Individuals with and without severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had similar rates of intrauterine fetal death, neonatal death, and Apgar score less than 7 at 5 minutes.
OBJECTIVE:
To compare the risk of intrauterine fetal death (20 weeks of gestation or later) and neonatal death among individuals who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) compared with those who tested negative for SARS-CoV-2 on admission for delivery.
DATA SOURCES:
MEDLINE, Ovid, EMBASE, Cumulative Index to Nursing and Allied Health, and Cochrane Library were searched from their inception until July 17, 2020. Hand search for additional articles continued through September 24, 2020. ClinicalTrials.gov was searched on October 21, 2020.
METHODS OF STUDY SELECTION:
The inclusion criteria were publications that compared at least 20 cases of both pregnant patients who tested positive for SARS-CoV-2 on admission to labor and delivery and those who tested negative. Exclusion criteria were publications with fewer than 20 individuals in either category or those lacking data on primary outcomes. A systematic search of the selected databases was performed, with co-primary outcomes being rates of intrauterine fetal death and neonatal death. Secondary outcomes included rates of maternal and neonatal adverse outcomes.
TABULATION, INTEGRATION, AND RESULTS:
Of the 941 articles and completed trials identified, six studies met criteria. Our analysis included 728 deliveries to patients who tested positive for SARS-CoV-2 and 3,836 contemporaneous deliveries to patients who tested negative. Intrauterine fetal death occurred in 8 of 728 (1.1%) patients who tested positive and 44 of 3,836 (1.1%) who tested negative (P=.60). Neonatal death occurred in 0 of 432 (0.0%) patients who tested positive and 5 of 2,400 (0.2%) who tested negative (P=.90). Preterm birth occurred in 95 of 714 (13.3%) patients who tested positive and 446 of 3,759 (11.9%) who tested negative (P=.31). Maternal death occurred in 3 of 559 (0.5%) patients who tested positive and 8 of 3,155 (0.3%) who tested negative (P=.23).
CONCLUSION:
The incidences of intrauterine fetal death and neonatal death were similar among individuals who tested positive compared with negative for SARS-CoV-2 when admitted to labor and delivery. Other immediate outcomes of the newborns were also similar among those born to individuals who tested positive compared with negative for SARS-CoV-2.
SYSTEMATIC REVIEW REGISTRATION:
PROSPERO, CRD42020203475.
In December 2019, the first documented case of human coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was described.1 By October 2020, there were more than 40 million confirmed cases of SARS-CoV-2 globally, with more than 1 million COVID-19–associated deaths.2 Coronavirus disease during pregnancy is considered a risk factor for adverse outcomes such as perinatal death, preterm birth, and cesarean delivery.3 Data from the Centers for Disease Control and Prevention indicate that pregnant individuals might be at increased risk for severe COVID-19 illness.4 Although there are a multitude of reports5–9 on pregnancy outcomes among individuals who tested positive for SARS-CoV-2, few reports provide outcomes on a contemporaneous control group (ie, outcome data on individuals who tested negative for the novel virus). Without the comparator group, assessing whether the adverse outcomes differ among those with the infection is difficult to ascertain.
In this systematic review and meta-analysis, we compared outcomes among individuals undergoing delivery who tested positive for SARS-CoV-2 on admission to labor and delivery compared with those who tested negative. The co-primary objectives of this systematic review and meta-analysis were to compare the risks of fetal death (20 weeks of gestation or later) and neonatal death in pregnancies in the two groups. We hypothesized there would be higher rates of fetal and neonatal death among individuals who tested positive for SARS-CoV-2. The secondary objectives were to compare the adverse outcomes for maternal-neonatal dyad among pregnancies with and without SARS-CoV-2 infection.
SOURCES
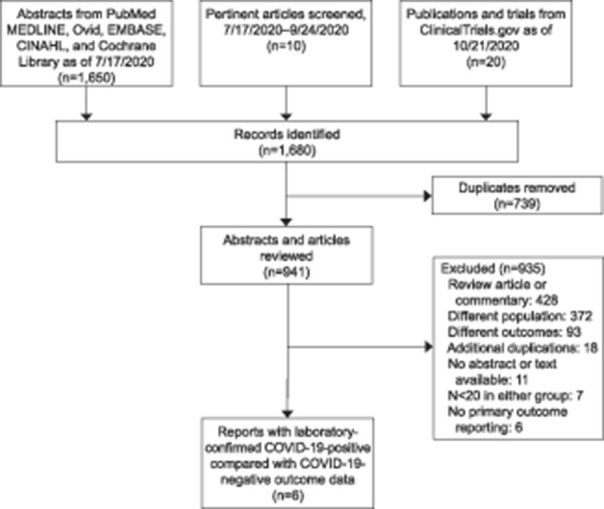
We performed this review based on guidelines designed for performing a systematic review and meta-analysis.10–12 Before data extraction, the review was registered with the PROSPERO International Prospective Register of Systematic Reviews (registration number: CRD42020203475). To avoid ascertainment bias, an experienced librarian (ES) developed searches for Medline Ovid, EMBASE, Cumulative Index to Nursing and Allied Health, and Cochrane Library from inception until July 17, 2020. The main search was conducted in Medline Ovid using the MeSH Headings as well as equivalent key words and phrases. These terms were then tested for relevancy and the main search was finalized in Medline on July 16, 2020. These search terms were then translated into EMBASE on July 17, 2020, CINAHL on July 17, 2020, and Cochrane Library on July 17, 2020, yielding a total of 911 de-duplicated results that were provided to authors for further review. The keywords details and full search strategy used are provided in Appendix 1, available online at http://links.lww.com/AOG/C238. Hand searching until September 24, 2020, was used to include three additional articles.13–15 Unanimous consensus among coauthors was to include one article13 from medRxiv, a non–peer-reviewed preprint server for health sciences. This article accounted for approximately one third of the total number of test-positive patients who were analyzed. A final search of completed trials on ClinicalTrials.gov was performed on October 21, 2020. A total of 941 publications and completed trials underwent two-person independent review; 428 review articles were excluded, 372 additional publications were not specific to the population of interest and were excluded, and 93 articles were excluded on the basis of publishing outcomes that did not pertain to this analysis (Fig. 1). Search results were not limited by language. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines were followed.
