Abstract
Background:
The unanimous method of screening cervical cancer is a cervical smear stained with Papanicolaou stain. However, in spite of the various modifications, the staining procedure takes 20 min and uses substantial amount of alcohol which is highly priced. The aim of the study was to assess and analyze the quality of staining of cervical smears stained with Rapid Economical Acetic acid Papanicolaou (REAP) as compared to conventional pap stain in order to establish REAP as an alternative to conventional pap stain.
Methods and Material:
In this prospective study, a total of two smears each were collected from 50 females who visited the gynecology outpatient department. One of the smears was stained with conventional pap and the other with REAP. The conventional pap and REAP smears were evaluated and compared for the quality of staining.
Results:
The cervical smears stained with REAP showed optimal cytoplasmic and nuclear staining in 86% and 90% cases, respectively. The cytological findings and diagnosis of REAP stained smears correlated with their corresponding smears stained with conventional pap in 96% of cases. The turnaround time and cost per smear was much low for REAP as compared to conventional pap stain.
Conclusion:
The present study was able to establish REAP as an appropriate alternative to conventional pap stain. The staining by REAP was comparable to conventional pap stain.
Keywords: Acetic acid, alcohol, conventional pap, economical, papanicolaou stain
INTRODUCTION
In 1943, Papanicolaou first described the Papanicolaou (Pap) stain and since then it has been commonly used as a screening test despite being time consuming and necessitating the use of generous amount of alcohol.[1] The initial Pap stain was later modified by him in 1954 and 1960. It yields a polychromatic, transparent staining reaction with crisp nuclear and cytological features, which can be easily interpreted by a trained pathologist.[2] The Pap stain has been most frequently used for screening of cervical cancer. The pap smear can detect a continuum of pathologic changes, ranging from atypical squamous cells of undetermined significance to precancerous conditions like low-grade squamous intraepithelial lesions (LSIL) and high-grade squamous intraepithelial lesions (HSIL) to invasive cancer, thus providing an opportunity for screening and detection of the disease in the preinvasive phase allowing early treatment leading to reduction in morbidity and mortality.[3] The conventional pap stain is a 20 min procedure and uses a significant amount of absolute alcohol as the dehydrating agent, which is highly expensive and difficult to obtain in a developing country like India.[4] Though the use of other alcohols like isopropyl alcohol which are cheaper than absolute alcohol may give comparable results, in a developing country like India, where most of the peripheral laboratories are deprived of both human and material resources, a conventional pap stain may not be always feasible for mass cervical screening.
Hence for laboratories with restricted resources a rapid, cost-effective, and technician friendly staining procedure needs to be implemented for a more widespread and effective cervical screening programme. Many authors have standardized and compared a variety of alterations in the conventional pap stain to decrease the turnaround time and to reduce the use of alcohol. Various modifications of pap stain such as Ultra-Fast and rapid pap have reduced the time to a minimum of 90 s, but the issues of ethanol and color preservation still remains.[5,6]
In 2008, Biswas et al. standardized a rapid and more economical modification of conventional pap referred to as Rapid Economical Acetic acid Papanicolaou (REAP) stain. The total staining time was reduced to 3 min with this customized technique. It is cost-effective as the low-cost acetic acid replaces the more expensive absolute alcohol, except for fixation and last step dehydration. In addition, it gives excellent nuclear and cytoplasmic staining with good color intensity and long-term color preservation.[7]
Our aim in the present study was to assess and analyze whether an economical and rapid Papanicolaou staining technique (REAP) can serve as an appropriate alternative to the conventional Papanicolaou staining.
MATERIALS AND METHODS
This was a prospective cross-sectional study where a total of 100 smears were collected from 50 sexually active female patients who attended the gynaecology OPD during 1 month as part of routine cervical screening. From each patient's sample two smears were prepared and fixed in 95% ethyl alcohol after administering the informed consent to each patient separately. Patients from whom only one smear was obtained or whose smears were unsatisfactory were excluded from the study.
The two sets of cervical smears were labelled independently. One set of smears was stained by conventional Pap stain and the other by REAP in which absolute alcohol was replaced by 1% acetic acid in all steps except during fixation and prior to mounting.
The procedure for conventional pap and REAP staining is given in Table 1.[7]
Table 1.
