Abstract
Research has been increasingly focusing on the selection of novel and effective biological control agents (BCAs) against soil-borne plant pathogens. The large-scale application of BCAs requires fast and robust screening methods for the evaluation of the efficacy of high numbers of candidates. In this context, the digital technologies can be applied not only for early disease detection but also for rapid performance analyses of BCAs. The present study investigates the ability of different Trichoderma spp. to contain the development of main baby-leaf vegetable pathogens and applies functional plant imaging to select the best performing antagonists against multiple pathosystems. Specifically, sixteen different Trichoderma spp. strains were characterized both in vivo and in vitro for their ability to contain R. solani, S. sclerotiorum and S. rolfsii development. All Trichoderma spp. showed, in vitro significant radial growth inhibition of the target phytopathogens. Furthermore, biocontrol trials were performed on wild rocket, green and red baby lettuces infected, respectively, with R. solani, S. sclerotiorum and S. rolfsii. The plant status was monitored by using hyperspectral imaging. Two strains, Tl35 and Ta56, belonging to T. longibrachiatum and T. atroviride species, significantly reduced disease incidence and severity (DI and DSI) in the three pathosystems. Vegetation indices, calculated on the hyperspectral data extracted from the images of plant-Trichoderma-pathogen interaction, proved to be suitable to refer about the plant health status. Four of them (OSAVI, SAVI, TSAVI and TVI) were found informative for all the pathosystems analyzed, resulting closely correlated to DSI according to significant changes in the spectral signatures among health, infected and bio-protected plants. Findings clearly indicate the possibility to promote sustainable disease management of crops by applying digital plant imaging as large-scale screening method of BCAs' effectiveness and precision biological control support.
Keywords: BCAs, Sclerotinia sclerotiorum, Sclerotium rolfsii, Rhizoctonia solani, Diplotaxis tenuifolia, Lactuca sativa, fresh-cutting vegetables, plant reflectance
Introduction
Baby leaf vegetables constitute the major ingredient of ready-to-eat salads, very appreciated worldwide by consumers looking for healthy diets rich in fibers and low in calories, with organoleptic and nutraceutical traits particularly enhanced in pigmented varieties. Currently, in Italy, which is among the top European producers of these crops, it is estimated that more than 4,500 hectares are devoted, both in tunnels and, marginally, in open field, to grow baby salads for the high convenience food chain (Morra et al., 2017). A rather large group of different leafy vegetable species are included under this appellation, although by far, wild rocket [Diplotaxis tenuifolia (L.) DC.] and baby lettuce (Lactuca sativa L. var. acephala) are the most extensively cultivated. Because of the intensive exploitation of soils, continuous cropping, cultivars susceptibility to pathogens and reduced use of synthetic fungicides, those crops are dramatically prone to several diseases occurring in the humid and temperate microclimate of the sprinkler-irrigated tunnels/fields (Caruso et al., 2018; Gilardi et al., 2018a,b; Gullino et al., 2019). The soil-borne fungi Rhizoctonia solani Kuhn, Sclerotinia sclerotiorum (Lib.) de Bary and Sclerotium rolfsii Sacc., belonging to the Phylum Basidiomycota, are parenchymatic, polyphagous, necrotrophic pathogens of different salad crops, causing huge economic losses and symptoms ranging from the simple rotting of the attacked organs to the damping-off. Their non-chemical counteraction is particularly requested under sustainable management systems pursuing the zero residues goal, while it is mandatory according to the organic farming rules (Giménez et al., 2019). To this scope, the integrated disease management people are exploring alternative approaches to synthetic fungicides, including the implementation of effective microbes able to control phytopathogenic attacks, referred as biological control agents (BCAs).
Soil microbiota represents a precious reservoir of biocontrol microorganisms to impact plant health, growth and productivity in agricultural applications. Several fungal species belonging to the genus Trichoderma (Ascomycota) are known to suppress soil-borne and foliar plant diseases directly by mechanisms against the host pathogen (competition for space and nutrients, antibiosis, and mycoparasitism) and indirectly by the induction of a resistance responses in the colonized plants (Howell, 2003). Because of their crucial role as antagonists, Trichoderma spp. are among the most effective and commercialized biological control agents, registered as Plant Protection Products to manage a broad-spectrum of plant pathogens (Sharma et al., 2019). A number of Trichoderma spp. antagonistic strains are sourced from several telluric environments carrying disease control-related functions, including suppressive composts, to gain increasing efficacy firstly due to the niche-competence shared with the targeted soil-borne pathogens (Wang et al., 2019). The selection of novel and effective BCAs requires fast and robust screening methods suitable to evaluate high numbers of candidates. In this context, digital technologies, such as remote sensing, could play a pivotal role not only for early disease detection but also for the rapid performance analyses of BCAs and in the prediction of the biocontrol efficacy.
Hyperspectral imaging is a non-destructive and powerful digital technology to directly identifying biochemical and physiological shifts occurring in plants in response to external stimuli, including pathological prodding (Thomas et al., 2018). It involves the pixel-by-pixel analysis of an image containing spatially distributed the reflectance spectrum captured in the visible (VIS, spectral range 400–700 nm) and near infrared (NIR 700–1,000 nm) regions as hypercube dataset resulting by the interaction of the canopy with the incident light (Liu H. et al., 2020). Several previous hyperspectral studies pointed up broad/narrow extracted band indices, called vegetation indices (VIs) that have been used to associate the spectral information to several crop characteristics (Thenkabail et al., 2000), including plant health (Xue and Su, 2017). For example, the best known one, Normalized Difference Vegetation Index (NDVI) that is predictive of the vegetative growth and the general plant status (Rouse et al., 1973), recently was also proposed to refer about the Vitis vinifera – Botrytis cinerea interaction (Pañitrur-De la Fuente et al., 2020). The sensitivity of hyperspectral VIs about disease grade of the canopy, was also proposed to automatically evaluate the performances of disease control methods as innovative functional application (Martins et al., 2018). In this view, hyperspectral imaging may additionally help the fine scouting of new effective microbial antagonists under selection by configuring a standard quantitative analytic method to follow biocontrol dynamics that can be usefully implemented in a perspective definition of precision biological control guidelines.
The aim of this work was to select new useful antagonistic strains of Trichoderma able to protect wild rocket and baby lettuce from deleterious soil-borne pathogens. R. solani and S. sclerotiorum infections are very diffuse among these cultivations while S. rolfsii is going emerging importance on baby-leaf because of its attitude to grow at high temperature regime, as under greenhouse. Additionally, computing the reflectance data from the canopy of the bio-treated plants, this study can lead to the identification of high-performing vegetative indices (VIs) functional to the large-scale evaluation of the biocontrol effectiveness and, furthermore, to discriminate between healthy and infected plants.
Materials and Methods
Isolation of Trichoderma Strains
The sixteen Trichoderma strains characterized here, were isolated from a high suppressive rocket and fennel-derived compost (Pane et al., 2020; Scotti et al., 2020) and stored in the fungal collection of CREA-Centro di ricerca Orticoltura e Florovivaismo (Pontecagnano Faiano, Italy CREA-OF). Isolates were subjected to monosporic culturing by serial ten-fold dilution. For the strain characterization, macroscopic features (medium pigmentation, colony color, colony edge shape, smell) were evaluated after 7 days of growth on potato dextrose agar (PDA, Condalab, Madrid, Spain) medium at 25°C. Microscopic parameters (conidium length, width and shape) were also measured under light microscopy at 40× magnification with the optical microscope (Nikon Eclipse 80i, Nikon, Melville, NY, USA) in 0.05% Tween® 20 considering n = 40 conidia. All the isolates were maintained on PDA at 4°C and sub-cultured weekly.
Identification of Trichoderma Strains
Isolates were grown in potato dextrose broth (PDB, Condalab, Madrid, Spain) on a rotary shaker at 120 rpm for 96 h at 25°C. Fresh mycelium was collected after vacuum filtration through No. 4 Whatman filter paper (Whatman Biosystems Ltd., Maidstone, UK), then frozen in liquid nitrogen, ground to a fine powder and immediately processed. Total genomic DNA was extracted from 100 mg of ground mycelium by using the PureLink® Plant Total DNA Purification Kit (Invitrogen™, ThermoFisher Scientific, Waltham, MA, USA) according to the manufacturer's protocol. PCR amplification of internal transcribed spacers and translation elongation factor 1α (TEF1) was performed in a Biorad C1000 Thermal Cycler (Bio-Rad, Hercules, CA) following PCR program: denaturation at 96°C for 2 min; 35 cycles of denaturation at 94°C for 30 s; annealing at 55°C for 30 s; extension at 68°C for 75 s; final extension at 68°C for 10 min. Primers ITS1 (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) were used to amplify a fragment (~0.6 kb) of rDNA including ITS1 and ITS2 and the 5.8S rDNA gene (White et al., 1990; Gardes and Bruns, 1993) while the 5′ portions of translation elongation factor 1α (~0.8kb) coding region and introns were amplified with primers TEF1-F (5′- ATGGGTAAGGARGACAAGAC- 3′) and TEF1-R (5′-GGARGTACCAGTSATCATGTT-3′), which prime within conserved exons (O'Donnell et al., 1998). Amplicons were separated by gel electrophoresis in 1% w/v agarose supplemented with SYBR Safe DNA Gel Stain (Invitrogen, Paisley, UK). Amplicon sizes were determined against a 100 bp DNA ladder (Invitrogen™, ThermoFisher Scientific, Waltham, MA, USA). PCR products were purified by PureLink™ PCR Purification Kit (Invitrogen™, ThermoFisher Scientific, Waltham, MA, USA) following the manufacturer's instructions, quantified with a NanoDrop™ system (NanoDrop Technologies Inc., Wilmington, DE, USA) and sent to Sanger sequencing.
Phylogenetic Reconstruction
Phylogenetic relationships of the 16 Trichoderma strains were investigated based on ITS and TEF1 sequences. DNA sequences were blasted against the NCBI GenBank database using default parameters and then aligned with the more related Trichoderma isolates by the Clustal W algorithm (Thompson et al., 1994) with MEGA7 software (Kumar et al., 2016). Multiple alignments parameters were gap penalty = 10 and gap length penalty = 10. The default parameters (Ktuple = 2, gap penalty = 5, window = 4, and diagonals saved = 4) were used for the pairwise alignment. Final alignment adjustments were made manually in order to remove artificial gaps, as reported by Ospina-Giraldo et al. (1999). The analysis was conducted on the two gene partial sequences separately. Aligned sequences were then concatamerized to a total length of 1,667 nucleotides. The evolutionary history was inferred using the maximum likelihood method. The evolutionary distances were computed using the Tamura-Nei model. The confidence of the branching was estimated by bootstrap (BP) analysis with 1,000 replications (1000 BP). T. atroviride sequences DAOM 233966, DAOM 231423, DAOM 233456, T. longibrachiatum sequences DAOM 231854, DAOM 232019 and DAOM 231850 and T. harzianum sequences DAOM 233458, DAOM 232032 and DAOM 232055, were used as references. All sequences were deposited in GenBank under the accession numbers reported in Table 1.
Table 1.
List of Trichoderma strains identified in this study with the GenBank accession numbers of the internal transcribed spacer (ITS) and translation elongation factor 1α (TEF1) sequences.
| Strains | ITS | TEF1 | Species |
|---|---|---|---|
| Ta100 | MW191738 | MW201694 | Trichoderma atroviride |
| Ta104 | MW191739 | MW201695 | Trichoderma atroviride |
| Ta104C | MW191740 | MW201689 | Trichoderma atroviride |
| Ta104S | MW191741 | MW201690 | Trichoderma atroviride |
| Ta105 | MW191742 | MW201692 | Trichoderma atroviride |
| Ta116 | MW191743 | MW201693 | Trichoderma atroviride |
| Ta117 | MW191744 | MW201691 | Trichoderma atroviride |
| Tl35 | MW191751 | MW201701 | Trichoderma longibrachiatum |
| Ta56 | MW191737 | MW201688 | Trichoderma atroviride |
| TaIC12 | MW191745 | MW246311 | Trichoderma atroviride |
| Tat11 | MW191747 | MW201697 | Trichoderma atroviride |
| Tat3C1 | MW191746 | MW201696 | Trichoderma atroviride |
| ThCB | MW191749 | MW201699 | Trichoderma harzianum |
| ThRP | MW191750 | MW201700 | Trichoderma harzianum |
| Th23 | MW191748 | MW201698 | Trichoderma harzianum |
| Tl41 | MW191752 | MW201702 | Trichoderma longibrachiatum |
In vitro Dual Confrontation Assay
The ability of the sixteen Trichoderma strains to contain the development of R. solani, S. sclerotiorum and S. rolfsii in vitro, was evaluated by the dual culture technique. These phytopathogenic fungi were stored in the fungal collection of CREA-OF, maintained on PDA slants. Mycelial plugs of 5-mm diameter, obtained from the periphery of 7-days old cultures of both pathogen and Trichoderma strains were placed simultaneously on the border of the plate (9 cm diameter), about 0.25 mm from the edges at opposite sides. The Petri dishes containing PDA medium inoculated only with the pathogen were used as reference controls. All plates were incubated at 25°C and the radial growth was recorded 7-days post-inoculation. The growth inhibition percentage was calculated by using the formula:
where C = pathogen radial growth in the control and T = pathogen radial growth of the in the dual culture.
In vivo Biocontrol Activity Assays
The biocontrol activity of Trichoderma strains was assessed in vivo against R. solani on wild rocket, S. sclerotiorum on green baby lettuce and S. rolfsii on red baby lettuce.
One L flasks containing 150 g of common millet seeds were saturated with a 0.1 × PDB (w/w) and autoclaved. Flasks were then inoculated with 15 plugs 5 mm diameter obtained from one-week-old plates of each pathogen maintained on PDA, and incubated for 21 days at 25°C. At the end of incubation, the inoculum was ground and added to sterilized peat soil at the final concentration of 1% (w/w) for R. solani and S. rolfsii, and 2% (w/w) for S. sclerotiorum, respectively, according to the pathogen virulence. In the uninfected pots, non-inoculated common millet prepared as described above, was added. Trichoderma spp. spore suspensions were obtained from one-week-old cultures maintained on PDA at 25°C. For each isolate, the conidia were harvested by washing the plates with sterilized water using a sterile brush. The suspension was filtered and collected in a 50 mL Falcon® tube (Falcon, USA). The spore suspension concentration was measured by a Burker chamber (Brand, Germany) and adjusted at 1 × 107 spore mL−1. Seeds of wild rocket cv. Tricia (Enza Zaden, Italy), green baby lettuce cv 166 (Sementi Dom Dotto, Italy) and red baby lettuce cv. Pamela (Maraldi, Italy) were sown in vermiculite-filled 500 mL bowls, germinated in the dark at 25°C and then maintained in a growth chamber at 22°C with a 12-h photoperiod. The irrigation was manually performed daily and a basal NPK mix liquid fertilization was applied twice a week. After 15 days, plants were transplanted in plastic pots (7 cm diameter and 100 mL volume capacity) filled with sterile peat, infected as described above. Each treatment consisted of three pots (replicates) containing 5 plants each for baby lettuces, and 10 plants per pot for rocket. After that, Trichoderma suspension treatments were applied by soil drenching reaching a final concentration of 1 × 106 spore mL−1. Untreated infected pots and healthy pots were used as reference controls.