Fig. 1. Flowchart of the study selection process. COVID-19, coronavirus disease 2019.
Huntley. Adverse Pregnancy Outcomes With and Without SARS-CoV-2. Obstet Gynecol 2021.
STUDY SELECTION
Inclusion criteria for this study were case series, observational studies, or completed clinical trials consisting of at least 20 individuals who tested positive for SARS-CoV-2 at the time of hospital admission for delivery at a gestational age greater than 20 weeks that also included a contemporaneous control group of at least 20 additional pregnant patients who tested negative for SARS-CoV-2 during the same study period. This threshold was set to avoid selection bias of rare outcomes. Exclusion criteria included publications whose data were outside the scope of the declared primary or secondary outcomes, as well as articles lacking laboratory-confirmed SARS-CoV-2 diagnosis. We also excluded publications lacking primary data (eg, consensus documents, narrative reviews). Articles were not excluded based on country of origin or language. To minimize the effects of publication bias, one of this study’s authors (S.P.C.) emailed the corresponding authors from seven14,16–21 published articles that met inclusion criteria but lacked primary outcome data; emails were sent to all authors requesting unpublished data for secondary outcomes. Three authors14,17,18 replied with additional unpublished data that were incorporated into our analysis. Despite meeting all other inclusion criteria, six articles were excluded from consideration owing to lack of fetal and neonatal death rates.6,19–23
METHODS
Maternal, obstetric, and perinatal outcomes were abstracted. The maternal outcomes we compared were maternal death; maternal respiratory support; intensive care unit (ICU) admission; sepsis; venous thromboembolism; blood transfusion; combined antepartum and postpartum hemorrhage; intrapartum fever; postpartum fever; preeclampsia with and without severe features; and postpartum readmission to the hospital. The obstetric outcomes we compared were rates of operative vaginal delivery and cesarean delivery stratified by indication. The additional neonatal adverse outcomes we compared were neonatal intensive care unit (NICU) admission, preterm birth, small for gestational age (as defined by the original publication authors), large for gestational age (as defined by the original publication authors), 5-minute Apgar score less than 7, ventilation longer than 6 hours, hypoglycemia, seizure, intraventricular hemorrhage grade III or IV, bronchopulmonary dysplasia, necrotizing enterocolitis (NEC) class 2 or 3, and sepsis. Data for average birth weight and neonatal length of stay were not aggregated owing to variation in reporting among articles considered (eg, mean with standard deviation vs mode with interquartile range). Given inconsistent reporting of similar data types, we calculated pooled proportions of combined rates of all forms of hypertensive disorders of pregnancy mentioned in each publication.
To minimize the effects of information bias, two authors (B.J.F.H., I.A.M.) reviewed all abstracts independently. Agreement regarding potential relevance or inconsistencies was reached by consensus or resolved by discussion with a third reviewer (S.P.C.). The same reviewers independently extracted relevant data from full-text copies of applicable articles, compared findings, and likewise resolved inconsistencies by discussion with the third reviewer.
Data analysis was performed with Review Manager 5.3. All analyses were carried out using the random effects model (of DerSimonian and Laird, assuming that the data being analyzed were drawn from a hierarchy of different populations). Owing to the small number of cases of the outcomes explored, univariate comparisons of dichotomous data were performed using Fisher exact test. Heterogeneity was measured using I-squared (Higgins I2).
Quality assessment of the included studies was performed using the Newcastle-Ottawa Scale for case–control studies.24 According to the Newcastle-Ottawa Scale, each study is judged on three broad perspectives: the selection of the study groups; the comparability of the groups; and the ascertainment of the exposure. Assessment of the selection of a study includes the evaluation of the adequacy of the case definition, the representativeness of the cases, and the selection and definition of the control group. Assessment of the comparability of the study includes the evaluation of the case and control groups on the basis of the design or analysis. Finally, the exposure perspective includes the ascertainment of the exposure, the homogeneity in the ascertainment for both the case and control groups, and the nonresponse rate. According to the Newcastle-Ottawa Scale, a study can be awarded a maximum of one star for each numbered item within the selection and exposure categories. A maximum of two stars can be given for comparability (Appendix 2, available online at http://links.lww.com/AOG/C238). In the absence of original research and in keeping with historical convention, this study was considered exempt from IRB approval.
