Abstract
Background
We prepared a meta-analysis on case reports in children with COVID-19, aiming to identify potential risk factors for severe illness and to develop a prediction model for risk assessment.
Methods
Literature retrieval, case report selection, and data extraction were independently completed by two authors. STATA software (version 14.1) and R programming environment (v4.0.2) were used for data handling.
Results
This meta-analysis was conducted based on 52 case reports, including 203 children (96 boys) with COVID-19. By severity, 26 (12.94%), 160 (79.60%), and 15 (7.46%) children were diagnosed as asymptomatic, mild/moderate, and severe cases, respectively. After adjusting for age and sex, 11 factors were found to be significantly associated with the risk of severe illness relative to asymptomatic or mild/moderate illness, especially for dyspnea/tachypnea (odds ratio, 95% confidence interval, P: 6.61, 4.12–9.09, <0.001) and abnormal chest X-ray (3.33, 1.84–4.82, <0.001). A nomogram modeling age, comorbidity, cough, dyspnea or tachypnea, CRP, and LDH was developed, and prediction performance was good as reflected by the C-index.
Conclusions
Our findings provide systematic evidence for the contribution of comorbidity, cough, dyspnea or tachypnea, CRP, and LDH, both individually and jointly, to develop severe symptoms in children with asymptomatic or mild/moderate COVID-19.
Impact
We have identified potential risk factors for severe illness in children with COVID-19.
We have developed a prediction model to facilitate risk assessment in children with COVID-19.
We found the contribution of five risk factors to develop severe symptoms in children with asymptomatic or mild/moderate COVID-19.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), originated in Wuhan and has rapidly spread to 220 countries, areas, or territories globally, infecting nearly 56 million people of all ages and causing over 1344,003 deaths as of November 20, 2020 (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). Epidemiologic evidence indicates that children are less likely to develop severe COVID-19 than adults, and infected children typically have a good prognosis,1 with “trained immunity” as a potential explanation.2 However, controversies remain, as a study from China reported that infants and younger children are more likely to develop severe clinical manifestations than older children, likely due to immature immune system.3 Despite children represents about 1–2% of total COVID-19 burden,4 the actual fatality figure is relatively high. In the medical literature, reports regarding the clinical features of children with COVID-19 are scarce, with the majority arising from studies of cases report and case series. Thus the aggregation of these cases via a meta-analysis may help to better understand its clinical features and risk profiles. Although most pediatric COVID-19 patients are not severe,3,5–7 a serious COVID-19 illness could result in severe outcomes, including an intensive care unit (ICU) admission and even death in children. Considering the fact that the respiratory structural characteristics and immune response system differ remarkably between children and adults,8–10 one of the most urgent tasks facing us is to seek potential predictors that can assist in improving clinical management of children with COVID-19.
To yield more information, we prepared a meta-analysis on case reports in children with COVID-19, aiming to test the hypothesis that some potential risk factors can lead to severe COVID-19 of children, and if this hypothesis is confirmed, we further develop a prediction model to facilitate risk assessment.
Methods
Literature search
Case reports, published in English or Chinese language, were identified by retrieving PubMed, EMBASE (Excerpt Medica Database), and Web of Science databases before May 1, 2020, using key terms “COVID-19,” “2019-nCoV,” “SARS-CoV-2,” “children,” and “pediatric.” Literature search was independently done by two authors (B.Z. and Y.Y.), and disagreement was resolved by consensus.
Diagnosis
For each eligible case report or case series, COVID-19 should be diagnosed by high-throughput sequencing or real-time PCR kit for nasal/pharyngeal swab specimens.
Clinical classification of COVID-19
The severity of COVID-19 was defined according to clinical features, laboratory testing, and chest X-ray imaging, including asymptomatic infection, mild, moderate, severe, and critical cases.11
Asymptomatic infection: without any clinical symptoms and signs and the chest imaging is normal, while the 2019-nCoV nucleic acid test is in a positive period.
Mild: symptoms of acute upper respiratory tract infection, including fever, fatigue, myalgia, cough, sore throat, runny nose, and sneezing. Physical examination shows congestion of the pharynx and no auscultory abnormalities. Some cases may have no fever or have only digestive symptoms, such as nausea, vomiting, abdominal pain, and diarrhea.
