Abstract
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the coccidiostat Nilablend™ 200G containing lasalocid A sodium and nicarbazin. Nilablend™ 200G is not safe for chickens for fattening at the proposed maximum use level of 50 mg lasalocid A sodium + 50 mg nicarbazin/kg complete feed. Concurrent administration of Nilablend™ 200G (containing lasalocid A sodium) with tiamulin and certain other medicinal substances should be avoided. Lasalocid A sodium has antimicrobial activity against Gram‐positive bacterial species while many Enterobacteriaceae are naturally resistant. Induction of resistance and/or cross‐resistance was not observed in experimental conditions. No information on the interactions of nicarbazin with feed materials, other approved additives or medicinal products have been provided. No data were submitted on the microbiological safety of nicarbazin. The toxicological package for lasalocid A sodium and nicarbazin identified no observed adverse effect levels (NOAELs) that could be the basis for setting health‐based guidance values (e.g. an acceptable daily intake (ADI)). The Panel concluded that a concern for the genotoxicity of nicarbazin in Nilablend™ 200G cannot be excluded and that clarification on the mechanism of action of the test items would be needed. Therefore, the FEEDAP Panel is not in the position to establish an ADI for DNC on which to base the assessment of consumer safety. Nilablend™ 200G is considered toxic by inhalation, corrosive and irritant to eyes, slightly irritant to the skin but not a skin sensitiser. Inhalation exposure is considered a risk to persons handling the additive. The FEEDAP Panel cannot conclude on the safety of Nilablend™ 200G for the environment due to a possible risk for aquatic compartment (freshwater) for DNC. The efficacy of Nilablend® 200G was demonstrated.
Keywords: coccidiostats, lasalocid A sodium, nicarbazin, DNC, HDP, chickens for fattening, safety, efficacy
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from Zoetis Belgium SA2 for authorisation of the product lasalocid A sodium and nicarbazin (Nilablend™ 200G), when used as a feed additive for chickens for fattening (category: coccidiostats and histomonostats).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). The particulars and documents in support of the application were considered valid by EFSA as of 29 October 2019.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product lasalocid A sodium and nicarbazin (Nilablend™ 200G), when used under the proposed conditions of use (see Section 3.1.4).
1.2. Additional information
The feed additive containing two active substances lasalocid A sodium and nicarbazin (Nilablend™ 200G) has never been assessed by EFSA and it is not authorised in the European Union.
Lasalocid A sodium (Avatec® 150G) is authorised for use in chickens for fattening and chickens reared for laying,3 turkeys,4 pheasants, guinea fowl, quails and partridges other than laying birds5 with a withdrawal period of 5 days. Maximum residue limits (MRLs) are in force for lasalocid A sodium6 in poultry as follows: 60 μg/kg muscle, 300 μg/kg liver, 300 μg/kg skin/fat, 150 μg/kg kidney and 150 μg/kg egg.
EFSA issued seven opinions on the feed additive Avatec® 150G: two opinions on the re‐evaluation of the product for chickens for fattening and chickens reared for laying in accordance with article 9G of Council Directive 70/524/EEC (EFSA, 2004a,b), one opinion on a new formulation of Avatec® 150G in accordance with Regulation (EC) No 1831/2003 (EFSA, 2005), one opinion on the re‐evaluation of the product for turkeys in accordance with Regulation (EC) No 1831/2003 (EFSA FEEDAP Panel, 2010a) and one opinion on the safety and efficacy for pheasants, partridges, quails and guinea‐fowl (EFSA FEEDAP Panel, 2011); one opinion adopted in 2017, dealing with the re‐evaluation of the product under Regulation (EC) No 1831/2003 for chickens for fattening and chickens reared for laying and the assessment of the compliance with MRLs established by Implementing Regulation (EU) No 1277/20146 for chickens for fattening, chickens reared for laying, turkeys for fattening and other minor avian species, except laying birds (EFSA FEEDAP Panel, 2017a); the last opinion, adopted by the FEEDAP Panel in 2020 (EFSA FEEDAP Panel, 2020), assessed the new tolerance and efficacy studies in chickens for fattening to address the concerns identified by the FEEDAP Panel in its former opinion in 2017.
Nicarbazin is also authorised for chickens for fattening alone (Koffogran®)7 and in combination with narasin (Maxiban®).8 Nicarbazin in combination with monensin sodium (Monimax®)9 has been authorised for chickens for fattening, chickens reared for laying and turkeys for fattening.
Koffogran has been assessed by the FEEDAP Panel (EFSA, 2004c; EFSA FEEDAP Panel, 2010b). The FEEDAP Panel has adopted three opinions on Maxiban® (EFSA FEEDAP Panel, 2010c, 2016, 2019a). The FEEDAP Panel also adopted three opinions on Monimax (EFSA FEEDAP Panel, 2017b, 2018a, 2019b).
The MRLs in force for nicarbazin (dinitrocarbanilide (DNC) as the marker residue) in chicken tissues are 15,000 μg DNC/kg of fresh liver, 6,000 μg DNC/kg of fresh kidney, 4,000 μg DNC/kg of fresh muscle and 4,000 μg DNC/kg fresh skin + fat. The withdrawal time before slaughter is one day for nicarbazin from Koffogran and zero day for nicarbazin from Maxiban®.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier10 in support of the authorisation request for the use of lasalocid A sodium and nicarbazin (Nilablend™ 200G), as a feed additive.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports and experts’ elicitation knowledge, to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the lasalocid A sodium and nicarbazin in animal feed/marker residue in tissues. The Executive Summary of the EURL report can be found in Annex A.11
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of lasalocid A sodium and nicarbazin (Nilablend™ 200G) is in line with the principles laid down in Regulation (EC) No 429/200812 and the relevant guidance documents: Guidance for assessing the safety of feed additives for the environment (EFSA, 2008), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012), Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEDAP Panel, 2017c), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017d), Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017e), Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018b) and Guidance on the assessment of the efficacy of feed additives (EFSA FEEDAP Panel, 2018c).
3. Assessment
The present opinion assesses the safety and efficacy of the coccidiostat consisting of lasalocid A sodium and nicarbazin (Nilablend™ 200G) when used as feed additive for chickens for fattening.
3.1. Characterisation
3.1.1. Characterisation of the active substances
3.1.1.1. Lasalocid A sodium
Lasalocid A sodium is a monocarboxylic polyether ionophore obtained via fermentation using a non‐genetically modified strain of Streptomyces lasalocidi. ■■■■■
Lasalocid A sodium (sodium 6‐3R, 4S,5S,7R)‐7‐[2S,3S,5S)‐5‐ethyl‐5‐[(2R,5R,6S)‐5‐ethyl‐5‐hydroxy‐6‐methyltetrahydro‐2H‐pyran‐2‐yl]‐tetrahydro‐3‐methyl‐2‐furyl]‐4‐hydroxy‐3,5‐dimethyl‐6‐oxononyl]‐2,3‐cresotate; molecular formula C34H53O8Na; molecular weight 612.77 g/mol) has the CAS No. 25999‐20‐6.
Lasalocid sodium homologues B, C, D and E are also present. The structural formula of lasalocid sodium and its five forms is given in Figure 1.
Figure 1.
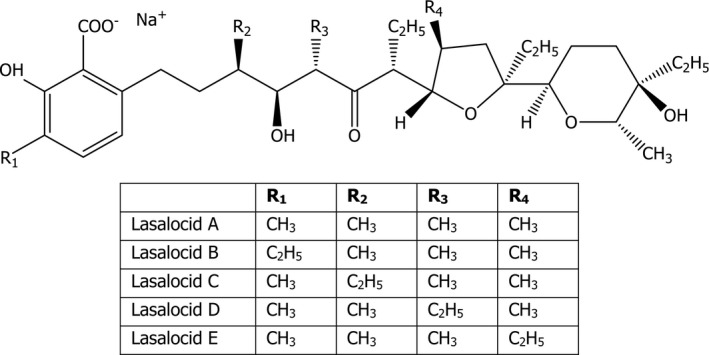
Structural formula of lasalocid sodium
The specifications for lasalocid A sodium content are set as ≥ 80% and for the homologues ≤ 10%. Data of three batches of the active substance showed that lasalocid A sodium amounted to 88.7–90.4% and the homologues were between 2.7 and 2.9%, which sum up to a total of 91.5–93.3%.14 Ethyl acetate content was between 48.9 and 101.0 mg/kg (specification is < 5,000 mg/kg). The FEEDAP Panel notes that the remaining unidentified substances may be fermentation by‐products.
Lasalocid A sodium is a white to brown powder, with melting point of 180°C; it is soluble in water (1.06 g/L) and readily soluble in organic solvents (2.0 g/L in ethanol, 500 g/L in chloroform, 93 g/L in acetone).
Characterisation of the production organism
The active substance lasalocid A sodium is produced by fermentation using a non‐genetically modified strain of Streptomyces lasalocidi. ■■■■■
■■■■■ Recently, a new species of the genus Streptomyces, Streptomyces lasalocidi, has been described as the valid taxonomic name with standing‐in prokaryotic nomenclature (Erwin et al., 2020). The type strain of this species is (ATCC 31180T = NRRL 3382T = DSM 46487T).
The susceptibility of the production strain to the antibiotics recommended by the FEEDAP Panel for ‘Corynebacterium and other Gram+’ (EFSA FEEDAP Panel, 2018a,b,c) was tested by broth microdilution following the method of the Clinical and Laboratory Standards Institute (CLSI)).17 All minimum inhibitory concentration (MIC) values were lower than the cut‐off values established in the guidance, except for ampicillin (8 μg/mL vs 1 μg/mL) and tetracycline (4 μg/mL vs 2 μg/mL). Exceedance of the cut‐off by one dilution to tetracycline is considered within the normal variation of the antimicrobial testing by microdilution. The strain should be considered resistant to ampicillin.
The whole genome sequence (WGS) of the production strain was interrogated■■■■■ for the presence of antimicrobial resistance (AMR) genes, using different databases.■■■■■ The search identified two hits: gimA gene (94% coverage and 80% identity to macrolide‐inactivating glycosyl transferases) and aac(3)‐Xa_1 gene (97% coverage and 74% identity to aminoglycoside acetyl transferases). Genes related to ampicillin resistance, an antibiotic to which Streptomyces spp. are considered intrinsically resistant (Hamid, 2011), were not identified.
3.1.1.2. Nicarbazin
Nicarbazin (CAS‐No: 330‐95‐0) is an equimolar complex formed by 1,3‐bis(4‐nitrophenyl)urea, also known as N,N’‐bi(4‐nitrophenyl)urea or 4,4′‐dinitrocarbanilide (DNC, molecular formula C13H10N4O5, molecular weight 302.25 g/mol) and 4,6‐dimethylpyrimidin‐2‐ol, also known as 2‐hydroxy‐4,6‐dimethylpyrimidine (HDP, molecular formula C6H8N2O, molecular weight 124.14 g/mol). The structural formula of nicarbazin is given in Figure 2.
Figure 2.
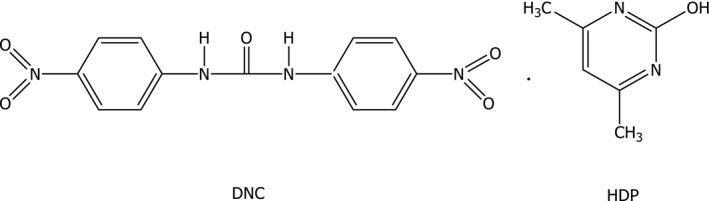
Structural formula of nicarbazin
■■■■■
Nicarbazin is a yellow to yellow green powder having a melting point of 260–265°C. It is slightly soluble in dimethyl formamide and insoluble in water.
Nicarbazin contains by specification 67.4–73.0% DNC and 27.7–30% HDP. Analytical data of three batches showed values of 70.5–70.7% for DNC and 28.6% for HDP. The content of free HDP is specified as ≤ 2.5%; values measured were 0.9–1.3%.20 Impurities were measured in three batches.20 The specification for PNA is ≤ 0.1%; values measured were 0.02–0.07%. Another impurity, methyl(4‐nitrophenyl) carbamate, was specified with ≤ 0.4%; values measured amounted to 0.16–0.35%. Any single unspecified impurity was specified as ≤ 0.2% (measured 0.03% in the three batches); total impurities was specified as ≤ 1.5% (measured 0.23–0.48% in the three batches). The residual solvents methanol and methylbenzene were specified as ≤ 3,000 mg/kg and ≤ 890 mg/kg (measured 234–944 mg/kg and 153–286 mg/kg, respectively).
3.1.2. Characterisation of the additive
The product Nilablend™ 200G is obtained by mixing the active substances with calcium sulfate dihydrate and povidone and subsequent granulation.
The composition of Nilablend™ 200G is summarised in Table 1.
Table 1.
Composition of Nilablend™ 200G
| Ingredients | g/kg Nilablend™ |
|---|---|
| Active ingredients | |
| Lasalocid A sodium1 | 100 |
| Nicarbazin2 | 100 |
| Other ingredients | |
| Povidone (polyvinyl pyrrolidone) | 60 |
| Calcium sulfate dihydrate | q.s. 1000 |
From lasalocid sodium produced by fermentation with a minimum content of 80% lasalocid A sodium.
From nicarbazin produced by chemical synthesis with a minimum content of 67.4% DNC and 27.7% HDP.
The content of the active substances in Nilablend™ 200G is set in the specifications as: 92.5–107.5 g/kg lasalocid A sodium and 90.0–110.0 g/kg nicarbazin.
Batch to batch consistency was confirmed by analysis of six batches with mean lasalocid A concentration of 99.0 g/kg (range: 98.6–99.2 g/kg) and mean nicarbazin concentration of 100.7 g/kg (range: 99.7–101.6 g/kg).21
Three batches of Nilablend™ 200G were analysed for heavy metals, arsenic and fluorine, dioxins and dioxin‐like PCBs, aflatoxin B1 and microbial contamination.22 Results showed that heavy metals, arsenic and fluorine were below the limit of quantification (LOQ): arsenic < 0.50 mg/kg, cadmium < 0.20 mg/kg, lead < 0.50 mg/kg, mercury < 0.02 mg/kg and fluorine < 40 mg/kg. Values for dioxins (polychlorinated dibenzo‐p‐dioxins and dibenzofurans (PCDD/F)) were between 0.17 and 0.18 ng WHO‐PCDD/F‐TEQ/kg, the sum of dioxins and dioxin‐like polychlorinated biphenyls (DL‐PCBs) was 0.31 ng WHO‐PCDD/F‐DL‐PCB‐TEQ/kg and non‐dioxin‐like PCBs were 2 μg/kg. Aflatoxin B1 was below the LOQ (0.6 μg/kg). Salmonella spp. were not detected in 25 g samples, Enterobacteriaceae were below the limit of detection (LOD) (10 colony‐forming units (CFU)/g) and mould and yeast were also below LOD (100 CFU/g). None of the amounts of these impurities were of concern.
The presence of viable cells of the production strain of lasalocid A sodium was analysed in three batches of the active substance ■■■■■■■■■■■■■■■ No cells of the production strain were detected.
The presence of DNA from the production strain of lasalocid A sodium was analysed in 1 g of three batches of the final product analysed in triplicate.24 The primers targeted the junction region between an integrated giant linear plasmid and the chromosome, with an expected amplicon size of 625 bp. The protocol included a lysis step. Positive and negative controls were included. No DNA was detected in the nine analysed samples in a polymerase chain reaction (PCR) experiment having an LOD < 10 ng DNA/g of product.
Nilablend™ 200G is a granulated product of yellow to brownish yellow colour with an average bulk density of 860 kg/m3 and average tap density of 950 kg/m3. Sieve analysis of six batches of Nilablend™ 200G showed that only 1–3% of the particles are passing through a sieve of 105 μm mesh size. Dusting potential of Nilablend™ 200G determined in six batches ranged between 0 and 0.03 g/m3;25 no information was provided on the content of the active substances in the dust.
3.1.3. Stability and homogeneity
3.1.3.1. Shelf‐life
For the study of the shelf‐life of the additive, three batches of Nilablend™ 200G was stored in multi‐wall (five‐layer) Kraft polyethylene lined bags for 24 months at 25°C/60% relative humidity (RH), 30°C/65% RH, 30°C/75% RH and for 12 months at 40°C/75% RH. Recoveries of lasalocid A sodium were above 99%, 98%, 96% after 24 months storage at the first three conditions, respectively. Recoveries after 12 months at 40°C/75% RH were above 96%. Recoveries of nicarbazin were above 99% at all conditions.26
3.1.3.2. Stability in premixtures and feedingstuffs
Nilablend™ 200G was incorporated in a vitamin/mineral premixture (containing choline chloride) for poultry. Two concentrations were prepared providing 2,000 + 2,000 mg and 800 + 800 mg lasalocid A sodium + nicarbazin/kg premixture.27 The premixtures were stored under 25°C/60% RH and 30°C/65% RH for 6 months. The applicant provided the acceptance criteria for the recovery (80–110% for both active substances) and a detailed analytical report of the results. Recoveries were above 80% indicating that mineral premixtures containing Nilablend™ 200G can be stored up to 6 months when stored under 25°C/60% RH or 30°C/65% RH.
The applicant submitted three studies on the stability of the additive in feed.
In the first study, Nilablend™ 200G was incorporated in a commercial feed for chickens for fattening providing 40 mg each of lasalocid A sodium and nicarbazin/kg feed.28 Samples of pelleted/crumbled feed (three batches) were stored at 25°C/60% RH for up to 90 days and at 30°C/65% RH and 40°C/75% RH for up to 28 days. Final recoveries ranged from 87.1% to 104.5% of the target concentration for nicarbazin and 83–118% of the target concentration for total lasalocid indicating that Nilablend™ 200G incorporated in pelleted/crumbled feed is stable for at least 90 days when stored under 25°C/60% RH and at least 28 days when stored under 30°C/65% RH or 40°C/75% RH.
In the second study, Nilablend™ 200G was incorporated in a complete poultry diet based mainly on corn and soybean.29 Two batches of mash feed were prepared providing 40 mg each of lasalocid A sodium and nicarbazin per kg feed. One batch of mash feed was prepared providing 50 mg each of lasalocid A sodium and nicarbazin per kg; a part of this batch was pelleted and crumbled to produce a fourth batch. Samples of the above feeds were stored at 25°C/60% RH and 30°C/65% RH up to 90 days and at 40°C/75% RH up to 28 days. After 3‐month storage of the mash feed, recoveries for lasalocid A sodium ranged from 96.8% to 100% at 25°C/60% RH and from 89.2% to 101% at 30°C/65% RH; recoveries for nicarbazin were between 92.4% and 108% at 25°C/60% RH and between 94.7% and 107% at 30°C/65% RH. The values corresponding to 4 weeks storage at 40°C/75% RH were 98.6–107% for lasalocid A sodium and 90–112% for nicarbazin. After 3 months storage of the pelleted feed at 25°C/65% RH, recoveries were 96.1% for lasalocid A sodium and 93% for nicarbazin; at 30°C/65% RH, recoveries were 99% for lasalocid A sodium and 110% for nicarbazin. In the pelleted feed after 4‐week storage at 40°C/75% RH, recoveries were 87% for lasalocid A sodium and 96.5% for nicarbazin.
In the third study, Nilablend™ 200G was incorporated in a complete poultry diet (based mainly on corn and soybean meal).30 A total of five batches of feed were prepared: one batch (mash) providing 40 mg each of lasalocid A sodium and nicarbazin per kg feed; two batches (mash) and two batches (pelleted/crumbled) providing 50 mg each of lasalocid A sodium and nicarbazin per kg feed. After 3‐month storage of the mash feed at 25°C/65% RH and 30°C/65%, RH recoveries for lasalocid A sodium were between 99.7 and 101% and 76.9 and 85%, respectively; the recoveries for nicarbazin at 25°C/65% RH and 30°C/65% RH were between 99.1 and 110% and 101 and 102%, respectively. The values corresponding to 4 weeks storage at 40°C/75% RH were between 94.4 and 97.9 for lasalocid A sodium and 91.6–94% for nicarbazin. After 3‐month storage of the pelleted feed at 25°C/65% RH, the recovery for lasalocid A sodium was 91.5%, while they were not measured at 30°C/65% RH. The recovery of nicarbazin was 104% at 25°C/65% RH. In the pelleted feed after 4‐week storage at 40°C/75% RH, recoveries were 88.1–93.5% for lasalocid A sodium and 88.2–93.3% for nicarbazin.
3.1.3.3. Homogeneity
The homogeneous distribution of the active substances in premixture and in feed was studied by taking 10 samples from each of the premixture and feed batches prepared for stability testing (described under Section 3.1.3.2) and analysed for lasalocid A sodium and nicarbazin content. The coefficients of variation (CV) were in all cases ≤ 15%.
3.1.4. Conditions of use
Nilablend™ 200G is intended to be used to prevent coccidiosis in chickens for fattening up to slaughter. The inclusion level of lasalocid A sodium and nicarbazin from the additive Nilablend™ 200G (combination of the two active substances in a 1:1 ratio), in complete feed for chickens for fattening is 40 + 40 to 50 + 50 mg lasalocid A sodium + nicarbazin/kg. The applicant proposes a withdrawal period of 2 days. Nilablend™ 200G shall be incorporated in compound feed in form of a premixture. The MRLs proposed for edible chicken tissues (on fresh matter basis) are:
-
–
for lasalocid A sodium, 300 μg/kg liver, 150 μg/kg kidney, 60 μg/kg muscle and 300 μg/kg skin/fat31;
-
–
for nicarbazin, (dinitrocarbanilide (DNC) as the marker residue) 15,000 μg DNC/kg of liver, 6,000 μg DNC/kg of kidney, 4,000 μg DNC/kg of muscle and 4,000 μg DNC/kg skin + fat.
3.2. Safety
The FEEDAP Panel has assessed the safety of Lasalocid A sodium for use in chickens for fattening in 2017 (EFSA FEEDAP Panel, 2017a). For the current evaluation, the applicant mentioned the conclusions in this previous assessment and performed a literature search covering the period 2015–2019 to complement the previous assessment. The literature search was performed according to the principles set in the FEEDAP guidance on the renewal of feed additives and all the details of the search were provided and covered: the safety for the target animals (plus other poultry species), safety for the consumers (i.e. toxicity, including genotoxicity and mutagenicity), workers/users and the environment, interactions/incompatibilities (e.g. with drugs, feed components etc.), antimicrobial resistance (cross‐resistance) and shedding of enteropathogens. The FEEDAP Panel assessed the outcome of the literature review and the relevant papers are described in the assessment below. In addition, the applicant provided specific studies performed with nicarbazin (and its two moieties, DNC and HDP), and the combination of nicarbazin and lasalocid, which are described below.
3.2.1. Safety of the production strain of lasalocid A sodium
The identity of the production strain as Streptomyces lasalocidi has been confirmed. Based on the WGS data provided, the production strain harbours two AMR‐like genes, with homology to a macrolide‐inactivating glycosyl transferase and an aminoglycoside acetyl transferase, respectively. Viable cells of the production strain were not detected in lasalocid A sodium and its DNA was not detected in the final product Nilablend™ 200G. Consequently, the Panel concludes that Nilablend™ 200G does not raise safety concerns as regards the production strain of lasalocid A sodium.
3.2.2. Absorption, distribution, metabolism, excretion (ADME) and residues
Nilablend™ 200G is a mixture of lasalocid A sodium and nicarbazin. Nicarbazin is entirely split in the intestinal tract of birds into its two constituents, DNC and HDP. Consequently, the three constituents (lasalocid A sodium, DNC and HDP) must be assessed separately. The potential interaction of lasalocid A sodium with nicarbazin/DNC/HDP should also be considered.
The ADME and residue studies with lasalocid A sodium in chickens for fattening were already assessed by the FEEDAP Panel in its previous opinions on Avatec® (EFSA, 2004a,b, 2017a). New ADME and residue studies have been carried out with [14C]‐HDP‐nicarbazin and [14C]‐DNC‐ nicarbazin alone and in association with lasalocid. A study of the comparative metabolic fate of nicarbazin in chicken and rat was performed. A marker residue study following Nilablend™ 200G administration was also provided.