RESULTS
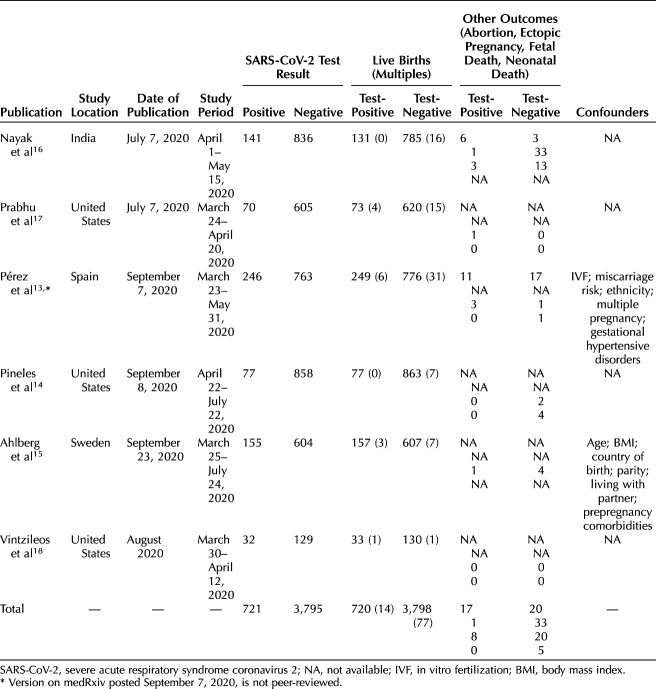
Of 1,650 abstracts resulting from the database search, 739 were identified as duplicates and removed by the librarian. Ten additional hand-searched manuscripts and 20 publications associated with 11 completed clinical trials were further assessed. Of the remaining 941 articles, 18 duplicates were subsequently identified and removed from consideration, 428 narrative publications were excluded, and 372 articles that reported on cohorts other than our preidentified study population were excluded. An additional 93 articles were removed from consideration after title and abstract review indicated that outcomes reported did not overlap with our preidentified outcomes of interest. Eleven articles were removed that lacked abstract or full-text availability. Seven articles had fewer than 20 patients in both testing cohorts and were hence excluded. Six remaining articles lacked primary outcomes data and were also excluded (Fig. 1). Email correspondence with authors enabled inclusion of one additional article18 and permitted the incorporation of additional primary outcomes data from another article.17 There were six articles with at least 20 gravid individuals with laboratory-confirmed SARS-CoV-2 and 20 contemporaneous gravid individuals who tested negative for the novel virus on admission for delivery with fetal and neonatal death rate data. All six publications were observational studies that reported universal screening for SARS-CoV-2 on admission for delivery. These six articles originated from four countries, with study period spanning from March 2020 to July 2020 (Table 1). Our final analysis included 728 deliveries (including 14 multiples) to patients who tested positive for SARS-CoV-2 and 3,836 deliveries (including 77 multiples) to contemporaneous patients who tested negative.
Table 1.
General Characteristics of Studies and Cohorts
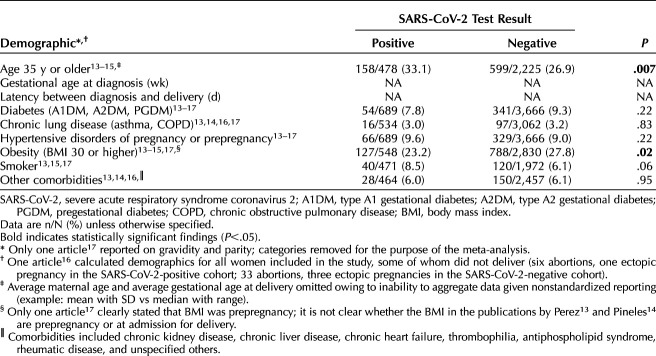
Individuals who tested positive for SARS-CoV-2 were significantly more likely to be 35 years or older (P=.007) (Table 2). There was no significant difference between the test-positive compared with the test-negative cohorts in rates of diabetes, chronic lung disease (combined asthma and chronic obstructive pulmonary disease), or hypertensive disorders of pregnancy (combined chronic hypertension, gestational hypertension, and preeclampsia with and without severe features). Obesity (body mass index [BMI, calculated as weight in kilograms divided by height in meters squared] 30 or higher) was less likely in the test-positive cohort (23.2% vs 27.8%, P=.02). The average maternal age and average gestational age at delivery were unable to be calculated owing to variations in reporting. Although outcomes were reported among women who delivered beyond 20 weeks of gestation, one article16 reported demographics for all women included in the study, some of whom did not deliver viable offspring (43 abortions or ectopic pregnancies accumulated between both cohorts). Maternal characteristics associated with these 43 pregnancy outcomes were included in the overall maternal demographic analysis.
Table 2.
Maternal Demographics
Among the 4,473 pregnant individuals who delivered and were included in the analysis, 714 (16.0%) had laboratory-confirmed SARS-CoV-2 infection. Accounting for higher-order deliveries, there was a total of 4,564 births included in this study: 728 deliveries by patients who tested positive for SARS-CoV-2 compared with 3,836 deliveries by patients who tested negative. Two authors13,18 categorized COVID-19 disease severity according to the World Health Organization classification scheme.25 The combined asymptomatic rate among test-positive individuals from these two cohorts was 86.0% (239/278), mild 11.5% (32/278), moderate 1.1% (3/278), severe 1.4% (4/278), and critical 0.0% (0/278).
