Abstract
Purpose: Propranolol, a nonselective B1/B2 adrenoceptor antagonist, promotes the regression of infantile hemangiomas likely through suppression of vascular endothelial growth factor (VEGF), which prompted its use for the prevention of retinopathy of prematurity. We tested the hypothesis that topical ocular propranolol is safe and effective for reducing the severity of oxygen-induced retinopathy (OIR) in the neonatal rat intermittent hypoxia (IH) model.
Methods: At birth (P0), rat pups were randomly assigned to room air or neonatal intermittent hypoxia (IH) consisting of 50% O2 with brief episodes of hypoxia (12% O2) from P0 to P14, during which they received a single daily dose of oral propranolol (1 mg/kg/day in 50 μL in sterile normal saline) or topical ocular propranolol (0.2% in 10 μL in normal saline) from P5 to P14. Placebo-controlled littermates received 50 μL oral or 10 μL topical ocular sterile normal saline. Retinal vascular and astrocyte integrity; retinal histopathology and morphometry; and angiogenesis biomarkers were determined.
Results: Topical ocular propranolol improved retinal vascular damage and preserved the astrocytic template, but did not completely prevent OIR. The beneficial effects of propranolol were associated with reduced ocular VEGF and increased endogenous soluble inhibitor, sVEGFR-1, when administered topically.
Conclusions: Propranolol failed to completely prevent severe OIR, however, it prevented astrocyte degeneration resulting from neonatal IH-induced damage. We conclude that the mechanisms of propranolol's beneficial effects in neonatal IH may involve in part, astrocyte preservation.
Keywords: angiogenesis, hypoxia, propranolol, retina, neovascularization
Introduction
Retinopathy of prematurity (ROP) is a blinding disease resulting from pathological angiogenesis in the incompletely vascularized retina of extremely low gestational age neonates (ELGANs). Despite current therapeutic strategies, ROP still represents a significant cause of morbidity and disability in childhood, and as many as ∼50,000 preterm infants worldwide become blind or visually impaired from ROP each year.1 ROP is characterized by 2 phases: a vaso-obliterative phase and a vasoproliferative phase of pathologic neovascularization that may lead to retinal detachment and blindness.2 Hyperoxia, fluctuations in arterial oxygen saturation, or neonatal intermittent hypoxia (IH), are important factors associated with ROP3–7 that impact retinal vascular development by increasing the production of angiogenic factors such as vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF)-I, resulting in pathologic angiogenesis.8,9
In this regard, anti-VEGF therapies are increasingly being used to treat aggressive posterior ROP.10,11 However, its use is associated with many unwanted adverse effects.12–15 The primary focus of our laboratory is to develop age-appropriate, noninvasive, and safe therapies that are less painful, but with equal or more therapeutic benefits; with particular reference to neonatal IH, or frequent, brief hypoxia. Our previous studies have shown that neonatal IH episodes increase the risk for severe oxygen-induced retinopathy (OIR) regardless of resolution in room air (RA) or hyperoxia between episodes.16 We also showed that topical ocular ophthalmic solutions of ketorolac (Acuvail) combined with systemic caffeine citrate was beneficial for reducing the severity of OIR in a rat model of neonatal IH.17 However, although the combination therapy improved the OIR, it did not fully prevent it.
Propranolol is a beta-blocker that has recently been suggested for prevention and treatment of ROP and OIR.18–24 The mechanism of its inhibitory effects may involve suppression of angiogenesis factors, which are also involved in proliferating hemangiomas.25 The similarities between regulation of growth of infantile hemangiomas and development of ROP have prompted the use of propranolol for ROP.26 Propranolol is used in children to treat a variety of diseases, including hypertension, cardiac arrhythmia, obstructive heart disease, thyrotoxicosis, and migraine headache, and is generally well tolerated. However, clinically relevant adverse events, such as hypotension, bradycardia, heart block, hypoglycemia, and bronchospasm, have been reported with systemic propranolol administration. These adverse effects were noted in patients treated with higher doses of up to 3.0 mg/kg/day for 3–6 months for infantile hemangioma.27 A recent systematic review of oral propranolol for ROP showed dose ranges of up to 2 mg/kg/day every 6 h.28 However, serious adverse effects were reported.29 Alternative routes of administration of propranolol have been considered to reduce the risk of adverse events. The use of 0.1% propranolol eye drops for ROP has proven to be safe but ineffective in treating ROP.18 A clinical trial using higher topical ocular doses of 0.2% propranolol was well tolerated and resulted in more beneficial effects for reducing ROP.30
Given that over 90% of ELGANs experience frequent, brief, recurrent episodes of IH during oxygen supplementation, it is important to study its effects on the efficacy of topical ocular propranolol. Due to lack of robust preclinical investigations, we conducted a series of experiments to test the hypothesis that topical ocular propranolol at the currently proposed dose for ROP, is safe and effective for reducing the severity of IH-induced OIR. Our hypothesis was tested with the following objectives: (1) To compare the efficacy of topical ocular versus oral propranolol for the prevention of severe IH-induced OIR; (2) To examine the effects of oral and topical ocular propranolol on IH-induced angiogenesis biomarkers in the systemic and ocular compartments; and (3) To establish whether propranolol worsens or reduces IH-induced OIR in the recovery/reoxygenation phase following neonatal IH exposure. The primary outcome was retinal vascular and astrocyte integrity, and the secondary outcomes were retinal histopathology and morphometry, and angiogenesis biomarkers.