3.2.2.1. Lasalocid A sodium
The main conclusions of the previous FEEDAP opinions on Avatec® 150G (EFSA, 2004a,b, 2017) are summarised as follows: (i) lasalocid is extensively absorbed and metabolised in chickens mainly by oxidation to mono‐ and multi‐hydroxy metabolites; excretion occurs essentially through the bile and droppings as unchanged lasalocid (about 10%) and a great number of metabolites of increasing polarity corresponding to several un‐resolved peaks (HPLC) representing each less than 5% of the whole radioactivity excreted; (ii) lasalocid is the major component (26% for both sexes) in the liver of chickens, whereas a major metabolite (15%) has been identified as a monohydroxy‐lasalocid (hydroxylation position not established); other metabolites of increasing polarity, representing each less than 10%, have been separated but not identified; (iii) unchanged lasalocid A appeared to be the marker residue and the liver the target tissue and (iv) complementary data showed the similarity of the metabolic fate in the chicken and the rat where monohydroxy‐ and dihydroxy‐lasalocid were identified in the urine.
3.2.2.2. Nicarbazin
Two studies were performed following the same experimental protocol (link: study 210).32 Four groups of chickens for fattening (36 days of age, 4 birds per gender) were fed for seven consecutive days a complete feed supplemented with either [14C]‐HDP‐nicarbazin plus lasalocid A sodium (two groups) or [14C]‐DNC‐nicarbazin plus lasalocid (two groups) at the maximum levels proposed for use, i.e. 50 mg nicarbazin and 50 mg lasalocid/kg (analytically confirmed). [14C]‐labelling was located on chemically stable positions of the molecules (pyrimidine ring for HDP, benzene ring for DNC) and the radiopurity was checked. In each study, birds of the two groups were euthanised at day 42 and 43, after 6 and 24 h withdrawal of the supplemented feed, respectively. Tissues (liver, kidney, muscle and skin/fat) and bile were sampled. Excreta were collected daily and individually from day 1 (pre dosage) to day 6/7 of the experiment. Total radioactive residues were measured in all samples collected. Excreta and duplicate tissue samples were pooled by gender and sampling time point to study the metabolic profiling of [14C]‐HDP derived metabolites, the other performing the profiling of [14C]‐DNC‐derived metabolites.33 , 34
Metabolic profiles of [14C]‐DNC‐nicarbazin from tissues and excreta were obtained after extraction (acetonitrile/TFA (trifluoroacetic acid)) followed by HPLC fractionation and radioactivity detection.34 Extractability (minus analytical losses) of total residues was 76%, 94%, 85%, 93% and 93% in the excreta, liver, kidney, muscle and skin/fat, respectively. Identification of DNC and metabolites was limited to the comparative chromatographic behaviour of standard DNC and standard metabolites M1 (4,4′‐diacetamidocarbanilide), M2 (N,N’‐1,4‐phenylenebisacetamide) and M3 (N‐[4‐[[[(4‐nitrophenyl)amino]carbonyl]amino]phenyl]acetamide). In the pooled excreta from day 6 and day 7, i.e. at steady state, DNC amounted to 26% (average value of males and females) of the whole radioactivity, M1 to 39%, minor metabolites were each less than 10% (M2, 7% and M3, 3%). [14C]‐DNC profiling in tissues collected after 6‐h withdrawal showed that DNC was the main residue with average values (males and females) of 90%, 66%, 83% and 84% of the whole radioactivity of the liver, kidney, muscle and skin/fat, respectively. These values were retained as ratio marker to total residue. No metabolite amounting to ˃ 10% was identified in all tissues, with the exception of kidney where metabolite M1 amounted to 25%.
Metabolic profiles of [14C]‐HDP‐nicarbazin were obtained from tissues and excreta following extraction (acetonitrile/water) using radio‐chromatographic detection/separation and liquid chromatography with tandem mass spectrometry (LC–MS/MS) identification (study 215).33 Extractability of total residues was ≥ 85% in the excreta and ≥ 92% in tissues. HDP was the major residue amounting to 63–64% (male and female) of the total radioactivity in the liver, 84% in the kidney (pooled gender), 75–79% in the muscle, 84–67% in the skin/fat; after 6‐ and 7‐day total exposure, HDP represented 78% (males) and 63% (females) of the radioactivity of the excreta. None of the metabolites detected amounted to more than 10% each of the total radioactivity. Bile analysis showed considerable radioactivity excretion in the first hours in the two studies that indicating an active first pass metabolism of nicarbazin.
Total radioactive residues determined in tissue samples of birds from the two studies are reported on Table 2.
Table 2.
Total residue concentrations1 measured in tissues/organs from chickens (four males and four females) fed for 6 days 50 mg [14C]‐DNC‐nicarbazin or 50 mg [14C]‐HDP‐nicarbazin associated with 50 mg lasalocid A sodium/kg feed, following a 0.25‐day withdrawal period
| Treatment | Moiety measured | Liver | Kidney | Muscle | Skin/fat |
|---|---|---|---|---|---|
| 14 C‐DNC-nicarbazin + lasalocid | mg equivalent DNC/kg fresh tissue | 14.020 ± 3.918 (21.896) | 9.843 ± 2.274 (14.391) | 2.183 ± 0.718 (3.619) | 2.298 ± 0.802 (3.902) |
| 14 C‐HDP-nicarbazin + lasalocid | mg equivalent HDP/kg fresh tissue | 0.061 ± 0.021 (0.103) | 0.094 ± 0.029 (0.152) | 0.042 ± 0.014 (0.070) | 0.024 ± 0.009 (0.042) |
Mean concentrations ± SD, two times SD in parenthesis.
Total residues in tissues derived from [14C]‐DNC‐nicarbazin were much higher (50‐ to 200‐fold) than those measured with [14C]‐HDP‐nicarbazin.
Potential interaction nicarbazin/lasalocid
The potential influence of lasalocid on the metabolic behaviour of nicarbazin was studied based on the comparison of the metabolic fate and residues of nicarbazin in the presence32 or in the absence of lasalocid sodium.
The metabolic fate of [14 C]‐HDP nicarbazin was studied35 on seven groups of chickens (three males and three females per group), which entered the study at an age of 14 days and were kept on control feed until 35 days of age. The test substance at a nominal level of 50 mg/kg feed (analytically confirmed) was then given to for seven consecutive days (until 43‐day‐old), followed by different withdrawal times (0.25, 1, 3, 4, 7 and 10 days). One group served as donor animals for blood samples in 24‐h intervals along the longest withdrawal period. At the end of the withdrawal periods, the animals were euthanised and tissues sampled. Excreta were sampled from the group with 7‐day withdrawal. Total radioactive residues were determined in all samples. Duplicate samples were used to perform metabolite profiling using liquid chromatography with tandem mass spectrometry (LC–MS/MS) analysis.
Plasma whole radioactivity reached steady state after 1‐day administration, only after 2–3 days in the excreta, that justifies the 7 days duration of the chicken exposure. Total residue concentration in edible tissues after 6 h withdrawal, expressed as mg equivalent HDP/kg fresh tissue amounted to 0.060 ± 0.024 in the liver, 0.104 ± 0.044 in the kidney, 0.047 ± 0.024 in the muscle and 0.024 ± 0.009 in the skin/fat. These values are not significantly different from those determined when [14C]‐HDP‐nicarbazin was administered together with lasalocid sodium. Metabolic profiles confirmed that HDP was the major radioactive residue in all tissues, excreta and plasma.36 None of the other metabolites amounted to more than 10% of the whole radioactivity, whatever the withdrawal period, with the exception of one metabolite (not identified) that was found in a range of values of 11–17% total residues in the liver of males and females after 6 h of withdrawal.
A study following an identical protocol was performed with [14 C]‐DNC nicarbazin.37 Total residue concentration in edible tissues after 6 h withdrawal, expressed as mg equivalent DNC/kg fresh tissue amounted to 15.294 ± 3.089 in the liver, 11.000 ± 2.122 in the kidney, 2.335 ± 0.454 in the muscle and 2.593 ± 0.566 in the skin/fat. These values are not significantly different from those determined when [14C]‐DNC‐nicarbazin was given together with lasalocid A sodium. Metabolic profiles confirmed that DNC was the major radioactive residue in all tissues, excreta and plasma.38 None of the other metabolites amounted to more than 10% of the whole radioactivity, whatever the withdrawal period.
It can be concluded that lasalocid A sodium does not interfere with the metabolic fate of HDP and DNC in chicken.
The FEEDAP Panel noted that the potential interaction of nicarbazin on the metabolic fate of lasalocid sodium was not studied.
3.2.2.3. Marker residue study
Residues of lasalocid A and DNC were determined in the tissues of chickens administered Nilablend™ 200G at a dose corresponding to 50 mg nicarbazin and 50 mg lasalocid sodium/kg feed (analytically confirmed).39 Three groups of eight birds (1‐day‐old, four males and four females) were fed the supplemented complete feed for 42 days then slaughtered after withdrawal periods of 1, 2 or 3 days. Tissues (liver, kidney, muscle and skin/fat) were sampled, preserved (–70°C) and analysed for lasalocid A40 and DNC contents.41 Lasalocid A was measured using a validated LC‐MS/MS method with monensin sodium as internal standard.42 Tissues were analysed for DNC content using a validated LC‐MS/MS analytical method with deuterated DNC‐d8 as internal standard.43
The results relevant for the assessment are summarised in Tables 3 and 4.
Table 3.
Marker residue concentrations1 of lasalocid A (mg/kg fresh tissue) measured in tissues from chickens (four males and four females) administered for 42 days Nilablend™ 200G at a dose corresponding to 50 mg nicarbazin and 50 mg lasalocid A sodium/kg feed, at different withdrawal periods
| Withdrawal | Liver | Kidney | Muscle | Skin/fat |
|---|---|---|---|---|
| 1 day 2 | 0.136 ± 0.122 (0.380) | 0.051 ± 0.045 (0.141) | 0.051 ± 0.045 (0.141) | 0.160 ± 0.128 (0.416) |
| 2 days 3 | 0.036 ± 0.049 (0.134) | 0.019 ± 0.021 (0.061) | 0.014 ± 0.011 (0.036) | 0.025 ± 0.029 (0.083) |
Mean concentrations ± SD, two times SD in parenthesis.
Results including one and two values below the LOQ for kidney and muscle, respectively, and accounting each to 0.010 mg/kg tissue.
Results including 5, 6 and 4 values below the LOQ for kidney, muscle and skin/fat, respectively, and accounted each to 0.010 mg/kg tissue.
Table 4.
Marker residue concentrations1 of nicarbazin (mg DNC/kg fresh tissue) measured in tissues from chickens (four males and four females) administered for 42 days a Nilablend™ 200G dose corresponding to 50 mg nicarbazin and 50 mg lasalocid A sodium, at different withdrawal periods
| Withdrawal | Liver | Kidney | Muscle | Skin/fat |
|---|---|---|---|---|
| 1 day | 8.570 ± 1.432 (11.435) | 3.510 ± 1.120 (5.750) | 1.640 ± 0.294 (2.228) | 1.950 ± 0.257 (2.464) |
| 2 days | 6.090 ± 0.597 (7.284) | 1.390 ± 0.373 (2.136) | 1.040 ± 0.170 (1.380) | 1.220 ± 0.207 (1.634) |
Mean concentrations ± SD, two times SD in parenthesis.
3.2.2.4. DNC metabolic fate in laboratory animals
Two studies in rats were provided. The purpose of the first study44 was to determine the pharmacokinetics of nicarbazin and the components of nicarbazin (DNC and HDP) at varying concentrations and ratios after a single oral dose to rats and make comparisons between the dose levels. These data were not considered relevant for consumer assessment.
The second study45 was done to quantify the metabolites of DNC after a single oral dose administration to rats. A single dose of DNC (450 mg/kg body weight (bw)) was administered by gavage to six males and six females (240–300 g bw, 9 and 13 weeks old). Blood was collected from the jugular vein 2, 4, 6, 12, 24, 32 and 48 h post dose. Individual plasma samples were analysed for the presence of metabolites M1, M2 and M3 using an LC‐MS/MS method.46 The three metabolites were detected and identified in all plasma samples and reached a maximum concentration after 9–12 h for M1, 2–4 h for M2 and 4 h for M3. These data indicate a commonality of metabolic pathway of DNC in the rat and chicken.
The FEEDAP Panel noted that the applicant also submitted a pharmacokinetic study in dog, which was not considered relevant for consumer assessment.47
3.2.2.5. Conclusion on ADME and residues
Lasalocid is extensively absorbed and metabolised; unchanged lasalocid A is the marker residue and liver is the target tissue. The DNC moiety is extensively metabolised, but DNC is the major residue. The HDP moiety of nicarbazin is metabolised to a limited extent and HDP is the main residue in target tissues. The concentration of DNC residues in tissues is two orders of magnitude higher than that of HDP. DNC should be considered as the marker residue of nicarbazin and liver is the target tissue. Lasalocid does not interfere with the metabolic fate and residual status of DNC and HDP. The commonality of metabolic pathways of lasalocid and DNC in the chicken and the rat is established.
3.2.3. Safety for the target species
3.2.3.1. Safety for chickens for fattening
The applicant submitted two studies to demonstrate the safety of the simultaneous administration of lasalocid A sodium and nicarbazin in chickens for fattening. For the exploratory study, commercially available sources of lasalocid A sodium and nicarbazin were mixed at appropriate doses. Although this study does not comply with the FEEDAP requirements (shorter duration, small number of replicates and low final body weight), it is fully described below since it provides essential information for the assessment. The pivotal study is a tolerance study performed in line with the FEEDAP Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017d); the study was conducted with the final product, i.e. the combination of lasalocid A sodium and nicarbazin at a proportion of 1:1.
Exploratory study 48
A total of 80 1‐day‐old Ross 708 chickens (40 males and 40 females) was randomly allocated to four treatment groups which were fed diets supplemented for 28 days with 0, 50 + 45, 100 + 90 and 150 + 135 mg lasalocid sodium49 and nicarbazin/kg complete feed (from two separate commercial products) corresponding to 0, 1x, 2x and 3x of the maximum proposed (use) level for lasalocid A sodium and nicarbazin. Analytical data (see Table 5) confirm the intended levels. Group size was 20 birds per treatment (two male replicates and two female replicates with five birds each).
Table 5.
Least square means of the most relevant parameters from the exploratory study in chickens for fattening fed lasalocid sodium and nicarbazin compared to the control group1
| Control | 1x | 2x | 3x | |
|---|---|---|---|---|
| Lasalocid sodium + nicarbazin (mg/kg feed) | ||||
| Intended | 0 | 50 + 45 | 100 + 90 | 150 + 135 |
| Analysed, starter | < 0.1 + 1.5 | 46.6 + 43.2 | 106 + 78.4 | 165 + 111.5 |
| Analysed, grower | 1.6 + 2.0 | 42.7 + 39.1 | 105 + 85.9 | 112 + 138.2 |
| Mortality 2 | 0 | 0 | 0 | 1 3 |
| Performance parameters | ||||
| Final body weight (g) | 1,008 | 1,041 | 1,055* | 952* |
| Adjusted average feed to gain ratio | 1.63 | 1.54 | 1.54 | 1.65 |
| Serum Chemistry | ||||
| Alkaline phosphatase (U/L) | 29,489 | 21,094* | 17,229* | 13,040* |
| Total Protein (g/dL) | 2.6 | 2.5* | 2.4 | 2.8 |
| Chloride (mmol/L) females | 115 | 115 | 114 | 112* |
| Chloride (mmol/L) males | 113 | 112 | 117* | 114 |
| Pathology (Organ weight) | ||||
| Heart weight (g) | 6.83 | 6.39 | 6.47 | 5.82* |
Values with * in the same row are different (p ≤ 0.1) from control values.
28 days duration, 4 replicates with 5 birds each per treatment, two birds per replicate for serum values and pathology.
n out of 20 per treatment group.
Dehydration due to osteomyelitis of the femur.
The basal diet consisted mainly of maize and methionine supplemented soybean meal; the starter formulation was calculated to contain 23% crude protein (CP, analysed 23.2%), 0.59% methionine (Met) and 13.4 MJ metabolisable energy (ME)/kg; the grower formulation 20% CP (analysed 20.3%), 0.58% Met and 13.6 MJ ME/kg.50 The starter was fed as crumbles for the first 21 days, the grower as pellets until study completion. The birds had ad libitum access to feed and water.
Bird health, litter conditions and mortality were recorded daily. Bird weight and feed consumption were measured in weekly intervals. Zootechnical parameters (weight gain and average daily gain, feed intake, feed to gain ratio) were calculated for the corresponding phases. Blood samples were taken for clinical biochemistry51 from eight randomly selected birds (4 males + 4 females) per treatment on day 28. The same birds were necropsied, organ and tissue samples52 collected, and preserved for histology. All tissues were processed to slides from the control and the high dose group (3x), for the two other groups only heart, skeletal muscle, liver, brain, eyes with optic nerve and spinal cord.
An additional histopathology review53 was done later, since the pivotal study (made about 5 years after the exploratory study) had detected some effects on the central nervous system. The previously made slides of the brain, eyes with optic nerve and spinal cord from the control and the three treated groups were evaluated.
The pen was the experimental unit for statistical analysis. Body weight and average weekly feed consumption were analysed using a general linear mixed model (GLMM) for repeated measures in which fixed effects were sex, treatment, time and the corresponding interactions. Clinical pathology variables and organ weights were analysed by GLMM considering as fixed effects the sex, treatment and its interaction. Statistical summaries and analyses were based on the 0.1 level of significance using two‐sided test comparing only each treatment group against the control and not between the different treatment groups. Polynomial contrasts (linear or quadratic) were not analysed.54 Mortality rates were summarised by treatment groups.
The main results of the study are summarised in Table 5.
At study completion, the value of the final body weight mid dose group (2x) was higher than the control while the high‐dose group (3x) was lower. Regarding feed intake, an interaction between treatment and sex, and between treatment, sex and time was observed.55 No significant differences were observed for feed to gain ratio. The changes in alkaline phosphatase (ALP) showed a clear inverse relationship to the dose, whereas changes in total protein (TP) and chloride (interaction treatment and sex) were not considered treatment related. The only significant changes in organ weight were seen for absolute heart weight with a reduction in the high‐dose group. Organ weights relative to body weight were not provided.
Treatment‐related microscopic findings were present in lymphoid tissues of males. Minimal or mild decreased lymphoid cellularity was seen in the spleen of the high‐dose males. Minimal or mild increased lymphoid apoptosis was present in the thymus in the intermediate and high dose males. In the Bursa of Fabricius, increased lymphoid apoptosis was observed in the high‐dose males. The findings in thymus and Bursa of Fabricius were considered treatment related due to an increase in incidence and severity (thymus only). Increased perivascular vacuolation of the heart was found in the intermediate and high dose groups in both sexes (and in the low dose males too). This effect is considered treatment related.
Treatment‐related findings were observed in the brain, optic nerve and eye. Vacuolation of white matter tracts in the brain was present in virtually all animals administered the test articles. Severity ranged from minimal (grade 1) to marked (grade 4), with dose‐related increased incidence and severity. Vacuolation was present at increased incidence and severity in animals given the test article, and thus considered treatment related.
Vacuolation of the optic nerves was limited to the intermediate (2x) and high (3x) dose groups and ranged from minimal (grade 1) to moderate (grade 3) with dose‐related increased incidence and severity. Test article‐related findings in the eye were limited to minimally decreased ganglion cells in the retina of a single male in the high dose group.
Tolerance study 56
A total of 600 (plus 30 reference)57 1‐day‐old Ross 308 chickens (males) were randomly allocated to five treatment groups which were fed diets for 40 days supplemented with 0, 50 + 50, 75 + 75, 100 + 100 and 150 + 150 mg lasalocid A sodium + nicarbazin/kg complete feed (from the final product) corresponding to 0, 1x, 1.5x, 2x and 3x of the maximum proposed (use) level. The intended levels of lasalocid A sodium and nicarbazin and those analytically confirmed in each treatment diet are shown in Table 6. From day 41 to 47, all birds were fed control feed (recovery phase). Group size was 80 birds per treatment (8 replicates with 10 birds each (+ five spare birds for the first week)).
Table 6.
Least square means of the most relevant parameters from the tolerance study in chickens for fattening fed the final combination of lasalocid A sodium and nicarbazin compared to the control group1
| Control | 1x | 1.5x | 2x | 3x | |
|---|---|---|---|---|---|
| Lasalocid A sodium + nicarbazin (mg/kg feed) | |||||
| Intended | 0 | 50 + 50 | 75 + 75 | 100 + 100 | 150 + 150 |
| Analysed, starter | 59.7 + 54.4 | 87.6 + 80.0 | 116 + 117 | 174 + 169 | |
| Analysed, grower | 48.6 + 47.1 | 71.9 + 71.1 | 100 + 96.0 | 151 + 144 | |
| Analysed finisher 1 | 52.3 + 47.3 | 81.4 + 74.9 | 107 + 102 | 152 + 137 | |
| Mortality 2 | |||||
| Total mortality (n) | 3 | 13* | 18* | 14* | 29* |
| Potentially treatment related mortality (n)3 | 4 | 7 | 8 | 6 | 17 |
| Performance parameters | |||||
| Final body weight (g) | 2,599 | 2,554 | 2,417* | 2,268* | 1,637* |
| Average feed intake (g/d) | 90 | 89 | 85* | 81* | 63* |
| Adjusted average feed to gain ratio | 1.52 | 1.54* | 1.57* | 1.60* | 1.74* |
| Haematology | |||||
| Mean corpuscular haemoglobin (pg) | 43.4 | 44.1 | 44.6* | 45.7* | 46.0* |
| Mean corpuscular volume (fl) | 122.0 | 122.6 | 122.7 | 124.7 | 127.0* |
| Serum Chemistry | |||||
| Total cholesterol (mg/dL) | 135 | 149 | 154* | 157* | 187* |
| Albumin (g/dL) | 1.2 | 1.2 | 1.3 | 1.3 | 1.5* |
| Magnesium (mg/dL) | 2.3 | 2.4 | 2.3 | 2.3 | 2.6* |
| Sodium (mmol/L) | 163 | 165 | 164 | 162 | 169* |
| Calcium (mg/dL) | 9.6 | 9.6 | 9.4 | 9.1* | 10.1* |
| Total protein (g/dL) | 3.3 | 3.5 | 3.4 | 3.5 | 3.9* |
| Creatine kinase (U/L) | 16,673 | 17,357 | 17,633 | 21,358 | 10,627* |
| Pathology (organ weight) | |||||
| Testes (g) | 0.49 | 0.39* | 0.37* | 0.39* | 0.21* |
| Relative to body weight | 0.0184 | 0.0150 | 0.0154 | 0.0166 | 0.0140 |
| Heart (g) | 14.85 | 13.63 | 13.14 | 11.45* | 9.76* |
| Relative to body weight | 0.5592 | 0.54158 | 0.5419 | 0.4839 | 0.7091 |
| Liver (g) | 60.09 | 69.18* | 63.98 | 60.66 | 44.19* |
| Relative to body weight | 2.2620 | 2.7228* | 2.6437* | 2.5555* | 2.8548* |
| Spleen (g) | 3.77 | 3.72 | 3.28 | 3.01 | 2.24* |
| Relative to body weight | 0.1421 | 0.1478 | 0.1358 | 0.1258 | 0.1439 |
| Brain (g) | 2.78 | 2.82 | 2.90 | 2.88 | 2.65 |
| Relative to body weight | 0.1050 | 0.1111 | 0.1202* | 0.1217* | 0.1916* |
Nd: not detected.
Values with * in the same row are different (p ≤ 0.05) from control values.
40 days duration, 8 replicates per treatment, one bird per replicate for haematology, serum values and pathology.
n out of 120 for the first week, afterwards out of 80 per treatment group.
From day 0 to 47.
The basal diet consisted mainly of maize and methionine supplemented soybean meal. The starter diet was fed as crumbles for the first 14 days, the grower as pellets for two subsequent weeks followed by finisher 1 until day 40 (completion of the tolerance study) and finisher 2 for the one‐week recovery period. The starter formulation was calculated to contain 22% CP (analysed 21.8%), 0.71% Met and 12.7 MJ ME/kg; the grower formulation 19.9% CP (analysed 19.2%), 0.63% Met, and 13.1 MJ ME/kg; the finisher formulation 17.9% CP (analysed 17.6% for finisher 1 and 17.2% for finisher 2), 0.56% Met and 13.3 MJ ME/kg. The birds had ad libitum access to feed and water.
Bird health, litter conditions and mortality were recorded daily. Birds were weighed per pen on days 1, 14, 28, 40 and 47, feed remaining in each pen was weighed and recorded on days 14, 28, 40 and 47. Other zootechnical parameters (weight gain and average daily gain, feed intake, feed to gain ratio) were calculated accordingly. Blood samples were taken for haematology58 and clinical biochemistry59 from one animal per pen, each on days 40 and 47. The same birds (8 birds on days 40 and 8 birds on day 47) were necropsied, organ and tissue samples60 collected, and preserved for histology. Birds which died during the study or required unscheduled euthanasia were also necropsied.