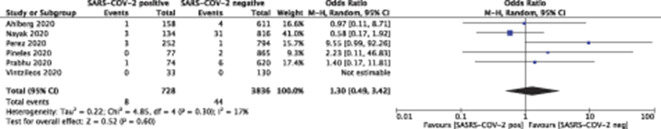
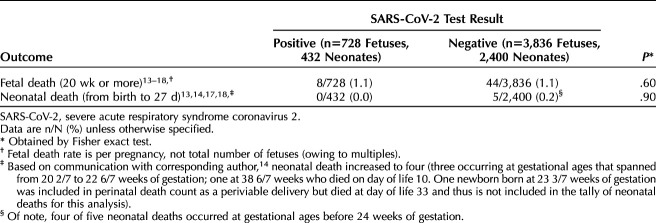
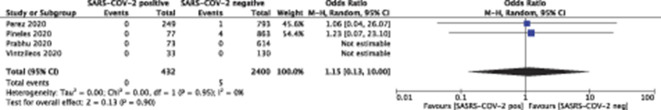
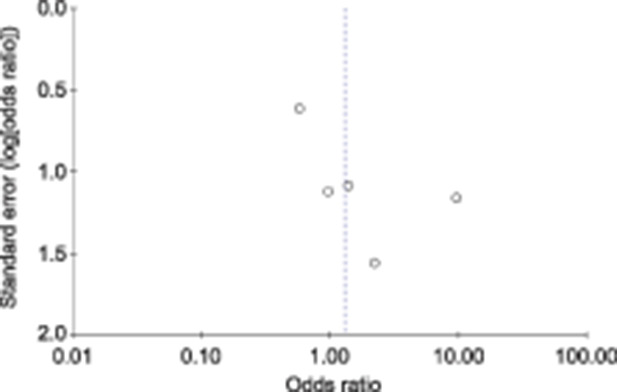
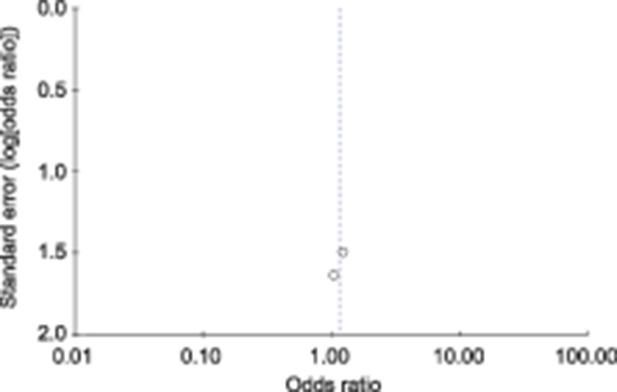
Fetal death occurred in 8 of 728 (1.1%) patients who tested positive for SARS-CoV-2 and in 44 of 3,836 (1.1%) who tested negative (P=.60) (Figs. 2 and 3). Neonatal death occurred in 0 of 432 (0.0%) live births to patients who tested positive for SARS-CoV-2 and in 5 of 2,400 (0.2%) live births to those who tested negative (P=.90) (Table 3) (Figs. 4 and 5).
Fig. 2. Forest plot for fetal death. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; M-H, Mantel-Haenszel.
Huntley. Adverse Pregnancy Outcomes With and Without SARS-CoV-2. Obstet Gynecol 2021.
Fig. 3. Funnel plot for fetal death.
Huntley. Adverse Pregnancy Outcomes With and Without SARS-CoV-2. Obstet Gynecol 2021.
Table 3.
Primary Outcomes
Fig. 4. Forest plot for neonatal death. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; M-H, Mantel-Haenszel.
Huntley. Adverse Pregnancy Outcomes With and Without SARS-CoV-2. Obstet Gynecol 2021.
Fig. 5. Funnel plot for neonatal death.
Huntley. Adverse Pregnancy Outcomes With and Without SARS-CoV-2. Obstet Gynecol 2021.
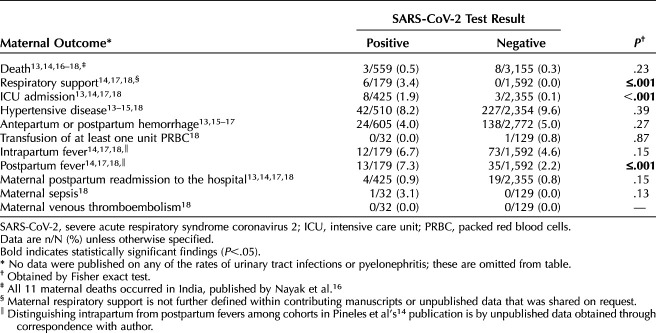
Statistically significant differences in secondary maternal outcomes were observed in maternal respiratory support, maternal ICU admission, and postpartum fever comparisons (P≤0.001 for all three outcomes). Among the studies that met the eligibility criteria, there was no statistically significantly difference in the rate of maternal death associated with SARS-CoV-2 carriage (P=.27; Table 4).
Table 4.
Maternal Adverse Outcomes
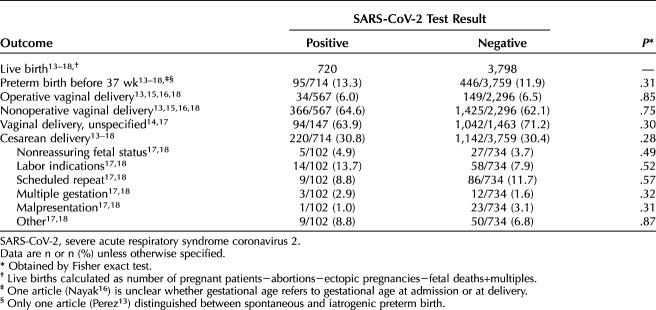
Thirty percent of each cohort underwent cesarean delivery (30.8% test-positive vs 30.4% test-negative) (Table 5).
Table 5.
