Abstract
Aim
To present the first case series of patients with Langerhans cell histiocytosis (LCH) also affected by Crohn's disease (CD), both of which are granulomatous diseases, and in LCH investigate the role of interleukin (IL)‐23, which is a well‐described disease mediator in CD.
Methods
A case series of three patients with LCH and CD were described; a cohort of LCH patients (n = 55) as well as controls (n = 55) were analysed for circulating IL‐23 levels; and the relation between the percentage of LCH cells in lesions and circulating IL‐23 levels was analysed in seven LCH patients.
Results
Differential diagnostic challenges for these two granulomatous diseases were highlighted in the case series, and it took up to 3 years to diagnose CD. Elevated IL‐23 levels were found in LCH patients. The amount of lesional LCH cells correlated with the levels of circulating IL‐23.
Conclusion
Both CD and LCH should be considered in patients with inflammatory gastrointestinal involvement. The IL‐23 pathway is a common immunological trait between these two granulomatous diseases.
Keywords: Crohn's disease, interleukin‐23, Langerhans cell histiocytosis
Abbreviations
- CD
Crohn's disease
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- IL
interleukin
- LCH
Langerhans cell histiocytosis
- STAT3
signal transducer and activator of transcription 3
- Th
T helper
- TNF
tumour necrosis factor
Key notes.
We present the first case series report of LCH patients that subsequently developed Crohn's disease (CD) or vice versa.
Both LCH and CD should be considered in patients with inflammatory gastrointestinal involvement, also in patients already affected by one of these diseases.
The IL‐23 pathway is a common immunological trait between LCH and CD.
1. INTRODUCTION
Paul Langerhans (1847‐1888) first described Langerhans cells in the skin, today known as tissue‐resident macrophages of embryonic origin. Langerhans cell histiocytosis (LCH) is an inflammatory myeloid neoplasia with genetic alterations in the mitogen‐activated protein kinase pathway and a minimal incidence in children of 4.6‐8.9 per million with a slight male predominance (1.2:1), and with the highest incidence so far reported in a population‐based study from Sweden. 1 , 2 LCH has a broad clinical presentation, ranging from single system self‐resolving disease in bone and skin, to multisystem risk organ disease that is life threatening. 2 Based on an autopsy, Keeling and Harries first reported a case of gastrointestinal (GI)‐LCH, 3 which may present with failure to thrive, anaemia, abdominal pain, bloody stool, diarrhoea and hypoalbuminemia, in paediatric patients, typically associated with systemic disease and poor prognosis. 4 GI‐LCH presents diagnostic and therapeutic challenges related to the diffuse and heterogeneous nature of its GI symptomatology, overlapping with other GI disorders such as Crohn's disease (CD).
There have been large increases in the incidence of both paediatric CD and ulcerative colitis over the last 50 years that now appear to be stabilising in the western world (Western Europe, USA, Canada, Australia, New Zealand). Incidence rates for paediatric CD have increased up to 9 or 10 per 100 000 person‐years in parts of Europe, including Scandinavia. 5 As with LCH, a moderate male predominance has been observed for CD. 6
Here, we present the first case series of LCH patients also affected by CD, both granulomatous disorders, and investigate underlying immunological disease mechanisms with focus on interleukin (IL)‐23 signalling. IL‐23 is an upstream regulator that, through signal transducer and activator of transcription 3 (STAT3) signalling, orchestrate T helper (h) 17 cell responses and downstream cytokines such as IL‐22 and IL‐17A. 7 Myeloid cells constitute a well‐recognised source of IL‐23 in inflammatory and neoplastic settings, but its role in LCH is unclear. Notably, drugs targeting the two subunits of IL‐23, namely p40 and p19, are being tested in trials for multiple inflammatory conditions. 7 Ustekinumab (targeting p40) is today widely licensed for CD treatment and used especially in patients that do not respond to blockage of tumour necrosis factor (TNF), a cytokine also targeted in LCH and with, except for single case report studies, no definitively established effect. 8 , 9 , 10 While genetic mechanisms underlying LCH have led to significant advances in understanding LCH pathogenesis, immunobiology studies focusing on contributions of individual clinically targetable cytokines, such as IL‐23, are warranted. Here, in addition to reporting the first case series of patients with both LCH and CD, we therefore also have addressed the role of IL‐23 in LCH.