The pen was the experimental unit for statistical analysis. Body weight and average weekly feed consumption were analysed using a general linear mixed model (GLMM) for repeated measures in which fixed effects were sex, treatment, time and the corresponding interactions. Clinical pathology variables and organ weights were analysed by GLMM considering as fixed effects the sex, treatment and its interaction. Statistical summaries and analyses were based on the 0.05 level of significance using two‐sided test comparing only each treatment group against the control and not between the different treatment groups. Polynomial contrasts (linear or quadratic) were not analysed.61 Mortality rates were summarised by treatment groups.
The main results are summarised in Table 6. A significant increase in mortality was observed between control group and all other treatment groups. Different causes were recorded for the mortalities, including bacterial infection, ascites, dehydration, sudden death syndrome, splayed legs or unknown cause. The mortalities caused by bacterial infection and ascites maybe considered not treatment related. All other causes may be considered as potentially treatment related. The highest losses were seen in the 3x group with similar lower values for the other treatment groups.
Growth depression by treatment became significant after 28 days for the groups 1.5x, 2x and 3x. After 40 days, the treated groups reached 98, 93, 87 and 63% of the 40‐day body weight of the control group. Feed intake was significantly reduced for the 1.5x, 2x and 3x groups. Consequently, feed to gain ratio was adversely affected in the supplemented groups as compared to the control group. The significant differences observed for the overdose groups did not disappear during a 7‐day recovery phase.
Statistically significant treatment‐related changes in haematology were found for higher mean corpuscular haemoglobin values in 1.5x, 2x and 3x groups when compared with the control groups. Higher mean corpuscular volume was seen for the 3x group. These findings are considered not biologically meaningful and not adverse due to the small magnitude of change and lack of systemic changes between correlated variables.
In serum chemistry, total cholesterol was significantly higher in the 1.5x, 2x and 3x groups than the control group. The clinical significance of this change is unknown, but unlikely to be adverse given the lack of correlating findings (the lack of dose dependent liver to body weight ratio, and the lack of treatment‐related changes in liver histopathology). Other statistically significant treatment‐related changes compared to the control group included higher albumin, magnesium, sodium and total protein and lower creatine kinase values and were found only in the 3x group. Lack of correlating clinical findings may suggest these changes may not be biologically relevant. Significantly higher calcium values were observed in the 3x group and significantly lower calcium values in the 2x group compared to the control. Given that changes are of small magnitude and not in the same direction, findings are considered most likely consistent with biological variation.
A statistically significant decrease in organ weight was observed for the testes in all treated groups, for the heart in the 2x and the 3x overdose groups, and spleen and liver in the 3x overdose group compared to control. An increased liver weight (p ≤ 0.05) was also observed in the use level group (1x). A significant increase in relative liver weight in comparison to control group was seen in all treated groups.
Relative brain weight was significantly higher in all overdose groups (1.5x, 2x and 3x) in comparison to the untreated control, while the increase in the use level group was not significant. On day 47 necropsy, there were no differences in absolute or relative liver weights of all treated groups in comparison to the control group.
Histopathology central nervous system
At day 40 necropsy, treatment‐related microscopic findings were seen in the brain, spinal cord, optic nerve and eyes (Table 7). In the brain, spinal cord and optic nerve, findings consisted of minimal to severe vacuolations, primarily in the white matter and occasionally in the grey matter. In the brain, vacuolation occurred in the cerebrum, midbrain, cerebellum and brainstem, with the greatest incidence and severity in the midbrain, especially targeted were the white matter tracts of the optic tectum. These findings increased in incidence and severity with increased test article inclusion, and all groups treated with the test article were affected.
Table 7.
Summary of selected microscopic findings in chickens for fattening fed the final combination of lasalocid A sodium and nicarbazin1
| Control | 1x | 1.5x | 2x | 3x | |
|---|---|---|---|---|---|
| Brain | |||||
| Brainstem, white matter, vacuolation | 0 | 0 | 0 | 0 | 3 |
| Cerebellum, white matter, vacuolation | 1 | 3 | 4 | 3 | 4 |
| Cerebrum, white matter, vacuolation | 0 | 0 | 3 | 5 | 6 |
| Midbrain, white matter, vacuolation | 0 | 4 | 8 | 8 | 8 |
| Minimal to mild | – | 4 | 5 | 1 | 1 |
| Moderate to severe | – | – | 3 | 7 | 7 |
| Spinal cord, thoracic | |||||
| White matter, vacuolation | 0 | 1 | 0 | 5 | 5 |
| Optic nerves, vacuolation | 0 | 2 | 4 | 4 | 8 |
| Minimal | – | 2 | 4 | 3 | 5 |
| Mild to moderate | – | – | – | 1 | 3 |
| Eyes, retina, ganglion cells decreased | |||||
| Bilateral, minimal to marked | 0 | 0 | 0 | 0 | 6 |
| Unilateral, minimal | 0 | 0 | 0 | 1 | 0 |
8 male birds per group sacrificed at day 40.
One bird of the control group had minimal vacuolation in the cerebellar white matter, but this was attributed to background artefact. Apart from this bird, vacuolation occurred exclusively in the birds treated with the additive. Particularly frequent vacuolation was seen in the midbrain white matter with a dose‐dependent increase in severity. There was also minimal to mild vacuolation in the spinal cord white matter of the 2x and 3x birds (and one bird of the use level group). Minimal to moderate vacuolation of the optic nerve occurred in all treated groups, with incidence and severity increasing in a dose‐response pattern. In the eye, a bilateral decrease in retinal ganglion cells was seen almost exclusively in the 3x birds, and a single 2x bird, in one eye only. This finding is not generally spontaneous and was not seen in the lowest dose or control birds, so it was considered to be treatment related.
Using a special staining, no axonal degeneration or degenerating neurons in the brain were observed. Brain and optic nerve vacuoles remained clear, suggesting that myelin splitting was caused by intramyelinic oedema. Intramyelinic oedema represents the result of fluid accumulation within the cytoplasm of oligodendroglial cells and, in the absence of associated demyelination or of axonal or cellular degeneration, would be expected, at most, to result in a potential decrease in the speed of nerve impulse transmission within the affected fibres.
Treatment‐related, dose‐dependent, histopathological findings included brain and spinal cord vacuolation, that was the most severe in 2x and 3x birds, and a loss of ganglion cells in the retinas of primarily 3x birds. Special staining suggested that the white matter vacuolations are attributable to myelin splitting caused by intramyelinic oedema, and is in fact related to treatment, and not a background artefact. This vacuolation occurred in areas of the brain in which axons of the retinal ganglion cells synapse, suggesting that it is possible that brain and ocular lesions contributed to the decreased feed consumption and weight gain that occurred in a dose‐dependent manner, especially 3x birds, via potentially impaired vision and/or neurological function.
In addition, the Pathology Consultation Report53 adds that intramyelinic oedema, restricted primarily to the optic tracts, might be expected to have an impact on the visual acuity of affected chickens, but not to directly result in sickness or pain. However, if weight loss is seen in affected chickens, this might potentially represent the result of decreased visual acuity, thus making it difficult for the chickens to see or to accurately peck at granular feed.
Synopsis on the studies submitted on the safety for the target species
Three times the highest Nilablend™ 200G concentration applied (50 mg lasalocid A sodium + 50 mg nicarbazin/kg complete feed) resulted in a severe growth depression in a 40‐day study as well in another 28‐day study. In the longer lasting study, also the 2x and the 1.5x overdoses significantly reduced growth compared to an untreated control. The mortality was treatment related and very high except for 1x. With increasing exposure time, lower additive concentrations reduced feed intake; after 40 days, the effect was obvious in all overdose groups. This may explain why the 2.0x overdose did not negatively affect body weight after 28 days, but had an effect after 40 days. The increase in relative liver weight, seen for all treated groups in the 40‐day study, was not related to any microscopic finding and disappeared after a 7‐day recovery phase. No other haematology or clinical biochemistry observations gave an indication for relevant treatment‐related adverse effects. The FEEDAP Panel noted that the statistical analysis provided did not allow the detection of dose‐related effects which is considered a limitation of the studies.
Relative brain weight was significantly increased in all overdose groups in comparison to the untreated control, while the increase in the use level group was not significant. Histopathological examination indicated a treatment‐related increase of intramyelinic oedema, of which incidence and severity was dose dependent, starting already at the use level of the additive. Furthermore, the intramyelinic oedema was primarily restricted to the optic tracts in the tolerance and the exploratory study and might be expected to have an impact on visual acuity.
The intramyelinic oedema may not cause distress or pain to the affected chickens. In both studies, there were not abnormal events or general health observations recorded that might indicate the loss of visual acuity. But it cannot be excluded that the weight loss seen in chickens treated with the additive might potentially represent the result of decreased visual acuity, thus making it difficult for the chickens to see or to accurately peck at granular feed.
The FEEDAP Panel considers that a reduction of visual acuity affects animal welfare. The occurrence of intramyelinic oedema in chickens is therefore regarded as an adverse effect.
Since intramyelinic oedema was already seen in birds receiving the use level, it is concluded that Nilablend™ 200G at the proposed maximum use level of 50 mg lasalocid A sodium + 50 mg nicarbazin/kg complete feed is not safe for chickens for fattening.
3.2.3.2. Interactions
Data on the interactions of lasalocid A sodium with feed materials, other approved additives or medicinal products have been assessed by the FEEDAP Panel in the past (EFSA, 2004a,b, 2005) and it was concluded that:
‘The data submitted regarding the possible interactions between lasalocid sodium and tiamulin used in chicken husbandry were insufficient. Consequently, the FEEDAP Panel considers that it would be safer to avoid concurrent administration of lasalocid with tiamulin and certain other medicinal substances, and that the current advice should be retained’.
In its recent opinion on the re‐evaluation of Avatec® 150 G (containing lasalocid A sodium) for chickens for fattening (EFSA FEEDAP Panel, 2017a), the FEEDAP Panel updated its previous assessment in the light of new information found in the literature and reiterated its former conclusions. In the absence of new information, the FEEDAP Panel applies the same conclusions to the lasalocid A sodium contained in Nilablend™ 200G. Therefore, the contra‐indications identified for lasalocid A sodium would apply to Nilablend™ 200G.
No information on the interactions of nicarbazin with feed materials, other approved additives or medicinal products have been provided.
3.2.3.3. Microbiological safety of the additive
Data concerning the microbial safety of lasalocid A sodium were submitted in former dossiers on Avatec® 150 G and were assessed by the FEEDAP Panel (EFSA, 2004a,b, 2005; EFSA FEEDAP Panel, 2017a). For the current evaluation, the applicant submitted a literature review on the safety of lasalocid A sodium covering the period 2015–2019 (for details, see Section 3.2).62 The review addressed the shedding of enteropathogens related to lasalocid A sodium and the antimicrobial cross‐resistance.
In its recent opinion on the re‐evaluation of Avatec® 150 G for chickens for fattening (EFSA FEEDAP Panel, 2017a), the FEEDAP Panel concluded that ‘lasalocid sodium has a selective antimicrobial activity against Gram‐positive bacterial species while many Enterobacteriaceae are naturally resistant. Induction of resistance and/or cross‐resistance was not observed in experimental conditions.’
The literature review identified no new data requiring consideration in the latest opinion and the FEEDAP Panel reiterates its previous conclusions on the microbial safety of lasalocid A sodium.
No data were submitted on the microbiological safety of nicarbazin.
3.2.3.4. Conclusions on safety for the target species
The additive consisting of lasalocid A sodium and nicarbazin (Nilablend™ 200G) is not safe for chickens for fattening at the proposed maximum use level of 50 mg lasalocid A sodium + 50 mg nicarbazin/kg complete feed.
Concurrent administration of Nilablend™ 200G (containing lasalocid) with tiamulin and certain other medicinal substances should be avoided. No information on the interactions of nicarbazin with feed materials, other approved additives or medicinal products have been provided.
Lasalocid A sodium has antimicrobial activity against Gram‐positive bacterial species, while many Enterobacteriaceae are naturally resistant. Induction of resistance and/or cross‐resistance was not observed in experimental conditions. No conclusions can be drawn on the microbiological safety of nicarbazin.
3.2.4. Toxicological studies
3.2.4.1. Lasalocid A sodium
In previous FEEDAP Panel opinions (EFSA, 2004a,b; EFSA FEEDAP Panel, 2010a), it was concluded that: ‘Absence of mutagenic effects at the gene level both in bacteria and in mammalian cells was demonstrated. There was no evidence for in vitro genotoxicity of lasalocid A sodium. The results of chronic oral toxicity/carcinogenicity studies carried out in rats and dogs suggest no evidence of carcinogenicity. Fetotoxicity in rabbits was observed but was related to developmental and maternal toxicity. No specific teratogenicity study was available in rodents. However, relevant data from the rat two‐generation study is considered sufficient supporting evidence for the lack of teratogenicity of lasalocid’.
‘A lowest NOAEL of 0.5 mg/kg bw per day was established from the 2‐year chronic oral toxicity study in rats and maternal toxicity study in rabbits. A toxicological ADI of 0.005 mg/kg bw (or 0.3 mg/60 kg person per day) has been derived applying a safety factor of 100′.
The FEEDAP Panel in 2004 noted that lasalocid A sodium caused positive inotropic effects after single intravenous dosing to dogs. Since these effects have been previously accepted as being relevant to consumer risk assessment, particularly when occurring at doses lower than identified as a toxicological no observed adverse effect level (NOAEL), the applicant was requested to perform an acute study in dogs, by the oral route, investigating these effects to identify whether they would be critical to the risk assessment for this product.
After the submission of a new cardiovascular study in dogs, the FEEADP Panel updated the previous conclusions (EFSA FEEDAP Panel, 2017a) on the toxicological profile of lasalocid A sodium as follows: ‘The newly conducted cardiovascular study in dogs indicates an acute NOAEL for lasalocid A sodium of 1 mg/kg bw per day, with only limited effects seen at 3 mg/kg. Since this NOAEL is above the lowest NOAEL previously identified of 0.5 mg/kg bw per day, observed in a 2‐year toxicity study in rats and a developmental study in rabbits, there is no reason to consider cardiovascular effects in the risk assessment. The previously identified lowest NOAEL of 0.5 mg/kg bw per day is concluded to be an appropriate base for establishing an Acceptable Daily Intake (ADI).’
The literature search provided by the applicant in the current dossier did not identify any papers/data that would lead to a modification of the conclusion previously reached on the toxicological profile of lasalocid A sodium.
3.2.4.2. Nicarbazin
The toxicity of nicarbazin was investigated for nicarbazin or the combination of DNC and HDP.
Genotoxicity studies
In order to investigate the potential of nicarbazin to induce gene mutations in bacteria, the Ames test was performed according to OECD Test Guideline (TG) 471 (1997) and following Good Laboratory Practice (GLP) in Salmonella typhimurium strains TA98, TA100, TA1535, TA1537 and E.coli strain WP2uvrA.63 The active components of nicarbazin, DNC (purity 99.6%) and HDP (purity 100%) were tested in two independent experiments applying the plate incorporation in the presence and absence of metabolic activation and pre‐incubation methods only in the presence of S9‐mix. DNC was dissolved in dimethyl sulfoxide (DMSO) and tested at least at six concentrations ranging from 0.5 to 500 μg/plate (maximum concentration was limited by solubility). HDP, dissolved in purified water, was tested at seven concentrations ranging from 5 to 5,000 μg/plate. Appropriate positive and negative controls were evaluated concurrently. All positive control chemicals induced significant increases in revertant colony numbers, confirming the sensitivity of the tests and the efficacy of the S9‐mix. Precipitation was observed at the highest concentration tested (i.e. 500 μg/plate) only after DNC treatment; toxicity was not detected in any experimental condition. No increase in the mean number of revertant colonies was observed at any tested concentration in any tester strains with or without S9‐mix. The Panel concluded that DNC and HDP did not induce gene mutations in bacteria under the experimental conditions employed in this study.
An in vitro micronucleus test64 was performed according to OECD TG 487 (2016) and following GLP to evaluate the potential of the active components of nicarbazin, DNC (purity 99.6%) and HDP (purity 100%) to induce chromosome damage in human lymphoblastoid TK6 cells in the absence and presence of metabolic activation. A short treatment (3 + 27 h of recovery) with and without S9‐mix and a continuous treatment (30 + 0 h recovery) without S9‐mix were the experimental conditions applied. Cytochalasin B was added to the cultures at a final concentration of 3 μg/mL per culture. DNC, dissolved in DMSO, was tested up to 50 μg/mL due to precipitation observed at 50 μg/mL and above. HDP, dissolved in purified water, was tested up to 1,242 μg/mL, equivalent to 10 mmol/L, an acceptable maximum concentration recommended by OECD TG 487. Appropriate positive and negative control chemicals were used; the results obtained in Experiment 1 following the 3 + 27 h DNC treatment with S9‐mix showed the failure of the positive control compound to produce a positive response; thus, the short‐term treatments with DNC were repeated in Experiment 2. No significant changes in cell proliferation were observed after treatments compared to negative vehicle controls in any experimental condition. DNC induced a statistically significant increase in the frequency of micronuclei (MN) after short‐term treatment both in the presence and absence of S9‐mix. The increases were not dose‐related being detected only at an intermediate concentration (10 μg/mL) and not reproduced in Experiment 2. The Panel considered them not biologically relevant and concluded that DNC did not induce chromosome damage under the experimental conditions employed in this study. The frequency of micronucleated cells observed after continuous treatment with DNC was comparable to the value detected in the concurrent vehicle control.
HDP did not induce increase in the frequency of MN after short‐term treatment, while statistically significant increases (p < 0.01) were detected after continuous treatment at all three concentrations analysed (800, 1,000 and 1,242 μg/mL); the increase was dose‐related and a statistically significant linear trend was observed (p < 0.01). The Panel concluded that HDP induced chromosome damage in mammalian cells under the experimental conditions employed in this study.
A micronucleus test65 was performed in bone marrow cells of male Wistar WI (Han) rats according to OECD TG 474 (2016) to evaluate the potential of nicarbazin (purity stated as DNC 70.9%, HDP 28.7%) to induce chromosomal damage after oral administration. Animals were treated by gavage with 500, 1,000 and 2,000 mg/kg bw of nicarbazin at 0 and 24 h and sacrificed 24 h after dosing. No clinical signs of toxicity and mortality were reported, with the exception of treatment‐related weight loss from day 1 to Day 3. Bioanalysis detected DNC and HDP in plasma and confirmed that animals were systemically exposed to nicarbazin at all dose levels. Eight thousand polychromatic erythrocytes (PCEs) were scored for each animal for the analysis of micronuclei. Positive and negative control values of micronucleus frequency were within the historical control ranges of the laboratory confirming the sensitivity of the assay. A small dose‐related decrease in group mean of %PCE values was observed, not statistically significant. The frequency of micronuclei was significantly higher (p < 0.01) in treated animals compared to control groups at 1,000 and 2,000 mg/kg bw. The increased values were within the 95th percentile of the historical vehicle control range. Based on these data, the Panel considered the results of the in vivo micronucleus assay equivocal since not all the criteria for a positive response were clearly fulfilled.
Conclusions on genotoxicity
The two active components of nicarbazin (DNC and HDP) were tested in vitro separately; both compounds did not induce gene mutations in bacteria; in mammalian cells, in vitro DNC did not induce chromosome damage, while HDP caused a statistically significant increase of micronuclei. Equivocal results were obtained for nicarbazin in the in vivo micronucleus assay. Overall, the Panel concluded that a concern for genotoxicity cannot be excluded and that clarification on the mechanism of action of the test items would be needed.
Subchronic oral toxicity
The applicant performed short‐term oral dose range‐finding studies in rats and dogs with a duration ranging from 7 days to 4 weeks66 to determine the appropriate drug‐in‐diet palatability and starting doses prior to the subchronic studies submitted.
The first subchronic study was performed in RccHan®:WIST rats. Groups of 13 rats of each sex received a diet for 13 weeks containing nicarbazin (3:1 mixture of DNC and HDP), at dose levels of 0, 50 + 17, 150 + 50 or 300 + 100 mg/kg bw per day (DNC+HDP).67 The carrier (control article) was Certified Rodent Meal 2016CM (Envigo RMS, Inc.). The test article doses are hereafter presented in the text as 3:1 ratios of DNC+HDP. Three rats of each sex per group were assigned to toxicokinetic studies. The study was conducted according to OECD TG 408. There was no difference between control and treated groups in the clinical observations including ophthalmoscopy, mortality, body weight, food consumption, functional observational battery (FOB), locomotor activity, clinical pathology (including clinical chemistry and haematology), organ weight or macroscopic or microscopic findings. Since no test article‐related observations were seen in this study, the no observed adverse effect level (NOAEL) is concluded by the FEEDAP Panel to be 300 + 100 mg/kg bw per day DNC + HDP, the highest dose tested.
The second subchronic study was performed with Beagle dogs.68 Groups of four dogs of each sex received nicarbazin (3:1 mixture of DNC and HDP), dissolved in aqueous 0.5% methylcellulose, at dose levels of 0, 60 + 20, 180 + 60 or 600 + 200 mg/kg bw per day (DNC+HDP) by gavage for 90 days. The study design was based on OECD TG 409. There was no difference between control and treated groups in the observations including body weight, clinical pathology (haematology, coagulation, clinical chemistry and urine), organ weights, gross or microscopic pathology findings. Since no test article‐related observations were seen in this study, the NOAEL is considered by the FEEDAP Panel to be 600 + 200 mg/kg bw per day DNC + HDP, the highest dose tested.
Chronic oral toxicity
In a GLP study69 based on OECD TG 452, RccHan®:WIST rats (20 males and 20 females per group) were fed a diet (certified rodent meal 2016CM) with 0 (controls), 52.5 + 17.5, 150 + 50 or 300 + 100 mg/kg bw per day of a 3:1 mixture of the two individual components of nicarbazin (DNC and HDP) ad libitum for up to 52 weeks. No test article‐related deaths occurred and no test article‐related clinical or ophthalmic observations, changes in body weight or food consumption or coagulation effects were noted. On day 365, males administered ≥ 150 + 50 mg DNC + HDP/kg bw per day and females administered 300 + 100 mg DNC + HDP/kg bw per day had minimally to moderately higher urea nitrogen and/or creatinine concentrations. These treatment‐related changes were more pronounced in males. Haematological changes on day 365 in animals administered 300 + 100 mg DNC + DHP/kg bw per day were consistent with a test article‐related inflammatory response and correlated microscopically with chronic inflammation in the kidneys. These findings included minimally to mildly higher absolute neutrophil count and minimally lower mean corpuscular haemoglobin in both sexes and minimally lower haemoglobin concentration, mean corpuscular volume and red blood cell distribution width in males. The only treatment‐related urinalysis effect was minimally lower urine pH on day 365 in males administered 300 + 100 mg DNC + HDP/kg bw per day, which correlated microscopically with increased incidences and/or severities of tubular degeneration/regeneration, chronic inflammation, and crystal accumulation in the kidneys. Test article‐related microscopic findings were noted in the kidneys of animals administered ≥ 150 + 50 mg/kg bw per day and consisted of increased severities and/or incidences of tubular degeneration/regeneration, chronic inflammation, and crystal accumulations. Chronic inflammation in the kidneys correlated macroscopically with rough surface and/or tan discolouration in animals administered ≥ 150 + 50 mg DNC + HDP/kg bw per day and with increased kidney weights in males administered 300 + 100 mg DNC + HDP/kg bw per day. In general, the renal changes observed were more prominent in males than in females. Based on the above observations, the FEEDAP Panel considered the NOAEL to be 52.5 + 17.5 mg DNC + HDP/kg bw per day.