Moderate COVID-19: with pneumonia, frequent fever and cough, mostly dry cough, followed by productive cough, some may have wheezing, but no obvious hypoxemia such as shortness of breath, and from the lungs one can hear sputum or dry snoring and/or wet snoring. Some cases may have no clinical signs and symptoms, but chest CT shows lung lesions, which are subclinical.
Severe COVID-19: early respiratory symptoms such as fever and cough and may be accompanied by gastrointestinal symptoms, such as diarrhea. The disease usually progresses around 1 week, and dyspnea occurs, with central cyanosis. Oxygen saturation is <92%, with other hypoxia manifestations.
Critical COVID-19: children can quickly progress to acute respiratory distress syndrome or respiratory failure and may also have shock, encephalopathy, myocardial injury or heart failure, coagulation dysfunction, and acute kidney injury. Organ dysfunction can be life threatening.
Selection process
For each retrieved case report or case series, two authors (B.Z. and Y.Y.) independently reviewed title and abstract for initial selection, and if necessary full text for eligibility. Disagreement was discussed between the two authors until a consensus on eligibility was attained. In case of more than one report of the same patients, we abstracted data from the most complete report.
Data extraction
Data from each eligible patient were independently extracted by two authors (B.Z. and Y.Y.), and they were typed into a predesigned Microsoft Office ExcelTM spreadsheet, including first author’s name, year of publication, country where participants were enrolled, study type, sex, age, clinical symptoms and signs, laboratory findings, imaging features, and severity. Extracted data were compared for consistency using the kappa statistic, and any divergence was resolved by a third author (W.N.).
Quality assessment
The Joanna Briggs Institute Meta-Analysis of Statistics Assessment and Review Instrument (JBI-MAStARI)12 scale was employed for critical appraisal, and this scale ranges from 0 (the worst) to 16 points (the best).
Statistical analysis
Based on the severity of COVID-19, all study children were classified into three groups (asymptomatic, mild/moderate, and severe/critical). Continuous variables were tested for normality by using skewness and kurtosis test. Skewed continuous variables are expressed as median (interquartile range) and normally distributed variables as mean (standard deviation or SD). Categorical variables are expressed as number (percentage). Between-group comparisons were implemented by rank-sum test or χ2 test, where appropriate. Potential risk factors for severity were selected by logistic regression analyses at a statistical significance level of 5% before and after adjusting for confounding factors, including age and sex. The magnitude of risk association is quantified by odds ratio (OR) and 95% confidence interval (95% CI).
Finally, on the basis of significant risk factors, a prediction nomogram, generated by the R programming environment version 3.5.2 for Windows, was established to enhance clinical application, and calibration curve and the C-index were used to assess prediction performance.
Unless otherwise reported, statistical analyses were completed using the STATA software version 14.0 (Stata Corp, TX) for Windows. Two-sided P value <5% was reported to be statistically significant. The power to detect statistical significance was estimated by using the PS Power and Sample Size Calculations software version 3.0.
Results
Baseline characteristics
After comprehensive literature search, 52 case reports were eligible for inclusion,4,13–63 including 203 children (mean age: 5.46 years, 96 boys and 98 girls) with COVID-19. By severity, 26 (12.94%), 160 (79.60%), and 15 (7.46%) children were diagnosed as asymptomatic, mild/moderate, and severe cases, respectively, and their baseline characteristics are provided in Table 1. As assessed by the JBI-MAStARI scale,12 the quality score of all case reports ranged from 13 to 16.
Table 1.
The baseline characteristics of study children with COVID-19 from 52 case reports.
