Abstract
Background and purpose
Biomarkers reflecting the course of patients suffering from anti‐N‐methyl‐D‐aspartate receptor encephalitis (anti‐NMDARE) are urgently needed. Neurofilament light chains (NfL) have been studied as potential markers for neuroaxonal injury mainly in neuroinflammatory diseases, but so far there have been only in a few small reports on anti‐NMDARE. We aimed to compare the longitudinal course of cerebrospinal fluid (CSF)‐NfL levels and anti‐N‐methyl‐D‐aspartate receptor (anti‐NMDAR) antibodies with clinical parameters in six patients with anti‐NMDARE.
Methods
Longitudinal measurement of CSF‐NfL levels and CSF anti‐NMDAR antibodies in six patients suffering from anti‐NMDARE was performed.
Results
The major finding of this study is that most of our patients showed highly elevated NfL, with peak levels considerably delayed to clinical nadir. High NfL levels were associated with hippocampal atrophy but not with tumors detected. Furthermore, we did not find a clear relationship between NfL levels, CSF antibody titer, and CSF inflammatory markers.
Conclusions
CSF‐NfL levels do not predict short‐term outcome but rather are associated with intensive care unit stay and extreme delta brushes. However, high CSF‐NFL levels were associated with long‐term outcome. Our data suggest early aggressive immunotherapy to avoid primary and secondary neuroaxonal damage.
Keywords: anti‐N‐methyl‐D‐aspartate receptor encephalitis, encephalitis, neurodegeneration, neurofilament light
Clinical course, NFl levels and treatment regimen in anti‐NMDARE patients.

INTRODUCTION
Anti‐N‐methyl‐D‐aspartate receptor encephalitis (anti‐NMDARE) has a broad clinical spectrum with moderate to severe disease courses [1]. Biomarkers that correlate with disease activity are urgently needed to guide treatment decisions [2]. Inflammatory cerebrospinal fluid (CSF) alterations are typical findings in anti‐NMDARE, though besides clinical deterioration, antibody titer may be the only indicator for disease activity [1, 3, 4]. The latter is being proposed as a useful marker for predicting long‐term outcome and relapses. The usefulness of antibody titers in comatose patients is clinically difficult to assess, and serial CSF findings have been sparsely reported [5, 6, 7, 8]. Long‐lived plasma cells, hard to attack with immunotherapy, may survive in different niches in various organs and cause a steady‐state antibody level, reflected by the persistence of serum and CSF antibodies even years after clinical recovery [7, 9, 10, 11]. Nevertheless, CSF and serum titers are significantly higher in patients with poor outcome as well as in patients with underlying neoplasms [2, 12]. Monitoring of CSF parameters and antibody titers is, especially in the case of normal cerebral magnetic resonance imaging (cMRI) and electroencephalography (EEG), often used as a decision‐making tool to escalate or discontinue immunotherapy. Considering the frequently observed dichotomy between clinical findings and antibody titers, there is a need for additional biomarkers guiding the clinical decision‐making process, especially in comatose patients. Neurofilament light chains (NfL) are a reliable indicator of axonal damage and emerging biomarkers in neuroinflammatory diseases of the central nervous system [13]. Their value in anti‐NMDARE is far from understood. In this study, we aimed to report on longitudinal CSF Nfl and autoantibody levels of patients suffering from anti‐NMDARE.
METHODS
CSF‐NfL were measured by SIMOA Nf‐light kit in the immunoassay analyzer, Simoa (Quanterix, Boston, MA, USA). Anti‐N‐methyl‐D‐aspartate receptor (anti‐NMDAR) antibodies were determined using an in‐house cell‐based assay as reported earlier [14, 15]. Ethical approval was gained by the local ethics committee (Medical University of Vienna; 1773/2016). Values are given as medians and minimum–maximum. We retrospectively reviewed patients treated at our department between 2015 and 2020. Any other differential diagnosis than NMDARE had to be excluded, including negative polymerase chain reaction for the herpes virus or any other antibodies in the CSF or serum. Relapse was defined as clinical deterioration of prior symptoms or any new symptom after 2 months of clinical stabilization or improvement.
RESULTS
Clinical signs and symptoms
We report six patients (Figure 1a–f) with anti‐NMDARE, hospitalized between 2015 and 2020, and with follow‐up intervals from 15 to 55 months (median 29 months). Median age of the predominantly female patients (5/6) was 26.5 years. Initial symptoms were auditory and/or visual hallucinations (4/6), agitation and/or aggression (5/6), and delusions and catatonia (2/6). Epileptic seizures as the first presenting symptom were seen in three patients. Three months after initial symptoms were recognized, all patients fulfilled diagnostic criteria for anti‐NMDARE [16]. All patients required intensive care unit (ICU) monitoring due to dysautonomia and/or epileptic seizures between 5 days and 4 weeks after disease onset (median 18 days).