Pot distribution was arranged randomly in the growth chamber at the same conditions described above. After 7-days incubation, each pot was assessed for hyperspectral images, disease incidence (DI%) and severity index (DSI). DI was calculated as the percentage of plants with disease symptoms on the total. Disease severity was assessed using a 1–3 scale adapted from Larkin and Honeycutt (2006): 0: no symptom; 1: foliar discoloration; 2: plant withering and visible lesion(s); 3: severe infection and plant dead.
DI% and DSI were calculated according to Yang et al. (2009). The experiment was performed twice.
Hyperspectral Imaging
Hyperspectral images were acquired by using the SPECIM IQ camera (Specim, Spectral Imaging Ltd., Oulu, Finland) working in the range of 400–1,000 nm on a total of 204 wavelengths with a spectral resolution of 4 nm. The camera carries a CMOS sensor with a spatial sampling of 512 pixels and an image resolution of 512 × 512 pixel. The pixel size is 17.58 × 17.58 μm. Reflectance value was calculated automatically by the camera software. The images were captured under natural light conditions (Irradiance range 800–1,000 W/m2). One image per replicate (pot) was acquired, each containing all conditions (treatments) analyzed. Relative reflectance of hyperspectral images was simultaneously computed by the camera software. White reference, dark frame and raw data, were acquired during the measurements. The equation applied for the computation of the raw reflectance was as follows:
where White is the white reference, t1 and t2 are integration times (used for a highly reflective white reference), and Dark represents a target with low reflectance.
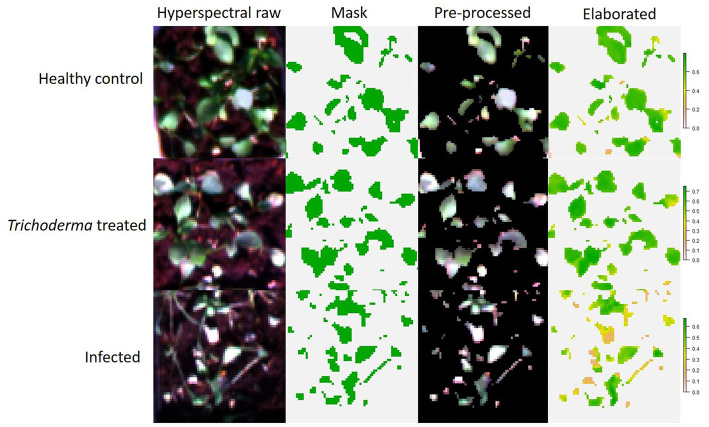
The elaboration of the hyperspectral images was carried out with the R software. Raster R package (Hijmans et al., 2015) was used to visualize and extract the hypercube dataset, successively elaborated into a typical spectral graphic. The unsupervisioned classification of the images was performed with Cluster R package to remove background once separated the objects “X” into “K” clusters. K-means clustering algorithm is a partitional or non-hierarchical clustering method (MacQueen, 1967; Anderberg, 1973), that here highlighted two clusters, background and plants (Figure 1). Then, the background cluster was deleted from the image, while the plant cluster was submitted to the extraction of the 46 hyperspectral VIs by imaging, averaging the pixel values for each replicate per treatment.
Figure 1.
Workflow of data processing in hyperspectral imaging.
Hyperspectral Vegetation Indices and Statistical Analysis
Measurements of the pathogen growth inhibitions in vitro, disease incidence and disease severity percentages, were subjected to the statistical analysis by GraphPad Prism Software. Ordinary one-way ANOVA was applied to test the effects of the Trichoderma strains on the assessed parameters. In all cases, the statistical analysis of variance was corrected for multiple comparisons by the Bonferroni hypothesis test, considering a p-value ≤ 0.05. Since experiment effect was not observed, data from the repeated experiments were pooled.
The same procedure was applied to evaluate the indices calculated on the hyperspectral dataset. Moreover, in order to select the most informative ones, they were analyzed, in relation to the observed disease severity in each pathosystem, by Multiple Variable analysis, applying the Pearson's correlation coefficient. The high-performing VIs that resulted commons to all the three host-pathogen target systems, were filtered on the base of a stringent statistical grid (p-value ≤ 0.05 and R2 > 0.5) and highlighted by using Venn diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/). The heatmap visualization and the hierarchical clustering analysis of the selected indices were obtained applying ClustVis online software (https://biit.cs.ut.ee/clustvis). Unit variance scaling was applied to rows and columns and they were clustered using correlation distance and average linkage. Furthermore, the Principal Component Analysis (PCA) of vegetative indices / disease index for each pathosystem was performed with the pca function of the R Factoextra package (Kassambara and Mundt, 2017). Data were log-normalized and disease severity index was converted to “factor” by grouping in classes according to the following 0–4 scale: 0 = 0 ≤ DSI ≤ 0.2; 1 = 0.21 ≤ DSI ≤ 0.4; 2 = 0.41 ≤ DSI ≤ 0.6; 3 = 0.61 ≤ DSI ≤ 0.8, 4 = 0.81 ≤ DSI ≤ 1. Then, lm function (R package) was applied to fit linear models.
Results
Colony and Conidium Morphological Characteristics
The morphological characterization of the sixteen Trichoderma isolates studied in this work was carried out based on the inoculated medium appearance and pigmentation, color and edge of colonies, culture smell, shape and size of the conidia. After 5-days incubation at 25°C, the growth and sporulation patterns of the Trichoderma isolates showed significant differences. During the growth, due to the release of secondary metabolites, medium pigmentation varied significantly among the Trichoderma isolates, ranging from colorless to bright yellow and yellow-brownish to amber. Some of them showed a profuse production of conidia with coloration ranging from white to dark green (Figure 2). Furthermore, microscopic observations allowed highlighting differences in terms of conidia size and shape. In fact, the conidia of Ta56, Ta117, Ta105, ThRP, and Tat3C1 isolates, showed spherical shape with length-to-width ratio around 1, while the conidia of all the remaining strains, resulted ellipsoidal with length-to-width ratio > 1. The morphological colony and conidium features are summarized in Table 2.
Figure 2.
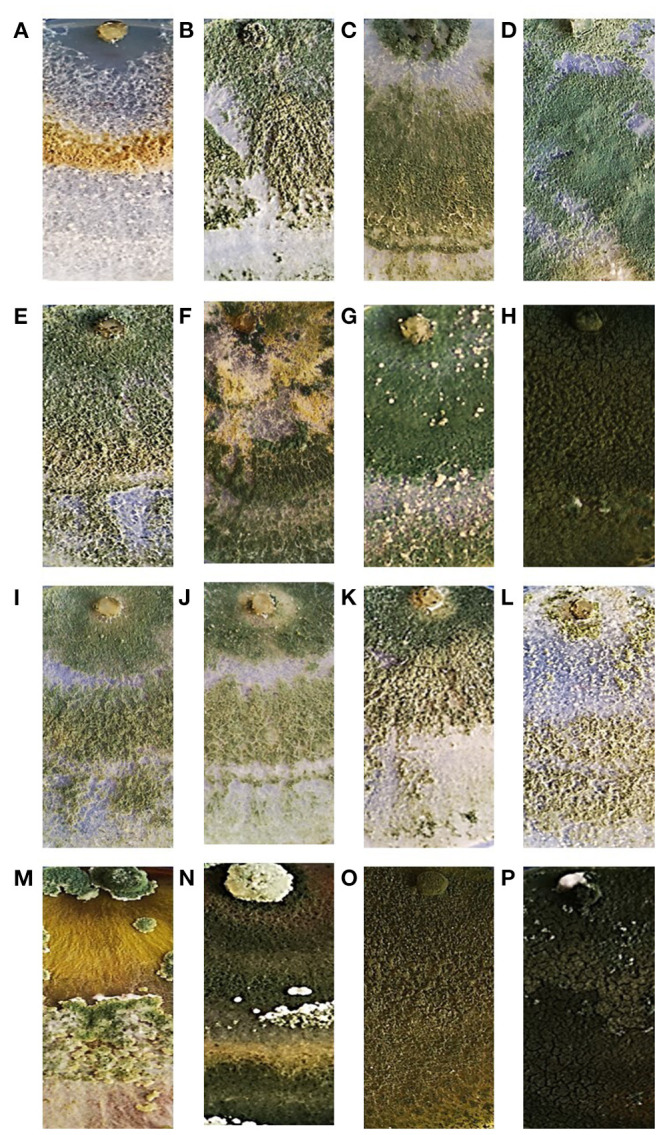
Colony appearance of the 16 Trichoderma strains used in this study after 7-days growth on PDA medium at 25°C. T. atroviride Ta100 (A); T. atroviride Ta104 (B); T. atroviride Ta104C (C); T. atroviride Ta104S (D); T. atroviride Ta105 (E); T. atroviride Ta116 (F); T. atroviride Ta117 (G); T. longibrachiatum Tl35 (H); T. atroviride Ta56 (I); T. atroviride TaIC12 (J); T. atroviride Tat11 (K); T. atroviride Tat3C1 (L); T. harzianum ThCB (M); T. harzianum ThRP (N); T. harzianum Th23 (O); T. longibrachiatum Tl41 (P).
Table 2.
Main morphological features of colonies (medium pigmentation, color, edge and smell) and conidia (length, width, length-to-width ratio and shape) of the Trichoderma strains used in this study.
| Strains | Colonies | Conidia | ||||||
|---|---|---|---|---|---|---|---|---|
| Medium pigmentation | Color | Edge | Smell | Length (μm) | Width (μm) | L/W ratio | Shape | |
| Ta100 | Uncolored | White | Smooth | - | 4.5 ± 0.3 | 4.09 ± 0.2 | 1.13 ± 0.20 | Ellipsoidal |
| Ta104 | Uncolored | Dull green to bluish green | Smooth | - | 3.9 ± 0.2 | 3.3 ± 0.3 | 1.22 ± 0.31 | Ellipsoidal |
| Ta104C | Uncolored | Dull green to bluish green | Smooth | - | 4.5 ± 0.3 | 4.1 ± 0.3 | 1.16 ± 0.22 | Ellipsoidal |
| Ta104S | Uncolored | Dark green | Smooth | - | 4.3 ± 0.3 | 3.77 ± 0.4 | 1.17 ± 0.16 | Ellipsoidal |
| Ta105 | Uncolored | Dull green to bluish green | Smooth | - | 3.8 ± 0.3 | 3.8 ± 0.3 | 1.02 ± 0.21 | Sphaeric |
| Ta116 | Uncolored | Yellow to dark green | Smooth | - | 4.5 ± 0.3 | 3.6 ± 0.4 | 1.27 ± 0.24 | Ellipsoidal |
| Ta117 | Uncolored | Dark green | Smooth | - | 4.3 ± 0.2 | 4.2 ± 0.3 | 1.04 ± 0.21 | Sphaeric |
| Tl 35 | Yellow-greenish | Dark green-white tufts | Smooth | - | 4.7 ± 0.5 | 3 ± 0.1 | 1.39 ± 0.28 | Ellipsoidal |
| Ta56 | Uncolored | Dull green to bluish green | Smooth | coconut | 4,0 ± 0.4 | 4,1 ± 0,3 | 1.00 ± 1.15 | Sphaeric |
| TaIC12 | Uncolored | Dull green to bluish green | Smooth | coconut | 4.6 ± 0.4 | 4.1 ± 0.3 | 1.17 ± 0.27 | Ellipsoidal |
| Tat11 | Uncolored | Dull green to bluish green | Smooth | coconut | 5.4 ± 0.3 | 4.8 ± 0.2 | 1.14 ± 0.24 | Ellipsoidal |
| Tat3C1 | Uncolored | White-green | Wavy | - | 4.2 ± 0.1 | 4 ± 0.2 | 1.09 ± 0.21 | Sphaeric |
| ThCB | Amber | Scattered in tufts, yellow green | Wavy | - | 3.7 ± 0.2 | 3.3 ± 0.2 | 1.14 ± 0.24 | Ellipsoidal |
| ThRP | Yellow-brownish | Dark green | Smooth | - | 4.9 ± 0.5 | 4.8 ± 0.5 | 1.05 ± 0.26 | Sphaeric |
| Th 23 | Bright yellow | Dark green-white tufts | Smooth | - | 4.1 ± 0.2 | 3 ± 0.4 | 1.41 ± 0.25 | Ellipsoidal |
| Tl41 | Yellow-orange | Dark green | Smooth | - | 4 ± 0.4 | 3.5 ± 0.3 | 1.20 ± 0.32 | Ellipsoidal |
Determination of Trichoderma Species
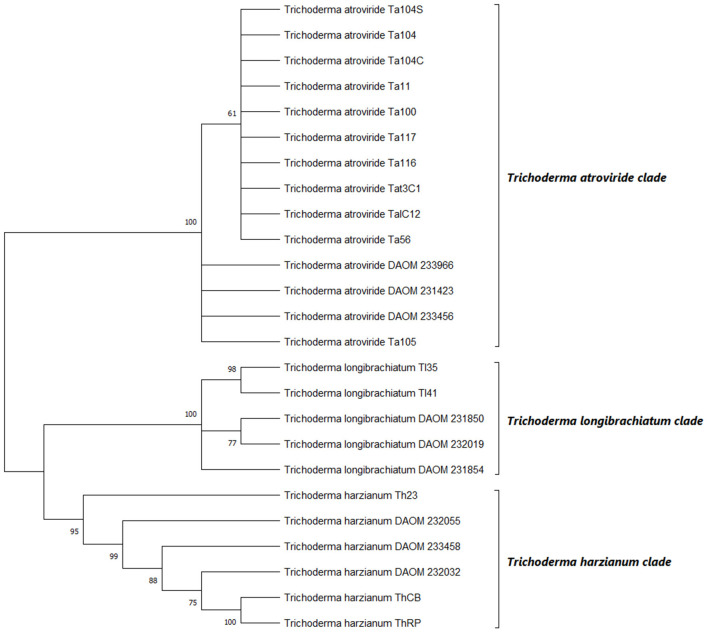
The multi-locus sequence analysis is suggested for a better distribution of Trichoderma spp. in a phylogenetic tree (Samuels et al., 2010). Therefore, in the present work, concatemers of the ITS-TEF1 genes were used to contract the phylogenetic tree inferred by neighbor-joining method, as reported by Ospina-Giraldo et al. (1999). rDNA region and partial translated elongation factor locus amplifications, yielded products of ~600 and 800 bp, respectively, as estimated by agarose gel electrophoresis. Loci were analyzed separately, aligned and manually adjusted. Sequences were then grouped in concatamers and subjected to the phylogenetic analysis. This analysis involved 26 nucleotide sequences with a total of 1,667 positions in the final dataset. Based on the bootstrap values, the 16 Trichoderma strains were arranged into three distinct groups, belonging to T. atroviride, T. longibrachiatum and T. harzianum species (Figure 3). The strains Ta100, Ta104, Ta104C, Ta104S, Ta105, Ta116, Ta117, Ta56, Tat11, TaIC12, and Tat3C1, clustered within the T. atroviride clade, resulting aligned with the reference strains (DAOM 233966, DAOM 231423, and DAOM 233456). On the other hand, Tl35 and Tl41 strains were identified as T. longibrachiatum, showing a strictly association with the reference isolates (DAOM 231854, DAOM 232019, and DAOM 231850), while the strains Th23, ThRP and ThCB clustered in the T. harzianum clade. These identifications resulted well-supported by bootstrap tests, with values >60%.
