Abstract
Introduction
We evaluated the clinical profile of the orexin receptor antagonist suvorexant for treating insomnia in patients with mild‐to‐moderate probable Alzheimer's disease (AD) dementia.
Methods
Randomized, double‐blind, 4‐week trial of suvorexant 10 mg (could be increased to 20 mg based on clinical response) or placebo in patients who met clinical diagnostic criteria for both probable AD dementia and insomnia. Sleep was assessed by overnight polysomnography in a sleep laboratory. The primary endpoint was change‐from‐baseline in polysomnography‐derived total sleep time (TST) at week 4.
Results
Of 285 participants randomized (suvorexant, N = 142; placebo, N = 143), 277 (97%) completed the trial (suvorexant, N = 136; placebo, N = 141). At week 4, the model‐based least squares mean improvement‐from‐baseline in TST was 73 minutes for suvorexant and 45 minutes for placebo; (difference = 28 minutes [95% confidence interval 11‐45], p < 0.01). Somnolence was reported in 4.2% of suvorexant‐treated patients and 1.4% of placebo‐treated patients.
Discussion
Suvorexant improved TST in patients with probable AD dementia and insomnia.
Keywords: Alzheimer's disease, insomnia, randomized clinical trial, suvorexant
1. INTRODUCTION
Sleep disturbance and insomnia are common in Alzheimer's disease (AD), affecting ≈40% of patients.1 There is emerging evidence that poor sleep may contribute to the development of AD and impair memory function.2, 3, 4, 5, 6, 7, 8, 9 Options for effective pharmacological treatment of insomnia in AD are limited, with inconsistent or poor‐quality evidence for efficacy of melatonin,10, 11, 12, 13 second‐generation antipsychotics (which are primarily used to target other neuropsychiatric and behavioral symptoms associated with AD),14, 15, 16 and sedating antidepressants.17, 18, 19 Furthermore, potential for adverse events and worsening of cognitive impairment and functional decline is an important concern in treating sleep problems in patients with AD using antipsychotics and sedatives.20, 21, 22
Suvorexant is an orexin receptor antagonist that enables sleep to occur via selective antagonism of wake‐promoting endogenous orexin neuropeptides at orexin receptors OX1R and OX2R.23, 24 It has been demonstrated to be effective for treating insomnia in adults, including elders.25, 26, 27, 28, 29 In clinical trials of 3‐months’ duration, next‐day somnolence was the most common adverse event (6.7% for suvorexant vs 3.3% for placebo) but usually did not result in treatment discontinuation.27 An important question for clinicians is whether the safety and efficacy profile of suvorexant for treating insomnia in non‐demented elders is similar for treating insomnia in those with AD, given the brain changes that occur in AD, including possible dysregulation of orexin signaling.30, 31, 32, 33, 34 We report here a randomized, double‐blind, placebo‐controlled trial testing the efficacy and safety of suvorexant for treating insomnia in patients with mild‐to‐moderate probable AD dementia.
2. METHODS
Full details of the trial methods are provided in the trial protocol available in the appendix.
2.1. Participants
Eligible patients were between 50 and 90 years of age and met National Institute on Aging‐Alzheimer's Association and Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) clinical criteria for probable AD dementia, as well as DSM‐5 criteria for insomnia.35, 36 The diagnoses were made or confirmed by the investigator based on interviews with the patient and their trial partner along with other assessments as detailed in the trial protocol. This included a structured diagnostic interview and sleep history for the diagnosis of insomnia. All patients had a score of 12 to 26 on the Mini‐Mental State Exam (MMSE, range 0–30, lower indicating worse performance) at the screening visit,37 corresponding to dementia of mild to moderate severity. Insomnia had to be confirmed by a mean total sleep time (TST) of <6 hours over screening and baseline sleep laboratory polysomnography (PSG) visits, with neither night >6.5 hours. All patients had to have a competent trial partner who resided with the patient overnight. Patients were excluded if they lived in a nursing home or had evidence of significant neurological, psychiatric, or other sleep disorders that might have confounded the diagnosis of AD dementia or insomnia. Patients who had PSG evidence of significant/severe sleep‐related breathing disorder (defined in this trial as >30 apnea/hypopnea episodes per hour) or periodic limb movement disorder (defined in this trial as >30 periodic leg movements associated with an arousal per hour) on the screening or baseline PSG nights were excluded, as were patients who used continuous positive airway pressure. Use of sedating medications was prohibited before and during the trial, and there were restrictions on the use of alcohol, caffeine, and tobacco. Patients could be taking an acetylcholinesterase inhibitor and/or memantine provided they were taking stable doses prior to screening. Written informed consent was provided by the patient or his/her legal representative.
RESEARCH IN CONTEXT
Systematic review: We searched MEDLINE for randomized controlled trials of medications to treat insomnia or sleep disturbance in Alzheimer's disease (AD). Our search identified six randomized controlled trials that evaluated sleep medications but no trials that evaluated an orexin receptor antagonist or that assessed sleep using polysomnography, considered the best objective assessment.
Interpretation: Suvorexant was effective at increasing total sleep time as assessed by polysomnography in patients with mild‐to‐moderate probable AD dementia. Suvorexant was well‐tolerated with no evidence for worsening of the underlying cognitive impairment.
Future directions: Additional studies could explore: (1) whether the effects of suvorexant differ in individuals with more severe dementia, or those with less typical patterns of sleep disturbance related to disease progression; and (2) possible beneficial effects of suvorexant on the underlying pathophysiology of AD.
HIGHLIGHTS
We used polysomnography to assess suvorexant for treating insomnia in Alzheimer's disease (AD).
Suvorexant improved total sleep time in patients with probable AD dementia and insomnia.
Suvorexant did not worsen the underlying cognitive impairment.
2.2. Trial design
The trial (MSD Protocol 061; clinicaltrials.gov NCT02750306) was conducted at 35 centers—primarily memory clinics and contract research clinics with experience in neurology studies—in eight countries from May 2016 to September 2018. Investigators are listed in the appendix. The trial consisted of a 3‐week screening period with a 2‐week single‐blind placebo run‐in, followed by a 4‐week double‐blind randomized treatment period (Figure 1). Sleep was primarily assessed by PSG, since this is considered the best objective assessment and is particularly valuable where self‐reports are likely to be unreliable, as is the case for AD patients. Overnight PSG in a sleep laboratory starting at the patient's habitual bedtime and with a fixed duration of 8 hours (patients who were asleep at 8 hours were woken) was performed at a screening visit 14 days before randomization, at a baseline visit 7 days before randomization, and at the end of the 4‐week double‐blind treatment period. Patients had to meet TST criteria over the screening and baseline PSG nights to be eligible for the double‐blind treatment period (see Participants section). In the 4‐week double‐blind treatment period, patients were randomly assigned 1:1 to nightly oral suvorexant or matching placebo administered 30 minutes before the patient's bedtime. Consistent with the U.S. product label, the starting dose of suvorexant was 10 mg. At the week 2 clinic visit, this dose could be escalated, in a blinded fashion, to the maximum recommended dose of 20 mg (or matching placebo) if there was insufficient response as indicated by a Clinical Global Impression–Severity (CGI‐S)38 for insomnia of mildly ill or worse and the tolerability of the current dose was acceptable in the investigator's judgment. The patient and caregiver were not told if the dose was increased. The trial was conducted in accordance with principles of Good Clinical Practice and approved by institutional review boards.
