Stress-induced depression is common worldwide. NAc, a “reward” center, is recently reported to be critical to confer the susceptibility to chronic social defeat stress (CSDS) and the depression-related outcome. However, the underlying molecular mechanisms have not been well characterized.
Keywords: CDK5, depression, NAc, nNOS, social defeat
Abstract
Stress-induced depression is common worldwide. NAc, a “reward” center, is recently reported to be critical to confer the susceptibility to chronic social defeat stress (CSDS) and the depression-related outcome. However, the underlying molecular mechanisms have not been well characterized. In this study, we induced depression-like behaviors with CSDS and chronic mild stress in male mice to mimic social and environmental factors, respectively, and observed animal behaviors with social interaction test, tail suspension test, and sucrose preference test. To determine the role of neuronal nitric oxide synthase (nNOS) and its product nitric oxide (NO), we used brain region-specifically nNOS overexpression and stereotaxic injection of NO inhibitor or donor. Moreover, the downstream molecular cyclin-dependent kinase 5 (CDK5) was explored by conditional KO and gene mutation. We demonstrate that nNOS-implicated mechanisms in NAc shell (NAcSh), including increased cell number, increased protein expression levels, and increased specific enzyme activity, contribute the susceptibility to social defeat and the following depression-like behaviors. NAcSh nNOS does not directly respond to chronic mild stress but facilitates the depression-like behaviors. The increased NAcSh nNOS expression after CSDS leads to the social avoidance and depression-like behaviors in defeated mice, which is dependent on the nNOS enzyme activity and NO production. Moreover, we identify the downstream signal in NAcSh. S-nitrosylation of CDK5 by NO contributes to enhanced CDK5 activity, leading to depression-related behaviors in susceptible mice. Therefore, NAcSh nNOS mediates susceptibility to social defeat stress and the depression-like behaviors through CDK5.
SIGNIFICANCE STATEMENT Stress-induced depression is common worldwide, and chronic exposure to social and psychological stressors is important cause of human depression. Our study conducted with chronic social defeat stress mice models demonstrates that nNOS in NAcSh is crucial to regulate the susceptibility to social defeat stress and the following depression-like behaviors, indicating NAcSh nNOS as the responding molecule to social factors of depression. Moreover, we discover the downstream mechanism of NAcSh nNOS in mediating the susceptibility is NO and S-nitrosylation of CDK5. Thus, NAcSh nNOS mediates susceptibility to social defeat stress through CDK5 is a potential mechanism for depression, which may interpret how the brain transduces social stress exposure into depression.
Introduction
Major depressive disorder (MDD) is one of the greatest public health problems in the world, and >320 million people (∼4.7% of the world's population) suffered from depression globally (World Health Organization, 2017). Based on the monoaminergic hypothesis, which was established a half-century ago, monoamine reuptake inhibitors are widely used in clinic for depression treatment, although these drugs have a limited efficacy and a slow onset of therapeutic action (Thompson et al., 2015; Perez-Caballero et al., 2019). Researchers have made numerous efforts for better understanding the mechanisms underlying depression and developing originally new antidepressants.
An excitatory synapse hypothesis of depression has been proposed. Chronic stress- and genetic susceptibility-caused changes in the glutamatergic synapses strength at the PFC, hippocampus, and NAc are thought to result in a dysfunction of corticomesolimbic reward circuitry that underlies many of the symptoms of depression (Thompson et al., 2015). The NMDAR antagonists are regarded as the prospectively therapeutic alternative for MDD in the future (Lang et al., 2018), especially after the finding that ketamine has rapid, robust, and sustained antidepressant-like effects in humans (Berman et al., 2000) and animals (N. Li et al., 2011). Neuronal nitric oxide synthase (nNOS), catalyzing the formation of nitric oxide (NO), can bind with NMDAR through postsynaptic density-95 (PSD-95) (Christopherson et al., 1999; Aarts et al., 2002). Both the enzyme and its production are reported to be closely related to neuropsychiatric disorders, including depression, anxiety, and bipolar disorders (Q. G. Zhou et al., 2018). We have demonstrated that hippocampal nNOS is essential for chronic mild stress (CMS)-induced depressive behaviors in mice (Q. G. Zhou et al., 2007, 2011). Recently, more evidence from animal and clinical studies supports the implication of NO in the pathophysiology of depression (Dhir and Kulkarni, 2011; Baranyi et al., 2015), and nNOS inhibitors (intraperitoneal or intrahippocampal injection) showed antidepressant-like properties (Q. G. Zhou et al., 2018). Thus, nNOS might be a novel therapeutic target for depressive disorder other than NMDAR. However, knowledge about how nNOS mediates depression is limited, especially nNOS located outside hippocampus.
NAc (also called ventral striatum), a “reward” center in the mesolimbic dopamine (DA) circuit, influencing the motivation to seek rewarding stimuli and motor behaviors (Smith et al., 2011; Floresco, 2015), has been repeatedly reported to be involved in depression and related mood disorders (Russo and Nestler, 2013; Thompson et al., 2015; Francis and Lobo, 2017). Since NAc integrates the information associated with motivation and reward (Stuber et al., 2011; Britt et al., 2012), it might possess some different properties in mediating depression compared with hippocampus (Belleau et al., 2019). Depression-like behaviors arising from environmental factors and social conflicts are well reproduced by CMS and chronic social defeat stress (CSDS) model in rodents, respectively (Venzala et al., 2013). NAc is critical to control the susceptibility to CSDS and the depression-like behaviors (Chaudhury et al., 2013; Francis et al., 2015; Heshmati et al., 2018). Regarding the regulation of depressive behaviors by NAc, great focus has been placed on classic transmitter DA and its receptors (Chaudhury et al., 2013; Tye et al., 2013; Francis et al., 2015; Heshmati et al., 2018), while the role of gas transmitter NO and its enzyme nNOS has not been characterized.
In NAc, nNOS staining is localized to aspiny interneurons, moderate in the core and robust in the shell subregion (Hoque and West, 2012). Although nNOS interneurons make up only 1%-2% of the neuronal population, they are thought to exert an important functional impact on neurotransmission through NO diffusion (West, 2002; West and Grace, 2004; Sagi et al., 2014). Here, we explored the function of NAc nNOS and its product NO in regulating susceptibility to social defeat stress and the depression-like behaviors in mice, revealed that nNOS in NAc responded to social stress (CSDS) and environmental stress (CMS) differently with hippocampal nNOS, and explored the possible downstream signal of NAc nNOS mediating CSDS-induced depression.
Materials and Methods
Animals
Male adult C57BL/6 mice (7-8 weeks) and male CD-1 mice (4 months, sexually experienced retired breeders) were purchased from Model Animal Research Center of Nanjing University (Nanjing, China) and Animal Core Facility of Nanjing Medical University (Nanjing, China), respectively. Homozygous nNOS KO mice (B6;129S4-Nos1tm1Plh/J, nNOS−/−, stock #002633, RRID:IMSR_JAX:002633, The Jackson Laboratory) and their WT control with similar genetic background (B6129SF2, nNOS+/+) were maintained in Model Animal Research Center of Nanjing University. Floxed Cdk5 (cyclin-dependent kinase 5 [CDK5]) mice (B6.129S4(Cg)-Cdk5tm1.1Lht/J, Cdk5flox/flox; stock #014156, RRID:IMSR_JAX:014156, The Jackson Laboratory) were gifts from Zhi-Gang Xu (Shandong University, Qingdao, China). Animals were housed at controlled temperature (20 ± 2°C) and 12:12 h light/dark cycle (lights on at 07:00) with access to food and water ad libitum. All animal experiments were conducted in accordance with the Institutional Animal Care and Use Committee of Nanjing Medical University.
Depression models
Exposure to stress increases the risk for depression in humans, so we used two animal models, CSDS and CMS, to induce depression-like behaviors in mice. CSDS involves repeated bouts of social subordination, whereas CMS involves a series of repeated physical stresses (Nestler and Hyman, 2010; Russo and Nestler, 2013).
CSDS
CSDS was conducted as described previously (Golden et al., 2011). Briefly, retired male breeder CD1 mice (singly housed) were screened for 3 consecutive days before the experiment to verify their aggressive characteristics. Over the following 10 consecutive days, each experimental C57BL/6 male mouse (intruder) was introduced into the home cage of a novel aggressive CD1 mouse (resident) for 10 min physical attack and basic observation throughout the defeat sessions was made to ensure that high-quality aggressive bouts occur. In cases in which repeated defeats lead to the development of open wounds exceeding 1 cm, the intruder mouse was immediately removed from the experiment and euthanasia is done. The offending aggressor mouse was removed too. After the 10 min physical attack, the intruders were kept in sensory contact with the residents for 24 h until the next defeat, using a perforated Plexiglas partition to divide the resident home cage in two compartments. Control mice were housed in pairs, one on each side of a perforated Plexiglas partition, and they were never in physical or sensory contact with CD1 mice. The animals underwent behavioral tests 24 h after 10 or 7 d CSDS.
CMS
The procedure of CMS was designed as we described previously (Q. G. Zhou et al., 2011). In brief, the CMS protocol consists of the sequential application of a variety of mild stressors, including restraint, forced swimming, water and/or food deprivation 24 h and following placing the empty bottles 1 h, housing in wet sawdust, reversal of the light/dark cycle, an electric shock (0.75 mA, 3 s) in 3 min, and exposure to the same device without shock in the same period time on next day, in a schedule that lasts for 4 weeks. The schedule is repeated thereafter from week 1 and lasted for 4 weeks to induce depressive behaviors. The protocol of subthreshold CMS was the same as CMS, except that the total duration was 2 weeks and no obvious behaviors alteration appeared in stressed mice.
Behavioral tests
We conducted three behavioral tests to measure the depression-like behaviors in mice, social interaction test (SIT, social avoidance), tail suspension test (TST, behavioral despair), and sucrose preference test (SPT, anhedonia) (Nestler and Hyman, 2010; Golden et al., 2011).
SIT
After the last stress, the experimental mice were singly housed and were tested in the SIT 24 h later. This test was composed of two 2.5 min phases as described previously (Isingrini et al., 2016). In the first 2.5 min, each experimental mouse was placed in an open field (45 cm wide × 45 cm deep × 45 cm high) with a Plexiglas wire mesh enclosure (10 cm wide × 6.5 cm deep × 42 cm high) in which the wire mesh was empty (no target). In the second 2.5 min, an unfamiliar CD1 mouse (target) was placed inside the wire mesh. The interaction zone of the test arena encompasses a 27 cm wide × 15 cm deep rectangular area projecting 8.5 cm around the wire-mesh enclosure. The corner zones encompass a 9 cm × 9 cm area projecting from both corner joints opposing the wire-mesh enclosure. The behaviors of the experimental mouse were recorded and analyzed with TopScan LITE software (RRID:SCR_014494, Clever Sys), and the proximity of the body to interaction zone was regarded as the interaction. Between the two phases, the experimental mouse was returned to its home cage for 30 s. The ratio of interaction was calculated by dividing time spent in the interaction zone with target (CD1 mouse) by that without target.
TST
After SIT, mice were allowed to rest in their home cage for 30 min, and were then assessed in TST as we described previously (Q. G. Zhou et al., 2011). Mice were suspended by their tail with adhesive tape to a hook in a soundproof box. The test session was videotaped for 6 min, and the immobility was analyzed using TopScan LITE software (RRID:SCR_014494, Clever Sys).
SPT
The SPT was performed as we reported (Liu et al., 2018). Mice were allowed to rest in their home cage for 30 min after TST, and then placed into SPT apparatus individually, given two regular bottles for 24 h: one containing 1% (wt/vol) sucrose water and one containing tap water (regular water). Then, the mice were deprived of both food and water for 12 h. Immediately after that, all animals were given 10 h access to one tube of 1% (wt/vol) sucrose solution and one tube of regular water. Each tube was weighed before and after the test. At the end of the test, the mice were returned to group housing with ad libitum food and water. The sucrose preference for each mouse was evaluated as follows: [sucrose consumption/(sucrose consumption + regular water consumption)] × 100%.
Recombinant virus and stereotaxic infection
To specifically manipulate the target protein, the recombinant virus was infused into the desired brain region with a stereotaxic apparatus. The recombinant viruses used in this study, including adeno-associated virus AAV-hSyn-nNOS-eGFP (2.8 × 1012 vg/ml), AAV-hSyn-inactive nNOS-eGFP (2.8 × 1012 vg/ml), AAV-hSyn-eGFP (2.8 × 1012 vg/ml), AAV-CMV-CDK5-C83/157S-3Flag-T2A-eGFP (1.3 × 1013 vg/ml), and AAV-CMV-3Flag-T2A-eGFP (1.3 × 1013 vg/ml), were produced by GeneChem. AAV-hSyn-Cre (1.0 × 1013 vg/ml, catalog #CN867) was purchased from Neuron Biotech. AAV-hSyn-nNOS-eGFP expressed the fusion protein of mouse full-length nNOS and fluorescent protein eGFP. AAV-hSyn-inactive nNOS-eGFP expressed the fusion protein of eGFP and mouse nNOS truncation mutants, while mouse nNOS truncation mutants is a catalytically inactive protein with deletion of aminoacids 1373-1429 of nNOS (Hallmark et al., 1999). AAV-CMV-CDK5-C83/157S-3Flag-T2A-eGFP expresses 3Flag-tagged mouse CDK5 with two point mutations: Cys 83 Ser and Cys 157 Ser. This mutant CDK5 could not be activated by S-nitrosylation (Qu et al., 2011). Desired AAV was microinjected into NAc or ventral hippocampus of control, susceptible, or resilient mice 3 weeks before behavioral tests. Mice were anesthetized, and desired virus in appropriate volume (1 μl for AAV-hSyn-nNOS-eGFP, AAV-hSyn-inactive nNOS-eGFP, and AAV-hSyn-eGFP, 0.4 μl for AAV-hSyn-Cre, AAV-CMV-CDK5-C83/157S-3Flag-T2A-eGFP, and AAV-CMV-3Flag-T2A-eGFP) was microinjected into the shell of NAc (NAcSh) or ventral hippocampus bilaterally using a stereotaxic instrument for mice (Stoelting) at a rate of 2 nl/s. The The coordinates for NAcSh are as follows: AP, 1.6 mm; ML, ±1.3 mm; DV, −4.5 mm; hippocampus: AP, −2.9 mm; ML, ±2.8 mm; DV, −3.2 mm. Following injection, the needle was withdrawn over a course of 10 min to assure even distribution of the virus.
Cannula implantation and drug microinjection
Cannula was pre-implanted when drug microinjection was needed. Five days before behavioral tests, stainless-steel guide cannula (26 gauge, 4.0 mm, RWD Life Science) were implanted into NAcSh (AP, 1.6 mm; ML, ±1.3 mm) and fixed to the skull with adhesive luting cement and acrylic dental cement. Following surgery, a stainless-steel obturator was inserted into the guide cannula to avoid obstruction until microinjection was made. Thirty minutes before behavioral tests, mice were briefly head-restrained while the stainless-steel obturator was removed and an injection tube (30 gauge, 4.5 mm, RWD Life Science) was inserted into the guide cannula. A dose of L-VNIO (Alexis Biochemicals, catalog #270-216), C-PTIO (Sigma Millipore, catalog #C221), DETA/NONOate (Sigma Millipore, catalog #A5581), or roscovitine (ROS; Sigma Millipore, catalog #R7772) was slowly infused bilaterally at a flow rate of 0.2 μl/min to a total volume of 1 μl. The concentration of L-VNIO (1.5 mm), C-PTIO (10 μm), and DETA/NONOate (100 μm) was the same as we used in our published work (Luo et al., 2010; Liang et al., 2020), and the concentration of ROS (50 μm) was referred to literature (K. Li et al., 2016). The injection tube was left in place for 5 min after injection to reduce backflow and then was slowly withdrawn. The stainless-steel obturator was reinserted into the guide cannula.