Methods
Animals
All experiments were approved by the State University of New York, Downstate Medical Center Institutional Animal Care and Use Committee, Brooklyn, NY (Protocol #18-10456). Animals were treated humanely, in accordance with the guidelines outlined by the United States Department of Agriculture and the Guide for the Care and Use of Laboratory Animals. Certified infection-free, timed-pregnant Sprague–Dawley rats were purchased from Charles River Laboratories (Wilmington, MA) at 18 days gestation. The animals were housed in an animal facility with a 12-h day/12-h night cycle and provided standard laboratory diet and water ad libitum until delivery of their pups. All procedures were performed in accordance with the Association for Research in Vision and Ophthalmology statement on the Use of Animals in Ophthalmic and Vision Research and the American Veterinary Medical Association Guidelines for the Euthanasia of Animals.
Experimental design
Within 2–4 h of birth, newborn rat pups delivering on the same day were pooled (8 litters per experimental day; 4 groups at one time) and randomly assigned to expanded litters of 18 pups/litter. The expanded litter size was used to simulate relative postnatal malnutrition of ELGANs who are at increased risk for severe ROP. The pups were assigned to either: (1) IH (50%–12% O2) from P0 to P14; (2) IH from P0 to P14 followed by RA recovery/reoxygenation from P14 to P21; (3) RA from P0 to P14; or (4) RA from P0 to P21. The RA groups served as controls. Animals were studied at P14 to determine immediate effects of IH, or P21, to determine whether the responses to IH resolve following reoxygenation/recovery in RA for 1 week. Animals were examined on a daily basis for adverse events. The cecal period (conception to eye opening) was recorded to determine the effects of IH and/or propranolol on retinal neural circuitry maturation.31–33 Percentage changes in body weight, organ to body weight ratios (brain, heart, lungs, liver, kidneys), and body length were assessed at P14 and P21. Mixed arterial and venous blood samples were collected for serum levels of angiogenesis biomarkers (VEGF, soluble VEGFR-1, soluble VEGFR-2, and IGF-I). Eyes were collected for vascular and astrocyte integrity, retinal layer integrity, retinal morphometry, corneal thickness, and angiogenesis biomarkers.
Groups
Pups were randomized to the following groups (n = 18 pups/group): (1) saline oral P14 RA; (2) saline oral P21 RA; (3) saline oral P14 IH; (4) Saline oral P21 IH; (5) propranolol oral P14 RA; (6) propranolol oral P21 RA; (7) propranolol oral P14 IH; (8) propranolol oral P21 IH; (9) saline topical ocular P14 RA; (10) saline topical ocular P21 RA; (11) saline topical ocular P14 IH; (12) Saline topical ocular P21 IH; (13) propranolol topical ocular P14 RA; (14) propranolol topical ocular P21 RA; (15) propranolol topical ocular P14 IH; and (16) propranolol topical ocular P21 IH.
IH profile
Animals randomized to IH were placed with the dams in specialized oxygen chambers attached to an oxycycler (BioSpherix, New York). The animal chambers housed 2 rat cages, were optimized for gas efficiency, and provided adequate ventilation for the animals in a controlled atmosphere with minimal gas usage. Oxygen content inside the chamber was continuously monitored and recorded on a Dell Computer. Carbon dioxide in the chamber was monitored and removed from the atmosphere by placing soda lime within the chamber. The IH paradigms consisted of an initial exposure to hyperoxia (50% O2) for 30 min followed by 3 brief, 1-min, clustered hypoxic events (12% O2), with a 10-min recovery in 50% O2 between each IH events. This model represents infants who recover from IH in hyperoxia between each IH event. Reoxygenation in 50% O2 followed each clustered IH event for 2.5 h for a total of 8 clustered IH episodes per day for 14 days, as previously described.15–17,34 Animals randomized to RA were placed in an oxycycler with the oxygen environment kept at 21% O2. Dams were switched every 2 days between RA and IH.
Administration of propranolol or placebo saline
For administration of topical ocular propranolol or placebo saline, animals were placed in the oxycycler from P0 to P5. Treatment began at P5 because the eyelids of neonatal rats are fused and the skin above the eyes becomes loose only at P5 to allow for the formation of a “tent” for injection under the skin.10 Before injection, the eyelids were first cleansed with betadine solution. A tent was created by gently pinching the skin above the eyes and propranolol or placebo saline was injected by inserting the ultra-fine needle (31 gauge attached to a 0.3-mL syringe; Becton Dickinson, NJ) into the tent in an upward angle so as to prevent damage to the eyes. Ten microliters of propranolol (0.2%) was administered above the eye once a day from P5 to P14. The dose of topical ocular propranolol is consistent with that used in preterm infants with ROP.30 The eyelids were gently massaged to disperse the solution and cleansed with betadine after injection. For administration of oral propranolol (1 mg/kg/day) or placebo saline, the rats were gently restrained using the left hand with the mouth facing upward. Using a feeding needle (24G-1n;1′′ Straight 1.25 mm ball), 50 μL propranolol or placebo saline was placed into the mouth once a day from P5 to P14. The dose of oral propranolol was consistent with that previously used in preterm infants with ROP.28,29
Sample collection and processing
For serum biomarkers of angiogenesis, whole blood was collected in Eppendorf tubes with no preservatives and placed on ice for 30 min. The samples were centrifuged at 3,500 rpm for 20 min at 4°C. The resulting serum was transferred to a clean Eppendorf tube and frozen at −20°C until assay for VEGF, sVEGFR-1, sVEGFR-2, and IGF-I. For assessment of ocular angiogenesis biomarkers (retina, choroid, and vitreous fluid), eyes were enucleated and rinsed in ice-cold phosphate-buffered saline (PBS). The vitreous fluid was collected by first piercing the eyes and placing them in Eppendorf collection tubes. The eyes were centrifuged at 3,000 rpm for 20 min. The vitreous fluid was collected in the outer collection tube. The retinas and choroids were then excised under a dissecting microscope (Olympus) and placed in sterile Lysing Matrix D tubes (2.0 mL) containing 1.4 mm ceramic spheres (MP Biomedicals, Santa Ana, CA) and 1.0 mL PBS before snap-freezing in liquid nitrogen. Samples were stored at −80°C until analysis. For retinal and astrocyte integrity, eyes were enucleated and rinsed in ice-cold PBS (pH 7.4) on ice. Enucleation was performed with the use of iris forceps and scissors for separation of the eyes from the surrounding connective tissue, nerves, and muscle. Whole eyes were placed in 4% paraformaldehyde (PFA) on ice for 120 min then flatmounted for adenosine diphosphatase (ADPase), glial fibrillary acid protein (GFAP), and isolectin B4 staining. For retinal layer integrity and retinal morphometry, whole eyes were fixed in situ in 10% neutral-buffered formalin (NBF). After 2 days, the eyes were enucleated, marked for orientation, placed in 4% NBF, and sent to the SUNY Downstate Pathology Department (Brooklyn, NY) for tissue processing and Hematoxylin and Eosin (H&E) staining using standard laboratory techniques. Cross-sections (5 μM) were cut through the optic disk and stained with H&E using standard techniques. All samples were analyzed on the same day. On the day of analyses, the tubes were allowed to defrost on ice and placed in a high-speed FastPrep-24 instrument (MP Biomedicals). The homogenates were then centrifuged at 4°C at 10,000 rpm for 20 min. The supernatant was filtered, and the filtrate was used for the assays. A portion of the filtrate (10 μL) was used to determine total cellular protein levels.