Figure 3.
Phylogenetic relationships among the 16 strains of Trichoderma spp. inferred by analysis of rDNA (ITS) and translation elongation factor 1-α (TEF1) concatemers. The evolutionary history was inferred using the maximum likelihood method. The optimal tree is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The evolutionary distances were computed by applying Tamura-Nei model. DAOM 233966. DAOM 231423 and DAOM 233456 (T. atroviride); DAOM 231854. DAOM 232019 and DAOM 231850 (T. longibrachiatum); DAOM 233458. DAOM 232032 and DAOM 232055 (T. harzianum) are the reference sequences.
In vitro Dual Challenge Assay
The dual culture assay was optimized to compare the inhibition activity of the 16 Trichoderma strains against the three soil-borne fungal pathogens. Since no significant differences were observed in the timing of growth among Trichoderma strains, S. sclerotiorum, R. solani, and S. rolfsii, the fungi were co-inoculated. As reported in Figure 4, all Trichoderma strains determined around 60% inhibition of S. sclerotiorum and R. solani radial growth. Only slight differences were observed among the different Trichoderma strains in inhibiting those phytopathogenic fungi. Furthermore, all the biocontrol strains, except Ta100 and Th23, reached the pathogen in 4–5 days and overgrew it in 9–10 days. On the other hand, most of the Trichoderma strains showed the ability to inhibit S. rolfsii radial growth up to 70%. Additionally, significant differences were observed among the different Trichoderma strains in containing this pathogen. In fact, a profuse overgrowth was observed for Ta116, ThRP, Ta105, Tat11, ThCB, Ta104C, Ta56, TaIC12, and Ta104S after 9 days, while Tl35 and Th23 resulted less effective in reducing the in vitro fungus development.
Figure 4.
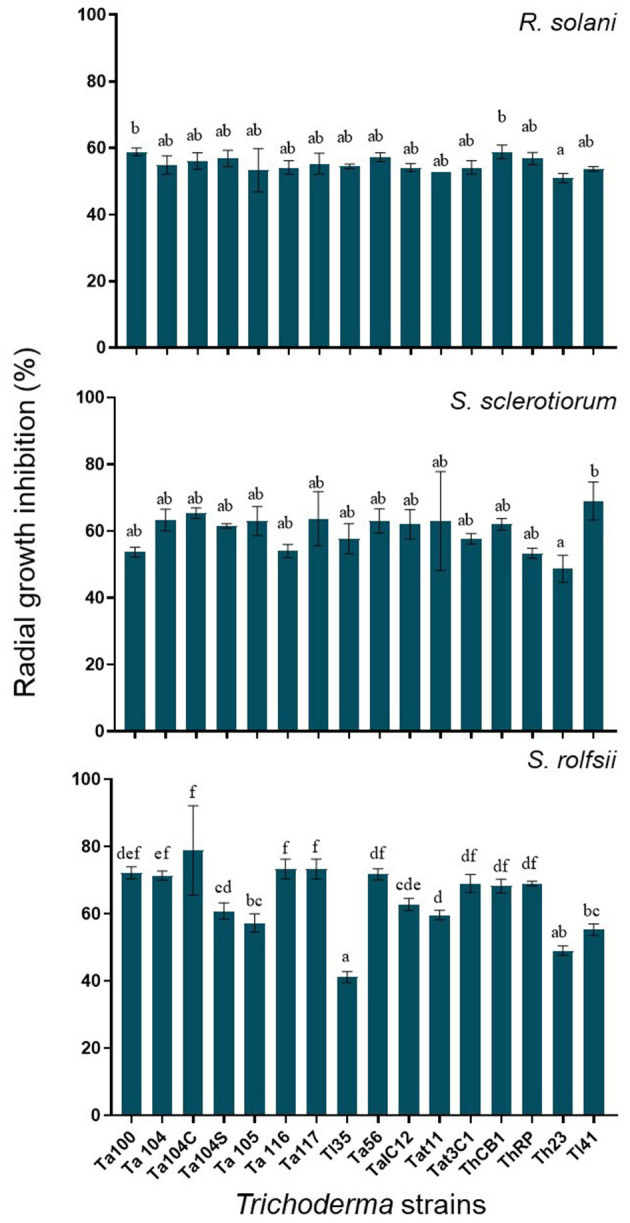
R. solani. S. sclerotiorum and S. rolfsii radial growth inhibition observed in dual culture assay. Values are expressed as inhibition percentage of pathogen radial growth. Each value is the average of three replicates. Bars with different letters are significantly different (p-value ≤ 0.05) according to ANOVA and Bonferroni correction test for multiple comparisons.
In vivo Biocontrol Activity
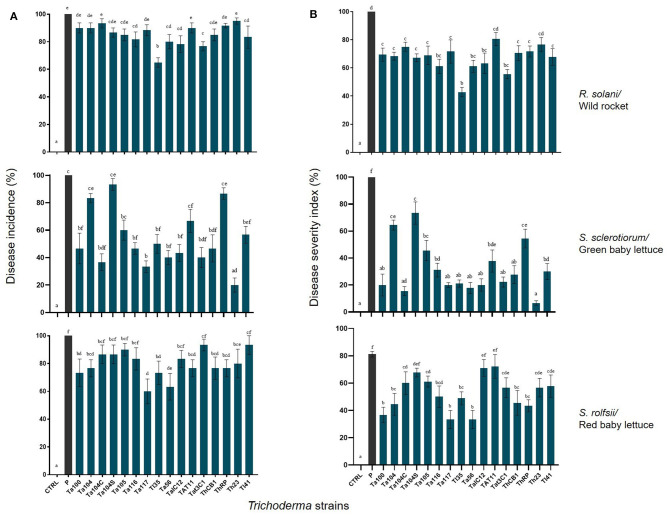
The ability of the different Trichoderma strains to protect plants was investigated by in vivo assays with R. solani on wild rocket, S. sclerotiorum on green baby lettuce and S. rolfsii on red baby lettuce. On all cases, disease incidence percentages (Figure 5 left) and disease severity index (Figure 5 right) were assessed. Overall, a significant Trichoderma treatment effect was found (p-value <0.001), as well as the interaction between factor Trichoderma strain × plant/pathogen system (p-value < 0.001). The application of Ta116, Tl35, Ta56, TaIC12, Tat3C1, and Tl41, on wild rocket significantly reduced the percentage of Rhizoctonia disease incidence detected 120 h post-inoculation, in comparison with the infected control. In fact, only the 60% of Tl35 treated plants showed disease symptoms; for all the other treatments, the disease incidence was around 80%. Interestingly, all Trichoderma strains, except for Tat11 and Th23, contained the severity of the disease: the bio-treated plants displayed mild disease symptoms or were almost healthy.
Figure 5.
Disease incidence (A) and disease severity (B) percentages observed on Trichoderma treated wild rocket. green baby lettuce and red baby lettuce infected with R. solani. S. sclerotiorum and S. rolfsii. respectively. compared with the infected untreated control. Bars with different letters are significantly different (p-value ≤ 0.05) according to ANOVA and Bonferroni correction test for multiple comparisons.
On the other hand, the BCAs reduced Sclerotinia disease incidence on green baby lettuce, excepted for Ta104, Ta104S and ThRP; the number of plants with symptoms was significantly lower than that observed in the infected control and a consistent reduction in the disease severity index was also observed.
Trichoderma harzianum Th23 resulted the best one in containing Sclerotinia disease development. The strains Ta100, Ta104, Ta117, Tl35, Ta56, Tat11, ThCB, ThRP, and Th23, were able to control S. rolfsii on red baby lettuce determining a meaningful reduction of disease incidence. Furthermore, all Trichoderma treated plants, excepted for Ta104S, TaIC12, and Tat11 interactions, showed a significant lower disease severity index than the infected control.
Hyperspectral Imaging
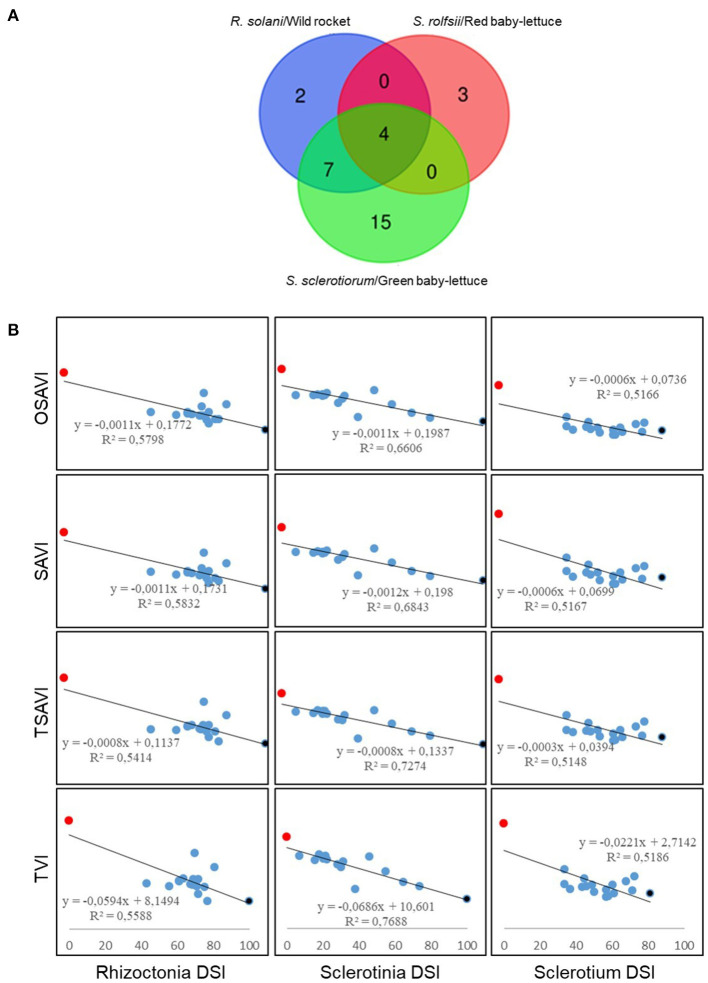
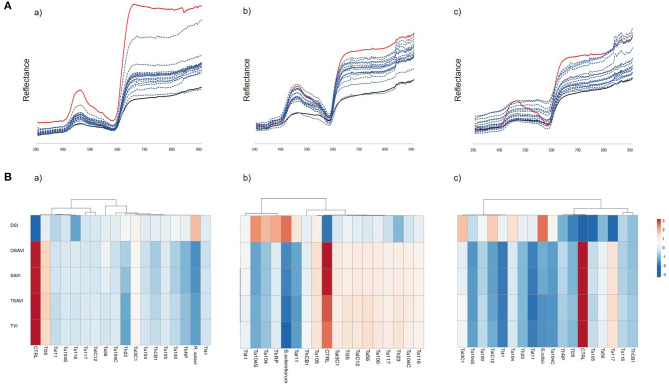
Plants infected with the three soil-borne pathogens and exposed to the biocontrol treatment with Trichoderma, were subjected to hyperspectral imaging analysis in order to capture the spectral changes that occurred during the plant-pathogen-antagonist relation. As reported in Figure 5A, out of the 46 analyzed hyperspectral indices, 13 significantly cross-correlated with Rhizoctonia disease on rocket, 26 with Sclerotinia drop on green baby lettuce and 7 with Sclerotium rotting on red baby lettuce. Interestingly, four indices, OSAVI, SAVI, TSAVI and TVI, resulted shared by the three assayed pathosystems. The Multiple Variable analysis showed the score of their negative cross-correlation with the disease severity index for each plant/pathogen systems, with samples distributing between the two extremes, full healthy and full diseased (Figure 6B), coherently with changes visualized in the spectral signatures among non-inoculated, infected and infected but bio-treated plants (Figure 7A). Hence, heatmap visualization of the VIs/DSI hierarchical clustering quickly identified the most effective biocontrol agents in relation to the specific pathosystem (Figure 7B).
Figure 6.
Selection of the significant (p-value ≤ 0.05 and R2 > 0.5) cross-correlated hyperspectral vegetation indices with disease severity index (DSI) for R. solani/wild rocket. S. sclerotiorum/green baby-lettuce and S. rolfsii/red baby-lettuce compatible interactions. (A) Venn diagram showing the number of the vegetative indices significantly cross-correlated with disease severity. common and not common to all the pathosystems. (B) Pearson's correlation between the common hyperspectral vegetative indices (OSAVI. SAVI. TSAVI and TVI) and DSIs. Non-inoculated healthy controls (red); infected plants (black); infected plants treated with Trichoderma strains (blue).
Figure 7.
(A) Spectral signatures of wild rocket (a). green baby lettuce (b) and red baby lettuce (c) assayed with R. solani. S. sclerotiorum and R. rolfsii. respectively. and treated with the Trichoderma strains (blue). compared to the non-inoculated (red) and infected (black) controls. (B) Hierarchical clustering of OSAVI. SAVI. TSAVI. TVI in relation to the observed disease severity index (DSI) in the systems R. solani-wild rocket (a). S. sclerotiorum-green baby-lettuce (b). and S. rolfsii-red baby-lettuce (c). Rows were centered and unit variance scaling was applied. Columns were clustered using correlation distance and average linkage. Analysis was performed by ClustVis software.
PCA analysis of VIs detected in the three different pathosystems showed their consistent ability to discriminate among different disease levels (Figure 8). Furthermore, OSAVI, SAVI and TSAVI resulted quite redundant, probably due to they differ only in the algorithm used for combining spectral data, while the distinct contribution in explaining the variance along PC1 (93.9%) was associated to TVI (Figure 8).
