Abstract
Objectives
We aimed to evaluate the feasibility of using three dimensional‐quantitative coronary angiography (3D‐QCA) based fractional flow reserve (FFR) (vessel fractional flow reserve [vFFR], CAAS8.1, Pie Medical Imaging) and to correlate vFFR values with intravascular ultrasound (IVUS) for the evaluation of intermediate left main coronary artery (LMCA) stenosis.
Background
3D‐QCA derived FFR indices have been recently developed for less invasive functional lesion assessment. However, LMCA lesions were vastly under‐represented in first validation studies.
Methods
This observational single‐center cohort study enrolled consecutive patients with stable angina, unstable angina, or non‐ST‐segment elevation myocardial infarction and nonostial, intermediate grade LMCA stenoses who underwent IVUS evaluation. vFFR was computed based on two angiograms with optimal LMCA stenosis projection and correlated with IVUS‐derived minimal lumen area (MLA).
Results
A total of 256 patients with intermediate grade LMCA stenosis evaluated with IVUS were screened for eligibility; 147 patients met the clinical inclusion criteria and had a complete IVUS LMCA footage available, of them, 63 patients (63 lesions) underwent 3D‐QCA and vFFR analyses. The main reason for screening failure was insufficient quality of the angiogram (51 patients,60.7%). Mean age was 65 ± 11 years, 75% were male. Overall, mean MLA within LMCA was 8.77 ± 3.17 mm2, while mean vFFR was 0.87 ± 0.09. A correlation was observed between vFFR and LMCA MLA (r = .792, p = .001). The diagnostic accuracy of vFFR ≤0.8 in identifying lesions with MLA < 6.0 mm2 (sensitivity 98%, specificity 71.4%, area under the curve (AUC) 0.95, 95% confidence interval (CI) 0.89–1.00, p = .001) was good.
Conclusions
In patients with good quality angiographic visualization of LMCA and available complete LMCA IVUS footage, 3D‐QCA based vFFR assessment of LMCA disease correlates well to LMCA MLA as assessed by IVUS.
Keywords: 3D‐QCA‐based FFR, angiography‐based FFR, intravascular ultrasound, left main disease, multimodality diagnostics, vessel fractional flow reserve
Abbreviations
- CABG
coronary artery bypass grafting
- FFR
fractional flow reserve
- IVUS
intravascular ultrasound
- LMCA
left main coronary artery
- MLA
minimum lumen area
- NSTEMI
non‐ST‐segment elevation myocardial infarction
- %DS
percentage diameter stenosis
- SD
standard deviation
- 3D‐QCA
three dimensional‐quantitative coronary angiography
- vFFR
vessel fractional flow reserve
1. INTRODUCTION
Evaluation of left main coronary artery (LMCA) lesion remains challenging and often warrants a multimodality approach, including intravascular imaging and functional assessment. 1 , 2 , 3 Concomitantly, reliable invasivefractional flow reserve (FFR) assessment of LMCA also carries some risks and limitations and is still underused in clinical practice despite strong recommendations in current revascularization guidelines. 1 , 4 , 5
Recently, three dimensional three dimensional‐quantitative coronary angiography (3D‐QCA) derived FFR indices have been developed for less invasive functional lesion assessment, demonstrating a high linear correlation with invasively measured FFR and a high accuracy to detect the lesions with FFR ≤0.8. 6 , 7 , 8 , 9 , 10 , 11 However, patients with LMCA lesions were vastly under‐represented in first validation studies. 7 , 8 , 9 , 10 , 11 , 12
While pressure wire based FFR measurement demonstrated low‐to‐moderate correlation with intravascular ultrasound (IVUS) measurements in nonleft main coronary stenosis (also dependent on the vessel size), 13 a good correlation between IVUS LMCA quantitative lumen measurements and FFR values have been reported. 14 , 15 , 16 , 17
Given this background, we aimed to evaluate the feasibility of using 3D‐QCA based FFR for left main disease and to correlate vFFR values (CAAS 8.1 Workstation, Pie Medical Imaging) 7 with IVUS measurements for evaluation of intermediate to severe LMCA stenosis.
2. MATERIALS AND METHODS
2.1. Study population
This observational, retrospective, single‐center study included consecutive patients presenting with stable angina, unstable angina, and non‐ST‐segment elevation myocardial infarction (NSTEMI) with nonostial LMCA stenoses who underwent IVUS evaluation between September 2008 and December 2016.