Retinal vascular and astrocyte integrity
Eyes were enucleated and placed in 4% PFA on ice for 120 min. The corneas, lens, vitreous, and sclera were removed, and the retinas were cut in quadrants, and flattened and immersed in 4% PFA overnight at 4°. Following several washes in tris maleate buffer (pH 7.2) and incubation in ADPase, retinas were stained with ammonium sulfide, washed, and mounted on slides with PBS/glycerin. For astrocyte integrity, retinal flatmounts were stained with GS-lectin and GFAP on the same day of harvesting. Retinal flatmounts were fixed in methanol for 20 min, followed by permeabilization and blocking in PermBlock (PBS +0.3% Triton X-100 + 0.2% bovine serum albumin) in 5% goat serum for 1 h. After washing in PBS/Triton X-100 (TXPBS), flatmounts were then incubated with rabbit GFAP primary antibody (Cell Signaling Technologies, Danvers, MA) overnight at 4°C. Following several washes with TXPBS, the flatmounts were incubated with Alexa Fluor 488 goat anti-rabbit fluorescent secondary antibodies, and Alexa Fluor 594 Isolectin B4 (Thermo Fisher Sci/Life Technologies, Grand Island, NY) overnight at 4°C. The flatmounts were washed with TXPBS and mounted on slides with ProLong Antifade fluorescent mounting media. Images were captured at 20 × magnification using an Olympus BX53 microscope, DP72 digital camera, and CellSens imaging software attached to a Dell Precision T3500 computer (Olympus America, Inc., Center Valley, PA).
Retinal morphometry
ADPase-stained retinal flatmounts were used to determine the tortuosity index and size of the arteries and veins. For tortuosity index, a line was traced along the tortuous arteries using the freehand line tool of the CellSens software (Olympus America, Inc.) and compared with a straight line traced from the vessel origin at the optic disk to the branch point as previously described.16 Vessel diameter at the optic disk was quantified using the straight line tool. Neovascularization at the periphery was quantified in the ADPase-stained images (10 × magnification) using the count and measure on ROI tool of the CellSens software. The number of endothelial cells (ECs) present in the nerve fiber layer (NFL)/ganglion cell layer (GCL), the total retinal thickness, and the thickness of the NFL/GCL, inner plexiform layer (IPL), inner nuclear layer (INL), outer nuclear layer (ONL), corneal thickness, and number of corneal epithelial cells were quantified in the H&E-stained sections using the count and measure tool of CellSens Dimension software (Olympus America, Inc.).
Assay of angiogenesis biomarkers
VEGF sVEGFR-1, sVEGFR-2, and IGF-I levels in the serum, vitreous fluid, and retinal and choroid homogenates were assayed using the commercially available Quantikine Enzyme-Linked Immunosorbent Assay Kits from R&D Systems (Minneapolis, MN). All assays were conducted according to the manufacturer's protocol. All tissue data were standardized using total cellular protein levels.
Total cellular protein levels
On the day of assays, an aliquot (10 μL) of the retinal and choroid homogenates was utilized for total cellular protein levels using the Bradford method (Bio-Rad, Hercules, CA) with bovine serum albumin as a standard.
Statistical analyses
To achieve our objectives, data were analyzed in 2 ways: (1) 1-way ANOVA to determine differences within each mode of administration (eye drops or oral); and (2) unpaired t-test (saline vs. propranolol; topical ocular vs. oral; and RA vs. IH). For 1-way ANOVA, we used the Bartlett's test for normality of variances. Normally distributed data were analyzed using 1-way ANOVA with Bonferroni's multiple comparison post hoc tests. For non-normally distributed data, we used the Kruskal–Wallis nonparametric test, with Dunn's post hoc test.
For comparison between 2 groups, the Levene's test for normality of variances was first conducted. Normally distributed data were analyzed using unpaired t-test. Non-normally distributed data were analyzed using Mann–Whitney U nonparametric test. Data are presented as mean ± standard error of the mean and a P value of <0.05 was considered as statistically significant, using IBM SPSS version 26.0 (IBM, Inc., Chicago, IL). Graphs were prepared using GraphPad Prizm version 7.03 (GraphPad, San Diego, CA).