Figure 1.
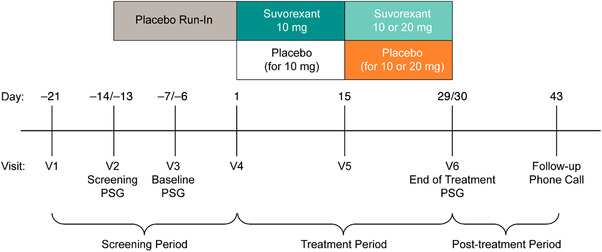
Trial design. Polysomnography (PSG) was recorded overnight (8‐hours duration) in a sleep laboratory. The Mini‐Mental State Exam and Digit Symbol test were administered the morning after PSG recording nights. The patient's partner completed an e‐diary of the patient's sleep each morning throughout the treatment period, and the Sleep Disorders Inventory weekly in the e‐diary starting at visit 4. Partners rated their own sleep using the single‐item Sleep Quality Scale at visits 4, 5, and 6. The Neuropsychiatric Inventory was completed by an interview with the partner and scored by a qualified trained rater at visits 4 and 6. Clinicians completed the Clinical Global Impression–Severity for insomnia at visits 4, 5, and 6
2.3. Randomization and masking
An interactive response system randomly assigned patients to suvorexant or placebo in a 1:1 ratio according to a computer‐generated assignment schedule. Randomization was stratified according to dementia severity as indexed by screening MMSE score (moderate =12–20, mild = 21–26), with the intention to enroll ≈30% of patients in the moderate stratum. All treatments were administered as identical‐appearing tablets.
2.4. Outcomes
Sleep stage scoring of the PSG recordings was performed for each 30‐second epoch according to 2007 American Academy of Sleep Medicine scoring conventions39, 40 by a certified sleep technician at a central sleep scoring laboratory. Each 30‐second epoch was scored as wake; rapid eye movement (REM) sleep; or non‐REM sleep stages N1, N2, or N3/slow wave sleep. The primary efficacy endpoint was the change‐from‐baseline to week‐4 in TST over the 8‐hour PSG recording period, measured in minutes (higher score corresponds to improved sleep). The secondary efficacy endpoint was wake after persistent sleep onset (WASO) measured in minutes, defined as the total wake time over the PSG recording period after the first period of continuous sleep lasting at least 10 minutes (lower score corresponds to improved sleep). Exploratory PSG assessments are detailed in the statistical analysis plan in the appendix and included: (1) the percentage of responders achieving an improvement in TST ≥50 minutes; (2) TST and WASO by thirds of the night; (3) additional sleep endpoints—sleep efficiency, latency to persistent sleep, the number of arousals adjusted for TST, and the number of awakenings adjusted for TST; (4) PSG sleep architecture measures (the percentage of TST spent in REM, N1, N2, and N3/slow wave sleep; latency to REM sleep).
In addition to PSG measures we also assessed the following exploratory subjective endpoints: (1) partner‐rated assessments of the patient's sleep including time spent in bed, waking up earlier than planned, sleep quality (overall impression taking into account the amount of sleep and number and duration of awakenings),41 and total score on the Sleep Disorders Inventory, which includes items assessing getting to sleep, waking during the night, getting up during the night, early waking, and next day sleepiness41; (2) clinician's global impression of severity of patient's insomnia (CGI‐S)38; and (3) partner‐rated assessment of their own sleep quality and distress. Further details of these assessments are provided in the statistical analysis plan in the appendix. Actigraphy measures were also recorded via an activity/sleep watch worn by the patient and will be the subject of a separate report.
Safety was assessed by adverse event reports, laboratory analyses, electrocardiography, and physical examinations performed as stated in the protocol, available in the appendix. A guidance document listing adverse events pre‐specified as events of clinical interest for which additional information was to be collected was provided to investigators, including those of interest for centrally active drugs (eg, suicidality), those of relevance for sleep medications (eg, sleep paralysis, sleep walking), those potentially relevant to orexin antagonism (eg, cataplexy), and those of concern in an AD population (eg, confusion). An independent committee blind to treatment assignment comprising three experts in neurology, psychiatry, and sleep, respectively, adjudicated all events of clinical interest potentially suggestive of intrusion of REM into wakefulness (cataplexy) or at initiation of sleep (sleep‐onset paralysis). Falls were similarly adjudicated to ascertain whether they were due to a possible episode of cataplexy. Cognition and psychomotor performance was assessed by objective tests comprising the MMSE37 and Digit Symbol (DS) test42 administered the morning after PSG recording nights. Neuropsychiatric symptoms (eg, agitation, delusions) were assessed by the trial partner using the 10‐item version of the Neuropsychiatric Inventory (NPI‐10).43
2.5. Statistical analysis
The modified intent‐to‐treat approach was used for the primary and secondary efficacy endpoints, in which treated patients with both a baseline measurement and at least one post‐randomization observation were included. We used an analysis of covariance (ANCOVA) general linear model to analyze all change‐from‐baseline scores. The model adjusted for the following covariates that could have potentially impacted the results: baseline value, geographic region, treatment, sex, age category (<65, ≥65) and baseline dementia severity (MMSE 12–20 or 21–26). The estimated week 4 change‐from‐baseline mean treatment differences (suvorexant – placebo), corresponding 95% confidence intervals (CIs), and two‐sided p values were calculated from this model. Statistical significance for comparison of the primary and secondary efficacy endpoints (TST and WASO) between treatments was based on a fixed sequential multiplicity strategy to control the overall type I error at the two‐sided 5% significance level. This required the comparison of the primary efficacy endpoint (TST) between treatments to be significant at the 5% significance level in order for the comparison of the secondary endpoint (WASO) between treatments to be evaluated at the 5% level of significance. A sensitivity analysis using multiple imputation in conjunction with a tipping point method was conducted to assess the effect of missing data. Details of this analysis and the analytic methods for the exploratory endpoints are provided in the statistical analysis plan available in the appendix. For all exploratory analyses, all p values cited should be considered nominal.
All treated patients were included in the safety analyses. The percentages of patients with adverse events were calculated. All statistical analyses were performed using SAS Versions 9.3 and 9.4 (SAS Institute, Cary, NC).