Figure 8.
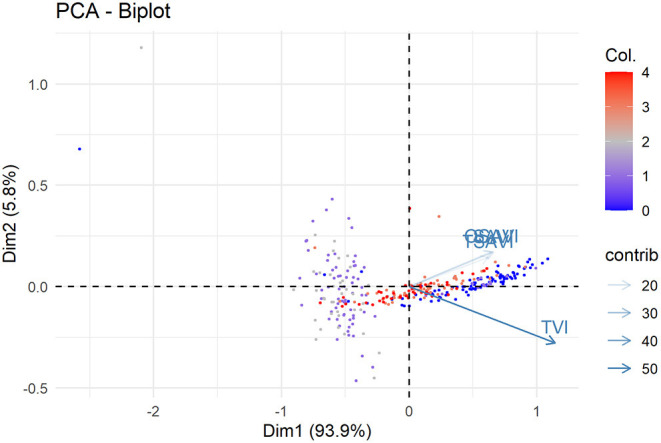
Principal component analysis of OSAVI. SAVI. TSAVI and TVI indices measured in wild rocket/R. solani. green baby lettuce/S. sclerotiorum and red baby lettuce/S. rolfsii pathosystems associated to the relative DSI. PC1 and PC2 explain the 93.9% and 5.8% of the total variability. respectively. Col indicate the distribution of DSI values in classes as follow: 0 = 0 ≤ DSI ≤ 0.2; 1 = 0.21 ≤ DSI ≤ 0.4; 2 = 0.41 ≤ DSI ≤ 0.6; 3 = 0.61 ≤ DSI ≤ 0.8. 4 = 0.81 ≤ DSI ≤ 1.
In order to fit a linear model, DSI data and selected indices were analyzed for multiple regression (Table 3). Based on the PCA results, SAVI indices (OSAVI, SAVI and TSAVI) computed together and TVI were submitted to linear regression analysis. OSAVI index was excluded since OSAVI:TVI interaction was found not significant in the resulting linear model. Results showed that F-statistic was highly significant (<3.5e-10) meaning that at least, one of the predictor is significantly related to the outcome variable. All the coefficients, including the interaction term coefficients, were statistically significant, suggesting that there is an interaction between the two predictor variables TSAVI + SAVI and TVI. On the other hand, these last are able to provide information about the biological observations although R-squared value was low. Thereby, statistical outputs corroborated the visualization by VIs images of the effects of Trichoderma strains on the disease symptom expressions over the cultivars.
Table 3.
Summary of statistical values associated to linear regression model for prediction of DSI using SAVI (OSAVI, SAVI and TSAVI) and TVI vegetative indices.
| Residuals | |||||
|---|---|---|---|---|---|
| Min | 1Q | Median | 3Q | Max | |
| −0.53359 | −0.24166 | −0.02817 | 0.22259 | 0.63078 | |
| Coefficients | |||||
| Estimate | Std. Error | tvalue | Pr(>|t|) | Signif. | |
| (Intercept) | 0.23261 | 0.05218 | 4.458 | 1.15E-05 | *** |
| TSAVI | −14.1965 | 4.65149 | −3.052 | 0.002465 | ** |
| SAVI | 10.91309 | 2.58815 | 4.217 | 3.24E-05 | *** |
| TVI | 0.06265 | 0.02652 | 2.362 | 0.018777 | * |
| TSAVI:TVI | 1.56296 | 0.61288 | 2.55 | 0.011235 | * |
| SAVI:TVI | −1.45497 | 0.4281 | −3.399 | 0.000763 | *** |
| Residual standard error: 0.2812 on 318 degrees of freedom. Multiple R-squared: 0.1526. Adjusted R-squared: 0.1393. F-statistic: 11.46 on 5 and 318 DF. p-value: 3.5e-10. | |||||
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001.
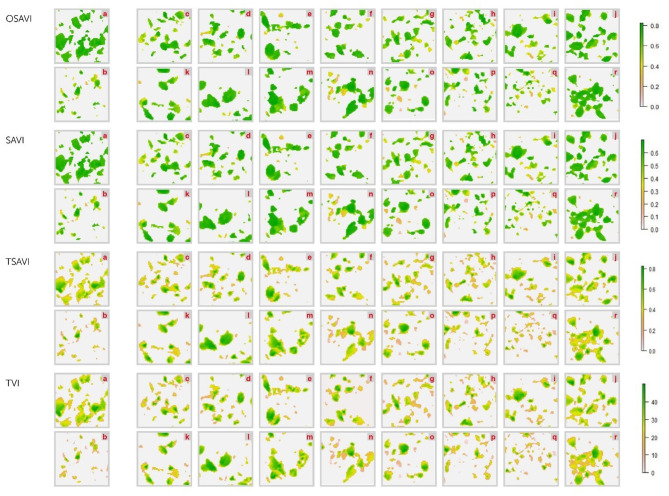
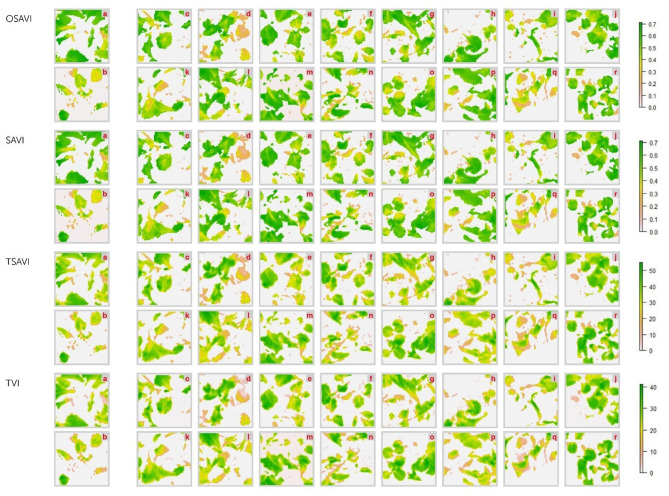
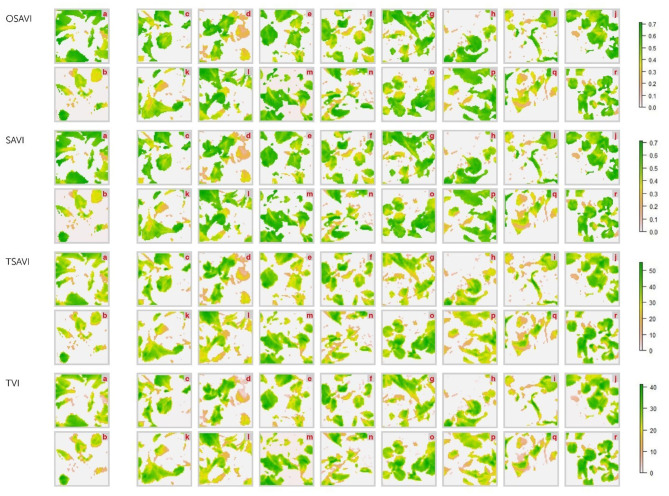
Differences between healthy and diseased controls resulted, actually, perceptible on OSAVI, SAVI, TSAVI and TVI images, and the BCA treated plants displayed intermediate collocations (Figures 9–11). However, the correlational analysis identified disease-specific indices as reported in Figure 6A. Indeed, MCARI and SRPI resulted effective to track the R. solani/wild rocket interaction, other 15 indices (ARI, CAR, LRDSI, msr705, NDVI, PRI, PSSRc, R705, RDVI, RGRcn, RVI, RSVI, SIPI, TCARI, VARI-Green) were found significantly correlated to the S. sclerotiorum infection degree of green baby lettuce, while LIC3, VOG2, VOG3 were found suitable for following the S. rolfsii/red baby lettuce interaction. A summarization of Pearson's analysis involving all the VIs, is reported in Table 4.
Figure 9.
Hyperspectral vegetative indices (OSAVI. SAVI. TSAVI and TVI) images of wild rocket plants infected with R. solani and treated with Trichoderma strains (c–r) compared to non-infected (a) and infected (b) controls. acquired by Specim IQ hyperspectral camera. The description of the vegetative indices features are reported in Table 2. The list of Trichoderma treatments is as follow: T. atroviride Ta100 (c); T. atroviride Ta104 (d); T. atroviride Ta104C (e); T. atroviride Ta104S (f); T. atroviride Ta105 (g); T. atroviride 116 (h); T. atroviride Ta117 (i); T. longibrachiatum Tl35 (j); T. atroviride Ta56 (k); T. atroviride TaIC12 (l); T. atroviride Tat11 (m); T. atroviride Tat3C1 (n); T. harzianum ThCB (o); T. harzianum ThRP (p); T. harzianum Th23 (q); T. longibrachiatum Tl41 (r).
Figure 11.
Hyperspectral vegetative indices (OSAVI. SAVI. TSAVI and TVI) images of red baby lettuce plants infected with S. rolfsii and treated with Trichoderma strains (c–r) compared to non-infected (a) and infected (b) controls. acquired by Specim IQ hyperspectral camera. The description of the vegetative indices features are reported in Table 2. The list of Trichoderma treatments is as follow: T. atroviride Ta100 (c); T. atroviride Ta104 (d); T. atroviride Ta104C (e); T. atroviride Ta104S (f); T. atroviride Ta105 (g); T. atroviride 116 (h); T. atroviride Ta117 (i); T. longibrachiatum Tl35 (j); T. atroviride Ta56 (k); T. atroviride TaIC12 (l); T. atroviride Tat11 (m); T. atroviride Tat3C1 (n); T. harzianum ThCB (o); T. harzianum ThRP (p); T. harzianum Th23 (q); T. longibrachiatum Tl41 (r).
Table 4.
List of the hyperspectral vegetation indices from the literature that were used in this study for estimating their informative degree about disease severity.
| Index | Formula | Target | References | Significance on R. solani/Wild rocket | Significance on S. sclerotiorum/Green baby lettuce | Significance on S. rolfsii/Red baby lettuce | |
|---|---|---|---|---|---|---|---|
| AI | Anthocyanin index (AI) | (R600-R699)/(R500-R599) | Anthocyanin | Gamon and Surfus, 1999 | ns | ns | ns |
| ARI | Anthocyanin Reflectance Index (ARI) | (1/R550)-(1/R700) | Carotenoids | Gitelson et al., 2001 | ns | **** | ns |
| CAR | Simple ratio 515/570 | R515/R570 | Carotenoids | Hernández-Clemente et al., 2012 | ns | *** | ns |
| DVI | Difference Vegetation Index | R782- R675 | Plant vitality. Chlorophyll | Tucker, 1979 | *** | *** | ns |
| FWBI1 | Floating-position water band index (FWBI1) | R900/min (R930-R980) | Water | Harris et al., 2006 | ns | ns | ns |
| FWBI2 | Floating-position water band index (FWBI2) | R920/min (R960-R1000) | Water | Harris et al., 2006 | ns | ns | ns |
| G | Simple Ratio 550/670 Greenness Index | R550/ R670 | Plant vitality. chlorophyll | Smith et al., 1995 | *** | **** | ns |
| GI | Greeness index | R539/ R682 | Plant vitality | Zarco-Tejada et al., 2005 | *** | **** | ns |
| Green- NDVI | Green Normalized Difference Vegetation Index | (R750- R550)/(R750+ R550) | Vegetation | Buschmann and Nagel, 1993 | ns | ns | ns |
| HVI | Hyperspectral Vegetation Index | R743/ R692 | Plant vitality | Gitelson et al., 1996 | ns | ns | ns |
| LIC3 | Simple Ratio 440/740 Lichtenthaler indices 3 | R440/R740 | Carotenoids | Lichtenthaler et al., 1996 | ns | ns | *** |
| LRDSI | Leaf rust disease severity index (LRDSI) | 6.9 × (R605/R455)−1.2 | Rust severity | Ashourloo et al., 2014b | ns | **** | ns |
| MCARI | Modified Chlorophyll Absorption in Reflectance Index | R712x(R712-R682)−0.2x(R712- R539)] / R682 | Chlorophyll | Daughtry, 2000 | *** | ns | ns |
| MCARI1 | Modified Chlorophyll Absorption in Reflectance Index 1 | 1.2 x [2.5 x (R800- R670)−1.3 x (R800- R550)] | Plant vitality. Chlorophyll | Haboudane, 2004 | ns | ns | ns |
| MSAVI | Modified Soil Adjusted Vegetation Index hyper | 0.5x[2 x R800+ 1 –√(2 x R800+1)x 2 – 8 x (R800- R670)] | Healthy vegetation. Reduces soil noise | Qi et al., 1994 | *** | *** | ns |
| mSR705 | Modified Simple Ratio 705 | (R750- R445)/(R705+ R445) | Vegetation | Wu et al., 2008 | ns | **** | ns |
| NDVI | Normalized Difference Vegetation Index (NDVI) | (R800-R670)/(R800+R670) | Plant vitality. Chlorophyll | Rouse et al., 1973 | ns | **** | ns |
| NPQI | Normalized Difference 415/435 Normalized Phaeophytinization Index | R415-R435/ R415+R435 | Vegetation. Chlorophyll. Stress | Barnes et al., 1992 | ns | ns | ns |
| OSAVI | Optimized Soil Adjusted Vegetation Index | (1+0.16) x(R800-R670) / (R800+ R670+ 0.16) | Relatively sparse vegetation | Rondeaux et al., 1996 | *** | **** | *** |
| PRI | Photochemical reflectance index (PRI) | (R531-R570)/(R531+R570) | Photochemical activity | Gamon et al., 1992 | ns | **** | ns |
| PRI515 | Normalized Difference 515/531 Photochemical Reflectance Index 515/531 | (R515- R531)/ (R515+R531) | Carotenoids | Hernández-Clemente et al., 2011 | *** | **** | ns |
| PSSRa | Pigment-specific simple ratio (PSSR) | R800/R680 | Chlorophyll a | Blackburn, 1998 | *** | *** | ns |
| PSSRb | Pigment-specific simple ratio (PSSR) | R800/R635 | Chlorophyll b | Blackburn, 1998 | ns | ns | ns |
| PSSRc | Pigment-specific simple ratio (PSSR) | R800/R470 | Carotenoids | Blackburn, 1998 | ns | *** | ns |
| PVI | Perpendicular Vegetation Index | (R800-0.2R670-0.6)/1.019 | Vegetation | Wiegand and Hatfield, 1988 | ns | ns | ns |
| R705/(R717+R491) | New vegetation index | R705/(R717+R491) | Vegetation. Chlorophyll | Tian et al., 2011 | ns | *** | ns |
| RARS | Simple Ratio 760/500 Ratio Analysis of Reflectance Spectra | R746/R513 | Carotenoids | Chappelle et al., 1992 | ns | ns | ns |
| RDVI | Renormalized Difference Vegetation Index | (R800-R670)/(R800+R670)∧0.5 | Plant vitality. Chlorophyll | Roujean and Breon, 1995 | ns | **** | ns |
| Red Edge NDVI | Red Edge Normalized Difference Vegetation Index | R750-R705/ R750+R705 | Vegetation | Gitelson and Merzlyak, 1994 | ns | ns | ns |
| RGRcn | Red green ratio chlorophyll content (RGRcn) | (R612+R660)/(R510+R560) | Chlorophyll content | Steddom et al., 2003 | ns | **** | ns |
| RVI | Ratio vegetation index (RVI) | R493/R678 | Plant vitality | Tilley et al., 2003 | ns | **** | ns |
| RVSI | Red-Edge Stress Vegetation Index | R800-R670/(R800+ R670)0.5 | Plant vitality | Roujean and Breon, 1995 | ns | **** | ns |
| SAVI | Soil Adjustment Vegetation Index | ((R782-R675)/(R782+R675+0.2)) (1.2) | Crop Parameters | Baret and Guyot, 1991 | *** | **** | *** |
| SIPI | Structure Intensive Pigment Index 1 | R800- R445/R800/R680 | Vegetation. Chlorophyll | Peñuelas et al., 1995 | ns | *** | ns |
| SRI | Simple Ratio Index | R800/R680 | Vegetation. Chlorophyll. Leaf area index | Jordan, 1969 | *** | *** | ns |
| SRPI | Simple Ratio Pigment Index (SRPI) | R430/R680 | Vegetation. Chlorophyll | Peñuelas et al., 1994 | *** | ns | ns |
| TCARI | Transformed Chlorophyll Absorbtion Ratio | 3 × [(R700-R670)−0.2 × (R700- R550) x R700/ R670)] | Plant vitality. Chlorophyll | Haboudane et al., 2002 | ns | **** | ns |
| TSAVI | Trasformed Soil Adjustment Vegetation Index | R782-R675 | Crop Parameters | Baret and Guyot, 1991 | *** | **** | *** |
| TVI | Triangular Vegetation Index | 0.5 × [120 x (R750-R550)−200 × (R670- R550)] | Plant vitality. Chlorophyll | Broge and Leblanc, 2001 | *** | **** | *** |
| VARIgreen | Visible atmospherically resistant index (VARIgreen) | (RGreen-RRed)/(RGreen+RRed-RBlue) | Vegetation | Gitelson et al., 2002 | ns | **** | ns |
| VOG1 | Simple Ratio 740/720 | R740/R720 | Plant vitality. Chlorophyll | Vogelmann et al., 1993 | ns | ns | ns |
| VOG2 | Simple Ratio 734/747 Vogelmann indices | (R734-R747)/(R715+ R726) | Plant vitality. Chlorophyll | Vogelmann et al., 1993 | ns | ns | *** |
| VOG3 | Simple Ratio 734/747 Vogelmann indices 3 | (R734-R747)/(R715+ R720) | Plant vitality. Chlorophyll | Vogelmann et al., 1993 | ns | ns | *** |
| WBI | Water band index (WBI) | R950/R900 | Water | Peñuelas et al., 1993 | ns | ns | ns |
| WI | Water Index | R900/R970 | Water | Peñuelas et al., 1997 | ns | ns | ns |
| ZTM | Zarco - Tejada - Miller Index | R750/R710 | Chlorophyll | Zarco-Tejada et al., 2001 | ns | ns | ns |
For each one the formula, functional target, literature reference and the significance level (ns: P > 0.05; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001) of the cross-correlation with disease severity index for the three different pathosystems were reported.
