Abstract
Introduction
We investigated and compared associations of objective estimates of sleep and 24‐hour activity rhythms using actigraphy with risk of dementia.
Methods
We included 1322 non‐demented participants from the prospective, population‐based Rotterdam Study cohort with valid actigraphy data (mean age 66 ± 8 years, 53% women), and followed them for up to 11.2 years to determine incident dementia.
Results
During follow‐up, 60 individuals developed dementia, of which 49 had Alzheimer's disease (AD). Poor sleep as indicated by longer sleep latency, wake after sleep onset, and time in bed and lower sleep efficiency, as well as an earlier “lights out” time, were associated with increased risk of dementia, especially AD. We found no associations of 24‐hour activity rhythms with dementia risk.
Discussion
Poor sleep, but not 24‐hour activity rhythm disturbance, is associated with increased risk of dementia. Actigraphy‐estimated nighttime wakefulness may be further targeted in etiologic or risk prediction studies.
Keywords: 24‐hour activity rhythms, actigraphy, Alzheimer's disease, cohort, dementia, epidemiology, longitudinal, population‐based, prospective, rest‐activity rhythms, sleep
1. INTRODUCTION
Sleep is essential to the brain as it supports learning and memory, regulates synaptic plasticity, and enhances waste clearance from the brain. 1 , 2 Conversely, disturbed sleep may harm the brain through increased neuro‐inflammation 3 or atherosclerosis, 4 or by accumulation of detrimental proteins involved in Alzheimer's disease (AD) pathology. 1 , 5 Against this background, sleep disturbances have been associated with incident dementia 6 , 7 and as such may be regarded as a potential risk factor, or as an early feature of disease before a diagnosis can be made.
Sleep is closely related to the circadian timing system, 8 functioning of which is reflected behaviorally in 24‐hour rhythms of physical activity. Disturbed 24‐hour activity rhythms have also been linked to dementia risk. 9 , 10 , 11 Yet, it remains unknown how sleep and 24‐hour activity rhythms compare with respect to dementia risk, and to what extent these aspects contribute to risk independent from each other. 12 Also, we need to consider relevant interactions, such as that of sleep disturbances with presence of the apolipoprotein E ε4 (APOE ε4) allele on risk of AD. 13 Last, only a minority of population‐based studies investigated objectively measured sleep in relation to dementia risk, while most studies 6 , 7 , 14 measured sleep using self‐report measures as these are feasible to obtain in large study populations. Although important for evaluating sleep, 15 self‐report measures may hamper attributing associations to sleep per se as they rely on cognitive and affective factors that determine the subjective appraisal of sleep. 16
Sleep and 24‐hour activity rhythms may be independently inferred from physical activity measurements over multiple days using actigraphy. In this study, we investigated associations of actigraphy‐derived sleep and 24‐hour activity rhythm parameters with the risk of dementia, using data from the population‐based Rotterdam Study cohort. We followed 1322 middle‐aged and elderly individuals (mean age 66 years) for more than 11 years. We compared sleep and 24‐hour activity rhythm parameters using mutually adjusted models and investigated effect‐modification by APOE ε4 status.
2. METHODS
2.1. Study setting and population
This study is embedded in the Rotterdam Study, a prospective population‐based cohort starting in 1990. Participants were recruited from inhabitants of a representative suburban district of a large Dutch city, independent of health‐care seeking. 17 Participants underwent a 2‐hour interview at home, and subsequently underwent an extensive set of examinations (a total of 5 hours) during 2 visits to a dedicated research center strategically placed in the district center. Rounds are repeated every 4 to 5 years. In parallel, incident disease is assessed continuously with electronic linkage between the study database and medical records.
The Rotterdam Study has been approved by the Medical Ethics Committee of the Erasmus Medical Center. All participants provided written informed consent for participation and to have medical information obtained from their treating physicians. We reported this study in line with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (see checklist in supporting information).
Between September 2004 and March 2007 (baseline of the current study), we invited participants who visited the research center and were deemed able to understand instructions to keep an actigraph for 7 days and also complete a daily sleep diary. A 7‐day recording duration was chosen to balance an incrementally lower added value of extra days of recording 18 with a prolonged participant burden in this population‐based study. For this first assessment of actigraphy in our cohort, we invited a subset of 2632 participants, of whom 2063 (78%) aged 62.4 ± 9.4 years accepted. We excluded participants with: (a) actigraph malfunctioning (n = 197); (b) less than 96 hours of consecutive recording (n = 109); (c) measurements during daylight savings (n = 23); (d) missing information on dementia status (n = 54). Last, we excluded persons aged <55 years at baseline, as those were considered not at risk for dementia in a population‐based setting (n = 358; see Figure S1 in supporting information for a flowchart of participant selection). 19 The 1322 included individuals were on average 2.5 years younger, 8% less likely to be female, and had a 0.4 higher Mini‐Mental Status Examination score, but did not differ in questionnaire‐assessed sleep or bedtimes compared to invited persons aged ≥55 who did not participate (n = 768). Included participants were followed until onset of dementia; loss to follow‐up; death; or January 1, 2016. Follow‐up totaled to 11,630 person‐years (95% of the possible total without loss to follow‐up 20 ; 8.8 years per person on average).