FIGURE 1.
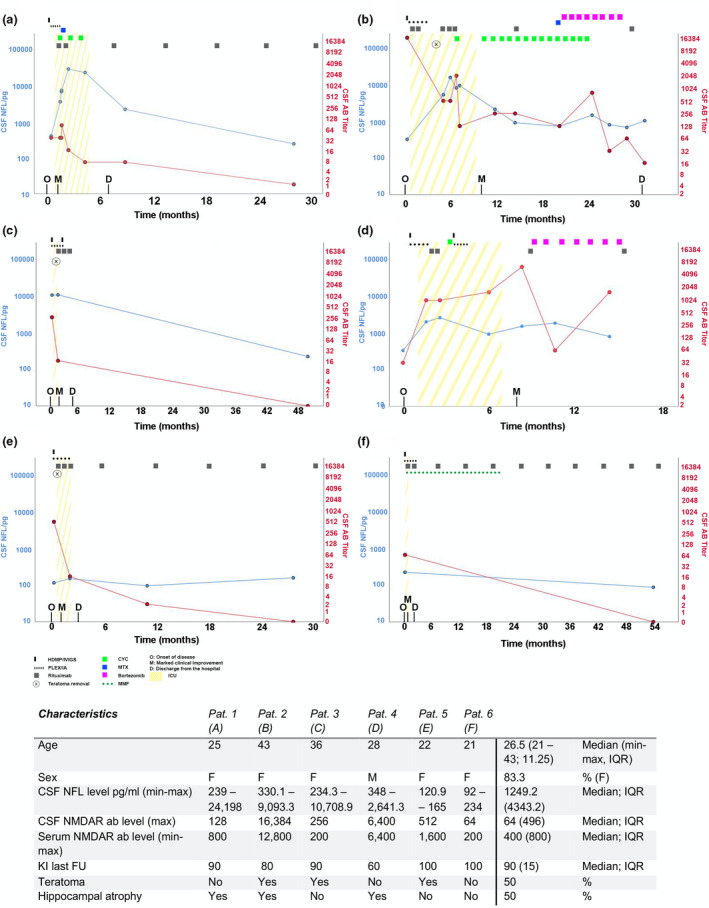
Clinical course and treatment regimen in anti‐N‐methyl‐D‐aspartate receptor encephalitis patients. (a–f) Disease course, treatment regimens, and clinical milestones. Y‐axes are scaled logarithmically. AB, antibody; CSF, cerebrospinal fluid; CYC, cyclophosphamide; D, discharge from the hospital; F, female; FU, follow‐up; HDMP, high‐dose methylprednisolone; ICU, intensive care unit; IQR, interquartile range; IVIG, intravenous immunoglobulin; KI, Karnofsky index; M, marked clinical improvement; MTX, methotrexate; MMF, mycophenolate mofetil; NFL, neurofilament light chains; NMDAR, anti‐N‐methyl‐D‐aspartate receptor; O, onset of disease; PLEX/IA, plasma exchange or immunoadsorption [Colour figure can be viewed at wileyonlinelibrary.com]
Initial diagnostic workup
Initial cMRI was unremarkable in four patients. Hippocampal T2‐signal alteration was noted in one patient, and unspecific juxtacortical T2‐signal alterations were found in another patient. EEG gave evidence of epileptic seizures in three patients. On admission, CSF studies showed a median 57 cells (6–115 cells), four patients had total protein elevation to 51.4 mg/dl, whereas intrathecal immunoglobulin G (IgG) production and positive oligoclonal bands (OCBs) were detected in four patients. In three female patients, ovarian teratoma was found, followed by resection between 3 weeks to 4 months after symptom onset. Tumor screening was conducted at least once during follow‐up.
Treatment
All patients received first‐line immunotherapy (steroids, immunoglobulins, and plasma exchange) within 10 to 24 days (median 13 days) and, if clinically indicated, second‐line therapy within 3 to 6 weeks (minimum‐maximum, median 4.5 weeks) after disease onset. Second‐line therapy included rituximab (750 mg/m2 body‐surface area) and/or cyclophosphamide (375 mg/m2 body surface area). Intrathecal methotrexate (1/6) and bortezomib subcutaneously (2/6) was used as escalation therapy in patients with a long comatose state [17, 18] .