Immunofluorescence and cell counting
To determine the number of nNOS+ cells or confirm the effective infection of recombinant virus, immunofluorescent labeling was performed as we previously did (Luo et al., 2010, 2014; L. Zhou et al., 2010). The 4 or 5 mice whose social interaction ratio was nearest to the average value of each group were chosen for immunofluorescence. Mice were transcardially perfused with 4% PFA (w/v) in PB (0.1 m, pH 7.4), and brains were removed and postfixed overnight. Serial vibratome sections (40 μm) were processed. After blocking in PBS containing 3% normal goat serum, 0.3% (w/v) Triton X-100, and 0.1% BSA at room temperature for 1 h, slices were incubated in primary antibody diluted in blocking solution overnight at 4°C and washed. Slices were then incubated in secondary antibody for 2 h at room temperature. The primary antibodies used were as follows: rabbit anti-nNOS (1:400; Thermo Fisher Scientific, catalog #61-7000, RRID:AB_2313734), rabbit anti-GFP (1:1000, Abcam, catalog #ab290, RRID:AB_303395), rabbit anti-CDK5 (1:100; Abcam, catalog #ab40773, RRID:AB_726779), guinea pig anti-c-Fos (1:200; Synaptic Systems, catalog #226-004, RRID:AB_2619946), and mouse anti-NeuN (1:500; Millipore, catalog #MAB377, RRID:AB_2298772). Secondary antibodies used were as follows: goat anti-rabbit Cy3 (1:400; Jackson ImmunoResearch Laboratories, catalog #111-165-003, RRID:AB_2338000), goat anti-rabbit Dylight 488 (1:400; Jackson ImmunoResearch Laboratories, catalog #111-545-003, RRID:AB_2338046), goat anti-mouse Cy3 (1:200; Jackson ImmunoResearch Laboratories, catalog #115-165-003, RRID:AB_2338680), and goat anti-guinea pig Alexa-488 (1:200; Abcam, catalog #ab150185, RRID:AB_2736871). Finally, slices were mounted onto slides, and images were acquired with a fluorescence microscope (Axio Imager, Carl Zeiss) or a confocal laser-scanning microscope (LSM700, Carl Zeiss).
For nNOS+ cells counting, the immunofluorescent images were analyzed with UTHSCSA ImageTool 3.0 software. The analysis was conducted on every third section in a series of 40 µm coronal sections throughout NAc (from AP 1.94 mm to AP 0.86 mm, ∼9 sections per mouse). To determine the total number of nNOS+ cells per NAc, the counts from sampled sections were averaged, and the mean values were multiplied by the total number of sections. The product was regarded as the final value of 1 mouse. This is an approximation and not an unbiased stereological estimate or an accurate count.
Biochemical assays
After the last test, mice were decapitated under anesthesia, and the brains were quickly placed on the ice; 1 mm brain slices were cut by two knife blades, which held together with a 1 mm interstice. After removal of the olfactory bulb, the second 1 mm coronal section (beginning from bregma ∼1.94 mm) was selected to dissect bilateral NAc and mPFC, while the seventh 1 mm coronal section (beginning from bregma ∼−2.92 mm) was selected to dissect bilateral VTA. According to mouse brain atlas hires, bilateral hippocampus tissues were dissected using forceps before the dissection of VTA. Samples were stored at −80°C until further use.
Western blot
To determine the change of protein levels of nNOS, CDK5, and S-nitrosylated CDK5 in CSDS-induced depression, Western blot was performed as we did previously (Luo et al., 2010, 2014; L. Zhou et al., 2010). Tissues from bilateral NAc were lysed in 100 mm HEPES containing 200 mm NaCl, 10% glycerol, 2 mm Na4P2O7, 2 mm DTT, 1 mm EDTA, 1 mm benzamidine, 0.1 mm Na3VO4, 1 μm pepstatin, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μm PMSF at PH 7.4. After lysis for 30 min in ice, samples were centrifuged at 12,000 × g, 4°C for 15 min. The samples containing equivalent amounts of protein were applied to acrylamide denaturing gels (SDS-PAGE). The separated proteins were transferred onto Immobilon-P Transfer Membranes (Millipore). Blotting membranes were incubated with blocking solution [7.5% nonfat dried milk powder dissolved in PBST buffer (pH 7.5, 10 mm Tris-HCl, 150 mm NaCl, and 0.1% Tween 20)] for 1 h at room temperature, washed 3 times, and then were incubated with primary antibodies in PBST overnight at 4°C. The primary antibodies were as follows: rabbit anti-nNOS (1:1000; Thermo Fisher Scientific, catalog #61-7000, RRID:AB_2313734), mouse anti-Flag (1:4000; Sigma Millipore, catalog #F1804, RRID:AB_262044), and rabbit anti-CDK5 (1:800; Abcam, catalog #ab40773, RRID:AB_726779). Internal control was performed using mouse anti-GAPDH (1:4000; KangChen Bio-tech, catalog #KC-5G4, RRID:AB_2493106), rabbit anti-β-actin (1:2000; Bioss, catalog #bs-0061R, RRID:AB_10855480), or mouse anti-tubulin (1:5000; Bioworld Technology, catalog #BS1482M). After several washes with PBST buffer, the membranes were incubated for 2 h with appropriate HRP-linked secondary antibody [goat anti-mouse HRP (1:8000, Multi Science, catalog #70-GAM0072, RRID:AB_2827834) or goat anti-rabbit HRP (1:6000, Multi Science, catalog #70-GAR0072)]. The membranes were then processed with enhanced chemiluminescence Western blotting detection reagents (Bio-Rad. The films were scanned with ChemiDOCTM MP Imaging System (Bio-Rad), avoiding densitometric saturation, and densitometry was performed using Image LabTM software (Bio-Rad).
nNOS-specific activity assay
To explore the role of nNOS in regulating the susceptibility to CSDS, the specific enzyme activity of nNOS was determined. Bilateral NAc was collected and homogenized in ice-cold PBS, pH 7.4, and analyzed using the Nitric Oxide Assay Kit (Beyotime Biotech, catalog #S0023). Because NO has an extremely short half-life, we quantified total NOS (including nNOS, eNOS, and iNOS) activity by measuring the concentrations of the two stable NO products nitrate and nitrite. According to the protocol of the manufacturer, the assay included a process to convert nitrate to nitrite and then to use a Greiss reaction to measure the nitrite concentrations. Absorbance of the samples was measured at 540 nm. To calculate nNOS activity, the measured value of total NOS activity of nNOS KO group was regarded as background from eNOS and iNOS, and was subtracted from the total NOS activity value of each group. Finally, the nNOS-specific activity was expressed as pmol nitrate and nitrite/min/mg of protein.
CDK5-specific activity assay
To explore the role of CDK5 in regulating the susceptibility to CSDS, the specific enzyme activity of CDK5 was determined. Analysis was conducted by a GENMED in vitro CDK5 Kinase Activity Kit (catalog #GMS50151.2.3v.A, GENMED Scientifics) with tissues from bilateral NAc. According to the instructions, ADP was produced with the oxidation of NADH using phosphoenolpyruvate catalyzed by lactic dehydrogenase and pyruvate kinase. In brief, a 100 µl mixture containing tissue lysate (250 µg) was incubated for 5 min at 30°C with the peptide substrate PKTPKKAKKL and the pyruvate kinase/lactic dehydrogenase system in the presence of the selective CDK5/p25 inhibitor BML259. The oxidation of NADH was measured by examining the absorbance at 340 nm using a spectrophotometer. The CDK5-specific activity was expressed as nmol NADH/min/mg of protein.
Biotin-switch assay
To determine the S-nitrosylation of CDK5 by NO, biotin-switch assay was conducted. Biotin-Switch Assay Kit (catalog #10006518, Cayman) was used to complete the biotin-switch of tissue lysate until the biotinylated proteins were precipitated with acetone. The assay was performed in the dark according to the manufacturer's instructions. Briefly, tissues from bilateral NAc were lysed and incubated in wash buffer containing blocking reagent and 1% Triton X-100 for 30 min at 4°C. Proteins were precipitated with acetone for 1 h at −20°C. After adding Buffer B containing 1% Triton X-100, reducing and labeling reagents, proteins were incubated at room temperature for 1 h. Proteins were precipitated with acetone for 1 h at −20°C. Then, biotinylated proteins were resuspended in 250 μl HENS buffer plus 500 μl neutralization buffer (20 mm HEPES, 100 mm NaCl, 1 mm EDTA, 0.5% Triton X-100) and precipitated with 50 μl prewashed avidin-affinity resin beads (Sigma Millipore) at room temperature for 1 h or overnight at 4°C. The beads were washed 5 times at 4°C using wash buffer (0.05 m HEPES buffer, pH 7.1, containing 0.15% Triton X-100, 0.15 m NaCl, and 0.1 × 10−3 m sodium orthovanadate). Biotinylated proteins were eluted using 30 μl elution buffer (20 mm HEPES, 100 mm NaCl, 1 mm EDTA, 100 mm β-mercaptoethanol) and heated at 100°C for 5 min in reducing SDS-PAGE loading buffer. Finally, biotinylated proteins were detected by immunoblotting using rabbit anti-CDK5 (1:800; Abcam, catalog #ab40773, RRID:AB_726779).
Experimental design and statistical analyses
Most experiments in this study were performed with a single cohort, except two experiments (see Figs. 7I-L, 8L-O). For these two experiments, data from two cohorts were pooled together. No sample-size calculation was performed in this study. Sample size was 8-18 mice for animal behavioral tests and 4 or 5 mice for biochemical measurements, according to previously published studies (Chaudhury et al., 2013; Tye et al., 2013; Cheng et al., 2019) and our prior experience (Hu et al., 2012; L. J. Zhu et al., 2014; Liang et al., 2020). The accurate sample size is given in each figure legend. For biochemical measurements, no data were excluded from the analysis unless the data were not successfully obtained for technical or accidental reasons. For behavioral tests, animals were excluded from the analysis if accidental events occurred, such as no movement in SIT, fall or climb in TST, water leaking in SPT, and escape. The data from the mice whose brain region was not been correctly targeted in microinjection were also excluded. The details of excluded animals, if any, are provided in each figure legend. Investigators were blind to group treatment when assessing the outcome.
Figure 7.
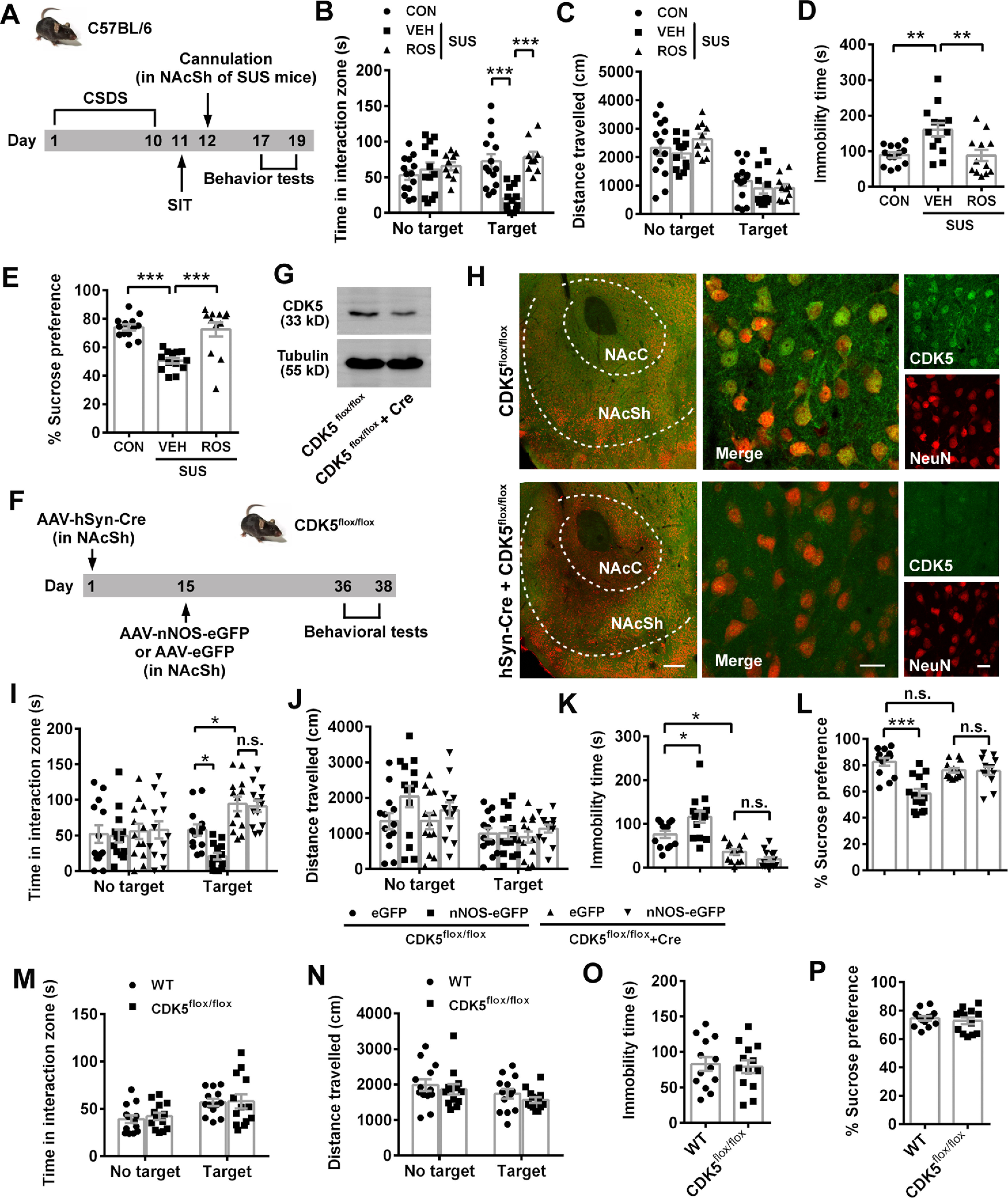
CDK5 activity is required for NAcSh nNOS-mediated susceptibility to CSDS. A, Scheme illustrating experimental design for B-E. B-E, Scatter plots showing time spent in interaction zone (B) and total distance traveled (C) in SIT (n = 14, n = 13, and n = 10, respectively), immobility time in TST (D, n = 13, n = 13, and n = 12, respectively), and sucrose preference percentage in SPT (E, n = 13, n = 13, and n = 12, respectively) of control (CON), vehicle-treated (VEH), or ROS-treated susceptible mice. Two SUS+ROS mice in SIT were excluded because of no movement. One CON mouse climbed up in TST and was excluded. One CON mouse in SPT was excluded because of water leaking. F, Experimental designed scheme for G-L. G-H, Representative Western blots (G) and immunofluorescent images (H) showing effective KO of CDK5 in neurons of NAcSh. Scale bars: left, 200 μm; middle, 20 μm; right, 20 μm. I-L, Scatter plots showing time spent in interaction zone (I) and total distance traveled (J) in SIT, immobility time in TST (K), and sucrose preference percentage in SPT (L). n = 13, n = 14, n = 13, and n = 13, respectively. Three mice (one in eGFP+CDK5flox/flox group, one in eGFP+CDK5flox/flox+Cre group, and one in nNOS-eGFP+CDK5flox/flox+Cre group) were excluded from analysis because brain region was not correctly targeted in microinjection. M-P, Scatter plots showing time spent in interaction zone (M) and total distance traveled (N) in SIT, immobility time in TST (O), and sucrose preference percentage in SPT (P). n = 13 for each group. Data are mean ± SEM. *p < 0.05. **p < 0.01. ***p < 0.001. n.s., not significant.
Figure 8.