With ≈130 patients randomized per treatment group, the trial had ≈80% power to detect a 25‐minute difference in change from baseline in TST between treatment groups, corresponding to a standardized effect size of 0.4. This calculation assumed a standard deviation of 68 minutes for change from baseline in TST (based on PSG data from insomnia patients ≥65 years in the two pivotal phase three trials of suvorexant25), a two‐sided α = 0.05, and a dropout rate of 10% at week 4. Subgroup analyses were pre‐specified but the trial was not powered for such analyses. No interim analyses were performed.
2.6. Role of the funding source
The funder contributed to the trial design; the collection, analysis, and interpretation of the data; the writing of the report; and the decision to submit for publication. The corresponding author had full access to all the data in the trial.
3. RESULTS
3.1. Patient characteristics and disposition
Of 285 patients enrolled, 142 were assigned to suvorexant and 143 were assigned to placebo. Patient characteristics were similar across the two trial groups (Table 1); 79% of the trial population had AD dementia of mild severity (21% moderate severity) based on MMSE score, 71% were ≥65 years, 65% were women, and 64% were Hispanic/Latino. Baseline scores on efficacy measures were also similar across trial groups (Table 2). At the week 2 visit, 109/142 (77%) assigned to suvorexant and 104/143 (73%) assigned to placebo had their dose increased to 20 mg or matching placebo. Of the enrolled patients, 136/142 in the suvorexant group and 141/143 in the placebo group completed the trial, with patient withdrawal being the main reason for discontinuation (Figure 2). One patient re‐enrolled at a different site after completing the trial; demographic, efficacy, and safety data from the second enrollment were excluded from all data analyses. Seven (5%) of 142 patients in the suvorexant group and 4 (3%) of 143 patients in the placebo group were excluded from the population used for the efficacy analyses (Figure 2).
Table 1.
Baseline characteristics of treated patients
| Suvorexant N = 142 | Placebo N = 143 | |
|---|---|---|
| Age | ||
| Mean (SD), years | 69.6 (8.7) | 69.1 (8.5) |
| <65 years, n (%) | 39 (27.5) | 44 (30.8) |
| ≥65 years, n (%) | 103 (72.5) | 99 (69.2) |
| Sex, n (%) | ||
| Women | 91 (64.1) | 95 (66.4) |
| Men | 51 (35.9) | 48 (33.6) |
| Body mass index | ||
| Mean (SD), kg/m2 | 27.1 (4.1) | 26.9 (3.7) |
| Underweight (<18.5), n (%) | 1 (0.7) | 0 (0) |
| Normal (18.5–24.9), n (%) | 44 (31.0) | 44 (30.8) |
| Overweight (25–30), n (%) | 68 (47.9) | 70 (49.0) |
| Obese (>30), n (%) | 29 (20.4) | 28 (19.6) |
| Race, n (%) | ||
| White | 86 (60.6) | 80 (55.9) |
| Black | 24 (16.9) | 22 (15.4) |
| Other | 32 (22.5) | 41 (28.7) |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 89 (62.7) | 93 (65.0) |
| Non‐Hispanic/Latino | 52 (36.6) | 50 (35.0) |
| Geographical location, n (%) | ||
| North America | 96 (67.6) | 92 (64.3) |
| Europe | 16 (11.3) | 11 (7.7) |
| Other | 30 (21.1) | 40 (28.0) |
| MMSE | ||
| Mean (SD) | 22.5 (3.0) | 22.3 (3.3) |
| Mild (21–26), n (%) | 113 (79.6) | 113 (79.0) |
| Moderate (12–20), n (%) | 29 (20.4) | 30 (21.0) |
| APOE ε4 genotype, n (%) | ||
| Positive | 40 (28.2) | 53 (37.1) |
| Negative | 86 (60.6) | 74 (51.7) |
| Ambiguous | 3 (2.1) | 2 (1.4) |
| Missing | 13 (9.2) | 14 (9.8) |
| PSG measures, mean (SD) | ||
| AHI | 10.1 (8.2) | 8.8 (7.3) |
| PLMAI | 2.2 (4.4) | 2.1 (3.9) |
| Taking AD medication, n (%) | ||
| Donepezil | 47 (33.1) | 51 (35.7) |
| Memantine | 23 (16.2) | 19 (13.3) |
| Rivastigmine | 6 (4.2) | 4 (2.8) |
| Taking SSRI/SNRI, n (%) | ||
| Any | 23 (16.2) | 25 (17.5) |
Abbreviations: AD, Alzheimer's disease; AHI, Apnea/Hypopnea Index (number of apneas or hypopneas per hour assessed during PSG); APOE ε4, apolipoprotein ε4 gene variant; MMSE, Mini‐Mental State Exam; PLMAI, Periodic Leg Movement Arousal Index (number of leg movements associated with an arousal per hour assessed during PSG); PSG, polysomnography; SD, standard deviation; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin and norepinephrine reuptake inhibitor.
Table 2.
Summary of results for PSG sleep measures
| Observed baseline mean (SD) | Observed week 4 mean (SD) | Model‐based LS mean change from baseline (95% CI)a | Difference in LS mean suvorexant versus placebo | ||||
|---|---|---|---|---|---|---|---|
| Measure | Suvorexant | Placebo | Suvorexant | Placebo | Suvorexant | Placebo | (95% CI), p valueb |
| Primary | |||||||
| TST, min | 277.7 (76.9) | 274.1 (84.3) | 349.4 (71.9) | 321.0 (85.2) | 73.4 (61.3, 85.5) | 45.2 (33.3, 57.2) | 28.2 (11.1, 45.2), 0.001 |
| Secondary | |||||||
| WASO, min | 134.3 (59.5) | 142.3 (61.3) | 92.5 (55.5) | 109.8 (55.1) | −45.0 (−53.8, −36.3) | −29.4 (−38.1, −20.7) | −15.7 (−28.1, −3.3), 0.014 |