| Characteristics | Asymptomatic COVID-19 (n = 26) | Mild/moderate COVID-19 (n = 160) | Severe COVID-19 (n = 15) | P |
|---|---|---|---|---|
| Demographic information | ||||
| Age (months) | 72 (12.96, 120) | 48 (12, 108) | 12.96 (8.04, 96) | 0.458 |
| Age | 0.594 | |||
| ≤1 year | 5 (19.2%) | 37 (23.9%) | 5 (33.3%) | |
| >1 year | 21 (80.8%) | 118 (76.1%) | 10 (66.7%) | |
| Boys | 10 (40%) | 74 (48.1%) | 12 (80%) | 0.037 |
| Day to negative | 8.5 (6.5, 15) | 10 (5, 13) | 5 (5,5) | 0.658 |
| Comorbidity | 0 (0, 0) | 0 (0, 0) | 0 (0,1) | <0.001 |
| Temperature | 36.4 (36, 36.7) | 38.1 (37.6, 38.55) | 39 (37.9, 40) | <0.001 |
| Clinical symptoms | ||||
| Fever | 0 (0%) | 97 (60.6%) | 11 (73.3%) | <0.001 |
| Nose symptoms | 0 (0%) | 20 (12.5%) | 0 (0%) | 0.058 |
| Throat symptoms | 0 (0%) | 22 (13.8%) | 2 (13.3%) | 0.132 |
| Cough | 0 (0%) | 58 (36.3%) | 11 (73.3%) | <0.001 |
| Phlegm sputum | 0 (0%) | 3 (1.9%) | 4 (26.7%) | <0.001 |
| Asthma/wheeze | 0 (0%) | 4 (2.5%) | 0 (0%) | 0.593 |
| Short of breath | 0 (0%) | 2 (1.3%) | 1 (6.7%) | 0.203 |
| Dyspnea/tachypnea | 0 (0%) | 2 (1.3%) | 13 (86.7%) | <0.001 |
| diarrhea | 0 (0%) | 12 (7.5%) | 5 (33.3%) | 0.001 |
| Nausea/vomiting | 0 (0%) | 13 (8.1%) | 6 (40%) | <0.001 |
| Abdominal pain | 0 (0%) | 3 (1.9%) | 0 (0%) | 0.677 |
| Headache | 0 (0%) | 4 (2.5%) | 1 (6.7%) | 0.418 |
| Fatigue symptoms | 0 (0%) | 6 (3.8%) | 2 (13.3%) | 0.104 |
| Chest radiography features | ||||
| Abnormal chest X-ray | 1 (4.3%) | 81 (65.3%) | 12 (92.3%) | <0.001 |
| Normal chest X-ray/CT | 22 (95.7%) | 43 (34.7%) | 1 (7.7%) | <0.001 |
| X-ray/CT unilateral injury | 0 (0%) | 23 (18.5%) | 3 (23.1%) | |
| X-ray/CT bilateral injury | 0 (0%) | 15 (12.1%) | 7 (53.8%) | |
| GGO (CT) | 0 (0%) | 43 (26.9%) | 7 (46.7%) | 0.002 |
| Laboratory biomarkers | ||||
| WBC (×109/L) | 8.14 (6.5, 9.42) | 6.7 (5.45, 8.6) | 9 (4.43, 11.96) | 0.173 |
| LYMPH (%) | 59.9 (25.4, 66.7) | 51 (42.3, 56.5) | NA | 0.933 |
| LYMPH (×109/L) | 2.89 (2.4, 3.7) | 2.69 (1.75, 4.05) | 1.76 (0.8, 2.47) | 0.042 |
| NEUT (%) | 27.8 (25.4, 32.1) | 34.2 (25, 42) | NA | 0.568 |
| NEUT (×109/L) | 3.2 (2.1, 5.56) | 2.49 (1.3, 3.6) | 3.8 (1.27, 7.77) | 0.226 |
| MONO | NA | 1.26 (0.95, 7.45) | 0.89 (0.08, 1.69) | 0.643 |
| HGB (g/L) | 133 (128, 142) | 126 (115, 136) | 108 (100, 153) | 0.378 |
| PLT (×109/L) | 278 (260, 358) | 256 (188, 311) | 193 (183.5, 216) | 0.131 |
| CRP (mg/mL) | 0.71 (0.2, 3.58) | 2.01 (0.5, 8) | 24.6 (6.48, 24.8) | 0.019 |
| PCT (ng/mL) | 0.08 (0.04, 0.14) | 0.07 (0.03, 0.1) | 0.25 (0.11, 0.43) | 0.070 |
| ALT (U/L) | 15.74 (13, 25.7) | 15.74 (13, 23) | 20 (12, 88) | 0.787 |
| AST (U/L) | 33.21 (23.19, 42) | 31.36 (24, 40.8) | 63 (63, 63) | 0.373 |
| Cr (μmol/L) | 78 (75, 81) | 29 (22.8, 48) | 224 (45, 224.5) | 0.005 |
| LDH (U/L) | 175 (159.4, 218) | 229.35 (177.1, 367.5) | 485 (361, 609) | 0.104 |
| CK (U/L) | 68 (68, 85) | 75 (46, 112) | 62 (50.5, 80.4) | 0.540 |