RESEARCH IN CONTEXT
Systematic review: We reviewed the literature through PubMed and references of relevant articles on the association of actigraphy‐estimated sleep and 24‐hour activity rhythms with incident cognitive decline or dementia: longitudinal studies were scarce. None investigated both sleep and 24‐hour activity rhythm parameters or compared associations. Relevant potential interactions, such as those with apolipoprotein E4 (APOE‐ɛ4), should be taken into account.
Interpretation: Findings indicate that actigraphy‐estimated nighttime wakefulness, but not a fragmented or unstable 24‐hour activity rhythm, plays a role in dementia etiology. A phase advance of sleep and “lights out” time may indicate prodromal dementia.
Future directions: Future studies may (a) focus on the role of actigraphy‐estimated poor sleep, specifically nighttime wakefulness, in dementia etiology; (b) further investigate potential effect‐modification by APOE‐ɛ4; and (c) further investigate potential prodromal features such as a phase advance of sleep or earlier “lights out” time.
2.2. Sleep and 24‐hour activity rhythms
Participants wore an actigraph around the wrist (ActiWatch model AW4, Cambridge Technology Ltd) for 138 ± 14 hours (median = 144) and completed a sleep diary during the same time period. 21 The Actiwatch is a research‐grade actigraphy device, used to assess both sleep and 24‐hour activity rhythms in a research and clinical setting, which has been validated against polysomnography. 22 , 23 Participants pressed a marker button on the device to denote “lights out” time (time intending to go to sleep) and getting up time. Missing marker times (21% of all time values) were imputed from the sleep diary, or estimated by inspecting actigraphy recordings when sleep diaries were missing. Within the defined time in bed, total sleep time and wakefulness were estimated using a validated algorithm with a threshold of 20 activity counts. 22 Counts were summed per 30‐second epochs. We defined “sleep onset” as the midpoint of the first immobile period lasting ≥10 minutes after “lights out” with ≤1 epoch of movement. Sleep‐onset latency was calculated as the time from “lights out” to sleep onset, and wake after sleep onset was calculated as the wakefulness after sleep onset. Sleep efficiency was calculated as total sleep time/time in bed × 100%.
We calculated the following indicators of the 24‐hour activity rhythm 24 , 25 : Intradaily variability, which quantifies the amount of alterations of activity‐inactivity; interdaily stability, which quantifies how activity profiles across days resemble each other; and the average time of day when the least active 5 consecutive hours started (L5 onset) indicating phase of most inactivity. We chose these non‐parametric indicators of the 24‐hour activity rhythm as these are thought to better represent the non‐sinusoidal form of the rhythm in older adults. 26
Correlations among sleep and 24‐hour activity rhythm parameters at baseline in a similar study population have been reported previously. 27
2.3. Dementia
Diagnosing dementia involved cognitive screening for all participants visiting the research center. We further assessed individuals scoring a Mini‐Mental State Examination <26 or Geriatric Mental Schedule organic level >0 with the Cambridge Mental Disorders of the Elderly Examination, including a spouse or informant interview. Regardless of attending the research center, for all participants we surveilled medical records of general practitioners and the regional institute for outpatient mental health care for dementia. As a result, dates of diagnoses were not centered around examination rounds but distributed throughout the follow‐up. A consensus panel adjudicated diagnoses according to standard criteria. In this study, we considered the outcomes of all‐cause dementia (Diagnostics and Statistical Manual of Mental Disorders—3rd Edition Revised [DSM‐III‐R]; hereafter: dementia), and AD (National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association [NINCDS—ADRDA]; including confirmed, probable, and possible AD).
2.4. Covariates
For our etiological study, we selected potential confounders, or proxies for unmeasured confounders, based on theoretical knowledge as recommended in the disjunctive cause criterion. 28 We considered age, sex, education (categorized as primary, secondary/lower vocational, intermediate vocational, and higher vocational/university), paid employment, self‐reported physical activity, 29 , 30 habitual alcohol consumption, body mass index, positive history of cardiovascular disease (transient ischemic attack [TIA], stroke, heart disease), smoking status, presence of hypertension, and presence of diabetes mellitus as potential confounders. Measurements of covariates took place during home interviews or at research center visits and are described in detail elsewhere. 31 For the sensitivity analyses we assessed depressive symptoms 32 (Centre for Epidemiological Studies–Depression Scale [CES‐D]), possible sleep apnea (two questions of the Pittsburgh Sleep Quality Index [PSQI]), 33 napping (napping per day during daytime and evenings according to the sleep diary), and number of APOE ε4 alleles. 31
2.5. Statistical analysis
We used Cox proportional hazards regression models to associate sleep and 24‐hour activity rhythm parameters (independent variable) with incident dementia and AD (dependent variable). We adjusted analyses for confounders by adding as independent variables age and sex in model 1, and age, sex, educational level, employment status, physical activity, alcohol consumption, body mass index, smoking status, history of cardiovascular disease, presence of hypertension, and presence of diabetes mellitus in model 2. We also investigated non‐linearity in associations for total sleep time and time in bed by modeling a quadratic term. We additionally adjusted all associations of sleep and bedtime parameters observed in the main analysis for the 24‐hour activity rhythm variables, to evaluate their independence. In sensitivity analysis, we separately adjusted analyses for possible sleep apnea, napping, and number of APOE ε4 alleles, and restricted analyses to persons without clinically relevant depressive symptoms (CES‐D ≤16).