2. MATERIALS AND METHODS
2.1. Patient recruitment
The three case series patients were recruited via the network of the Histiocyte Society, at the Department of Pediatric Oncology, Karolinska University Hospital, Stockholm, Sweden; Fiona Elsey Cancer Research Institute, Ballarat, Australia; and Department of Internal Medicine, Hôpital Pitié‐Salpêtrière, Paris, France. For plasma analysis, LCH patients with biopsy‐proven diagnosis and controls were enrolled at the Karolinska University Hospital, Stockholm. Patients with LCH were enrolled at the Department of Pediatric Oncology (n = 51) and the Department of Hematology (n = 4), while healthy adult volunteers were enrolled at the Blood Transfusion Clinic (n = 48) and healthy children at the Pediatric Day Surgery Clinic (n = 7). For correlation to LCH cell frequencies, LCH patients with active disease were recruited at the Department of Pediatric Oncology, Karolinska University Hospital, Stockholm (n = 4) and Fiona Elsey Cancer Research Institute, Ballarat (n = 3). For intestinal biopsy analysis, treatment naïve children undergoing diagnostic colonoscopy for suspected inflammatory bowel disease were recruited at the Department of Pediatric Gastroenterology, Hepatology and Nutrition, Karolinska University Hospital, Stockholm (n = 26). After clinical and pathologic evaluation, which included clinical examination, radiology, laboratory tests, endoscopy and histopathology review, patients that were not diagnosed with inflammatory bowel disease were considered as controls, and patients with other inflammatory or infectious disease were excluded. Further details on the inflammatory bowel disease (IBD) cohort are provided in a previous report. 11 The studies were approved by the Regional Review Board in Stockholm (2009/1937‐31/1, 2010/32‐31/4, 2011/803‐31/3), and written informed consents were obtained from patients and controls, as well as their parents when the participant was younger than 18 years of age.
2.2. Immunological assays
For real‐time polymerase chain reaction, ribonucleic acid was extracted from snap‐frozen intestinal biopsies of patients undergoing colonoscopy, and the cytokines IL‐23, IL‐22 and IL‐17A were analysed as previously described. 11 IL‐23 in plasma was detected using enzyme‐linked immunosorbent assay, performed using an IL‐23 DuoSet ELISA kit (R&D Systems) as previously described 12 and LEGENDplex (BioLegend). Briefly, the plasma concentrations of IL‐23 and IL‐12p40 in patients with active LCH were measured using the LEGENDplex as per manufacturer's instructions, and LEGENDplex Data Analysis software (BioLegend) was used to determine the concentration of cytokines by comparing them against a set of standard curves. Flow cytometry was performed as previously described, 13 using a surrogate gating strategy to identify lymphocyte subsets. 14 Levels of LCH cells in lesional LCH cell suspensions (Figure 2B) were calculated as the percentage of CD1a positive cells among the cells positive for the myeloid mononuclear phagocyte marker CD11c, in the CD3‐CD19‐gate. Algorithms used for dimensionality reduction were UMAP (https://github.com/lmcinnes/umap) and Phenograph (https://github.com/JinmiaoChenLab/Rphenograph).
FIGURE 2.