Figure 10.
Hyperspectral vegetative indices (OSAVI. SAVI. TSAVI and TVI) images of green baby-lettuce plants infected with S. sclerotiorum and treated with Trichoderma strains (c–r) compared to non-infected (a) and infected (b) controls. acquired by Specim IQ hyperspectral camera. The description of the vegetative indices features are reported in Table 2. The list of Trichoderma treatments is as follow: T. atroviride Ta100 (c); T. atroviride Ta104 (d); T. atroviride Ta104C (e); T. atroviride Ta104S (f); T. atroviride Ta105 (g); T. atroviride 116 (h); T. atroviride Ta117 (i); T. longibrachiatum Tl35 (j); T. atroviride Ta56 (k); T. atroviride TaIC12 (l); T. atroviride Tat11 (m); T. atroviride Tat3C1 (n); T. harzianum ThCB (o); T. harzianum ThRP (p); T. harzianum Th23 (q); T. longibrachiatum Tl41 (r).
Discussion
Trichoderma spp. include a plethora of isolates with biocontrol activity against phytopathogens (Kumar et al., 2017) that can also give additional benefits to the plants, such as increase the nutrient uptake, enhance the photosynthetic activity and stimulate different metabolic processes that positively affect yields and quality of the treated crops (El Enshasy et al., 2020). Recently, it has been shown that soil treatment with Trichoderma gave biostimulant effects on wild rocket and baby lettuce, ranging from the increase of leaf yield, fresh and dry weight, to the improvement of leaf nutritional status, resulting in a premium quality of the fresh-cuttings with higher lipophilic antioxidant activity and total ascorbic acid content (Fiorentino et al., 2018; Caruso et al., 2020; Di Mola et al., 2020; Rouphael et al., 2020). However, expressing the full biocontrol potential in these contexts, Trichoderma-based formulates can successfully integrate disease management protocols for producing baby leaf vegetables with high added value in terms of sustainability, decreasing the dependence on synthetic fungicides.
This study recruited sixteen new Trichoderma antagonistic strains assigned, on the base of the variations of the rRNA ITS and translation elongation factor 1-α gene partial sequences, to three different species, T. longibrachiatum, T. atroviride, and T. harzianum. Several stains of these species are well-known as BCAs of many pathogens affecting vegetables, including our targets (Bastakoti et al., 2017): they are proposed alone, being part of complex microbial consortia or activating suppressive organic amendments (Kareem et al., 2016; Wang et al., 2019; Chilosi et al., 2020). The macroscopic and microscopic examination of the selected strains showed interesting characters such as the profuse sporulation, the ability to secrete secondary metabolites in the medium changing its pigmentation and the capability of some to produce a volatile compound with the typical coconut-like aroma. This last specific character was detected in the strains Ta56, TaIC12, and Tat11 and could be putatively associated to the production of 6-pentyl-α-pyrone, a bioactive unsaturated δ-lactone with interesting properties involved in the microbial antagonism (Bonnarme et al., 1997; Serrano-Carreón et al., 2004; Longo and Sanromán, 2006; Ramos et al., 2008; Penha et al., 2012; Pascale et al., 2017). However, to clarify these aspects, further metabolomic investigations are necessary.
All the new identified antagonists significantly inhibited the mycelial growth of the pathogens in the dual culture assay. The main mechanism of control resulted to be the mycoparasitism, highlighted by the overgrowth of the BCAs onto the pathogen mycelia, observed already after 9–10 days of incubation. Mycoparasitism is one of the major weapons displayed by Trichoderma spp. against phytopathogens (Sachdev and Singh, 2020) allowing them to parasitize and kill the fungal host after the direct contact. During this intimate interaction, the beneficial fungus produces antibiotics and a huge array of cell degrading enzyme (protease, as β-glucanase, chitinase) necessary for the parasitism process (Steyaert et al., 2003).
In vivo biocontrol assays classified the Trichoderma BCA-candidates for the substantial ability to protect wild rocket, red and green baby lettuces from their most feared telluric fungal pathogens. Contrary to what was observed in in vitro assays, the in planta trials showed meaningful differences in biocontrol intensity among the strains in relation to the target pathosystem.
Specifically, Tl35, Ta56, Ta116, TaIC12, and Tat3C1 resulted the most effective strains in controlling Rhizoctonia damping-off of wild rocket, determining a significant reduction in terms of DSI (roughly 60%) compared with infected control under high disease pressure (100%). Rhizoctonia crown and root rot is a problematic disease of wild rocket for the ready-to-eat produces in the Italian cropping areas (Nicoletti et al., 2004). For their biological control, only the hyperparasite Clonostachys rosea has been noticed in literature (Nicoletti et al., 2007). Genetic resistance to this pathogen is not available yet (Pane et al., 2017), while wild rocket waste meals are proposed as amendments to promote the soil general suppressiveness providing antifungal molecules contained into the grounded plant tissues (Schlatter et al., 2017). Our results suggest that Trichoderma spp. can reduce the incidence and the severity of the disease and earn a chance as effective antagonist in Rhizoctonia damping-off management.
On the other hand, the biological control of Sclerotinia species, in particular S. sclerotiorum, has received increasing attention on adult lettuce inasmuch as chemical control of this pathogen is usually difficult, because the ascospores can infect any part of the head and sclerotia resist in the soil and can occur after prolonged wet periods (Patterson and Grogan, 1985; Elias et al., 2016; Subbarao et al., 2017). Therefore, the antagonists play a crucial role in the lettuce drop management because of their ability to parasitize the sclerotia in deep soil layers (Subbarao, 1998). As Sclerotinia drop biocontrol agents, Coniothyrium minitans Campbell, Clonostachys rosea (Schroers et al., 1999) and Trichoderma spp. resulted effective either in laboratory or in soil assays (Turner and Tribe, 1976; Phillips, 1989; Whipps and Budge, 1990; Jones et al., 2003, Bonini et al., 2020). Furthermore, against the compatible interaction between Sclerotinia spp. and adult lettuces, Trichoderma biocontrol agents have been reported to effectively reduce the seedlings drop (Elias et al., 2016), promote plant growth under infection (da Silva et al., 2019) and delay the symptoms appearance by emitting volatiles (da Silva et al., 2021). Accordingly, our results on baby lettuces demonstrated that Trichoderma strains Ta100, Ta104C, Ta117, Tl35, Ta56, TaIC12, Tat3C1, ThCB, and Th23, belonging to different species, can contain Sclerotinia drop disease, reaching a significant reduction of DSI (around 30%) compared to untreated control (DSI 100%).
Many previous studies reported the effectiveness of Trichoderma volatile compounds in inhibiting S. rolfsii growth (Hirpara et al., 2017; Marques et al., 2018; Sridharan et al., 2020). The culture filtrates with the highest antifungal effect against S. rolfsii were those of T. brevicompactum (Marques et al., 2018), T. harzianum (Saxena et al., 2014), T. viride (Darvin, 2013) and T. virens (Srinivasa and Devi, 2014). Wonglom et al. (2019) selected a Trichoderma strain with higher biocontrol properties against Sclerotium stem rot on red oak lettuce, due to its capability to produce volatile antifungal compounds (phenylethyl alcohol and epi-cubenol) and the cell wall degrading enzyme β-1,3-glucanase. Similarly, our results showed that Trichoderma strains Ta100, Ta104, Ta116, Ta117, Tl35, Ta56, ThCB1, and ThRP, highly suppressed lettuce Sclerotium rotting with a reduction of DSI around 50%, on average, compared to untreated infected control.
Interestingly, T. atroviride strain TA56 and T. longibrachiatum strain TA35 resulted to be multi-suppressive, namely highly effective in containing all the three diseases of the baby-leaf vegetables, demonstrating positive performances both in vitro and in vivo. The ability of these two BCAs to control the main disease of baby leaf make them promising candidates for a wide-spectrum application in preventive and/curative biological control practices in fresh-cut salad cropping, especially under soil sickness conditions.
Hyperspectral imaging was used here to objectively assess the biocontrol effectiveness of the comparing Trichoderma spp., interpreting the canopy reflectance response to the bio-treatments acquired by a hyperspectral sensor and summarized by VIs, thus, quantifying the functional effects. Four out of the 46 tried VIs, previously calibrated on plant physiological and structural shifts (Mishra et al., 2017), displayed significant (p-value < 0.05, highest coefficient of determination R2) positive relationships with plant health, as variably modulated by the biological control treatments contemporary in all the three target systems. The indices OSAVI, SAVI, TSAVI and TVI were able to highlight the most effective BCAs in controlling multiple soil-borne diseases of baby leaf vegetables. This result confirmed that selected indices can be applied as highly-informative tool for both BCA selections and disease monitoring in the presence of soil-borne pathogens generally associated to root and collar rot and, in advancing, leaf withering and plant death.
Therefore, the disease progression significantly affects the vegetation vitality and also the chlorophyll content. OSAVI, SAVI and TSAVI are soil adjusted vegetation indices, also defined as soil-line indices descriptive for sparse vegetation covering (Ren et al., 2018) as baby-leaf crops are. They have been used for grading wheat powdery mildew disease severity trough satellite-acquired scenes (Gröll et al., 2007; Feng et al., 2016; Ma et al., 2018). Recently, SAVI has been applied for the field estimation of the severity of cotton root rot caused by the fungus Phymatotrichopsis omnivora (Zhao et al., 2020), while OSAVI has been used to sense Fusarium Head Blight on wheat by computing Sentinel-2 multispectral data (Liu L. et al., 2020). Similarly to our findings, OSAVI has been found highly correlated with Rhizoctonia crown and root rot severity on sugar beet assessed with a non-imaging remote sensing approach (Reynolds et al., 2012). On the other hand, TVI is the triangular vegetation index associated to leaf chlorophyll content (Cui et al., 2019) and plant vitality (Broge and Leblanc, 2001). It has been calibrated for the leaf area index estimation (Xing et al., 2020) and is also known for describing spectral variations due to wheat leaf rust symptoms caused by Puccinia triticina (Ashourloo et al., 2014a,b).
To the best of our knowledge, this is the first study that retrieved hyperspectral VIs with high discriminatory capability for the biocontrol ability of Trichoderma against developing soil-borne diseases of leafy vegetables. Previously, Silva et al. (2018) have tried to apply a laser speckle based on a light signal at 632 nm to assess the efficacy of maize seed treatments with T. harzianum on the germination, vigor and sanitation of seedlings. Instead, Pishchik et al. (2016) have calculated VIs on VIS, RED (red-edge), NIR and MID (middle infrared) spectral information acquired with a field pulse photometer, to tentatively track the synergistic effect of the plant growth promoting bacteria, Bacillus subtilis and a humic fertilizer on lettuce plants quality and vitality.
The four indices of this study, each applying its own peculiar algorithm, work in the spectral range 550–800 nm, just on the border between VIS and NIR regions, suggesting that this part of the spectrum could be sensitive to the reflectance shifts occurring at canopy level during the plant-pathogen-antagonist interaction. Marín-Ortiz et al. (2020) have found in the VIS/NIR range 448–995 nm the distinctive spectral response of tomato to the Fusarium oxysporum infection that has been also associated to changes in the leaf concentration of chlorophyll and carotene. Similarly, the soil-borne pathogens studied in our systems could bring to the decline of chlorophyll and other pigments, as-well-as growth reduction conditioning the reflectance reaction.