Post‐hoc, we restricted analyses to persons without paid employment. Also, we presented stratified results for all parameters by APOE ε4 genotype (≥1 ε4 allele versus no ε4‐alleles), age (≤75 vs >75), and sex on risk of dementia, and formally tested multiplicative interaction by modeling a product term. We evaluated statistical significance of interaction terms at P < .0016, defined by applying a Bonferroni correction for testing 10 parameters across three stratifications (P = .05/30).
Last, we explored whether associations depended on follow‐up time to provide some insight into possible reverse causation. 34 We performed analyses in increasingly longer epochs of follow‐up time from baseline (eg, baseline to 2 years, baseline to 4 years, etc), using Firth's penalized Cox regression to account for the smaller number of events. 35
Testing the proportional hazards assumption of the main analyses using Schoenfeld residuals indicated a violation for L5 onset. Please note that this non‐proportionality was not removed, but made insightful with aforementioned analysis. 34
Sleep variables were winsorized (ie, values of outliers changed toward the mean) to 3 standard deviations (SD) and subsequently standardized to facilitate comparison. Missing values on covariates (a median of 1% missing [interquartile range 0%‐7%]) were imputed using five multiple imputations, except APOE genotype, performed with IBM SPSS Statistics version 24 (IBM Corp, Armonk, NY, USA). Statistical analyses were performed with R software (packages: survival, coxphf).
3. RESULTS
We included 1322 participants at baseline (Table 1) aged 66.1 ± 7.6 years. During 11.2 years of follow‐up (median = 9.5), 60 individuals developed dementia, including 47 with AD.
TABLE 1.
Characteristics of study population at baseline
| Characteristic (unit) | Values (N = 1322) |
|---|---|
| Age at baseline (years) | 66.1 ± 7.6 |
| Female | 699 (53%) |
|
Educational level Primary education Lower/intermediate or lower vocational Higher or intermediate vocational Higher vocational or university |
109 (8%) 585 (44%) 390 (30%) 238 (18%) |
| Paid employment | 274 (21%) |
| Physical activity (MET‐hours/wk) | 62 (19‐96) |
| Alcohol consumption (g/d) | 9 (1‐20) |
|
Smoking status Never Former Current |
413 (31%) 695 (53%) 214 (16%) |
| Body mass index (kg/m2) | 28.0 ± 4.0 |
| History of cardiovascular disease | 1.1 (0.3–32.0) |
| Presence of hypertension | 888 (67%) |
| Presence of diabetes mellitus | 104 (8%) |
| Depressive symptoms (CES‐D score) | 3 (1‐7) |
| Possible sleep apnea | 369 (28%) |
| Napping (number of naps) | 1 (0‐3) |
| Presence of ≥1 APOE ε4 allele a | 346 (26%) |
| Total sleep time (hours) | 6.4 ± 0.9 |
| Sleep efficiency (%) | 79 (74‐83) |
| Wake after sleep onset (hours) | 1.1 (0.9‐1.4) |
| Sleep latency (minutes) | 13 (7‐22) |
| Time in bed (hours) | 8.2 ± 0.9 |
| Bedtime (“lights out”; hh:mm) | 23:50 ± 00:50 |
| Time getting up (hh:mm) | 08:05 ± 00:50 |
| Intradaily variability (score) | 0.40 (0.33‐0.49) |
| Interdaily stability (score) | 0.83 (0.76‐0.88) |
| Onset least active consecutive 5 hours (hh:mm) | 01:50 ± 01:08 |
Note: Characteristics of the study population at baseline. Values are expressed as No. (%) for categorical variables and mean ± standard deviation or median (1st quartile–3rd quartile) for continuous variables, unless specified otherwise. Includes imputed values for covariates.
Abbreviations: APOE, apolipoprotein E; CES‐D, Center for Epidemiological Studies–Depression Scale; MET, metabolic equivalent of task; N, sample size.
Missing 71 participants, including 3 persons with incident Alzheimer's disease.
3.1. Associations of sleep and 24‐hour activity rhythms with dementia risk
Longer sleep‐onset latency (hazard ratio [HR] per SD increase 1.44, 95% confidence interval [CI] 1.13‐1.83) and longer time in bed (HR 1.40, 95% CI 1.04‐1.88) were associated with an increased risk of dementia. A higher sleep efficiency (HR 0.72, 95% CI 0.55‐0.93) and later “lights out” time were associated with decreased dementia risk (HR 0.56, 95% CI 0.41‐0.76). For AD, aforementioned associations were stronger, including an association for longer wake after sleep onset (Table 2). In contrast, total sleep time was not associated with the risk of dementia (HR 0.97, 95% CI 0.74‐1.29) or AD (HR 0.92, 95% CI 0.68‐1.26, Table 2). Estimates were not meaningfully different when only adjusted for age and sex (Table 2).
TABLE 2.