Exclusion criteria involved: severe valvular heart disease, left ventricle ejection fraction <30%, previous coronary artery bypass grafting (CABG), insufficient quality of angiogram precluding vFFR computation (i.e., absence of a minimum of two angiographic projections with views of at least 30° apart, substantial foreshortening or overlap of the vessel, ostial LMCA stenosis, inadequate contrast flush), insufficient quality of IVUS pullback precluding quantitative luminal assessment, deep catheter intubation into LMCA precluding complete stenosis visualization, unavailability of baseline aortic root blood pressure required for vFFR computation, and significant downstream disease in both daughter arteries (>50% stenosis by visual estimation). 18 , 19
2.2. vFFR analyses
Computation of vFFR was performed offline by trained analysts blinded to the IVUS measurements using a validated software CAAS workstation 8.1 (Pie Medical Imaging, Maastricht, the Netherlands). Within CAAS Workstation vFFR the pressure drop is calculated instantaneously by applying physical laws including viscous resistance and separation loss effects present in coronary flow behavior, as previously described. 7
A total of two 2‐D angiograms with optimal visualization of LMCA stenosis were loaded into the software. Optimal visualization of LMCA stenosis was defined as angiograms visualizing the LMCA stenosis without overlap or significant foreshortening including the projection with the highest angiographic percentage diameter stenosis (%DS).
Although temporal alignment of the cardiac cycle between the two angiograms was performed automatically by electrocardiography triggering, manual frame selection was allowed. Contour detecting was performed semiautomatically, delineating the vessel contour from the ostium up to 3 cm distal to the LMCA lesion in either the left anterior descending (LAD) or left circumflex artery (LCX), depending on which was least diseased distally. This approach followed the methodology of invasive FFR for LMCA disease described in prior studies. 14 , 15 In case of distal LMCA stenoses and true bifurcation lesions, vFFR was analyzed up to 3 cm distal to the LMCA lesion in both LAD and LCX artery; the lower vFFR value and the corresponding IVUS measurements from the pullback acquired in the same daughter artery were included in the correlation analysis 14 , 15 .
vFFR was calculated automatically incorporating the invasively measured aortic root pressure and automatically generated 3D‐QCA values. The %DS was determined from the generated 3D models.
2.3. IVUS analyses
The LMCA segments were examined with an IVUS system with automatic pullback at 0.5 mm/s (OptiCross, Boston Scientific, Natick, MA; Eagle Eye, Volcano Corp, Rancho Cordova, CA; TVC Insight, Infra‐ReDx, Burlington, MA) or 2.5 mm/sec (Kodama, Acist Medical, Eden Prairie, MN). IVUS imaging assessment was performed off‐line in fixed 0.5 mm intervals between the LMCA ostium and its distal bifurcation using dedicated software (QCU‐CMS, Leiden University Medical Center, LKEB, Division of Image Processing, version 4.69) by two dedicated academic intravascular imaging specialists, blinded to the vFFR results.
The proximal border of the LMCA, the ostium, was defined as the first frame, that contained a 360° luminal border of the LMCA.
The minimum lumen area (MLA) and external elastic membrane area were measured at the site within the LMCA coronary segment above the carina at which the lumen was smallest. The plaque burden at the MLA site was calculated as (external elastic membrane area–lumen area)/external elastic membrane area × 100 (%). Percent of area stenosis was also calculated as (reference lumen area − MLA)/reference lumen area×100 (%).
2.4. Statistical analyses
The data distribution was assessed by Kolmogorov – Smirnov analysis. Normally distributed continuous variables are presented as the mean ± SD, and were compared using the Student t test. Non‐normally distributed continuous variables are presented as median (25th–75th percentile), and were compared using the Mann–Whitney test. Categorical variables are displayed as counts and percentages, and were compared using chi‐square or Fisher exact tests as appropriate. The correlation between vFFR and MLA and remaining IVUS‐derived parameters was assessed calculating the Pearson R or Spearman's rank correlation coefficients, for variables with normal and non‐normal distribution, respectively. Receiver‐operating curve analyses were performed to assess the discriminative power of the vFFR and 3D‐QCA based %DS to detect an IVUS derived MLA <6.0 mm2. 1 , 14 , 17 Finally, exploratory analysis of the optimal cutoff values of vFFR for IVUS derived MLA <6.0 mm2 was conducted; the cut‐off was identified as the values for which the sum of the sensitivity and specificity was greatest. All statistical analyses were performed using SPSS (version 25.0, SPSS, Inc., Chicago, Illinois). A p value of < .05 was considered as statistically significant.