Method of Conventional Pap stain and REAP staining[7]
| Conventional Pap Stain | REAP | ||
|---|---|---|---|
| 95% alcohol (for fixation) | 95% alcohol (for fixation) | ||
| 95% alcohol | 10 dips | 1% acetic acid | 10 dips |
| 80% alcohol | 10 dips | – | |
| 70% alcohol | 10 dips | - | |
| 50% alcohol | 10 dips | – | |
| Tap water | 10 dips | - | |
| Harris haematoxylin | 2 minutes | Harris Haematoxylin, preheated60°C | 10 dips |
| Scott’s Tap water | 3-5 min | Tap water | 10 dips |
| 50% alcohol | 10 dips | – | |
| 70% alcohol | 10 dips | – | |
| 80% alcohol | 10 dips | 1% acetic acid | 10 dips |
| 95% alcohol | 10 dips | – | |
| OG 6 | 1 min | OG 6 | 10 dips |
| 95% alcohol | 10 dips | – | |
| 95% alcohol | 10 dips | 1% acetic acid | 10 dips |
| EA | 10 min | EA | 10 dips |
| 95% alcohol | 40 dips | 1% acetic acid | 10 dips |
| 95% alcohol | 40 dips | – | |
| 100% alcohol | 10 dips | Methanol | 10 dips |
| Xylene | 10 dips | Xylene | 10 dips |
| Xylene | 10 dips | – | |
| Xylene | 10 dips | – | |
| DPX mount | Coverslip | DPX mount | Coverslip |
In conventional pap stain, the fixed smears are run through a series of descending ethyl alcohol grades (10 dips each) before nuclear staining. These ethyl alcohol grades are replaced by single 1% acetic acid step (10 dips) in REAP. The time with nuclear stain, Harris hematoxylin was reduced from 1 min in conventional pap to 10 dips in REAP, as the stain is preheated to 60°C. Heating haematoxylin to 60°C before staining facilitates rapid penetration of the stain. In REAP the bluing agent, Scott's tap water was replaced by ordinary tap water and the time was reduced from 3–5 min to 10 dips. The timings in OG-6 and EA were reduced from 1 min and 10 min, respectively, to 10 dips in REAP. After staining both REAP and Conventional Papanicolaou smears were mounted using DPX.
The smears stained with conventional pap were evaluated by the reporting consultant and a report with diagnosis was handed over to the patient. The smears stained by REAP were examined blindly by both the investigators for cytoplasmic and nuclear staining and a detailed report along with diagnosis was prepared. The conventional pap stained smears were reviewed and the staining, cytological findings, and diagnosis of REAP stained smears were compared with that of the conventional pap. Also, the cost-effectiveness and rapid turnaround time of REAP were evaluated in comparison to conventional pap stain.
The study was carried out after approval from the institutional ethics committee.
RESULTS
The age of the patients ranged from 22 to 60 years with a mean age of 38.3 years. A comparison of the staining quality of REAP and conventional pap stained smears is shown in Table 2.
Table 2.
Result of staining quality by conventional pap stain and REAP
| Procedure | Optimal |
Suboptimal |
||
|---|---|---|---|---|
| Cytoplasmic | Nuclear | Cytoplasmic | Nuclear | |
| Conventional Pap stain | 48 | 50 | 02 | - |
| REAP | 43 | 45 | 07 | 05 |
The cytoplasmic differentiation and transparency were optimal in 43 (86%) smears out of the 50 stained by REAP technique. In seven smears (14%), the cytoplasmic stain penetration was suboptimal in thick areas and overlapping cell clusters. The nuclear details and chromatin pattern were clear and crisp in 45 (90%) smears stained by REAP. In five smears (10%) nuclear staining was suboptimal.
The staining reaction of non-epithelial cells such as white and red blood cells was similar in both the techniques. There was no difference in staining of bacteria, however out of the 50 smears stained with REAP technique, the diagnosis of candidiasis was missed in two cases. In 48 (96%) out of 50 cases the findings and diagnosis of smears stained by REAP correlated with their corresponding smears stained with conventional pap stain.
The turnaround time for staining by REAP technique was 4 min as compared to 20 min in conventional pap stain. The cost per smear with REAP was much less than the cost of conventional pap smear. The cost of acetic acid was approximately 1/6thof the total cost of absolute alcohol.
DISCUSSION
For more than 50 years, Papanicolaou stain as introduced and modified by George N Papanicolaou has been used as the universal screening test for detection of cervical cancer.[1] Over the years, the conventional method has undergone various modifications in different laboratories in search of a rapid Pap stain that is as fast as Diff- Quik and provides cytomorphological features as exquisite as Pap stain.[8]
In the present study, an attempt was made to look for a rapid and economical alternative to conventional pap, which can be used for mass screening of cervical cancer. The study compared the staining quality, cytomorphological findings, and cost-effectiveness of REAP and conventional pap.
The quality of staining was evaluated on the basis of cytoplasmic and nuclear staining.
In the present study, 86% cases showed optimal cytoplasmic staining, whereas 14% cases demonstrated a suboptimal staining reaction with REAP. The suboptimal staining could have been due to presence of thick areas, overlapping of cells and partial air drying. The stain could not sufficiently penetrate the individual cells in areas with overlapping clusters. The results in the present study were comparable to those of previous studies where the optimal cytoplasmic staining was seen in 80–90% of cases.[7,9,10] The cytoplasmic differentiation and transparency in smears stained with REAP [Figure 1a] was comparable to those stained by conventional pap [Figure 1b].