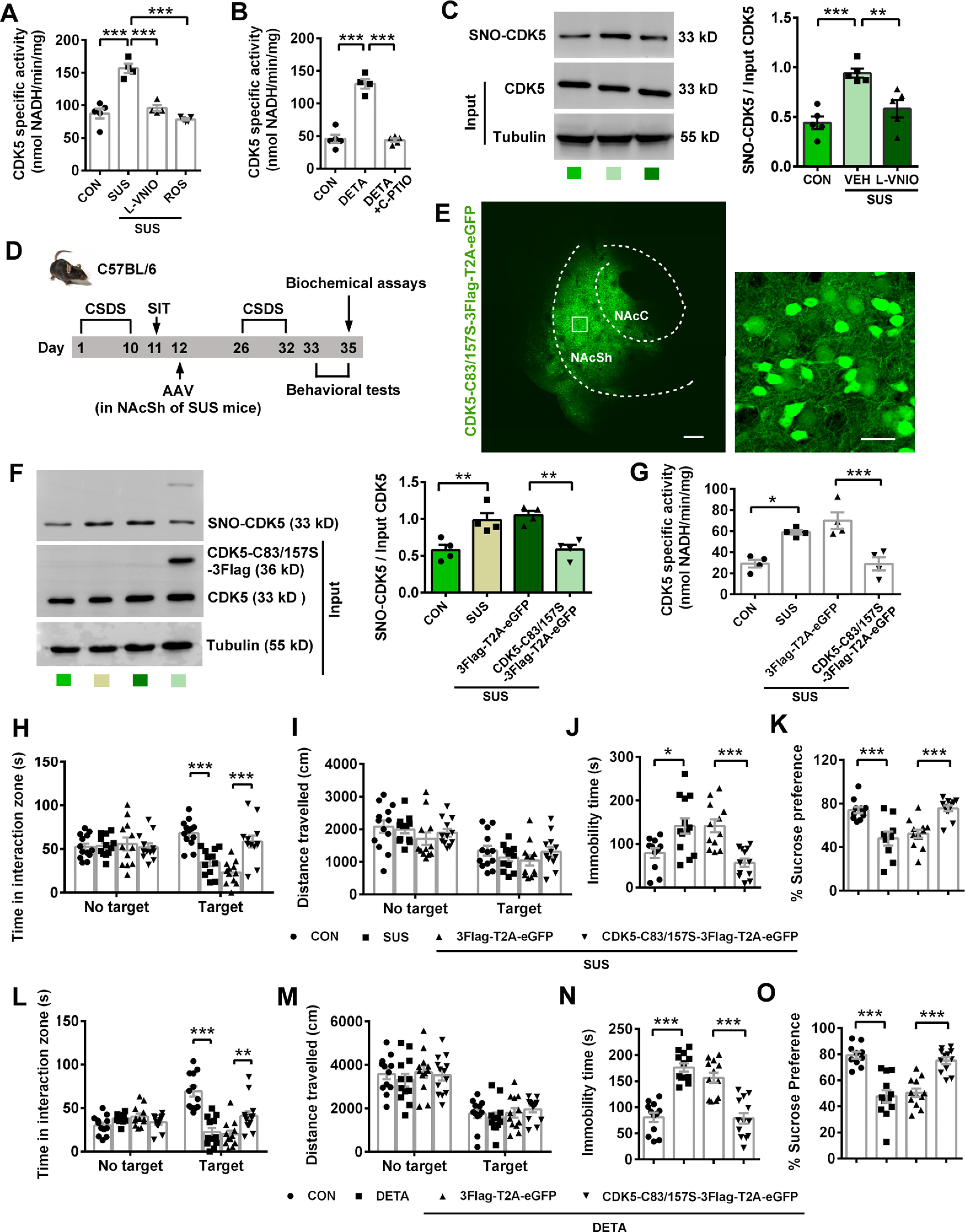
NAcSh nNOS controls susceptibility to CSDS through elevated CDK5 activity by S-nitrosylation of CDK5. A, Scatter plot showing CDK5-specific activity of control (CON) and susceptible (SUS) mice exposed to CSDS. L-VNIO or ROS was delivered into bilateral NAcSh through an implanted cannula. n = 5, n = 4, n = 4, and n = 5, respectively. B, Scatter plot showing CDK5-specific activity of control (CON), DETA/NONOate, and DETA/NONOate+C-PTIO groups. DETA/NONOate with or without C-PTIO was delivered into bilateral NAcSh through an implanted cannula 30 min before measurement. n = 5, n = 4, and n = 5, respectively. C, Representative blots (left) and scatter plot (right) showing S-nitrosylation of CDK5 in control (CON), vehicle-treated (VEH), and L-VNIO-treated susceptible mice. n = 5 for each group. D, Experimental design for E-K. AAV-CMV-CDK5-C83/157S-3Flag-T2A-eGFP or its control AAV-CMV-3Flag-T2A-eGFP was bilaterally microinjected into NAcSh of susceptible mice. Mice in CON and SUS groups were not treated with any viral injection. E, Representative fluorescent images showing effective AAV-CMV-CDK5-C83/157S-3Flag-T2A-eGFP infection in NAcSh. The extent of AP spread of the virus was ∼1.2 mm and almost covered the entire NAc. Scale bars: left, 20 μm; right, 200 μm. F, Representative blots of biotin-switch assay (left) and scatter plots (right) showing S-nitrosylated level of CDK5 (SNO-CDK5) in NAcSh. n = 4 for each group. G, Scatter plot showing CDK5 activity in NAcSh. n = 4 for each group. H-K, Scatter plots showing time spent in interaction zone (H) and total distance traveled (I) in SIT (n = 13, n = 12, n = 12, and n = 12, respectively), immobility time in TST (J, n = 11, n = 13, n = 12, and n = 12, respectively), and sucrose preference percentage in SPT (K, n = 11, n = 9, n = 12, and n = 12, respectively). One SUS mouse in SIT was excluded from analysis because of no movement. Two CON mice climbed up in TST and were excluded. Two CON mice and 2 SUS mice escaped during SPT, and 2 SUS mice were excluded from the analysis because of water leaking in SPT. L-O, DETA/NONOate-induced social avoidance and depression-like behaviors are dependent on S-nitrosylation of CDK5. Scatter plots showing time spent in interaction zone (L) and total distance traveled (M) in SIT (n = 12, n = 12, n = 12, and n = 13, respectively), immobility time in TST (N, n = 12, n = 12, n = 12, and n = 13, respectively), and sucrose preference percentage in SPT (O, n = 10, n = 12, n = 12, and n = 12, respectively). Two mice in the CON group and 1 mouse in the DETA+ CDK5-C83/157S-3Flag-T2A-eGFP group in SPT were excluded from analysis because of water leaking. AAV-CMV-CDK5-C83/157S-3Flag-T2A-eGFP or AAV-CMV-3Flag-T2A-eGFP was microinjected into bilateral NAcSh, and cannula was implanted. Three weeks later, DETA/NONOate was delivered through the cannula. Data are mean ± SEM. *p < 0.05. **p < 0.01. ***p < 0.001.
Appropriate parametric statistics were used to test our hypothesis. Comparisons among multiple groups were made by Tukey's post hoc test following one-way or two-way ANOVA, and comparisons between two groups were made by Sidak's post hoc test following two-way ANOVA, according to the recommendation of GraphPad Prism 6 software (RRID:SCR_002798). Two independent variables in SIT were target and treatment or genotype or phenotype, two independent variables in nNOS+ cells counting experiment were location and phenotype, and one independent variable in other experiments was treatment or genotype or phenotype. Comparisons between two groups were made with unpaired two-tail Student's t test (GraphPad Prism 6 software, RRID:SCR_002798). Data are presented as mean ± SEM. p < 0.05 is considered statistically significant. Statistical details of experiments are shown in results, figures, and figure legends.
Results
nNOS is specifically upregulated in NAc shell of susceptible mice exposed to CSDS
In order to investigate the contribution of social factor to depression, we used the mouse CSDS model (Fig. 1A) and divided the defeated mice into susceptible and resilient subtypes by social interaction ratio (Fig. 1B), according to a previous report (Golden et al., 2011). Susceptible mice spent less time in the interaction zone with target than control and resilient mice (Fig. 1C; ANOVA, F(2,78) = 11.34, p < 0.0001 for interaction; F(1,78) = 2.838, p = 0.096 for target; F(2,78) = 2.565, p = 0.083 for phenotype). Post hoc test following ANOVA, p < 0.0001 for CON vs SUS, p = 0.0008 for SUS vs RES and p = 0.0838 for CON vs RES with target; p = 0.261 for CON vs SUS, p = 0.369 for SUS vs RES and p = 0.977 for CON vs RES with no target, whereas the locomotor activities among groups were similar with or without target (Fig. 1D; ANOVA, F(2,78) = 0.057, p = 0.945 for interaction; F(1,78) = 35.93, p < 0.0001 for target; F(2,78) = 0.814, p = 0.447 for phenotype). Post hoc test following ANOVA, p = 0.991 for CON vs SUS, p = 0.847 for SUS vs RES and p = 0.811 for CON vs RES with target; p = 0.992 for CON vs SUS, p = 0.586 for SUS vs RES and p = 0.570 for CON vs RES with no target. Behavioral despair (TST) and anhedonia (SPT) were successfully induced in susceptible mice (Fig. 1E,F; post hoc test following ANOVA, F(2,30) = 5.848, p = 0.028 for CON vs SUS, p = 0.011 for SUS vs RES, and p = 0.951 for CON vs RES in Fig. 1E; F(2,33) = 10.43, p = 0.002 for CON vs SUS, p = 0.0009 for SUS vs RES, and p = 0.993 for CON vs RES in Fig. 1F). Yet, there was still substantial overlap between susceptible and resilient mice in TST and SPT. As well as the different behavioral phenotypes, susceptible and resilient mice showed upregulated and unchanged nNOS expression levels in NAc, respectively (Fig. 1G; post hoc test following ANOVA, F(2,9) = 20.47, p = 0.0007 for CON vs SUS, p = 0.002 for SUS vs RES, p = 0.808 for CON vs RES). nNOS upregulation was not found in hippocampus, mPFC, or VTA of susceptible mice (Fig. 1H; ANOVA: F(2,9) = 0.102, p = 0.904 for hippocampus; F(2,9) = 0.041, p = 0.960 for mPFC; F(2,9) = 0.040, p = 0.961 for VTA), which were known regions related to modulation of depressive behaviors. The results indicated that nNOS in NAc was specifically sensitive to CSDS-induced depression. Moreover, because NAc consists of two primary segments, a medial “core” and a more lateral “shell” (Zahm and Brog, 1992), and they may subserve distinct behavioral functions (Floresco, 2015), we examined in situ nNOS expression by immunofluorescence. As shown in Figure 1I, J, the number of nNOS+ neurons in NAc core (NAcC) was similar in control, susceptible and resilient mice, but significantly increased in NAcSh of susceptible mice (ANOVA, F(2,24) = 44.71, p < 0.0001 for interaction; F(1,24) = 454.0, p < 0.0001 for location; F(2,24) = 62.24, p < 0.0001 for phenotype). Post hoc test following ANOVA, p = 0.510 for CON vs SUS and p = 0.620 for SUS vs RES in core; p < 0.0001 for CON vs SUS and p < 0.0001 for SUS vs RES in shell. Thus, upregulated nNOS expression in susceptible mice specifically occurred in NAcSh. We next investigated whether the activity of nNOS enzyme in nNOS-expressing neurons in NAcSh was important to mediate susceptibility to CSDS. In agreement with the protein expression and cell counting, nNOS-specific activity in NAc was obviously enhanced in susceptible, but not resilient, mice (Fig. 1K; post hoc test following ANOVA, F(2,12) = 55.62, p < 0.0001 for CON vs SUS, p < 0.0001 for SUS vs RES, p = 0.696 for CON vs RES). We labeled neurons with nNOS and c-Fos (a widely used indicator of cell activation) antibodies. Unexpectedly, there was no nNOS+ neuron in NAcSh colabeled with c-Fos 60 min after SIT, TST, or SPT (Fig. 1L), suggesting that the regulation of depression-related behaviors by NAcSh nNOS may be independent of nNOS+ cell activity.
Figure 1.
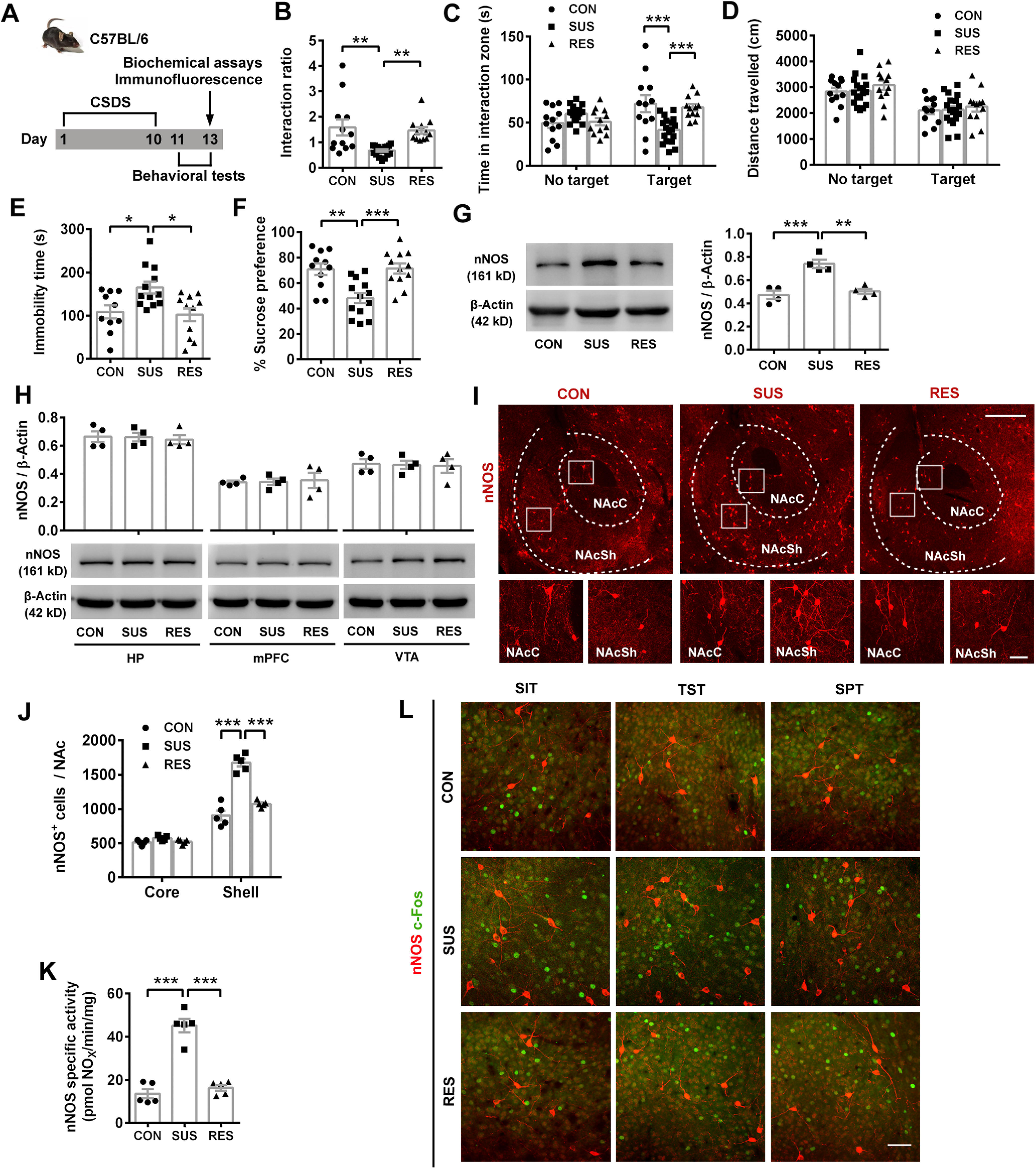
CSDS causes increased nNOS expression specifically in NAc shell. A, Scheme illustrating experimental design for B-K. B-D, Scatter plots showing social interaction ratio (B), time spent in interaction zone (C), and total distance traveled (D) of undefeated (control, CON, n = 12) and defeated (susceptible, SUS, n = 18; resilient, RES, n = 12) mice in SIT after 10 d CSDS. E, F, Scatter plots showing immobility time in TST (E, n = 10, n = 12, and n = 11, respectively), and sucrose preference percentage (F, n = 11, n = 13, and n = 12, respectively). One CON mouse escaped after SIT, and one video with tri-channel recordings for 1 mouse in each group of TST was damaged. G, Representative blots (left) and scatter plot (right) showing nNOS expression in NAc. n = 4 for each group. H, Scatter plot (top) and representative blots (bottom) showing nNOS expression in hippocampus (HP), mPFC, and VTA; n = 4 for each group. I, Representative immunofluorescent images labeled with nNOS antibody. Scale bars: top, 400 μm; bottom, 50 μm. J, Scatter plot showing the number of nNOS-positive neurons in shell and core of NAc. n = 5 for each group. K, Scatter plot showing nNOS-specific activity. n = 5 for each group. L, Representative immunofluorescent images of NAcSh labeled with nNOS and c-Fos antibodies. Mice were subjected to SIT, TST, or SPT, and killed 60 min after each test. Similar results were obtained with 3 mice for each group. Scale bar, 50 μm. Data are mean ± SEM. *p < 0.05. **p < 0.01. ***p < 0.001.
nNOS overexpression in NAcSh promotes susceptibility to depression
Next, we determined whether nNOS in NAcSh alone was sufficient to lead to depression-related behaviors. To avoid the influence of nNOS background, we overexpressed full-length nNOS with a recombinant AAV (AAV-hSyn-nNOS-eGFP) in NAcSh neurons of nNOS gene KO mice (Fig. 2A). Three weeks after the virus microinjection, the effective and accurate infection of NAcSh (Fig. 2B) and considerable nNOS-eGFP expression were confirmed (Fig. 2C). Compared with AAV-hSyn-eGFP-infected mice, AAV-hSyn-nNOS-eGFP-infected mice spent much less time in the interaction zone with target (Fig. 2D; ANOVA, F(1,46) = 19.99, p < 0.0001 for interaction; F(1,46) = 4.561, p = 0.038 for target; F(1,46) = 14.03, p = 0.0005 for treatment). Post hoc test following ANOVA, p < 0.0001 for eGFP vs nNOS-eGFP with target; p = 0.848 for eGFP vs nNOS-eGFP with no target. This active social avoidance was not because of different locomotor activities, as total distances traveled in both groups were equivalent (Fig. 2E; ANOVA, F(1,46) = 0.004, p = 0.952 for interaction; F(1,46) = 3.387, p = 0.245 for target; F(1,46) = 0.120, p = 0.731 for treatment). Consistent with augmented social avoidance, overexpression of nNOS resulted in increased immobility time in TST (Fig. 2F; t test: t(22) = 6.262, p < 0.0001) and reduced sucrose preference in SPT (Fig. 2G; t test: t(22) = 3.567, p = 0.002). Thus, nNOS overexpression in NAcSh caused depression-like behaviors.