3. RESULTS
A total of 256 patients with intermediate grade LMCA stenosis evaluated with IVUS were screened for eligibility; 147 patients met the clinical inclusion criteria and had a complete IVUS LMCA footage available, of them, 63 patients (63 lesions) underwent 3D‐QCA and vFFR analyses (Figure 1). The main reason for screening failure was insufficient quality of the angiogram (51 patients, 60.7%).
FIGURE 1.
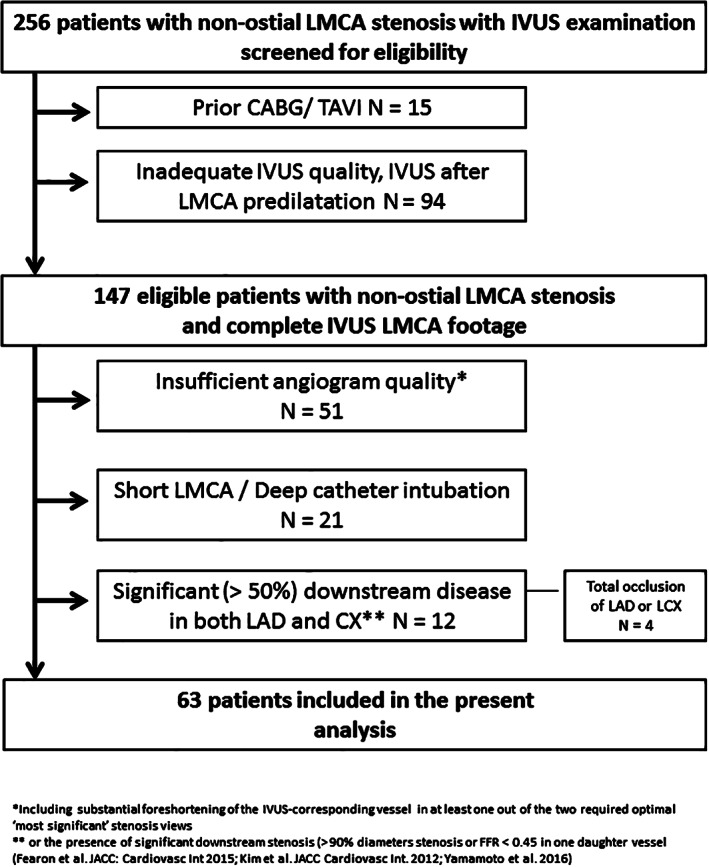
Study flow chart
Mean age was 65 ± 11 years, 75% were male. Thirty‐three patients presented with stable angina, 10 patients with unstable angina, and 20 patients with NSTEMI. Overall, mean MLA within LMCA was 8.77 ± 3.17 mm2, while mean vFFR was 0.87 ± 0.09. Baseline clinical, IVUS and angiographic characteristics are presented in Table 1.
TABLE 1.
Baseline clinical, intravascular ultrasound (IVUS) and angiographic characteristics
| N = 63 patients | |
|---|---|
| Clinical characteristics | |
| Age, years (± SD) | 65 ± 11 |
| Male, n (%) | 47 (74.6) |
| BMI, kg/m2 (± SD) | 26.3 ± 4.7 |
| Diabetes mellitus, n (%) | 13 (20.6) |
| Hypercholesterolemia | 27 (42.8) |
| Hypertension | 36 (57.1) |
| Current smoking | 17 (27.0) |
| Family history of CVD | 20 (31.7) |
| Quantitative IVUS parameters | |
| Area stenosis at MLA, % (± SD) | 55.6 ± 10.6 |
| MLA, mm2 (± SD) | 8.8 ± 3.2 |
| Mean lumen area, mm2 (± SD) | 13.3 ± 3.0 |
| MLD, mm (± SD) | 3.5 ± 0.4 |
| Vessel area, mm2 (± SD) | 23.5 ± 5.8 |
| Plaque burden area, mm2 (± SD) | 10.1 ± 3.8 |
| 3D‐QCA and vFFR | |
| %DS | 36.0 (18.0–53.0) |
| vFFR (± SD) | 0.87 ± 0.09 |
Note: Data presented as mean ± standard deviation (SD) or median (25th–75th percentile).