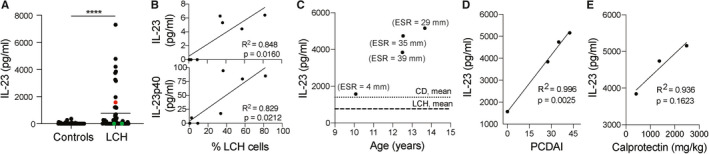
IL‐23 in LCH and in Patient A. A, IL‐23 plasma levels of LCH patients (n = 55) and healthy controls (n = 55), red dot indicates Patient A, green dots patients with history of intestinal LCH. B, IL‐23 and IL‐23p40 plasma levels (detected using a complimentary method, LEGENDplex) correlated with % of LCH cells, defined as CD1a+, within lesional CD11c + cells (n = 7). C‐E, IL‐23 plasma levels in Patient A and in relation (C) to erythrocyte sedimentation rate (ESR) over time, mean values of CD and LCH patients indicated, (D) to Pediatric Crohn's Disease Activity Index (PCDAI), and (E) to calprotectin. Differences between two groups in the distributions of continuous variables were evaluated using Mann‐Whitney U test. Pearson correlation coefficients were calculated in order to evaluate correlations between continuous variables. P values: *P < .05, ***P < .001, ****P < .0001; mean values indicated when presenting more than one data point
2.3. Whole‐genome sequencing
Genomic deoxyribonucleic acid was isolated from blood of patient A, and his brother and parents according to standard procedures. Whole‐genome sequencing libraries were prepared using the TruSeq PCR free protocol (Illumina) and sequenced on a HiSeq X machine (Illumina) to obtain around 30X genome coverage for each sample. Fastq files were analysed with the MIP pipeline (v.5.0.11) to generated annotated and ranked list of variants. Variants were visualised in SCOUT (https://github.com/Clinical‐Genomics/scout) for analysis of 366 genes associated with primary immunodeficiency and 51 genes associated with monogenic inflammatory bowel disease. Further analysis according to putative inheritance model for monogenic disorders with high penetrance was performed using GEMINI v.0.20.1.
2.4. Statistical analyses
Differences between two groups in the distributions of continuous variables were evaluated using Mann‐Whitney U test. Pearson correlation coefficients were calculated in order to evaluate correlations between continuous variables. P values below 5% were considered statistically significant.
3. RESULTS
3.1. Case series
Patient A presented with a forearm swelling in the absence of trauma at the age of 15 months, diagnosed with unifocal bone LCH, which developed into multifocal bone disease and responded well to 1 year of chemotherapy. At 11 years, he presented with gastrointestinal symptoms and weight loss of 6 kg. Histopathological examination revealed morphologic features suggestive of CD including presence of colonic CD1a and CD207‐negative granulomas typical for CD, and he was diagnosed with CD at 12 years (Figure 1A). The patient received multiple treatment regimens, including immunomodulatory therapies, until anti‐TNF therapy, namely infliximab, was introduced and a good, long‐lasting response was achieved. His brother, also diagnosed with paediatric CD, responded to anti‐TNF therapy as well. Given the history of the granulomatous diseases LCH and CD in childhood, whole‐genome sequencing of all four family members, that is patient, brother, mother, father, was performed, but did not uncover a monogenic disease explanation.
FIGURE 1.