Figure 2.
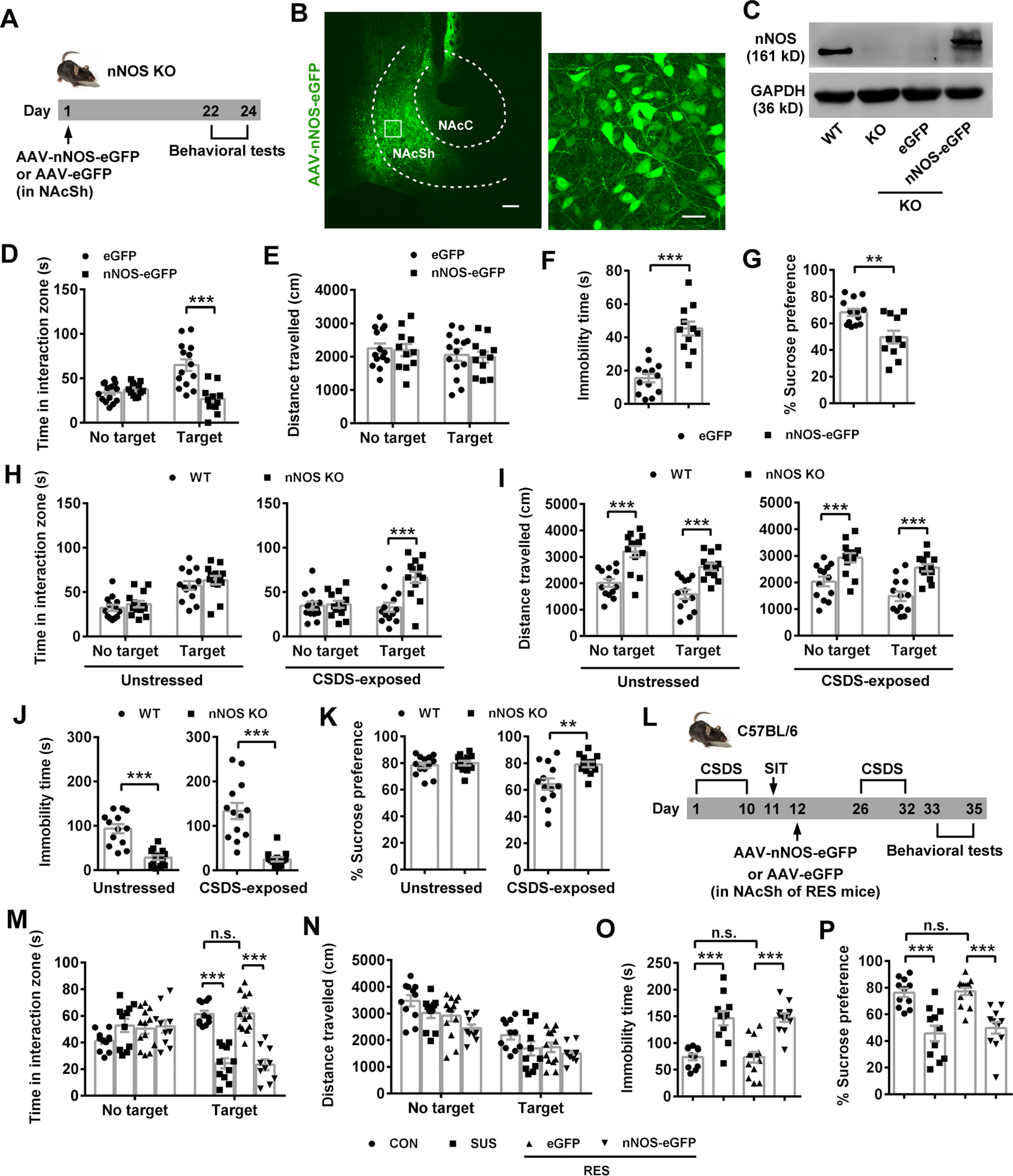
nNOS overexpression in NAcSh is sufficient to induce social avoidance and depression-related behaviors. A, Experimental design for B-G. B, C, Representative fluorescent images (B) and blots (C) showing nNOS-eGFP expression in NAcSh of nNOS gene KO mice infected by AAV-hSyn-nNOS-eGFP. The extent of AP spread of the virus was ∼1.2 mm and almost covered the entire NAc. Scale bars: left, 200 μm; bottom, 20 μm. D-G, Scatter plots showing time spent in interaction zone (D) and total distance traveled (E) in SIT (n = 14 and n = 11, respectively), immobility time in TST (F, n = 13 and n = 11, respectively), and sucrose preference percentage in SPT (G, n = 13 and n = 11, respectively). One mouse in the eGFP group escaped after SIT. Three mice in the nNOS-eGFP group were excluded because brain region was not correctly targeted in microinjection. H-K, Scatter plots showing time spent in interaction zone (H) and total distance traveled (I) in SIT (n = 13 for each group), immobility time in TST (J, n = 13 for each group), and sucrose preference percentage in SPT (K, n = 13 for each group) of nNOS KO and WT mice. L, Scheme illustrating experimental design for M-P. M-P, Scatter plots showing time spent in interaction zone (M) and total distance traveled (N) in SIT (n = 11, n = 11, n = 13, and n = 10, respectively), immobility time in TST (O, n = 11, n = 11, n = 13, and n = 12, respectively), and sucrose preference percentage in SPT (P, n = 11, n = 11, n = 13, and n = 12, respectively). Two mice in the RES+nNOS-eGFP group of SIT were excluded from the analysis because of no movement. Data are mean ± SEM. **p < 0.01. ***p < 0.001. n.s., not significant.
Moreover, we compared the behaviors of nNOS KO mice with WT mice to clarify the results from nNOS overexpression in nNOS KO mice. Under physiological (unstressed) condition, nNOS KO mice spent equivalent time in interaction zone with target to WT mice (Fig. 2H, left; ANOVA, F(1,48) = 0.042, p = 0.838 for interaction; F(1,48) = 36.62, p < 0.0001 for target; F(1,48) = 1.404, p = 0.242 for genotype). Post hoc test following ANOVA, p = 0.552 for WT vs nNOS KO with target. However, total distance traveled by nNOS KO mice was markedly increased with or without target (Fig. 2I, left; ANOVA, F(1,48) = 0.199, p = 0.658 for interaction; F(1,48) = 9.018, p = 0.004 for target; F(1,48) = 44.09, p < 0.0001 for genotype). Post hoc test following ANOVA, p = 0.0001 for WT vs nNOS KO with target, p < 0.0001 for WT vs nNOS KO with no target, indicating the enhanced locomotor activity of nNOS KO mice. Dramatically reduced immobility time showed by nNOS KO mice in TST (Fig. 2J, left; t test: t(24) = 5.742, p < 0.0001) may result from the enhanced locomotor activity, at least partly. The sucrose preference was equal between nNOS KO and WT mice (Fig. 2K, left; t test: t(24) = 0.597, p = 0.556). Under CSDS-exposed condition, WT mice tended to avoid the interaction with target, but nNOS KO mice did not. As shown in Figure 2H (right), nNOS KO mice spent much more time in interaction zone with target than WT mice (ANOVA, F(1,48) = 10.10, p = 0.003 for interaction; F(1,48) = 8.031, p = 0.007 for target; F(1,48) = 11.99, p = 0.001 for genotype). Post hoc test following ANOVA, p < 0.0001 for WT vs nNOS KO with target. After CSDS, nNOS KO mice also traveled much longer in SIT (Fig. 2I, right; ANOVA, F(1,48) = 0.268, p = 0.607 for interaction; F(1,48) = 7.735, p = 0.008 for target; F(1,48) = 34.90, p < 0.0001 for genotype). Post hoc test following ANOVA, p < 0.0001 for WT vs nNOS KO with target, p = 0.0008 for WT vs nNOS KO with no target and struggled much longer in TST (Fig. 2J, right; t test: t(24) = 5.763, p < 0.0001), compared with WT mice. Finally, CSDS-exposed nNOS KO mice showed higher sucrose preference than CSDS-exposed WT mice (Fig. 2K, right; t test: t(24) = 3.128, p = 0.005).
Further, we microinjected AAV-hSyn-nNOS-eGFP or AAV-hSyn-eGFP into resilient C57BL/6 mice to observe whether the resilient phenotype could be reversed to the susceptible phenotype (Fig. 2L). The behavioral results showed that the mice resilient to the first 10 d CSDS became susceptible to the second 7 d CSDS when they were infected with AAV-hSyn-nNOS-eGFP, whereas AAV-hSyn-eGFP-infected mice maintained their resilient phenotype (Fig. 2M; ANOVA, F(3,82) = 21.85, p < 0.0001 for interaction; F(1,82) = 5.476, p = 0.0217 for target; F(3,82) = 11.54, p < 0.0001 for phenotype or treatment). Post hoc test following ANOVA, p < 0.0001 for CON vs SUS, p = 0.9997 for CON vs RES+eGFP, and p < 0.0001 for RES+eGFP vs RES+nNOS-eGFP with target. The locomotor activities among groups were similar in the presence of target (Fig. 2N; ANOVA, F(3,82) = 0.350, p = 0.789 for interaction; F(1,82) = 75.57, p < 0.0001 for target; F(3,82) = 6.373, p = 0.0006 for phenotype or treatment). Post hoc test following ANOVA, p = 0.271 for CON vs SUS, p = 0.322 for CON vs RES+eGFP, and p = 0.813 for RES+eGFP vs RES+nNOS-eGFP with target. Overexpression of nNOS in NAcSh also produced behavior despair and anhedonia in resilient mice (Fig. 2O,P; post hoc test following ANOVA, F(3,43) = 19.23, p < 0.0001 for CON vs SUS, p > 0.9999 for CON vs RES+eGFP and p < 0.0001 for RES+eGFP vs RES+nNOS-eGFP in Fig. 2O; F(3,43) = 16.55, p < 0.0001 for CON vs SUS, p = 0.998 for CON vs RES+eGFP and p < 0.0001 for RES+eGFP vs RES+nNOS-eGFP in Fig. 2P). Therefore, nNOS overexpression in NAcSh promoted susceptibility to depression in resilient mice exposed to CSDS.
NAcSh nNOS does not directly respond to CMS but facilitates the depression-like behaviors
Although we identified nNOS in NAcSh as the key to mediate susceptibility to CSDS and induce depression-related behaviors here, we also reported that hippocampal nNOS mediated CMS-induced depression in mice (Q. G. Zhou et al., 2011). Is there any distinction between the action of nNOS in hippocampus and NAcSh? Given that CSDS did not change nNOS expression in hippocampus (Fig. 1H), we then detected NAc nNOS expression after CMS (Fig. 3A). The result showed that CMS had no obvious effect on nNOS protein level in NAc (t test: t(8) = 0.196, p = 0.850), although it produced ∼2.6-fold increase in hippocampus (t test: t(8) = 3.809, p = 0.005) (Fig. 3B). From this discrepancy, we hypothesized that NAc nNOS reacted to social stress rather than environmental stress. Indeed, CMS led to marked behavioral despair in TST (Fig. 3C; t test: t(26) = 5.447, p < 0.0001) and anhedonia in SPT (Fig. 3D; t test: t(26) = 4.892, p < 0.0001), but it did not cause social avoidance (Fig. 3E; ANOVA, F(1,48) = 0.003, p = 0.957 for interaction; F(1,48) = 41.00, p < 0.0001 for target; F(1,48) = 0.338, p = 0.564 for treatment). Post hoc test following ANOVA, p = 0.917 for CON vs CMS with target and p = 0.881 for CON vs CMS with no target or change locomotor activity (Fig. 3F; ANOVA, F(1,48) = 0.630, p = 0.431 for interaction; F(1,48) =6.629, p = 0.013 for target; F(1,48) = 1.894, p = 0.175 for treatment). Post hoc test following ANOVA, p = 0.246 for CON vs CMS with target and p = 0.899 for CON vs CMS with no target. To compare with the effects of nNOS overexpression in NAc, we delivered AAV-hSyn-nNOS-eGFP or AAV-hSyn-eGFP into hippocampus of nNOS KO mice (Fig. 3G). Three weeks later, effective infection in ventral hippocampus was illustrated (Fig. 3H). As expected, nNOS-eGFP mice showed depression-like behaviors in TST (Fig. 3I; t test: t(22) = 3.484, p = 0.002) and SPT (Fig. 3J; t test: t(22) = 5.705, p < 0.0001), whereas their social interaction is as follows (Fig. 3K; ANOVA, F(1,44) = 0.950, p = 0.335 for interaction; F(1,44) = 12.21, p = 0.001 for target; F(1,44) = 0.852, p = 0.361 for treatment). Post hoc test following ANOVA, p = 0.338 for eGFP vs nNOS-eGFP with target and p = 0.999 for eGFP vs nNOS-eGFP with no target and locomotor activity (Fig. 3L; ANOVA, F(1,44) = 0.0003, p = 0.987 for interaction; F(1,44) = 4.066, p = 0.050 for target; F(1,44) = 0.054, p = 0.818 for treatment) was normal. The results suggested the particular role of NAc nNOS in social stress-induced depression.
Figure 3.
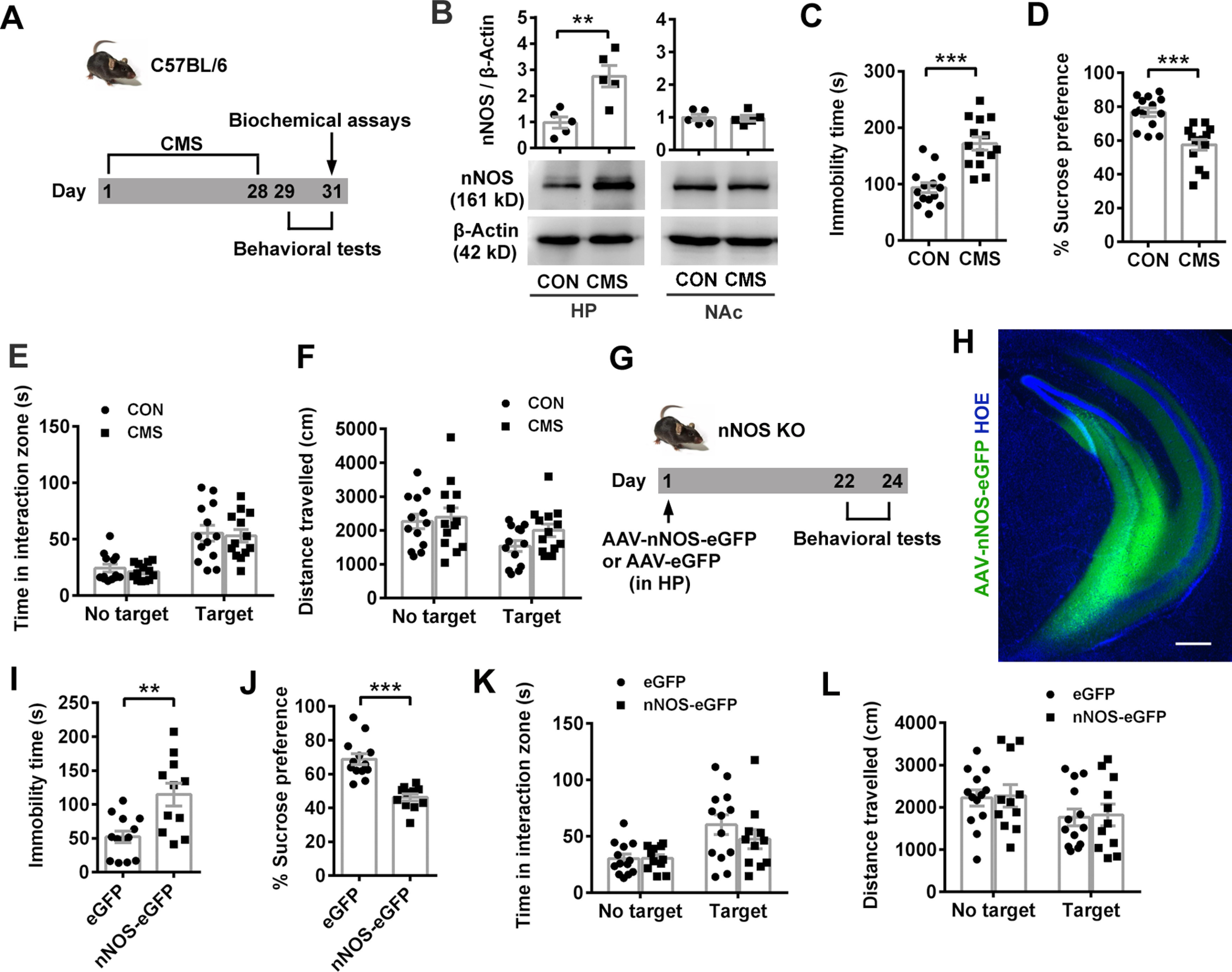
NAcSh nNOS does not directly respond to CMS. A, Scheme illustrating experimental design for B–F. B, Scatter plots (top) and representative blots (bottom) showing nNOS expression. n = 5 for each group. C-F, Scatter plots showing immobility time in TST (C, n = 14 for each group), sucrose preference percentage in SPT (D, n = 14 for each group), time spent in interaction zone (E), and total distance traveled (F) in SIT (n = 13 for each group) after 28 d CMS. One mouse in each group of SIT was excluded from the analysis because of no movement. G, Scheme illustrating experimental design for H-L. H, Representative fluorescent image showing ventral hippocampus infected with AAV-hSyn-nNOS-eGFP. Scale bar, 200 μm. I-L, Scatter plots showing immobility time in TST (I), sucrose preference percentage in SPT (J), time spent in interaction zone (K), and total distance traveled (L) in SIT. n = 13 for eGFP and 11 for nNOS-eGFP. Data are mean ± SEM. **p < 0.01. ***p < 0.001.