Abbreviations: %DS, percentage diameter stenosis; 3D‐QCA, three‐dimensional quantitative coronary angiography; BMI, body mass index; CVD, cardiovascular disease; MLA, minimum lumen area; MLD, minimum lumen diameter; vFFR, vessel fractional flow reserve.
A good correlation was observed between vFFR and LMCA MLA (r = .792, p = .001) (Figures 2 and 3a). The observed correlation remained significant regardless of clinical presentation (stable angina: r = .79, p = .001, acute coronary syndrome (unstable angina or NSTEMI): r = .80, p = .001). There was a moderate correlation between vFFR and mean lumen area (r = .622, p = .001) and average plaque burden area (r = .420, p = .003). The diagnostic accuracy of vFFR ≤0.8 in identifying lesions with MLA < 6.0 mm2 (sensitivity 98%, specificity 71.4%, area under the curve [AUC] 0.95, 95% confidence interval [CI] 0.89–1.00, p = .001) was good (Figure 3b). An inverse correlation was found between %DS by QCA and vFFR (r = −.485, p = .001). No significant correlation was observed between %DS by IVUS and vFFR (r = −.183, p = .212).
FIGURE 2.
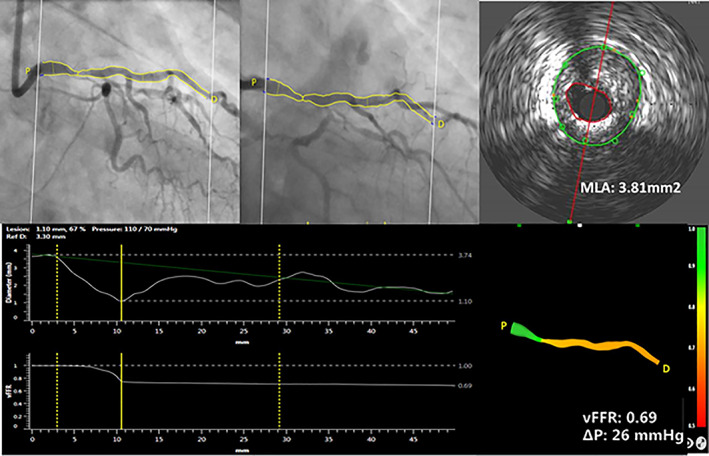
Case example of three‐dimensional reconstruction of left main coronary artery (LMCA) and computation of vessel fractional flow reserve (vFFR), using two angiographic projections with at least 30° apart and invasively measured aortic root blood pressure. Quantitative lumen assessment by intravascular ultrasound in LMCA [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
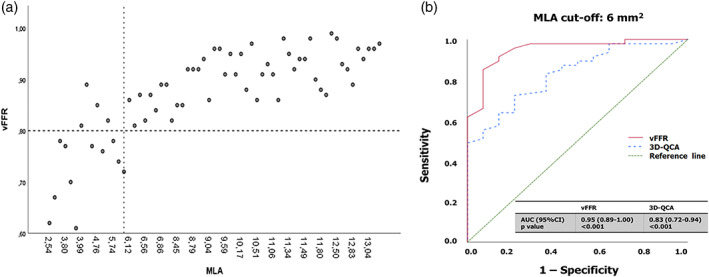
(a) Scatter plot illustrating corresponding 3D quantitative angiography based vessel fractional flow reserve (vFFR) and intravascular ultrasound (IVUS)‐derived minimal lumen area (MLA) measurements in the left main coronary artery stenosis. (b) Receiver operating curve (ROC) for vFFR and 3D‐QCA based percentage of diameter stenosis. Comparison is made with an IVUS‐MLA below 6.0 mm2 [Color figure can be viewed at wileyonlinelibrary.com]
Overall, revascularization was performed in 17 LMCA lesions: in 13 (93%) of 14 lesions with IVUS defined MLA < 6.0 m2 and in 4 (8%) out of 49 lesions with IVUS defined MLA ≥6.0 m2, corresponding to 10 (91%) of 11 lesions with vFFR ≤0.8, and 7 (14%) of 52 lesions with vFFR >0.8.
3.1. Exploratory analyses of optimal vFFR threshold compared with IVUS luminal assessment
Compared with the IVUS MLA threshold of 6.0 mm2 as a reference, a vFFR value of ≤0.83 had the highest sensitivity and specificity (91.8 and 85.7%, respectively).