Clinical course and follow‐up
Discharge from the ICU was possible after 10 days to 9 months (median 11 weeks) after first symptoms occurred. Marked clinical improvement such as holding eye contact or opening eyes on command was documented between 2 weeks and 10 months (median 7.5 weeks) after onset, and a Karnofsky index score of 50 was reached the soonest at 6 weeks and the latest at 22 months (median 4.5 months) after onset. Over the course, magnetic resonance imaging (MRI) showed hippocampal atrophy in three patients (Figure 1a,b,d), and of those the median ICU stay was 7 months (4–9 months). EEG extreme delta brushes (EDB) were recorded in 5/6 patients. EDB were seen for a median of 7 weeks (3–12 weeks) before the peak of CSF‐NfL levels. EEG studies revealed repeated epileptic status documented over a period of 4 weeks in two patients. The median time interval between last EEG‐confirmed seizure and NfL peak level was 7 weeks (10 days–9 weeks). Orofacial dyskinesia without EEG correlate was observed in five patients and thus not considered an epileptic seizure. In all patients, no relapse or worsening of symptoms was observed during the entire follow‐up period (median 29 months, 15–55 months).
NFL/CSF
In patients with at least three NfL assessments (five patients), levels peaked between 8 weeks to 6 months (median 2.5 months) after onset. In contrast, CSF anti‐NMDAR antibody titer maximum was reached between 2 weeks to 9 months after onset (median 4 weeks). Lumbar puncture (LP) was performed a median of six times (2–12). The median time interval from initial LP to normalization of cell count was 13 weeks (5–58 weeks), and the time to negative OCB (four patients) was a median of 30 weeks (6–32 weeks). We observed CSF‐NfL peak levels and negative intrathecal IgG either simultaneously or NfL peak levels following absence of intrathecal IgG within 1 month in four patients. In three of those four patients, normalization of CSF cell counts was observed within 1 month prior to CSF‐NfL peaks.
DISCUSSION
To our knowledge, this is the first description of patients with anti‐NMDARE with longitudinal follow‐up (median 29 months) simultaneously assessing NfL and anti‐NMDAR antibodies in the CSF. Similar to previous findings, anti‐NMDAR antibodies are detected in the CSF with higher sensitivity than in serum, and the CSF titer was shown to predict relapses more reliable than serum antibodies [2, 12]. For this reason, we chose to correlate CSF antibodies with CSF‐Nfl levels to avoid interference of Nfl levels by peripheral neurological comorbidities. NfL are emerging biomarkers especially in inflammatory central nervous system disorders; however, their value in anti‐NMDARE is still unclear. In our study, we made several interesting observations: (i) Although in all cases early treatment was initiated, CSF‐NfL peaked with delay in most cases within the first 6 months after disease onset, and in most cases, CSF antibody maximum titers preceded NfL peak levels. (ii) All patients with EDB detected prior to LP had significantly increased CSF‐NfL levels (>2.500 pg/ml) and at least 10‐fold higher levels than patients without EDB. (iii) A clear relationship between CSF‐NfL levels and clinical course or anti‐NMDAR antibody titer could not be observed, and clinical nadir was reached before CSF‐NfL levels peaked. (iv) MRI hippocampal atrophy was seen in half of the patients and in 75% of patients with CSF‐NfL levels above 2.500 pg/ml. In two patients, CSF‐NfL peak was associated with hippocampal atrophy rather than hippocampal T2 signal alteration in MRI, which is contradictory to previous findings [19] (v) Reduction of inflammatory markers in CSF (cell count and intrathecal IgG synthesis) preceded CSF‐NfL peaks. (vi) Detection of teratoma did not seem to influence CSF‐NfL levels. In an earlier small study, a correlation between serum‐NfL and clinical course was found; however, we could not confirm this observation [20]. Otherwise, our results are in line with an anti‐NMDARE case report showing by repetitive measures that CSF‐NfL levels peak 4 months after symptom onset, far beyond anti‐NMDAR antibody maximum titers[21]. Given the fact that released NfL reflect neuroaxonal damage, our data support the assumption that severe axonal damage in anti‐NMDARE is not caused primarily by immune‐mediated mechanisms including anti‐NMDAR antibodies, but rather is related to epileptic seizures and intensive care treatment (days onset to ICU range 10–28), the latter being also a negative predictor of outcome[4]. EDB occurred several weeks before CSF‐NfL peak levels and may thus have influenced NfL levels prior to maximum. Delayed neuroaxonal injury, as mirrored by delayed detection of CSF‐NfL levels, may be due to synaptic degeneration. Limitations of our study that need to be noted are the small sample size, the retrospective analysis, and the arbitrarily chosen LP intervals according to clinical assessment. Hence, we were not able to rule out status epilepticus or epileptic seizures as possibly confounding factors and potential cause for elevated CSF‐NfL levels. Nevertheless, we could not observe epileptic seizures confirmed clinically or by EEG timely linked to the NfL apex.
Our data suggest that CSF‐NfL levels do not correlate with short‐term outcome, but higher CSF Nfl levels were associated with worse long‐term outcome in our cohort of patients with anti‐NMDARE, especially in those with a protracted clinical course. Nevertheless, neuroaxonal damage reflected by high CSF‐NfL levels favors early aggressive immunotherapy in anti‐NMDARE to potentially prevent neuroaxonal injury and avoid secondary complications. To establish NfL as a reliable biomarker in anti‐NMDARE, further studies are warranted with larger sample sizes and standardized repeated measurements.