| Exploratory | |||||||
| LPS, min | 76.9 (82.5) | 70.8 (71.3) | 44.7 (50.2) | 56.0 (69.8) | −29.5 (−39.3, −19.7) | −17.4 (−27.1, −7.7) | −12.1 (−25.9, 1.7), 0.085 |
| LREM, min | 164.4 (101.2) | 147.3 (90.1) | 121.9 (79.0) | 121.6 (78.9) | −36.8 (−49.5, −24.1) | −31.4 (−44.1, −18.8) | −5.4 (−23.4, 12.7), 0.559 |
| SE, % | 57.9 (15.9) | 57.1 (17.6) | 72.8 (14.9) | 67.0 (17.8) | 15.2 (12.7, 17.8) | 9.5 (7.0, 12.0) | 5.7 (2.2, 9.3), 0.002 |
| NAW ratio | 4.7 (2.3) | 4.8 (2.2) | 3.8 (2.0) | 3.8 (2.1) | −1.0 (−1.3, −0.6) | −0.9 (−1.3, −0.6) | −0.0 (−0.5, 0.5), 0.991 |
| NOA ratio | 6.8 (4.2) | 6.6 (5.0) | 6.9 (4.4) | 6.1 (3.5) | 0.1 (−0.5, 0.7) | −0.5 (−1.1, 0.0) | 0.7 (−0.1, 1.5), 0.110 |
| REM, % | 15.8 (7.3) | 17.3 (8.4) | 19.3 (7.6) | 18.8 (9.3) | 3.1 (1.9, 4.4) | 1.9 (0.6, 3.1) | 1.3 (−0.5, 3.0), 0.163 |
| NREM, % | 84.2 (7.3) | 82.7 (8.4) | 80.7 (7.6) | 81.2 (9.3) | −3.1 (−4.4, −1.9) | −1.9 (−3.1, −0.6) | −1.3 (−3.0, 0.5), 0.163 |
| N1, % | 16.2 (8.5) | 16.9 (12.6) | 14.5 (8.7) | 14.2 (9.8) | −1.9 (−3.3, −0.6) | −2.5 (−3.8, −1.2) | 0.6 (−1.2, 2.5), 0.518 |
| N2, % | 60.8 (9.8) | 58.8 (11.2) | 59.7 (9.5) | 60.1 (10.8) | −0.4 (−2.0, 1.2) | 0.6 (−0.9, 2.2) | −1.0 (−3.2, 1.2), 0.367 |
| N3, % | 7.1 (7.8) | 7.0 (8.2) | 6.5 (7.1) | 6.9 (7.1) | −0.7 (−1.5, 0.2) | −0.1 (−0.9, 0.8) | −0.6 (−1.8, 0.6), 0.300 |
Abbreviations: CI, confidence interval; LPS, latency to persistent sleep; LREM, latency to rapid eye movement sleep; LS, least squares; NAW, number of awakenings (ratio of awakenings after persistent sleep vs TST x 100); NOA, number of arousals (ratio of arousals vs TST x 100); NREM (N1, N2, N3), non‐rapid eye movement sleep stages 1, 2, and 3 (N3 is often referred to as slow wave sleep) expressed as percent of TST; REM, rapid eye movement sleep expressed as percent of TST; SD, standard deviation; SE, sleep efficiency (% of time in bed spent asleep); TST, total sleep time; WASO, wake after persistent sleep onset.
Results based on an ANCOVA model including terms for baseline value, baseline severity category (MMSE score of 12 to 20, 21 to 26), age (non‐elders, elders), sex, region, and treatment.
Per the pre‐specified multiplicity testing strategy, only the primary and secondary endpoints were formally tested, p values for exploratory endpoints should be considered nominal.
Figure 2.
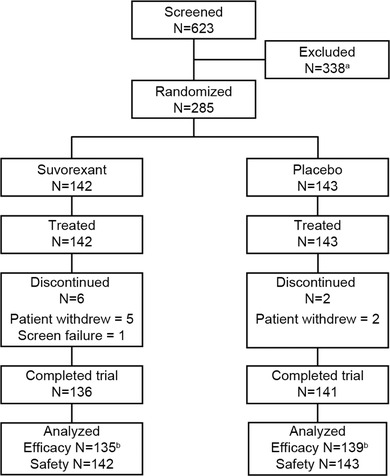
Patient disposition. aThere were 43 reasons for exclusion, and an individual patient could be excluded for multiple reasons. The main reasons for exclusions were the following: underlying pathology of sleep identified during the screening polysomnography (PSG) night, N = 116; Mini‐Mental State Exam outside the 12 to 26 range, N = 19; total sleep time (TST) >6.5 hours during the screening PSG night, N = 19; and mean TST over the screening and baseline PSG nights >6 hours, N = 15. bThe number of patients in the full‐analysis‐set for the primary endpoint of change from baseline in TST at week 4. In the suvorexant group, seven patients were excluded due to missing PSG data. In the placebo group, two patients were excluded due to missing PSG data and two patients were excluded due to Good Clinical Practice noncompliance issues at one site
3.2. PSG outcomes
Results for the PSG outcomes are shown in Table 2. Mean (SD) baseline TST was 278 (77) minutes for suvorexant and 274 (84) minutes for placebo. At week 4, the model‐based least squares mean change‐from‐baseline in TST was 73 minutes for suvorexant and 45 minutes for placebo (difference = 28 minutes [95% CI 11‐45], p < 0.01). Results were similar in the pre‐specified sensitivity analysis (Figure A.1 in the Appendix). The number (%) of patients with ≥50 minute improvement in TST at week 4 was 83 (62%) of 135 in the suvorexant group and 62 (45%) of 139 in the placebo group (estimated odds ratio = 2.2 [95% CI 1.3‐3.6], p = 0.002). Results were similar in a post hoc analysis of the number of patients with ≥60 minute improvement: 74 (55%) of 135 in the suvorexant group and 55 (40%) of 139 in the placebo group (estimated odds ratio = 2.0 [95% CI 1.2‐3.3], p = 0.006). In an exploratory analysis, the increase in TST with suvorexant versus placebo appeared particularly pronounced during the last third of the night (Figure 3A; difference = 13 minutes [95% CI 4‐21], p = 0.003). Further exploratory subgroup analyses did not suggest effects of baseline age, sex, race, region, ethnicity, MMSE severity, apolipoprotein E (APOE) ε4 gene carrier status, or number of apnea/hypopnea episodes on the observed treatment difference in TST based on overlapping confidence intervals (Figure 4).
Figure 3.
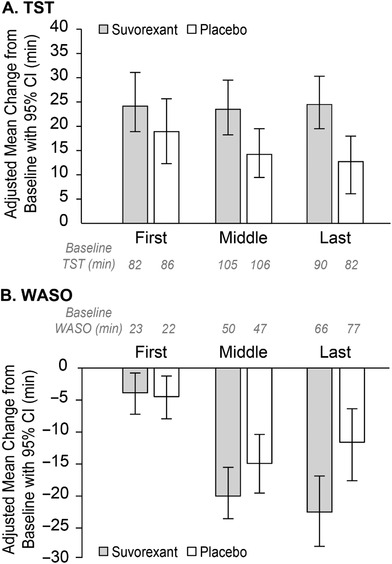
Change from baseline in total sleep time (TST) and wake after persistent sleep onset (WASO) by thirds of the night (first, middle, and last) with baseline values in minutes
Figure 4.