Results
Somatic growth
Low postnatal weight accretion is a strong predictor of severe ROP.35,36 The effect of propranolol on percentage change in body weight, as well as the effects of IH and comparisons between topical ocular and oral administration at P14 and P21 are presented in Tables 1 and 2, respectively. At P14 (the time when both treatment and IH exposure ended), topical ocular propranolol decreased weight gain and body length substantially in RA and IH, whereas oral administration resulted in lower weight gain in RA, but not in IH. Body length was also lower with topical ocular propranolol in RA and IH, but not with oral treatment. Organ weight is considered one of the most sensitive indicators of toxicity and potential harmful effects of an experimental compound.37 Evaluation of organ-to-body weight ratios revealed that at P14, only the lung/body weight ratios were affected and this was consistent with severe lung hemorrhage. In RA, oral propranolol increased lung/body weight ratios in RA and IH (Table 1). At P21 (the time of recovery/reoxygenation in RA), treatment with propranolol in RA and IH resulted in higher weight gain. All organ weights were affected by propranolol and IH at P21. In RA, only liver/body weight ratios declined with oral propranolol. In IH, organ/body weight ratios were increased with saline and propranolol regardless of route of administration (Table 2).
Table 1.
Somatic Growth at P14
| RA |
IH |
|||||||
|---|---|---|---|---|---|---|---|---|
| Topical ocular |
Oral |
Topical ocular |
Oral |
|||||
| Saline | Prop | Saline | Prop | Saline | Prop | Saline | Prop | |
| % Change body wt. (P0–P14) | 252.6 ± 14.9 | 208.8 ± 14.9** | 286.2 ± 7.8# | 258.9 ± 14.8# | 252.0 ± 18.8 | 159.3 ± 18.2**# | 214.5 ± 18.2§§ | 244.9 ± 10.8##§§ |
| % Change length (P0–P14) | 57.1 ± 2.1 | 35.7 ± 8.5** | 54.6 ± 2.7 | 53.5 ± 2.4## | 55.5 ± 6.6 | 36.3 ± 3.7** | 55.4 ± 2.6 | 61.1 ± 2.4##§ |
| Brain/body wt. ratio | 0.046 ± 0.002 | 0.049 ± 0.0016 | 0.047 ± 0.001 | 0.044 ± 0.006 | 0.044 ± 0.001 | 0.05 ± 0.004 | 0.049 ± 0.003 | 0.044 ± 0.001 |
| Heart/body wt. ratio | 0.006 ± 0.0003 | 0.006 ± 0.0005 | 0.007 ± 0.0003 | 0.007 ± 0.007 | 0.007 ± 0.0003 | 0.007 ± 0.0007 | 0.007 ± 0.0005 | 0.006 ± 0.0003 |
| Lung/body wt. ratio | 0.018 ± 0.0009 | 0.018 ± 0.0009 | 0.018 ± 0.0001 | 0.02 ± 0.001** | 0.018 ± 0.0007 | 0.024 ± 0.001** | 0.018 ± 0.001 | 0.019 ± 0.001##§§ |
| Liver/body wt. ratio | 0.028 ± 0.001 | 0.027 ± 0.002 | 0.027 ± 0.0009 | 0.028 ± 0.003 | 0.028 ± 0.002 | 0.028 ± 0.002 | 0.03 ± 0.002 | 0.03 ± 0.001 |
| Kidney/body wt. ratio | 0.013 ± 0.0005 | 0.013 ± 0.0007 | 0.014 ± 0.0007 | 0.014 ± 0.001 | 0.014 ± 0.0005 | 0.014 ± 0.001 | 0.015 ± 0.0004 | 0.014 ± 0.0005 |
P < 0.05, **P < 0.01 saline versus propranolol; #P < 0.05, ##P < 0.01 topical ocular versus oral; §P < 0.05, §§P < 0.01 RA versus IH (n = 18 pups/group).
IH, intermittent hypoxia; Prop, propranolol; RA, room air; Wt., weight.
Table 2.
Somatic Growth at P21
| RA |
IH |
|||||||
|---|---|---|---|---|---|---|---|---|
| Topical ocular |
Oral |
Topical ocular |
Oral |
|||||
| Sal | Prop | Saline | Prop | Saline | Prop | Saline | Prop | |
| % Change body wt. (P0–P21) | 350.1 ± 14.4 | 429.1 ± 14.5** | 475.1 ± 32.4## | 482.3 ± 7.2## | 514.0 ± 11.6§§ | 532.0 ± 22.1§§ | 388.0 ± 21.3##§§ | 458.6 ± 11.3**## |
| % Change length (P0–P21) | 84.6 ± 1.4 | 95.2 ± 4.1* | 97.4 ± 3.6## | 99.7 ± 1.2 | 84.1 ± 2.2 | 84.9 ± 2.2§ | 73.1 ± 1.8##§§ | 79.1 ± 1.2#§§ |
| Brain/body wt. ratio | 0.041 ± 0.001 | 0.034 ± 0.001** | 0.034 ± 0.002## | 0.03 ± 0.0006 | 0.052 ± 0.001§§ | 0.056 ± 0.001§§ | 0.039 ± 0.001## | 0.034 ± 0.0009**## |
| Heart/Body wt. ratio | 0.006 ± 0.004 | 0.006 ± 0.0003 | 0.005 ± 0.0002 | 0.006 ± 0.0006 | 0.01 ± 0.0003§§ | 0.01 ± 0.004§§ | 0.007 ± 0.0003## | 0.005 ± 0.0002**## |
| Lung/body wt. ratio | 0.01 ± 0.0004 | 0.01 ± 0.0005 | 0.01 ± 0.001 | 0.01 ± 0.0005 | 0.019 ± 0.0002§§ | 0.019 ± 0.005§§ | 0.013 ± 0.0003## | 0.012 ± 0.0004## |
| Liver/body wt. ratio | 0.034 ± 0.002 | 0.037 ± 0.002 | 0.039 ± 0.002 | 0.03 ± 0.002*# | 0.067 ± 0.003 | 0.061 ± 0.003§§ | 0.035 ± 0.002##§§ | 0.034 ± 0.001## |
| Kidney/body wt. ratio | 0.013 ± 0.0006 | 0.012 ± 0.0006 | 0.012 ± 0.0006 | 0.013 ± 0.0002 | 0.021 ± 0.0006§§ | 0.019 ± 0.0005*§§ | 0.011 ± 0.0006## | 0.012 ± 0.0004## |
P < 0.05, **P < 0.01 saline versus propranolol; #P < 0.05, ##P < 0.01 topical ocular versus oral §P < 0.05, §§P < 0.01 RA vs. IH (n = 18 pups/group).