As a matter of fact, decreases in chlorophyll content has been noticed in Rhizoctonia diseased carrot (Ahmad et al., 2019), in cucumber affected both by R. solani and S. rolfsii (Kotasthane et al., 2015) and in soybean attacked by S. sclerotiorum (Vitorino et al., 2020). On the contrary, Trichoderma can enhance the phothosynthetic performances of the colonized plants by increasing their chlorophyll content and, at the same time, determining an improvement of their general physiological status (Singh et al., 2013; Doley et al., 2014; Kotasthane et al., 2015) exerting an antagonistic action with respect to the pathogen in promoting the vitality of the plant. Therefore, according to these inferences the plant functional imaging as applied here may return valuable information about how the biocontrol agents is working.
Findings of the present study indicate the potential to boost the sustainability of disease management protocols trough high-performing hyperspectral VIs that can drive the biocontrol practices, such as, for example, the microbial augmentation, based on the early recognition of the worsening of the plant state and of the possible effectiveness reduction of the adopted plant protection strategy. Functional plant imaging can be used to track the plant progression under biocontrol effect using a restricted number of bands. The digital imaging has been proposed for the early diagnosis of plant diseases (Lowe et al., 2017), for the real-time field estimation of phytopathological conditions (Golhani et al., 2018) and to provide useful information for pest and disease control (Yao et al., 2011). Here, it helped to scout effective biological control agents against baby-leaf salad pathogens, demonstrating the potential to sense the biocontrol making on developing soil-borne diseases. The association between BCAs and hyperspectral imaging, concurring at reducing chemical pressure of fungicides on the environment and avoiding crop losses for uncontrolled pathogenic attacks, opens to the concept of precision biological control. The availability of digital tools for the automatized large-scale evaluation of biocontrol evolution will be useful both in field/greenhouse systems to rapidly assess the success of biological measures against phytopathogens as well as Susič et al. (2020) have recently pointed up for pest control.
Conclusions
The high-effective Trichoderma strains identified in this study are able for protecting baby-leaf vegetables from a wide-spectrum of soil-borne pathogens, such as R. solani, S. sclerotiorum, and R. rolfsii. Strains belonging to T. longibrachiatum, T. atroviride, and T. harzianum are suitable for large-scale preventive applications in greenhouses that host wild rocket and baby-lettuces in succession and/or in rotation and have a perspective to work in consortia since they sourced from a unique niche. The scenario of applying digital imaging as innovative scheme to boost biological control, from the high throughput screening of the microorganisms to their field application, is highlighted. OSAVI, SAVI, TSAVI, and TVI, that were found highly correlated to disease severity, are promising and informative hyperspectral VIs to track biological control activity against multiple soil-borne pathogens of baby leaf vegetables. In future studies, digital imaging will be able to integrate metabolomic linked to transcriptomic analyses, which, supported by machine learning processing, can contribute to further improve the accuracy of the forecasting models by imaging applied to the plant protection practices.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, MW191738; https://www.ncbi.nlm.nih.gov/genbank/, MW191739; https://www.ncbi.nlm.nih.gov/genbank/, MW191740; https://www.ncbi.nlm.nih.gov/genbank/, MW191741; https://www.ncbi.nlm.nih.gov/genbank/, MW191742; https://www.ncbi.nlm.nih.gov/genbank/, MW191743; https://www.ncbi.nlm.nih.gov/genbank/, MW191744; https://www.ncbi.nlm.nih.gov/genbank/, MW191751; https://www.ncbi.nlm.nih.gov/genbank/, MW191737; https://www.ncbi.nlm.nih.gov/genbank/, MW191745; https://www.ncbi.nlm.nih.gov/genbank/, MW191747; https://www.ncbi.nlm.nih.gov/genbank/, MW191746; https://www.ncbi.nlm.nih.gov/genbank/, MW191749; https://www.ncbi.nlm.nih.gov/genbank/, MW191750; https://www.ncbi.nlm.nih.gov/genbank/, MW191748; https://www.ncbi.nlm.nih.gov/genbank/, MW191752; https://www.ncbi.nlm.nih.gov/genbank/, MW201694; https://www.ncbi.nlm.nih.gov/genbank/, MW201695; https://www.ncbi.nlm.nih.gov/genbank/, MW201689; https://www.ncbi.nlm.nih.gov/genbank/, MW201690; https://www.ncbi.nlm.nih.gov/genbank/, MW201692; https://www.ncbi.nlm.nih.gov/genbank/, MW201693; https://www.ncbi.nlm.nih.gov/genbank/, MW201691; https://www.ncbi.nlm.nih.gov/genbank/, MW201701; https://www.ncbi.nlm.nih.gov/genbank/, MW201688; https://www.ncbi.nlm.nih.gov/genbank/, MW201697; https://www.ncbi.nlm.nih.gov/genbank/, MW201696; https://www.ncbi.nlm.nih.gov/genbank/, MW201699; https://www.ncbi.nlm.nih.gov/genbank/, MW201700; https://www.ncbi.nlm.nih.gov/genbank/, MW201698; https://www.ncbi.nlm.nih.gov/genbank/, MW201702; https://www.ncbi.nlm.nih.gov/genbank/, MW246311.
Author Contributions
GM and CP conceived and designed the study and wrote the initial manuscript. GM, NN, MC, and CP conducted the experiments. GM and NN analyzed data. CP assisted in data analysis and interpretation of results. MZ and TC reviewed and edited the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Diana Perrotti and Stefano Guarracino for the graphical support about the representation of hyperspectral images.
Footnotes
Funding. This research was funded by the Italian Ministry of Agriculture, Food and Forestry Policies (MiPAAF), project AgriDigit, sub-project Tecnologie digitali integrate per il rafforzamento sostenibile di produzioni e trasformazioni agroalimentari (AgroFiliere) (DM 36503.7305.2018 of 20/12/2018).
References
- Ahmad L., Siddiqui Z. A., Abd_Allah E. F. (2019). Effects of interaction of Meloidogyne incognita, Alternaria dauci and Rhizoctonia solani on the growth, chlorophyll, carotenoid and proline contents of carrot in three types of soil. Acta Agric. Scand. B Soil Plant Sci. 69, 1–8. 10.1080/09064710.2019.1568541 [DOI] [Google Scholar]
- Anderberg M. R. (1973). Cluster Analysis for Applications. New York, NY; London: Academic Press. [Google Scholar]
- Ashourloo D., Mobasheri M., Huete A. (2014a). Evaluating the effect of different wheat rust disease symptoms on vegetation indices using hyperspectral measurements. Remote Sens. 6, 5107–5123. 10.3390/rs6065107 [DOI] [Google Scholar]
- Ashourloo D., Mobasheri M. R., Huete A. (2014b). Developing two spectral disease indices for detection of wheat leaf rust (Puccinia triticina). Remote Sens. 6, 4723–4740. 10.3390/rs6064723 [DOI] [Google Scholar]
- Baret F., Guyot G. (1991). Potentials and limits of vegetation indices for LAI and APAR assessment. Remote Sens. Environ. 35, 161–173. 10.1016/0034-4257(91)90009-U [DOI] [Google Scholar]
- Barnes J. D., Balaguer L., Manrique E., Elvira S., Davison A. W. (1992). A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 32, 85–100. 10.1016/0098-8472(92)90034-Y [DOI] [Google Scholar]
- Bastakoti S., Belbase S., Manandhar S., Arjyal C. (2017). Trichoderma species as biocontrol agent against soil borne fungal pathogens. Nepal J. Biotechnol. 5:39. 10.3126/njb.v5i1.1849231372559 [DOI] [Google Scholar]
- Blackburn G. A. (1998). Quantifying chlorophylls and caroteniods at leaf and canopy scales. Remote Sens. Environ. 66, 273–285. 10.1016/S0034-4257(98)00059-5 [DOI] [Google Scholar]
- Bonini P., Rouphael Y., Cardarelli M., Ceccarelli A. V., Colla G. (2020). Effectiveness of Trichoderma application through drip-irrigation to reduce Sclerotinia disease incidence and improve the growth performance of greenhouse lettuce. Acta Hortic. 1268, 199–204. 10.17660/ActaHortic.2020.1268.26 [DOI] [Google Scholar]
- Bonnarme P., Djian A., Latrasse A., Féron G., Giniès C., Durand A., et al. (1997). Production of 6-pentyl-α-pyrone by Trichoderma sp. from vegetable oils. J. Biotechnol. 56, 143–150. 10.1016/S0168-1656(97)00108-9 [DOI] [Google Scholar]
- Broge N. H., Leblanc E. (2001). Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 76, 156–172. 10.1016/S0034-4257(00)00197-8 [DOI] [Google Scholar]
- Buschmann C., Nagel E. (1993). In vivo spectroscopy and internal optics of leaves as basis for remote sensing of vegetation. Int. J. Remote Sens. 14, 711–722. 10.1080/01431169308904370 [DOI] [Google Scholar]
- Caruso G., El-Nakhel C., Rouphael Y., Comite E., Lombardi N., Cuciniello A., et al. (2020). Diplotaxis tenuifolia (L.) DC. Yield and quality as influenced by cropping season, protein hydrolysates, and trichoderma applications. Plants 9:697. 10.3390/plants9060697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Parrella G., Giorgini M., Nicoletti R. (2018). Crop systems, quality and protection of Diplotaxis tenuifolia. Agriculture 8:55. 10.3390/agriculture8040055 [DOI] [Google Scholar]
- Chappelle E. W., Kim M. S., McMurtrey J. E. (1992). Ratio analysis of reflectance spectra (RARS): an algorithm for the remote estimation of the concentrations of chlorophyll a, chlorophyll b, and carotenoids in soybean leaves. Remote Sens. Environ. 39, 239–247. 10.1016/0034-4257(92)90089-3 [DOI] [Google Scholar]
- Chilosi G., Aleandri M. P., Luccioli E., Stazi S. R., Marabottini R., Morales-Rodríguez C., et al. (2020). Suppression of soil-borne plant pathogens in growing media amended with espresso spent coffee grounds as a carrier of Trichoderma spp. Sci. Hortic. 259:108666. 10.1016/j.scienta.2019.108666 [DOI] [Google Scholar]
- Cui B., Zhao Q., Huang W., Song X., Ye H., Zhou X. (2019). A new integrated vegetation index for the estimation of winter wheat leaf chlorophyll content. Remote Sens. 11:974. 10.3390/rs11080974 [DOI] [Google Scholar]
- da Silva G. B. P., Heckler L. I., Durigon M., dos Santos R. F., Lovato M., Finger G., et al. (2019). Biological control of white mold (Sclerotinia sclerotiorum) in lettuce using Brazilian Trichoderma spp. strains. Aust. J. Crop Sci. 13, 803–809. 10.21475/ajcs.19.13.06.p1214 [DOI] [Google Scholar]
- da Silva L. R., Valadares-Inglis M. C., Peixoto G. H. S., Gonçalves deLuccas B. E., CostaMuniz P. H. P., Martins Magalhães D., et al. (2021). Volatile organic compounds emitted by Trichoderma azevedoi promote the growth of lettuce plants and delay the symptoms of white mold. Biol. Control. 152:104447. 10.1016/j.biocontrol.2020.104447 [DOI] [Google Scholar]
- Darvin G. (2013). Effect of plant extracts on radial growth of Sclerotium rolfsii Sacc. causing stem rot of groundnut. Int. J. Appl. Biol. Pharm. Technol. 4, 69–73. [Google Scholar]
- Daughtry C. (2000). Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 74, 229–239. 10.1016/S0034-4257(00)00113-9 [DOI] [Google Scholar]
- Di Mola I., Ottaiano L., Cozzolino E., Senatore M., Sacco A., El-Nakhel C., et al. (2020). Trichoderma spp. and mulching films differentially boost qualitative and quantitative aspects of greenhouse lettuce under diverse N conditions. Horticulturae 6:55. 10.3390/horticulturae6030055 [DOI] [Google Scholar]
- Doley K., Dudhane M., Borde M., Jite P. (2014). Effects of Glomus fasciculatum and Trichoderma asperelloides in roots of groundnut (Cv. Western-51) against pathogen Sclerotium rolfsii. Int. J. Phytopathol. 3, 89–100. 10.33687/phytopath.003.02.0809 [DOI] [Google Scholar]
- El Enshasy H. A., Ambehabati K. K., El Baz A. F., Ramchuran S., Sayyed R. Z., Amalin D., et al. (2020). Trichoderma: biocontrol agents for promoting plant growth and soil health, in Agriculturally Important Fungi for Sustainable Agriculture, eds A. Yadav, S. Mishra, D. Kour, N. Yadav, A. Kumar (Cham: Springer; ), 239–259. 10.1007/978-3-030-48474-3_8 [DOI] [Google Scholar]
- Elias L. M., Domingues M. V. P., Moura K. E. D., Harakava R., Patricio F. R. A. (2016). Selection of Trichoderma isolates for biological control of Sclerotinia minor and S. sclerotiorum in lettuce. Summa Phytopathol. 42, 216–221. 10.1590/0100-5405/2147 [DOI] [Google Scholar]
- Feng W., Shen W., He L., Duan J., Guo B., Li Y., et al. (2016). Improved remote sensing detection of wheat powdery mildew using dual-green vegetation indices. Precis. Agric. 17, 608–627. 10.1007/s11119-016-9440-2 [DOI] [Google Scholar]
- Fiorentino N., Ventorino V., Woo S. L., Pepe O., De Rosa A., Gioia L., et al. (2018). Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve n uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 9:743. 10.3389/fpls.2018.00743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamon J. A., Peñuelas J., Field C. B. (1992). A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 41, 35–44. 10.1016/0034-4257(92)90059-S [DOI] [Google Scholar]
- Gamon J. A., Surfus J. S. (1999). Assessing leaf pigment content and activity with a reflectometer. New Phytol. 143, 105–117. 10.1046/j.1469-8137.1999.00424.x [DOI] [Google Scholar]