Obstetric Adverse Outcomes
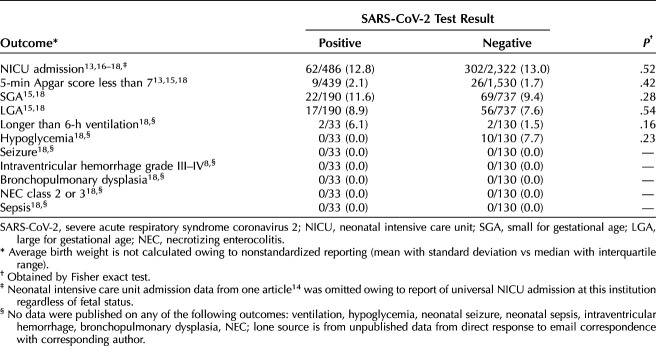
No neonatal secondary outcomes were significantly different. The rate of preterm birth (before 37 weeks of gestation) was similar 95 of 714 (13.3%) among test-positive patients compared with 446 of 3,759 test-negative (P=.31). Neonatal intensive care unit admission rates among publications that did not implement universal NICU admissions were similar between cohorts, with 12.8% of neonates born to patients who tested positive for SARS-CoV-2 compared with 13.0% born to those who tested negative being admitted to the NICU. Rates of Apgar score less than 7 at 5 minutes were similar between the two groups (2.1% of those born to patients who tested positive for SARS-CoV-2 compared with 1.7% born to those who tested negative, P=.42). The rates of small for gestational age and large for gestational age neonates were similar between groups, with P=.28 and P=.54, respectively (Table 6). None of the publications reported on neonatal ventilation longer than 6 hours, hypoglycemia, seizures, intraventricular hemorrhage, bronchopulmonary dysplasia, NEC, or sepsis. Data on rates of neonatal ventilation longer than 6 hours and rates of neonatal hypoglycemia were shared by one author.18 Shared data from the same author indicate no cases of neonatal seizure, intraventricular hemorrhage grade III or IV, bronchopulmonary dysplasia, NEC class 2 or 3, or neonatal sepsis occurred in either group.
Table 6.
Neonatal Adverse Outcomes
DISCUSSION
Of the 4,473 pregnant people assessed for the novel virus on labor and delivery in this systematic review and meta-analysis, the co-primary outcomes of fetal and neonatal death occurred at similarly low rates (approximately 1% risk of fetal death, and near-0% risk for neonatal death) regardless of SARS-CoV-2 carrier status. The rate of fetal death in our analysis may seem high, however the rate reported by Khalil et al26 of 0.9% was similar to the rate calculated in this meta-analysis.
Notably, only one publication specified the gestational ages at which fetal death occurred (one fetal death at 37 weeks of gestation among asymptomatic patients who tested positive for SARS-CoV-2 and six fetal deaths among patients who tested negative occurring between 20 and 25 weeks), hence there is insufficient data to generalize the gestational age that fetal deaths occurred. As for exploring the underlying etiology of fetal death, Prabhu et al17 assessed placental pathology and found higher rates of fetal vascular malperfusion and meconium staining of the placenta among births to patients who tested positive for SARS-CoV-2. However, the placental pathology associated with the lone fetal death in the cohort of patients who tested positive for SARS-CoV-2 was reportedly normal. Although a publication by Patberg et al27 also found an association between maternal SARS-CoV-2 carriage and increased rates of placental histopathologic abnormalities such as fetal vascular malperfusion and villitis, there remains insufficient reporting among articles meeting inclusion criteria for this meta-analysis to comment on putative etiologies of fetal death in pregnancies that were and were not affected by the virus. Although transplacental infection of the fetus can occur,28,29 causing thrombotic and vascular changes similar to other inflammatory conditions,30,31 this appears to be uncommon32 and provides putative explanation for why the neonatal outcomes were similar among cohorts in this systematic review.
The rate of neonatal mortality identified among neonates born to patients who tested positive for SARS-CoV-2 in publications meeting inclusion criteria for this meta-analysis (0.0%, 0/432) was consistent with data from both the Centers for Disease Control and Prevention33 reporting the neonatal death rate of 0.2% (9/4,495) and a prior meta-analysis on this topic that reported neonatal death occurring in 0.3% of live births (1/313).5
Neonatal morbidity, as assessed by an Apgar score less than 7 at 5 minutes, admission to the NICU, ventilation for at least 6 hours, hypoglycemia, and seizure, also occurred with similar frequency among those who tested positive compared with negative for SARS-CoV-2. None of the six publications meeting inclusion criteria for this meta-analysis reported on the outcomes of neonatal seizure, intraventricular hemorrhage grade III or IV, bronchopulmonary dysplasia, NEC class 2 or 3, or sepsis. Data shared by Vintzileos et al have 33 deliveries among test-positive patients and 130 deliveries among test-negative patients yet report none of the secondary neonatal outcomes of interest. Given the small number of cases, this was not statistically significant.
Obstetric practice patterns, such as decreased in-person prenatal visits and ultrasonograms, were altered during the early response to the pandemic,34–36 and may have contributed to the early rise in adverse outcomes. The observed preterm birth rate of 12–13% and cesarean delivery rate of approximately 30% were similar between those with and without the virus in this review, suggesting that previously ascertained increased rates of preterm birth (20%)5 and cesarean delivery (85%)5 might not have reflected disease process so much as they were influenced by transient changes in obstetric management. This observation was shared by a corresponding author of one of the original publications from China.37 A recent analysis of more than 1.5 million births also showed substantial reductions in preterm birth rates after implementation of interventions to decrease communal spread of SARS-CoV-2.38 Compared with the prepandemic period, however, data suggest there has been a significant decrease in rates of preterm birth overall during the pandemic.39
Consistent with trends observed in nonpregnant patients who tested positive for SARS-CoV-2,40 pregnant patients who tested positive in our review were more likely to experience fever, respiratory support, and admission to the ICU compared with their test-negative counterparts. Pathophysiologic mechanisms of advanced respiratory disease have been described in autopsy studies of nonpregnant populations and include diffuse alveolar thickening with mononuclear cell and macrophage infiltration; interstitial edema; and eventual pulmonary edema followed by hyaline membrane formation reflective of early acute respiratory distress syndrome.41 Such immunologic mechanisms have yet to be validated in the pregnant population.