Associations of sleep, bedtime, and 24‐hour activity rhythm parameters with incident dementia and Alzheimer's disease
| Dementia HR (95% CI) | Alzheimer's disease HR (95% CI) | |||
|---|---|---|---|---|
| Cases/N = 60/1322 | Cases/N = 49/1322 | |||
| Determinant (per SD increase) | Model 1 | Model 2 | Model 1 | Model 2 |
| Sleep | ||||
| Total sleep time | 0.99 (0.76‐1.30) | 0.97 (0.74‐1.29) | 0.95 (0.70‐1.28) | 0.92 (0.68‐1.26) |
| Sleep‐onset latency | 1.38 (1.10‐1.74) | 1.44 (1.13‐1.83) | 1.42 (1.11‐1.83) | 1.45 (1.11‐1.89) |
| Wake after sleep onset | 1.17 (0.92‐1.51) | 1.23 (0.95‐1.59) | 1.30 (1.00‐1.70) | 1.38 (1.05‐1.81) |
| Time in bed | 1.34 (1.00‐1.80) | 1.40 (1.04‐1.88) | 1.40 (1.01‐1.95) | 1.49 (1.06‐2.10) |
| Sleep efficiency | 0.78 (0.60‐1.00) | 0.72 (0.55‐0.93) | 0.72 (0.54‐0.94) | 0.66 (0.50‐0.87) |
| Bedtimes | ||||
| Time “lights out” | 0.57 (0.42‐0.76) | 0.56 (0.41‐0.76) | 0.55 (0.40‐0.76) | 0.53 (0.37‐0.74) |
| Time getting up | 0.79 (0.59‐1.06) | 0.79 (0.58‐1.08) | 0.81 (0.58‐1.13) | 0.79 (0.56‐1.13) |
| 24‐hour rhythm | ||||
| Intradaily variability | 1.06 (0.82‐1.38) | 1.07 (0.82‐1.40) | 1.04 (0.78‐1.40) | 1.05 (0.78‐1.41) |
| Interdaily stability | 0.93 (0.71‐1.22) | 0.92 (0.70‐1.20) | 0.90 (0.67‐1.21) | 0.87 (0.65‐1.17) |
| L5 onset | 0.88 (0.69‐1.13) | 0.92 (0.72‐1.17) | 0.85 (0.65‐1.12) | 0.88 (0.67‐1.16) |
Note: Hazard ratios (HRs) were obtained with Cox regression models. HRs above 1.00 denote that, with a higher value of the determinant, the hazards of dementia increases, while HRs below 1.00 indicate that dementia risk decreases. To compare HRs across determinants with different units, HRs are expressed per standard deviation increase. Model 1 is adjusted for age and sex. Model 2 is additionally adjusted for educational level, employment status, physical activity, alcohol consumption, body mass index, smoking status, history of cardiovascular disease, presence of hypertension, and presence of diabetes mellitus.
CI, confidence interval; HR, hazard ratio; L5, least active consecutive 5 hours of the day; N, sample size; SD, standard deviation.
We found no statistically significant non‐linearity after fitting quadratic terms for the associations of total sleep time (P value = .95) or time in bed (P value = .27) with dementia risk, nor with AD risk (P value = .44; P value = .30, respectively).
The 24‐hour activity rhythms were not associated with dementia risk (Table 2). Aforementioned associations of sleep parameters with dementia risk were also not affected by further adjustment for 24‐hour activity rhythm parameters (Table 3).
TABLE 3.
Associations of sleep parameters with incident dementia and Alzheimer's disease, additionally adjusted for 24‐hour activity rhythm parameters
| Determinant (per SD increase) | Dementia HR (95% CI) Cases/N = 60/1322 | Alzheimer's disease HR (95% CI) Cases/N = 49/1322 |
|---|---|---|
| Sleep | ||
| Total sleep time | 1.00 (0.74‐1.34) | 0.93 (0.67‐1.29) |
| Sleep‐onset latency | 1.52 (1.17‐1.97) | 1.53 (1.14‐2.05) |
| Wake after sleep onset | 1.25 (0.95‐1.64) | 1.42 (1.07‐1.90) |
| Time in bed | 1.44 (1.06‐1.95) | 1.52 (1.07‐2.15) |
| Sleep efficiency | 0.70 (0.52‐0.93) | 0.63 (0.46‐0.86) |
Note: Hazard ratios were obtained with Cox regression models, adjusted for main analysis confounder and additionally for intradaily variability, interdaily stability, and time of onset of the least active consecutive 5 hours of the day. Hazard ratios (HRs) above 1.00 denote that, with a higher value of the determinant, the hazards of dementia increases, while HRs below 1.00 indicate that dementia risk decreases. To compare HRs across determinants with different units, HRs are expressed per standard deviation increase. Confounders included age, sex, educational level, employment status, physical activity, alcohol consumption, body mass index, smoking status, history of cardiovascular disease, presence of hypertension, and presence of diabetes mellitus. In all models, no 24‐hour activity rhythm parameter was statistically significant at P value <.05. We observed no multicollinearity: All variance inflation factors were lower than 2.
Abbreviations; CI, confidence interval; HR, hazard ratio; N, sample size; SD, standard deviation.
Estimates remained similar after separate further adjustment for possible sleep apnea, number of naps, or number of APOE ε4 alleles (Table S1 in supporting information). Also, restricting analyses to persons without clinically relevant depressive symptoms did not substantially affect estimates (Table S2 in supporting information). Post‐hoc restriction to individuals without paid employment also showed no meaningful influence on our estimates (Table S3 in supporting information).