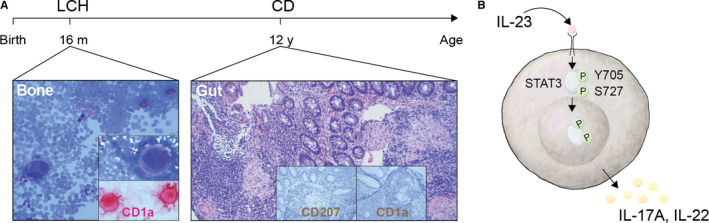
Timeline and illustration of IL‐23 signalling pathway. A, Timeline of LCH and CD development in Patient A with histological and immunohistochemical analysis of diagnostic specimens of bone aspirate (Bone) and colon biopsies (Gut) showing CD1a (red in Bone, brown in Gut) and CD207 (brown in Gut). B, Illustration of IL‐23 signalling pathway: transcription factor STAT3, its phosphorylation sites Y705 and S727, and downstream cytokines IL‐17A and IL‐22; p indicates phosphorylation
Patient B presented with a lesion in the cranial vault and was diagnosed with unifocal bone LCH at the age of 13 years, treated with curettage. When presenting with intestinal symptoms 7 years later, LCH was suspected and vinblastine and corticosteroids were administered for 1 year. After initial improvement, there was progressive worsening of the intestinal symptoms and additional weight loss of 10 kg. Trimethoprim/sulphamethoxazole for suspected Whipple disease had no effect. When positive for QuantiFERON, that is interferon‐gamma release assay, anti‐tuberculosis treatment with prednisone was introduced with signs of amelioration, but intestinal symptoms reappeared when prednisone was tapered. Finally diagnosed with CD based on endoscopic and histological findings at the age of 23‐years, the patient has been in partial remission since the introduction of infliximab.
Patient C presented with weight loss, abdominal pain, fatigue and diarrhoea, and was diagnosed with terminal ileal CD at the age of 13 years. At the age of 40, he presented with bone pain with skin lesions and was diagnosed with multisystem multifocal LCH, treated with vinblastine and corticosteroids for 6 months with good response. Two months following completion of therapy for LCH, he developed abdominal pain, diarrhoea and fatigue and had a flare‐up of Crohn's disease requiring resection of the terminal ileum. Since then, he has remained well (Table 1).
Table 1.
Clinical and laboratory findings in patients with LCH and CD
| Patient A | Patient B | Patient C | |
|---|---|---|---|
| Sex | Male | Male | Male |
| Ethnic origin | Sweden | France/Morocco | Canada/Australia |
| BRAFV600E, type of specimen tested | Negative, colon | Negative, colon | Negative, bone |
| Langerhans cell histiocytosis | |||
| Age at LCH onset | 15 m | 13 y | 40 y |
| Age at LCH diagnosis | 16 m | 13 y | 40 y |
| Presentation | Forearm swelling without trauma | Cranial vault lesion | Skin, subcutaneous and bone lesions |
| CD1a/CD207 positive specimen | Bone | Bone | Skin, bone |
| Organs involved since diagnosis | Bone | Bone | Skin, bone |
| Disease progression | SS/UF to SS/MF | No | No |
| Maximal extent of disease | SS/MF RO‐ | SS/UF RO‐ | MS/MF RO‐ |
| Treatment duration | VB/Pred, 1 y | surgical | VB/Pred, 6 m |
| Treatment response | Good | Good | Good |
| Haemoglobin, g/L | 99 | NA | 139 |
| Platelets, 109/L | 560 | NA | 367 |
| Albumin, g/L | NA | NA | 35 |
| Erythrocyte sedimentation rate, mm/h | 29 | NA | 57 |
| C‐reactive protein, mg/L | NA | NA | 78.5 |
| Comments/other diseases/manifestations | Childhood autism, ADHD, atopic eczema, dermatitis | Atopic dermatitis, Gilbert syndrome | Angiolipomas |
| Crohn's disease | |||
| Age at CD onset | 11 y | 20 y | 13 y |
| Age at CD diagnosis | 12 y | 23 y | 13 y |
| Presentation | Weight loss 6 kg, fatigue, nausea, anorexia | Weight loss 10 kg, abdominal pain, diarrhoea | Weight loss, abdominal pain, fatigue, diarrhoea |
|
Histopathology: CD1a/CD207 staining Epithelioid granulomas Giant cells |
Negative Yes Yes |
Negative Yes Yes |
Negative Yes Yes |
| Intestinal location | Ileocolonic | Ileocolonic | Distal ileum |
| Treatments | Steroids, 5‐ASA, AZA, MTX, IFX | Surgery, steroids, Inh/Rif/Pza/Emb a , TMP/SMX, AZA, IFX | Steroids, surgery |
| Haemoglobin, g/L | 113 | 128 | 154 |
| Platelets, 109/L | 456 | 340 | 259 |
| Albumin, g/L | 30 | 35 | 36 |
| Erythrocyte sedimentation rate, mm | 39 | NA | NA |
| C‐reactive protein, mg/L | 22 | 41 | 120 |
| Faecal calprotectin, mg/kg | 1366 | NA | 634 |
| Extra‐intestinal manifestations/complications | Arthritis | None/Pneumoperitoneum | None |
All laboratory values reported at active disease.