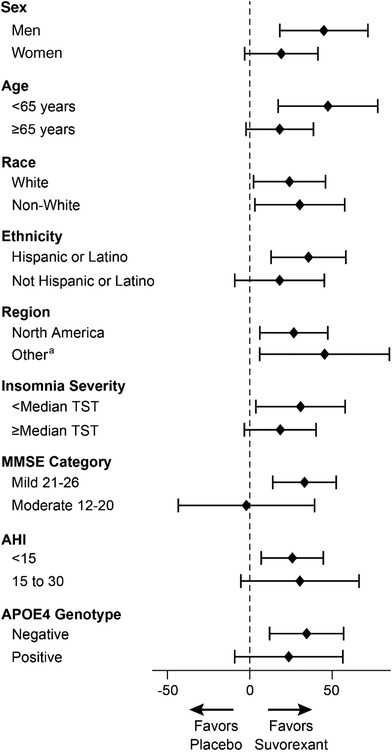
Subgroup analyses based on patient characteristics at baseline: point estimates and 95% confidence intervals for the difference between suvorexant and placebo in change from baseline in total sleep time (TST; minutes) at week 4. aRegions were North America, Europe, and Other. The confidence interval for Europe was not calculated because there were fewer than 20 participants per treatment group. Abbreviations: AHI, Apnea/Hypopnea Index (number of apneas or hypopneas per hour assessed during PSG); APOE ɛ4, apolipoprotein ɛ4 gene variant; MMSE, Mini‐Mental State Exam
Mean (SD) baseline WASO was 134 (60) minutes for suvorexant and 142 (61) minutes for placebo. At week 4, the model‐based least squares mean change‐from‐baseline in WASO was −45 minutes for suvorexant and −29 minutes for placebo (difference = −16 minutes [95% CI −28 to −3], p < 0.05). In an exploratory analysis, the decrease in WASO with suvorexant versus placebo appeared particularly pronounced during the last third of the night (Figure 3B; difference = −11 minutes [95% CI −19 to −3], p = 0.009).
The only exploratory PSG measure that showed a nominally significant difference from placebo was sleep efficiency (p < 0.01), indicating greater sleep efficiency with suvorexant. There was a mean 12‐minute decrease in the sleep‐onset endpoint (latency to persistent sleep) for suvorexant versus placebo, but this was not significant. There were no significant differences between suvorexant and placebo in the sleep architecture profile, that is, the percentage of TST spent in the different sleep stages (REM and non‐REM).
3.3. Trial partner and clinician ratings
Results for the trial partner and clinician ratings are shown in Table 3. Nominally significant improvements for suvorexant versus placebo were seen for the partner's rating of the patient's sleep quality (p < 0.05), and the CGI‐S (p < 0.01). No nominally significant differences between suvorexant and placebo were seen for the other endpoints.
Table 3.
Summary of results for trial partner and clinician ratings
| Observed baseline mean (SD) | Observed week 4 mean (SD) | Model‐based LS mean change from baseline (95% CI)a | Difference in LS mean suvorexant versus Placebo | ||||
|---|---|---|---|---|---|---|---|
| Measure | Suvorexant | Placebo | Suvorexant | Placebo | Suvorexant | Placebo | (95% CI), p valuea |
| Partner's assessment of patient's sleep | |||||||
| sNTIBm, minb | 468.6 (116.4) | 449.0 (107.3) | 498.1 (107.8) | 477.2 (92.1) | 34.0 (20.0, 48.0) | 23.6 (9.8, 37.5) | 10.4 (−9.4, 30.1), 0.302 |
| sEARLIER, % yesc | ‐ | ‐ | 83 (64.3) | 87 (66.4) | ‐ | ‐ | OR 0.90 (0.52,1.57), 0.712 |
| sSQRm, 0–4 scaleb | 1.6 (0.7) | 1.6 (0.7) | 2.5 (0.8) | 2.3 (0.8) | 0.9 (0.7, 1.0) | 0.7 (0.5, 0.8) | 0.2 (0.0, 0.4),d 0.021 |
| SDI total, 0–12 scalec | 1.2 (1.2) | 1.0 (1.0) | 0.2 (0.5) | 0.3 (0.6) | −0.9 (−0.9, −0.8) | −0.7 (−0.8, −0.6) | −0.1 (−0.2, 0.0), 0.102 |
| Clinician's global impression of patient's insomnia severity | |||||||
| CGI‐S, 1–7 scalec | 4.1 (0.7) | 4.1 (0.8) | 2.6 (1.0) | 2.8 (1.2) | −1.5 (−1.7, −1.4) | −1.2 (−1.4, −1.0) | −0.3 (−0.6, −0.1), 0.010 |
| Partner's assessment of their own sleep quality | |||||||
| SSQC, 0–10 scaleb | 4.8 (2.2) | 4.5 (2.0) | 6.7 (2.2) | 6.5 (1.9) | 2.1 (1.7, 2.4) | 1.8 (1.5, 2.2) | 0.2 (−0.2, 0.7), 0.328 |
| Partner's assessment of their own distress | |||||||
| SDI distress, 0–5 scalec | 1.1 (0.7) | 1.0 (0.6) | 0.5 (0.4) | 0.5 (0.5) | −0.5 (−0.6, −0.4) | −0.5 (−0.6, −0.4) | −0.1 (−0.2, 0.1), 0.470 |
| NPI‐10 distress, 0–50 scalec | 6.6 (6.6) | 4.9 (4.2) | 4.7 (5.4) | 3.5 (3.6) | −1.7 (−2.5, −0.8) | −1.5 (−2.3, −0.7) | −0.1 (−1.3, 1.1), 0.841 |
| Partner's assessment of patient's neuropsychiatric symptoms | |||||||
| NPI‐10 total, 0–120 scalec | 4.4 (9.6) | 2.9 (5.5) | 3.0 (7.1) | 2.3 (5.3) | −1.2 (−1.9, −0.4) | −0.9 (−1.7, −0.2) | −0.2 (−1.3, 0.8), 0.651 |
Abbreviations: CGI‐S, clinical global impression of severity of insomnia; CI, confidence interval; LS, least squares;
NPI‐10, neuropsychiatric inventory 10‐Item version; OR, odds ratio; SD, standard deviation; SDI, sleep disorders inventory; sEARLIER, subjective report of whether the patient woke up earlier than planned (yes/no response); sNTIBm subjective nighttime time in bed (mean computed from daily assessments over week 4); SSQC, single‐item sleep quality scale—caregiver; sSQRm,; subjective sleep quality rating (mean computed from daily assessments over week 4).
For all measures except sEARLIER and NPI‐10, analyses are based on a longitudinal data analysis model with terms for baseline value, baseline severity category (MMSE score of 12 to 20, 21 to 26), age category (non‐elders, elders), sex, region, treatment, time point, and treatment‐by‐time point interaction as covariates. sEARLIER analysis is based on a generalized mixed‐effects model including terms for baseline value, baseline severity category (MMSE score of 12 to 20, 21 to 26), age (non‐elders, elders), sex, region, treatment, with treatment difference expressed as an odds ratio (OR). NPI‐10 analyses are based on an ANCOVA model including terms for baseline value, baseline severity category (MMSE score of 12 to 20, 21 to 26), age (non‐elders, elders), sex, region, and treatment. p values should be considered nominal.
For these endpoints a higher score indicates less impairment.
For these endpoints a lower score indicates less impairment.