4. DISCUSSION
The present study assessed for the first time a combined wire‐free 3D‐QCA based functional (vFFR) and IVUS evaluation of LMCA stenoses. In a selected patient population with sufficient angiogram quality, vFFR demonstrated a good linear correlation with IVUS‐derived MLA and a good sensitivity to detect lesions with IVUS‐confirmed significant disease. Our findings are of note, as LMCA lesions have been vastly under‐represented or explicitly excluded in previous validation studies of 3D‐QCA based FFR indices, including vFFR, quantitative flow ratio (qFR), or FFR angio. 6 , 7 , 8 , 9 , 10 , 11 , 20 LMCA lesions have been also excluded in several trials using invasive FFR or iFR. 21 , 22 , 23
We compared vFFR against IVUS as (a) it is a guideline‐advocated (class II a, Level B) imaging modality for left main disease assessment, 1 and (b) a good correlation between invasive FFR and IVUS has been demonstrated for LMCA stenoses 14 , 15 .
Notably, the strength of correlation between vFFR and IVUS‐derived MLA appeared similar to those previously reported in studies with invasive FFR and IVUS evaluation of LMCA 14 , 15 .
Given the limited number of concomitantly available data on appropriate invasive FFR, angiography, and IVUS evaluation, we could not compare vFFR directly against invasive FFR in this cohort. Nevertheless, in order to facilitate an indirect comparison between vFFR and FFR for LMCA assessment we used the same methodology as described preciously in invasive FFR studies, including the length of the 3D reconstruction within the LAD or Cx, at least 30 mm from the distal lesion border, resembling location of pressure wire sensor 14 , 15 . Furthermore, we compared vFFR sensitivity and specificity to identify IVUS‐confirmed LMCA disease using MLA threshold of 6.0 mm2 that was indicative of significant LMCA stenosis by invasive FFR. 1 , 14 , 17
Jasti et al. analyzed 55 patients with ambiguous LMCA stenoses with both IVUS and invasive FFR concluding that an MLA of 5.9 mm2 had the highest sensitivity and specificity (93 and 95%, respectively) for determining a functionally significant LMCA stenosis, defined as FFR < 0.75. 14 Park et al. showed that an IVUS‐derived MLA of ≤4.5 mm2 is a useful index of an invasive FFR of ≤0.80 (77% sensitivity, 82% specificity, AUC: 0.83, 95% CI: 0.76– 0.96; p < .001) 15 ; these values, however, were obtained in an Asian population, and are not generally applicable to other populations. 1 A prospective study showed that a MLA ≥6 mm2 is a safe threshold for deferring LMCA revascularization. 17 Using the latter MLA cut‐off, we identified a vFFR cut‐off value of ≤0.83 as having the highest diagnostic accuracy to reveal IVUS confirmed significant left main disease with the sensitivity and specificity (91.8 and 85.7%, respectively) similar to studies that identified this MLA cut‐off using invasive FFR as a reference. 14 , 15
The potentially higher threshold for vFFR for LMCA evaluation in this analysis needs to be interpreted cautiously and strictly as exploratory, considering that the challenges of angiographic visualization of severely stenotic LMCA 4 , 5 , such as catheter wedging, intubation depth, risk of lesion dissection (in particular with repeated intubations) could impact the analyzability rates and the selection of patients in this retrospective analysis in favor of nonsignificant LMCA stenosis; as a consequence, it could also influence the identified highest diagnostic accuracy cut‐off point to >0.80.
Nevertheless, the observed correlation between 3D‐QCA based vFFR assessment of LMCA disease and LMCA MLA as assessed by IVUS in patients with good quality angiographic visualization of LMCA and available complete LMCA IVUS footage, warrants confirmation in larger dedicated clinical outcome trials.
Since we were forced to exclude a significant number of cases due to insufficient quality of the angiogram, this limitation could at least partially be addressed in a prospective study with protocol‐mandated angiogram acquisition respecting appropriate projections of >30° apart, along with a brisk contrast injection. Nevertheless, LMCA segments remain particularly challenging for optimal angiographic visualization, and if short, or in overlap with tortuous proximal segments of LAD and/or Cx precluding reliable contour tracing, might not be appropriate for vFFR, even in the setting of a dedicated, prospective study.
Cases included in our study involved LMCA disease of rather moderate severity. Nevertheless, MLA and %DS values were comparable to previous studies on the topic. 14 , 15 Future studies might provide more detailed data on the MLA – vFFR correlation in more severe LMCA lesions.