Reproduction toxicity studies including prenatal developmental toxicity
The applicant performed dose‐range finding studies70 to select the appropriate doses prior to the studies assessed below. In a dietary two‐generation reproductive toxicity study71 based upon OECD TG 416, four groups of male and female Crl:CD(SD) rats (25/sex per group) were administered either basal diet or the test article, the components of nicarbazin (a 3:1 mixture of DNC and HDP) in the diet. Target concentrations for the Fo and F1 generations were 0, 52.5 + 17.5, 150 + 50 and 300 + 100 mg/kg bw per day DNC + HDP, which were equivalent to nicarbazin concentrations of 0, 70, 200 and 400 mg/kg bw given per day, respectively. The target concentrations were exceeded slightly throughout the study. The test article was given continuously in the diet for at least 70 consecutive days prior to mating. F0 animals were approximately 6 weeks of age at the initiation of test diet administration. Offspring (1/sex per group) from the pairing of the F0 animals were selected on PND (post‐natal day) 21 to constitute the F1 generation and received the test diet from that point onwards. The F0 and F1 males continued to receive the test article throughout mating and continuing through the day of euthanasia. The F0 and F1 females continued to receive the test article throughout mating, gestation and lactation, and through the day of euthanasia. For both generations (F1 and F2), 8 pups/litter (4 pups/sex, when possible) were selected on PND 4 to reduce the variability among the litters. F0 males and females were exposed for 130–134 consecutive days, and F1 males and females were exposed for 132–140 consecutive days. All animals were observed twice daily for mortality and moribund condition. Clinical observations, body weights and food consumption were recorded at appropriate intervals for males and females throughout the study (including during gestation and lactation for females). Vaginal lavages were performed daily for determination of oestrous cycles beginning 21 days prior to cohabitation. All F0 and F1 females were allowed to deliver and rear their pups until weaning on Lactation Day 21. Clinical observations, body weights and sexes for F1 and F2 pups were recorded at appropriate intervals. Developmental landmarks (balanopreputial separation and vaginal patency) were evaluated for the F1 rats selected to constitute the F1 generation. Non‐selected F1 pups and all surviving F2 pups were necropsied on PND 21. Selected organs were weighed for 1 pups/sex per litter from both F1 and F2 pups that were necropsied on PND 21. Each surviving F0 and F1 parental animal received a complete detailed gross necropsy following the completion of weaning of the F1 and F2 pups, respectively; selected organs were weighed. Spermatogenic endpoints (sperm motility, morphology and numbers) were recorded for all F0 and F1 males, and ovarian primordial/primary follicle counts were recorded for F1 females in all groups. Designated tissues were examined microscopically from all F0 and F1 parental animals in the control and high exposure groups and from all parental animals that were found dead or euthanised in extremis. Blood samples for bioanalysis were collected from F0 males and females (5/group) on Study Day 65 and F1 culled pups (5 litters/group) on PND 4. There were four occasions during the study when animals how many? in all groups? were found dead or were euthanised in extremis, but these showed no relationship to treatment. There were no differences between control and treated groups at any stage of the study in clinical observations, body weight or food consumption. No differences were observed between groups on F0 and F1 male and female mating and fertility, male copulation and female conception indices, oestrous cycle lengths, pre‐coital intervals, gestation length, the process of parturition and spermatogenesis parameters (motility, progressive motility, testicular and epididymal sperm concentration, sperm production rate and the percentage of morphologically normal sperm) at any exposure level. There were no differences between control and treated groups at any stage of the study in macroscopic or microscopic findings, organ weights, F1 female primordial follicle counts, mean numbers of pups born, live litter size on PND 0, percentage of males or postnatal survival. There were no test article‐related effects on attainment of balanopreputial separation and vaginal patency or body weights at attainment of these developmental landmarks in the F1 pups at any exposure level. Since no test article‐related observations were seen in this study, the NOAEL is concluded by the FEEDAP Panel to be of 300 + 100 mg/kg bw per day DNC+HDP, the highest level tested. This NOAEL applies to parental systemic toxicity and to neonatal toxicity.
In an oral embryo/foetal development study72 based upon OECD TG 414, four groups of 24 time‐mated female New Zealand White [Hra:(NZW)SPF] rabbits received the components of nicarbazin (DNC and HDP) by gavage (5 mL/kg) in the vehicle (0.5% aqueous methylcellulose) once daily from gestation days 7 to 28, at doses of 0, 22.5 + 7.5, 45 + 15 and 90 + 30 mg/kg bw per day DN + /HDP (equivalent to 30, 60 and 120 mg/kg bw per day nicarbazin, respectively). The females were approximately 7 months of age at the initiation of dose administration. All animals were observed twice daily for mortality and moribund condition. Clinical observations, body weights, and food consumption were recorded. On gestation day 29, a laparohysterectomy was performed on each surviving female. The uteri, placentae, and ovaries were examined, and the numbers of foetuses, early and late resorptions, total implantations and corpora lutea were recorded. Gravid uterine weights were recorded. The fetuses were weighed, sexed and examined for external, visceral and skeletal malformations and developmental variations. There were no differences between treated and control groups in clinical observations, mortality, body weight, food consumption, gravid uterine weight or macroscopic necropsy findings. No differences were found in any of the measurements and observations made on the fetuses. Since no test article‐related observations were seen in this study, the NOAEL is concluded by the FEEDAP Panel to be at or above the highest dose tested of 90 + 30 mg/kg bw per day DNC + HDP. This NOAEL applies to maternal systemic toxicity and embryo/fetal development.
In a GLP oral embryo/foetal development study73 based upon OECD TG 414, four groups of time‐mated female Crl:CD(SD) rats (25/group) were administered either the vehicle (0.5% aqueous methylcellulose) or the components of the test article, nicarbazin (a 3:1 mixture of DNC and HDP) orally by gavage (10 mL/kg) once daily from Gestation Days 6 to 20. Dosage levels were 0, 52.5 + 17.5, 150 + 50 and 450 + 150 mg/kg bw per day DNC + HDP, which was equivalent to nicarbazin doses of 0, 70, 200 and 600 mg/kg bw per day. The females were approximately 10–11 weeks of age at the initiation of dose administration. All animals were observed twice daily for mortality and morbidity. Clinical observations, body weights and food consumption were recorded at appropriate intervals. On gestation day 21, a laparohysterectomy was performed on each female. The uteri, placentae and ovaries were examined, and the numbers of fetuses, early and late resorptions, total implantations and corpora lutea were recorded. Gravid uterine weights were recorded, and net body weights and net body weight changes were calculated. The fetuses were weighed, sexed and examined for external, visceral and skeletal malformations and developmental variations. No differences were seen between the groups in clinical observations, mortality, body weight, food consumption, gravid uterine weights or macroscopic findings at the scheduled necropsy. No differences were found in any of the measurements and observations made on the fetuses. Since no test article‐related observations were seen in this study, the no observed adverse effect level (NOAEL) is concluded by the FEEDAP Panel to be at or above the highest dose tested of 450 + 150 mg/kg bw per day DNC + HDP. This NOAEL applies to maternal systemic toxicity and embryo/fetal development.
3.2.4.3. DNC and HDP and lasalocid A sodium
Genotoxicity
No genotoxicity studies were performed with the combination of the active substances under assessment.
Subchronic oral toxicity
A combination of lasalocid A sodium and the individual components of nicarbazin (DNC and HDP), was administered for 13 weeks to rats in the diet at dose levels of 0, 2.12/1.52/0.60, 5.19/3.71/1.48 or 10.54/7.47/3.07 and 5.14/0/0 mg lasalocid/DNC/HDP/kg bw per day for males of group 1, 2, 3, 4 and 5 and 0, 2.16/1.55/0.62, 5.16/3.69/1.47 or 10.91/7.73/3.18 and 5.09/0/0 mg lasalocid/DNC/HDP/kg bw per day for females of group 1, 2, 3, 4 and 5. The study74 was performed under GLP and according to OECD TG 408.
No unscheduled deaths occurred during the study. There were no treatment‐related clinical signs in any treated males or females administered up to 10.54/7.47/3.07 mg lasalocid/DNC/HDP/kg bw per day for males or 5.16/3.69/1.47 mg lasalocid/DNC/HDP/kg bw per day for females. Females given actual achieved doses of 10.91/7.73/3.18 mg lasalocid/DNC/HDP/kg per day were thin and exhibited body weight losses, reductions in mean body weight gain (–45% vs control), and reductions in mean food consumption. No effects on the FOB were observed compared to the control. There were limited to slight, possibly treatment‐related changes in haematology and clinical chemistry parameters in females of group 3, 4 and 5, but the values were only slightly above or below the historical controls. No test article‐related gross pathology findings were observed, and no test article‐related organ weight differences. Dose‐related minimal to mild increased haematopoiesis was observed in the spleen of females of group 3 and 4 (≥ 5.16/3.69/1.47 mg lasalocid/DNC/HDP/kg bw per day) as well as in females of group 5 (lasalocid A sodium alone at 5.09 mg/kg bw per day). Haematopoiesis in the bone marrow was unaffected. Based on the minimal to slight severity, the lack of correlating gross and microscopic pathology, organ weight and haematological findings, the applicant considered the minimal to mild increased haematopoiesis in the spleen as non‐adverse. The FEEDAP Panel agreed with this conclusion. Based on the aforementioned results, the NOAEL was considered to be 10.54/7.47/3.07 mg lasalocid/DNC/HDP/kg bw per day for males and 5.16/3.69/1.47 mg lasalocid/DNC/HDP/kg bw per day for females.
Reproduction toxicity studies including prenatal developmental toxicity
A combined 28‐day repeated dose oral (dietary) toxicity study75 with the reproduction/developmental toxicity screening test of lasalocid A sodium and nicarbazin (DNC and HDP) was performed in rats. The study was performed under GLP and according OECD TG 422. One hundred 10‐ to 11‐week‐old rats (50 males and 50 females) were allocated in four groups given an unsupplemented basal diet (control; group 1) or the basal diet with a combination of lasalocid A sodium and the components of nicarbazin (DNC and HDP) (groups 2–4) or lasalocid A sodium only (group 5). Initial dose levels were 0, 2/1.42/0.58, 5/3.55/1.45, 10/7.09/2.91 or 5/0/0 mg lasalocid/DNC/HDP/kg bw per day. The level of group 4 was lowered to 7.5/5.32/2.18 mg lasalocid/DNC/HDP/kg bw per day on study day 14 due to mortality and moribund condition, body weight loss, and reduced food consumption at 10/7.09/2.91 mg lasalocid/DNC/HDP/kg bw per day. Males were exposed for 14 days prior to mating and continuously throughout the mating period through 1 day prior to euthanasia for a total of 29 days. Females were exposed for 14 days prior to pairing and continuously through lactation day 13 for a total of 50–63 days; females that failed to deliver were exposed through the day prior to euthanasia (PND 25) for a total of 40–43 days. All F0 females were allowed to deliver and rear their pups until lactation day 13.
Treatment‐related lower male and female fertility and copulation/conception indices and/or longer female oestrous cycle length were noted at 5/3.55/1.45 and 7.5/5.32/2.18 mg lasalocid/DNC/HDP/kg bw per day, which likely contributed to the poor reproductive outcome, including lower mean numbers of pups born and implantation sites, as well as reduced live litter sizes at PND 0. In addition, treatment‐related lower mean pup body weights and body weight gains were noted at 7.5/5.32/2.18 and 5/0/0 mg lasalocid/DNC/HDP/kg bw per day. Based on these results, the FEEDAP Panel considered the dose of 2/1.42/0.58 mg lasalocid/DNC/HDP/kg bw per day as the NOAEL for F0 male and F0 female reproductive toxicity and F1 neonatal toxicity.
Treatment‐related clinical observations and body weight deficits during the pre‐mating period were observed for F0 males at 10/7.09/2.91 mg lasalocid/DNC/HDP/kg bw per day, which resulted in the moribund condition of a single male at this exposure level. In addition, compound‐related effects on haematology parameters (higher red blood cell and haemoglobin distribution widths), higher adrenal gland weights, lower thymus weights and increase extramedullary haematopoiesis in the spleen were observed in F0 males at 7.5/5.32/2.18 mg lasalocid/DNC/HDP/kg bw per day. Based on these observations, the NOAEL for systemic toxicity in F0 males was considered to be 5/3.55/1.45 mg lasalocid/DNC/HDP/kg bw per day. Treatment‐related mortality, clinical observations, lower mean body weights and body weight gains, and reduced food intake were noted for F0 females at 10/7.09/2.91 mg lasalocid/DNC/HDP/kg bw per day during the pre‐mating period, and at 7.5/5.32/2.18 mg lasalocid/DNC/HDP/kg bw per day during gestation and lactation. Test article‐related effects on haematology parameters (RBC parameters, higher reticulocytes and lower platelet counts), lower thymus weights, increased extramedullary haematopoiesis in the spleen, lower incidence of increased mucification in vagina, squamous epithelial hyperplasia of the cervix and vagina and decreased ovarian corpora lutea were observed at 2/1.42/0.58, 5/3.55/1.45, 7.5/5.32/2.18 and/or 5/0/0 mg lasalocid/DNC/HDP/kg per day. Based on these observations, the NOAEL for systemic toxicity in F0 females could not be established.
Under the conditions of this screening study, there was no evidence of enhanced toxicity of the nicarbazin components DNC/HDP plus lasalocid A sodium over an equivalent dose of lasalocid A sodium alone.
3.2.4.4. Impurities in the active substance nicarbazin
The FEEDAP Panel notes that no information on the toxicity/genotoxicity of p‐nitroaniline (PNA) and methyl(4‐nitrophenyl) carbamate (M4NPC) was submitted in the dossier.
3.2.4.5. Conclusion on toxicology
The FEEDAP Panel reiterates the conclusions on the toxicology profile of lasalocid A sodium already adopted in 2017: ‘Absence of mutagenic effects at the gene level both in bacteria and in mammalian cells was demonstrated. There was no evidence for in vitro genotoxicity of lasalocid sodium. The results of chronic oral toxicity/carcinogenicity studies carried out in rats and dogs suggest no evidence of carcinogenicity. Fetotoxicity in rabbits was observed but was related to developmental and maternal toxicity. No specific teratogenicity study was available in rodents. However, relevant data from the rat two‐generation study is considered sufficient supporting evidence for the lack of teratogenicity of lasalocid’. ‘A lowest NOAEL of 0.5 mg/kg bw per day was established from the 2‐year chronic oral toxicity study in rats and maternal toxicity study in rabbits.’
The two active components of nicarbazin (DNC and HDP) were tested in vitro separately; both compounds did not induce gene mutations in bacteria; in mammalian cells, in vitro DNC did not induce chromosome damage, while HDP caused a statistically significant increase of micronuclei. Equivocal results were obtained for nicarbazin in the in vivo micronucleus assay. Overall, the Panel concludes that a concern for genotoxicity cannot be excluded and that clarification on the mechanism of action of the test items would be needed.
The lowest NOAEL identified in a 52‐week study in rat using DNC + HDP was 52.5 + 17.5 mg DNC + HDP/kg bw per day based on chronic inflammation in the kidneys which correlated macroscopically with rough surface and/or tan discolouration in animals exposed to ≥ 150 + 50 mg DNC + HDP/kg bw per day and with increased kidney weights in males given or exposed to 300 + 100 mg DNC + HDP/kg bw per day.
Based on the available toxicological data (subchronic oral toxicity study and a combined 28‐day repeated dose oral toxicity study with the reproduction/developmental toxicity screening test), the FEEDAP Panel concludes that there is no evidence for any interaction between DNC + HDP and lasalocid. The Panel noted that no genotoxicity studies were performed with the combination of the active substances under assessment.
3.2.5. Safety for the consumer
Based on the toxicological package provided, the FEEDAP Panel identified NOAELs that could be used for setting health‐based guidance values (e.g. acceptable daily intake (ADI)). Since the lack of genotoxic potential of nicarbazin in Nilablend™ 200G has not been adequately demonstrated, the FEEDAP Panel is not in the position to establish an ADI for DNC on which to base the assessment of consumer safety. The FEEDAP Panel cannot conclude on the safety of Nilablend™ 200G for the consumer and the proposal for MRLs and withdrawal time made by the applicant cannot be verified.
3.2.6. Safety for user
For the current assessment, the applicant has not provided studies performed with Nilablend™ 200G, apart from information on the physical characteristics of the additive (see Section 3.1.2). Instead, information/data on its individual components were submitted.
3.2.6.1. Lasalocid A sodium
Data concerning the user safety of lasalocid A sodium were submitted in former dossiers on Avatec® and were previously assessed by the FEEDAP Panel (EFSA, 2004a,b, 2005; EFSA FEEDAP Panel, 2010a,b,c, 2017a,b,c,d,e). For the current evaluation, no new data have been submitted; however, a literature review covering the period 2015–2019 (for details, see Section 3.2) and addressing the user safety of lasalocid A sodium was provided.62
On the basis of studies performed with the active substance, the FEEDAP Panel noted in its former opinion (EFSA, 2005) that: ‘Lasalocid sodium dust has the potential to cause local toxicity to the respiratory tract and also systemic toxicity to other organs. The acute dermal toxicity was low. Lasalocid sodium did not cause skin irritation or sensitisation but did cause eye irritation.’ The literature search provided by the applicant in the current dossier did not identify any papers/data that would lead to a modification of the above considerations.
3.2.6.2. Nicarbazin
Effects on eyes and skin
An acute dermal toxicity study in rats was submitted.76 Ten Sprague Dawley rats (five animals per sex) were dosed dermally with a 3:1 mixture of DNC and HDP to equal 2,000 mg/kg of body weight (information on compliance with GLP or OECD Guideline was not provided). The test article mixture was kept in contact with the skin for 24 h. Dermal responses were recorded at 24 h post‐dosing and on day 14. Animals were observed for mortality, toxicity and pharmacological effects at 1‐ and 4‐h post‐dosing and once daily thereafter for 14 days. Body weights were recorded pre‐test, weekly and at termination. All animals were examined for gross pathology. There were no deaths. Abnormal physical signs observed included chromorhinorrhea, chromodacryorrhea and wetness of the anogenital area. Two females lost body weight from day 0 to 7 and one female from day 7 to 14. All animals gained bodyweight by study termination. Immediately following unwrapping, erythema was absent or very slight with test article staining. On day 14, erythema was absent; however, test article staining remained. There were no observable gross abnormalities except for yellow staining observed on the treated skin of all animals. Based on these results, the FEEDAP Panel considered that the acute dermal toxicity of a mixture of DNC and HDP is low.
The potential of DNC/HDP (in a 3:1 ratio) for skin and eye irritation was investigated following OECD TG 40477 and 405.78 DNC/HDP was classified as a very slightly irritant when applied dermally to rabbits. DNC/HDP was classified as severe irritant and corrosive when applied to rabbit eyes.
The skin sensitisation potential of DNC/HDP was studied by the murine local lymph node assay (LLNA) (OECD TG 429)79 and showed that DNC/HDP is not a skin sensitiser.
Conclusions on the effects on eyes and skin
The mixture of DNC/HDP (in a 3:1 ratio) was classified as a very slight irritant to skin and as severe irritant and corrosive to eyes, but it is not skin sensitiser. The FEEDAP Panel concludes that these data can be considered as supportive only. Studies performed with nicarbazin and with the final additive Nilablend™ 200G, would be needed to conclude on the safety for the user of nicarbazin and of Nilablend™ 200G.
Effects on the respiratory system
No studies were submitted to assess the effects on the respiratory system of nicarbazin.
3.2.6.3. Inhalation exposure
The dusting potential of Nilablend™ 200G determined in six batches ranged between 0 and 0.03 g/m3, but no data were submitted on the concentration of the active substances in the dust and on the particle size of the dust.
The potential exposure of users by handling the additive to inhaled lasalocid A sodium and nicarbazin was calculated according to the Technical Guidance on User safety (EFSA FEEDAP Panel, 2012) and reported in Appendix A. In the absence of data, for the purpose of the inhalation exposure assessment, it is assumed that the concentration of lasalocid A sodium and nicarbazin in the dust is the same as in the additive (100 g/kg each80) and it is also assumed that 100% of the particles in the dust is in the respirable fraction. From dusting potential, the concentration of the active substances in the inhaled air could be calculated as 3 mg/m3 each, resulting in inhalation exposure of 0.42 mg per person during an 8‐h working day for each active substance.
Considering the respiratory toxicity of lasalocid A sodium and its potential to cause systemic toxicity, it is concluded that the exposure by inhalation indicates a risk to persons handling Nilablend™ 200G.
3.2.6.4. Conclusions on safety for the user
Nilablend™ 200G is considered toxic by inhalation, corrosive and irritant to eyes, slightly irritant to the skin but not a skin sensitiser. Inhalation exposure is considered a risk to persons handling the additive. Since the lack of genotoxic potential of nicarbazin has not been adequately demonstrated, it should be considered as an additional potential concern to users handling the additive.
3.2.7. Safety for the environment
The applicant submitted an updated environmental risk assessment including i) studies already assessed in former EFSA FEEDAP opinions (EFSA FEEDAP Panel, 2010a,b,c, 2017a,b,c,d,e), ii) new studies and iii) a specific literature review on the safety of lasalocid A sodium covering the period 2015–2019 (for details, see Section 3.2).62
The active substance is not a physiological/natural substance of established safety for the environment. Consequently, according to Regulation (EC) No 429/200881, the Phase I assessment has to be continued to determine the predicted environmental concentration (PEC).
In Phase I and II initially a total residues approach will be taken, meaning that the PECs will be calculated, based on the assumption that the additive is excreted 100% as parent compound.
LASALOCID A SODIUM
The environmental risk of lasalocid A sodium when used in chickens for fattening at a dose of 125 mg/kg complete feed was assessed in 2017 (EFSA FEEDAP Panel, 2017a) In that opinion, the FEEDAP Panel concluded that no risk was expected for the environment. Nilablend™ 200G is proposed for use in chickens for fattening at a maximum dose of 50 mg lasalocid A sodium/kg complete feed, therefore no risk is expected for the environment.
NICARBAZIN (DNC and HDP)
3.2.7.1. Phase I – Nicarbazin (DNC and HDP)
Physico‐chemical properties
The physico‐chemical properties of DNC and HDP are summarised in Tables 8 and 9.
Table 8.
Physico‐chemical properties of DNC
| Property | Value | Unit |
|---|---|---|
| Octanol/water partition coefficient (log Pow)1 | 3.97 (pH 7) | – |
| Water solubility2 | 0.04 | mg/L |
| Dissociation constant (pKa)3 | Not given | – |
| Vapour pressure4 | 1.6E‐08 at 25°C | Pa |
Technical dossier/Supplementary information March 2020/Reference 14.
Technical dossier/Supplementary information March 2020/Reference 11.
Technical dossier/Supplementary information March 2020/Reference 12.
Technical dossier/Supplementary information March 2020/Reference 13.
Table 9.
Physical‐chemical properties of HDP
| Property | Value | Unit |
|---|---|---|
| Octanol/water partition coefficient (log Pow)1 | –0.93 (pH 7) | – |
| Water solubility2 | 71200 mg/L at pH 7 | mg/L |
| Dissociation constant (pKa)3 | pKa1 3.55, pKa2 10.26 | – |
| Vapour pressure4 | 5.3E‐05 at 25°C | Pa |
Technical dossier/Supplementary information March 2020/Reference 21.
Technical dossier/Supplementary information March 2020/Reference 18.
Technical dossier/Supplementary information March 2020/Reference 19.
Technical dossier/Supplementary information March 2020/Reference 20.
Fate and behaviour
Fate in soil
Adsorption/desorption in soil
DNC
A GLP‐compliant study82 was conducted using [14C]‐DNC and five soil types, in accordance with OECD TG 106. The soils used to construct isotherms in this test are reported in Table 10.
Table 10.
Soil characteristics
| Name | Texture | pH (in 0.01 mol/L CaCl2) | Organic Carbon (%) |
|---|---|---|---|
| H1 | Clay | 5.5 | 7.5 |
| J1 | Clay Loam | 7.2 | 7.1 |
| E1 | Silt Loam | 5.2 | 3.4 |
| E3 | Loam | 5.1 | 3.26 |
| B2 | Loamy Sand | 4.6 | 1.13 |
Preliminary testing indicated that DNC is adsorbed to the glass test vessels. Therefore, the concentration of DNC in soil (Cs) was measured through a direct method, by combustion analysis in all samples. DNC was shown to have a strong affinity for soils, and therefore, a soil/solution ratio of 1:20 and an adsorption equilibrium period of 2 h were selected. Equilibrium in the desorption phase was reached after 4 h.
14C‐DNC had a very strong affinity for each of the soil types, with only 1–7% of the applied radioactivity recovered in the adsorption supernatants. Following completion of the desorption phase, only 1–4% of applied radioactivity was subsequently desorbed in each of the soils.
Adsorption and desorption distribution coefficients (Kd and Koc) and Freundlich isotherms at five test concentrations of [14C]‐DNC in 0.01 M aqueous calcium chloride (0.0002, 0.001, 0.002, 0.010 and 0.020 μg/g) were determined. The mean adsorption distribution coefficients, expressed in terms of soil organic carbon (Koc), are summarised in Table 11.
Table 11.