Furthermore, we demonstrated whether NAc nNOS could facilitate CMS-induced depression-like behaviors. We found microinjection of 1.4 × 109 vg AAV-hSyn-nNOS-eGFP in NAcSh of C57BL/6 mice to produce moderate overexpression of nNOS (Fig. 4A,B) without direct emergence of depression-like behaviors (Fig. 4C–F) (Fig. 4C; ANOVA, F(3,60) = 4.827, p = 0.004 for interaction; F(1,60) = 4.793, p = 0.032 for target; F(3,60) = 5.372, p = 0.002 for treatment). Post hoc test following ANOVA, p = 0.991 for nNOS-eGFP (0.7 × 109 vg) vs eGFP, p = 0.231 for nNOS-eGFP (1.4 × 109 vg) vs eGFP, and p < 0.0001 for nNOS-eGFP (2.8 × 109 vg) vs eGFP with target. Fig. 4D; ANOVA, F(3,60) = 0.202, p = 0.895 for interaction; F(1,60) = 8.953, p = 0.004 for target; F(3,60) = 0.568, p = 0.638 for treatment. Post hoc test following ANOVA, p = 0.939 for nNOS-eGFP (0.7 × 109 vg) vs eGFP, p > 0.9999 for nNOS-eGFP (1.4 × 109 vg) vs eGFP and p = 0.969 for nNOS-eGFP (2.8 × 109 vg) vs eGFP with target. Fig. 4E; ANOVA, F(3,30) = 7.733, p = 0.0006. Post hoc test following ANOVA, p > 0.9999 for nNOS-eGFP (0.7 × 109 vg) vs eGFP, p = 0.741 for nNOS-eGFP (1.4 × 109 vg) vs eGFP, and p = 0.001 for nNOS-eGFP (2.8 × 109 vg) vs eGFP. Fig. 4F: ANOVA, F(3,30) = 12.57, p < 0.0001. Post hoc test following ANOVA, p = 0.942 for nNOS-eGFP (0.7 × 109 vg) vs eGFP, p = 0.563 for nNOS-eGFP (1.4 × 109 vg) vs eGFP, and p < 0.0001 for nNOS-eGFP (2.8 × 109 vg) vs eGFP. In the experiment designed as Figure 4G, 3 weeks after AAV-nNOS-eGFP (1.4 × 109 vg) or AAV-eGFP infusion, C57BL/6 mice were subjected to a subthreshold CMS. Although moderate nNOS overexpression in NAcSh or subthreshold CMS alone did not result in depression-related behaviors in TST or SPT, the combination of these two conditions did (Fig. 4H,I; post hoc test following ANOVA, F(3,44) = 3.207, p = 0.882 for eGFP vs nNOS-eGFP, p = 0.975 for eGFP vs eGFP+Subthreshold CMS, p = 0.087 for eGFP+Subthreshold CMS vs nNOS-eGFP+Subthreshold CMS, and p = 0.0329 for eGFP vs nNOS-eGFP+Subthreshold CMS in Fig. 4H; F(3,44) = 22.84, p = 0.704 for eGFP vs nNOS-eGFP, p = 0.787 for eGFP vs eGFP+Subthreshold CMS, p < 0.0001 for eGFP+Subthreshold CMS vs nNOS-eGFP+ Subthreshold CMS, and p < 0.0001 for eGFP vs nNOS-eGFP+ Subthreshold CMS in Fig. 4I). All groups had the similar social interaction behaviors (Fig. 4J; ANOVA, F(3,84) = 0.045, p = 0.987 for interaction; F(1,84) = 10.96, p = 0.001 for target; F(3,84) = 0.368, p = 0.776 for treatment) and locomotor activities (Fig. 4K; ANOVA, F(3,84) = 0.173, p = 0.914 for interaction; F(1,84) = 23.09, p < 0.0001 for target; F(3,84) = 0.053, p = 0.984 for treatment). The findings suggested that moderate overexpression of NAcSh nNOS does not directly respond to CMS but facilitates the depression-like behaviors.
Figure 4.
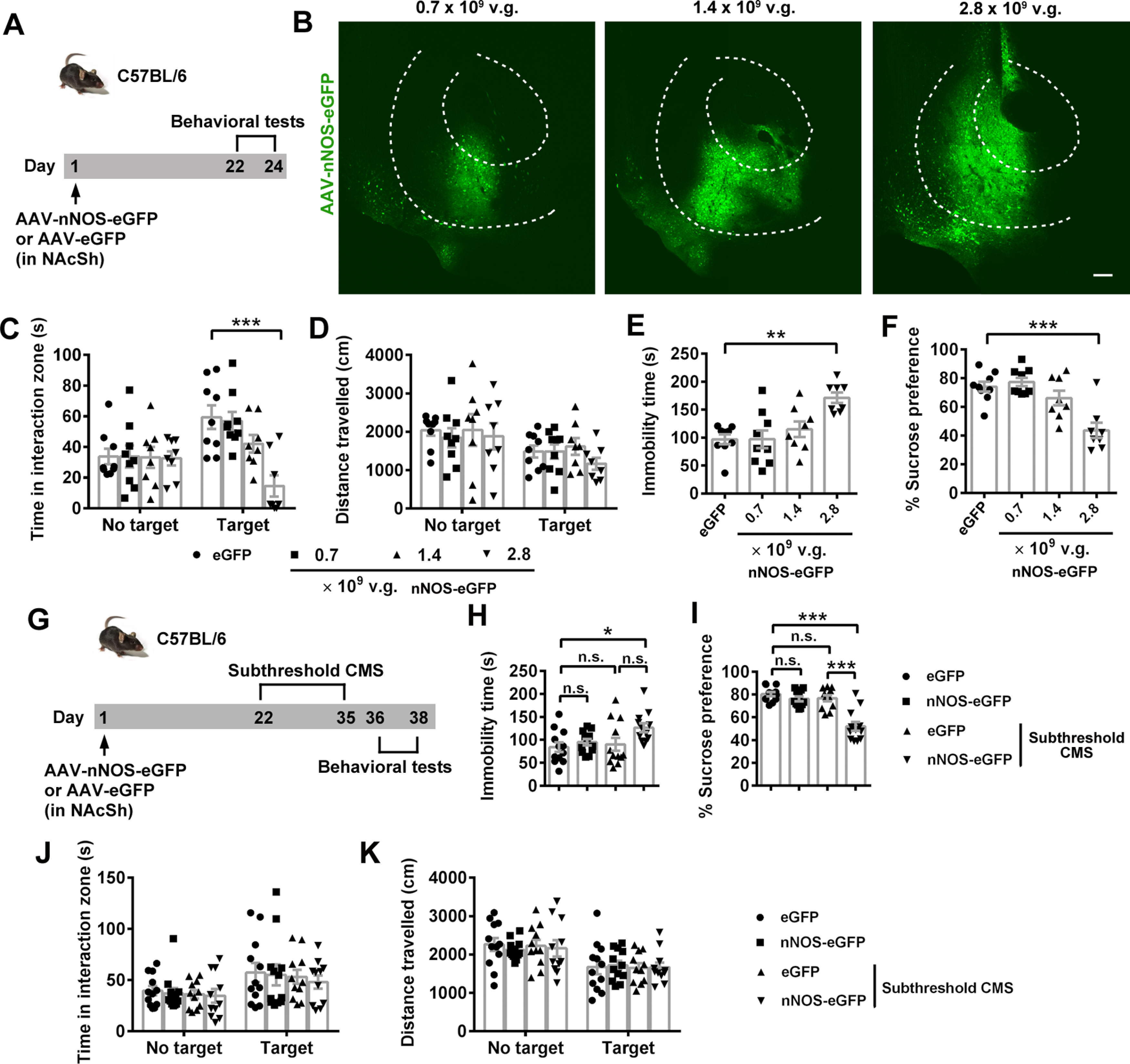
NAcSh nNOS facilitates CMS-induced depression-like behaviors. A, Experimental design for B–F. B, Representative immunofluorescent images showing NAc infected with 0.25, 0.5, and 1 μl AAV-hSyn-nNOS-eGFP (0.7 × 109, 1.4 × 109, and 2.8 × 109 vg, respectively). The extent of AP spread of the virus at 0.25, 0.5, and 1 μl was appropriate at 0.3, 0.6, and 1.2 mm within NAc, respectively. Scale bar, 200 μm. C-F, Scatter plots showing time spent in interaction zone (C) and total distance traveled (D) in SIT, immobility time in TST (E), and sucrose preference percentage in SPT (F). n = 9, n = 9, n = 8, and n = 8, respectively. One mouse in the eGFP group, 1 mouse in the nNOS-eGFP (0.7 × 109) group, 2 mice in the nNOS-eGFP (1.4 × 109) group, and 2 mice in the nNOS-eGFP (2.8 × 109) group were excluded because brain region was not correctly targeted in microinjection. G, Experimental design for H-K. H-K, Scatter plots showing immobility time in TST (H, n = 12 for each group), sucrose preference percentage in SPT (I, n = 12 for each group), time spent in interaction zone (J), and total distance traveled (K) in SIT (n = 12, n = 12, n = 11, and n = 11, respectively). One mouse of the eGFP+subthreshold CMS group and 1 mouse of the nNOS-eGFP+subthreshold CMS group in SIT were excluded because of no movement. Data are mean ± SEM. *p < 0.05. **p < 0.01. ***p < 0.001. n.s., not significant.
NO production and nNOS enzyme activity in NAcSh are necessary for susceptibility to CSDS-induced depression-like behaviors
Given that nNOS expression and specific activity increased in NAcSh of SUS mice but not RES mice, the most possible mechanism for nNOS-mediating depression-like behaviors after CSDS was the NO production. To test this hypothesis, we used nNOS-specific inhibitor L-VNIO, NO scavenger C-PTIO, and NO donor DETA/NONOate (Luo et al., 2010). Each drug was delivered into NAcSh through an implanted bilateral cannula 30 min before the behavioral tests (Fig. 5A). Bilateral cannulation in NAcSh did not significantly influence the behaviors in SIT (Fig. 5B; ANOVA, F(1,42) = 0.505, p = 0. 481 for interaction; F(1,42) = 22.31, p < 0.0001 for target; F(1,42) = 0.005, p = 0.944 for treatment). Post hoc test following ANOVA, p = 0.827 for CON vs IMP with target and p = 0.880 for CON vs IMP with no target, TST (Fig. 5C; t test: t(19) = 0.333, p = 0.743) and SPT (Fig. 5D; t test: t(20) = 0.592, p = 0.560). The results showed that either L-VNIO (Fig. 5E–H) or C-PTIO (Fig. 5I–L) reversed susceptible phenotype of mice exposed to CSDS. The drug-treated mice had higher social interaction time, reduced immobility time, and stronger sucrose preference compared with vehicle-treated ones (Fig. 5E; ANOVA, F(2,56) = 10.97, p < 0.0001 for interaction; F(1,56) = 2.068, p = 0.156 for target; F(2,56) = 5.816, p = 0.005 for treatment). Post hoc test following ANOVA, p < 0.0001 for CON vs SUS+VEH, p < 0.0001 for SUS+VEH vs SUS+L-VNIO with target. Figure 5F: ANOVA, F(2,56) = 0.544, p = 0.583 for interaction; F(1,56) = 33.59, p < 0.0001 for target; F(2,56) = 1.385, p = 0.259 for treatment. Post hoc test following ANOVA, p = 0.298 for CON vs SUS+VEH, p = 0.940 for SUS+VEH vs SUS+L-VNIO with target. Figure 5G: post hoc test following ANOVA, F(2,31) = 9.761, p = 0.0005 for CON vs SUS+VEH and p = 0.012 for SUS+VEH vs SUS+L-VNIO. Figure 5H: post hoc test following ANOVA, F(2,30) = 9.008, p = 0.002 for CON vs SUS+VEH, and p = 0.003 for SUS+VEH vs SUS+L-VNIO. Figure 5I: ANOVA, F(2,72) = 7.715, p = 0.0009 for interaction; F(1,72) = 1.678, p = 0.199 for target; F(2,72) = 6.884, p = 0.002 for treatment. Post hoc test following ANOVA, p < 0.0001 for CON vs SUS+VEH, p = 0.034 for SUS+VEH vs SUS+C-PTIO, p = 0.015 for CON vs SUS+C-PTIO with target. Figure 5J: ANOVA, F(2,72) = 2.130, p = 0.126 for interaction; F(1,72) = 60.58, p < 0.0001 for target; F(2,72) = 1.087, p = 0.343 for treatment. Post hoc test following ANOVA, p = 0.893 for CON vs SUS+VEH, p = 0.970 for SUS+VEH vs SUS+C-PTIO with target. Figure 5K: post hoc test following ANOVA, F(2,37) = 13.07, p = 0.012 for CON vs SUS+VEH, p < 0.0001 for SUS+VEH vs SUS+C-PTIO. Figure 5L: post hoc test following ANOVA, F(2,33) = 5.56, p = 0.012 for CON vs SUS+VEH, p = 0.023 for SUS+VEH vs SUS+C-PTIO. By contrast, DETA/NONOate could induce depression-like behaviors in unstressed mice (Fig. 5M–Q) (Fig. 5N: ANOVA, F(1,42) = 60.22, p < 0.0001 for interaction; F(1,42) = 0.036, p = 0.850 for target; F(1,42) = 30.97, p < 0.0001 for treatment). Post hoc test following ANOVA, p < 0.0001 for CON vs DETA with target. Figure 5O: ANOVA, F(1,42) = 0.683, p = 0.413 for interaction; F(1,42) = 23.87, p < 0.0001 for target; F(1,42) = 0.938, p = 0.338 for treatment. Post hoc test following ANOVA, p = 0.994 for CON vs DETA with target. Figure 5P: t test: t(19) = 4.039, p = 0.0007. Figure 5Q: t test: t(22) = 4.750, p < 0.0001. Together, nNOS-derived NO in NAcSh played a critical role in susceptibility to CSDS.
Figure 5.