Lower bound of 95% CI was >0; “0.0” is due to reporting to 1 decimal place.
“‐“ not applicable.
3.4. Safety
Adverse events occurred in 22.5% of patients in the suvorexant group and 16.1% of patients in the placebo group (Table 4). One serious adverse event was reported in the suvorexant group (ankle fracture following a fall). One patient in each group discontinued treatment due to an adverse event (ankle fracture in the suvorexant group and diarrhea in the placebo group). Somnolence was the most common adverse event (4.2% of patients in the suvorexant group and 1.4% of patients in the placebo group) and was of mild‐to‐moderate severity (Table 4). A total of four falls occurred in three patients (2.1%) in the suvorexant group and no patients in the placebo group (Table 4). All falls were tripping/stumbling‐type events, which occurred during wakefulness, without preceding symptoms or loss of consciousness, while the patient was walking or getting into or out of bed; one fall resulted in the ankle fracture described above. None of the patients who fell reported somnolence and none of the falls were adjudicated as being due to cataplexy. None of the falls were considered to be drug‐related by the investigators (while blinded to treatment). Three falls occurred in patients receiving placebo during the run‐in period. There were no differences between treatments on the objective cognitive and psychomotor tests (MMSE and DS) administered the morning after the overnight PSG at week 4 (Table 5), or in the trial partner's assessment of the patient's neuropsychiatric symptoms (NPI‐10; Table 3). There were no findings of note for vital signs, physical exams, electrocardiography or laboratory measures.
Table 4.
Summary of adverse events over 4 weeks, within 14 days of last dose—number (%) of patients
| Suvorexant N = 142 | Placebo N = 143 | |
|---|---|---|
| General categories of events | ||
| ≥1 Adverse event | 32 (22.5) | 23 (16.1) |
| ≥1 Drug‐related adverse eventa | 15 (10.6) | 11 (7.7) |
| ≥1 Serious adverse event | 1 (0.7) | 0 (0) |
| ≥1 Serious drug‐related adverse eventa | 0 (0) | 0 (0) |
| Discontinued drug due to adverse event | 1 (0.7) | 1 (0.7) |
| Specific events ≥2% in any group | ||
| Somnolence | 6 (4.2) | 2 (1.4) |
| Headache | 5 (3.5) | 6 (4.2) |
| Fall | 3 (2.1) | 0 (0) |
| Dry mouth | 3 (2.1) | 1 (0.7) |
| Diarrhea | 0 (0) | 4 (2.8) |
| Pre‐specified events of clinical interest | ||
| Suicidal ideation/behavior | 0 (0) | 0 (0) |
| Events indicative of abuse potentialb | 2 (1.4) | 1 (0.7) |
| Complex sleep‐related behaviors | 0 (0) | 0 (0) |
| Hypnagogic/hypnopompic hallucination | 1 (0.7) | 0 (0) |
| Somnolence resulting in dose reductionc | 1 (0.7) | 0 (0) |
| Sleep paralysis | 0 (0) | 0 (0) |
| Sleep‐onset paralysis (adjudicated) | 0 (0) | 0 (0) |
| Cataplexy (adjudicated) | 0 (0) | 0 (0) |
| Falls/ataxia/worsening of balanced | 3 (2.1) | 0 (0) |
| Agitation | 0 (0) | 0 (0) |
| Confusion or cognitive impairmente | 0 (0) | 0 (0) |
Determined by the investigator to be related to the drug (determination made while blinded).
Terms included depersonalization, derealization, dissociation, euphoric mood, mania, hallucination, and potential study medication misuse.
The prespecified term was somnolence resulting in dose reduction or discontinuation of trial medication; however, no patients discontinued trial medication due to somnolence.
Falls were adjudicated to determine whether they were suggestive of cataplexy.
Daytime or nighttime.
Table 5.
Summary of results for cognition and psychomotor endpoints
| Observed baseline mean (SD) | Observed week 4 mean (SD) | Model‐based LS mean change from baseline (95% CI)a | Difference in LS mean suvorexant versus placebo | ||||
|---|---|---|---|---|---|---|---|
| Measure | Suvorexant | Placebo | Suvorexant | Placebo | Suvorexant | Placebo | (95% CI), p valuea |
| MMSE, 0–30 scale | 23.4 (2.8) | 22.9 (3.0) | 24.3 (3.2) | 23.9 (3.2) | 0.9 (0.6, 1.3) | 0.9 (0.6, 1.3) | −0.0 (−0.5, 0.5), 0.952 |
| DS, attempts | 28.2 (12.1) | 26.4 (11.3) | 29.8 (11.3) | 28.7 (12.8) | 1.8 (0.5, 3.0) | 2.1 (0.9, 3.4) | −0.4 (−2.1, 1.4), 0.692 |
| DS, correct | 27.1 (12.1) | 25.4 (11.8) | 28.9 (11.4) | 27.6 (13.4) | 1.9 (0.6, 3.1) | 2.0 (0.8, 3.2) | −0.1 (−1.9, 1.6), 0.873 |
Abbreviations: CI, confidence interval; DS, Digit Symbol test; LS, least squares; MMSE, Mini‐Mental State Exam; SD, standard deviation.
Analyses are based on a longitudinal data analysis model with terms for baseline value, baseline severity category (MMSE score of 12 to 20, 21 to 26), age category (non‐elders, elders), sex, region, treatment, time point, and treatment‐by‐time point interaction as covariates. p values should be considered nominal.
4. DISCUSSION
In this study of patients with clinically diagnosed mild‐to‐moderate probable AD dementia and insomnia, suvorexant improved sleep on the primary endpoint of TST and the secondary endpoint of WASO at week 4, as assessed by PSG. The mean difference from placebo in TST of 28 minutes exceeded the American Academy of Sleep Medicine consensus criteria of 20 minutes for a clinically significant mean treatment difference versus placebo.44 Furthermore, patients taking suvorexant were twice as likely as those on placebo to show an improvement of ≥60 minutes in TST. Suvorexant appeared to have its largest effect on sleep maintenance during the latter part of the night, in line with observations from insomnia patients without dementia.26 Although suvorexant increased TST and reduced WASO, it did not appear to significantly alter the underlying sleep architecture profile (time spent in different sleep stages expressed as percentage of TST) compared with placebo, consistent with previous findings in insomnia patients without dementia.45 It has been reported previously that suvorexant might slightly increase the proportion of REM sleep and reduce the time to REM sleep in patients with insomnia45; similar directional trends were observed in the present trial but the confidence intervals overlapped zero.
PSG measures are considered the best objective assessment of sleep and are particularly valuable where self‐reports are likely to be unreliable, as is the case for AD patients. Ideally, improvements in PSG measures should be supported by improvements in subjective endpoints but there was insufficient prior data to determine the validity of patient self‐reports or partner/clinician assessments for detecting treatment effects in this population. Nevertheless, exploratory analyses of trial partner and clinician ratings support that suvorexant improved patient's sleep quality as assessed by their trial partner and reduced overall severity of insomnia as assessed by their trial clinician. However, these findings should be treated with caution as they were not formally tested or adjusted for multiplicity.