DCN sorption coefficients – Adsorption
| Soil identification | Mean partition coefficient | Freundlich coefficients | |||
|---|---|---|---|---|---|
| Kd (L/kg) | Koc (L/kg) | Kf | Kfoc | 1/n | |
| H1 (Clay) | 337 | 4,491 | 715 | 9,533 | 1.09 |
| J1 (Clay loam) | 557 | 7,844 | 274 | 3,863 | 0.92 |
| E1 (Silt Loam) | 434 | 12,764 | 1152 | 33,886 | 1.11 |
| E3 (Loam) | 398 | 12,197 | 446 | 13,674 | 1.01 |
| B2 (Loamy Sand) | 121 | 10,683 | 210 | 18,553 | 1.07 |
| Mean | 369 | 9,596 | 559 | 15,902 | 1.04 |
| Geomean | 330 | 8,986 | 462 | 12,592 | |
The mean desorption Koc was in the order clay (27,625 L/kg) < loamy sand (30,279 L/kg) < clay loam (34,399 L/kg) < loam (54,387 L/kg) < silt loam (58,092 L/kg). The results indicated that adsorption was not reversible.
Under the classification scheme proposed by McCall et al.,83 DNC would be classified as being immobile. The geometric mean adsorption coefficient for DNC (Koc = 8,986 L/kg) will be used for risk assessment purposes. The geometric mean Freundlich adsorption coefficient for DNC (Kfoc = 12,592 L/kg, 1/n= 1.04) can be used for groundwater assessment refinement.
HDP
A GLP‐compliant study84 was conducted using [14C]‐HDP and five soil types, in accordance with OECD TG 106. The same soils used for determining adsorption of DNC were used.
A series of preliminary experiments was performed in order to select optimal conditions for use in the isotherm test. A soil/solution ratio of 1:5 was selected for the clay soil and 1:1 for clay loam, silt loam, loam and loamy sand soils. Equilibrium in the adsorption phase was achieved after 48 h and equilibrium in the desorption phase was reached after 8 h.
14C‐HDP adsorbed to each of the soil types investigated, with 8–37% of the applied radioactivity recovered in the adsorption supernatants. Following completion of the desorption phase, 6–18% of applied radioactivity was subsequently desorbed in each of the soils.
Adsorption and desorption distribution coefficients (Kd and Koc) and Freundlich isotherms at five test concentrations of [14C]‐HDP in 0.01 mol/L aqueous calcium chloride (0.1, 0.5, 1, 5 and 10 μg/g) were determined. The results indicated that HDP adsorbed to each of the soil types investigated. The mean adsorption distribution coefficients, expressed in terms of soil organic carbon (Koc), are summarised in Table 12.
Table 12.
HDP sorption coefficients – Adsorption
| Soil identification | Mean partition coefficient | Freundlich coefficients | |||
|---|---|---|---|---|---|
| Kd (L/kg) | Koc (L/kg) | Kf | Kfoc | 1/n | |
| H1 (Clay) | 8.40 | 112 | 7.21 | 96 | 0.85 |
| J1 (Clay loam) | 3.92 | 55 | 3.61 | 51 | 0.95 |
| E1 (Silt Loam) | 3.47 | 102 | 3.03 | 89 | 0.91 |
| E3 (Loam) | 2.89 | 89 | 2.65 | 81 | 0.93 |
| B2 (Loamy Sand) | 3.50 | 311 | 2.63 | 232 | 0.82 |
| Mean | 4.44 | 134 | 3.82 | 110 | 0.89 |
| Geomean | 4.10 | 112 | 3.53 | 96 | 0.89 |
The mean desorption Koc was in the order clay (295 L/kg) < loam (401 L/kg) < silt loam (423 L/kg) < clay loam (702) < loamy sand (881). The results indicated that adsorption was partially reversible.
Under the classification scheme proposed by McCall et al., HDP would be classified as having low mobility. The geometric mean adsorption coefficient for HDP (Koc = 112 L/kg) will be used for risk assessment purposes. The geometric mean Freundlich adsorption coefficient for HDP (Kfoc = 96 L/kg, 1/n = 0.89) can be used for groundwater assessment refinement.
Degradation in soil
DNC
The degradation of [14C]‐DNC was investigated in a GLP‐compliant study,85 in accordance with OECD TG 307. The rate of degradation and transformation pathway was evaluated in sandy loam D soil treated with [14C]‐DNC at an application rate of 410 μg/kg soil (dry weight). The rate of transformation was determined in sandy loam B1, silt loam and clay loam soils at an application rate of 410 μg/kg soil. The characteristics of the four soils are reported in Table 13.
Table 13.
Soil characteristics
| Name | Sandy Loam (D) | Sandy Loam (B1) | Silt Loam (E1) | Clay Loam (J1) |
|---|---|---|---|---|
| Particle Size (% w/w): | ||||
| Clay (< 2 μm) | 16 | 11 | 21 | 34 |
| Silt (50‐2 μm) | 12 | 14 | 56 | 33 |
| Sand (2,000‐50 μm) | 72 | 75 | 23 | 33 |
| Texture (USDA) | Sandy Loam | Sandy Loam | Silt Loam | Clay Loam |
| pH (water) | 5.3 | 3.8 | 5.7 | 7.6 |
| pH (0.01M CaCl2) | 4.7 | 3.4 | 5.2 | 7.2 |
| Organic Matter (% w/w)1 | 4.52 | 5.9 | 5.86 | 12.3 |
| Organic Carbon (% w/w) | 2.62 | 3.4 | 3.40 | 7.1 |
| CEC (meq/100 g soil)2 | 12.7 | 11.3 | 17.0 | 33.9 |
| Maximum Water Holding Capacity pF 2.0 (% w/w) | 30.6 | 22.0 | 48.0 | 54.4 |
Organic carbon (OC) % = organic matter (OM) %/1.724.
CEC = cation exchange capacity.
Following treatment with [14C]‐DNC, the soil samples were incubated in the dark at 20°C for up to 120 days, under aerobic conditions. The test system was maintained under negative pressure throughout the incubation period. At intervals, duplicate soil samples were removed and extracted, and the extracts submitted to chromatographic analysis; non‐extractable residues and volatile evolution were also quantified.
Quantitative mass balance in each of the soil types under investigation was in the range 90–107% recovery. Extraction efficiency in each of the soils at zero time was generally high (79–96%), but then declined as the study progressed (48–80% at Day 120). Non‐extractable residues accounted for 32% in sandy loam D soil, 20% in sandy loam B1 soil, 44% in silt loam soil and 48% in clay loam soil at 120 day. Levels of 14CO2 and non‐specific [14C]‐volatile compounds were negligible throughout the study, representing less than 1% of applied radioactivity at the end of the study.
Chromatographic analysis of the soil extracts was performed by HPLC with radiochemical detection. In three of the soils (sandy loam D, sandy loam B1 and silt loam), only DNC was detected and in the fourth soil (clay loam), a minor unidentified component was also detected from Day 56 onwards. It was evident from the chromatographic analysis results that the time by which 50% of DNC had disappeared from three of the soils exceed the 120 days incubation period; in the fourth soil (clay loam rate soil), 46.9% DNC remained at 120 days.
The DT50 and DT90 values for DNC were calculated using non‐linear regression and a single first order model using CAKE. DT50 values of 293, 537, 217 and 152 days were estimated for sandy loam D, sandy loam B1, silt loam and clay loam, respectively, incubated at 20°C, with a mean value of 300 days (geomean 268 days); the mean DT50 was equivalent to 637 days when normalised to a temperature of 12°C according to the Arrhenius equation. The corresponding DT90 values (20°C) were 973, 1,780, 721 and 506 days.
The fit of this model may represent a physical phenomenon rather than biological as it is related to the decline in extraction efficiency over time. As the DegT50 values were considerably longer than the in‐life phase of the study, these values should be viewed with caution. A generic DT50 of 1,000 days is proposed for further exposure assessment.
HDP
The degradation of [14C]‐HDP was investigated in a GLP‐compliant study,86 in accordance with OECD TG 307. The same soils used for the evaluation of DNC degradation were used. The rate of degradation and transformation pathway was evaluated in the sandy loam D soil treated with [14C]‐HDP at an application rate of 210 μg/kg soil (dry weight). The rate of transformation was determined in the sandy loam B1, silt loam and clay loam soils at an application rate of 210 μg/kg soil. Following treatment with [14C]‐HDP, the soil samples were incubated in the dark at 20°C for up to 120 days, under aerobic conditions. The test system was maintained under negative pressure throughout the incubation period. At intervals, duplicate soil samples were removed and extracted, and the extracts submitted to chromatographic analysis; non‐extractable residues and volatile evolution were also quantified.
Quantitative mass balance in sandy loam D, silt loam and clay loam soil was in the range 93–96% recovery, but a lower mass balance was determined for the sandy loam B1 soil, probably due to a leak in the volatile trapping system (combined traps were used) as the recovery of 14C‐CO2 in the traps was considerably lower in this soil type, compared with the other soils. Extraction efficiency in each of the soils declined as the incubation progressed, accounting for 5–27% at the end of the incubation period. Non‐extractable residues increased to a peak of 67% at Day 10 in the route soil (sandy loam D) and subsequently declined to 49% at Day 120. Similar trends occurred in the other soil types. Levels of 14CO2 recovered increased as the incubation period progressed, representing 37, 16, 37 and 22% of applied radioactivity by study termination in sandy loam D, sandy loam B1, silt loam and clay loam, respectively. Non‐specific [14C]‐volatile compounds were low throughout the study, representing less than 3% of applied radioactivity at study termination.
Chromatographic analysis of those soil extracts containing more than 10% of applied radioactivity was performed by HPLC with radiochemical detection. In each of the soils, HDP was the principal component detected and levels declined in line with lower extraction efficiency as the incubation progressed. In sandy loam D, only 5% of parent was present at Day 28 (last sampling interval where analysis of extracts was performed). In the other soils, the final sampling interval levels of HDP were 25% in sandy loam B1 (Day 120), 6% in silt loam (Day 28) and 10% in clay loam (Day 14). Up to 12 minor unidentified components were detected in the sample extracts at intervals throughout the incubation period, with each representing less than 10% of applied radioactivity.
The DT50 and DT90 values for HDP were modelled using CAKE; the appropriate kinetic model was identified based on a combination of best‐fit, Χ2 error values and visual assessment of the data. DT50 values of 6.3 days (first order multi‐compartment; FOMC), 118 days (double first order in parallel), 9 days (FOMC) and 6.3 days (FOMC) were estimated for sandy loam D, sandy loam B1, silt loam and clay loam, respectively, with a geometric mean value of 14 days. This value, normalised to a temperature of 12°C according to the Arrhenius equation, results in a DT50 of 30 days. The corresponding DT90 values (20°C) were 21, 315, 30 and 21 days, with a geometric mean value of 45 days; this was equivalent to 96 days when normalised to a temperature of 12°C. Thus, it is considered that HDP will not persist in soil and there is no requirement to assess accumulation in soil (DT90 < 1 year). The reference DT50 value to be used for exposure assessment is 30 days.
Fate in water
The hydrolytic stability of DNC was investigated during the analytical method validation to support the analysis of aquatic ecotoxicological studies with DNC and HDP.87 , 88 It was shown that DNC and HDP were stable in aqueous solution at ambient temperature for 6 days when stored in the dark.
Conclusion on fate and behaviour
For DNC, a DT50 of 1,000 days will be used for the assessment together with the geometric mean adsorption coefficient Koc = 8,986 L/kg. The geometric mean Freundlich adsorption coefficient for DNC (Kfoc = 12,592 L/kg, 1/n = 1.04) can be used for groundwater assessment refinement.
For HDP, a DT50 of 30 days will be used for the assessment together with the geometric mean adsorption coefficient Koc = 112 L/kg. The geometric mean Freundlich adsorption coefficient for HDP (Kfoc = 96 L/kg, 1/n= 0.89) can be used for groundwater assessment refinement.
Predicted environmental concentrations (PECs)
The calculated PEC initial values for both DNC and HDP are given in Table 14.
Table 14.
Predicted Environmental Concentration (PECs) of DNC and HDP, in soil, groundwater, surface water and sediment
| Input | Value | |
|---|---|---|
| DNC | HDP | |
| Dose (mg/kg feed) | 35 | 15 |
| Molecular weight | 302.24 | 124.14 |
| Vapour Pressure (Pa) (at 25°C) | 1.6 × 10−8 | 5.3 × 10−5 |
| Solubility (mg/L) | 0.04 | 71,200 |
| Koc (L/kg) | 8,986 | 112 |
| DT50 in soil at 12°C (days) | 1,000 | 30 |
| Output | ||
| PECsoil (μg/kg) | 182 | 78 |
| PECgroundwater (μg/L) | 1 | 33 |
The Phase I PEC trigger values are exceeded; therefore, a Phase II assessment is considered necessary.
3.2.7.2. Phase II – Nicarbazin (DNC and HDP)
Exposure assessment
PECs calculation refined in Phase II
DNC – refinement of PEC soil for persistent compounds
The DT90 for DNC was determined to be greater than 1 year, therefore the PECs refined at steady state was calculated (Table 15) according to the FEEDAP technical guidance for assessing the safety of feed additives for the environment (EFSA, 2008).
Table 15.
Plateau Predicted Environmental Concentration (PECplateau) of DNC in soil, groundwater, surface water and sediment
| Compartment | PECplateau (DNC) |
|---|---|
| Soil (μg/kg) | 813 |
| Ground water (μg/L) | 5.1 |
| Surface water (μg/L) | 1.7 |
| Sediment (μg/kg) | 770 |
No refinement is necessary for HDP; the Phase II PEC are reported in Table 16.
Table 16.
Phase II Predicted Environmental Concentration (PEC) of HDP in soil, groundwater, surface water and sediment
| Compartment | PEC (HDP) |
|---|---|
| Soil (μg/kg) | 78 |
| Ground water (μg/L) | 33 |
| Surface water (μg/L) | 11 |
| Sediment (μg/kg) | 89 |
DNC and HDP – PEC groundwater refined
The relationship KOM > –5.9 + 3.8 DT50 can be used to ensure that the leaching concentrations is below the trigger value of 0.1 μg/L. For both DNC and HPD, taking into account the application rate (0.55 kg/ha for DNC, 0.23 kg/ha for HDP), the geomean DT50 calculated according SFO at 20°C (1,000 days for DNC, 14 days for HDP), the geomean Koc (8,986 for DNC, 112 L/kg for HDP), the inequality is respected. Therefore, no risk of leaching into groundwater is expected either for DNC or for HDP.
Conclusions on PEC used for calculation
The following values are used for the assessment: for DNC a PECsoil of 813 μg/kg, a PECsurface water of 1.7 μg/L and a PECsediment of 770 μg/kg; for HDP a PECsoil of 78 μg/kg, PECsurface water of 11 μg/L and PECsediment of 89 μg/kg.
Ecotoxicity studies
Toxicity of DNC and HDP to soil organisms
Effects on plants – DNC
The effect of DNC on terrestrial plants was investigated in a GLP study in accordance with OECD TG 208 (concentration range of 6.25–100 mg/kg, spacing factor of 2).89 The study was conducted using sandy loam soil and six plant species: maize, mung bean, radish, ryegrass, sunflower and tomato. Due to the lack of effect of DNC, no reliable ECx values could be determined for emergence, survival or growth; the EC50 values were greater than the highest concentration tested (100 mg DNC/kg soil).
Effects on plants – HDP
The effect of HDP on terrestrial plants was investigated in a GLP study in accordance with OECD TG 208 (concentration range of 6.25–100 mg/kg, spacing factor of 2).90 The study was conducted using sandy loam soil and six plant species: maize, mung bean, radish, ryegrass, sunflower and tomato. Due to the lack of effect of HDP, no reliable ECx values could be determined for emergence, survival or growth; the EC50 values were greater than the highest concentration tested (100 mg HDP/kg soil).
Effect on earthworms – DNC
The effect of DNC on the reproductive performance of earthworms was investigated in a GLP‐compliant study performed according to OECD TG 222.91 Earthworms were exposed to DNC in artificial soil over a 28‐day exposure period, at concentrations equivalent to 62.5, 125, 250, 500 and 1,000 mg/kg dry soil. Adult earthworms were sorted and removed from test soil and assessed for mortality, weight change and sublethal effects after 28 days exposure. Soil was replaced in the test container and juveniles were allowed to grow for a further 28 days, after which they (and any cocoons produced) were removed from the soil, counted and sublethal effects assessed. The data obtained from the study were subjected to statistical evaluation and it was concluded that there was no significant adult mortality noted during the test, nor effects on adult body weight. A dose‐response was evident for reproduction and the no effect concentration (NOEC) value was determined as 500 mg/kg. The EC50 values for adult mortality and growth over the 28‐day period were both > 1,000 mg/kg, with NOEC values of 1,000 mg/kg for both parameters. The lowest NOEC of 500 mg/kg has been selected for the assessment.
Effect on earthworms – HDP
The effect of HDP on the reproductive performance of earthworms was investigated in a GLP‐compliant study performed according to OECD TG 222. The study designed followed was the same as described above. HDP had a slight effect on mortality and growth, with a NOEC of 250 mg/kg and 500 mg/kg, respectively, and an EC50 of > 1,000 mg/kg for both parameters. A dose‐response was evident for reproduction and the NOEC value was determined as 62.5 mg/kg. The lowest NOEC of 62.5 mg/kg has been selected for the assessment.
Effects on soil micro‐organisms – DNC
The effect of DNC on soil nitrogen transformations was investigated, in accordance with OECD TG 216, in a GLP‐compliant study. The study was performed at 400 and 4,000 μg/kg soil (dry weight basis), equivalent to 1x and 10x the provisional maximum predicted environmental concentration in soil. Sandy loam soil was amended with ground Lucerne prior to treatment with DNC; weighed quantities of DNC were combined with quartz sand, which were then mixed into batches of soil. A corresponding control sample was prepared without the addition of DNC. The soils were incubated at about 20°C in the dark; on Day 0, 7, 14 and 28, subsamples of soil were removed for the determination of nitrate concentration. After 28 days, the amount of nitrate in the treated soils did not differ from that in the control soil by more than 25% (0.6% and –0.1% for the 1x and 10x PEC, respectively). Therefore, there were no biologically important effects on nitrogen transformation by soil microflora at either concentration. However, it should be noted that the highest concentration tested is below 10 × PEC as required by the FEEDAP technical guidance for assessing the safety of feed additives for the environment (EFSA, 2008).
Effects on soil micro‐organisms – HDP
The effect of HDP on soil nitrogen transformations was investigated, in accordance with OECD TG 216, in a GLP‐compliant study. The study was performed at 200 and 2,000 μg/kg soil (dry weight basis), equivalent to 1x and 10x the provisional maximum predicted environmental concentration in soil. Sandy loam soil was amended with ground Lucerne prior to treatment with HDP (dissolved in water, to prepare two treatment solutions). A corresponding control sample was prepared without the addition of HDP. The soils were incubated at ca. 20°C in the dark, and on Day 0, 7, 14 and 28, subsamples of soil were removed for the determination of nitrate concentration. After 28 days, the amount of nitrate in the treated soils did not differ from that in the control soil by more than 25% (1.1% and –3.3% at the PEC and 10x PEC, respectively). Therefore, there were no biologically important effects on nitrogen transformation by soil microflora at either concentration. However, it should be noted that the highest concentration tested is below 10 × PEC as required by the FEEDAP technical guidance for assessing the safety of feed additives for the environment (EFSA, 2008).
Toxicity of DNC and HDP to aquatic organisms
Effects on algae – DNC
In a GLP study (OECD TG 201), the growth inhibition of algae (Raphidocelis subcapitata) by DNC was investigated under static conditions.92 The test was conducted at nominal concentrations of 2, 4, 7, 12 and 20 μg DNC/L (three replicates for each treatment group), together with a solvent control (acetone) and an untreated control (six replicates for both of them). Concentrations of DNC were maintained during the 72‐h exposure, and therefore, results were based on nominal concentrations. The validity criteria of the study were met. Under the conditions of the study, the ErC50 (growth rate) values of DNC of R. subcapitata were established as > 20 μg/L and the NOEC value was 20 μg/L. NOEC value was selected for the assessment.
Effects on algae – HDP
The growth inhibition of algae (Raphidocelis subcapitata) by HDP, under static conditions, was investigated in a GLP compliant study (OECD TG 201).93 The test was conducted at nominal concentrations of 12.5, 25, 50, 100 and 200 mg/L (three replicates for each treatment group), together with an untreated control (six replicates). Concentrations of HDP were maintained during the 72‐h exposure period, and therefore, results were based on nominal concentrations. The validity criteria of the study were met. Under the conditions of the study, the ErC50 of HDP on the growth of R. subcapitata was established as > 200 mg/L and the corresponding NOEC value was 50 mg/L. ErC50 was selected for the assessment.
Effects on crustaceans – DNC
The acute toxicity of DNC to D. magna, under static conditions, was investigated in a GLP‐compliant study (OECD TG 202).94 Daphnids were exposed to nominal DNC concentrations of 4, 6, 9, 13 and 20 μg/L over a 48 h period; a solvent control (acetone) and untreated control were included in the test. Exposure concentrations, measured by HPLC, were slightly lower than nominal, and therefore, results were based on mean measured concentrations. The corresponding mean measured concentration of DNC was 3.14, 4.76, 7.01, 10.22 and 14.50 μg/L. Four replicates each containing 200 mL of treated or control medium, as appropriate, were prepared for each treatment group. Five D. magna neonates were added to each test vessel. No toxicity to daphnids was evident during the exposure period, and thus, the EC50 value for DNC was concluded to be > 14.5 μg/L.
A GLP compliant study following the OECD TG 211 was performed to investigate the effect of DNC on the reproductive capacity of D. magna.95 This chronic study was conducted over a 21‐day period, under semi‐static conditions, at nominal DNC concentrations of 5, 7, 10, 14 and 20 μg/L, plus solvent (acetone) and untreated controls. The validity criteria specified in OECD TG 211 were met. Analytical verification of test concentrations in freshly prepared and expired solutions indicated that concentrations of DNC were not maintained within ± 20% of the initial measured values during the 3‐day period between test medium renewal. The corresponding geometric mean measured concentrations of [14C]‐DNC were calculated as 4.86, 6.64, 10.76, 13.63 and 18.22 μg/L. The lack of significant adult immobilisation or reduction in offspring production indicated that DNC had no effect on D. magna survival and reproduction at any of the concentrations tested. Therefore, the 21‐day reproduction EC10 for DNC was concluded to be > 18.2 μg/L based upon geometric mean measured concentrations. EC10 value was selected for the assessment.
Effect on crustaceans – HDP
The acute toxicity of HDP to D. magna, under static conditions, was investigated in an OECD GLP‐compliant study (OECD TG 202).96 Daphnids were exposed to nominal HDP concentrations of 6.25, 12.5, 25, 50 and 100 mg/L over a 48 h period; an untreated control was also included in the test. Four replicates of treated or control were prepared (five D. magna neonates were added to each test vessel). Exposure concentrations, measured by HPLC, were comparable with nominal and therefore, results were based on nominal concentrations. The validity criteria were met. No toxicity to daphnids was evident during the exposure period, and thus, the EC50 value for HDP was concluded to be > 100 mg/L.
Effects on fish – DNC
The acute toxicity of DNC to rainbow trout (Oncorhynchus mykiss) was investigated in a GLP compliant study (OECD TG 203), under semi‐static conditions.97 The test was conducted at nominal concentrations of 4, 6, 9, 13 and 20 μg DNC/L, together with a solvent control (acetone) and an untreated control group (48 h renewal period). Concentrations of DNC were maintained during the 96‐h exposure, and therefore, results were based on nominal concentrations. No mortality was evident under the conditions of the study, and thus, the LC50 value for DNC was > 20 μg/L.
Effects on fish – HDP
The acute toxicity of HDP to rainbow trout (Oncorhynchus mykiss) was investigated in a GLP‐compliant study following the OECD TG 203 (96 h, semi‐static conditions).98 The test was conducted at nominal concentrations of 62.5, 125, 250, 500 and 1,000 mg HDP/L, together with a solvent control and an untreated control group (48 h renewal period). Concentrations of HDP were maintained during the 96‐h exposure, and therefore, results were based on nominal concentrations. No significant deviations from the study plan were noted. No mortality was evident under the conditions of the study, and thus, the LC50 value for HDP was > 1,000 mg/L.
Effects on sediment‐dwelling organisms – DNC
The toxicity of DNC to chironomids was investigated in a GLP compliant study (OECD TG 218),99 using sediment, which had been fortified with 14C‐DNC. Larvae of Chironomus riparius were exposed to nominal 14C‐DNC concentrations of 6.25, 12.5, 25, 50 and 100 mg equivalents/kg sediment (dry weight basis), together with a solvent and untreated control (test duration: 28 days, under static conditions). The number and sex of emerged adult chironomids were recorded daily during the exposure period. The distribution of radioactivity in the test system at Day 0 and Day 28 was determined by measuring levels of radioactivity in the sediment, interstitial and overlying water. As measured concentrations were comparable with nominal, the results were based on nominal concentrations. The validity criteria of the test were met. Due to the lack of a toxicological response to DNC from chironomids, it was not possible to determine ECx values. The most sensitive endpoint was mortality and the NOEC was established as 50 mg DNC equivalents/kg dry weight.