CONFLICT OF INTEREST
Patrick Altmann received a research grant from Quanterix International and was awarded a combined sponsorship from Biogen, Merck, Sanofi‐Genzyme, Roche, and Teva for a clinical study. Stefan Macher, Tobias Zrzavy, Romana Höftberger, Paulus Rommer, Fritz Zimprich, Thomas Berger, and Ekatarina Pataraia have nothing to disclose.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Dalmau J, Armangué T, Planagumà J, et al. An update on anti‐NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18:1045‐1057. [DOI] [PubMed] [Google Scholar]
- 2. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long‐term outcome in patients with anti‐NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Espinola‐Nadurille M, Bautista‐Gomez P, Flores J, et al. Non‐inflammatory cerebrospinal fluid delays the diagnosis and start of immunotherapy in anti‐NMDAR encephalitis. Arq Neuropsiquiatr. 2018;76:2‐5. [DOI] [PubMed] [Google Scholar]
- 4. Balu R, McCracken L, Lancaster E, Graus F, Dalmau J, Titulaer MJ. A score that predicts 1‐year functional status in patients with anti‐NMDA receptor encephalitis. Neurology. 2019;92:e244‐e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Behrendt V, Krogias C, Reinacher‐Schick A, Gold R, Kleiter I. Bortezomib treatment for patients with anti‐N‐methyl‐d‐aspartate receptor encephalitis. JAMA neurology. 2016;73:1251‐1253. [DOI] [PubMed] [Google Scholar]
- 6. Kataoka H, Kobayashi Y, Ueno S. Four‐year course of serum and cerebrospinal fluid antibody titers in a patient with anti‐NMDAR encephalitis. J Genet Syndr Gene Ther. 2014;5:1. [Google Scholar]
- 7. Hansen H‐C, Klingbeil C, Dalmau J, Li W, Weißbrich B, Wandinger K‐P. Persistent intrathecal antibody synthesis 15 years after recovering from anti–N‐methyl‐D‐aspartate receptor encephalitis. JAMA Neurol. 2013;70:117‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomas A, Rauschkolb P, Gresa‐Arribas N, Schned A, Dalmau JO, Fadul CE. Anti–N‐Methyl‐d‐aspartate receptor encephalitis: a patient with refractory illness after 25 months of intensive immunotherapy. JAMA Neurol. 2013;70:1566‐1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lightman SM, Utley A, Lee KP. Survival of long‐lived plasma cells (LLPC): piecing together the puzzle. Front Immunol. 2019;10:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pollok K, Mothes R, Ulbricht C, et al. The chronically inflamed central nervous system provides niches for long‐lived plasma cells. Acta Neuropathol Commun. 2017;5:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alexopoulos H, Kosmidis ML, Dalmau J, Dalakas MC. Paraneoplastic anti‐NMDAR encephalitis: long term follow‐up reveals persistent serum antibodies. J Neurol. 2011;258:1568‐1570. [DOI] [PubMed] [Google Scholar]
- 12. Gresa‐Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow‐up of anti‐NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13:167‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577‐589. [DOI] [PubMed] [Google Scholar]
- 14. Macher S, Zimprich F, DeSimoni D, Höftberger R, Rommer PS. Management of autoimmune encephalitis: an observational monocentric study of 35 patients. Front Immunol. 2018;9:2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ricken G, Schwaiger C, De Simoni D, et al. Detection methods for autoantibodies in suspected autoimmune encephalitis. Front Neurol. 2018;9:841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scheibe F, Prüss H, Mengel AM, et al. Bortezomib for treatment of therapy‐refractory anti‐NMDA receptor encephalitis. Neurology. 2017;88:366‐370. [DOI] [PubMed] [Google Scholar]
- 18. Yang X‐Z, Zhu H‐D, Ren H‐T, et al. Utility and safety of intrathecal methotrexate treatment in severe anti‐N‐methyl‐D‐aspartate receptor encephalitis: a pilot study. Chin Med J. 2018;131:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Körtvelyessy P, Prüss H, Thurner L, et al. Biomarkers of neurodegeneration in autoimmune‐mediated encephalitis. Front Neurol. 2018;9:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mariotto S, Gajofatto A, Zuliani L, et al. Serum and CSF neurofilament light chain levels in antibody‐mediated encephalitis. J Neurol. 2019;266:1643‐1648. [DOI] [PubMed] [Google Scholar]
- 21. Sveinsson O, Granqvist M, Forslin Y, Blennow K, Zetterberg H, Piehl F. Successful combined targeting of B‐and plasma cells in treatment refractory anti‐NMDAR encephalitis. J Neuroimmunol. 2017;312:15‐18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


