups of the placebo group, respectively.
4. DISCUSSION
The ZOE studies included participants across a range of frailty statuses, with 11.3% considered frail in the overall study population aged ≥50 years, increasing to 22.7% in those over age ≥80 years. VE against HZ was >90% across all levels of frailty, and immune responses were robust for all three frailty subgroups. Reactogenicity tended to decrease with increasing frailty in the RZV group, and no safety concerns were identified in any frailty subgroup.
Despite the lack of pre‐specified frailty measure in the parent ZOE studies, it was possible to generate a FI measure based on data collected at baseline in those clinical trials, using an approach that we previously validated. 21 Indeed, the FI could be calculated for 99.5% of participants who were included in the present study. In the case of the ZOE studies, even without specific attempts to recruit frail participants, use of relatively nonrestrictive in/exclusion criteria resulted in a broad range of participants. Given increasing awareness of the importance of frailty for responses to therapies and vulnerability to outcomes, the retrospective evaluation of frailty has potential applicability for analyses of other clinical trials.
In this study, results in the placebo group were consistent with published literature on frailty indices in which frailty increased with age, was higher in women than men, and adverse outcomes increased with increasing frailty (for solicited AEs such as headache, myalgia, fatigue and gastrointestinal symptoms, unsolicited AEs, SAEs, and death). Notably, we found that solicited AEs reports within 7 days, and unsolicited AE reports within 30 days of vaccination did not increase with frailty in the RZV group, whereas they did increase as expected in the placebo group. This observation is interesting since, based on literature, one expects to have more AEs with increasing frailty, also in the vaccine group. The finding suggests that the rate of AEs is driven by reactogenicity, and as such decreases with increasing frailty, in line with the lower observed reactogenicity in frailer participants. This result is consistent with decreasing vaccine reactogenicity with increasing age observed in the parent ZOE studies. 15 , 16 Overall, occurrence of SAEs, pIMDs, and deaths were similar in the vaccine and placebo groups in all frailty subgroups. The safety profile in all subgroups was clinically acceptable and comparable to the known safety profile of RZV vaccine.
RZV VE against HZ and against HZ ZBPI BOI were >90% and >85%, respectively, in all frailty subgroups. Of note, in the placebo group, the HZ ZBPI BOI was twice as high in frail participants compared to non‐frail participants; consequently, although the VE tended to be lower in frail participants, the absolute decrease in HZ ZBPI BOI was higher in frail participants compared to pre‐frail or non‐frail participants. This suggests that during the course of the ZOE studies, with a mean follow‐up of approximately 4 years, frail individuals may benefit at least as much from RZV as non‐frail individuals. In a previous publication, we estimated that the HZ ZBPI BOI score was 1.932 in individuals aged 80 years of age and older in the placebo group, 26 compared with a score of 2.330 in frail participants in the placebo group in this analysis. This reaffirms the findings of previous studies, that frailty is a better predictor of clinical outcomes than chronological age. 18
We identified robust immunogenicity responses to RZV across frailty subgroups which persisted at least 36 months post‐RZV dose two. Previous studies with other vaccines, for example, for influenza and pneumococcal disease, demonstrated a decline in vaccine effectiveness with age and with frailty. 10 , 11 , 12 , 13 , 14 , 25 Frailty has been theorized to be associated with an age‐related decline in innate and adaptive humoral and cell‐mediated immunity that impairs the ability to resist infection and respond to vaccination, contributing to immunosenescence. 28 , 29 However, the persistence of strong gE‐specific CD4 T‐cell responses, and the associated VE estimates of >90% in all frailty subgroups, seen with RZV, suggests that the vaccine can overcome immunosenescence to provide protection against HZ, including in frail individuals. 27 , 30 Adjuvants, such as AS01B present in RZV, have the potential to improve the efficacy of other vaccines that are intended for use in older adults and other populations that may otherwise have a lower response to vaccination.
In conclusion, adjuvanted RZV was highly effective in reducing the risk of HZ and associated BOI for all frailty subgroups. There was a trend for reduced reactogenicity with increasing frailty, and there were no safety concerns in pre‐frail or frail individuals. This study may help inform older adults, their health care providers, and policy makers regarding the benefits of vaccination against HZ with the recombinant zoster vaccine.