Abbreviations: 5‐ASA, 5‐aminosalicylic acid; ADHD, attention deficit hyperactivity disorder; AZA, Azathioprine; CD, Crohn's disease; IFX, Infliximab; Inh/Rif/Pza/Emb, isoniazid/rifampicin/pyrazinamine/ethambutol; LCH, Langerhans cell histiocytosis; m, months; MF, multifocal; MS, multisystem; MTX, methotrexate; NA, data not available; Pred, prednisolone; RO‐, no risk organ involvement; SS, single system; TMP/SMX, trimethoprim/sulphamethoxazole; UF, unifocal; VB, vinblastine; y, years.
Tuberculosis treatment.
3.2. Immunological studies
Interleukin (IL)‐23 signalling (Figure 1B) promotes chronic inflammatory processes and intestinal inflammation in particular. Patient A, even prior to the diagnosis of CD (Figure 2A), depicted as red dot, and those with LCH had higher IL‐23 levels compared to controls (Figure 2A). In contrast, patients with a history of intestinal LCH had undetectable levels of IL‐23 (Figure 2A), depicted as green dots. Plasma levels of both IL‐23 and one of its subunits p40 showed positive correlation with LCH tumour cells, defined as CD1a + among mononuclear myeloid CD11c + cells in the lesions, in patients with active LCH (Figure 2B). When IL‐23 levels were longitudinally assessed in patient A, they were associated with higher erythrocyte sedimentation rate and were above the mean levels of other CD and LCH patients (Figure 2C). Plasma IL‐23 displayed a strong correlation with Pediatric CD Activity Index (Figure 2D), and an association with faecal calprotectin (Figure 2E). Defects in cytokine receptor signalling can cause primary immunodeficiencies with high plasma levels of specific cytokines. Since plasma IL‐23 showed association with disease activity, we examined IL‐23 receptor signalling in T cells from patient A and family members (Figure S1A‐D). Isolated peripheral blood mononuclear cells were stimulated with IL‐23 or IL‐21, the latter serving as a positive control, and activation was quantified using antibodies specific for STAT3 pY705 and pS727 phosphorylation sites (Figure S1B), but no functional defects were detected. Of note, an expansion of Th17 and Th17/Th1 cells was detected (Figure S1C‐D), as well as elevated levels of IL‐23 and its downstream partners in affected intestinal tissue (Figure S1E).
4. DISCUSSION
This LCH‐CD case series report has both clinical and biological implications. It highlights an important differential diagnosis to consider in LCH patients with intestinal symptoms, namely CD. Gastrointestinal LCH in patients with intestinal manifestations has previously been described, 15 , 16 , 17 , 18 , 19 , 20 as well as cases with CD1a‐negative LCH in children, 21 and was also considered in our patients, along with Whipple disease, intestinal tuberculosis and non‐LCH myeloid neoplasms, including multiple treatment attempts. Following adequate treatment, all three patients achieved remission. However, it took up to 3 years to diagnose CD, illustrating diagnostic challenges, despite CD1a and CD207 negativity in intestinal biopsies. During this time, the patients lost weight, underwent multiple treatment attempts and suffered complications. It is therefore important to be aware of the possibility of these two diseases coexisting, even though the combination of LCH and CD seems very rare. Of note, Patient C presented with a different diagnostic task, where the LCH diagnosis was established on an active CD background.