Considering the challenges in LMCA lesion evaluation and recognized limitations of IVUS and FFR/iFR in LMCA assessment, relevant is the search of novel strategies that could reinforce the currently available diagnostic options used in guideline‐recommended multimodality approach to LMCA disease. 1 , 4 , 5 It is conceivable that less invasive and relatively straightforward nature of 3D‐QCA derived FFR estimation could facilitate routine vFFR screening of all angiographically ambiguous LMCA lesions thereby aiding identification of patients requiring additional intravascular imaging or/and invasive pressure wire based physiological lesion assessment.
5. LIMITATIONS
The study has the following limitations: This was a single‐centre, retrospective study. Although consecutive patients were screened for eligibility, selection bias cannot be excluded and presented correlations need to be confirmed in a larger, prospective study. Secondly, no invasive FFR measurements were available in majority of patients, precluding correlation of vFFR versus invasively measured FFR in LMCA. Finally, vFFR was assessed offline without independent core lab. The ongoing FAST II study will provide further insights on vFFR role and clinical utility in more complex lesions, including nonostial LMCA stenoses, with all vFFR being conducted by a dedicated core laboratory (NCT03791320).
6. CONCLUSIONS
In patients with good quality angiographic visualization of LMCA and available complete LMCA IVUS footage, 3D‐QCA based vFFR assessment of LMCA disease correlates with LMCA MLA as assessed by IVUS.
CONFLICT OF INTERESTS
M. T. acknowledges funding received from the European Society of Cardiology in form of an ESC 2018 Grant. K. M. received institutional grant support from Acist Medical. L. J. C. V. Z. received institutional research grant support from Acist Medical. N. V M. received research grant support from Edwards, Medtronic, Abbott, Boston Scientific, Pulse Cath, Acist Medical and Essential Medical. J. D. received institutional grant/research support from Abbott Vascular, Boston Scientific, Acist Medical, Medtronic and PulseCath, and consultancy and speaker fees from Acist medical, Boston Scientific, ReCor Medical, Medtronic and Pulse Cath. The remaining authors have nothing to disclose.
Tomaniak M, Masdjedi K, van Zandvoort LJ, et al. Correlation between 3D‐QCA based FFR and quantitative lumen assessment by IVUS for left main coronary artery stenoses. Catheter Cardiovasc Interv. 2021;97:E495–E501. 10.1002/ccd.29151
REFERENCES
- 1. Neumann FJ, Sousa‐Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87‐165. [DOI] [PubMed] [Google Scholar]
- 2. Mintz GS, Lefevre T, Lassen JF, et al. Intravascular ultrasound in the evaluation and treatment of left main coronary artery disease: a consensus statement from the European bifurcation Club. EuroIntervention. 2018;14:e467‐e474. [DOI] [PubMed] [Google Scholar]
- 3. Cameron A, Kemp HG Jr, Fisher LD, et al. Left main coronary artery stenosis: angiographic determination. Circulation. 1983;68:484‐489. [DOI] [PubMed] [Google Scholar]
- 4. Ramadan R, Boden WE, Kinlay S. Management of left main coronary artery disease. J Am Heart Assoc. 2018;7(7):e008151. 10.1161/JAHA.117.008151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamilos M, Muller O, Cuisset T, et al. Long‐term clinical outcome after fractional flow reserve‐guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation. 2009;120:1505‐1512. [DOI] [PubMed] [Google Scholar]
- 6. Collet C, Onuma Y, Sonck J, et al. Diagnostic performance of angiography‐derived fractional flow reserve: a systematic review and Bayesian meta‐analysis. Eur Heart J. 2018;39:3314‐3321. [DOI] [PubMed] [Google Scholar]
- 7. Masdjedi K, van Zandvoort LJC, Balbi MM, et al. Validation of 3‐dimensional quantitative coronary angiography based software to calculate fractional flow reserve: fast assessment of stenosis severity (FAST)‐study. EuroIntervention. 2019. May 14;EIJ‐D‐19‐00466. doi: 10.4244/EIJ‐D‐19‐00466. Online ahead of print. [Google Scholar]