Supporting information
Supplementary File S1: Frailty assessment
Supplementary TableS1: Details of components of frailty index
Supplementary Table S2: Detail of medical history search items
Supplementary Table S3: Vaccine efficacy estimates (modified total vaccinated cohort)
Supplementary Table S4: Number of participants in the various cohorts (total vaccinated cohort)
Supplementary Table S5: Summary of demographic characteristics
Supplementary Table S6: Incidence and nature of symptoms (solicited only) reported during the 7‐day (days 0–6) post‐vaccination period overall per participant by frailty status (total vaccinated cohort ‐ diary card subset)
Supplementary Figure S1: Percentages of participants reporting any unsolicited adverse events within the 30‐day (days 0–29) post‐vaccination by frailty status (total vaccinated cohort)
ACKNOWLEDGMENTS
The authors thank the study participants, investigators, and study teams involved in this trial. We thank Quentin Deraedt, PhD (Modis c/o GSK), for editorial assistance and manuscript coordination. KES was also supported by the National Institute on Aging, Duke Pepper Older Americans Independence Center P30AG028716. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding for this study was provided by GlaxoSmithKline Biologicals SA.
Members of the Zoster‐064 Study Group: Australia: Ktut Arya, Michael Crookes, Ferdinandus de Looze, Wilfred Yeo. Brazil: Clóvis Cunha, Antônio Tarcísio Freire, José Luiz Neto, Thiago Silva, Lily Weckx, Cristiano Zerbini. Canada: Laurie Breger, Marc Dionne, Jan Dutz, Murdo Ferguson, Jean‐Sebastien Gauthier, Wayne Ghesquiere, Iris Gorfinkel, Ken Heaton, Pierre Lachance, Shelly McNeil, Calvin Powell, Eric St‐Amour, Guy Tellier, Azhar Toma. Czechia: Roman Chlibek. Estonia: Airi Poder, Irina Zahharova. Finland: Miia Virta. France: Elisabeth Barberan, Alain Baty, Jean Beytout, Hervé Bosquet, Loïc Boucher, Alain Boye, François Brault, Benoit Daguzan, Pierre André Ferrand, Christophe Genies, Pascal Hanrion, Philippe Remaud, Patrick Robert, Dominique Saillard, Denis Taminau. Germany: Juergen Berger‐Roscher, Antje Dahmen, Rolf Dominicus, Tamara Eckermann, Meral Esen, Beatrice Gerlach, Christine Grigat, Josef Grosskopf, Monika Hamann, Susanne Hoeltz‐Roehrig, Gabriele Illies, Alen Jambrecina, Thomas Jung, Gerd Kahrmann, Claus Keller, Christiane Klein, Uwe Kleinecke‐Pohl, Hans‐Joachim Koenig, Christine Kosch, Maximilian Kropp, Anneliese Linnhoff, Beate Moeckesch, Michael Mueller, Georg Plassmann, Felix Proepper, Joachim Sauter, Axel Schaefer, Isabelle Schenkenberger, Juergen Schmidt, Bernhard Schmitt, Christian Schubert, Tino Schwarz, Juergen Stockhausen, Nicole Toursarkissian, Juergen Wachter, Karl Wilhelm. Hong Kong: David Shu Cheong Hui, Edmund Kwok Yiu Sha. Italy: Piero Barbanti, Giancarlo Icardi, Guglielmo Migliorino, Angelo Pellegrino, Graziella Soldato, Pasquale Sordillo, Tommaso Staniscia. Japan: Masahiro Endo, Takashi Eto, Kenjiro Nakamura, Yuji Naritomi, Hiroaki Ogata, Yusuke Saruta, Shin Suzuki. Republic of Korea: Hee Jin Cheong, Eun‐Ju Choo, Hyo Youl Kim, Jacob Lee, Jin‐Soo Lee, Dae Won Park, Kyong Ran Peck, Young Goo Song. Mexico: Jose‐Fernando Barba‐Gómez, Juan Carlos Tinoco. Spain: Marta Aldea Novo, Carles Brotons Cuixart, Covadonga Caso, Javier Díez‐Domingo, Xavier Farrés Fabré, Pyrene Martínez Piera, Silvia Narejos Pérez, Concepción Núñez López, Mercè Pérez Vera, Alex Rodríguez Badia, Maria Luisa Rodríguez de la Pinta, Manuel Terns Riera. Sweden: Niklas Bengtsson, Katarina Berndtsson Blom, Dan Curiac, Pekka Koskinen, Bo Liu, Martin Lundvall, Abul Kashem Munir, Karlis Pauksens, Lars Rombo, Johan Berglund. Taiwan: Hsiao‐Ting Chang, Huey‐Shinn Cheng, Kuo‐Chin Huang, Chiu‐Shong Liu. United Kingdom: Yieng Huong, Angela Macari, Damien McNally, Michael Nagle, Janice Patrick, Samir Purnell‐Mullick, Michael Redmond. United States: Michael Adams, Charles Andrews, Mira Baron, Herman Jackson Downey, John Earl, William Ellison, Cecil Farrington, Matthew Finneran, David Francyk, George Freeman, Paul Hartley, Patricia Houser, Jeff Jacqmein, Leslie Klaff, Robert Lipetz, Srikanth Malempati, Mary Beth Manning, Richard Mills, Terry Poling, Stephanie Powell, George Raad, Bruce Rankin, Ernie Riffer, Robert Rosen, Shari Rozen, John Scott, Gerald Shockey, Sylvia Shoffner, Jonathan Staub, Mark Turner, Victor Vidals, Jonathan Wilson.