Although vulnerability to the SARS-CoV-2 virus appears to be disproportionately distributed along racial lines,42 with Black and Latino individuals bearing an unusually high burden of adverse outcomes,43 these disparities must be understood in the context of social determinants of health to avoid the inappropriate conclusions that would further health inequities. For instance, in a study on hospitalization and mortality rates due to COVID-19 in nonpregnant patients,44 being of Black race was not associated with higher in-hospital mortality than being of White race when adjusting for sociodemographic and clinic characteristics on admission. Unfortunately, among the three publications included in this meta-analysis that commented on racial and ethnic demographics,13,14,17 none of the articles described who classified individuals’ race or ethnicity or whether the options were defined by the investigator or the participant. Furthermore, one author reported on a nonspecific category of “Other” without commenting on whether this was a prespecified formal category in a database or providing detail to describe which patients were included in that category. Thus, we are unable to appropriately assess whether race disproportionately affects maternal, neonatal and obstetric health outcomes among pregnant individuals with and without SARS-CoV-2.
The strengths of our analysis include data from four countries, which make the findings generalizable. With control groups of individuals who tested negative for SARS-CoV-2, we were able to compare the outcomes during the pandemic and associated changes in practice.34,45,46 Although there have been many systematic reviews on the topic,5,47–52 there is a paucity of literature directly comparing outcomes of deliveries to patients who tested positive compared with negative for the novel virus. A thorough review of ClinicalTrials.gov, for example, revealed that there are no completed trials that compare maternal and neonatal outcomes among individuals who tested positive compared with negative for SARS-CoV-2. In addition, our comparative group consisted of a contemporaneous cohort of individuals who tested negative for SARS-CoV-2, setting this analysis apart from previously published meta-analyses.53 Another strength is that we incorporated unpublished data from corresponding authors of prior publications to provide a nuanced understanding of the risk and rates of adverse outcomes given SARS-CoV-2 infection. Lastly, by setting a moderately high threshold for inclusion, the analysis avoids detection of rare adverse outcomes owing to selection bias.5
We acknowledge the limitations. The small number of cases in some of the included studies, their retrospective nonrandomized design, the lack of standardized criteria for the surveillance, and the heterogeneity of some of the baseline characteristics among women who tested positive for SARS-CoV-2 compared with those who tested negative are limitations of this systematic review. Because we had no access to original raw data of all the included studies, it was not possible to perform an individual patient meta-analysis nor a multivariate logistic regression, because the influence of known and suspected measured confounding factors was not universally reported in the original publications, except for two studies (Table 1). Most publications did not provide data on the severity of the infection, which ranged from asymptomatic to critical.54 Thus, although adverse outcomes due to COVID-19 during pregnancy are known to be associated with increased disease severity,55 we are unable to link disease severity to clinically relevant outcomes. Some authors either did not respond to email inquiries16,19–21 or understandably chose not to share yet unpublished data. Lastly, we note that one significant limitation is that lack of international standards in reporting prohibits aggregation of some data (eg, mean vs median gravidity, parity, newborn weight, maternal age).
In conclusion, in this meta-analysis, we did not find that SARS-CoV-2 positivity changed the rates of intrauterine fetal death or neonatal death. Rates of maternal ICU admission and need for respiratory support were greater among those who tested positive for SARS-CoV-2 compared with those who did not. Larger studies comparing these two groups are warranted to ensure that uncommon morbidities are not more common among individuals who test positive for SARS-CoV-2. Lastly, a large multicenter study comparing the pregnancy outcomes before and during the pandemic is warranted to see whether the changes in obstetric practice have influenced the outcomes.
Footnotes
Financial Disclosure The authors did not report any potential conflicts of interest.
The authors thank Emma Silva, librarian at the University of Texas Health Sciences Center at Houston, for her assistance with initiating the literature search.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews are available at http://links.lww.com/AOG/C239.
Contributor Information
Isabelle A. Mulder, Email: Isabelle.A.Mulder@uth.tmc.edu.
Daniele Di Mascio, Email: dani.dimascio@gmail.com.
William S. Vintzileos, Email: William.Vintzileos@nyulangone.org.
Anthony M. Vintzileos, Email: Anthony.Vintzileos@nyulangone.org.
Vincenzo Berghella, Email: vincenzo.berghella@jefferson.edu.
Suneet P. Chauhan, Email: Suneet.P.Chauhan@uth.tmc.edu.