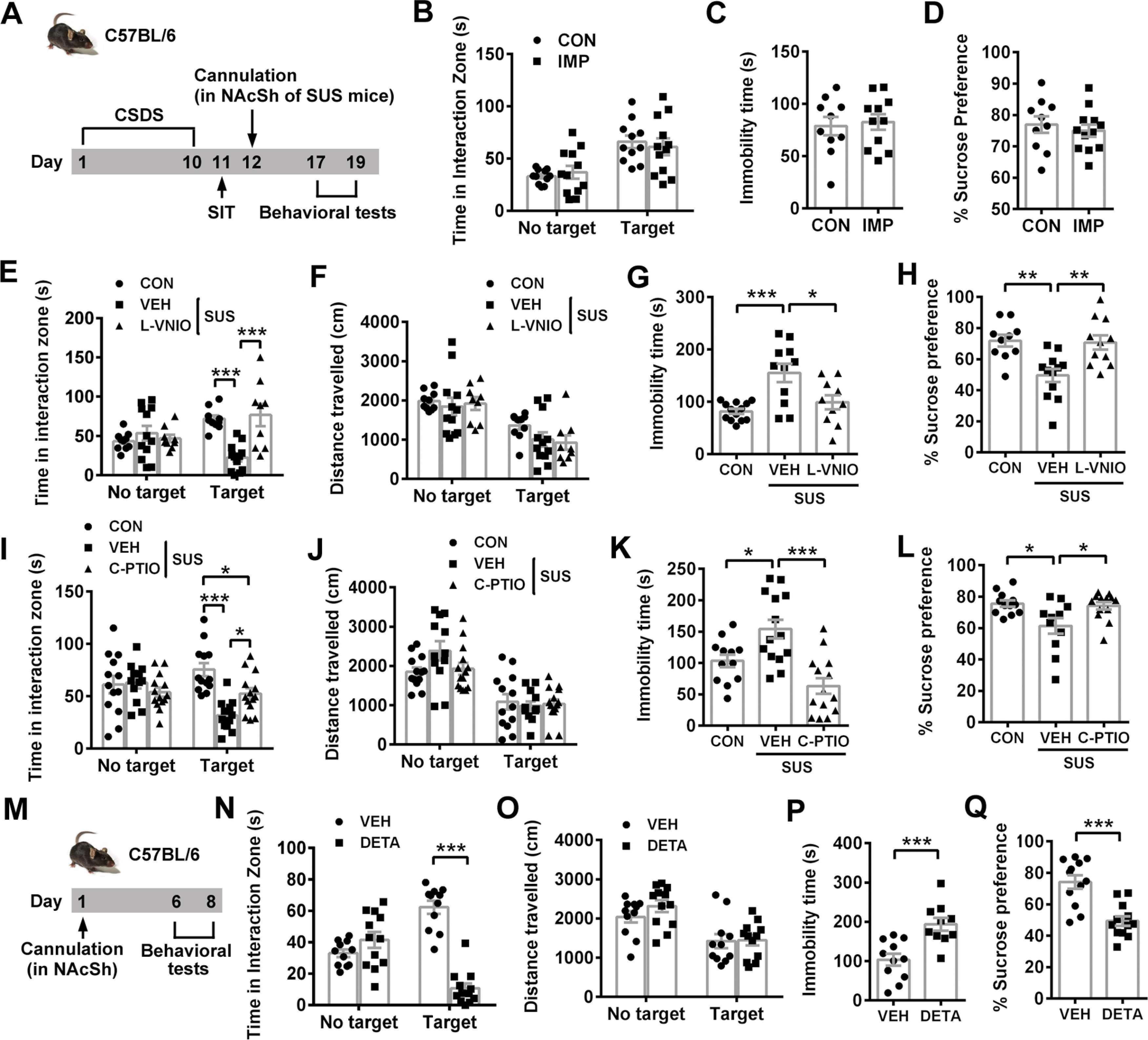
NAcSh NO availability accounts for susceptibility to CSDS-induced depression-like behaviors. A, Experimental design for B-L. B-D, Scatter plots showing time spent in interaction zone in SIT (B, n = 11 and n = 12, respectively), immobility time in TST (C, n = 10 and n = 11, respectively), and sucrose preference percentage in SPT (D, n = 10 and n = 12, respectively) of control (CON) and cannula-implanted (IMP) mice. One CON mouse escaped after SIT, and 1 IMP mouse in TST was excluded from the analysis because of a fall. E-H, Scatter plots showing time spent in interaction zone (E) and total distance traveled (F) in SIT (n = 10, n = 12, and n = 9, respectively), immobility time in TST (G, n = 13, n = 11, and n = 10, respectively), and sucrose preference percentage in SPT (H, n = 10, n = 12, and n = 11, respectively) of control (CON), vehicle-treated (VEH), or L-VNIO-treated susceptible mice. Three CON mice and 2 SUS+L-VNIO mice in SIT were excluded from analysis because of no movement. One SUS+VEH mouse and 1 SUS+L-VNIO mouse climbed up in TST and were excluded. Three CON mice in SPT were excluded from the analysis because of water leaking. I-L, Scatter plots showing time spent in interaction zone (I) and total distance traveled (J) in SIT (n = 13, n = 12, and n = 14, respectively), immobility time in TST (K, n = 12, n = 14, and n = 14, respectively), and sucrose preference percentage in SPT (L, n = 12, n = 11, and n = 13, respectively) of control (CON), vehicle-treated (VEH), or C-PTIO-treated susceptible mice. Two SUS+VEH mice in SIT were excluded from analysis because of no movement. One CON mouse fell down in TST and was excluded from analysis. One CON mouse, 3 VEH+SUS mice, and 1 SUS+C-PTIO mouse in SPT were excluded because of water leaking. M, Experimental design for N-Q. N-Q, Scatter plots showing time spent in interaction zone (N) and total distance traveled (O) in SIT (n = 11 and n = 12, respectively), immobility time in TST (P, n = 11 and n = 10, respectively), and sucrose preference percentage in SPT (Q, n = 12 for each group) of vehicle-treated (VEH) or DETA/NONOate-treated mice. One VEH mouse in SIT was excluded from analysis because of no movement. One VEH mouse and 2 DETA mice fell down or climbed up in TST and were excluded. Data are mean ± SEM. *p < 0.05. **p < 0.01. ***p < 0.001.
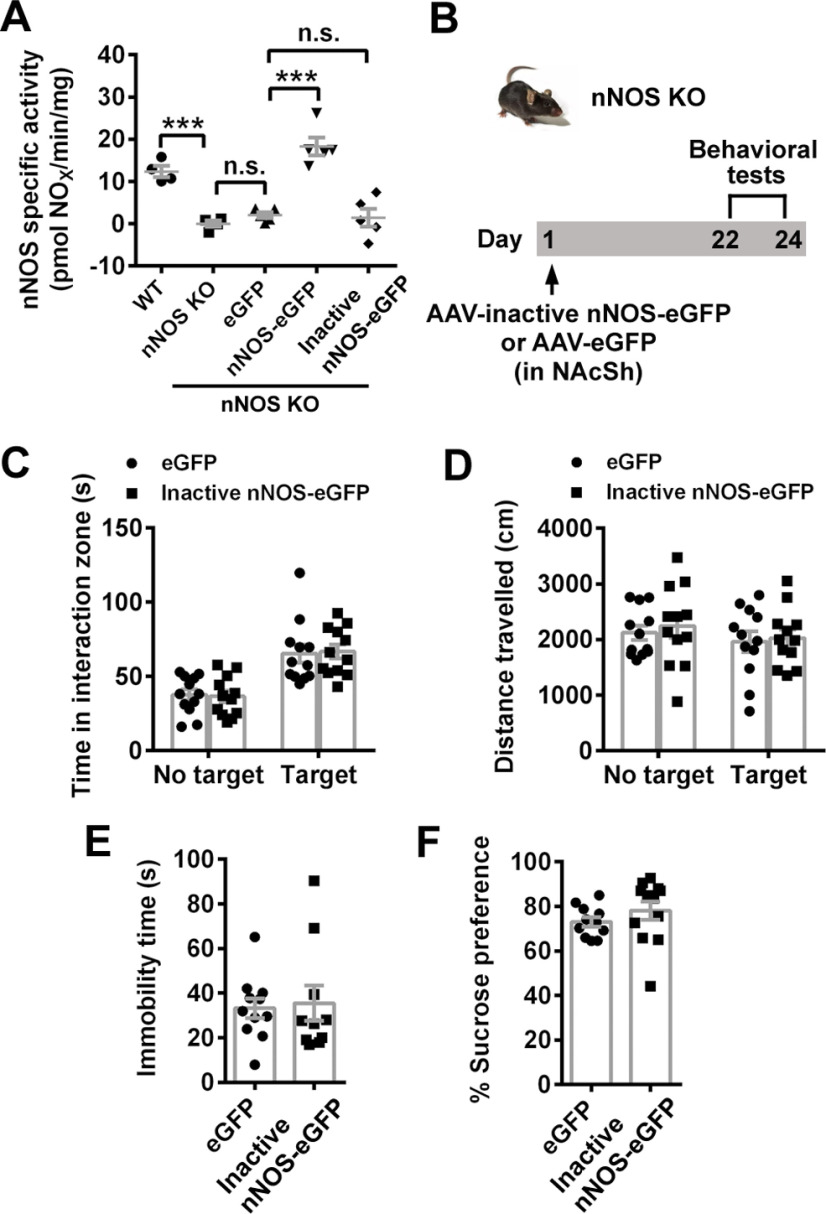
Moreover, we generated a recombinant AAV to express an inactive nNOS (Hallmark et al., 1999). Compared with AAV-hSyn-eGFP-infected KO mice, AAV-hSyn-nNOS-eGFP-infected KO mice had marked increase of nNOS-specific activity, but AAV-hSyn-inactive nNOS-eGFP-infected KO mice showed similar nNOS-specific activity at the low level 3 weeks after infection (Fig. 6A; post hoc test following ANOVA, F(4,18) = 26.08, p = 0.0006 for WT vs nNOS KO, p = 0.899 for nNOS KO vs nNOS KO+eGFP, p < 0.0001 for nNOS KO+eGFP vs nNOS KO+nNOS-eGFP, p = 0.999 for nNOS KO+eGFP vs nNOS KO+inactive nNOS-eGFP). We also found that, unlike full-length nNOS, inactive nNOS overexpression in NAcSh (Fig. 6B) did not change the social interaction (Fig. 6C; ANOVA, F(1,44) = 0.072, p = 0.789 for interaction; F(1,44) = 38.03, p < 0.0001 for target; F(1,44) = 0.0001, p = 0.979 for treatment). Post hoc test following ANOVA, p = 0.973 for eGFP vs inactive nNOS-eGFP with target and p = 0.982 for eGFP vs inactive nNOS-eGFP with no target, locomotor activities (Fig. 6D; ANOVA, F(1,44) = 0.024, p = 0.877 for interaction; F(1,44) = 1.198, p = 0.280 for target; F(1,44) = 0.287, p = 0.595 for treatment), immobility time (Fig. 6E; t test: t(19) = 0.262, p = 0.796) and sucrose preference (Fig. 6F; t test: t(21) = 1.068, p = 0.298), compared with eGFP control. Therefore, the contribution of overexpressed or upregulated nNOS in NAcSh to the depression-related behaviors was dependent on enzyme activity and the product NO.
Figure 6.
nNOS enzyme activity in NAcSh is necessary for mediating CSDS-induced depression. A, Scatter plot showing nNOS-specific activity. n = 4, n = 4, n = 5, n = 5, and n = 5, respectively. Mean measured value of nNOS KO mice was regarded as the background and subtracted from measured value of each other group. B, Experimental design for C-F. C-F, Scatter plots showing time spent in interaction zone (C) and total distance traveled (D) in SIT (n = 12 for each group), immobility time in TST (E, n = 11 and n = 10, respectively), and sucrose preference percentage in SPT (F, n = 11 and n = 12, respectively). One mouse escaped after SIT. Two mice in inactive nNOS-eGFP group climbed up in TST and were excluded. Data are mean ± SEM. ***p < 0.001. n.s., not significant.
CDK5 activity is required for NAcSh nNOS-mediated susceptibility to CSDS
Subsequently, we determined the downstream signal through which NAcSh nNOS mediated susceptibility to CSDS. Conditional KO (cKO) of CDK5 in ventral striatum produced antidepressant-like effects and promoted resistance to CSDS (Plattner et al., 2015). Moreover, pathophysiological NO could S-nitrosylate CDK5, stimulating its activity (Qu et al., 2011). There is a possibility that CDK5 is involved in NAcSh nNOS-mediated susceptibility to CSDS. To verify this possibility, we bilaterally microinjected a CDK5 inhibitor ROS into NAcSh of susceptible mice 30 min before behavior tests (Fig. 7A), and found that the decreased social interaction in susceptible mice was reversed by ROS treatment completely (Fig. 7B; ANOVA, F(2,68) = 8.886, p = 0.0004 for interaction; F(1,68) = 0.165, p = 0.686 for target; F(2,68) = 8.062, p = 0.0007 for treatment). Post hoc test following ANOVA, p < 0.0001 for CON vs SUS+VEH, p < 0.0001 for SUS+VEH vs SUS+ROS with target, but total distance traveled in presence of the target was similar among groups (Fig. 7C; ANOVA, F(2,68) = 1.069, p = 0.349 for interaction; F(1,68) = 72.32, p < 0.0001 for target; F(2,68) = 1.105, p = 0.337 for treatment). Post hoc test following ANOVA, p = 0.574 for CON vs SUS+VEH, p = 0.996 for SUS+VEH vs SUS+ROS with target. Consistently, ROS-treated mice struggled longer in the TST (Fig. 7D; post hoc test following ANOVA, F(2,35) = 7.795, p = 0.005 for CON vs SUS+VEH, p = 0.005 for SUS+VEH vs SUS+ROS) and favored sucrose over water much more (Fig. 7E; post hoc test following ANOVA, F(2,35) = 17.13, p < 0.0001 for CON vs SUS+VEH, p < 0.0001 for SUS+VEH vs SUS+ROS) than vehicle-treated susceptible mice. Then, we examined whether CDK5 was necessary for the depression-like behaviors induced by NAcSh nNOS overexpression. Neuron-specific knockdown of CDK5 was achieved by microinjecting AAV-hSyn-Cre into NAcSh of homozygous floxed CDK5 mice (Fig. 7F). Two weeks later, AAV-hSyn-nNOS-eGFP was delivered by microinjection to overexpress full-length nNOS. Five weeks later, the validity of CDK5 cKO was confirmed (Fig. 7G,H), and animal behaviors were tested. In agreement with the behavioral alterations resulting from nNOS overexpression in nNOS KO mice, AAV-hSyn-nNOS-eGFP-infected CDK5flox/flox mice spent less time in the interaction zone with target compared with AAV-hSyn-eGFP-infected ones (Fig. 7I; ANOVA, F(3,98) = 5.230, p = 0.002 for interaction; F(1,98) = 3.236, p = 0.075 for target; F(3,98) = 7.996, p < 0.0001 for treatment). Post hoc test following ANOVA, p = 0.036 for eGFP+CDK5flox/flox vs nNOS-eGFP+CDK5flox/flox, p = 0.045 for eGFP+CDK5flox/flox vs eGFP+CDK5flox/flox+Cre, p = 0.995 for eGFP+CDK5flox/flox+ Cre vs nNOS-eGFP+CDK5flox/flox+Cre with target, although their locomotor activities were equivalent (Fig. 7J; ANOVA, F(3,98) = 1.129, p = 0.341 for interaction; F(1,98) = 16.27, p = 0.0001 for target; F(3,98) = 1.718, p = 0.168 for treatment). Post hoc test following ANOVA, p > 0.9999 for eGFP+CDK5flox/flox vs nNOS-eGFP+CDK5flox/flox, p = 0.990 for eGFP+CDK5flox/flox vs eGFP+CDK5flox/flox+Cre, p = 0.852 for eGFP+CDK5flox/flox+ Cre vs nNOS-eGFP+CDK5flox/flox+Cre with target. In CDK5flox/flox mice, AAV-hSyn-nNOS-eGFP infection also led to longer immobility time in TST (Fig. 7K; post hoc test following ANOVA, F(3,49) = 24.02, p = 0.0126 for eGFP+CDK5flox/flox vs nNOS-eGFP+ CDK5flox/flox, p = 0.016 for eGFP+CDK5flox/flox vs eGFP+ CDK5flox/flox+Cre, p = 0.532 for eGFP+CDK5flox/flox+Cre vs nNOS-eGFP+CDK5flox/flox+Cre) and lower sucrose preference (Fig. 7L; post hoc test following ANOVA, F(3,49) = 13.26, p < 0.0001 for eGFP+CDK5flox/flox vs nNOS-eGFP+ CDK5flox/flox, p = 0.425 for eGFP+CDK5flox/flox vs eGFP+ CDK5flox/flox+Cre, p = 0.999 for eGFP+CDK5flox/flox+Cre vs nNOS-eGFP+CDK5flox/flox+Cre). All these behavioral alterations induced by nNOS overexpression in CDK5flox/flox mice disappeared when nNOS was overexpressed in CDK5 cKO mice (Fig. 7I–L). Moreover, the results from Figure 7I–L also suggested that cKO of CDK5 had antidepressant-like effects, shown by comparison between eGFP+CDK5flox/flox and eGFP+CDK5flox/flox+Cre. However, CDK5flox/flox mice exhibited similar behaviors with WT mice in all tests (Fig. 7M–P) (Fig. 7M: ANOVA, F(1,48) = 0.040, p = 0.842 for interaction; F(1,48) = 11.15, p = 0.002 for target; F(1,48) = 0.171, p = 0.681 for genotype). Post hoc test following ANOVA, p = 0.986 for WT vs CDK5flox/flox with target and p = 0.889 for WT vs CDK5flox/flox with no target. Figure 7N: ANOVA, F(1,48) = 0.043, p = 0.836 for interaction; F(1,48) = 4.005, p = 0.051 for target; F(1,48) = 1.166, p = 0.286 for genotype. Post hoc test following ANOVA, p = 0.599 for WT vs CDK5flox/flox with target and p = 0.789 for WT vs CDK5flox/flox with no target. Figure 7O: t test: t(24) = 0.298, p = 0.768. Figure 7P: t test: t(24) = 0.591, p = 0.560).