Trademark: Shingrix is a trademark owned by or licensed to the GSK group of companies.
Author Contributions
Melissa K. Andrew, Desmond Curran, Joon Hyung Kim, Myron J. Levin, Shelly A. McNeil, Lidia Oostvogels, Kenneth E. Schmader and Anne E. Schuind were involved in the conception or design of the study. Anthony L. Cunningham, Myron J. Levin, and Shelly A. McNeil contributed to data collection or data generation. All authors contributed to data analysis or data interpretation. All authors reviewed the draft critically, approved the final version to be submitted, and take accountability for all aspects of the published work.
Conflict of Interest
Dr Andrew reports grants from the GSK group of companies (GSK) during the study, as well as grants from GSK, the Canadian Frailty Network, the Canadian Institutes of Health Research, the Foundation for Influenza Epidemiology, and grants and personal fees from Sanofi and Pfizer outside the submitted work. Dr Cunningham reports receiving support from GSK to attend conferences, and for a collaborative discovery science project on the vaccine adjuvant used in RZV outside the submitted work. Dr Levin reports grants and fees for Advisory Board from GSK during the study, and is serving as an Advisory Board member for GSK and Merck. Mr Matthews is a freelance consultant for GSK. Dr Schmader reports grants from GSK during the study. Dr McNeil reports grants, personal fees and support for the conduct of clinical trials from GSK and Pfizer, personal fees and support for the conduct of clinical trials from Sanofi Pasteur, as well as personal fees from Merck outside the submitted work. Dr Kim, Mr Dessart, Dr Riley, Dr Schuind and Dr Curran are employees and Dr Oostvogels is a former employee of GSK. Dr Kim, Dr Schuind, Dr Oostvogels and Dr Curran own GSK stock options or (restricted) shares. Dr Oostvogels is employee of CureVac AG and is inventor on a patent owned by GSK and relevant to RZV.
Sponsor's Role
GlaxoSmithKline Biologicals SA was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. GlaxoSmithKline Biologicals SA does not veto ongoing publications or control the decision about what journal to submit to, with ultimate decision on the target made by the coauthors. GlaxoSmithKline Biologicals SA covered all costs associated with developing and publishing this article.
Data Sharing Statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com (study ID 204878). The study protocol, statistical analysis plan and results summary are posted on ClinicalTrials.gov (NCT03563183).
This article was published online on 16 November 2020. Few errors were subsequently identified in the article. This notice is included in both the online and print versions to indicate that they have been corrected on 23 November 2020.