3.2. Effect modification by APOE ε4, age, and sex
Stratifying by APOE ε4 suggested that associations of sleep parameters with increased risk of dementia were present only in ε4‐negative individuals (Table 4), but when formally tested no sleep‐by‐APOE ε4 interaction term survived multiple testing.
TABLE 4.
Effect‐modification of associations of sleep, bedtime, and 24‐hour activity rhythm parameters with risk of dementia by APOE ε4
| Dementia HR (95% CI), APOE‐stratified | |||
|---|---|---|---|
| Determinant (per SD increase) | ε4 carriers Cases/N a = 21/346 | ε4 non‐carriers Cases/N a = 36/905 | Interaction P |
| Sleep | |||
| Total sleep time | 1.04 (0.65‐1.66) | 0.96 (0.68‐1.35) | .89 |
| Sleep‐onset latency | 1.28 (0.82‐2.01) | 1.51 (1.10‐2.06) | .38 |
| Wake after sleep onset | 0.83 (0.49‐1.39) | 1.63 (1.19‐2.25) | .01 |
| Time in bed | 1.14 (0.72‐1.82) | 1.73 (1.16‐2.57) | .02 |
| Sleep efficiency | 0.95 (0.57‐1.56) | 0.61 (0.44‐0.84) | .04 |
| Bedtimes | |||
| ”Lights out” time | 0.52 (0.31‐0.87) | 0.42 (0.27‐0.65) | .12 |
| Getting up time | 0.55 (0.31‐0.98) | 0.84 (0.56‐1.26) | .20 |
| 24‐hour activity rhythm | |||
| Intradaily variability | 0.75 (0.41‐1.36) | 1.16 (0.85‐1.60) | .03 |
| Interdaily stability | 1.36 (0.75‐2.47) | 0.85 (0.62‐1.18) | .07 |
| L5 onset | 0.70 (0.42‐1.16) | 0.94 (0.69‐1.29) | .48 |
Note: Hazard ratios were obtained from Cox regression models, adjusted for age and sex (if applicable), and educational level, employment status, physical activity, alcohol consumption, body mass index, smoking status, history of cardiovascular disease, presence of hypertension, and presence of diabetes mellitus. We tested interaction through modeling a product term of the unstandardized determinant with the number of APOE ε4 alleles.
Abbreviations: APOE, apolipoprotein E gene; CI, confidence interval; L5, least active consecutive 5 hours of the day; N, sample size.
Missing data on APOE ε4 genotype for 71 individuals in total, of whom 3 had incident Alzheimer's disease.
Age‐stratified analyses did not show a consistent pattern of differences in associations across age, and we found no statistically significant multiplicative interactions with age (Table S4 in supporting information).
Sex‐stratified analyses showed shorter total sleep time was associated with lower dementia risk in women, opposite to the direction of the point estimate in men. Vice versa, longer time in bed was associated with increased dementia risk only in men (Table S4). Yet, we found no statistically significant interactions with sex.
3.3. Increasing epochs of follow‐up time
For the sleep parameters, hazard ratio estimates remained mostly similar over increasing follow‐up time (Figure 1A). The strong association of later “lights out” with lower dementia risk in the first 2 years of follow‐up (HR 0.27, 95% CI 0.10‐0.73) attenuated with increasing follow‐up time (Figure 1B). Later L5 onset was associated with lower dementia risk in the first 2 years of follow‐up only (HR 0.23, 95% CI 0.09‐0.61; Figure 1C). Incident cases in this period all had AD. Overall, findings were similar for AD.
FIGURE 1.
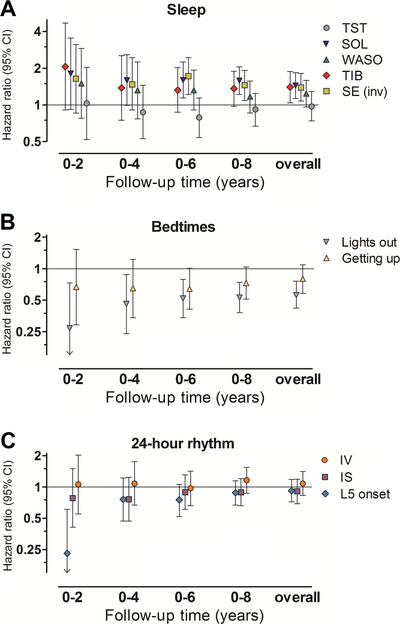
Associations of sleep, bedtime, and 24‐hour activity rhythm parameters with incident dementia, over increasing epochs of follow‐up time. NOTE. Associations of (A) sleep, (B) bedtimes, and (C) 24‐hour activity rhythm parameters with risk of dementia are shown for increasing epochs of follow‐up time within the study timeframe. Hazard ratios for epochs in shorter follow‐up time were obtained using multivariate Firth's penalized Cox regression models. We obtained estimates after censoring all participants still at risk at 2 years (8 incident dementia cases), 4 years (15 cases), 6 years (28 cases), 8 years (47 cases), and after the total follow‐up of 11.2 years after baseline (60 cases). Hazard ratios are adjusted for age, sex, educational level, employment status, physical activity, alcohol consumption, body mass index, smoking status, history of cardiovascular disease, presence of hypertension, and presence of diabetes mellitus, are expressed per standard deviation increase in the parameter, and plotted at a log2‐scale. Please note that estimates obtained for sleep efficiency were inversed (transformed as 1/estimate depicting sleep “inefficiency”) for graphical comparison of effect sizes of sleep parameters. CI, confidence interval; IS, interdaily stability; IV, intradaily variability; L5, least active consecutive 5 hours of the day; SE, sleep efficiency; SOL, sleep‐onset latency; TIB, time in bed; TST, total sleep time; WASO, wake after sleep onset
4. DISCUSSION
In the general population, actigraphy‐estimated longer sleep‐onset latency, longer wake after sleep onset, longer time in bed, and lower sleep efficiency, as well as earlier “lights out” time, were associated with a higher risk of dementia. In contrast, 24‐hour activity rhythm fragmentation or stability did not influence dementia risk.