Eye opening at P14
Rats are born with fused eyes and immature retinas. Retinas continue to mature postnatally, consistent with adult retinas by postnatal day 14, correlating with maturation of the retinal neural circuitry, but it continues to mature in the following 2–3 weeks.33 Table 3 shows the percentage of eyes opened at P14 for each group. In the pups receiving oral saline, there was a ∼40% reduction in the percentage of eyes opened in pups who were exposed to IH conditions compared with RA. Results only achieved statistical significance for opening of the left eye, although a 40%–50% reduction also occurred for the right eye and both eyes. Topical propranolol resulted in delayed eye opening in comparison to topical saline, in RA and IH conditions. However, this was not evident in the oral propranolol groups. Oral propranolol-treated pups had improved eye opening percentages in IH conditions compared with oral saline controls in IH, although no statistical significance was achieved.
Table 3.
Eye Opening at P14
| Ambient air (21% O2) |
50%–12% O2 (IH) |
|||||
|---|---|---|---|---|---|---|
| Left eye | Right eye | Both eyes | Left eye | Right eye | Both eyes | |
| Saline eye drops | 14 (77%) | 12 (67%) | 12 (67%) | 11 (61%) | 12 (67%) | 11 (61%) |
| Propranolol eye drops | 10 (56%) | 11 (61%) | 10 (56%) | 12 (67%) | 10 (56%) | 10 (56%) |
| Saline oral | 14 (77%) | 12 (67%) | 12 (67%) | 6 (33%)* | 7 (39%) | 6 (33%) |
| Propranolol oral | 9 (50%) | 12 (67%) | 12 (67%) | 10 (56%) | 9 (50%) | 9 (50%) |
P < 0.05 versus saline oral in ambient air (n = 18 pups/group).
Serum growth factors
The effect of IH and/or propranolol on systemic growth factors are presented in Table 4. At P14, in RA, VEGF was lower with oral treatment than topical ocular treatment with saline or propranolol, although significant differences were achieved between the saline groups. An opposite effect was seen in IH. At P21, systemic VEGF levels were generally lower than at P14, and levels in both oral groups were higher than topical ocular groups with significant differences between the propranolol groups in RA. In IH, VEGF levels in the topical ocular propranolol group were higher than saline and not detected in both oral groups. We studied 2 types of endogenous soluble VEGF receptors: sVEGFR-1 and sVEGFR-2. Both isoforms are splice variants of their membrane type. sVEGFR-1 is believed to trap VEGF and decrease its angiogenic effect. Membrane type VEGFR-2 is the main signaling pathway through which VEGF contributes to the angiogenic process.38 At P14, all IH-exposed groups had lower levels of sVEGFR-1 compared with RA. In RA, topical ocular propranolol decreased sVEGFR-1 compared with topical ocular saline, while oral propranolol increased sVEGFR-1 compared with saline oral. Similar findings were noted in IH. The RA effects remained sustained at P21. However, in IH, oral propranolol substantially decreased sVEGFR-1 levels compared with oral saline. sVEGFR-2 levels, on the other hand, were significantly lower than sVEGFR-1 levels. No differences in sVEGFR-2 levels were noted between the saline and propranolol groups RA or IH at P14, although lower levels were noted between the RA and IH oral groups. At P21, topical ocular propranolol lowered sVEGFR-2 levels, but oral propranolol increased it in RA. In IH, levels were higher with topical ocular propranolol than saline. IGF-I is highly associated with postnatal growth and development of ROP. Studies have shown that infants who developed severe ROP are deficient in IGF-I.39 IGF-I is also a permissive factor for VEGF and is required for maximum VEGF activation.40 In our study, IGF-I levels did not appreciably change in response to IH or propranolol treatment at P14 or P21.
Table 4.
Serum Growth Factors
| RA |
IH |
|||||||
|---|---|---|---|---|---|---|---|---|
| Topical ocular |
Oral |
Topical ocular |
Oral |
|||||
| Sal | Prop | Saline | Prop | Saline | Prop | Saline | Prop | |
| P14: | ||||||||
| VEGF | 44.3 ± 6.8 | 36.8 ± 4.7 | 17.2 ± 3.0## | 25.2 ± 9.4 | 10.6 ± 3.3§§ | 17.2 ± 1.6§§ | 33.5 ± 4.2##§§ | 27.8 ± 8.0 |
| sVEGFR-1 | 394.5 ± 48.9 | 279.3 ± 16.3* | 182.8 ± 23.1## | 282.0 ± 50.0 | 160.2 ± 15.2§§ | 132.8 ± 11.9§§ | 121.5 ± 26.4 | 154.5 ± 21.9§ |
| sVEGFR-2 | 7.9 ± 0.08 | 7.3 ± 0.001 | 7.7 ± 0.11 | 7.65 ± 0.07 | 6.7 ± 0.02§§ | 7.0 ± 0.11 | 5.9 ± 0.05##§§ | 6.5 ± 0.03##§§ |
| IGF-I | 3506 ± 264 | 3051 ± 396 | 2565 ± 379 | 2792 ± 455 | 2994 ± 386 | 2824 ± 277 | 2012 ± 473 | 2714 ± 273 |
| P21 | ||||||||
| VEGF | 0.13 ± 0.09 | 5.2 ± 0.32**## | 11.3 ± 0.9 | 14.1 ± 0.21**## | 0.63 ± 0.05§§ | 10.1 ± 0.38**§§ | 0 ± 0##§§ | 0 ± 0##§§ |
| sVEGFR-1 | 219.8 ± 38.7 | 40.1 ± 26.4** | 128.1 ± 31.7 | 135.8 ± 30.7# | 70.3 ± 23.6§§ | 78.7 ± 27.8 | 101.7 ± 22.5 | 26.2 ± 22.8*§§ |
| sVEGFR-2 | 7.3 ± 0.04 | 6.3 ± 0.02** | 6.6 ± 0.09## | 6.9 ± 0.03** | 6.6 ± 0.03§§ | 7.1 ± 0.03**§§ | 7.5 ± 0.02##§§ | 7.1 ± 0.06 |
| IGF-I | 3095 ± 329 | 3706 ± 138 | 3722 ± 138 | 3741 ± 120 | 3755 ± 156 | 3804 ± 113 | 3501 ± 210 | 3652 ± 139 |
P < 0.05, **P < 0.01 saline versus propranolol; #P < 0.05, ##P < 0.01 topical ocular versus oral; §P < 0.05, §§P < 0.01 RA versus IH (n = 8 samples/group).