- 8. Westra J, Tu S, Winther S, et al. Evaluation of coronary artery stenosis by quantitative flow ratio during invasive coronary angiography: the WIFI II study (wire‐free functional imaging II). Circ Cardiovasc Imaging. 2018;11:e007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Westra J, Andersen BK, Campo G, et al. Diagnostic performance of in‐procedure angiography‐derived quantitative flow reserve compared to pressure‐derived fractional flow reserve: the FAVOR II Europe‐Japan study. J Am Heart Assoc. 2018;7(14):e009603. 10.1161/JAHA.118.009603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu B, Tu S, Qiao S, et al. Diagnostic accuracy of angiography‐based quantitative flow ratio measurements for online assessment of coronary stenosis. J Am Coll Cardiol. 2017;70:3077‐3087. [DOI] [PubMed] [Google Scholar]
- 11. Pellicano M, Lavi I, De Bruyne B, et al. Validation study of image‐based fractional flow reserve during coronary angiography. Circ Cardiovasc Interv. 2017;10(9):e005259. 10.1161/CIRCINTERVENTIONS.116.005259. [DOI] [PubMed] [Google Scholar]
- 12. Fearon WF, Achenbach S, Engstrom T, et al. Accuracy of fractional flow reserve derived from coronary angiography. Circulation. 2019;139:477‐484. [DOI] [PubMed] [Google Scholar]
- 13. Nascimento BR, de Sousa MR, Koo BK, et al. Diagnostic accuracy of intravascular ultrasound‐derived minimal lumen area compared with fractional flow reserve–meta‐analysis: pooled accuracy of IVUS luminal area versus FFR. Catheter Cardiovasc Interv. 2014;84:377‐385. [DOI] [PubMed] [Google Scholar]
- 14. Jasti V, Ivan E, Yalamanchili V, Wongpraparut N, Leesar MA. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation. 2004;110:2831‐2836. [DOI] [PubMed] [Google Scholar]
- 15. Park SJ, Ahn JM, Kang SJ, et al. Intravascular ultrasound‐derived minimal lumen area criteria for functionally significant left main coronary artery stenosis. JACC Cardiovasc Interv. 2014;7:868‐874. [DOI] [PubMed] [Google Scholar]
- 16. Kang SJ, Lee JY, Ahn JM, et al. Intravascular ultrasound‐derived predictors for fractional flow reserve in intermediate left main disease. JACC Cardiovasc Interv. 2011;4:1168‐1174. [DOI] [PubMed] [Google Scholar]
- 17. de la Torre Hernandez JM, Hernandez Hernandez F, Alfonso F, et al. Prospective application of pre‐defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J Am Coll Cardiol. 2011;58:351‐358. [DOI] [PubMed] [Google Scholar]
- 18. Fearon WF, Yong AS, Lenders G, et al. The impact of downstream coronary stenosis on fractional flow reserve assessment of intermediate left main coronary artery disease: human validation. JACC Cardiovasc Interv. 2015;8:398‐403. [DOI] [PubMed] [Google Scholar]
- 19. Yamamoto E, Saito N, Matsuo H, et al. Prediction of the true fractional flow reserve of left main coronary artery stenosis with concomitant downstream stenoses: in vitro and in vivo experiments. EuroIntervention. 2016;11:e1249‐e1256. [DOI] [PubMed] [Google Scholar]
- 20. Asano T, Katagiri Y, Chang CC, et al. Angiography‐derived fractional flow reserve in the SYNTAX II trial: feasibility, diagnostic performance of quantitative flow ratio, and clinical prognostic value of functional SYNTAX score derived from quantitative flow ratio in patients with 3‐vessel disease. JACC Cardiovasc Interv. 2019;12:259‐270. [DOI] [PubMed] [Google Scholar]
- 21. Berry C, McClure JD, Oldroyd KG. Meta‐analysis of death and myocardial infarction in the DEFINE‐FLAIR and iFR‐SWEDEHEART trials. Circulation. 2017;136:2389‐2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zimmermann FM, De Bruyne B, Pijls NHJ, et al. A protocol update of the fractional flow reserve versus angiography for multivessel evaluation (FAME) 3 trial: a comparison of fractional flow reserve‐guided percutaneous coronary intervention and coronary artery bypass graft surgery in patients with multivessel coronary artery disease. Am Heart J. 2019;214:156‐157. [DOI] [PubMed] [Google Scholar]
- 23. Escaned J, Collet C, Ryan N, et al. Clinical outcomes of state‐of‐the‐art percutaneous coronary revascularization in patients with de novo three vessel disease: 1‐year results of the SYNTAX II study. Eur Heart J. 2017;38:3124‐3134. [DOI] [PMC free article] [PubMed] [Google Scholar]