Several methodological considerations should be mentioned. Actigraphy‐derived behavioral rhythms do not necessarily equate to the endogenous circadian rhythm. Additionally, the gold standard for measuring sleep is polysomnography, which may especially classify sleep‐onset latency more accurately. Potential misclassification of sleep and circadian rhythms, and the low number of incident cases in this study, may have reduced our power to detect small effect sizes. Also, we could not assess the extent to which preclinical amyloid beta (Aβ) or tau pathology, which may affect sleep‐wake regulating brainstem regions 36 years before dementia diagnosis, 37 , 38 confounded associations with dementia risk. Last, selection bias may have influenced our findings, although characteristics of included and non‐included participants were largely similar.
Our study adds to previous actigraphy‐based studies 9 , 11 , 13 , 39 , 40 , 41 by showing that disturbed sleep is more predictive of developing dementia than disrupted 24‐hour activity rhythms. Instead of total sleep time, it was rather an increased amount of wakefulness when in bed, in line with previous findings, 9 , 39 and an advanced “lights out” time that determined dementia risk. We speculate that this indicates that a reduced capability to sleep when in bed drives dementia risk, rather than, for example, deliberate lifestyle choices to curtail sleep. Our findings suggest that individuals may have tried to adapt to such an “incapability” to sleep by increasing time in bed, mainly by advancing “lights out” time, to maintain a sufficient amount of sleep. Several mechanisms could underlie this incapability to sleep.
First, associations may indicate presence of an underlying disease process that both increases dementia risk and impairs sleep, for which accumulation of AD pathology 42 in the brain seems to be a likely 43 substrate. Such confounding, however, is not in line with the finding that associations for poor sleep seemed restricted to APOE ε4 non‐carriers, and not ε4‐carriers who are at increased risk of having more brain Aβ deposition at this age. 43 Also, a previous study in a sample with the same age distribution and similar sociodemographic characteristics found that high intradaily variability was related strongest to a cerebrospinal fluid biomarker profile suggestive of preclinical AD. 44 Yet, intradaily variability was unrelated to incident dementia or AD in our study. Also arguing against confounding by preclinical pathology are the time‐stratified analyses, which showed that poor sleep was not associated substantially more strongly with dementia risk in short versus longer follow‐up durations. If preclinical pathology would drive these associations, one would expect to see stronger associations when sleep is measured closer to the diagnosis. Second, the slowly progressing dementia process may impair sleep not directly but through emergence of prodromal features such as behavioral or neuropsychiatric symptoms. This mechanism may be less likely as associations were also present in persons without depressive symptoms, and independent of self‐reported napping. Third, sleep disorders, particularly the presence of sleep‐disordered breathing, may underlie some of the associations of poor sleep with dementia risk. 45 Sleep‐disordered breathing may instigate neurodegenerative processes through intermittent hypoxia and oxidative stress, or through cardiovascular or proteostatic mechanisms. 46 We could only account for such effects by adjusting for an estimation of possible sleep apnea based on two PSQI questions regarding snoring and breathing pauses. In future work, sleep‐disordered breathing should be taken into account by assessing these with respiratory sensors overnight. Further research to disentangle the specific roles of actigraphy‐estimated nighttime wakefulness and sleep‐disordered breathing in neurodegenerative or AD pathologies remains needed. Future studies may also consider relating changes in actigraphy‐estimated sleep or 24‐hour activity rhythms over repeated measurements, or specific measures of sleep fragmentation, with risk of dementia or related outcomes.
Another remark regarding our APOE‐stratified findings is that, interestingly, associations of sleep with dementia risk seemed restricted to APOE ε4 non‐carriers, although we found no statistically significant interactions after correcting for multiple testing. Possibly, disturbed sleep and carrying APOE ε4 impact dementia risk similarly, for example, through protein misfolding, 42 synaptic, 47 or hematopoietic effects. 4 The damage accumulated by carrying ε4 throughout life then marginalizes potential harmful effects that disturbed sleep, or what underlies it, may have on dementia risk. The discrepancy of our findings with previous work, 13 reporting that sleep fragmentation increases risk of AD only in APOE ε4‐carriers, is not readily explained. Possibly, survival bias in this previous study 13 through including old (mean age >80) ε4‐carriers, 48 , 49 or modeling poor sleep differently may have played a role.