| CKMB (U/L) | 52 (34, 76) | 23 (12.3, 30) | NA | 0.010 |
| D-dimer (mg/L) | 0.25 (0.16, 0.3) | 0.33 (0.25, 0.6) | NA | 0.095 |
| NEUT/LYMPH | 1.14 (1, 1.99) | 0.93 (0.46, 2.02) | 3.13 (0.02, 6.84) | 0.428 |
| PLT/LYMPH | 114.29 (77.42, 151.16) | 101.92 (61.67, 175.86) | 106.43 (74.14, 212.93) | 0.957 |
| LYMPH/MONO | NA | 2.32 (0.19, 2.87) | 4.11 (1.47, 6.75) | 0.563 |
| WBC/LYMPH | 2.13 (1.91, 2.64) | 2.27 (1.84, 3.17) | 4.83 (3.03, 8.2) | 0.089 |
The p value was calculated using the rank sum test or χ2 test where appropriate. Data are expressed as median (interquartile range) or count (percentage), if appropriate.
GGO ground-glass opacity, CT computed tomography, WBC white blood cell, NEUT neutrophil, LYMPH lymphocyte, MONO monocyte, HGB hemoglobin, PLT platelet, CRP C-reactive protein, PCT procalcitonin, ALT alanine transaminase, AST aspartate aminotransferase, Cr creatinine, LDH lactate dehydrogenase, CK creatine kinase, CKMB creatine phosphokinase-isoenzyme-MB.
Identification of potential risk factors
After adjusting for age and sex, 11 factors were found to be significantly associated with the risk of severe illness relative to asymptomatic or mild/moderate illness (Table 2), especially for dyspnea/tachypnea (OR, 95% CI, P: 6.61, 4.12–9.09, <0.001) and abnormal chest X-ray (3.33, 1.84–4.82, <0.001). The power to detect significance was >90% for the above comparisons.
Table 2.
Identification of significant factors in association with severe COVID-19 relative to mild/moderate or asymptomatic COVID-19.
| Significant factors | Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Comorbidity | 2.74 | 1.41–4.07 | <0.001 | 2.76 | 1.39–4.13 | <0.001 |
| Fever | 2.53 | 1.45–3.60 | <0.001 | 2.64 | 1.52–3.75 | <0.001 |
| Temperature | 2.29 | 1.00–3.60 | 0.001 | 2.15 | 0.81–3.49 | 0.002 |
| Cough | 2.39 | 1.29–3.48 | <0.001 | 2.27 | 1.15–3.38 | <0.001 |
| Phlegm/sputum | 3.12 | 1.51–4.72 | <0.001 | 2.89 | 1.09–4.69 | 0.002 |
| Dyspnea/tachypnea | 6.40 | 4.36–8.44 | <0.001 | 6.61 | 4.12–9.09 | <0.001 |
| Diarrhea | 2.08 | 0.92–3.25 | <0.001 | 1.95 | 0.61–3.29 | 0.004 |
| Abnormal chest X-ray | 3.32 | 1.84–4.79 | <0.001 | 3.33 | 1.84–4.82 | <0.001 |
| GGO (CT) | 1.59 | 0.68–2.50 | 0.001 | 1.63 | 0.65–2.59 | 0.001 |
| CRP | 2.23 | 0.57–3.89 | 0.009 | 2.17 | 0.51–3.84 | 0.011 |
| LDH | 1.97 | 0.02–4.15 | 0.007 | 1.60 | 0.94–4.51 | 0.017 |
The P value was calculated after adjusting for age and sex.
OR odds ratio, 95% CI 95% confidence interval, GGO ground-glass opacity, CT computed tomography, WBC white blood cell, LYMPH lymphocyte, CRP C-reactive protein, LDH lactate dehydrogenase.
Prediction nomogram model
Due to missing values or establishment of some radiographic features such as abnormal chest X-ray, five factors, including comorbidity, cough, dyspnea or tachypnea, C-reactive protein (CRP), and lactate dehydrogenase (LDH), were retained for further model development, together with age.