IGF, insulin-like growth factor; sVEGFR, soluble vascular endothelial growth factor receptor; VEGF, vascular endothelial growth factor.
Retinal vasculature
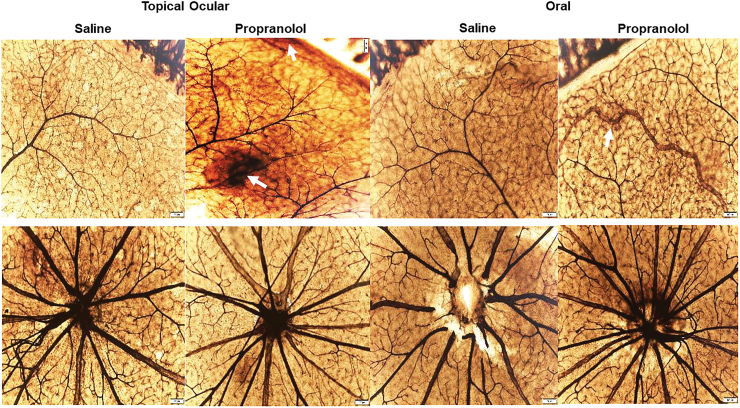
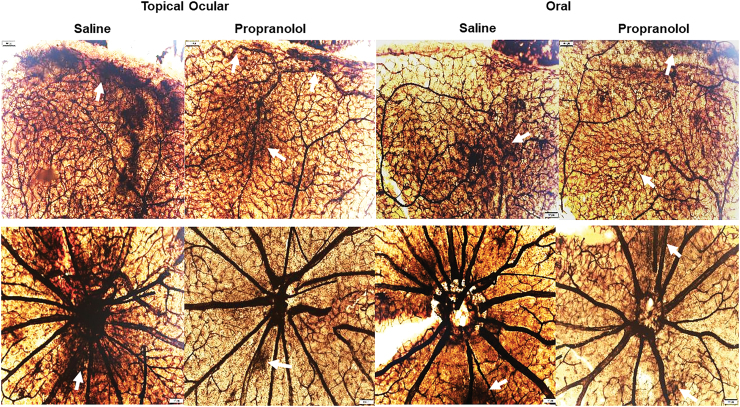
Figures 1 and 2 show the ADPase-stained retinal flatmounts at P21 in RA and IH, respectively. The upper panels for each figure represent the peripheral vessels and the lower panels represent the vessels at the optic disk. In Fig. 1 (RA), the flatmounts from topical ocular saline-treated pups showed well-preserved retinal vessel architecture at the periphery and optic disk. However, the pups treated with topical ocular propranolol showed evidence of hemorrhage at the periphery (arrows), but the optic disk was unaffected. Similarly, the groups exposed to oral saline showed no retinal vascular abnormalities at the periphery or optic disk. However, in the oral propranolol group, there was vascular enlargement and tortuosity at the periphery (arrow). IH exposure (Fig. 2) resulted in significant vascular pathology evidenced by increased hemorrhage, vascular tufts, vessel thickening, vascular tortuosity, and vessel overgrowth and disorganization at the periphery and optic disk (characteristics consistent with severe ROP). Propranolol treatment did not appear to appreciably reduce retinal vascular abnormalities. For a more objective assessment, we measured arterial and venous diameter, tortuosity index, and neovascularization. The images were coded and image analysis was conducted in a masked manner. The results are presented in Tables 5 and 6. There were no statistically significant differences among the groups for vessel diameter and tortuosity index. In contrast, at P14, vessel density was higher in all propranolol groups exposed to RA, but lower with treatment in neonatal IH compared with placebo saline. Neonatal IH groups treated with topical ocular and oral saline also had greater vessel abundance than their RA counterparts. At P21, vessel abundance in the saline groups exposed to neonatal IH remained elevated compared with RA, as did topical ocular propranolol groups exposed to neonatal IH compared with their RA littermates. In neonatal IH, both topical ocular and oral propranolol reduced vessel abundance compared with placebo saline.
FIG. 1.
Representative ADPase-stained retinas from rats raised in RA at postnatal day 21 (P21) and treated with topical ocular or oral saline or propranolol. The upper panels are peripheral vessels, and the lower panels are vessels at the optic disk. Pups were administered a single daily treatment of topical ocular or oral propranolol or saline occurred from P5 to P14. Topical ocular propranolol caused hemorhrage (arrow) and oral propranolol in caused tortuous vessels (arrow). Images are 10 × magnification, scale bar is 100 μm. ADPase, adenosine diphosphatase; RA, room air. Color images are available online.
FIG. 2.