We could not confirm the hypothesis that circadian disturbances, reflected by variability and stability of activity rhythms, are implicated 50 in dementia etiology. Yet, the association of earlier L5 onset with increased dementia risk in the next 2 years suggests a phase advance of nighttime inactivity as a prodromal feature of dementia and AD. Heterogeneity of activity rhythm findings in dementia risk, including ours, with regard to the direction of a prodromal phase shift 10 and use of different modeling strategies 11 , 51 should be further investigated.
We studied a relatively young age group (mean age 66 years), which we felt may provide insights particularly interesting for prediction or prevention. Importantly, age at baseline did not substantially modify our results, suggesting that our findings may be compared to that of previous studies that used samples with mean ages between 76 and 83 years. 9 , 11 , 13 , 39 , 40 , 41
In conclusion, actigraphy‐estimated nighttime wakefulness indicating an incapability to sleep is associated with an increased risk of dementia, especially AD. At the same time, circadian disturbances as reflected in 24‐hour activity rhythms played a limited role in dementia risk in this population of middle‐aged and elderly persons.
FUNDING INFORMATION
The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam; Netherlands Organization for the Health Research and Development (ZonMw); the Ministry of Education; Culture and Science; the Ministry for Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam.
This work was funded by a grant from the Netherlands Organization for Scientific Research (NWO‐VIDI: 017.106.370) to Henning Tiemeier.
No funding body influenced the study design; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the article for publication.
Thom S. Lysen and M. Arfan Ikram had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
AUTHOR CONTRIBUTIONS
Thom S. Lysen, Annemarie I. Luik, Henning Tiemeier, M. Arfan Ikram made substantial contributions to the conception and design of the work. Annemarie I. Luik, M. Kamran Ikram, Henning Tiemeier, M. Arfan Ikram supervised the acquisition of the data. Thom S. Lysen performed the data analysis. All authors contributed to interpreting the data. All authors contributed to drafting this manuscript and critically revising it for important intellectual content. All authors approved the final version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
The authors are grateful to the study participants, the staff from the Rotterdam Study, and the participating general practitioners and pharmacists.
Lysen TS, Luik AI, Ikram MK, Tiemeier H, Ikram MA. Actigraphy‐estimated sleep and 24‐hour activity rhythms and the risk of dementia. Alzheimer's Dement. 2020;16:1259–1267. 10.1002/alz.12122
REFERENCES
- 1. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cirelli C, Tononi G. The sleeping brain. Cerebrum. 2017;2017:cer‐07‐17. [PMC free article] [PubMed] [Google Scholar]
- 3. Bellesi M, de Vivo L, Chini M, Gilli F, Tononi G, Cirelli C. Sleep loss promotes astrocytic phagocytosis and microglial activation in mouse cerebral cortex. J Neurosci. 2017;37(21):5263‐5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McAlpine CS, Kiss MG, Rattik S, et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. 2019;566(7744):383‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holth JK, Fritschi SK, Wang C, et al. The sleep‐wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bubu OM, Brannick M, Mortimer J, et al. Sleep, cognitive impairment and Alzheimer's disease: a systematic review and meta‐analysis. Sleep. 2017;40(1). [DOI] [PubMed] [Google Scholar]
- 7. Shi L, Chen SJ, Ma MY, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta‐analysis. Sleep Med Rev. 2018;40:4‐16. [DOI] [PubMed] [Google Scholar]
- 8. Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195‐204. [PubMed] [Google Scholar]
- 9. Diem SJ, Blackwell TL, Stone KL, et al. Measures of sleep‐wake patterns and risk of mild cognitive impairment or dementia in older women. Am J Geriatr Psychiatry. 2016;24(3):248‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019;18(3):307‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70(5):722‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Musiek ES. Circadian clock disruption in neurodegenerative diseases: cause and effect? Front Pharmacol. 2015;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim AS, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein E epsilon4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol. 2013;70(12):1544‐1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lysen TS, Wolters FJ, Luik AI, Ikram MK, Tiemeier H, Ikram MA. Subjective sleep quality is not associated with incident dementia: the rotterdam study. J Alzheimers Dis. 2018;64(1):239‐247. [DOI] [PubMed] [Google Scholar]
- 15. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008;9(Suppl 1):S10‐S17. [DOI] [PubMed] [Google Scholar]
- 17. Ikram MA, Brusselle G, Ghanbari M, et al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol. 2020;35(5):483‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Someren EJW. Improving actigraphic sleep estimates in insomnia and dementia: how many nights? J Sleep Res. 2007;16(3):269‐275. [DOI] [PubMed] [Google Scholar]
- 19. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63‐75 e2. [DOI] [PubMed] [Google Scholar]
- 20. Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow‐up. Lancet. 2002;359(9314):1309‐1310. [DOI] [PubMed] [Google Scholar]