- Gardes M., Bruns T. D. (1993). ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol. 2, 113–118. 10.1111/j.1365-294X.1993.tb00005.x [DOI] [PubMed] [Google Scholar]
- Gilardi G., Gullino M. L., Garibaldi A. (2018a). Emerging foliar and soil-borne pathogens of leafy vegetable crops: a possible threat to Europe. EPPO Bull. 48, 116–127. 10.1111/epp.12447 [DOI] [Google Scholar]
- Gilardi G., Gullino M. L., Garibaldi A. (2018b). Emerging soil-borne and foliar diseases on leafy vegetables for fresh-cut production in northern Italy. Acta Hortic. 1209, 65–70. 10.17660/ActaHortic.2018.1209.10 [DOI] [Google Scholar]
- Giménez A., Fernández J. A., Pascual J. A., Ros M., López-Serrano M., Egea-Gilabert C. (2019). An agroindustrial compost as alternative to peat for production of baby leaf red lettuce in a floating system. Sci. Hortic. 246, 907–915. 10.1016/j.scienta.2018.11.080 [DOI] [Google Scholar]
- Gitelson A., Merzlyak M. N. (1994). Quantitative estimation of chlorophyll-a using reflectance spectra: experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. B. Biol. 22, 247–252. 10.1016/1011-1344(93)06963-4 [DOI] [Google Scholar]
- Gitelson A. A., Kaufman Y. J., Stark R., Rundquist D. (2002). Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 80, 76–87. 10.1016/S0034-4257(01)00289-9 [DOI] [Google Scholar]
- Gitelson A. A., Merzlyak M. N., Chivkunova O. B. (2001). Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 74:38. 10.1562/0031-8655(2001)074andlt;0038:OPANEOandgt;2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gitelson A. A., Merzlyak M. N., Lichtenthaler H. K. (1996). Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J. Plant Physiol. 148, 501–508. 10.1016/S0176-1617(96)80285-9 [DOI] [Google Scholar]
- Golhani K., Balasundram S. K., Vadamalai G., Pradhan B. (2018). A review of neural networks in plant disease detection using hyperspectral data. Inf. Process. Agric. 5, 354–371. 10.1016/j.inpa.2018.05.002 [DOI] [Google Scholar]
- Gröll K., Graeff S., Claupein W. (2007). Use of vegetation indices to detect plant diseases, in Agrarinformatik im Spannungsfeld zwischen Regionalisierung und globalen Wertschöpfungsketten – Referate der 27. GIL Jahrestagung, eds S. Böttinger, L. Theuvsen, S. Rank, and M. Morgenstern (Bonn: Gesellschaft für Informatik e. V; ), 91–94. [Google Scholar]
- Gullino M. L., Gilardi G., Garibaldi A. (2019). Ready-to-eat salad crops: a plant pathogen's heaven. Plant Dis. 103:9. 10.1094/PDIS-03-19-0472-FE [DOI] [PubMed] [Google Scholar]
- Haboudane D. (2004). Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: modeling and validation in the context of precision agriculture. Remote Sens. Environ. 90, 337–352. 10.1016/j.rse.2003.12.013 [DOI] [Google Scholar]
- Haboudane D., Miller J. R., Tremblay N., Zarco-Tejada P. J., Dextraze L. (2002). Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 81, 416–426. 10.1016/S0034-4257(02)00018-4 [DOI] [Google Scholar]
- Harris A., Bryant R. G., Baird A. J. (2006). Mapping the effects of water stress on Sphagnum: preliminary observations using airborne remote sensing. Remote Sens. Environ. 100, 363–378. 10.1016/j.rse.2005.10.024 [DOI] [Google Scholar]
- Hernández-Clemente R., Navarro-Cerrillo R. M., Suárez L., Morales F., Zarco-Tejada P. J. (2011). Assessing structural effects on PRI for stress detection in conifer forests. Remote Sens. Environ. 115, 2360–2375. 10.1016/j.rse.2011.04.036 [DOI] [Google Scholar]
- Hernández-Clemente R., Navarro-Cerrillo R. M., Zarco-Tejada P. J. (2012). Carotenoid content estimation in a heterogeneous conifer forest using narrow-band indices and PROSPECT+DART simulations. Remote Sens. Environ. 127, 298–315. 10.1016/j.rse.2012.09.014 [DOI] [Google Scholar]
- Hijmans R. J., Van Etten J., Cheng J., Mattiuzzi M., Sumner M., Greenberg J. A., et al. (2015). Package raster. R Package 734. [Google Scholar]
- Hirpara D. G., Gajera H. P., Hirpara H. Z., Golakiya B. A. (2017). Antipathy of Trichoderma against Sclerotium rolfsii Sacc.: evaluation of cell wall-degrading enzymatic activities and molecular diversity analysis of antagonists. J. Mol. Microbiol. Biotechnol. 27, 22–28. 10.1159/000452997 [DOI] [PubMed] [Google Scholar]
- Howell C. R. (2003). Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Dis. 87, 4–10. 10.1094/PDIS.2003.87.1.4 [DOI] [PubMed] [Google Scholar]
- Jones E. E., Stewart A., Whipps J. M. (2003). Use of Coniothyrium minitans transformed with the hygromycin B resistance gene to study survival and infection of Sclerotinia sclerotiorum sclerotia in soil. Mycol. Res. 107, 267–276. 10.1017/S0953756203007457 [DOI] [PubMed] [Google Scholar]
- Jordan C. F. (1969). Derivation of leaf-area index from quality of light on the forest floor. Ecology 50, 663–666. 10.2307/1936256 [DOI] [Google Scholar]
- Kareem T. K., Ugoji O. E., Aboaba O. O. (2016). Biocontrol of Fusarium wilt of cucumber with Trichoderma longibrachiatum NGJ167 (Rifai). Br. Microbiol. Res. J. 16, 1–11. 10.9734/BMRJ/2016/28208 [DOI] [Google Scholar]
- Kassambara A., Mundt F. (2017). Package Factoextra. Extract and Visualize the Results of Multivariate Data Analyses. 76. [Google Scholar]
- Kotasthane A., Agrawal T., Kushwah R., Rahatkar O. V. (2015). In-vitro antagonism of Trichoderma spp. against Sclerotium rolfsii and Rhizoctonia solani and their response towards growth of cucumber, bottle gourd and bitter gourd. Eu. J. Plant Pathol. 141, 523–543. 10.1007/s10658-014-0560-0 [DOI] [Google Scholar]
- Kumar G., Maharshi A., Patel J., Mukherjee A., Singh H. B., Sarma B. K. (2017). Trichoderma: a potential fungal antagonist to control plant diseases. SATSA Mukhapatra Annu. Tech. Issue 21:206. [Google Scholar]
- Kumar S., Stecher G., Tamura K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin R. P., Honeycutt C. W. (2006). Effects of different 3-year cropping systems on soil microbial communities and Rhizoctonia diseases of potato. Phytopathology 96, 68–79. 10.1094/PHYTO-96-0068 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler H. K., Lang M., Sowinska M., Heisel F., Miehé J. A. (1996). Detection of vegetation stress via a new high resolution fluorescence imaging system. J. Plant Physiol. 148, 599–612. 10.1016/S0176-1617(96)80081-2 [DOI] [Google Scholar]
- Liu H., Bruning B., Garnett T., Berger B. (2020). Hyperspectral imaging and 3D technologies for plant phenotyping: from satellite to close-range sensing. Comput. Electron. Agr. 175:105621. 10.1016/j.compag.2020.105621 [DOI] [Google Scholar]
- Liu L., Dong Y., Huang W., Du X., Ren B., Huang L., et al. (2020). Wheat fusarium head blight using sentinel-2 multispectral imagery. IEEE Access. 8, 52181–52191. 10.1109/ACCESS.2020.2980310 [DOI] [Google Scholar]
- Longo M. A., Sanromán M. N. (2006). Production of food aroma compounds: microbial and enzymatic methodologies. Food Technol. Biotechnol. 44, 335–353. 10.1201/9780429441837-15 [DOI] [Google Scholar]
- Lowe A., Harrison N., French A. P. (2017). Hyperspectral image analysis techniques for the detection and classification of the early onset of plant disease and stress. Plant Methods. 13:80. 10.1186/s13007-017-0233-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Jing Y., Huang W., Shi Y., Dong Y., Zhang J., et al. (2018). Integrating early growth information to monitor winter wheat powdery mildew using multi-temporal landsat-8 imagery. Sensors 18:3290. 10.3390/s18103290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen J. B. (1967). Some methods for classification and analysis of multivariate observations. Proc. Berkeley Symp. Math. Statist. Prob. 1, 281–297. [Google Scholar]
- Marín-Ortiz J. C., Gutierrez-Toro N., Botero-Fernández V., Hoyos-Carvajal L. M. (2020). Linking physiological parameters with visible/near-infrared leaf reflectance in the incubation period of vascular wilt disease. Saudi J. Biol. Sci. 27, 88–99. 10.1016/j.sjbs.2019.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques E., Martins I., de Mello S. C. M. (2018). Antifungal potential of crude extracts of Trichoderma spp. Biota Neotrop. 18:e20170418. 10.1590/1676-0611-bn-2017-0418 [DOI] [Google Scholar]
- Martins L. M., Neiro E., da S., Dias A. R., Roque C. G., Rojo Baio F. H., et al. (2018). Cotton vegetation indices under different control methods of ramularia leaf spot. Biosci. J. 34, 1706–1713. 10.14393/BJ-v34n6a2018-39975 [DOI] [Google Scholar]
- Mishra P., Asaari M. S. M., Herrero-Langreo A., Lohumi S., Diezma B., Scheunders P. (2017). Close range hyperspectral imaging of plants: a review. Biosyst. Eng. 164, 49–67. 10.1016/j.biosystemseng.2017.09.009 [DOI] [Google Scholar]
- Morra L., Cerrato D., Bilotto M., Baiano S. (2017). Introduction of sorghum [Sorghum bicolor (L.) Moench] green manure in rotations of head salads and baby leaf crops under greenhouse. Ital. J. Agron. 12:753. 10.4081/ija.2016.753 [DOI] [Google Scholar]
- Nicoletti R., Raimo F., Miccio G. (2004). First report of Rhizoctonia solani on Diplotaxis tenuifolia in Italy. Plant Pathol. 53, 811–811. 10.1111/j.1365-3059.2004.01078.x [DOI] [Google Scholar]
- Nicoletti R., Raimo F., Miccio G. (2007). Diplotaxis tenuifolia: biology, production and properties. Eur. J. Plant Sci. Biotechnol. 1, 36–43. [Google Scholar]
- O'Donnell K., Kistler H. C., Cigelnik E., Ploetz R. C. (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. 95, 2044–2049 10.1073/pnas.95.5.2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospina-Giraldo M. D., Royse D. J., Chen X., Romaine C. P. (1999). Molecular phylogenetic analyses of biological control strains of Trichoderma harzianum and other biotypes of Trichoderma spp. associated with mushroom green mold. Phytopathology 89, 308–313. 10.1094/PHYTO.1999.89.4.308 [DOI] [PubMed] [Google Scholar]
- Pane C., Sigillo L., Caputo M., Serratore G., Zaccardelli M., Tripodi P. (2017). Response of rocket salad germplasm (Eruca and Diplotaxis spp.) to major pathogens causing damping-off, wilting and leaf spot diseases. Arch. Phytopathol.Pflanzenschutz. 50, 167–177. 10.1080/03235408.2017.1285511 [DOI] [Google Scholar]
- Pane C., Sorrentino R., Scotti R., Molisso M., Di Matteo A., Celano G., et al. (2020). Alpha and beta-diversity of microbial communities associated to plant disease suppressive functions of on-farm green composts. Agriculture 10:113. 10.3390/agriculture10040113 [DOI] [Google Scholar]
- Pañitrur-De la Fuente C., Valdés-Gómez H., Roudet J., Verdugo-Vásquez N., Mirabal Y., Laurie V. F., et al. (2020). Vigor thresholded NDVI is a key early risk indicator of Botrytis bunch rot in vineyards. OENO One 52, 279–297. 10.20870/oeno-one.2020.54.2.2954 [DOI] [Google Scholar]
- Pascale A., Vinale F., Manganiello G., Nigro M., Lanzuise S., Ruocco M., et al. (2017). Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop Prot. 92, 176–181. 10.1016/j.cropro.2016.11.010 [DOI] [Google Scholar]
- Patterson C. L., Grogan R. G. (1985). Differences in epidemiology and control of lettuce drop caused by Sclerotinia minor and S. sclerotiorum. Plant Dis. 69, 766–770. 10.1094/PD-69-766 [DOI] [Google Scholar]
- Penha M. P., Rocha-Leao M. H. M., Leite S. G. F. (2012). Sugarcane bagasse as support for the production of coconut aroma by solid-state fermentation (SSF). Bioresources 7, 2366–2375. 10.15376/biores.7.2.2366-2375 [DOI] [Google Scholar]
- Peñuelas J., Baret F., Filella I. (1995). Semi-empirical indices to assess carotenoids/chlorophyll-a ratio from leaf spectral reflectance. Photosynthetica 31, 221–230. [Google Scholar]
- Peñuelas J., Filella I., Briel C., Serrano L., Savé R. (1993). The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 14, 1887–1905. 10.1080/01431169308954010 [DOI] [Google Scholar]
- Peñuelas J., Gamon J. A., Fredeen A. L., Merino J., Field C. B. (1994). Reflectance indices associated with physiological changes in nitrogen- and water-limited sunflower leaves. Remote Sens. Environ. 48, 135–146. 10.1016/0034-4257(94)90136-8 [DOI] [Google Scholar]
- Peñuelas J., Pinol J., Ogaya R., Filella I. (1997). Estimation of plant water concentration by the reflectance water index WI (R900/R970). Int. J. Remote Sens. 18, 2869–2875. 10.1080/014311697217396 [DOI] [Google Scholar]
- Phillips A. J. L. (1989). Fungi associated with sclerotia of Sclerotinia sclerotiorum in South Africa and their effects on the pathogen. Phytophylactica 21, 135–140. [Google Scholar]
- Pishchik V. N., Vorobyov N. I., Walsh O. S., Surin V. G., Khomyakov Y. V. (2016). Estimation of synergistic effect of humic fertilizer and Bacillus subtilis on lettuce plants by reflectance measurements. J. Plant Nutr. 39, 1074–1086. 10.1080/01904167.2015.1061551 [DOI] [Google Scholar]
- Qi J., Chehbouni A., Huete A. R., Kerr Y. H., Sorooshian S. (1994). A modified soil adjusted vegetation index. Remote Sens. Environ. 48, 119–126. 10.1016/0034-4257(94)90134-1 [DOI] [Google Scholar]