Figure 1.
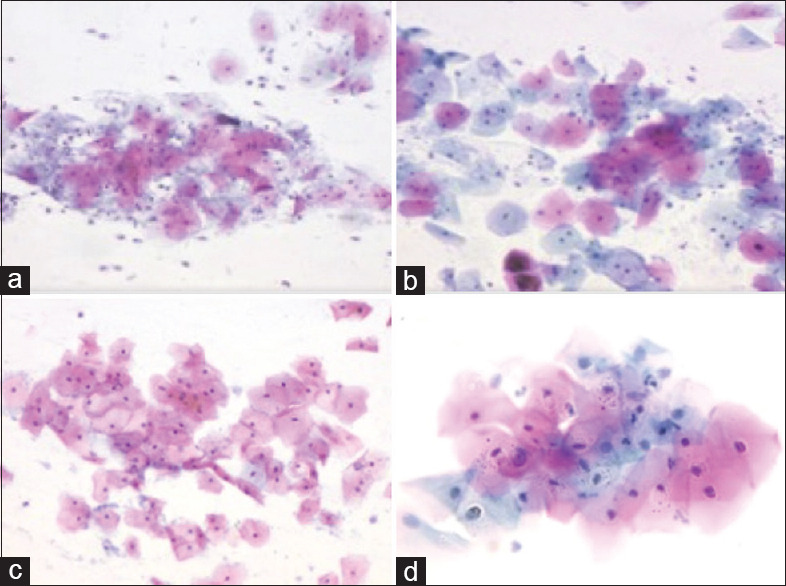
a. Squamous cells with eosinophilic and cyanophilic differentiation of cytoplasm (REAP, 200x), b. Conventional pap stained smear of the case in A (Pap, 200x), c. Squamous cells with vesicular nuclei (REAP, 200x), d. Conventional pap stained smear of the case in C (Pap, 200x)
In the present study, 90% cases showed optimal nuclear staining whereas 10% cases demonstrated a suboptimal staining reaction with REAP. The suboptimal staining could have been due to air drying artifacts. The results in the present study were comparable to those of Asthana et al. which showed 92% optimal nuclear staining with REAP. However, in the studies by Dighe et al. and Biswas et al., the suboptimal nuclear staining with REAP was much less. The nuclear staining in smears stained with REAP [Figure 1c] was comparable to those stained by conventional pap [Figure 1d].
The staining of endocervical cells was comparable by both REAP [Figure 2a and conventional pap [Figure 2b].
Figure 2.
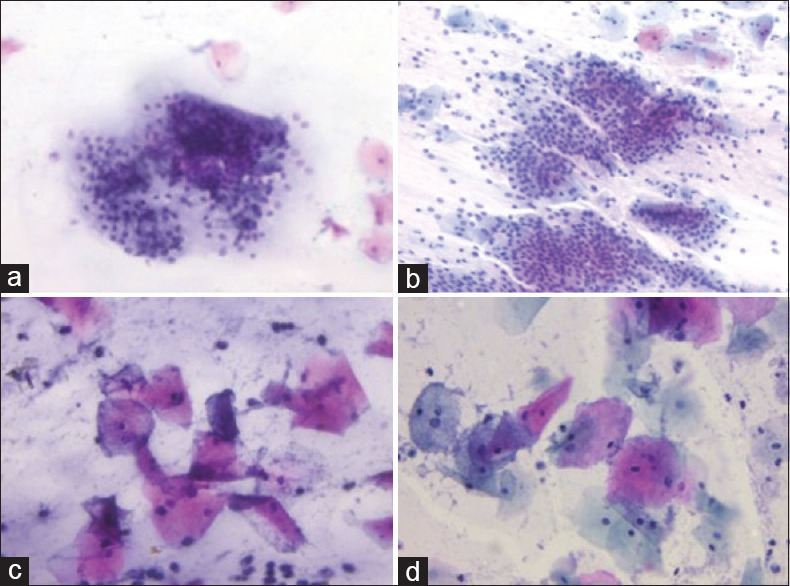
a. Endocervical cells in a honeycomb pattern with optimal cytoplasmic and nuclear staining (REAP, 200x), b. Conventional pap stained smear showing endocervical cells of the case in A (Pap, 200x), c. Smear showing coccobacilli and WBCs (REAP, 400x), d. Conventional pap stained smear showing coccobacilli of the case in C (Pap, 400x)
There was no difference in the staining reaction of non-epithelial cells and bacteria by REAP [Figure 2c] and conventional pap [Figure 2d].