REFERENCES
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Accessed October 22, 2020. https://covid19.who.int/
- 3.Di Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2020;2:100107. doi: 10.1016/j.ajogmf.2020.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep 2020;69:769–75. doi: 10.15585/mmwr.mm6925a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huntley BJF, Huntley ES, Di Mascio D, Chen T, Berghella V, Chauhan SP. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome coronavirus 2 (SARS-Co-V-2) infection: a systematic review. Obstet Gynecol 2020;136:303–12. doi: 10.1097/AOG.0000000000004010 [DOI] [PubMed] [Google Scholar]
- 6.Flaherman VJ, Afshar Y, Boscardin J, Keller RL, Mardy A, Prahl MK, et al. Infant outcomes following maternal infection with SARS-CoV-2: first report from the PRIORITY study. Clin Infect Dis 2020 Sep 18. [Epub ahead of print]. doi: 10.1093/cid/ciaa1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fassett MJ, Lurvey LD, Yasumura L, Nguyen M, Colli JJ, Volodarskiy M, et al. Universal SARS-Cov-2 screening in women admitted for delivery in a large managed care organization. Am J Perinatol 2020;37:1110–4. doi: 10.1055/s-0040-1714060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WAPM (World Association of Perinatal Medicine) Working Group on COVID-19. Maternal and perinatal outcomes of pregnant women with SARS-COV-2 infection. Ultrasound Obstet Gynecol 2020;57:232–41. doi: 10.1002/uog.23107 [DOI] [PubMed] [Google Scholar]
- 9.Di Mascio D, Sen C, Saccone G, Grünebaum A, Yoshimatsu J, Stanojevic M, et al. Risk factors associated with adverse fetal outcomes in pregnancies affected by Coronavirus disease 2019 (COVID-19): a secondary analysis of the WAPM study on COVID-19. J Perinat Med 2020;48:950–8. doi: 10.1515/jpm-2020-0355 [DOI] [PubMed] [Google Scholar]
- 10.Henderson LK, Craig JC, Willis NS, Tovey D, Webster AC. How to write a Cochrane systematic review. Nephrology (Carlton) 2010;15:617–24. doi: 10.1111/j.1440-1797.2010.01380.x [DOI] [PubMed] [Google Scholar]
- 11.NHS Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. Accessed December 3, 2016. Available at: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf
- 12.Welch V, Petticrew M, Petkovic J, Moher D, Waters E, White E, et al. Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): explanation and elaboration. J Clin Epidemiol 2016;70:68–89. doi: 10.1016/j.jclinepi.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 13.Perez OM, Rodriguez PP, Hernandez MM, Encinas Pardilla MB, Perez NP, Vila Hernandez MR, et al. The association between COVID-19 and preterm delivery: a cohort study with a multivariate analysis. medRxiv 2020. doi: 10.1101/2020.09.05.20188458 [DOI] [Google Scholar]
- 14.Pineles BL, Alamo IC, Farooq N, Green J, Blackwell SC, Sibai BM, et al. Racial-ethnic disparities and pregnancy outcomes in SARS-CoV-2 infection in a universally-tested cohort in Houston, Texas [letter]. Eur J Obstet Gynecol Reprod Biol 2020;254:329–30. doi: 10.1016/j.ejogrb.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahlberg M, Neovius M, Saltvedt S, Söderling J, Pettersson K, Brandkvist C, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA 2020;324:1782–5. doi: 10.1001/jama.2020.19124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nayak AH, Kapote DS, Fonseca M, Chavan N, Mayekar R, Sarmalkar M, et al. Impact of the coronavirus infection in pregnancy: a preliminary study of 141 patients. J Obstet Gynaecol India 2020;70:256–61. doi: 10.1007/s13224-020-01335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prabhu M, Cagino K, Matthews KC, Friedlander RL, Glynn SM, Kubiak JM, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG 2020;127:1548–56. doi: 10.1111/1471-0528.16403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vintzileos WS, Muscat J, Hoffmann E, John NS, Vertichio R, Vintzileos AM, et al. Screening all pregnant women admitted to labor and delivery for the virus responsible for coronavirus disease 2019. Am J Obstet Gynecol 2020;223:284–6. doi: 10.1016/j.ajog.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med 2020;382:2163–4. doi: 10.1056/nejmc2009316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldfarb IT, Clapp MA, Soffer MD, Shook LL, Rushfirth K, Edlow AG, et al. Prevalence and severity of coronavirus disease 2019 (COVID-19) illness in symptomatic pregnant and postpartum women stratified by Hispanic ethnicity. Obstet Gynecol 2020;136:300–2. doi: 10.1097/AOG.0000000000004005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller ES, Grobman WA, Sakowicz A, Rosati J, Peaceman AM. Clinical implications of universal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing in pregnancy. Obstet Gynecol 2020;136:232–4. doi: 10.1097/AOG.0000000000003983 [DOI] [PubMed] [Google Scholar]
- 22.Sakowicz A, Ayala AE, Ukeje CC, Witting CS, Grobman WA, Miller ES. Risk factors for severe acute respiratory syndrome coronavirus 2 infection in pregnant women. Am J Obstet Gynecol MFM 2020;2:100198. doi: 10.1016/j.ajogmf.2020.100198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haizler-Cohen L, Davidov A, Blitz MJ, Fruhman G. Severe acute respiratory syndrome coronavirus 2 antibodies in pregnant women admitted to labor and delivery units. Am J Obstet Gynecol 2021;224:112–4. doi: 10.1016/j.ajog.2020.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed October 20, 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 25.World Health Organization. Clinical management of COVID-19. Interim guidance 15 October 2020. Accessed October 20, 2020. https://www.who.int/publications/i/item/clinical-management-of-covid-19 [Google Scholar]