Besides similar presentation, granulomas with giant cells and histiocytes in intestinal tissue can be found in both LCH and CD. In the present study, we draw attention to one of the well‐established drivers of chronic tissue inflammation, namely IL‐23, used for treatment of CD and other inflammatory conditions. In the IL‐23 signalling axis, with an established role in CD pathogenesis, IL‐23 secreted by myeloid cells regulates the production of IL‐17A and IL‐22, cytokines produced by lymphocytes, such as T cells. An IL‐23 disease phenotype was detected in Patient A, and, interestingly, plasma levels of IL‐23 were also elevated in a cohort of LCH patients but seemed not to be related to GI‐LCH. While the role of IL‐23 in CD is established from the immunopathogenic and therapeutic perspectives, our findings highlight IL‐23 as one of the cytokines also contributing to LCH pathogenesis. In support of this, besides the elevated plasma levels, we found a correlation between IL‐23 and the percentage of lesional LCH cells. In line with our findings, higher levels of cytokines downstream of IL‐23, as well as the subunit p40, which is shared by IL‐12 and IL‐23, have previously been reported in LCH. 22 , 23 Further studies are warranted addressing the effects of IL‐23 on innate and adaptive immune cells populations, stretching beyond perpetuation of T‐cell responses, the master regulation of downstream cytokines and features that would be particular interesting for LCH, for example, its role in bone resorption through osteoclastogenesis, also in an IL‐17 independent manner. 7 In this context, it is also interesting to note that LCH cells carry transcripts for the receptor of one of the prominent IL‐23 downstream cytokines, namely IL‐22. 24 While more research and mechanistic approaches are needed before IL‐23 can be proposed as an additional LCH treatment target, it presents an interesting option given good treatment responses in other disorders with a chronic inflammatory component and few side effects. 7
One could speculate that LCH and CD might have a common immunological component possibly through extracellular signal‐regulated kinase‐dependent IL‐23 signalling 25 or through other consequences of activated extracellular signal‐regulated kinase—the key feature of LCH, 2 also detected in CD lesions. 26 Clearly, activation of extracellular signal‐regulated kinase, that in the case of LCH is well‐recognised to be constitutive due to activating mutations, can otherwise be achieved through multiple signalling pathways. 27
From the genetic point of view, and in contrast to single somatic driver mutations seen in LCH, CD typically, and except for a small group of monogenetic very‐early‐onset IBD cases, is caused by polygenetic germ‐line alterations with low penetrance and high frequency. 28 However, this view has recently been significantly complemented by demonstration of presence of somatic mutations in inflammatory genes in IBD colon in adults and contribution of somatic mosaicism and common genetic variation to the risk of very‐early‐onset IBD. 29 , 30 While germ line whole‐genome sequencing of the family A did not reveal a monogenetic disease explanation, and intestinal CD affected tissue in all three case series patients were negative for the most common mutation in LCH, namely BRAFV600E, other somatic alterations in the genes BRAF, MEK or other genes cannot be ruled out. Unfortunately, we were not able to detect a driver mutation in the original specimens where the LCH diagnosis was made. These historical samples were not available for further analysis, representing a major limitation of the study.
Finally, the German pathologist, physiologist and biologist Paul Langerhans (1847‐1888), who described not only the Langerhans cells and the cells that secrete insulin but also numerous marine invertebrates, and the American gastroenterologist Burrill Bernard Crohn (1884‐1983) never actually met as scientists. Both the diseases that bear their names occurred in the patients presented in this report. To conclude, these patients can remind us that lessons learned across paediatric subspecialties may improve clinical care and may also reveal immunological mechanisms underlining granulomatous inflammation in the gut and elsewhere.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Fig S1
ACKNOWLEDGEMENTS
We thank the patients and their families for generous participation. For sequencing and bioinformatics support, we thank Clinical Genomics facility at Scilifelab. The GEMINI analyses were performed on resources provided by SNIC through Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) under Project SNIC sens2017581. The study was supported by grants from the Swedish Children's Cancer Foundation (J‐IH, ML), the Stockholm County Council (ALF grant) (J‐IH), Dr Åke Olsson Foundation (ML), Robert Lundbergs Memorial Foundation (EK) and Mary Béves Foundation (EK).