The adverse event profile of suvorexant was generally similar to that observed in previous trials.25, 27, 28, 29 Somnolence was the most common adverse event with suvorexant but was not severe and did not result in discontinuation of trial medication. An increase in falls compared to placebo was not observed in previous randomized trials in elders.27 In the present trial, three patients in the suvorexant group (2.1%) fell/tripped in the treatment phase, whereas none in the placebo group did; three patients also reported falls on placebo during the run‐in period. Suvorexant did not appear to impair next‐day cognitive or psychomotor performance as assessed by objective tests, although these assessments do not constitute a comprehensive assessment of cognition.
This is the largest randomized controlled trial to date of the effects of a sleep medication on PSG sleep measures in a probable AD dementia population. The trial included a relatively high proportion (≈65%) of Hispanic/Latino patients, due to the use of study sites in Florida, California, and Peru, which have high Hispanic/Latino populations. Given the changes in the disease that occur over time, no single study can address the full range of sleep disturbance in AD. Our trial focused on patients with mild‐to‐moderate probable AD dementia being cared for at home whose sleep disturbance met diagnostic criteria for insomnia. In this group, suvorexant was effective and well‐tolerated, and the clinical outcomes were generally similar to those observed in previous trials of suvorexant in non‐demented patients with insomnia.26, 27 For example, the difference between suvorexant 10 to 20 mg and placebo in the present trial was 28 minutes compared with 35 minutes for suvorexant 15 to 20 mg after 4 weeks in the previous phase 3 trials.26 A high placebo response rate was also observed in the present trial (increase in TST of 45 minutes after 4 weeks), similar to that seen in the previous phase 3 trials (increase in TST of 43 minutes after 4 weeks).26
A limitation of our trial is that most patients (79%) had probable AD dementia of mild severity due to difficulties in recruiting patients with moderate severity. It is possible that the effects of suvorexant may differ in individuals with more severe dementia, or those with less typical patterns of sleep disturbance related to disease progression. Another limitation of the trial was that the diagnosis of probable AD dementia was clinical and was not confirmed by biomarkers (eg, positron emission tomography [PET] amyloid) or supported by magnetic resonance imaging (MRI) brain scans to rule out other causes, so some patients may have had other types of dementia. Data from previous trials have suggested that ≈25% of those clinically diagnosed with probable AD dementia do not have AD when biomarkers are assessed.46 However, clinical assessment corresponds to how most patients are currently diagnosed. While our trial had an intermediate duration of 4 weeks, data from a previous 1‐year trial, which included elders with insomnia, suggest that the early improvements in sleep seen with suvorexant are likely to be maintained provided that treatment is continued.29 Finally, we note that there are potentially important interactions between orexin, AD neuropathology (tau and amyloid β), sleep, and cognitive function, which our trial was not designed to evaluate.30, 31, 32, 33, 34 The possible influence of suvorexant on these interactions requires investigation in further studies. Our results do suggest that functional orexin signaling is sufficiently retained in patients with (predominantly mild) probable AD dementia, as suvorexant was able to competitively antagonize the action of endogenous orexin neuropeptides at orexin receptors to improve sleep in this population.
CONFLICT OF INTERESTS
W.J.H., P.C., E.S., K.B., J.H., J.S., C.L., and D.M. are current or former employees of Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and they own or owned stock options in Merck & Co., Inc., Kenilworth, NJ, USA. D.B. has acted as a consultant for Merck & Co., Inc. (Kenilworth, NJ USA), Jazz, Eisai, and Ferring.
Supporting information
Supporting information
AUTHOR CONTRIBUTIONS
W.J.H. and C.L. did the literature search and wrote the first draft of the paper. W.J.H., E.S., J.H., K.B., and D.M. contributed to the design of the trial. K.B., J.H., J.S., D.B., and W.J.H. contributed to the conduct and administration of the trial. P.C. performed the data analyses. All authors contributed to the interpretation of the data and the review and revision of the paper.
DATA SHARING STATEMENT
MSD's data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical trial data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
ACKNOWLEDGMENTS
This trial was supported by MSD. We thank the patients, caregivers, and families who participated in the trial, the site investigators and their staff, the members of the trial committee, and Theresa Taylor and Tien Dam of MSD for assistance with trial administration.
Herring WJ, Ceesay P, Snyder E, et al. Polysomnographic assessment of suvorexant in patients with probable Alzheimer's disease dementia and insomnia: a randomized trial. Alzheimer's Dement. 2020;16:541–551. 10.1002/alz.12035
Trial Registration: ClinicalTrials.gov, number NCT02750306
Funding information Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA
REFERENCES
- 1. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer's disease: systematic review and meta‐analysis. J Affect Disord. 2016;190:264‐271. [DOI] [PubMed] [Google Scholar]
- 2. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease? Trends Neurosci. 2016;39(8):552‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krause AJ, Simon EB, Mander BA, et al. The sleep‐deprived human brain. Nat Rev Neurosci. 2017;18(7):404‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holth J, Patel T, Holtzman DM. Sleep in Alzheimer's disease ‐ beyond amyloid. Neurobiol Sleep Circadian Rhythms. 2017;2:4‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shokri‐Kojori E, Wang GJ, Wiers CE, et al. beta‐Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A. 2018;115(17):4483‐4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lucey BP, McCullough A, Landsness EC, et al. Reduced non‐rapid eye movement sleep is associated with tau pathology in early Alzheimer's disease. Sci Transl Med. 2019;11(474). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holth JK, Fritschi SK, Wang C, et al. The sleep‐wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019;363(6429):880‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Macedo AC, Balouch S, Tabet N. Is sleep disruption a risk factor for Alzheimer's disease? J Alzheimers Dis. 2017;58(4):993‐1002. [DOI] [PubMed] [Google Scholar]
- 10. Asayama K, Yamadera H, Ito T, Suzuki H, Kudo Y, Endo S. Double blind study of melatonin effects on the sleep‐wake rhythm, cognitive and non‐cognitive functions in Alzheimer type dementia. J Nippon Med Sch. 2003;70(4):334‐341. [DOI] [PubMed] [Google Scholar]