- Ramos A. S., Fiaux S. B., Leite S. G. F. (2008). Production of 6-pentyl-α-pyrone by Trichoderma harzianum in solid-state fermentation. Braz. J. Microbiol. 39, 712–717. 10.1590/S1517-83822008000400022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H., Zhou G., Zhang F. (2018). Using negative soil adjustment factor in soil-adjusted vegetation index (SAVI) for aboveground living biomass estimation in arid grasslands. Remote Sens. Environ. 209, 439–445. 10.1016/j.rse.2018.02.068 [DOI] [Google Scholar]
- Reynolds G. J., Windels C. E., MacRae I. V., Laguette S. (2012). Remote sensing for assessing Rhizoctonia crown and root rot severity in sugar beet. Plant Dis. 96, 497–505. 10.1094/PDIS-11-10-0831 [DOI] [PubMed] [Google Scholar]
- Rondeaux G., Steven M., Baret F. (1996). Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 55, 95–107. 10.1016/0034-4257(95)00186-7 [DOI] [Google Scholar]
- Roujean J.-L., Breon F.-M. (1995). Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 51, 375–384. 10.1016/0034-4257(94)00114-3 [DOI] [Google Scholar]
- Rouphael Y., Carillo P., Colla G., Fiorentino N., Sabatino L., El-Nakhel C., et al. (2020). Appraisal of combined applications of Trichoderma virens and a biopolymer-based biostimulant on lettuce agronomical, physiological, and qualitative properties under variable N regimes. Agronomy 10:196. 10.3390/agronomy10020196 [DOI] [Google Scholar]
- Rouse J., Jr., Haas R., Schell J., Deering D. (1973). Monitoring vegetation systems in the Great Plains with ERTS, in Proceedings of the Third Earth Resources Technology Satellite-1 Symposium (Washington, DC: ). [Google Scholar]
- Sachdev S., Singh R. P. (2020). Trichoderma: a multifaceted fungus for sustainable agriculture, in Ecological and Practical Applications for Sustainable Agriculture, eds K. Bauddh, S. Kumar, R. Singh, and J. Korstad (Singapore: Springer; ), 261–304. 10.1007/978-981-15-3372-3_13 [DOI] [Google Scholar]
- Samuels G. J., Ismaiel A., Bon M. C., De Respinis S., Petrini O. (2010). Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia 102, 944–966. 10.3852/09-243 [DOI] [PubMed] [Google Scholar]
- Saxena D., Tewari A. K., Rai D. (2014). In vitro antagonistic assessment of T. harzianum PBT 23 against plant pathogenic fungi. J. Microbiol. Biotechnol. Res. 4, 59–65. [Google Scholar]
- Schlatter D., Kinkel L., Thomashow L., Weller D., Paulitz T. (2017). Disease suppressive soils: new insights from the soil microbiome. Phytopathology 107, 1284–1297. 10.1094/PHYTO-03-17-0111-RVW [DOI] [PubMed] [Google Scholar]
- Schroers H. J., Samuels G. J., Seifert K. A., Gams W. (1999). Classification of the mycoparasite Gliocladium roseum in Clonostachys as C. rosea, its relationship to Bionectria ochroleuca, and notes on other Gliocladium-like fungi. Mycologia 91, 365–385 10.1080/00275514.1999.12061028 [DOI] [Google Scholar]
- Scotti R., Mitchell A. L., Pane C., Finn R. D., Zaccardelli M. (2020). Microbiota characterization of agricultural green waste-based suppressive composts using omics and classic approaches. Agriculture 10:61. 10.3390/agriculture10030061 [DOI] [Google Scholar]
- Serrano-Carreón L., Flores C., Rodríguez B., Galindo E. (2004). Rhizoctonia solani, an elicitor of 6-pentyl-α-pyrone production by Trichoderma harzianum in a two liquid phases, extractive fermentation system. Biotechnol. Lett. 26, 1403–1406. 10.1023/B:BILE.0000045640.71840.b5 [DOI] [PubMed] [Google Scholar]
- Sharma S., Kour D., Rana K. L., Dhiman A., Thakur S., Thakur P., et al. (2019). Trichoderma: biodiversity, ecological significances, and industrial applications in Recent Advancement in White Biotechnology through Fungi, eds A. Yadav, S. Mishra, S. Singh, and A. Gupta (Cham: Springer; ), 85–120 10.1007/978-3-030-10480-1_3 [DOI] [Google Scholar]
- Silva G. M., Peixoto L. S., Fujii A. K., Parisi J. J. D., Aguiar R. H., Fracarolli J. A. (2018). Evaluation of maize seeds treated with Trichodermil® through biospeckle. J. Agr. Sci. Tech-IRAN. 8, 175–187. 10.17265/2161-6264/2018.03.004 [DOI] [Google Scholar]
- Singh S., Singh H., Singh D., Chandra B., Viswa K. (2013). Trichoderma harzianum and Pseudomonas sp. mediated management of Sclerotium rolfsii rot in tomato (Lycopersicon esculentum mill.). Bioscan. 8, 801–804. [Google Scholar]
- Smith R. C. G., Adams J., Stephens D. J., Hick P. T. (1995). Forecasting wheat yield in a mediterranean-type environment from the NOAA satellite. Aust. J. Agric. Res. 46:113. 10.1071/AR9950113 [DOI] [Google Scholar]
- Sridharan A. P., Thankappan S., Karthikeyan G., Uthandi S. (2020). Comprehensive profiling of the VOCs of Trichoderma longibrachiatum EF5 while interacting with Sclerotium rolfsii and Macrophomina phaseolina. Microbiol. Res. 236, 126–436. 10.1016/j.micres.2020.126436 [DOI] [PubMed] [Google Scholar]
- Srinivasa N., Devi T. P. (2014). Separation and identification of antifungal compounds from Trichoderma species BY GC-MS and their bio-efficacy against soil-borne pathogens. BIOINFOLET- Jo. L. Sci. 11, 255–257. [Google Scholar]
- Steddom K., Heidel G., Jones D., Rush C. M. (2003). Remote detection of rhizomania in sugar beets. Phytopathology 93, 720–726. 10.1094/PHYTO.2003.93.6.720 [DOI] [PubMed] [Google Scholar]
- Steyaert J. M., Ridgway H. J., Elad Y., Stewart A. (2003). Genetic basis of mycoparasitism: a mechanism of biological control by species of Trichoderma. New Zeal. J. Crop Hort. Sci. 31, 281–291. 10.1080/01140671.2003.9514263 [DOI] [Google Scholar]
- Subbarao K. V. (1998). Progress toward integrated management of lettuce drop. Plant Dis. 82, 1068–1078. 10.1094/PDIS.1998.82.10.1068 [DOI] [PubMed] [Google Scholar]
- Subbarao K. V., Davis R. M., Gilbertson R. L., Raid R. N. (eds.). (2017). Compendium of Lettuce Diseases and Pests. St. Paul, MN: APS Press, The American Phytopathological Society. [Google Scholar]
- Susič N., Žibrat U., Sinkovič L., Vončina A., Razinger J., Knapič M., et al. (2020). From genome to field—observation of the multimodal nematicidal and plant growth-promoting effects of Bacillus firmus I-1582 on tomatoes using hyperspectral remote sensing. Plants 9:592. 10.3390/plants9050592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thenkabail P. S., Smith R. B., De Pauw E. (2000). Hyperspectral vegetation indices and their relationships with agricultural crop characteristics. Rem. Sens. Environ. 71, 158–182. 10.1016/S0034-4257(99)00067-X [DOI] [Google Scholar]
- Thomas S., Kuska M. T., Bohnenkamp D., Brugger A., Alisaac E., Wahabzada M., et al. (2018). Benefits of hyperspectral imaging for plant disease detection and plant protection: a technical perspective. J. Plant Dis. Protect. 125, 5–20. 10.1007/s41348-017-0124-6 [DOI] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y. C., Yao X., Yang J., Cao W. X., Hannaway D. B., Zhu Y. (2011). Assessing newly developed and published vegetation indices for estimating rice leaf nitrogen concentration with ground- and space-based hyperspectral reflectance. Field Crops Res. 120, 299–310. 10.1016/j.fcr.2010.11.002 [DOI] [Google Scholar]
- Tilley D. R., Ahmed M., Son J. H., Badrinarayanan H. (2003). Hyperspectral reflectance of emergent macrophytes as an indicator of water column ammonia in an oligohaline, subtropical marsh. Ecol. Eng. 21, 153–163. 10.1016/j.ecoleng.2003.10.004 [DOI] [Google Scholar]
- Tucker C. J. (1979). Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 8, 127–150. 10.1016/0034-4257(79)90013-0 [DOI] [Google Scholar]
- Turner G. J., Tribe H. T. (1976). On Coniothyrium minitans and its parasitism of Sclerotinia species. Trans. Brit. Mycol. Soc. 66, 97–105. 10.1016/S0007-1536(76)80098-8 [DOI] [Google Scholar]
- Vitorino L. C., Silva F. O., da Cruvinel B. G., Bessa L. A., Rosa M., Souchie E. L., et al. (2020). Biocontrol potential of Sclerotinia sclerotiorum and physiological changes in soybean in response to Butia archeri palm rhizobacteria. Plants 9:64. 10.3390/plants9010064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmann J. E., Rock B. N., Moss D. M. (1993). Red edge spectral measurements from sugar maple leaves. Int. J. Remote Sens. 14, 1563–1575. 10.1080/01431169308953986 [DOI] [Google Scholar]
- Wang Z., Li Y., Zhuang L., Yu Y., Liu J., Zhang L., et al. (2019). A rhizosphere-derived consortium of Bacillus subtilis and Trichoderma harzianum suppresses common scab of potato and increases yield. Comput. Struct. Biotechnol. J. 17, 645–653. 10.1016/j.csbj.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipps J. M., Budge S. P. (1990). Screening for sclerotial mycoparasites of Sclerotinia sclerotiorum. Mycol. Res. 94, 607–612. 10.1016/S0953-7562(09)80660-6 [DOI] [Google Scholar]
- White T. J., Burns T., Lee S., Taylor J. (1990). Amplification and sequencing of fungal ribosomal RNA genes for phylogenetics, in PCR Protocols A Guide to Methods and Applications, eds M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (San Diego, CA: Academic Press; ), 315–322. [Google Scholar]
- Wiegand C. L., Hatfield J. L. (1988). The spectral-agronomic multisite-multicrop analyses (SAMMA) project, in Proceedings of the 16th International Society for Photogrammetry and Remote Sensing Congress (Kyoto: ), 696–706 [Google Scholar]
- Wonglom P., Daengsuwan W., Ito S., Sunpapao A. (2019). Biological control of Sclerotium fruit rot of snake fruit and stem rot of lettuce by Trichoderma sp. T76-12/2 and the mechanisms involved. Physiol. Mol. Plant Pathol. 107, 1–7. 10.1016/j.pmpp.2019.04.007 [DOI] [Google Scholar]
- Wu C., Niu Z., Tang Q., Huang W. (2008). Estimating chlorophyll content from hyperspectral vegetation indices: modeling and validation. Agr. Forest Meteorol. 148, 1230–1241. 10.1016/j.agrformet.2008.03.005 [DOI] [Google Scholar]
- Xing N., Huang W., Xie Q., Shi Y., Ye H., Dong Y., et al. (2020). A transformed triangular vegetation index for estimating winter wheat leaf area index. Remote Sens. 12:16. 10.3390/rs12010016 [DOI] [Google Scholar]
- Xue J., Su B. (2017). Significant remote sensing vegetation indices: a review of developments and applications. J. Sens. 2017, 1–17. 10.1155/2017/1353691 [DOI] [Google Scholar]
- Yang D., Wang B., Wang J., Chen Y., Zhou M. (2009). Activity and efficacy of Bacillus subtilis strain NJ-18 against rice sheath blight and sclerotinia stem rot of rape. Biol. Control. 51, 61–65. 10.1016/j.biocontrol.2009.05.021 [DOI] [Google Scholar]
- Yao H., Tang L., Tian L., Brown R. L., Bhatnagar D., Cleveland T. E. (2011). Using hyperspectral data in precision farming applications, in Hyperspectral Remote Sensing of Vegetation, eds P. S. Thenkabail, and J. G. Lyon (Boca Raton, FL; London; New York, NY: CRC Press/Taylor and Francis Group), 591–608. [Google Scholar]
- Zarco-Tejada P. J., Berjón A., López-Lozano R., Miller J. R., Martín P., Cachorro V., et al. (2005). Assessing vineyard condition with hyperspectral indices: leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 99, 271–287. 10.1016/j.rse.2005.09.002 [DOI] [Google Scholar]
- Zarco-Tejada P. J., Miller J. R., Noland T. L., Mohammed G. H., Sampson P. H. (2001). Scaling-up and model inversion methods with narrowband optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE Trans. Geosci. Remote Sens. 39, 1491–1507. 10.1109/36.934080 [DOI] [Google Scholar]
- Zhao H., Yang C., Guo W., Zhang L., Zhang D. (2020). Automatic estimation of crop disease severity levels based on vegetation index normalization. Remote Sens. 12:1930. 10.3390/rs12121930 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, MW191738; https://www.ncbi.nlm.nih.gov/genbank/, MW191739; https://www.ncbi.nlm.nih.gov/genbank/, MW191740; https://www.ncbi.nlm.nih.gov/genbank/, MW191741; https://www.ncbi.nlm.nih.gov/genbank/, MW191742; https://www.ncbi.nlm.nih.gov/genbank/, MW191743; https://www.ncbi.nlm.nih.gov/genbank/, MW191744; https://www.ncbi.nlm.nih.gov/genbank/, MW191751; https://www.ncbi.nlm.nih.gov/genbank/, MW191737; https://www.ncbi.nlm.nih.gov/genbank/, MW191745; https://www.ncbi.nlm.nih.gov/genbank/, MW191747; https://www.ncbi.nlm.nih.gov/genbank/, MW191746; https://www.ncbi.nlm.nih.gov/genbank/, MW191749; https://www.ncbi.nlm.nih.gov/genbank/, MW191750; https://www.ncbi.nlm.nih.gov/genbank/, MW191748; https://www.ncbi.nlm.nih.gov/genbank/, MW191752; https://www.ncbi.nlm.nih.gov/genbank/, MW201694; https://www.ncbi.nlm.nih.gov/genbank/, MW201695; https://www.ncbi.nlm.nih.gov/genbank/, MW201689; https://www.ncbi.nlm.nih.gov/genbank/, MW201690; https://www.ncbi.nlm.nih.gov/genbank/, MW201692; https://www.ncbi.nlm.nih.gov/genbank/, MW201693; https://www.ncbi.nlm.nih.gov/genbank/, MW201691; https://www.ncbi.nlm.nih.gov/genbank/, MW201701; https://www.ncbi.nlm.nih.gov/genbank/, MW201688; https://www.ncbi.nlm.nih.gov/genbank/, MW201697; https://www.ncbi.nlm.nih.gov/genbank/, MW201696; https://www.ncbi.nlm.nih.gov/genbank/, MW201699; https://www.ncbi.nlm.nih.gov/genbank/, MW201700; https://www.ncbi.nlm.nih.gov/genbank/, MW201698; https://www.ncbi.nlm.nih.gov/genbank/, MW201702; https://www.ncbi.nlm.nih.gov/genbank/, MW246311.