The suboptimal staining of cytoplasm and nucleus by REAP did not interfere with the interpretation of results. In 48 of 50 cases, the cytomorphological findings and final impression of corresponding smears stained by REAP and conventional pap stain correlated. In two out of 50 cases stained by REAP the diagnosis of vaginal candidiasis was missed due to low fungal load and suboptimal staining.
The present study demonstrated a considerable decrease in turnaround time for REAP as compared to conventional pap stain which was comparable to those of previous studies and ranged from 3 to 4 min.[7,9,10]
The modifications in the conventional pap stain with decrease in the turnaround time has been achieved in many previous studies but the REAP method is addition helps reduce the cost of the staining also.[11] The present study also highlighted the fact that REAP is less expensive and more economical than conventional pap as it decreases the cost of test to 1/6th. The modifications of conventional pap stain have been described by various authors but to the best of our knowledge comparison of REAP with conventional pap has been done only in one study in the past. Hence, this study establishes the utility of REAP as a suitable alternative to conventional pap.
The simplicity of the procedure (uniform 10 dips) also reduced the risk of human errors during staining because there is no variation of time or dips from one staining counter to the other unlike in the conventional Pap where each container has different timings during staining.
The relative cost and ease of availability of acetic acid used in REAP and the time required for staining procedure makes it an efficient alternative to conventional pap stain for screening of cervical smears especially in the rural areas and district hospital labs where the resources are limited.
To conclude, REAP is a rapid, inexpensive, and excellent technique both in terms of cytoplasmic and nuclear staining with minimum alcohol use, as compared to conventional pap stain. It is a technician friendly procedure without compromising on staining quality and diagnostic standards. It promises a role in mass cervical cancer screening in a developing country like India where the incidence is on the rise and resources are poor.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This study was carried out as a part of STS project with ICMR.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Thakur M, Guttikonda VR. Modified ultrafast Papanicolaou staining technique: A comparative: A comparative study. J Cytol. 2017;34:149–53. doi: 10.4103/JOC.JOC_23_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnan S, Madsen E, Porterfield D, Varghese B. Advancing cervical cancer prevention in India: Implementation science priorities. Oncologist. 2013;18(Suppl):13–25. doi: 10.1634/theoncologist.18-S2-13. [DOI] [PubMed] [Google Scholar]
- 3.Holowaty P, Miller AB, Rohan T, To T. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst. 1999;91:252–8. doi: 10.1093/jnci/91.3.252. [DOI] [PubMed] [Google Scholar]
- 4.Prasad PR. Short-duration Papanicolaou stain (SPS)—an alternative to conventional Papanicolaou stain in routine cytopathology? Comp Clin Pathol. 2017;26:1285–8. [Google Scholar]
- 5.Shree VR, Das S. Comparative evaluation of conventional Papanicolaou stain (PAP) stain efficacy versus modified ultrafast Papanicolaou stain (MUFP) stain. Arch Cytol Histopathol Res. 2019;4:116–7. [Google Scholar]
- 6.Gupta S, Chachra KL, Bhadola P, Sodhan P. Modified Papanicolaou staining protocol with minimum alcohol use: A cost-cutting measure for resource-limited settings. Cytopathology. 2010;21:229–33. doi: 10.1111/j.1365-2303.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- 7.Biswas RR, Paral CC, Ramprasad Dey R, Biswas SC. Rapid economic, acetic acid, papanicolaou stain (REAP)-Is it suitable alternative to standard PAP stain? Al Ameen J Med Sci. 2008;1:99–103. [Google Scholar]
- 8.Agarwal P, Toi PC, Subramaniam H, Apoorva Lakshmi S. Prospective comparison of cytological specimen adequacy assessment by different rapid staining techniques for rapid on-site evaluation in fine needle aspiration cytology and their cost-effectiveness. Diagn Cytopathol. 2019;47:469–74. doi: 10.1002/dc.24139. [DOI] [PubMed] [Google Scholar]
- 9.Dighe SB, Ajit D, Pathuthara S, Chinoy R. Papanicolaou stain is it economical to switch to economic, acetic acid, papanicolaou stain? Acta Cytologica. 2006;50:643–6. doi: 10.1159/000326034. [DOI] [PubMed] [Google Scholar]
- 10.Asthana A, Singh AK. Comparison of the routine Papanicolaou staining technique with the rapid, economic, acetic acid, Papanicolaou (REAP) technique. IJMDS. 2014;3:484–9. [Google Scholar]
- 11.Choudhary P, Sudhamani S, Pandit A, Kiri V. Comparison of modified ultrafast Papanicolaou stain with the standard rapid Papanicolaou stain in cytology of various organs. J Cytol. 2012;29:241–5. doi: 10.4103/0970-9371.103942. [DOI] [PMC free article] [PubMed] [Google Scholar]