Representative ADPase-stained retinas from rats exposed to neonatal IH at postnatal day 21 (P21) and treated with topical ocular or oral saline or propranolol. The upper panels are peripheral vessels, and the lower panels are vessels at the optic disk. Pups were administered a single daily treatment of topical ocular or oral propranolol or saline occurred from P5 to P14. Saline treatment in neonatal IH resulted in hemorrhage, vessel tortuosity, vascular abundance, and neovascularization (arrows) which were not prevented with propranolol (arrows). Images are 10 × magnification, scale bar is 100 μm. IH, intermittent hypoxia. Color images are available online.
Table 5.
Retinal Morphometry: P14
| RA |
IH |
|||||||
|---|---|---|---|---|---|---|---|---|
| Topical ocular |
Oral |
Topical ocular |
Oral |
|||||
| Saline | Prop | Saline | Prop | Saline | Prop | Saline | Prop | |
| Corneal thickness, μm | 142.0 ± 24.3 | 106.8 ± 8.0 | 109.8 ± 12.8 | 122.3 ± 22.1 | 146.8 ± 13.9 | 161.8 ± 31.9 | 122.3 ± 25.4 | 116.3 ± 12.1 |
| n = 12 | n = 18** | n = 12## | n = 21## | n = 16 | n = 10§§ | n = 12## | n = 12## | |
| No. corneal epithelial cells | 405.8 ± 9.7 | 162.6 ± 7.6 | 304.6 ± 18.5 | 180.9 ± 12.2 | 255.3 ± 24.3 | 171.2 ± 7.8 | 297.0 ± 16.9 | 187.9 ± 8.7 |
| n = 12 | n = 18** | n = 12## | n = 21**## | n = 16§§ | n = 10** | n = 12 | n = 12** | |
| Tortuosity index | 0.97 ± 0.02 | 0.96 ± 0.03 | 0.95 ± 0.03 | 0.94 ± 0.03 | 0.95 ± 0.02 | 0.94 ± 0.03 | 0.95 ± 0.03 | 0.95 ± 0.03 |
| n = 11 | n = 19 | n = 16 | n = 21 | n = 19 | n = 18 | n = 5 | n = 18 | |
| Artery diameter, μm | 25.5 ± 4 | 24.4 ± 3.8 | 27.9 ± 3.8 | 24 ± 3.6 | 29 ± 6.2 | 22.2 ± 4.5 | 30.5 ± 8.2 | 25.5 ± 6.3 |
| n = 20 | n = 23 | n = 24 | n = 30 | n = 28 | n = 23 | n = 13 | n = 24 | |
| Vein diameter, μm | 34.6 ± 8.1 | 36.3 ± 9.7 | 35.9 ± 8.3 | 38.2 ± 9.7 | 36.8 ± 8.3 | 34.8 ± 7 | 29.2 ± 4.6 | 34 ± 5.6 |
| n = 21 | n = 21 | n = 24 | n = 27 | n = 20 | n = 20 | n = 9 | n = 18 | |
| Neovascularization | 1063.7 ± 263.5 | 2126.1 ± 619.1** | 1354.0 ± 273.6 | 2013.6 ± 827.7* | 3112.3 ± 890.2§§ | 1661.3 ± 345.8** | 2176.6 ± 803.4##§§ | 1661.9 ± 237.3* |
| Number cells in NFL/GCL | 210 ± 73 | 194 ± 38 | 348 ± 80 | 412 ± 169 | 148.7 ± 54.5 | 307.2 ± 26.1 | 298.2 ± 93 | 241.3 ± 61.6 |
| n = 4 | n = 6 | n = 4 | n = 6 | n = 3 | n = 4* | n = 4 | n = 6 | |
| Total retinal thickness, μm | 292.5 ± 14 | 274.3 ± 51.7 | 305.9 ± 33.2 | 240 ± 31 | 229.8 ± 14.3 | 468.9 ± 11.3 | 561 ± 50 | 314 ± 46.9 |
| n = 4 | n = 10 | n = 6 | n = 8 | n = 4 | n = 4**§ | n = 4##§§ | n = 6** | |
| NFL/GCL thickness, μm | 41.2 ± 7 | 34.5 ± 12.2 | 44.5 ± 7.5 | 32.4 ± 4.7 | 37.8 ± 7.6 | 68.1 ± 7.9 | 82.3 ± 21.2 | 50.4 ± 7.2 |
| n = 4 | n = 10 | n = 6 | n = 8 | n = 4 | n = 4* | n = 4 | n = 6§ | |
| IPL thickness, μm | 38.8 ± 6 | 41.6 ± 10.9 | 50 ± 8.7 | 40.1 ± 2.6 | 41.2 ± 5.4 | 93.6 ± 4.3 | 73.9 ± 5.6 | 58.7 ± 10 |
| n = 4 | n = 10 | n = 6 | n = 8 | n = 4 | n = 4**§§ | n = 4 | n = 6# | |
| INL thickness, μm | 67.4 ± 7.8 | 65.8 ± 15.9 | 60.4 ± 13.9 | 50.1 ± 3.4 | 47.4 ± 9 | 110.1 ± 2.2 | 128.8 ± 9.4 | 77.6 ± 12.7 |
| n = 4 | n = 10 | n = 6 | n = 8 | n = 4 | n = 4** | n = 4##§§ | n = 6**§ | |
| ONL thickness, μm | 101.5 ± 11.4 | 94.1 ± 12.8 | 95.3 ± 4.5 | 77.5 ± 14.8 | 75.4 ± 7.6 | 109.8 ± 12.6 | 163.5 ± 14.1 | 98 ± 9.8 |
| n = 4 | n = 10 | n = 6 | n = 8 | n = 4 | n = 4* | n = 4##§§ | n = 6** | |
Data are mean ± SD. One-way ANOVA with Bonferroni's post hoc test was used to compare data among the topical ocular or oral groups. Unpaired t-test was used to compare RA versus IH.