Kvedaraite E, Lourda M, Han H, et al. Patients with both Langerhans cell histiocytosis and Crohn’s disease highlight a common role of interleukin‐23. Acta Paediatr. 2021;110:1315–1321. 10.1111/apa.15590
REFERENCES
- 1. Stålemark H, Laurencikas E, Karis J, Gavhed D, Fadeel B, Henter J‐I. Incidence of Langerhans cell histiocytosis in children: a population‐based study. Pediatr Blood Cancer. 2008;51(1):76‐81. [DOI] [PubMed] [Google Scholar]
- 2. Allen CE, Merad M, McClain KL. Langerhans‐cell histiocytosis. N Engl J Med. 2018;379(9):856‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keeling JW, Harries JT. Intestinal malabsorption in infants with histiocytosis X. Arch Dis Child. 1973;48(5):350‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singhi AD, Montgomery EA. Gastrointestinal tract langerhans cell histiocytosis: A clinicopathologic study of 12 patients. Am J Surg Pathol. 2011;35(2):305‐310. [DOI] [PubMed] [Google Scholar]
- 5. Roberts SE, Thorne K, Thapar N, et al. A systematic review and meta analysis of paediatric inflammatory bowel disease incidence and prevalence across Europe. J Crohns Colitis. 2020;14(8):1119–1148. [DOI] [PubMed] [Google Scholar]
- 6. Malmborg P, Grahnquist L, Lindholm J, Montgomery S, Hildebrand H. Increasing incidence of paediatric inflammatory bowel disease in northern Stockholm County, 2002–2007. J Pediatr Gastroenterol Nutr. 2013;57(1):29‐34. [DOI] [PubMed] [Google Scholar]
- 7. Moschen AR, Tilg H, Raine T. IL‐12, IL‐23 and IL‐17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol. 2019;16(3):185‐196. [DOI] [PubMed] [Google Scholar]
- 8. Henter JI, Karlén J, Calming U, Bernstrand C, Andersson U, Fadeel B. Successful treatment of Langerhans'‐cell histiocytosis with etanercept. N Engl J Med. 2001;345(21):1577‐1578. [DOI] [PubMed] [Google Scholar]
- 9. Chohan G, Barnett Y, Gibson J, Reddel SWR, Barnett MH. Langerhans cell histiocytosis with refractory central nervous system involvement responsive to infliximab. J Neurol Neurosurg Psychiatry. 2012;83(5):573‐575. [DOI] [PubMed] [Google Scholar]
- 10. Flores Legarreta A, Eckstein O, Burke TM, McClain KL. Anti TNF‐α therapy in patients with relapsed and refractory Langerhans cell histiocytosis: a phase II study. Pediatr Hematol Oncol. 2018;35(5‐6):362‐368. [DOI] [PubMed] [Google Scholar]
- 11. Kvedaraite E, Lourda M, Ideström M, et al. Tissue‐infiltrating neutrophils represent the main source of IL‐23 in the colon of patients with IBD. Gut. 2016;65(10):1632‐1641. [DOI] [PubMed] [Google Scholar]
- 12. Lourda M, Olsson‐Åkefeldt S, Gavhed D, et al. Adsorptive depletion of blood monocytes reduces the levels of circulating interleukin‐17A in Langerhans cell histiocytosis. Blood. 2016;128(9):1302‐1305. [DOI] [PubMed] [Google Scholar]
- 13. Schlums H, Cichocki F, Tesi B, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ma CS, Wong N, Rao G, et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J Allergy Clin Immunol. 2015;136(4):993‐1006.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee‐Elliott C, Alexander J, Gould A, Talbot R, Snook JA. Langerhan“s cell histiocytosis complicating small bowel Crohn”s disease. Gut. 1996;38(2):296‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nanduri VR, Kelly K, Malone M, Milla P, Pritchard J. Colon involvement in Langerhans' cell histiocytosis. J Pediatr Gastroenterol Nutr. 1999;29(4):462‐466. [DOI] [PubMed] [Google Scholar]