Effects on sediment‐dwelling organisms – HDP
The toxicity of HDP to chironomids was investigated in a GLP compliant study in accordance with OECD TG 218,100 using sediment which had been fortified with 14C‐HDP. C. riparius larvae were exposed to nominal 14C‐HDP concentrations of 0, 6.25, 12.5, 25, 50 and 100 mg equivalents/kg sediment (dry weight basis), over a 28‐day period, under static conditions (six replicates for each treatment group). The number and sex of emerged adult chironomids were recorded daily during the exposure period. The distribution of radioactivity in the test system at Day 0 and Day 28 was determined by measuring levels of radioactivity in the sediment, interstitial and overlying water. As measured concentrations were comparable with nominal, the results were based on nominal concentrations. Due to the lack of mortality, and of effects on emergence and overall development rate, the ECx values of HDP were reported as greater than the highest concentration tested (100 mg/kg dry weight basis). The most sensitive endpoint was female development rate and the NOEC was established as 25 mg HDP equivalents/kg dry weight.
Risk characterisation (PEC/PNEC ratio) for DNC and HDP
The tables below report the risk characterisation ratios for terrestrial (Table 17), freshwater (Table 18) and sediment (Table 19) compartments.
Table 17.
Risk characterisation (PEC/PNEC ratio) for DNC and HDP for the terrestrial compartment
| Taxa | PECsoil (μg/kg) | EC50 or NOEC (mg/kg) | AF | PNEC (μg/kg) | PEC/PNEC | |
|---|---|---|---|---|---|---|
| DNC | Earthworm | 813 | 5001 | 10 | 50,000 | 0.02 |
| Plants | > 1002 | 100 | 1,000 | 0.8 | ||
| HDP | Earthworm | 78 | 62.51 | 10 | 6,250 | 0.01 |
| Plants | > 1002 | 100 | 1,000 | 0.08 |
AF: assessment factor.
NOEC reproduction.
EC50.
Table 18.
Risk characterisation (PEC/PNEC ratio) for DNC and HDP for the freshwater compartment
| Taxa | PECsurfacewater (μg/L) | E(L)C50 or EC10/NOEC (μg/L) | AF | PNEC (μg/L) | PEC/PNEC | |
|---|---|---|---|---|---|---|
| DNC | Algae | 1.7 | 201 | 50 | 0.36 | 4.7 |
| Aquatic invertebrates | 18.21 | |||||
| Fish | 202 | |||||
| HDP | Algae | 11 | 200,0002 | 1,000 | 200 | 0.055 |
| Aquatic invertebrates | 100,0002 | |||||
| Fish | 1,000,0002 |
EC10/NOEC.
E(L)C50.
Table 19.
Risk characterisation (PEC/PNEC) for DNC and HDP for the sediment compartment
| Taxa | PECsed (μg/kg) | NOEC (mg/kg) | AF | PNEC (μg/kg) | PEC/PNEC | |
|---|---|---|---|---|---|---|
| DNC | C. riparius | 770 | 50 | 10 | 5,000 | 0.1 |
| HDP | C. riparius | 89 | 25 | 10 | 2,500 | 0.04 |
Since DNC is very persistent in soil (DT50 1,000 days), an AF of 100 will be considered for plants, even if 6 plants were provided in the study.
Bioaccumulation and secondary poisoning
In order to assess bioaccumulation and the risk for secondary poisoning, the method proposed in the relevant Guidance from the European Medicines Agency (EMA) has been considered (EMA, 2015). Based on the log KOW of 3.97 and high persistence, DNC has the potential for bioaccumulation in aquatic and terrestrial food chain.
The lipid‐normalised, growth‐corrected kinetic bioconcentration factor for DNC in whole fish was 61 L/kg. This value is lower than the threshold for bioaccumulation potential (BCF ≥ 100 L/kg) (ECHA, 2017). Hence, the substance is unlikely to bioaccumulate in fish and a risk for secondary poisoning for fish eating mammals and bird is not likely to occur.
Since there were no bioaccumulation data available for terrestrial organisms, the FEEDAP Panel made an assessment on secondary poisoning of DNC for terrestrial food chain. The lowest NOAEL identified from the toxicological data set has been identified in a 52‐week study in rat using DNC + HDP. It was determined as 52.5 + 17.5 mg DNC + HDP/kg bw per day based on chronic inflammation in the kidneys which correlated macroscopically with rough surface and/or tan discolouration in animals administered ≥ 150 + 50 mg DNC + HDP/kg bw per day and with increased kidney weights in males administered 300 + 100 mg DNC + HDP/kg bw per day. NOEC for DNC was 525 mg DNC/kg feed and was calculated from NOAEL taking into account conversion factor of 10 for rats. Using an assessment factor of 30, the corresponding PNECoral was equivalent to 17.5 mg/kg feed. This value is higher than the estimated concentration in worms of 0.61 mg/kg which is based on PECs presented in Table 15. The PEC/PNEC ratios for soil is presented in Table 20.
Table 20.
The assessment of secondary poisoning for DNC via the terrestrial food chain based on the 100% of the proposed recommended dose
| PECoral, predator (mg/kg) | PNECoral (mg/kg) | PEC/PNECoral | |
|---|---|---|---|
| DNC | 0.61 | 17.5 | 0.03 |
HDP, with log Kow < 3, does not have the potential for bioaccumulation; hence, there is no risk for secondary poisoning for this substance.
3.2.7.3. Conclusions on safety for the environment
Lasalocid A sodium from Nilablend™ 200G is not expected to pose a risk to the environment, at the proposed use level. Lasalocid A sodium is not considered to have a bioaccumulation potential.
DNC and HDP from Nilablend™ 200G do not pose a risk for groundwater. No risk for the terrestrial compartment is associated with DNC and HDP. No risk for the aquatic compartment is associated with HDP. A risk for aquatic compartment (freshwater) cannot be excluded for DNC. The bioaccumulation potential of HDP is low and no risk for secondary poisoning is identified. The high persistence and hydrophobicity of DNC indicate that there might be a risk for bioaccumulation but no risk for secondary poisoning was identified. The potential of DNC to accumulate in soil over the years should be investigated.
In summary, based on the available data, the FEEDAP Panel cannot conclude on the safety of Nilablend™ 200G for the environment due to a possible risk for aquatic compartment (freshwater) for DNC.
3.3. Efficacy
The applicant submitted four floor pen studies and 14 anticoccidial sensitivity tests (AST).
3.3.1. Floor pen studies
Four floor pen trials in chickens for fattening were submitted.101 The trials followed a similar design (Table 21). In each trial, one‐day‐old chickens (Ross 308; male and female in equal number) were penned and distributed into the experimental groups. In the first three trials, conducted consecutively in the same animal facility, the experimental groups were: an uninfected untreated control group (UUC), an infected untreated control group (IUC), an uninfected Nilablend™ 200G‐treated group (UTC), an infected Nilablend™ 200G‐treated group (IT) and an infected treated group with another coccidiostat. In the fourth trial, the groups were UUC, IUC and three IT groups (one treated with Nilablend™ 200G and two with another coccidiostat at different doses). In all four trials, the Nilablend™ 200G‐treated groups received feed containing 40 + 40 mg lasalocid A sodium + nicarbazin/kg feed, the lowest dose applied. The intended dietary concentrations were analytically confirmed (see Table 21). The experimental diets were fed for 35 days. In the infected groups, all birds were inoculated orally via a syringe with recent field isolates of pathogenic Eimeria species.102 Animal health and mortality were monitored daily. Feed intake and body weight of the animals were measured at day 0, 14, 21, 28 and 35, and feed to gain ratio was calculated. Samples of excreta were analysed for oocyst content. Intestinal lesions were scored on days 21 and 28 on five birds per pen, following the method of Johnson and Reid (1970) (0 = no lesion, 1 = very mild, 2 = mild, 3 = moderate and 4 = severe).
Table 21.
Experimental design of floor pen studies with chickens for fattening fed Nilablend™ 200G
| Trial no (study start) | Replicates per treatment (birds per replicate) | Inoculum characteristics | Feed analysis in diets lasalocid A Na+ nicarbazin (mg/kg feed)1 | |||
|---|---|---|---|---|---|---|
| Country and date of isolation | Intended dose (number of oocysts) and strain per bird | Day of inoculation | ||||
| 1 (03/2017) | 12 (30) | Belgium 01/2017 | 69,000 | E. acervulina | Day 14 | 39.1 + 37.4 37.6 + 34.4 40.1 + 37.3 |
| 30,000 | E. tenella | |||||
| 2,000 | E. maxima | |||||
| 900 | E. mitis | |||||
| 2 (04/2017) | 12 (30) | Italy 03/2017 | 65,000 | E. acervulina | Day 14 | 42.0 + 41.2 41.0 + 37.7 41.7 + 38.4 |
| 22,000 | E. tenella | |||||
| 4,000 | E. maxima | |||||
| 5,000 | E. mitis | |||||
| 2,000 | E. praecox/necatrix | |||||
| 3 (08/2017) | 12 (30) | France 06/2017 | 52,000 | E. acervulina | Day 15 | 42.6 + 37.5 41.2 + 38.3 42.7 + 34.0 |
| 17,500 | E. tenella | |||||
| 10,000 | E. maxima | |||||
| 1,000 | E. mitis | |||||
| 4 (08/2018) | 12 (18) | Denmark 01/2018 | 558,000 | E. acervulina | Day 15 | 43.3 + 37.8 39.7 + 40.8 41.4 + 42.7 |
Birds received starter diet from day 1 to 13/14, grower diet from day 14/15 to 28 and finisher diet from day 29 to 35.
The data were subjected to analysis of variance (ANOVA) using a general linear mixed model, including treatment as fixed effect (combined with time and its interaction depending on the parameter considered, e.g. BW or oocyst counts); and block as random effect. The pen was the experimental unit for statistical purposes. All hypotheses were tested at the 0.05 level of significance using two‐sided tests. If the treatment effect was significant, pairwise comparisons were made between all treatment groups (e.g. least significant difference). Oocyst counts were log‐normal transformed, whilst mortality data were arcsine square root transformed for the analysis.
The basal diet for all trials conducted was composed mainly of wheat, soybean meal and corn and was provided in pelleted form for ad libitum access.
In trials 1, 2 and 3, inoculation significantly increased mortality which was in turn significantly reduced by Nilablend™ 200G treatment (Table 22). In trial 4, only the total mortality was reported which was very low in all groups.
Table 22.
Coccidiosis‐related mortality and total mortality in floor pen trials1
| Trial | Number of birds per treatment | UUC | UTC | IUC | IT |
|---|---|---|---|---|---|
| 1 | 360 | 0c (12) | 0c (9) | 78a (89) | 12b (21) |
| 2 | 360 | 1b (17) | 0b (19) | 10a (30) | 3b (17) |
| 3 | 360 | 0b (9) | 0b (11) | 28a (41) | 1b (17) |
| 4 | 216 | Nr (6) | – | nr 1 | nr (9) |
nr: not reported.
–: no UTC group in this trial.
means in a row with different superscript letters are significantly different (p ≤ 0.05).
Total mortality is indicated in brackets.
The results of lesion scoring are summarised in Table 23. IT birds had statistically significantly lower mean intestinal lesion score of E. tenella compared to IUC birds on day 21 in trials 1, 2 and 3 and on day 28 in trials 1 and 3. Nilablend™ 200G also reduced E. maxima lesion scores on day 21 in trials 2 and 3 and on day 28 in trial 3. No improvement was noted on E. acervulina lesion scores of IT birds compared to IUC birds in any of the trials. In trial 4, lesion scores were not affected by the treatment.
Table 23.
Lesion scores for different Eimeria species at different study days in floor pen trials
| Trial | Group | E. acervulina | E. tenella | E. maxima | |||
|---|---|---|---|---|---|---|---|
| Day 21 | Day 28 | Day 21 | Day 28 | Day 21 | Day 28 | ||
| 1 | UUC | 0.1c | 1.2a | 0c | 0.1c | nr | nr |
| UTC | 0.1c | 0.9a | 0c | 0.1c | nr | nr | |
| IUC | 0.7b | 0.4b | 1.8a | 0.8a | nr | nr | |
| IT | 1.6a | 0.5b | 1.1b | 0.4b | nr | nr | |
| 2 | UUC | 0.6c | 1.3a | 0.1c | 0.1b | 0.8b | 0.6b |
| UTC | 0.4c | 1.0ab | 0.1c | 0.1b | 0.7b | 0.4b | |
| IUC | 1.7b | 0.8b | 1.8a | 0.8a | 1.7a | 1.1a | |
| IT | 2.2a | 1.1ab | 1.1b | 0.9a | 0.8b | 0.9a | |
| 3 | UUC | 0.09b | 1.27a | 0.10c | 0.12bc | 0.20c | 0.13c |
| UTC | 0.13b | 0.73b | 0.08c | 0.07b | 0.05d | 0.10c | |
| IUC | 1.41a | 0.78b | 2.42a | 0.42a | 1.91a | 0.95a | |
| IT | 1.82a | 1.43a | 0.95b | 0.20c | 0.53b | 0.62b | |
| 4 | UUC | 0.9b | 2.0 | ns | ns | ns | ns |
| IUC | 2.1a | 1.8 | ns | ns | ns | ns | |
| IT | 2.2a | 1.7 | ns | ns | ns | ns | |
means in a column with different superscript letters are significantly different (p ≤ 0.05).
nr: not reported in Trial 1 considering the low dose of E. maxima in the inoculum.
ns: not scored in trial 4 since the inoculum did not contain E. tenella and E. maxima.
The Eimeria oocyst counts are presented in Table 24. A significant positive effect in IT birds compared to IUC was seen only in trial 3 on days 20 and 22. However, an opposite effect (not significant) was seen on days 21 and 28. A cross contamination was generally observed in all trials at day 28 resulting in a significantly higher excretion in the UUC birds when compared to the treated groups in trials 1, 2 and 4.
Table 24.
Oocyst excretion (OPG) at different study days in floor pen trials
| Trial | Group | Day 20 | Day 21 | Day 22 | Day 28 |
|---|---|---|---|---|---|
| 1 | UUC | 3c | 2b | 1b | 84,548a |
| UTC | 35b | 2b | 0b | 38,074a | |
| IUC | 287,498a | 99,732a | 43,126a | 3,279b | |
| IT | 287,305a | 142,346a | 72,118a | 4,563b | |
| 2 | UUC | 1b | 3b | 4b | 5,529 |
| UTC | 2b | 1b | 2b | 814 | |
| IUC | 735,717a | 101,159a | 43,709a | 820 | |
| IT | 300,885a | 279,038a | 96,480a | 4,631 | |
| 3 | UUC | 3c | 33c | 1d | 30,076b |
| UTC | 19c | 6c | 22c | 7,400c | |
| IUC | 160,646a | 28,297b | 190,810a | 35,214b | |
| IT | 48,608b | 138,298ab | 108,489b | 104,407ab | |
| 4 | UUC | 3a | 14a | 17a | 40,275a |
| IUC | 278,135b | 3,559,568b | 98,622b | 660b | |
| IT | 458,236b | 1,730,974b | 79,230b | 1,191b |
means in a column with different superscript letters are significantly different (p ≤ 0.05).
The results concerning the zootechnical endpoints are summarised in Table 25. In all four experiments, performance (average daily weight gain and feed to gain ratio) of the IT birds was significantly improved compared to the IUC birds.
Table 25.
Zootechnical parameters of chickens for fattening fed Nilablend™ 200G in floor pen studies
| Trial | Group | Feed intake (g/d) | Weight gain (g/d) | Feed to gain ratio |
|---|---|---|---|---|
| 1 | UUC | 92a | 66a | 1.48c |
| UTC | 92a | 66a | 1.45c | |
| IUC | 79b | 60b | 1.57a | |
| IT | 91a | 66a | 1.52b | |
| 2 | UUC | 91a | 63b | 1.51b |
| UTC | 93a | 68a | 1.47a | |
| IUC | 85b | 56c | 1.63c | |
| IT | 90ab | 61b | 1.55b | |
| 3 | UUC | 90a | 67b | 1.44a |
| UTC | 90a | 69a | 1.41a | |
| IUC | 85b | 63c | 1.50b | |
| IT | 90a | 69a | 1.43a | |
| 4 | UUC | 79a | 62a | 1.40a |
| IUC | 73b | 57b | 1.45c | |
| IT | 76b | 61a | 1.42b |
means in a column with different superscript letters are significantly different (p ≤ 0.05).
Synopsis of the floor pen studies
In trials 1, 2 and 3 the challenge by Eimeria inoculation resulted in increased mortality, intestinal lesion scores and oocyst shedding (about one week after inoculation). These endpoints, except oocyst shedding, were significantly reduced by Nilablend™ 200G. Consequently, average daily gain and feed to gain ratio of the treated birds were significantly better compared to the untreated infected birds. None of the primary endpoints (mortality, lesion scores, oocyst excretion) were positively affected by Nilablend™ 200G treatment in trial 4. It should be noted that in all floor pen studies no effect of Nilablend™ 200G on intestinal lesion scores due to E. acervulina was found.
3.3.2. Anticoccidial sensitivity tests
A total of 14 anticoccidial sensitivity tests (AST) was submitted. Two ASTs were conducted in 2016,103 five in 2017104 and seven in 2018.105 Since studies should be conducted within 2 years before the submission of the application (FEEDAP Guidance on the assessment of the efficacy of feed additives, EFSA FEEDAP Panel, 2018a,b,c), the first two studies were not further considered for the purpose of the assessment.
The experimental design of the 12 ASTs (AST‐1 to AST‐12) is described in Table 26 (conducted in 2017) and Table 26 (conducted in 2018). At study start, 12‐days old chickens (Ross 308, male and female) were randomly allocated to the groups (UUC, IUC, and IT). The number of birds in all tests was 12 per replicate. Each treatment group had ten replicates except AST‐12 where the number of replicates was 10 in UUC and 9 in IUC and IT groups. The IT groups received feed containing Nilablend™ 200G (lasalocid A sodium + nicarbazin) for 8 days in all experiments. The intended dietary concentrations were analytically confirmed (Tables 26 and 27). The birds were individually inoculated on study day 1 or 2 using a syringe with recent field isolates of Eimeria species. The dose of the inoculum was derived from virulence dose titration studies (See Appendix B, Table B.1). Animal health and mortality were monitored. Feed and body weight of the animals were measured, feed to gain ratio was calculated. Samples of excreta were analysed for oocyst content. Intestinal lesions were scored following the method of Johnson and Reid (1970) (0 = no lesion, 1 = very mild, 2 = mild, 3 = moderate and 4 = severe) on day 6 or 7 post‐inoculation (PI).
Table 26.
Experimental design of anticoccidial sensitivity tests with chickens for fattening using Nilablend™ 200G conducted in 2017
| Trial no (month) | Inoculum characteristics | Feed analysis lasalocid A Na + nicarbazin (mg/kg feed)1) | ||
|---|---|---|---|---|
| Country and date of isolation | Intended dose (number of oocysts and strain per bird) | |||
| AST‐1 (Jan) | Netherlands Feb 2016 | 2,621 | E. acervulina | 27.9 + 29.8 39.1 + 38.6 47.8 + 52.2 |
| 93,339 | E. maxima | |||
| AST‐2 (Jul) | Germany May 2017 | 82,000 | E. acervulina | 31.4 + 28.6 43.2 + 38.9 53.1 + 48.1 |
| 62,000 | E. tenella | |||
| 14,000 | E. mitis | |||
| 8,000 | E. maxima | |||
| AST‐3 (Aug) | France May 2017 | 52,000 | E. acervulina | 31.4 + 28.6 43.2 + 38.9 53.1 + 48.1 |
| 17,500 | E. tenella | |||
| 10,000 | E. maxima | |||
| 1,000 | E. mitis | |||
| AST‐4 (Oct) | Belgium Aug 2017 | 101,000 | E. acervulina | 30.9 + 28.3 42.0 + 37.9 |
| 20,000 | E. tenella | |||
| 67,000 | E. maxima | |||
| AST‐5 (Nov) | UK June 2017 | 294,000 | E. acervulina | 30.9 + 28.3 |
| 54,000 | E. tenella | 42.0 + 37.9 | ||
| 2,000 | E. mitis | |||
Three infected treated groups (IT30, IT40 and IT50) in AST‐1, AST‐2 and AST‐3 two infected treated groups (IT30 and IT40) in AST‐4 and AST‐5.
Table 27.
Experimental design of anticoccidial sensitivity tests with chickens for fattening using Nilablend® 200G conducted in 2018
| Trial no (month) | Inoculum characteristics | Feed analysis lasalocid A Na + nicarbazin (mg/kg feed)1 | ||
|---|---|---|---|---|
| Country and date of isolation | Intended dose (number of oocysts) and strain per bird | |||
| AST‐6 (Jan) | Italy May 2017 | 108,000 | E. acervulina | 27.8/26.0 37.7/35.3 49.6/50.7 |
| 26,000 | E. tenella | |||
| AST‐7 (Apr) | Denmark Jan 2018 | 57,000 | E. acervulina | 28.0/27.6 41.5/38.2 |
| 43,000 | E. tenella | |||
| AST‐8 (Aug) | Belgium May 2018 | 46,000 | E. acervulina | 38.6/37.7 |
| 7,000 | E. maxima | |||
| 67,000 | E. tenella | |||
| 5,000 | E. brunetti | |||
| AST‐9 (Jun) | UK Nov 2017 | 3,600 | E. acervulina | 28.4/29.3 39.8/38.0 51.2/47.8 |
| 24,000 | E. maxima | |||
| 1,600 | E. tenella | |||
| AST‐10 (Jul) | Spain May 2018 | 34,500 | E. acervulina | 33.0/34.5 |
| 5,500 | E. maxima | |||
| 25,000 | E. tenella | |||
| AST‐11 (Nov) | Austria July 2018 | 310,000 | E. acervulina | 37.8/38.2 |
| 18,000 | E. maxima | |||
| 22,000 | E. mitis | |||
| AST‐12 (Dec) | Portugal Nov 2018 | 188,000 | E. acervulina | 36.4/36.6 |
| 18,000 | E. tenella | |||
| 2,000 | E. maxima | |||
| 18,000 | E. mitis | |||
Three infected treated groups (IT30, IT40 and IT50) in AST‐6 and AST‐9; two infected treated groups (IT30 and IT40) in AST‐7 and one infected treated group (IT40) in the other tests.
Table B.1.
Outcome of the virulence tests in ASTs
| Dose (number of oocysts and strain per bird) | Intestinal lesion scores 6 days PI | Intestinal lesion scores 7 days PI | Weight gain reduction (%) 6 days PI | Weight gain reduction (%) 7 days PI | Mortality (%) | ||
|---|---|---|---|---|---|---|---|
| AST‐1 | 4,000 | E. acervulina | 0 2.8 | 0.6 0.8 | 45 | nr | 0 |
| 99,000 | E. maxima | ||||||
| AST‐2 | 82,000 | E. acervulina | 2.0 3.0 1.6 nr | NA | nr | NA | 50 |
| 62,000 | E. tenella | ||||||
| 8,000 | E. maxima | ||||||
| 14,000 | E. mitis | ||||||
| AST‐3 | 52,000 | E. acervulina | 1.4 3.4 1.6 nr | 1.3 1.7 0 nr | nr | 44 | 20 |
| 17,500 | E. tenella | ||||||
| 10,000 | E. maxima | ||||||
| 1,000 | E. mitis | ||||||
| AST‐4 | 101,000 | E. acervulina | 1.6 0.6 1.4 | 1.8 0 2.2 | 47 | 35 | 0 |
| 20,000 | E. tenella | ||||||
| 67,000 | E. maxima | ||||||
| AST‐5 | 294,000 54,000 2,000 | E. acervulina E. tenella E. mitis | 1.8 0.4 0 | 2.2 1.4 0 | –2 | 2 | 0 |
| AST‐6 | 108,000 26,000 0 | E. acervulina E. tenella E. maxima | nr | 2.5 0 1.0 | nr | 43 | 0 |
| AST‐7 | 57,000 43,000 0 | E. acervulina E. tenella E. maxima | 2.2 2.6 1.4 | 2.0 2.6 1.6 | 15 | 29 | 0 |
| AST‐8 | 46,000 7,000 67,000 5,000 0 | E. acervulina E. maxima E. tenella E. brunetti E. necatrix | 3.0 0.6 2.6 0.2 0.4 | 2.6 1.4 2.4 0.4 0 | 23 | 22 | 0 |
| AST‐9 | 3,600 24,000 1,600 | E. acervulina E. maxima E. tenella | 1.6 2.8 0 | 2.0 1.8 0 | nr | 15 | 0 |
| AST‐10 | 34,500 5,500 25,000 | E. acervulina E. maxima E. tenella | 2.2 2.6 3.0 | 1.6 1.4 2.8 | 37 | 69 | 0 |
| AST‐11 | 129,000 17,000 8,000 3,000 | E. acervulina E. tenella E. maxima E. mitis | 2.7 1.0 0 – | 2.7 0.7 1.0 – | 50 | 50 | 0 |
| AST‐12 | 188,000 18,000 2,000 18,000 | E. acervulina E. tenella E. maxima E. mitis | 2.0 2.3 1.3 – | 2.3 1.3 0.3 – | 60 | 34 | 0 |
PI: post‐inoculation; nr: not reported.