- 26.Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O’Brien P, Magee L. Change in the incidence of Stillbirth and preterm delivery during the COVID-19 pandemic. JAMA 2020;324:705–6. doi: 10.1001/jama.2020.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patberg ET, Adams T, Rekawek P, Vahanian SA, Akerman M, Hernandez A, et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol 2020 Oct 19. [Epub ahead of print]. doi: 10.1016/j.ajog.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong L, Tian J, He S, Zhu C, Wang J, Liu C, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA 2020;323:1846–8. doi: 10.1001/jama.2020.4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patanè L, Morotti D, Giunta MR, Sigismondi C, Piccoli MG, Frigerio L, et al. Vertical transmission of COVID-19: SARS-CoV-2 RNA on the fetal side of the placenta in pregnancies with COVID-19 positive mothers and neonates at birth. Am J Obstet Gynecol MFM 2020;2:100145. doi: 10.1016/j.ajogmf.2020.100145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol 2020;154:23–32. doi: 10.1093/ajcp/aqaa089.PMID:32441303; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baergen RN, Heller DS. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatr Dev Pathol 2020;23:177–80. doi: 10.1177/1093526620925569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prochaska E, Jang M, Burd I. COVID-19 in pregnancy: placental and neonatal involvement. Am J Reprod Immunol 2020;84:e13306. doi: 10.1111/aji.13306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodworth KR, Olsen EO, Neelam V, Lewis EL, Galang RR, Oduyebo T, et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy—SET-NET, 16 jurisdictions, March 29–October 14, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1635–40. doi: 10.15585/mmwr.mm6944e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aziz A, Fuchs K, Nhan-Chang CL, Zork N, Friedman AM, Simpson LL. Adaptation of prenatal care and ultrasound. Semin Perinatol 2020;44:151278. doi: 10.1016/j.semperi.2020.151278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boelig RC, Manuck T, Oliver EA, Di Mascio D, Saccone G, Bellussi F, et al. Labor and delivery guidance for COVID-19. Am J Obstet Gynecol MFM 2020;2:100110. doi: 10.1016/j.ajogmf.2020.100110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boelig RC, Saccone G, Bellussi F, Berghella V. MFM guidance for COVID-19. Am J Obstet Gynecol MFM 2020;2:100106. doi: 10.1016/j.ajogmf.2020.100106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L, Zhao Y, Qiao J. More on clinical characteristics of pregnant women with Covid-19 in Wuhan, China [letter]. N Engl J Med 2020;383:697. doi: 10.1056/NEJMc2016881 [DOI] [PubMed] [Google Scholar]
- 38.Been J, Ochoa L, Bertens L, Schoenmakers S, Steegers E, Reiss I. Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health 2020;5:e604–11. doi: 10.1016/S2468-2667(20)30223-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berghella V, Burd J, Anderson K, Boelig R, Roman A. Decreased incidence of preterm birth during COVID-19 pandemic, Am J Obstet Gynecol MFM 2020;2:100258. doi: 10.1016/j.ajogmf.2020.100258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020;323:2052–9. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020;324:782–93. doi: 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 42.Chowkwanyun M, Reed AL, Jr. Racial health disparities and Covid-19—caution and context. N Engl J Med 2020;383:201–3. doi: 10.1056/NEJMp2012910 [DOI] [PubMed] [Google Scholar]
- 43.Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA 2020;323:2466–7. doi: 10.1001/jama.2020.8598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med 2020;382:2534–43. doi: 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.London V, McLaren R, Jr, Stein J, Atallah F, Fisher N, Haberman S, et al. Caring for pregnant patients with COVID-19: practical tips getting from policy to practice. Am J Perinatol 2020;37:850–3. doi: 10.1055/s-0040-1710539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greene NH, Kilpatrick SJ, Wong MS, Ozimek JA, Naqvi M. Impact of labor and delivery unit policy modifications on maternal and neonatal outcomes during the COVID-19 pandemic. Am J Obstet Gynecol MFM 2020;2:100234. doi: 10.1016/j.ajogmf.2020.100234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand 2020;99:823–9. doi: 10.1111/aogs.13867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akhtar H, Patel C, Abuelgasim E, Harky A. COVID-19 (SARS-CoV-2) infection in pregnancy: a systematic review. Gynecol Obstet Invest 2020;85:295–306. doi: 10.1159/000509290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Juan J, Gil MM, Rong Z, Zhang Y, Yang H, Poon LC. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol 2020;56:15–27. doi: 10.1002/uog.22088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res 2020;25:39. doi: 10.1186/s40001-020-00439-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khalil A, Kalafat E, Benlioglu C, O’Brien P, Morris E, Draycott T, et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EclinicalMedicine 2020;25:100446. doi: 10.1016/j.eclinm.2020.100446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Z, Wang M, Zhu Z, Liu Y. Coronavirus disease 2019 (COVID-19) and pregnancy: a systematic review. J Matern Fetal Neonatal Med 2020 Apr 30. [Epub ahead of print]. doi: 10.1080/14767058.2020.1759541 [DOI] [PubMed] [Google Scholar]
- 53.Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. doi: 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.National Institutes of Health. COVID-19 treatment guidelines. Accessed October 11, 2020. Available at: https://covid19treatmentguidelines.nih.gov/overview/management-of-covid-19/ [PubMed] [Google Scholar]
- 55.Brandt JS, Hill J, Reddy A, Schuster M, Patrick HS, Rosen T, et al. Epidemiology of coronavirus disease 2019 in pregnancy: risk factors and associations with adverse maternal and neonatal outcomes. Am J Obstet Gynecol 2020 Sep 25. [Epub ahead of print]. doi: 10.1016/j.ajog.2020.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]