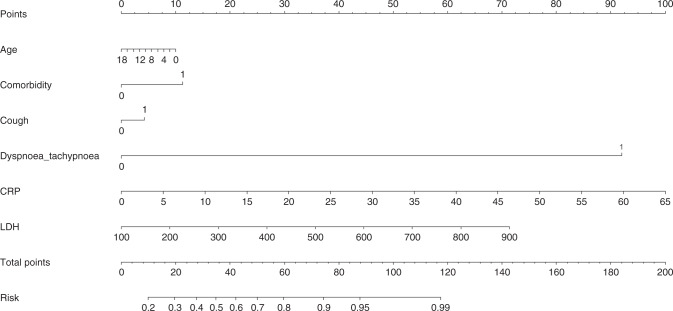
To facilitate clinical application, a nomogram model regressing age, comorbidity, cough, dyspnea or tachypnea, CRP, and LDH was developed (Fig. 1), and the prediction performance was good, as revealed by the calibration curve (Supplementary Fig. 1) and the C-index (80.1%).
Fig. 1. Development of a nomogram model in predicting the severe illness in children with COVID-19.
CRP C-reactive protein, LDH lactate dehydrogenase.
Taking the prediction nomogram model for pediatric COVID-19 severity as an example, assuming a child aged 9 years (5 points), with other comorbidities (11 points), with cough (5 points), with dyspnea or tachypnea (92 points), with circulating CRP of 30 mg/mL (45 point), and with LDH of 200 mg/mL (8 points), the probability of having severe pediatric COVID-19 was estimated to be >99%.
Discussion
The findings of this meta-analysis can enrich our understanding on the risk profiling of children with COVID-19 in predisposition to severe illness. In particular, we have identified five clinical characteristics or biomarkers in significant and independent association with COVID-19 severity, in line with the findings of some previous studies.64,65 Importantly, their prediction, together with age, was particularly evident in a nomogram model. To the best of our knowledge, this is the first study that has interrogated the possible risk factors of pediatric COVID-19 severity.
Although there are currently no effective antiviral drugs for SARS-CoV-2, prompt identification and early respiratory supportive care would provide relief in severe cases and reduce mortality. Recent studies estimated that 26–32% of adults were committed to ICU, and 1% patients with COVID-19 were asymptomatic.66 In contrast, we found only 7.5% of children with severe illness and 12.9% of children were completely asymptomatic, in agreement with a previous report.67 In this study, we found that children with COVID-19 who had clinical features such as fever, cough, phlegm/sputum, and dyspnea/tachypnea and chest radiography features such as abnormal chest X-ray, bilateral injury, or ground-glass opacity tended to develop into serious conditions, the findings consistent with that in adults.68–70
The findings of this present study, along with other studies,1,71 supported the note that children may have a better prognosis for COVID-19, when compared to adults. The reasons behind this claim are manifold, mainly because the SARS-CoV-2 S protein attaches to the angiotensin-converting enzyme 2,1,71,72 which is less developed at a younger age,73 and the percentage of children infected with COVID-19 having an exaggerated inflammatory response against the virus are not commonly described thus far.71,74
This meta-analysis of individual participant data was limited by the clinical heterogeneity of different reports, measurement bias of circulating biomarkers, and the limited number of assessable children with COVID-19, especially with severe illness.
Despite these limitations, our findings provide evidence for the contribution of comorbidity, cough, dyspnea or tachypnea, CRP, and LDH, both individually and jointly in a model, to develop severe symptoms in children with asymptomatic or mild/moderate COVID-19. Practically, we hope this meta-analysis will not represent just an endpoint of research but a start to construct the list of determinant factors in predicting the severe illness among children with COVID-19.
Supplementary information
Author contributions
W.N. planned and designed the study and directed its implementation. B.Z., Y.Y., Z.Z., S.W., M.Y., and X.D. contributed to data acquisition. B.Z. and Y.Y. conducted statistical analyses. B.Z., Y.Y., Z.Z., and X.D. performed the data preparation and quality control. B.Z. and W.N. wrote the manuscript. All authors read and approved the final manuscript prior to submission.
Data availability
Data involved in this meta-analysis are available upon reasonable request.
Competing interests
The authors declare no competing interests.