- 21. Van Den Berg JF, Van Rooij FJ, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population‐based study of elderly persons. J Sleep Res. 2008;17(3):295‐302. [DOI] [PubMed] [Google Scholar]
- 22. Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep‐disordered patients. Sleep Med. 2001;2(5):389‐396. [DOI] [PubMed] [Google Scholar]
- 23. Ancoli‐Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342‐392. [DOI] [PubMed] [Google Scholar]
- 24. Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest‐activity rhythm in aging and Alzheimer's disease. Biol Psychiatry. 1990;27(6):563‐572. [DOI] [PubMed] [Google Scholar]
- 25. Van Someren EJ. Actigraphic monitoring of sleep and circadian rhythms. Handb Clin Neurol. 2011;98:55‐63. [DOI] [PubMed] [Google Scholar]
- 26. Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. Bright light therapy: improved sensitivity to its effects on rest‐activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16(4):505‐518. [DOI] [PubMed] [Google Scholar]
- 27. Luik AI, Zuurbier LA, Direk N, Hofman A, Van Someren EJ, Tiemeier H. 24‐hour activity rhythm and sleep disturbances in depression and anxiety: a population‐based study of middle‐aged and older persons. Depress Anxiety. 2015;32(9):684‐692. [DOI] [PubMed] [Google Scholar]
- 28. VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol. 2019;34(3):211‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caspersen CJ, Bloemberg BP, Saris WH, Merritt RK, Kromhout D. The prevalence of selected physical activities and their relation with coronary heart disease risk factors in elderly men: the Zutphen Study, 1985. Am J Epidemiol. 1991;133(11):1078‐1092. [DOI] [PubMed] [Google Scholar]
- 30. Stel VS, Smit JH, Pluijm SM, Visser M, Deeg DJ, Lips P. Comparison of the LASA Physical Activity Questionnaire with a 7‐day diary and pedometer. J Clin Epidemiol. 2004;57(3):252‐258. [DOI] [PubMed] [Google Scholar]
- 31. Licher S, Heshmatollah A, van der Willik KD, et al. Lifetime risk and multimorbidity of non‐communicable diseases and disease‐free life expectancy in the general population: A population‐based cohort study. PLoS Med. 2019;16(2):e1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES‐D): results from a community‐based sample of older subjects in The Netherlands. Psychol Med. 1997;27(1):231‐235. [DOI] [PubMed] [Google Scholar]
- 33. Fogelholm M, Kronholm E, Kukkonen‐Harjula K, Partonen T, Partinen M, Harma M. Sleep‐related disturbances and physical inactivity are independently associated with obesity in adults. Int J Obes (Lond). 2007;31(11):1713‐1721. [DOI] [PubMed] [Google Scholar]
- 34. Hernan MA. The hazards of hazard ratios. Epidemiology. 2010;21(1):13‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heinze G, Dunkler D. Avoiding infinite estimates of time‐dependent effects in small‐sample survival studies. Stat Med. 2008;27(30):6455‐6469. [DOI] [PubMed] [Google Scholar]
- 36. Saper CB. The neurobiology of sleep. Continuum (Minneap Minn). 2013;19(1 Sleep Disorders):19‐31. [DOI] [PubMed] [Google Scholar]
- 37. Mander BA, Marks SM, Vogel JW, et al. beta‐amyloid disrupts human NREM slow waves and related hippocampus‐dependent memory consolidation. Nat Neurosci. 2015;18(7):1051‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Theofilas P, Dunlop S, Heinsen H, Grinberg LT. Turning on the light within: subcortical nuclei of the isodentritic core and their role in Alzheimer's disease pathogenesis. J Alzheimers Dis. 2015;46(1):17‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blackwell T, Yaffe K, Laffan A, et al. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community‐dwelling men: the MrOS sleep study. Sleep. 2014;37(4):655‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer's disease and cognitive decline in older persons. Sleep. 2013;36(7):1027‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rogers‐Soeder TS, Blackwell T, Yaffe K, et al. Rest‐activity rhythms and cognitive decline in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2018;66(11):2136‐2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease? Trends Neurosci. 2016;39(8):552‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta‐analysis. JAMA. 2015;313(19):1924‐1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, Ju YS. Circadian rest‐activity pattern changes in aging and preclinical Alzheimer disease. JAMA Neurol. 2018;75(5):582‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yaffe K, Laffan AM, Harrison SL, et al. Sleep‐disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buratti L, Luzzi S, Petrelli C, et al. Obstructive sleep apnea syndrome: an emerging risk factor for dementia. CNS Neurol Disord Drug Targets. 2016;15(6):678‐682. [DOI] [PubMed] [Google Scholar]
- 47. Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62(2):143‐150. [DOI] [PubMed] [Google Scholar]
- 48. Xu M, Zhao J, Zhang Y, et al. Apolipoprotein E gene variants and risk of coronary heart disease: a meta‐analysis. Biomed Res Int. 2016;2016:3912175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van der Lee SJ, Wolters FJ, Ikram MK, et al. The effect of APOE and other common genetic variants on the onset of Alzheimer's disease and dementia: a community‐based cohort study. Lancet Neurol. 2018;17(5):434‐444. [DOI] [PubMed] [Google Scholar]
- 50. Musiek ES. Circadian Rhythms in AD pathogenesis: a critical appraisal. Curr Sleep Med Rep. 2017;3(2):85‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li P, Yu L, Lim ASP, et al. Fractal regulation and incident Alzheimer's disease in elderly individuals. Alzheimers Dement. 2018;14(9):1114‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