S-nitrosylation contributes to increased CDK5 activity in NAcSh nNOS-mediated susceptibility to CSDS
Before establishing the relationship between S-nitrosylation of CDK5 and nNOS-mediating susceptibility to CSDS, we assessed CDK5-specific activity in NAcSh when nNOS was inhibited or NO was supplied. Consistent with the behavioral alterations, L-VNIO suppressed the CDK5-specific activity of susceptible mice as ROS did (Fig. 8A; post hoc test following ANOVA, F(3,14) = 35.58, p < 0.0001 for CON vs SUS, SUS vs SUS+L-VNIO, and SUS vs SUS+ROS), whereas DETA/NONOate resulted in enhanced CDK5-specific activity in unstressed mice (Fig. 8B; post hoc test following ANOVA, F(2,11) = 73.16, p < 0.0001 for CON vs DETA and DETA vs DETA+C-PTIO). Moreover, C-PTIO could abolish the effect of DETA/NONOate. Biotin-switch assay showed that susceptible mice had double S-nitrosylation level of CDK5 in NAcSh compared with control mice and L-VNIO suppressed this upregulation (Fig. 8C; post hoc test following ANOVA, F(2,12) = 14.33, p = 0.0006 for CON vs SUS+VEH, p = 0.008 for SUS+VEH vs SUS+L-VNIO). Thus, we hypothesized that the mechanism underlying the susceptibility regulation to CSDS by upregulated nNOS in NAcSh was elevated CDK5 activity through S-nitrosylation.
To further confirm this hypothesis, we generated a recombinant AAV-CMV-CDK5-C83/157S-3Flag-T2A-eGFP, which encoded mouse CDK5 with two point mutations: Cys 83 Ser and Cys 157 Ser. This mutant CDK5 could not be activated by S-nitrosylation (Qu et al., 2011). Then we examined the effects of this CDK5-C83/157S-overexpressing AAV as designed in Figure 8D. Mice in CON and SUS groups were not treated with any viral injection. The AAV efficiently infected NAcSh of susceptible mice (Fig. 8E) and expressed substantial CDK5-C83/157S (Fig. 8F, Input). Consistent with a previous report (Qu et al., 2011), the C83/157S mutation totally abrogated S-nitrosylation increase of CDK5 in susceptible mice exposed to CSDS (Fig. 8F, SNO-CDK5; post hoc test following ANOVA, F(3,12) = 11.70, p = 0.010 for CON vs SUS, p = 0.004 for SUS+3Flag-T2A-eGFP vs SUS+CDK5-C83/157S-3Flag-T2A-eGFP). Meanwhile, the CDK5 activity was also suppressed (Fig. 8G; post hoc test following ANOVA, F(3,12) = 14.81, p = 0.010 for CON vs SUS, p = 0.0009 for SUS+3Flag-T2A-eGFP vs SUS+CDK5-C83/157S-3Flag-T2A-eGFP). Most importantly, overexpression of CDK5-C83/157S in NAcSh of susceptible mice reversed the active social avoidance (Fig. 8H; ANOVA, F(3,90) = 10.47, p < 0.0001 for interaction; F(1,90) = 4.399, p = 0.039 for target; F(3,90) = 7.889, p < 0.0001 for phenotype or treatment). Post hoc test following ANOVA, p < 0.0001 for CON vs SUS and SUS+3Flag-T2A-eGFP vs SUS+CDK5-C83/157S-3Flag-T2A-eGFP with target, but the locomotor in the presence of target was not obviously altered (Fig. 8I; ANOVA, F(3,90) = 0.301, p = 0.825 for interaction; F(1,90) = 43.29, p < 0.0001 for target; F(3,90) = 1.716, p = 0.169 for phenotype or treatment). Post hoc test following ANOVA, p = 0.760 for CON vs SUS, p = 0.830 for SUS+3Flag-T2A-eGFP vs SUS+CDK5-C83/157S-3Flag-T2A-eGFP with target. The depression-related behaviors were also rescued in AAV-CMV-CDK5-C83/157S-3Flag-T2A-eGFP-infected mice, compared with control AAV-infected mice (Fig. 8J,K). Post hoc test following ANOVA, F(3,44) = 9.52, p = 0.017 for CON vs SUS, p = 0.0006 for SUS+3Flag-T2A-eGFP vs SUS+CDK5-C83/157S-3Flag-T2A-eGFP in Fig. 8J; F(3,40) = 13.37, p = 0.0003 for CON vs SUS, p = 0.0004 for SUS+3Flag-T2A-eGFP vs SUS+CDK5-C83/157S-3Flag-T2A-eGFP in Fig. 8K). Additionally, DETA/NONOate could not induce social avoidance and depression-like behaviors in mice infected with AAV-CMV-CDK5-C83/157S-3Flag-T2A-eGFP (Fig. 8L–O) (Fig. 8L: ANOVA, F(3,90) = 21.34, p < 0.0001 for interaction; F(1,90) = 0.848, p = 0.360 for target; F(3,90) = 9.898, p < 0.0001 for treatment). Post hoc test following ANOVA, p < 0.0001 for CON vs DETA, p = 0.002 for DETA + 3Flag-T2A-eGFP vs DETA+CDK5-C83/157S-3Flag-T2A-eGFP with target. Figure 8M: ANOVA, F(3,90) = 0.187, p = 0.905 for interaction; F(1,90) = 111.3, p < 0.0001 for target; F(3,90) = 0.713, p = 0.547 for treatment. Figure 8N: Post hoc test following ANOVA, F(3,45) = 29.82, p < 0.0001 for CON vs DETA and DETA + 3Flag-T2A-eGFP vs DETA+CDK5-C83/157S-3Flag-T2A-eGFP. Figure 8O: Post hoc test following ANOVA, F(3,42) = 22.13, p < 0.0001 for CON vs DETA and DETA + 3Flag-T2A-eGFP vs DETA+CDK5-C83/157S-3Flag-T2A-eGFP. Together, nNOS-derived NO in NAcSh mediated susceptibility to CSDS through elevated CDK5 activity by S-nitrosylation of CDK5.
Discussion
In this study, we demonstrate that nNOS in NAcSh, but not in hippocampus, is crucial to regulate the susceptibility to social defeat and the following depression-like behaviors. The increased NAcSh nNOS expression after CSDS contributes to the social avoidance and depression-like behaviors in defeated mice, and this effect is dependent on the nNOS enzyme activity and NO production. Moreover, we identify the downstream signal in NAcSh. S-nitrosylation of CDK5 by NO contributes to enhanced CDK5 activity, leading to depression-related behaviors in susceptible mice.
Clinic evidence that MDD patients showed elevated plasma nitrate levels compared with normal control subjects (Suzuki et al., 2001) and increased plasma NOx level associated with suicide attempt in depressive patients (Kim et al., 2006) suggests the possible relationship between NO and depression. A large number of animal studies with nNOS inhibitors establish the critical role of nNOS in mediating depression (Harkin et al., 1999; Volke et al., 2003; Ulak et al., 2010; Tomaz et al., 2014) as well as our findings (Q. G. Zhou et al., 2011). Along with the development of chemogenetics and optogenetics, a few studies focused on nNOS-expressing neurons. Recently, we demonstrated that the activity of mPFC nNOS-expressing neurons is necessary for mediation of another affective disorder anxiety (Liang et al., 2020), and Smith et al. (2017) discovered the stimulation of accumbens nNOS-expressing neurons in cocaine relapse. However, no papers about nNOS-expressing neurons and depression have been published, to our knowledge. In this study, we uniquely found three separate mechanisms by which nNOS regulation of depression-like behaviors after CSDS occurs in the NAc shell, that is, increase in cell number, increase in protein expression levels, and increase in specific activity. Our results suggest that these three mechanisms work together, independently of the nNOS-expressing neurons activity.
The early studies on the regulation of depression-like behaviors by nNOS usually used intraperitoneal injection, which was not region-specific. Recently, we and others verified that inhibiting hippocampal nNOS displayed antidepressant-like effects (Joca and Guimarães, 2006; Q. G. Zhou et al., 2011; Sherwin et al., 2017). Here, we focus on NAc nNOS and report the different roles of NAc nNOS and hippocampal nNOS in depression regulation. NAc nNOS specifically mediates susceptibility to the social component of CSDS-induced depression-like behaviors, whereas hippocampal nNOS is responsible for CMS-induced depression-like behaviors. Moreover, we found that social avoidance, but not sucrose preference, was differently influenced by CMS- and CSDS-induced mechanisms, which was also observed by other researchers (Venzala et al., 2013). The above differences may be because of the functional specialization of different brain regions and the internality of different stress procedures. CSDS involves subjecting normal rodents to repeated bouts of social subordination, whereas CMS involves subjecting rodents to a series of repeated physical stresses (Nestler and Hyman, 2010). Although a great number of studies on depression are performed within hippocampus or NAc, they are usually independent. The actual mechanisms underlying the distinct response of NAc and hippocampal nNOS to CSDS and CMS will not have easy answers, and further work is needed to clarify the underlying mechanisms. Despite distinct responses to social and environmental stress, nNOS upregulation either in NAc or in hippocampus can result in depression-like behaviors (despair and anhedonia) solely. Moreover, NAc nNOS could produce an additive or synergistic effect on depression-like behaviors with subthreshold CMS, indicating interaction of social and environmental factors in complex neurobiology of MDD.
Many studies have demonstrated the crucial role of NAc in the susceptibility to social stress and the depression-like behaviors (Newton et al., 2002; Chaudhury et al., 2013; Francis et al., 2015), and there is significant functional overlap between the shell and core subregion of NAc in encoding motivation and reward (Kalivas and Volkow, 2005; Floresco, 2015). Little is known about the specific role of NAcSh and NAcC in the pathophysiology of depression. It is well established that the NAcSh controls gating of behavioral responses to emotional stimuli and mediates social isolation-induced behavioral deficits (Barrot et al., 2002; Wallace et al., 2009). Until recently, cholinergic interneurons of NAcSh were discovered to regulate depression-like behaviors in CSDS mice (Cheng et al., 2019). The report that infusions of neuropeptide S into the NAcSh, but not the NAcC, exerted antidepressant-like effects in the learned helplessness rats also indicates the specific role NAcSh in depression (Shirayama et al., 2015). On the other hand, the NAcC is regarded as the key in mediating drug addiction for its prominent contribution to the cue-motivated behaviors (Kalivas and Volkow, 2005; Collins et al., 2019), although its implication in depression has also been supposed. Our finding in this study further supports the role of NAcSh in the depression-like behaviors.
Diverse molecular mechanisms are proposed to interpret the regulation of depression-like behaviors by NAc, including decreased neuroligin-2 selectively in DA D1-positive cells (Heshmati et al., 2018) and reduced HCN2 in cholinergic interneurons (Cheng et al., 2019). These meticulous works provide a basis for targeted, cell type-specific therapy of social stress-induced depression disorders. In our previous and present studies, we found that pharmacological inhibition of nNOS enzyme in NAc and hippocampus reduced susceptibility to CSDS and CMS, respectively. Thus, nNOS might be a more common and accessible target for treatment of both social stress- and environmental stress-induced depression. This is in agreement with interpretation to the excitatory synapse hypothesis of depression, as nNOS can bind with NMDARs and can be activated through calcium influx after NMDAR stimulation (Christopherson et al., 1999; Aarts et al., 2002). However, whether the nNOS effects described here are dependent or independent of an NMDAR mechanism remains unclear, based on our present study. It is reported that CSDS induces downregulation of NMDAR and PSD-95 in NAc neurons (Jiang et al., 2013). Fortunately, we have developed a small-molecule ZL006, which can dissociate nNOS from PSD-95 to disrupt the interaction between nNOS and NMDARs (L. Zhou et al., 2010; Luo et al., 2014), and recently it is reported to show antidepressant-like effect in unstressed mice (Doucet et al., 2013). Further studies are being performed in our laboratory, and the indirect intervention of NMDARs or nNOS is a promising strategy for depression disorder therapy.
NO-activated soluble guanylate cyclase and cyclic guanosine monophosphate is the most widely accepted downstream mechanism of nNOS (Q. G. Zhou et al., 2018). On the other hand, we have focused on S-nitrosylation of some functional proteins by NO over a long period (Luo et al., 2014; L. J. Zhu et al., 2014; Zhang et al., 2018). Now, we discovered the requirement of CDK5 and S-nitrosylation of CDK5 for the function of NAcSh nNOS in the susceptible mice exposed to CSDS. CDK5 is a proline-directed serine/threonine kinase with high activity in the brain. Unlike other CDK family members, it does not participate in cell cycle progression in proliferating cells but is fundamental to neurotransmission and synaptic plasticity (Chergui et al., 2004; Su et al., 2012; Rudenko et al., 2015). Dysfunction of CDK5 is implicated in several neuropsychiatric disorders, including depression (Barnett and Bibb, 2011; Su and Tsai, 2011). However, the regulation of CDK5 on depression is complicated, and the findings from various laboratories are not in complete agreement. Increased CDK5 activity in dentate gyrus mediates depressive-like behavior in rats (W. L. Zhu et al., 2012; Papadopoulou et al., 2015). cKO of CDK5 in VTA neurons induces depressive-like behaviors in mice (Zhong et al., 2014). cKO of CDK5 in NAc neurons produced antidepressant effects and promoted resistance to CSDS in mice (Plattner et al., 2015). Transcriptional activation of Cdk5 gene in NAc attenuates social avoidance of defeated mice (Heller et al., 2016). The discrepancy comes from different brain area aimed, as well as different methods used. With pharmacological inhibition and virus-dependent KO of CDK5 in NAc, we demonstrate that enhanced CDK5 function contributes to CSDS-induced depression, consistent with the finding from Plattner et al. (2015). Moreover, we provide evidence that nNOS expression and NO production in NAc mediate susceptibility to CSDS and depression-like behaviors through S-nitrosylation of CDK5. Nevertheless, more work is needed to clarify the role of CDK5 within different brain regions in depression regulation and to reveal the related mechanisms.
Footnotes
This work was supported by National Key R&D Program of China 2016YFC1306703, National Natural Science Foundation of China 31530091, Science and Technology Program of Guangdong 2018B030334001, Southeast University-Nanjing Medical University Joint Fund 2242017K3DN10, and Training Program for Distinguished Young Scholar of Nanjing Medical University 2017NJMURC003. We thank Dr. Zhi-Gang Xu (Shandong University, Qingdao, China) for the gifts of Floxed Cdk5 mice.
The authors declare no competing financial interests.