Patient consent
As it is a meta-analysis of individual participant data from case reports, patient consent was not required.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Bo Zhou, Yuan Yuan, Shunan Wang, Zhixin Zhang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41390-021-01429-2.
References
- 1.Castagnoli, R. et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 174, 882–889 (2020). [DOI] [PubMed]
- 2.Midulla, F. Cristiani, L. & Mancino, E. Will children reveal their secret? The coronavirus dilemma. Eur. Respir. J.55, 2000749 (2020). [DOI] [PMC free article] [PubMed]
- 3.Dong, Y. et al. Epidemiology of COVID-19 among children in China. Pediatrics145, e20200702 (2020). [DOI] [PubMed]
- 4.Li Y, et al. Insight into COVID-2019 for pediatricians. Pediatr. Pulmonol. 2020;55:E1–E4. doi: 10.1002/ppul.24386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu H, et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect. Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tagarro, A. et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 10.1001/jamapediatrics.2020.1346 (2020). [DOI] [PMC free article] [PubMed]
- 7.Ding Y, Yan H, Guo W. Clinical characteristics of children with COVID-19: a meta-analysis. Front. Pediatr. 2020;8:431. doi: 10.3389/fped.2020.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen K, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. World J. Pediatr. 2020;16:223–231. doi: 10.1007/s12519-020-00343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen KL, Yang YH. Diagnosis and treatment of 2019 novel coronavirus infection in children: a pressing issue. World J. Pediatr. 2020;16:219–221. doi: 10.1007/s12519-020-00344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen ZM, Fu JF, Shu Q. New coronavirus: new challenges for pediatricians. World J. Pediatr. 2020;16:222. doi: 10.1007/s12519-020-00346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Society of Pediatrics, Chinese Medical AssociationEditorial Board, Chinese Journal of Pediatrics. [Recommendations for the diagnosis, prevention and control of the 2019 novel coronavirus infection in children (first interim edition)] Zhonghua Er Ke Za Zhi. 2020;58:169–174. doi: 10.3760/cma.j.issn.0578-1310.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Munn Z, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Health. 2015;13:147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 13.Alzamora, M. C. et al. Severe COVID-19 during pregnancy and possible vertical transmission. Am. J. Perinatol.37, 861–865 (2020). [DOI] [PMC free article] [PubMed]
- 14.An, P. & Zhang, M. Novel coronavirus SARS-CoV-2: familial spread resulting in COVID-19 pneumonia in a pediatric patient. Diagn. Interv. Radiol.26, 262–263 (2020). [DOI] [PMC free article] [PubMed]
- 15.Cai JH, et al. [First case of 2019 novel coronavirus infection in children in Shanghai] Zhonghua Er Ke Za Zhi. 2020;58:E002. doi: 10.3760/cma.j.issn.0578-1310.2020.0002. [DOI] [PubMed] [Google Scholar]
- 16.Canarutto, D. et al. COVID-19 infection in a paucisymptomatic infant: Raising the index of suspicion in epidemic settings. Pediatr. Pulmonol.55, E4–E5 (2020). [DOI] [PMC free article] [PubMed]
- 17.Chen F, et al. [First case of severe childhood novel coronavirus pneumonia in China] Zhonghua Er Ke Za Zhi. 2020;58:E005. doi: 10.3760/cma.j.issn.0578-1310.2020.0005. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, et al. [COVID-19 with post-chemotherapy agranulocytosis in childhood acute leukemia: a case report] Zhonghua Xue Ye Xue Za Zhi. 2020;41:E004. doi: 10.3760/cma.j.issn.0253-2727.2020.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui, Y. et al. A 55-day-old female infant infected with COVID 19: presenting with pneumonia, liver injury, and heart damage. J. Infect. Dis.221, 1775–1781 (2020). [DOI] [PMC free article] [PubMed]
- 20.Dong, L. et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA323, 1846–1848 (2020). [DOI] [PMC free article] [PubMed]
- 21.Feng K, et al. [Analysis of CT features of 15 children with 2019 novel coronavirus infection] Zhonghua Er Ke Za Zhi. 2020;58:E007. doi: 10.3760/cma.j.issn.0578-1310.2020.0007. [DOI] [PubMed] [Google Scholar]
- 22.Genovese, G., Colonna, C. & Marzano, A. V. Varicella-like exanthem associated with COVID-19 in an 8-year-old girl: a diagnostic clue? Pediatr. Dermatol.37, 435–436 (2020). [DOI] [PMC free article] [PubMed]