NA: not applicable; due to high mortality, not enough animals were available to evaluate the parameter.
The data were subjected to analysis of variance (ANOVA) using a general linear mixed model including as fixed effects treatment, sex and their interaction. The pen (the bird for intestinal lesion score) was the experimental unit for statistical purposes. All hypothesis tests were conducted at the 0.05 level of significance using two‐sided tests. If the treatment effect was significant, pairwise comparisons between all treatment groups were made (e.g. least significant difference). Oocyst counts were log‐normal transformed and mortality (when occurred) was sinus square root transformed for the analysis.
Although different concentrations were tested in several studies, the assessment of ASTs refers only to the IT40 group (40 mg lasalocid A sodium + 40 mg nicarbazin/kg feed), since this concentration is the lowest applied for use, for which consequently efficacy has to be assessed. The results of the statistical evaluation with all experimental groups were taken for this consideration.
The Nilablend™ 200G treatment significantly reduced coccidiosis‐related mortality in four ASTs (Table 28), whereas in 8 of 12 trials no bird died due to coccidiosis (Appendix B, Table B.2).
Table 28.
Coccidiosis‐related mortality and total mortality in 4 out of 12 ASTs1
| Trial | Number of birds per treatment | UUC | IUC | IT40 |
|---|---|---|---|---|
| AST‐2 | 120 | 0b (1) | 13a (15) | 0b (4) |
| AST‐3 | 120 | 0b (1) | 5a (10) | 0b (1) |
| AST‐7 | 120 | 0b (0) | 17a (18) | 2b (4) |
| AST‐122 | 108 | 0b (0) | 3a (4) | 0b (0) |
Total mortality is indicated in brackets.
120 in UUC.
Table B.2.
Coccidiosis related and total mortality in ASTs
| Trial | Number of birds per treatment | UUC | IUC | IT40 |
|---|---|---|---|---|
| AST‐1 | 120 | 0 (0) | 0 (0) | 0 1 |
| AST‐2 | 120 | 0b (1) | 13a (15) | 0b (4) |
| AST‐3 | 120 | 0b 1 | 5a (10) | 0b 1 |
| AST‐4 | 120 | 0 1 | 0 (0) | 0 (2) |
| AST‐5 | 120 | 0 (12) | 0 1 | 0 (0) |
| AST‐6 | 120 | 0 (4) | 0 1 | 0 (3) |
| AST‐7 | 120 | 0b (0) | 17a (18) | 2b (4) |
| AST‐8 | 120 | 0 1 | 1 1 | 0 (0) |
| AST‐9 | 120 | 0 (0) | 0 (2) | 0 (4) |
| AST‐10 | 120 | 0 1 | 0 (0) | 0 (0) |
| AST‐11 | 120 | 0 1 | 0 (0) | 0 (0) |
| AST‐121 | 108 | 0b (0) | 3a (4) | 0b (0) |
120 in UUC.
Means in a row with different superscript letters are significantly different (p ≤ 0.05).
Table 28 shows the intestinal lesion scores of four ASTs conducted in 2017 on day 6 post‐inoculation (PI) (7 for AST‐4). No significant reduction of lesions scores by the Nilablend™ 200G treatment was seen in AST‐5. There was also no reduction of lesion scores due to E. acervulina in ASTs 2‐5 (not reported for AST‐1).
Table 29.
Intestinal lesion scores on day 6 PI (7 in AST‐4) of ASTsconducted in 2017
| Trial | Lesions due to | UUC | IUC | IT40 |
|---|---|---|---|---|
| AST‐1 | E. maxima | 0.3b | 1.4a | 0.4b |
| AST‐2 | E. maxima | 0.2c | 1.2a | 0.5b |
| E. tenella 1 | 0.0c/0.1c | 1.9a/1.5a | 1.0b/0.6b | |
| AST‐3 | E. maxima | 0.1c | 1.3a | 0.6b |
| E. tenella 1 | 0.1b/0.0c | 1.6a/1.6a | 1.2a/0.6b | |
| AST‐4 | E. maxima | 0.1b | 0.8a | 0.3b |
Means with different superscript letter in a row of the same trial (and of the same sex) are significantly different (p < 0.05).
Female/male.
Table 30 shows the intestinal lesion scores of six ASTs conducted in 2018 on day 7 post‐inoculation (PI) (6 for AST‐6 and AST‐9). No significant reduction of lesion scores by the Nilablend™ 200G treatment was seen in AST‐10.
Table 30.
Intestinal lesion scores on day 7 (6 in AST‐6 and AST‐9) of ASTs conducted in 2018
| Trial | Lesions due to | UUC | IUC | IT40 |
|---|---|---|---|---|
| AST‐6 | E. acervulina | 0.2c | 2.4a | 1.7b |
| E. maxima | 0.2b | 0.7a | 0.2b | |
| AST‐71 | E. acervulina | 0.1b/0.1c | 2.5a/2.1a | 2.2a/1.6b |
| E. tenella | 0.0c/0.0c | 2.5a/1.9a | 1.9b/1.2b | |
| AST‐8 | E. acervulina | 0.7c | 2.7a | 1.5b |
| E. tenella | 0.2b | 0.6a | 0.2b | |
| E. maxima | 0.5b | 1.0a | 0.6b | |
| AST‐9 | E. maxima | 0.4b | 2.5a | 0.5b |
| AST‐11 | E. acervulina | 0.3c | 2.8a | 1.4b |
| E. maxima | 0.4b | 0.9a | 0.5b | |
| AST‐12 | E. tenella | 0.1c | 1.2a | 0.6b |
Means with different superscript letter in a row of the same trial (and of the same sex) are significantly different (p < 0.05).
Female/male.
Data of oocyst excretion are summarised in Appendix B (Table B.3). Among the five studies conducted in 2017, four did not show a significant reduction of OPGs in the IT group compared to IUC. The highest number of oocysts was found for E. acervulina. A very small reduction of OPGs from E. maxima in AST‐4 was accompanied by an increase of E. acervulina oocysts in IT. AST‐5 showed a significant reduction in the number excreted oocysts for E. acervulina (and total oocyst).
Table B.3.
Oocyst excretion in ASTs
| E. acervulina | E. tenella | E. maxima | E. mitis | Total | ||
|---|---|---|---|---|---|---|
| AST‐1 | UUC | 3,384b | 3,418b | |||
| IUC | 137,256a | 140,904a | ||||
| IT30 | 46,322c | 47,686c | ||||
| IT40 | 96,255a | 99,976a | ||||
| IT50 | 124,293b | 128,116b | ||||
| AST‐2 | UUC | 12c | 3b | 0c | 13b | |
| IUC | 1,195,939a | 11,078a | 247ab | 1,239,013c | ||
| IT30 | 885,782ab | 3,705a | 464a | 921,509ac | ||
| IT40 | 893,462ab | 52,339a | 322a | 976,099ac | ||
| IT50 | 589,523b | 35,210a | 6bc | 640,231a | ||
| AST‐3 | UUC | 18b | 21b | 33b | ||
| IUC | 800,157a | 60,668a | 910,441a | |||
| IT30 | 619,716a | 100,703a | 748,296a | |||
| IT40 | 484,882a | 111,806a | 627,994a | |||
| IT50 | 568,572a | 99,451a | 683,172a | |||
| AST‐4 | UUC | 387c | 3b | 390 | ||
| IUC | 429,519a | 38,830c | 468,349 | |||
| IT30 | 657,033ab | 238a | 657,271 | |||
| IT40 | 942,563b | 11ab | 942,574 | |||
| AST‐5 | UUC | 111b | 0b | 3b | 113b | |
| IUC | 164,800c | 4,706c | 709a | 178,378c | ||
| IT30 | 31,052a | 97a | 82a | 34,076a | ||
| IT40 | 65,321a | 1,072ac | 642a | 71,253a | ||
| AST‐6 | UUC | 1b | 1b | |||
| IUC | 50,119a | 50,174a | ||||
| IT30 | 45,609a | 45,602a | ||||
| IT40 | 83,873a | 84,028a | ||||
| IT50 | 54,184a | 54,224a | ||||
| AST‐7 | UUC | 25c | 2c | 25c | ||
| IUC | 342,835ab | 64,158ab | 416,568ab | |||
| IT30 | 489,727a | 91,395a | 587,829a | |||
| IT40 | 257,007b | 22,101b | 299,845b | |||
| AST‐8 | UUC | 2,652a | 2a | 0a | 2,665a | |
| IUC | 110,289b | 1,019b | 26b | 133,241b | ||
| IT40 | 69,807b | 3a | 0a | 69,725b | ||
| AST‐9 | UUC | 75b | 0a | 75b | ||
| IUC | 12,206a | 50b | 14,423a | |||
| IT30 | 23,614a | 0a | 23,644a | |||
| IT40 | 17,266a | 0a | 17,773a | |||
| IT50 | 14,457a | 0a | 14,900a | |||
| AST‐101 | UUC | 21,120a/37a | 20 | 23,321a/37a | ||
| IUC | 168,560a/386,191b | 108 | 204,940a/387,180b | |||
| IT40 | 192,758a/251,930b | 700 | 252,760a/239,955b | |||
| AST‐111 | UUC | 149a/0a | 3a/0a | 0a | 668a/0a | |
| IUC | 265,548b/302,632b | 9,924b/38,195b | 68b | 294,738b/344,412b | ||
| IT40 | 59,027b/146,399b | 185c/32,345b | 14b | 61,182b/187,950b | ||
| AST‐12 | UUC | 4,220a | 160a | 0 | 30 | 4,917a |
| IUC | 313,589b | 93,317b | 31 | 157 | 448,734b | |
| IT40 | 360,542b | 84,597b | 5 | 854 | 473,608b |
Means with different superscript letter in a column in trial are significantly different (p < 0.05).
Female/male.
None of the seven ASTs conducted in 2018 showed a significant reduction of total oocyst excretion. Significantly lower numbers of OPG for E. tenella in AST‐8 and AST‐11 (males only) and for E. maxima in AST‐8 and AST‐9 were found the IT group compared to IUC, but they were too small to influence total oocyst excretion. These differences were not considered relevant.
The performance parameters feed intake, body weight gain and feed to gain ratio are listed in Appendix B (Table B.4). Four out of five ASTs conducted in 2017 showed a significant improvement of daily weight gain and three also of feed to gain ratio by the Nilablend™ 200G treatment. In all seven studies of the 2018 series, significantly better weight gain was observed and in four of them a better feed to gain ratio (males only in AST‐11).
Table B.4.
Zootechnical parameters in ASTs
| Average Daily Feed Intake (g/bird) | Average Daily Weight Gain (g/bird) | Feed to gain ratio | ||
|---|---|---|---|---|
| AST‐1 | UUC | 90a | 66a | 1.37 |
| IUC | 83b | 58b | 1.44 | |
| IT30 | 86b | 64a | 1.34 | |
| IT40 | 86ab | 65a | 1.32 | |
| IT50 | 87b | 65a | 1.35 | |
| AST‐21 | UUC | 91.6b/95.4a | 69.7c/70.7c | 1.34c |
| IUC | 75.8c/88.7b | 48.5d/56.9d | 1.58d | |
| IT30 | 81.3a/93.4ab | 55.7a/63.3a | 1.48a | |
| IT40 | 81.2a/92.9ab | 56.1ab/64.7ab | 1.45ab | |
| IT50 | 83.4a/95.5a | 59.8b/67.9bc | 1.42b | |
| AST‐3 | UUC | 94.7c | 73c | 1.30c |
| IUC | 85.6d | 56d | 1.53d | |
| IT30 | 90.0a | 63a | 1.43a | |
| IT40 | 90.2ab | 65ab | 1.38b | |
| IT50 | 92.2b | 67b | 1.41ab | |
| AST‐4 | UUC | 87b | 68b | 1.29b |
| IUC | 74c | 46c | 1.63c | |
| IT30 | 81a | 59a | 1.38a | |
| IT40 | 81a | 58a | 1.39a | |
| AST‐5 | UUC | 97b | 68 | 1.45b |
| IUC | 90a | 66 | 1.37a | |
| IT30 | 92a | 67 | 1.38a | |
| IT40 | 93ab | 67 | 1.38ab | |
| AST‐6 | UUC | 99 | 70b | 1.43b |
| IUC | 98 | 64c | 1.53c | |
| IT30 | 98 | 66ac | 1.48a | |
| IT40 | 98 | 67ab | 1.47a | |
| IT50 | 98 | 68ab | 1.47a | |
| AST‐7 | UUC | 83b | 60b | 1.41b |
| IUC | 75a | 47c | 1.62c | |
| IT30 | 77a | 51a | 1.52a | |
| IT40 | 77a | 53a | 1.46ab | |
| AST‐81 | UUC | 90 | 67a | 1.33a |
| IUC | 89 | 59b | 1.48b | |
| IT40 | 95 | 69a | 1.39c | |
| AST‐9 | UUC | 89c | 64a | 1.40 |
| IUC | 84b | 57b | 1.48 | |
| IT30 | 86ab | 63a | 1.40 | |
| IT40 | 87abc | 63a | 1.40 | |
| IT50 | 88ac | 64a | 1.41 | |
| AST‐10 | UUC | 88a | 67b | 1.33b |
| IUC | 82b | 57a | 1.44a | |
| IT40 | 89a | 65b | 1.38ab | |
| AST‐111 | UUC | 93 | 62a | 1.50ab/1.65a |
| IUC | 92 | 56b | 1.80b/1.49a | |
| IT40 | 95 | 63a | 1.44a/1.62a | |
| AST‐121 | UUC | 90a | 64a/67a | 1.34a/1.42a |
| IUC | 83b | 47b/53b | 1.77b/1.61a | |
| IT40 | 87ab | 51c/61c | 1.60b/1.50a |
Means with different superscript letter in a column in trial are significantly different (p < 0.05).
Female/male.
Synopsis of the AST studies
A summary on the effects of Nilablend® 200G at the lowest dose applied (40 mg lasalocid/kg + 40 mg nicarbazin/kg complete feed) in 12 ASTs conducted in 2017 and 2018 on the primary endpoints (reduction of mortality, intestinal lesion score and oocyst excretion) and on feed intake (FI) and body weight gain (WG) is provided in Tables 31 and 32. The coccidiostatic effect of the additive was tested against coccidia from different European countries.
Table 31.
Summary of the results of ASTs conducted in 2017
| AST | Mortality | ILS | Oocyst excretion | FI | WG | Oi | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E.a | E.t | E.m | E.a | E.t | E.m | E.mi | Tot | |||||
| 1 | – | nr | nr | + | – | nr | nr | nr | – | – | + | NL |
| 2 | + | – | + | + | – | – | nr | – | – | + | + | DE |
| 3 | + | – | + | + | – | – | nr | nr | – | + | + | FR |
| 4 | – | – | nr | + | – | nr | + | nr | – | + | + | BE |
| 5 | – | – | nr | – | + | – | nr | – | + | – | – | UK |
E.a: E. acervulina, E.t: E. tenella, E.m: E. maxima, E.mi: E. mitis, Tot: total, Oi: Origin of inoculum (country), FI: feed intake, WG: weight gain, nr: not reported.
+ significant improvement by treatment.
+ significant improvement in one gender only.
– no significant improvement by treatment.
Table 32.
Summary of the results of ASTs conducted in 2018
| AST | Mortality | ILS | Oocyst excretion | FI | WG | Oi | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E.a | E.t | E.m | E.a | E.t | E.m | E.mi | Tot | |||||
| 6 | – | + | nr | + | – | nr | nr | nr | – | – | + | IT |
| 7 | + | + | + | nr | – | – | nr | nr | – | – | + | DK |
| 8 | – | + | + | + | – | + | – | nr | – | – | + | BE |
| 9 | – | – | nr | + | – | nr | – | nr | – | – | + | UK |
| 10 | – | – | – | – | – | – | nr | nr | – | + | + | ES |
| 11 | – | + | nr | + | – | + | nr | – | – | – | + | AT |
| 12 | + | – | + | – | – | – | – | – | – | – | + | PT |
E.a: E. acervulina; E.t: E. tenella; E.m: E. maxima; E.mi: E. mitis; Tot: total; Oi: Origin of inoculum (country); FI: feed intake, WG: weight gain; nr: not reported.
+ significant improvement by treatment.
+ significant improvement in one gender only.
– no significant improvement by treatment.
Efficacy could convincingly be demonstrated in a total of seven out of 12 ASTs (AST‐2, AST‐3, AST‐7 and AST‐12 with reduced mortality and less severe intestinal lesions and AST‐6, AST‐8 and AST‐11 with lower ILS due to two Eimeria species). Another four ASTs showed lower ILS due to one Eimeria species only.
3.3.2.1. Conclusions on efficacy
Three floor pen trials demonstrate the coccidiostatic efficacy of Nilablend® 200G treatment in chickens for fattening when used at 40 mg lasalocid A sodium + 40 mg nicarbazin/kg complete feed. The efficacy of the same Nilablend® 200G feed concentration was also confirmed in 11 anticoccidial sensitivity tests made with recent inocula from nine different European countries.
3.4. Post‐market monitoring
Field monitoring of Eimeria spp. resistance chickens for fattening to lasalocid A sodium and nicarbazin should be undertaken, preferably during the latter part of the period of authorisation.
4. Conclusions
The additive consisting of lasalocid A sodium and nicarbazin (Nilablend™ 200G) is not safe for chickens for fattening at the proposed maximum use level of 50 mg lasalocid A sodium + 50 mg nicarbazin/kg complete feed.
Concurrent administration of Nilablend™ 200G (containing lasalocid A sodium) with tiamulin and certain other medicinal substances should be avoided. No information on the interactions of nicarbazin with feed materials, other approved additives or medicinal products have been provided. Lasalocid A sodium has antimicrobial activity against Gram‐positive bacterial species while many Enterobacteriaceae are naturally resistant. Induction of resistance and/or cross‐resistance was not observed in experimental conditions. No data were submitted on the microbiological safety of nicarbazin.
The toxicological package for lasalocid A sodium and nicarbazin identified NOAELs that could be the basis for setting health‐based guidance values (e.g. an acceptable daily intake (ADI)). The Panel concluded that a concern for the genotoxicity of nicarbazin in Nilablend™ 200G cannot be excluded and that clarification on the mechanism of action of the test items would be needed. Therefore, the FEEDAP Panel is not in the position to establish an ADI for DNC on which to base the assessment of consumer safety. The Panel cannot conclude on the safety of Nilablend™ 200G for the consumer and the proposal for MRLs and withdrawal time made by the applicant cannot be verified.
Nilablend™ 200G is considered toxic by inhalation, corrosive and irritant to eyes, slightly irritant to the skin but not a skin sensitiser. Inhalation exposure is considered a risk to persons handling the additive. Since the lack of genotoxic potential of nicarbazin has not been adequately demonstrated, it should be considered as an additional potential concern to users handling the additive.
The FEEDAP Panel cannot conclude on the safety of Nilablend™ 200G for the environment due to a possible risk for aquatic compartment (freshwater) for DNC.
Three floor pen trials demonstrate the coccidiostatic efficacy of Nilablend® 200G treatment in chickens for fattening when used at 40 mg lasalocid A sodium + 40 mg nicarbazin/kg complete feed. The efficacy of the same Nilablend® 200G feed concentration was confirmed in 11 anticoccidial sensitivity tests made with recent inocula from nine different European countries.
5. Documentation as provided to EFSA/Chronology
| Date | Event |
|---|---|
| 02/09/2019 | Dossier received by EFSA. Nilablend™ 200G (lasalocid A sodium and nicarbazin) for chickens for fattening. Submitted by Zoetis Belgium SA. |
| 11/09/2019 | Reception mandate from the European Commission |
| 29/10/2019 | Application validated by EFSA – Start of the scientific assessment |
| 20/12/2019 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issue: Characterisation of the additive |
| 14/01/2020 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 17/01/2020 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 29/01/2020 | Comments received from Member States |
| 29/01/2020 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: Characterisation of the additive, environmental safety, efficacy |
| 30/03/2020 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 08/04/2020 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: Characterisation of the additive |
| 04/05/2010 | Reception of spontaneous supplementary information. Characterisation and safety of the additive |
| 08/07/2020 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 19/10/2020 | Reception of spontaneous supplementary information. Characterisation of the additive |
| 21/10/2020 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: safety for the consumer |
| 07/01/2021 | Reception of supplementary information from the applicant – Scientific assessment re‐started |
| 13/01/2021 | Reception of spontaneous supplementary information. Metabolic and residue studies |
| 10/2/2021 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- ADFI
average daily feed intake
- ADG
average daily gain
- ADI
average daily intake
- AFC
EFSA Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food
- ANS
EFSA Scientific Panel on Additives and Nutrient Sources added to Food
- BW
body weight
- CAS
Chemical Abstracts Service
- CD
Commission Decision
- CDG
chemically defined group
- CEF
EFSA Scientific Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CFU
colony‐forming unit
- CG
chemical group
- CV
coefficient of variation
- DM
dry matter
- ECHA
European Chemicals Agency
- EMA
European Medicines Agency
- EURL
European Union Reference Laboratory
- FAO
Food Agricultural Organization
- FCR
feed conversion ratio
- FFAC
Feed Flavourings authorisation Consortium of FEFANA (EU Association of Specialty Feed Ingredients and their Mixtures)
- FGE
food group evaluation
- FLAVIS
The EU Flavour Information System
- FL‐no
FLAVIS number
- GC‐MS
gas chromatography‐mass spectrometry
- HACCP
hazard analysis and critical control points
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- LOD
limit of detection
- LOQ
limit of quantification
- Log Kow
logarithm of octanol‐water partition coefficient
- MCHC
mean corpuscular haemoglobin concentration
- MCV
mean corpuscular volume
- MIC
minimum inhibitory concentration
- MRL
maximum residue limit
- MSDI
maximised survey‐derived daily intake
- MW
molecular weight
- NOAEL
no observed adverse effect level
- NTP
National Toxicology Program
- RH
relative humidity
- SCAN
Scientific Committee on Animal Nutrition
- SCF
Scientific Committee on Food
- TTC
threshold of toxicological concern
- UF
uncertainty factor
- WHO
World Health Organization
Appendix A – Estimation of user exposure to lasalocid A sodium and nicarbazin from the additive Nilablend™ 200G including consideration of using a filter mask FF P2 or FF P3 as a preventative measure
1.
| Calculation | Identifier | Description | Amount | Source |
|---|---|---|---|---|
| a | Active substance in Nilablend™ 200G (each) (mg/g) | 100 | Technical dossier | |
| b | Dusting potential (g/m3) | 0.03 | Technical dossier | |
| a × b | c | Active substance in the air (each) (mg/m3) | 3 | |
| d | No of premixture batches prepared/working day | 10 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012) | |
| e | Time of exposure per production of one batch (s) | 20 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012) | |
| d × e | f | Total duration of daily exposure/worker (s) | 200 | |
| g | Uncertainty factor | 2 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012) | |
| f × g | h | Refined total duration of daily exposure/worker (s) | 400 | |
| h/3,600 | i | Refined total duration of daily exposure (h) | 0.11 | |
| j | Inhaled air per hour (m3) | 1.25 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012) | |
| j × i | k | Inhaled air during exposure (m3) | 0.14 | |
| c × k | l | Active substance inhaled during exposure per eight‐h working day (each) (mg) | 0.42 | |
| n/10 | o | Active substance inhaled per eight‐h working day (each) (mg) reduced by filter mask FF P2 (reduction factor 10) | 0.04 | |
| n/20 | p | Active substance inhaled per eight‐h working day (each) (mg) reduced by filter mask FF P3 (reduction factor 20) | 0.02 |
Appendix B – Efficacy
1.