- 11. Gehrman PR, Connor DJ, Martin JL, Shochat T, Corey‐Bloom J, Ancoli‐Israel S. Melatonin fails to improve sleep or agitation in double‐blind randomized placebo‐controlled trial of institutionalized patients with Alzheimer disease. Am J Geriatr Psychiatry. 2009;17(2):166‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serfaty M, Kennell‐Webb S, Warner J, Blizard R, Raven P. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. Int J Geriatr Psychiatry. 2002;17(12):1120‐1127. [DOI] [PubMed] [Google Scholar]
- 13. Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo‐controlled trial of melatonin for sleep disturbance in Alzheimer's disease. Sleep. 2003;26(7):893‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meguro K, Meguro M, Tanaka Y, Akanuma K, Yamaguchi K, Itoh M. Risperidone is effective for wandering and disturbed sleep/wake patterns in Alzheimer's disease. J Geriatr Psychiatry Neurol. 2004;17(2):61‐67. [DOI] [PubMed] [Google Scholar]
- 15. Onor ML, Saina M, Trevisiol M, Cristante T, Aguglia E. Clinical experience with risperidone in the treatment of behavioral and psychological symptoms of dementia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):205‐209. [DOI] [PubMed] [Google Scholar]
- 16. Yin Y, Liu Y, Zhuang J, et al. Low‐dose atypical antipsychotic risperidone improves the 5‐year outcome in Alzheimer's disease Patients with sleep disturbances. Pharmacology. 2015;96(3‐4):155‐162. [DOI] [PubMed] [Google Scholar]
- 17. Scoralick FM, Louzada LL, Quintas JL, Naves JO, Camargos EF, Nobrega OT. Mirtazapine does not improve sleep disorders in Alzheimer's disease: results from a double‐blind, placebo‐controlled pilot study. Psychogeriatrics. 2017;17(2):89‐96. [DOI] [PubMed] [Google Scholar]
- 18. Lopez‐Pousa S, Garre‐Olmo J, Vilalta‐Franch J, Turon‐Estrada A, Pericot‐Nierga I. Trazodone for Alzheimer's disease: a naturalistic follow‐up study. Arch Gerontol Geriatr. 2008;47(2):207‐215. [DOI] [PubMed] [Google Scholar]
- 19. Camargos EF, Louzada LL, Quintas JL, Naves JO, Louzada FM, Nobrega OT. Trazodone improves sleep parameters in Alzheimer disease patients: a randomized, double‐blind, and placebo‐controlled study. Am J Geriatr Psychiatry. 2014;22(12):1565‐1574. [DOI] [PubMed] [Google Scholar]
- 20. Salami O, Lyketsos C, Rao V. Treatment of sleep disturbance in Alzheimer's dementia. Int J Geriatr Psychiatry. 2011;26(8):771‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ellul J, Archer N, Foy CM, et al. The effects of commonly prescribed drugs in patients with Alzheimer's disease on the rate of deterioration. J Neurol Neurosurg Psychiatry. 2007;78(3):233‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elmstahl S, Stenberg I, Annerstedt L, Ingvad B. Behavioral disturbances and pharmacological treatment of patients with dementia in family caregiving: a 2‐year follow‐up. Int Psychogeriatr. 1998;10(3):239‐252. [DOI] [PubMed] [Google Scholar]
- 23. Cox CD, Breslin MJ, Whitman DB, et al. Discovery of the dual orexin receptor antagonist [(7R)‐4‐(5‐chloro‐1,3‐benzoxazol‐2‐yl)‐7‐methyl‐1,4‐diazepan‐1‐yl][5‐methyl‐2‐(2H ‐1,2,3‐triazol‐2‐yl)phenyl]methanone (MK‐4305) for the treatment of insomnia. J Med Chem. 2010;53(14):5320‐5332. [DOI] [PubMed] [Google Scholar]
- 24. Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant‐a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1‐2):52‐61. [DOI] [PubMed] [Google Scholar]
- 25. Herring WJ, Connor KM, Ivgy‐May N, et al. Suvorexant in patients with insomnia: results from two 3‐month randomized controlled clinical trials. Biol Psychiatry. 2016;79(2):136‐148. [DOI] [PubMed] [Google Scholar]
- 26. Herring WJ, Connor KM, Snyder E, et al. Suvorexant in patients with insomnia: pooled analyses of three‐month data from phase‐3 randomized controlled clinical trials. J Clin Sleep Med. 2016;12(9):1215‐1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herring WJ, Connor KM, Snyder E, et al. Suvorexant in elderly patients with insomnia: pooled analyses of data from phase III randomized controlled clinical trials. Am J Geriatr Psychiatry. 2017;25(7):791‐802. [DOI] [PubMed] [Google Scholar]
- 28. Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265‐2274. [DOI] [PubMed] [Google Scholar]
- 29. Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1‐year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double‐blind, placebo‐controlled trial. Lancet Neurol. 2014;13(5):461‐471. [DOI] [PubMed] [Google Scholar]
- 30. Fronczek R, van Geest S, Frolich M, et al. Hypocretin (orexin) loss in Alzheimer's disease. Neurobiol Aging. 2012;33(8):1642‐1650. [DOI] [PubMed] [Google Scholar]
- 31. Liguori C, Chiaravalloti A, Nuccetelli M, et al. Hypothalamic dysfunction is related to sleep impairment and CSF biomarkers in Alzheimer's disease. J Neurol. 2017;264(11):2215‐2223. [DOI] [PubMed] [Google Scholar]
- 32. Liguori C. Orexin and Alzheimer's disease. Curr Top Behav Neurosci. 2017;33:305‐322. [DOI] [PubMed] [Google Scholar]
- 33. Gabelle A, Jaussent I, Hirtz C, et al. Cerebrospinal fluid levels of orexin‐A and histamine, and sleep profile within the Alzheimer process. Neurobiol Aging. 2017;53:59‐66. [DOI] [PubMed] [Google Scholar]
- 34. Liguori C, Nuccetelli M, Izzi F, et al. Rapid eye movement sleep disruption and sleep fragmentation are associated with increased orexin‐A cerebrospinal‐fluid levels in mild cognitive impairment due to Alzheimer's disease. Neurobiol Aging. 2016;40:120‐126. [DOI] [PubMed] [Google Scholar]
- 35. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 37. Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 38. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28‐37. [PMC free article] [PubMed] [Google Scholar]
- 39. Silber MH, Ancoli‐Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3(2):121‐131. [PubMed] [Google Scholar]
- 40. Berry RB BR, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL and Vaughn BV for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.2. Darien, Illinois: American Academy of Sleep Medicine; 2015. [Google Scholar]
- 41. Tractenberg RE, Singer CM, Cummings JL, Thal LJ. The sleep disorders Inventory: an instrument for studies of sleep disturbance in persons with Alzheimer's disease. J Sleep Res. 2003;12(4):331‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jaeger J. Digit Symbol Substitution Test: The case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol. 2018;38(5):513‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308‐2314. [DOI] [PubMed] [Google Scholar]
- 44. Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(2):307‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Snyder E, Ma J, Svetnik V, et al. Effects of suvorexant on sleep architecture and power spectral profile in patients with insomnia: analysis of pooled phase 3 data. Sleep Med. 2016;19:93‐100. [DOI] [PubMed] [Google Scholar]
- 46. Siemers ER, Sundell KL, Carlson C, et al. Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2016;12(2):110‐120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