REFERENCES
- 1. Cohen JI. Clinical practice: herpes zoster. N Engl J Med. 2013;369:255‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levin MJ. Immune senescence and vaccines to prevent herpes zoster in older persons. Curr Opin Immunol. 2012;24:494‐500. [DOI] [PubMed] [Google Scholar]
- 3. Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis. 2010;51:197‐213. [DOI] [PubMed] [Google Scholar]
- 4. Drolet M, Brisson M, Schmader K, et al. Predictors of postherpetic neuralgia among patients with herpes zoster: a prospective study. J Pain. 2010;11:1211‐1221. [DOI] [PubMed] [Google Scholar]
- 5. Drolet M, Brisson M, Schmader KE, et al. The impact of herpes zoster and postherpetic neuralgia on health‐related quality of life: a prospective study. CMAJ. 2010;182:1731‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McElhaney JE. Herpes zoster: a common disease that can have a devastating impact on patients' quality of life. Expert Rev Vaccines. 2010;9:27‐30. [DOI] [PubMed] [Google Scholar]
- 7. Zorzoli E, Pica F, Masetti G, Franco E, Volpi A, Gabutti G. Herpes zoster in frail elderly patients: prevalence, impact, management, and preventive strategies. Aging Clin Exp Res. 2018;30:693‐702. [DOI] [PubMed] [Google Scholar]
- 8. Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2014;2:CD006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lang PO, Zarate‐Lagunes M, Pautex S. [Herpes zoster and post‐herpetic neuralgia in older adults]. Rev Med Suisse. 2008;4:2398‐2402, 2404. [PubMed] [Google Scholar]
- 10. Andrew MK, Shinde V, Ye L, et al. The importance of frailty in the assessment of influenza vaccine effectiveness against influenza‐related hospitalization in elderly people. J Infect Dis. 2017;216:405‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165‐1174. [DOI] [PubMed] [Google Scholar]
- 12. McElhaney JE, Dutz JP. Better influenza vaccines for older people: what will it take? J Infect Dis. 2008;198:632‐634. [DOI] [PubMed] [Google Scholar]
- 13. Ridda I, Macintyre CR, Lindley R, et al. Immunological responses to pneumococcal vaccine in frail older people. Vaccine. 2009;27:1628‐1636. [DOI] [PubMed] [Google Scholar]
- 14. Yao X, Hamilton RG, Weng NP, et al. Frailty is associated with impairment of vaccine‐induced antibody response and increase in post‐vaccination influenza infection in community‐dwelling older adults. Vaccine. 2011;29:5015‐5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087‐2096. [DOI] [PubMed] [Google Scholar]
- 16. Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019‐1032. [DOI] [PubMed] [Google Scholar]
- 17. Konrat C, Boutron I, Trinquart L, Auleley GR, Ricordeau P, Ravaud P. Underrepresentation of elderly people in randomised controlled trials. The example of trials of 4 widely prescribed drugs. PLoS One. 2012;7:e33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Song X, Mitnitski A, Rockwood K. Prevalence and 10‐year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681‐687. [DOI] [PubMed] [Google Scholar]
- 21. Curran D, Andrew MK, Levin MJ, et al. Evaluation of two frailty indices, with practical application in a vaccine clinical trial. Hum Vaccin Immunother. 2019;15:2960‐2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ware JE Jr. SF‐36 health survey update. Spine (Phila Pa 1976). 2000;25:3130‐3139. [DOI] [PubMed] [Google Scholar]
- 23. Rabin R, de Charro F. EQ‐5D: a measure of health status from the EuroQol group. Ann Med. 2001;33:337‐343. [DOI] [PubMed] [Google Scholar]
- 24. Coplan PM, Schmader K, Nikas A, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5:344‐356. [DOI] [PubMed] [Google Scholar]
- 25. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271‐2284. [DOI] [PubMed] [Google Scholar]
- 26. Curran D, Oostvogels L, Heineman T, et al. Quality of life impact of an adjuvanted recombinant zoster vaccine in adults aged 50 years and older. J Gerontol A Biol Sci Med Sci. 2019;74:1231‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cunningham AL, Heineman TC, Lal H, et al. Immune responses to a recombinant glycoprotein E herpes zoster vaccine in adults aged 50 years or older. J Infect Dis. 2018;217:1750‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnstone J, Parsons R, Botelho F, et al. Immune biomarkers predictive of respiratory viral infection in elderly nursing home residents. PLoS One. 2014;9:e108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cunningham AL, Heineman T. Vaccine profile of herpes zoster (HZ/su) subunit vaccine. Expert Rev Vaccines. 2017;16:1‐10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File S1: Frailty assessment
Supplementary TableS1: Details of components of frailty index
Supplementary Table S2: Detail of medical history search items
Supplementary Table S3: Vaccine efficacy estimates (modified total vaccinated cohort)
Supplementary Table S4: Number of participants in the various cohorts (total vaccinated cohort)
Supplementary Table S5: Summary of demographic characteristics
Supplementary Table S6: Incidence and nature of symptoms (solicited only) reported during the 7‐day (days 0–6) post‐vaccination period overall per participant by frailty status (total vaccinated cohort ‐ diary card subset)
Supplementary Figure S1: Percentages of participants reporting any unsolicited adverse events within the 30‐day (days 0–29) post‐vaccination by frailty status (total vaccinated cohort)