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Methods of Analysis for lasalocid A sodium and nicarbazin (Nilablend™ 200G)
1.
In the current application, authorisation is sought for NILABLEND™ 200G, under Article 4(1) for the category ‘coccidiostats and histomonostats’, according to the classification system of Article 6 of Regulation (EC) No 1831/2003. Authorisation is sought for chickens for fattening.
NILABLEND™ 200G is a preparation containing as active substances 100 g/kg of lasalocid A sodium and 100 g/kg of nicarbazin. NILABLEND™ 200G is intended to be incorporated in feedingstuffs through premixtures for chickens for fattening at levels of lasalocid A sodium:nicarbazin from 40:40 to 50:50 mg/kg feedingstuffs. The Applicant proposed maximum residue limits (MRLs) in chicken tissues ranging from 60 to 300 μg/kg of fresh tissue for lasalocid and ranging from 4000 to 15000 μg/kg of fresh tissue for 4,4‐dinitrocarbanilide (DNC), which is one of the components of nicarbazin.
The proposed MRLs for nicarbazin (as DNC) are not covered by Commission Regulation (EC) No 37/2010; therefore, the corresponding methods of analysis are evaluated by the EURL.
The Applicant proposed two single‐laboratory validated and further verified methods based on high‐performance liquid chromatography coupled to spectrophotometric detection (HPLC‐DAD) for the quantification of lasalocid A sodium and nicarbazin in the feed additive. For the quantification of the active substances in premixtures and feedingstuffs, the Applicant applied the ring‐trial validated method AOAC 2008.01 (equivalent to the European Union method described in Regulation (EC) No 152/2009) for lasalocid A sodium and a single‐laboratory validated and further verified method based on the ring‐trial validated method EN 15782 for nicarbazin.
Furthermore, the EURL is aware of another ring‐trial validated LC/MS‐MS method for the determination of various coccidiostats including lasalocid A and nicarbazin in feedingstuffs that has been recently published as CEN standard (EN 17299).
For the quantification of DNC residues in chicken tissues, the Applicant submitted a single‐laboratory validated method based on liquid chromatography coupled to a triple quadrupole mass spectrometer (LC‐MS/MS) that does not fully comply with the confirmatory requirements set by Commission Decision 2002/657/EC. However, in the frame of a previous nicarbazin dossier, the EURL already evaluated and recommended a similar method (AOAC 2013.07) validated for muscle, kidney, skin/fat and liver that complies with the criteria of Commission Decision 2002/657/EC.
Based on the acceptable method performance characteristics available, the EURL recommends for official control i) the single‐laboratory validated and further verified methods based on HPLC‐DAD for the quantification of lasalocid A sodium and nicarbazin in the feed additive; ii) the European Union method described in Regulation (EC) No 152/2009 for the quantification of lasalocid A sodium in premixtures and feedingstuffs; iii) the ring‐trial validated method EN 15782 for the quantification of nicarbazin in premixtures and feedingstuffs; iv) the ring‐trial validated method EN 17299 for the quantification of nicarbazin in feedingstuffs and v) the AOAC 2013.07 method or any equivalent method complying with the requirements set by Commission Decision 2002/657/EC, to enforce the MRLs for nicarbazin (as DNC) in the target tissues.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Durjava MF, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Aquilina G, Bories G, Brantom P, Cocconcelli PS, Gropp J, Rychen G, Holczknecht O and Vettori MV, 2021. Scientific Opinion on the safety and efficacy of a feed additive consisting of lasalocid A sodium and nicarbazin (Nilablend™ 200G) for chickens for fattening (Zoetis Belgium SA). EFSA Journal 2021;19(3):6466, 57 pp. 10.2903/j.efsa.2021.6466
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00591
Panel members: Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Fašmon Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission. The full output has been shared with the European Commission, EU Member States and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Commission.
Acknowledgments: The Panel wishes to acknowledge the contribution of Mohan Raj, the FEEDAP Working Group on Environment – Antonio Finzio, Andreas Focks and Ivana Teodorovic – and the Working Groupon Toxicology – Kettil Svensson and Luca Tosti – to this opinion. The Panel also wishes to thank Montserrat Anguita, Jaume Galobart, López Galvez Gloria, Matteo Lorenzo Innocenti, Elisa Pettenati, Fabiola Pizzo and Jordi Tarrés‐Call for the support provided to this scientific output.
Adopted: 10 February 2021
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Zoetis Belgium SA, Rue Laid Burniat 1, 1348 Louvain‐La‐Neuve, Belgium.
Commission Regulation (EC) No 2037/2005 of 14 December 2005 amending the condition for authorisation of a feed additive belonging to the group of coccidiostats. OJ L 328 of 15.12.2005, p. 21.
Commission Regulation (EU) No 874/2010 of 5 October 2010 concerning the authorisation of lasalocid A sodium as a feed additive for turkeys up to 16 weeks (holder of authorisation Alpharma (Belgium) BVBA) and amending Regulation (EC) No 2430/1999. OJ L 263, 6.10.2010, p. 1.
Commission Implementing Regulation (EU) No 900/2011 of 7 September 2011 concerning the authorisation of lasalocid A sodium as a feed additive for pheasants, guinea fowl, quails and partridges other than laying birds (holder of authorisation Alpharma (Belgium) BVBA). OJ L 231, 8.9.2011, p. 15.
Commission Implementing Regulation (EU) No 1277/2014 of 1 December 2014 amending Regulation (EU) No 37/2010, as regards the substance ‘lasalocid’. OJ L 346, 1.12.2014, p. 23. This regulation modified the maximum residue limits (MRLs) for lasalocid A in poultry as follows: 60 μg/kg (muscle), 300 μg/kg (liver), 300 µg/kg (skin/fat), 150 μg/kg (kidney), 150 μg/kg (eggs).
Commission Regulation (EU) No 875/2010 of 5 October 2010 concerning the authorisation for 10 years of a feed additive in feedingstuffs. OJ L 263, 6.10.2010, p. 4.
Commission Regulation (EU) No 885/2010 of 7 October 2010 concerning the authorisation of the preparation of narasin and nicarbazin as a feed additive for chickens for fattening (holder of the authorisation Eli Lilly and Company Ltd) amending Regulation (EC) No 2430/1999. OJ L 265, 8.10.2010, p. 5.
Commission Implementing Regulation (EU) 2020/994 of 9 July 2020 concerning the authorisation of monensin and nicarbazin (Monimax) as a feed additive for turkeys for fattening, chickens for fattening and chickens reared for laying (holder of authorization Huvepharma NV). OJ L 221, 10.7.2020, p. 79.
FEED dossier reference: FAD‐2019‐0056.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep-fad-2019-0056-nilablend.pdf
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
■■■■■
Technical dossier/Section II/Annex_II_9.
Technical dossier/Section II/Annex_II_14_Conf_Certificate of deposition ATCC 31180.conf.
■■■■■
■■■■■
Technical dossier/Supplementary information July 2020/annex‐ii‐17‐2‐conf‐wgs‐amr‐virul‐screening.
■■■■■
■■■■■
Technical dossier/Section II/Annex_II_2.
Technical dossier/Section II/Annex_II_4.
■■■■■
Spontaneous submission/October 2020/annex‐ii‐analyse‐presence‐of‐dna‐strain.
Technical dossier/Section II/Annex_II_8.
Technical dossier/Spontaneous submission May 2020/Annex‐II‐6‐B.
Technical dossier/Section II/Annex_II_21 and Supplementary information March 2020_Annexes_Sect II.
Technical dossier/Section II/Annex_II_20.
Technical dossier/Section II/Annex_II_22.
Technical dossier/Section II/Annex_II_23.
MRLs established by Implementing Regulation (EU) No 1277/2014.
Technical dossier/Section III/Annex_III_2_1_1_9_A412N‐GB‐17‐210_Study Report.
Technical dossier/Supplementary information May 2020/Annex_III_2_1_2_B_A412N‐GB‐17‐215_Total Residue study lasalocid‐nicarbazin.
Technical dossier/Supplementary information May 2020/Annex_III_21_1_15_A412N‐US‐17‐216_ Study Report.
Technical dossier/Section III/Annex_III_2_1_1_6_A412N‐GB‐16‐198_Study Report.
Technical dossier/Spontaneous submission May 2020/Annex_III_2_1_2_A_A412N‐GB‐17‐207_Total Residue study nicarbazin.
Technical dossier/Section III/Annex_III_2_1_1_3_A412N‐GB‐16‐164_Study Report.
Technical dossier/Section III/Annex_3_2_1_1_13_A412N‐US‐16‐166_Study Report.
Technical dossier/Supplementary information January 2121/Annex a413n‐gb‐18‐226‐study‐report and a413n‐gb‐18‐226‐study‐report‐amendment.
Technical dossier/Section II/Annex II_37.
Technical dossier/Supplementary information November 2020/Annex A416Z‐US‐16‐181 Study Report.
Technical dossier/Section III/Annex_III_2_1_2_A416N‐GB‐16‐154_Study Report.
Technical dossier/Section III/Annex_III_2_1_2_A416N‐GB‐16‐153_Study Report.
Technical dossier/Section III/Annex_III_2_1_1_18_A491R‐US‐17‐501_Study Report.
Technical dossier/Section III/Annex_III_2_1_1_20_A490N‐US‐18‐575_Study Report.
Technical dossier/Spontaneous submission January 2021/a496r‐us‐18‐519‐study‐report‐a.
Technical dossier/Section III/Annex a461n‐us‐18‐962‐study‐report and spontaneous submission January 2021/a466r‐us‐18‐924‐study‐report‐amendment.
Technical dossier/Section III/Annex_III_1_1_study A042.
Considering that lasalocid sodium contains at least 80% of lasalocid A sodium, the quantity indicated as lasalocid sodium corresponds to the maximum proposed use level of lasalocid A sodium.
Analytical data showed some deviations, such as 20.2% CP in the starter and 22.2% CP in the grower diet.
AST (aspartate aminotransferase), ALT (alanine aminotransferase), LDH (lactate dehydrogenase), GGT (gamma‐glutamyl transpeptidase), AP (alkaline phosphatase), CK (creatine kinase), total protein, total cholesterol, glucose, calcium, phosphorus, magnesium, sodium, potassium, chloride, uric acid, amylase, albumin and globulin.
The following samples were collected and placed into formalin and processed to slides: Thyroid glands, skin (neck), parathyroid glands, adrenal glands, thymus, pancreas, Bursa of Fabricius, oviduct, uterus, bone and marrow (distal femur), testes, epididymides, ovary, spinal cord, crop, oesophagus, proventriculus, ventriculus, duodenum, jejunum, ileum, colon, caeca, eyes, lungs, coelomic cavity air sac, trachea, spleen, kidneys, liver, heart, skeletal muscle (breast), brain and pituitary and all lesions found at necropsy.
Technical dossier/Section III/Annex_III_1_3_CVP.
Technical dossier/Section III/Annex III.1.1 study A042, p 166.
Technical dossier/Section III/Annex III.1.1 study A042, p. 508.
Technical dossier/Section III/Annex_III_1_2_study A1264.
For haematology and clinical biochemistry.
RBC (red blood count), haematocrit, haemoglobin, MCH (mean corpuscular haemoglobin), MCV (mean corpuscular volume), MCHC (mean corpuscular haemoglobin concentration), RDW (red cell distribution width), thrombocyte check, WBC (white blood cell count) and differentials, heterophils, eosinophils, basophils, monocytes and lymphocytes.
AST (aspartate aminotransferase), ALT (alanine aminotransferase), LDH (lactate dehydrogenase), GGT (gamma‐glutamyl transpetidase), AP (alkaline phosphatase), CK (creatine kinase), total protein, total cholesterol, BUN (blood urea nitrogen), glucose, calcium, phosphorus, magnesium, sodium, potassium, chloride, uric acid, albumin and globulin.
The following 40‐day samples were collected, weighed, and placed into formalin: liver, heart, spleen, kidneys, and Bursa of Fabricius. The following samples were collected and placed into formalin without being weighed: crop, gizzard, small intestine, caecum, colon sciatic nerve, pancreas and skeletal muscle. The following samples were collected, placed into formalin and held at study site for potential evaluation, until direction from the sponsor was received: brain, lung, proventriculus, adrenals, thymus, thyroid, and testis. The 47‐day samples consisted of liver tissue only.
Technical dossier/Section III/Annex III.1.1 study A1264, p. 16.
Technical dossier/Section III/Annex_III_Safety_Lasalocid_literature_review_report.
Technical dossier/Section III/Annex_III_2_2_2_A2 × 1N‐GB‐17‐451_Study Report.
Technical dossier/Section III/Annex_III_2_2_2_A2 × 1N‐GB‐17‐452_Study Report.
Technical dossier/Section III/Annex_III_2_2_3_A291N‐GB‐17‐499_Study Report and Supplementary information November 2020/summary analysis ‐ toxicological studies.pdf
Technical dossier/Section III/Annexes A261R‐US‐16‐638, A261N‐US‐16‐705, A291W‐US‐15‐327, A291R‐US‐16‐413, A291W‐US‐15‐342, A291R‐US‐16‐371, A291N‐US‐16‐428.
Technical dossier/Section III/Annex_III_2_2_3_A291N‐US‐16‐407_Study Report.
Technical dossier/Section III/Annex_III_2_2_3_A261N‐US‐16‐739_Study Report.
Technical dossier/Section III/Annex_III_2_2_4_A291N‐US‐17‐458_Study Report.
Technical dossier/Section III/Annexes A291R‐US‐16‐426, A2J1R‐US‐16‐036, and A291R‐US‐16‐375.
Technical dossier/Section III/Annex_III_2_2_5_A291N‐US‐17‐457_Study Report.
Technical dossier/Section III/Annex_III_2_2_5_A2J1N‐US‐16‐038_Study Report.
Technical dossier/Section III/Annex_III_2_2_5_A291N‐US‐16‐436_Study Report.
Technical dossier/Section III/Annex_III_2_2_3_A291N‐US‐16‐417_Study Report.
Technical dossier/Section III/Annex_III_2_2_5_A291N‐US‐16‐428_Study Report.
Technical dossier/Section III/Annex_III_3_1_2_2_A291N‐US‐17‐485_Study Report.
Technical dossier/Section III/Annex_III_3_1_2_2_A2J1N‐US‐17‐040_Study Report.
Annex_III_3_1_2_3_A2J1N‐US‐17‐039‐ Study Report.
Technical dossier/Section III/Annex_III_3_1_2_4_A291N‐US‐17‐486_Study Report.
From lasalocid sodium produced by fermentation with a minimum specification of 80% lasalocid A sodium and from nicarbazin produced by chemical synthesis with a minimum specification of 67.4% DNC and 27.7% HDP.
OJ L 133, 22.5.2008, p. 1.
Technical dossier/Supplementary information March 2020/Reference 15.
Technical dossier/Supplementary information March 2020/Reference 37.
Technical dossier/Supplementary information March 2020/Reference 22.
Technical dossier/Supplementary information March 2020/Reference 34.
Technical dossier/Supplementary information March 2020/Reference 35.
Technical dossier/Supplementary information March 2020/Reference 16.
Technical dossier/Supplementary information March 2020/Reference 23.
Technical dossier/Supplementary information March 2020/Reference 42.
Technical dossier/Supplementary information March 2020/Reference 43.
Technical dossier/Supplementary information March 2020/Reference 46.
Technical dossier/Supplementary information March 2020/Reference 53.
Technical dossier/Supplementary information March 2020/Reference 54.
Technical dossier/Supplementary information March 2020/Reference 49.
Technical dossier/Supplementary information March 2020/Reference 50.
Technical dossier/Supplementary information March 2020/Reference 51.
Technical dossier/Supplementary information March 2020/Reference 56.
Technical dossier/Supplementary information March 2020/Reference 57.
Technical dossier/Supplementary information March 2020/Reference 59.
Technical dossier/Supplementary information March 2020/Reference 60.
Trial 1: Technical dossier/Section IV/Annex IV 01. Trial 2: Technical dossier/Section IV/Annex IV 02. Trial 3: Technical dossier/Section IV/Annex IV 03. Trial 4: Technical dossier/Section IV/Annex IV 04.
The inocula used in floor pen studies were tested for its virulence in dose‐titration studies. The dose selected in trial 1 was based on results obtained with a dose of 82,800 E. acervulina and 36,000 E. tenella which resulted in a mortality of 50%, a weight gain reduction of 38% and in mean intestinal lesion scores of 4.0 (E. tenella) at day 6 post‐inoculation (PI), 0.4 (E. acervulina) and 3.2 (E. tenella) at day 7 PI. The dose selected in trial 2 (See Table 21) resulted in a mortality of 87.5%, a weight gain reduction of 70% and in mean intestinal lesion scores of 1.5 (E. acervulina), 3.5 (E. tenella) at day 6 PI and 1.0 (E. acervulina), 3.0 (E. tenella) at day 7 PI. The dose selected in trial 3 (See Table 21) resulted in a mortality of 20%, a weight gain reduction of 47% and in mean intestinal lesion scores of 1.4 (E. acervulina), 3.4 (E. tenella) at day 6 PI and 1.3 (E. acervulina), 1.7 (E. tenella) at day 7 PI. The dose selected in trial 4 (See Table 21) resulted in mean intestinal lesion scores of 2.0 and 1.2 at days 6 and 7 PI, respectively, and in a weight gain reduction of 39 and 43%, respectively.
Technical dossier/Section IV/Annex IV.05‐06.
Technical dossier/Section IV/Annex IV.07‐11.
Technical dossier/Section IV/Anex IV.12‐18.
References
- ECHA (European Chemicals Agency), 2017. Guidance on information requirements and chemical safety assessment.Chapter R.11: PBT/vPvB assessment. Available online: https://echa.europa.eu/documents/10162/13632/information_requirements_r11_en.pdf
- EFSA (European Food Safety Authority), 2004a. Opinion of the Scientific Panel on additives and products or substances used in animal feed on the re‐evaluation of coccidiostat Avatec in accordance with article 9G of Council Directive 70/524/EEC. EFSA Journal 2004;2(5):53, 44 pp. 10.2903/j.efsa.2004.53 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2004b. Update of an opinion of the Scientific Panel on additives and products or substances used in animal feed on the re‐evaluation of coccidiostat Avatec in accordance with article 9G of Council Directive 70/524/EEC. EFSA Journal 2004;2(7):77, 45 pp. 10.2903/j.efsa.2004.77 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2004c. Opinion of the Scientific Panel on additives and products or substances used in animal feed (FEEDAP) on the efficacy and safety of the coccidiostat Koffogran. EFSA Journal 2004;2(3):16, 40 pp. 10.2903/j.efsa.2004.16 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Panel on additives and products or substances used in animal feed on the change of terms of the authorisation of “Avatec 15%” as a feed additive, regarding a new formulation (Avatec® 150G) in accordance with Regulation (EC) No 1831/2003. EFSA Journal 2005;3(8):258, 8 pp. 10.2903/j.efsa.2005.258 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Technical Guidance for assessing the safety of feed additives for the environment Prepared by the Panel on Additives and Products or Substances used in Animal Feed. EFSA Journal 2008;842, 28 pp. 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2010a. Scientific Opinion on the safety and efficacy of Avatec® 150G (lasalocid A sodium) for turkeys. EFSA Journal 2010;8(4):1575, 25 pp. 10.2903/j.efsa.2010.1575 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products used in Animal Feed), 2010b. Scientific Opinion on the safety and efficacy of Koffogran (nicarbazin) as a feed additive for chickens for fattening. EFSA Journal 2010;8(3):1551, 40 pp. 10.2903/j.efsa.2010.1551 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products used in Animal Feed), 2010c. Scientific Opinion on the safety and efficacy of Maxiban® G160 (narasin and nicarbazin) for chickens for fattening. EFSA Journal 2010;8(4):1574, 45 pp. 10.2903/j.efsa.2010.1574 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011. Scientific Opinion on the safety and efficacy of Avatec® 150G (lasalocid A sodium) for pheasants, partridges, quails and guinea‐fowl. EFSA Journal 2011;9(4):2116, 18 pp. 10.2903/j.efsa.2010.2116 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Kolar B, Kouba M, López Puente S, López‐Alonso M, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, John Wallace RJ, Wester P, Brantom P, Halle I, van Beelen P, Holczknecht O, Vettori MV and Gropp J, 2016. Scientific Opinion on the modification of the terms of the authorisation regarding the formulation of Maxiban® G160 (narasin and nicarbazin) for chickens for fattening. EFSA Journal 2016;14(11):4614, 7 pp. 10.2903/j.efsa.2016.4614 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Brantom P, Halle I, van Beelen P, Holczknecht O, Vettori MV and Gropp J, 2017a. Scientific Opinion on the safety and efficacy of Avatec® 150G (lasalocid A sodium) for chickens for fattening and chickens reared for laying, and modification of the terms of authorisation for chickens for fattening, chickens reared for laying, turkeys for fattening, minor avian species (pheasants, guinea fowl, quails and partridges) except laying birds. EFSA Journal 2017;15(8):4857, 32 pp. 10.2903/j.efsa.2017.4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Brantom P, Halle I, van Beelen P, Holczknecht O, Vettori MV and Gropp J, 2017b. Scientific Opinion on the safety and efficacy of Monimax® (monensin sodium and nicarbazin) for turkeys for fattening. EFSA Journal 2017;15(12):5094, 49 pp. 10.2903/j.efsa.2017.5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J and Innocenti ML, 2017c. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(10):5023, 12 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017d. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017e. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechova A, Petkova M, Ramos F, Sanz Y, Villa R, Woutersen R, Aquilina G, Bories G, Brantom P, Cocconcelli PS, Halle I, Kolar B, van Beelen P, Wester P, Holczknecht O, Vittoria Vettori M and Gropp J, 2018a. Scientific Opinion on the safety and efficacy of Monimax® (monensin sodium and nicarbazin) for chickens for fattening and chickens reared for laying. EFSA Journal 2018;16(11):5459, 34 pp. 10.2903/j.efsa.2018.5459 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Glandorf B, Herman L, Kärenlampi S, Aguilera J, Anguita M, Brozzi R and Galobart J, 2018b. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal 2018;16(3):5206, 24 pp. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2018c. Guidance on the assessment of the efficacy of feed additives. EFSA Journal 2018;16(5):5274, 25 pp. 10.2903/j.efsa.2018.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances usedin Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Bories G, Brantom P, Gropp J, Finizio A, Focks A, Teodorovic I, Holczknecht O, Tarrés‐Call J, Vettori MV and Kouba M, 2019a. Scientific Opinion on the modification of the terms of authorisation regarding the maximum inclusion level of Maxiban® G160 (narasin and nicarbazin) for chickens for fattening. EFSA Journal 2019;17(8):5786, 36 pp. 10.2903/j.efsa.2018.5786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Finizio A, Focks A, Teodorovic I, Tarrés‐Call J, Vettori MV and Azimonti G, 2019b. Scientific Opinion on the safety for the environment of Monimax® (monensin sodium and nicarbazin) for chickens for fattening, chickens reared for laying and for turkeys for fattening. EFSA Journal 2019;17(11):5888, 12 pp. 10.2903/j.efsa.2019.5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, Lopez‐Alonso M, Lopez Puente S, Marcon F, Mayo B, Pechov_a A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Gropp J, Rychen G, Holczknecht O and Vettori MV, 2020. Scientific Opinion on the safety and efficacy of Avatec® 150G (lasalocid A sodium) as a feed additive for chickens for fattening and chickens reared for laying. EFSA Journal 2020;18(8):6202, 17 pp. 10.2903/j.efsa.2020. 6202 [DOI] [Google Scholar]
- EMA (European Medicines Agency), 2015. Guideline on the assessment of persistent, bioaccumulative and toxic (PBT) or very persistent and very bioaccumulative (vPvB) substances in veterinary medicinal products. EMA/CVMP/ERA/52740/2012. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/09/WC500193826.pdf
- Erwin GS, Heikkinen J, Halimaa P and Haber CL, 2020. Streptomyces lasalocidi sp. nov. (formerly ‘Streptomyces lasaliensis’), an actinomycete isolated from soil which produces the polyether antibiotic lasalocid. International Journal of Systematic and Evolutionary Microbiology, 70, 3076–3083. [DOI] [PubMed] [Google Scholar]
- Hamid ME, 2011. Variable antibiotic susceptibility patterns among Streptomyces species causing actinomycetoma in man and animals. Annals of Clinical Microbiology and Antimicrobials, 10, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J and Reid WM, 1970. Anticoccidial drugs: lesion scoring techniques in battery and floor pen experiments with chickens. Experimental Parasitology, 28, 30–36. [DOI] [PubMed] [Google Scholar]