- 23.Guiqing, H. E., Sun, W., Jing, W. U. & Cai, J. Serial computed tomography manifestations in a child with coronavirus disease (COVID-19) pneumonia. Indian Pediatr. 57, 467–468 (2020). [DOI] [PMC free article] [PubMed]
- 24.Hrusak O, et al. Flash survey on severe acute respiratory syndrome coronavirus-2 infections in paediatric patients on anticancer treatment. Eur. J. Cancer. 2020;132:11–16. doi: 10.1016/j.ejca.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji, L. N. et al. Clinical features of pediatric patients with COVID-19: a report of two family cluster cases. World J. Pediatr.16, 267–270 (2020). [DOI] [PMC free article] [PubMed]
- 26.Jiang, S. et al. Coinfection of SARS-CoV-2 and multiple respiratory pathogens in children. Clin. Chem. Lab. Med.58, 1160–1161 (2020). [DOI] [PubMed]
- 27.Kam, K. Q. et al. A well infant with coronavirus disease 2019 (COVID-19) with high viral load. Clin. Infect. Dis.71, 847–849 (2020). [DOI] [PMC free article] [PubMed]
- 28.Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect. Dis. 2020;52:427–429. doi: 10.1080/23744235.2020.1747634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kan, M. J., Grant, L. M. C., Muna, M. A. & Greenhow, T. L. Fever without a source in a young infant due to SARS-CoV-2. J. Pediatric Infect. Dis. Soc.10, 49–51 (2020). [DOI] [PMC free article] [PubMed]
- 30.Li, W. et al. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr. Radiol. 50, 796–799 (2020). [DOI] [PMC free article] [PubMed]
- 31.Lin, J. et al. The isolation period should be longer: lesson from a child infected with SARS-CoV-2 in Chongqing, China. Pediatr. Pulmonol. 55, E6–E9 (2020). [DOI] [PMC free article] [PubMed]
- 32.Liu H, et al. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J. Infect. 2020;80:e7–e13. doi: 10.1016/j.jinf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, M., Song, Z. & Xiao, K. High-resolution computed tomography manifestations of 5 pediatric patients with 2019 novel coronavirus. J. Comput. Assist. Tomogr.44, 311–313 (2020). [DOI] [PMC free article] [PubMed]
- 34.Liu W, et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N. Engl. J. Med. 2020;382:1370–1371. doi: 10.1056/NEJMc2003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paret, M. et al. SARS-CoV-2 infection (COVID-19) in febrile infants without respiratory distress. Clin. Infect. Dis.71, 2243–-2245 (2020). [DOI] [PMC free article] [PubMed]
- 36.Park JY, et al. First pediatric case of coronavirus disease 2019 in Korea. J. Korean Med. Sci. 2020;35:e124. doi: 10.3346/jkms.2020.35.e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian, G. et al. A COVID-19 transmission within a family cluster by presymptomatic infectors in China. Clin. Infect. Dis. 71, 861–862 (2020). [DOI] [PMC free article] [PubMed]
- 38.See, K. C. et al. COVID-19: four paediatric cases in Malaysia. Int. J. Infect. Dis. 94, 124–127 (2020). [DOI] [PMC free article] [PubMed]
- 39.Shen, Q. et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr. Pulmonol. 55, 1424–1429 (2020). [DOI] [PMC free article] [PubMed]
- 40.Su L, et al. The different clinical characteristics of corona virus disease cases between children and their families in China - the character of children with COVID-19. Emerg. Microbes Infect. 2020;9:707–713. doi: 10.1080/22221751.2020.1744483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song, R. et al. Clinical and epidemiological features of COVID-19 family clusters in Beijing, China. J. Infect. 81, e26–e30 (2020). [DOI] [PMC free article] [PubMed]
- 42.Zhu, L. et al. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatr. Pulmonol. 55, 1430–1432 (2020). [DOI] [PMC free article] [PubMed]
- 43.Zhou Y, et al. [Clinical features and chest CT findings of coronavirus disease 2019 in infants and young children] Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:215–220. doi: 10.7499/j.issn.1008-8830.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, Z. J. et al. Novel coronavirus infection in newborn babies under 28 days in China. Eur. Respir. J. 55, 2000697 (2020). [DOI] [PMC free article] [PubMed]
- 45.Zhang, T. et al. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J. Med. Virol.92, 909–914 (2020). [DOI] [PMC free article] [PubMed]