References
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M (2002) Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science 298:846–850. 10.1126/science.1072873 [DOI] [PubMed] [Google Scholar]
- Baranyi A, Amouzadeh-Ghadikolai O, Rothenhäusler HB, Theokas S, Robier C, Baranyi M, Koppitz M, Reicht G, Hlade P, Meinitzer A (2015) Nitric oxide-related biological pathways in patients with major depression. PLoS One 10:e0143397. 10.1371/journal.pone.0143397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett DG, Bibb JA (2011) The role of Cdk5 in cognition and neuropsychiatric and neurological pathology. Brain Res Bull 85:9–13. 10.1016/j.brainresbull.2010.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ (2002) CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci USA 99:11435–11440. 10.1073/pnas.172091899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleau EL, Treadway MT, Pizzagalli DA (2019) The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol Psychiatry 85:443–453. 10.1016/j.biopsych.2018.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. 10.1016/s0006-3223(99)00230-9 [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A (2012) Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76:790–803. 10.1016/j.neuron.2012.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, et al. (2013) Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493:532–536. 10.1038/nature11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Umschweif G, Leung J, Sagi Y, Greengard P (2019) HCN2 channels in cholinergic interneurons of nucleus accumbens shell regulate depressive behaviors. Neuron 101:662–672. 10.1016/j.neuron.2018.12.018 [DOI] [PubMed] [Google Scholar]
- Chergui K, Svenningsson P, Greengard P (2004) Cyclin-dependent kinase 5 regulates dopaminergic and glutamatergic transmission in the striatum. Proc Natl Acad Sci USA 101:2191–2196. 10.1073/pnas.0308652100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Hillier BJ, Lim WA, Bredt DS (1999) PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem 274:27467–27473. 10.1074/jbc.274.39.27467 [DOI] [PubMed] [Google Scholar]
- Collins AL, Aitken TJ, Huang IW, Shieh C, Greenfield VY, Monbouquette HG, Ostlund SB, Wassum KM (2019) Nucleus accumbens cholinergic interneurons oppose cue-motivated behavior. Biol Psychiatry 86:388–396. 10.1016/j.biopsych.2019.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir A, Kulkarni SK (2011) Nitric oxide and major depression. Nitric oxide 24:125–131. 10.1016/j.niox.2011.02.002 [DOI] [PubMed] [Google Scholar]
- Doucet MV, Levine H, Dev KK, Harkin A (2013) Small-molecule inhibitors at the PSD-95/nNOS interface have antidepressant-like properties in mice. Neuropsychopharmacology 38:1575–1584. 10.1038/npp.2013.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB (2015) The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol 66:25–52. 10.1146/annurev-psych-010213-115159 [DOI] [PubMed] [Google Scholar]
- Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, Brooks JM, Iñiguez SD, O'Donnell P, Kravitz A, Lobo MK (2015) Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry 77:212–222. 10.1016/j.biopsych.2014.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, Lobo MK (2017) Emerging role for nucleus accumbens medium spiny neuron subtypes in depression. Biol Psychiatry 81:645–653. 10.1016/j.biopsych.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, Berton O, Russo SJ (2011) A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6:1183–1191. 10.1038/nprot.2011.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmark OG, Phung YT, Black SM (1999) Chimeric forms of neuronal nitric oxide synthase identify different regions of the reductase domain that are essential for dimerization and activity. DNA Cell Biol 18:397–407. 10.1089/104454999315286 [DOI] [PubMed] [Google Scholar]
- Harkin AJ, Bruce KH, Craft B, Paul IA (1999) Nitric oxide synthase inhibitors have antidepressant-like properties in mice. Eur J Pharmacol 372:207–213. 10.1016/s0014-2999(99)00191-0 [DOI] [PubMed] [Google Scholar]
- Heller EA, Hamilton PJ, Burek DD, Lombroso SI, Peña CJ, Neve RL, Nestler EJ (2016) Targeted epigenetic remodeling of the Cdk5 gene in nucleus accumbens regulates cocaine- and stress-evoked behavior. J Neurosci 36:4690–4697. 10.1523/JNEUROSCI.0013-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshmati M, Aleyasin H, Menard C, Christoffel DJ, Flanigan ME, Pfau ML, Hodes GE, Lepack AE, Bicks LK, Takahashi A, Chandra R, Turecki G, Lobo MK, Maze I, Golden SA, Russo SJ (2018) Cell-type-specific role for nucleus accumbens neuroligin-2 in depression and stress susceptibility. Proc Natl Acad Sci USA 115:1111–1116. 10.1073/pnas.1719014115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque KE, West AR (2012) Dopaminergic modulation of nitric oxide synthase activity in subregions of the rat nucleus accumbens. Synapse 66:220–231. 10.1002/syn.21503 [DOI] [PubMed] [Google Scholar]
- Hu Y, Wu DL, Luo CX, Zhu LJ, Zhang J, Wu HY, Zhu DY (2012) Hippocampal nitric oxide contributes to sex difference in affective behaviors. Proc Natl Acad Sci USA 109:14224–14229. 10.1073/pnas.1207461109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isingrini E, Perret L, Rainer Q, Amilhon B, Guma E, Tanti A, Martin G, Robinson J, Moquin L, Marti F, Mechawar N, Williams S, Gratton A, Giros B (2016) Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat Neurosci 19:560–563. 10.1038/nn.4245 [DOI] [PubMed] [Google Scholar]
- Jiang B, Wang W, Wang F, Hu ZL, Xiao JL, Yang S, Zhang J, Peng XZ, Wang JH, Chen JG (2013) The stability of NR2B in the nucleus accumbens controls behavioral and synaptic adaptations to chronic stress. Biol Psychiatry 74:145–155. 10.1016/j.biopsych.2012.10.031 [DOI] [PubMed] [Google Scholar]
- Joca SR, Guimarães FS (2006) Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effects. Psychopharmacology (Berl) 185:298–305. 10.1007/s00213-006-0326-2 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413. 10.1176/appi.ajp.162.8.1403 [DOI] [PubMed] [Google Scholar]
- Kim YK, Paik JW, Lee SW, Yoon D, Han C, Lee BH (2006) Increased plasma nitric oxide level associated with suicide attempt in depressive patients. Prog Neuropsychopharmacol Biol Psychiatry 30:1091–1096. 10.1016/j.pnpbp.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Lang E, Mallien AS, Vasilescu AN, Hefter D, Luoni A, Riva MA, Borgwardt S, Sprengel R, Lang UE, Gass P, Inta D (2018) Molecular and cellular dissection of NMDA receptor subtypes as antidepressant targets. Neurosci Biobehav Rev 84:352–358. 10.1016/j.neubiorev.2017.08.012 [DOI] [PubMed] [Google Scholar]
- Li K, Liu FF, He CX, Huang HZ, Xie AJ, Hu F, Liu D, Wang JZ, Zhu LQ (2016) Olfactory deprivation hastens Alzheimer-like pathologies in a human tau-overexpressed mouse model via activation of cdk5. Mol Neurobiol 53:391–401. 10.1007/s12035-014-9007-z [DOI] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. 10.1016/j.biopsych.2010.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang HY, Chen ZJ, Xiao H, Lin YH, Hu YY, Chang L, Wu HY, Wang P, Lu W, Zhu DY, Luo CX (2020) nNOS-expressing neurons in the vmPFC transform pPVT-derived chronic pain signals into anxiety behaviors. Nat Commun 11:2501. 10.1038/s41467-020-16198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, Chen HS, Zhu DY, Zhou QG (2018) Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc 13:1686–1698. 10.1038/s41596-018-0011-z [DOI] [PubMed] [Google Scholar]
- Luo CX, Jin X, Cao CC, Zhu MM, Wang B, Chang L, Zhou QG, Wu HY, Zhu DY (2010) Bidirectional regulation of neurogenesis of neuronal nitric oxide synthase derived from neurons and neural stem cells. Stem Cells 28:2041–2052. 10.1002/stem.522 [DOI] [PubMed] [Google Scholar]
- Luo CX, Lin YH, Qian XD, Tang Y, Zhou HH, Jin X, Ni HY, Zhang FY, Qin C, Li F, Zhang Y, Wu HY, Chang L, Zhu DY (2014) Interaction of nNOS with PSD-95 negatively controls regenerative repair after stroke. J Neurosci 34:13535–13548. 10.1523/JNEUROSCI.1305-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE (2010) Animal models of neuropsychiatric disorders. Nat Neurosci 13:1161–1169. 10.1038/nn.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS (2002) Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci 22:10883–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou A, Siamatras T, Delgado-Morales R, Amin ND, Shukla V, Zheng YL, Pant HC, Almeida OF, Kino T (2015) Acute and chronic stress differentially regulate cyclin-dependent kinase 5 in mouse brain: implications to glucocorticoid actions and major depression. Transl Psychiatry 5:e578. 10.1038/tp.2015.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Caballero L, Torres-Sanchez S, Romero-López-Alberca C, González-Saiz F, Mico JA, Berrocoso E (2019) Monoaminergic system and depression. Cell Tissue Res 377:107–113. 10.1007/s00441-018-2978-8 [DOI] [PubMed] [Google Scholar]
- Plattner F, Hayashi K, Hernández A, Benavides DR, Tassin TC, Tan C, Day J, Fina MW, Yuen EY, Yan Z, Goldberg MS, Nairn AC, Greengard P, Nestler EJ, Taussig R, Nishi A, Houslay MD, Bibb JA (2015) The role of ventral striatal cAMP signaling in stress-induced behaviors. Nat Neurosci 18:1094–1100. 10.1038/nn.4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Nakamura T, Cao G, Holland EA, McKercher SR, Lipton SA (2011) S-Nitrosylation activates CDK5 and contributes to synaptic spine loss induced by beta-amyloid peptide. Proc Natl Acad Sci USA 108:14330–14335. 10.1073/pnas.1105172108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko A, Seo J, Hu J, Su SC, de Anda FC, Durak O, Ericsson M, Carlén M, Tsai LH (2015) Loss of cyclin-dependent kinase 5 from parvalbumin interneurons leads to hyperinhibition, decreased anxiety, and memory impairment. J Neurosci 35:2372–2383. 10.1523/JNEUROSCI.0969-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14:609–625. 10.1038/nrn3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi Y, Heiman M, Peterson JD, Musatov S, Scarduzio M, Logan SM, Kaplitt MG, Surmeier DJ, Heintz N, Greengard P (2014) Nitric oxide regulates synaptic transmission between spiny projection neurons. Proc Natl Acad Sci USA 111:17636–17641. 10.1073/pnas.1420162111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin E, Gigliucci V, Harkin A (2017) Regional specific modulation of neuronal activation associated with nitric oxide synthase inhibitors in an animal model of antidepressant activity. Behav Brain Res 316:18–28. 10.1016/j.bbr.2016.08.049 [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Ishima T, Oda Y, Okamura N, Iyo M, Hashimoto K (2015) Opposite roles for neuropeptide S in the nucleus accumbens and bed nucleus of the stria terminalis in learned helplessness rats. Behav Brain Res 291:67–71. 10.1016/j.bbr.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Smith AC, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, Spencer S, Garcia-Keller C, Stankeviciute NM, Smith RJ, Allen NP, Lorang MR, Griffin WC, Boger HA, Kalivas PW (2017) Accumbens nNOS interneurons regulate cocaine relapse. J Neurosci 37:742–756. 10.1523/JNEUROSCI.2673-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW (2011) Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci USA 108:E255–E264. 10.1073/pnas.1101920108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A (2011) Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475:377–380. 10.1038/nature10194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SC, Seo J, Pan JQ, Samuels BA, Rudenko A, Ericsson M, Neve RL, Yue DT, Tsai LH (2012) Regulation of N-type voltage-gated calcium channels and presynaptic function by cyclin-dependent kinase 5. Neuron 75:675–687. 10.1016/j.neuron.2012.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SC, Tsai LH (2011) Cyclin-dependent kinases in brain development and disease. Annu Rev Cell Dev Biol 27:465–491. 10.1146/annurev-cellbio-092910-154023 [DOI] [PubMed] [Google Scholar]
- Suzuki E, Yagi G, Nakaki T, Kanba S, Asai M (2001) Elevated plasma nitrate levels in depressive states. J Affect Disord 63:221–224. 10.1016/S0165-0327(00)00164-6 [DOI] [PubMed] [Google Scholar]
- Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X (2015) An excitatory synapse hypothesis of depression. Trends Neurosci 38:279–294. 10.1016/j.tins.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaz VS, Cordeiro RC, Costa AM, de Lucena DF, Nobre Júnior HV, de Sousa FC, Vasconcelos SM, Vale ML, Quevedo J, Macêdo D (2014) Antidepressant-like effect of nitric oxide synthase inhibitors and sildenafil against lipopolysaccharide-induced depressive-like behavior in mice. Neuroscience 268:236–246. 10.1016/j.neuroscience.2014.03.025 [DOI] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K (2013) Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493:537–541. 10.1038/nature11740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulak G, Mutlu O, Tanyeri P, Komsuoglu FI, Akar FY, Erden BF (2010) Involvement of serotonin receptor subtypes in the antidepressant-like effect of TRIM in the rat forced swimming test. Pharmacol Biochem Behav 95:308–314. 10.1016/j.pbb.2010.02.006 [DOI] [PubMed] [Google Scholar]
- Venzala E, García-García AL, Elizalde N, Tordera RM (2013) Social vs. environmental stress models of depression from a behavioural and neurochemical approach. Eur Neuropsychopharmacol 23:697–708. 10.1016/j.euroneuro.2012.05.010 [DOI] [PubMed] [Google Scholar]
- Volke V, Wegener G, Bourin M, Vasar E (2003) Antidepressant- and anxiolytic-like effects of selective neuronal NOS inhibitor 1-(2-trifluoromethylphenyl)-imidazole in mice. Behav Brain Res 140:141–147. 10.1016/s0166-4328(02)00312-1 [DOI] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iñiguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolaños CA, Barrot M, McClung CA, Nestler EJ (2009) CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci 12:200–209. 10.1038/nn.2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Grace AA (2004) The nitric oxide-guanylyl cyclase signaling pathway modulates membrane activity states and electrophysiological properties of striatal medium spiny neurons recorded in vivo. J Neurosci 24:1924–1935. 10.1523/JNEUROSCI.4470-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Galloway MP, Grace AA (2002) Regulation of striatal dopamine neurotransmission by nitric oxide: effector pathways and signaling mechanisms. Synapse 44:227–245. 10.1002/syn.10076 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2017) Depression and other common mental disorders: global health estimates. Geneva: World Health Organization. [Google Scholar]
- Zahm DS, Brog JS (1992) On the significance of sub territories in the 'accumbens' part of the rat ventral striatum. Neuroscience 50:751–767. 10.1016/0306-4522(92)90202-D [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhu Z, Liang HY, Zhang L, Zhou QG, Ni HY, Luo CX, Zhu DY (2018) nNOS-CAPON interaction mediates amyloid-β-induced neurotoxicity, especially in the early stages. Aging Cell 17:e12754. 10.1111/acel.12754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Liu X, Zhang Z, Hu Y, Liu SJ, Lezama-Ruiz M, Joksimovic M, Liu QS (2014) Cyclin-dependent kinase 5 in the ventral tegmental area regulates depression-related behaviors. J Neurosci 34:6352–6366. 10.1523/JNEUROSCI.3673-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Li F, Xu HB, Luo CX, Wu HY, Zhu MM, Lu W, Ji X, Zhou QG, Zhu DY (2010) Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat Med 16:1439–1443. 10.1038/nm.2245 [DOI] [PubMed] [Google Scholar]
- Zhou QG, Hu Y, Hua Y, Hu M, Luo CX, Han X, Zhu XJ, Wang B, Xu JS, Zhu DY (2007) Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J Neurochem 103:1843–1854. 10.1111/j.1471-4159.2007.04914.x [DOI] [PubMed] [Google Scholar]
- Zhou QG, Zhu LJ, Chen C, Wu HY, Luo CX, Chang L, Zhu DY (2011) Hippocampal neuronal nitric oxide synthase mediates the stress-related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptor. J Neurosci 31:7579–7590. 10.1523/JNEUROSCI.0004-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QG, Zhu XH, Nemes AD, Zhu DY (2018) Neuronal nitric oxide synthase and affective disorders. IBRO Rep 5:116–132. 10.1016/j.ibror.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LJ, Li TY, Luo CX, Jiang N, Chang L, Lin YH, Zhou HH, Chen C, Zhang Y, Lu W, Gao LY, Ma Y, Zhou QG, Hu Q, Hu XL, Zhang J, Wu HY, Zhu DY (2014) CAPON-nNOS coupling can serve as a target for developing new anxiolytics. Nat Med 20:1050–1054. 10.1038/nm.3644 [DOI] [PubMed] [Google Scholar]
- Zhu WL, Shi HS, Wang SJ, Xu CM, Jiang WG, Wang X, Wu P, Li QQ, Ding ZB, Lu L (2012) Increased Cdk5/p35 activity in the dentate gyrus mediates depressive-like behaviour in rats. Int J Neuropsychopharmacol 15:795–809. 10.1017/S1461145711000915 [DOI] [PubMed] [Google Scholar]