- 17. Shima H, Takahashi T, Shimada H. Protein‐losing enteropathy caused by gastrointestinal tract‐involved Langerhans cell histiocytosis. Pediatrics. 2010;125(2):e426‐e432. [DOI] [PubMed] [Google Scholar]
- 18. He H, Jiang Z, Sun L. A rare cause of intestinal ulcers masquerading as inflammatory bowel disease. Gastroenterology. 2014;147(2):283‐284. [DOI] [PubMed] [Google Scholar]
- 19. Cortazar JM, Kim AS. Incidental Langerhans cell histiocytosis of the colon with BRAF p. V600E mutation. Blood. 2017;130(16):1870. [DOI] [PubMed] [Google Scholar]
- 20. Therrien A, El Haffaf Z, Wartelle‐Bladou C, Côté‐Daigneault J, Nguyen BN. Langerhans cell histiocytosis presenting as Crohn's disease: a case report. Int J Colorectal Dis. 2018;33(10):1501‐1504. [DOI] [PubMed] [Google Scholar]
- 21. Powell P, Vitug G, Castro‐Silva F, Ray A. A rare case of CD1a‐negative Langerhans cell histiocytosis of the central nervous system in a child. Clin Case Rep. 2017;5(10):1664‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coury F, Annels N, Rivollier A, et al. Langerhans cell histiocytosis reveals a new IL‐17A–dependent pathway of dendritic cell fusion. Nat Med. 2008;14(1):81‐87. [DOI] [PubMed] [Google Scholar]
- 23. Morimoto A, Oh Y, Nakamura S, et al. Inflammatory serum cytokines and chemokines increase associated with the disease extent in pediatric Langerhans cell histiocytosis. Cytokine. 2017;97:73‐79. [DOI] [PubMed] [Google Scholar]
- 24. Halbritter F, Farlik M, Schwentner R, et al. Epigenomics and single‐cell sequencing define a developmental hierarchy in langerhans cell histiocytosis. Cancer Discov. 2019;9(10):1406‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brereton CF, Sutton CE, Lalor SJ, Lavelle EC, Mills KHG. Inhibition of ERK MAPK suppresses IL‐23‐ and IL‐1‐driven IL‐17 production and attenuates autoimmune disease. J Immunol. 2009;183(3):1715‐1723. [DOI] [PubMed] [Google Scholar]
- 26. Waetzig GH, Seegert D, Rosenstiel P, Nikolaus S, Schreiber S. p38 mitogen‐activated protein kinase is activated and linked to TNF‐alpha signaling in inflammatory bowel disease. J Immunol. 2002;168(10):5342‐5351. [DOI] [PubMed] [Google Scholar]
- 27. Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5(11):875‐885. [DOI] [PubMed] [Google Scholar]
- 28. McGovern DPB, Kugathasan S, Cho JH. Genetics of inflammatory bowel diseases. Gastroenterology. 2015;149(5):1163‐1176.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olafsson S, McIntyre RE, Coorens T, et al. Somatic Evolution in Non‐neoplastic IBD‐Affected Colon. Cell. 2020;182(3):672–684.e11. 10.1016/j.cell.2020.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Serra EG, Schwerd T, Moutsianas L, et al. Somatic mosaicism and common genetic variation contribute to the risk of very‐early‐onset inflammatory bowel disease. Nat Commun. 2020;11(1):995‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