- 46.Zhang GX, et al. [Twin girls infected with SARS-CoV-2] Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:221–225. doi: 10.7499/j.issn.1008-8830.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, B. et al. Positive rectal swabs in young patients recovered from coronavirus disease 2019 (COVID-19). J. Infect. 81, e49–e52 (2020). [DOI] [PMC free article] [PubMed]
- 48.Zeng LK, et al. [First case of neonate with COVID-19 in China] Zhonghua Er Ke Za Zhi. 2020;58:289–280. doi: 10.3760/cma.j.cn112140-20200212-00081. [DOI] [PubMed] [Google Scholar]
- 49.Zeng, L. et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 174, 722–725 (2020). [DOI] [PMC free article] [PubMed]
- 50.Yu, N. et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect. Dis. 20, 559–564 (2020). [DOI] [PMC free article] [PubMed]
- 51.Xu Y, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu R, et al. CT imaging of one extended family cluster of corona virus disease 2019 (COVID-19) including adolescent patients and “silent infection”. Quant. Imaging Med. Surg. 2020;10:800–804. doi: 10.21037/qims.2020.02.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu, P., Liang, L., Chen, C. & Nie, S. A child confirmed COVID-19 with only symptoms of conjunctivitis and eyelid dermatitis. Graefes Arch. Clin. Exp. Ophthalmol. 258, 1565–1566 (2020). [DOI] [PMC free article] [PubMed]
- 54.Wei, M. et al. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA323, 1313–1314 (2020). [DOI] [PMC free article] [PubMed]
- 55.Wang, S. et al. A case report of neonatal COVID-19 infection in China. Clin. Infect. Dis.71, 853–857 (2020). [DOI] [PMC free article] [PubMed]
- 56.Wang J, et al. [SARS-CoV-2 infection with gastrointestinal symptoms as the first manifestation in a neonate] Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:211–214. doi: 10.7499/j.issn.1008-8830.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, H. et al. Rehospitalization of a recovered coronavirus disease 19 (COVID-19) child with positive nucleic acid detection. Pediatr. Infect. Dis. J.39, e69–e70 (2020). [DOI] [PMC free article] [PubMed]
- 58.Tang, A. et al. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Infect. Dis.26, 1337–1339 (2020). [DOI] [PMC free article] [PubMed]
- 59.Tan YP, et al. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J. Clin. Virol. 2020;127:104353. doi: 10.1016/j.jcv.2020.104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan X, et al. [Clinical features of children with SARS-CoV-2 infection: an analysis of 13 cases from Changsha, China] Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:294–298. doi: 10.7499/j.issn.1008-8830.2003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun, D. et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J. Pediatr.16, 251–259 (2020). [DOI] [PMC free article] [PubMed]
- 62.Xing, Y. H. et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J. Microbiol. Immunol. Infect.53, 473–480 (2020). [DOI] [PMC free article] [PubMed]
- 63.Zheng, F. et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr. Med. Sci. 40, 275–280 (2020). [DOI] [PMC free article] [PubMed]
- 64.Li, Y. et al. Immune-related factors associated with pneumonia in 127 children with coronavirus disease 2019 in Wuhan. Pediatr. Pulmonol.55, 2354–2360 (2020). [DOI] [PMC free article] [PubMed]
- 65.Abdel-Mannan, O. et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 77, 1–6 (2020). [DOI] [PMC free article] [PubMed]
- 66.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 67.Wang Z, et al. Clinical characteristics of children with COVID-19: a rapid review and meta-analysis. Ann. Transl. Med. 2020;8:620. doi: 10.21037/atm-20-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen G, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li X, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu, C. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med.180, 934–943 (2020). [DOI] [PMC free article] [PubMed]
- 71.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wrapp D, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015;282:20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin. Chem. Lab. Med. 2020;58:1135–1138. doi: 10.1515/cclm-2020-0272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data involved in this meta-analysis are available upon reasonable request.