P < 0.05, **P < 0.01 saline versus propranolol; #P < 0.05, ##P < 0.01 topical ocular versus oral; §P < 0.05, §§P < 0.01 RA versus IH.
INL, inner nuclear layer; IPL, inner plexiform layer; NFL/GCL, nerve fiber layer/ganglion cell layer; ONL, outer nuclear layer; SD, standard deviation.
Table 6.
Retinal Morphometry – P21
| RA |
IH |
|||||||
|---|---|---|---|---|---|---|---|---|
| Topical ocular |
Oral |
Topical ocular |
Oral |
|||||
| Saline | Prop | Saline | Prop | Saline | Prop | Saline | Prop | |
| Corneal thickness, μm | 166.4 ± 9.9 | 190.5 ± 8.1 | 154.3 ± 4.3 | 137.5 ± 4.0 | 144.7 ± 13.5 | 151.1 ± 28.5 | 138.0 ± 13.5 | 130.6 ± 5.9 |
| n = 10 | n = 10** | n = 10## | n = 10**## | n = 16§§ | n = 10§§ | n = 16§§ | n = 10#§§ | |
| No. corneal epithelial cells | 244.5 ± 39.1 | 281.6 ± 35.8 | 256.0 ± 11.3 | 271.4 ± 11.9 | 387.1 ± 29.5 | 301.9 ± 9.3 | 445.6 ± 13.2 | 245.6 ± 13.4 |
| n = 10 | n = 10* | n = 10 | n = 10** | n = 16§§ | n = 10** | n = 12##§§ | n = 10##§ | |
| Tortuosity index | 0.96 ± 0.02 | 0.97 ± 0.028.1 | 0.91 ± 0.06 | 0.97 ± 0.03 | 0.94 ± 0.04 | 0.97 ± 0.009 | 0.92 ± 0.03 | 0.95 ± 0.03 |
| n = 28 | n = 21 | n = 8 | n = 19 | n = 17 | n = 10 | n = 22 | n = 22 | |
| Artery diameter, μm | 27.5 ± 6 | 25.7 ± 7 | 23.2 ± 5.4 | 26.6 ± 6.6 | 30.6 ± 4.9 | 29.5 ± 8.1 | 29.4 ± 4.5 | 30.9 ± 6.4 |
| n = 46 | n = 27 | n = 15 | n = 24 | n = 34 | n = 25 | n = 38 | n = 36 | |
| Vein diameter, μm | 37.1 ± 7.4 | 40.2 ± 7.8 | 38.8 ± 7.9 | 36.5 ± 7.5 | 36.7 ± 8.6 | 37 ± 5.6 | 41.1 ± 8.7 | 38 ± 5.8 |
| n = 39 | n = 22 | n = 12 | n = 22 | n = 21 | n = 15 | n = 32 | n = 23 | |
| Neovascularization | 1820.7 ± 823.5 | 1376.8 ± 258.7 | 1249.9 ± 504.5 | 1422.9 ± 348.5 | 2560.1 ± 364.2§ | 1979.6 ± 463.5**§§ | 2208.8 ± 419.8§§ | 1790.0 ± 362.6* |
| Number of cells in NFL/GCL | 145.3 ± 45.7 | 179.6 ± 59.8 | 168 ± 36.9 | 208.5 ± 39 | 173 ± 109 | 172.3 ± 56.2 | 134.7 ± 52.8 | 174.5 ± 44.9 |
| n = 3 | n = 3 | n = 5 | n = 6 | n = 9 | n = 9 | n = 9 | n = 9 | |
| Total retinal thickness, μm | 306.7 ± 43.1 | 350.5 ± 27.4 | 341.7 ± 63.4 | 335.3 ± 63.2 | 296.8 ± 48.8 | 322.9 ± 96.7 | 297.9 ± 55 | 292. ± 42.3 |
| n = 6 | n = 4 | n = 8 | n = 12 | n = 6 | n = 6 | n = 8 | n = 8 | |
| NFL/GCL thickness, μm | 39.1 ± 1.5 | 52.4 ± 18.8 | 37.8 ± 7.2 | 38.4 ± 9.2 | 33 ± 10.9 | 33 ± 6.2 | 38.2 ± 4.2 | 32.8 ± 6.3 |
| n = 6 | n = 4 | n = 8 | n = 12 | n = 6 | n = 6 | n = 8 | n = 8 | |
| IPL thickness, μm | 47.3 ± 8.7 | 62 ± 6.7 | 68.8 ± 14.1 | 73.8 ± 18.2 | 56.6 ± 13.2 | 64.1 ± 19.4 | 69.7 ± 18 | 52 ± 7.7 |
| n = 6 | n = 4 | n = 8 | n = 12 | n = 6 | n = 6 | n = 8 | n = 8 | |
| INL thickness, μm | 56.5 ± 10.1 | 64.5 ± 1.5 | 66 ± 14.9 | 72.7 ± 16.3 | 53.7 ± 12.3 | 67.9 ± 25.5 | 48 ± 23 | 51.3 ± 8.7 |
| n = 6 | n = 4 | n = 8 | n = 12 | n = 6 | n = 6 | n = 8 | n = 8 | |
| ONL thickness, μm | 90.4 ± 19.2 | 93.5 ± 3.2 | 95.9 ± 17.5 | 100.2 ± 15.8 | 91.8 ± 13.6 | 96.5 ± 24.8 | 83.4 ± 30.9 | 82.7 ± 12.8 |
| n = 6 | n = 4 | n = 8 | n = 12 | n = 6 | n = 6 | n = 8 | n = 8 | |
Data are mean ± SD. One-way ANOVA with Bonferroni's post hoc test was used to compare data among the topical ocular or oral groups. Unpaired t-test was used to compare RA versus IH.
P < 0.05; **P < 0.01 saline versus propranolol; #P < 0.05; ##P < 0.01 topical ocular versus oral; §P < 0.05, §§P < 0.01 RA versus IH.
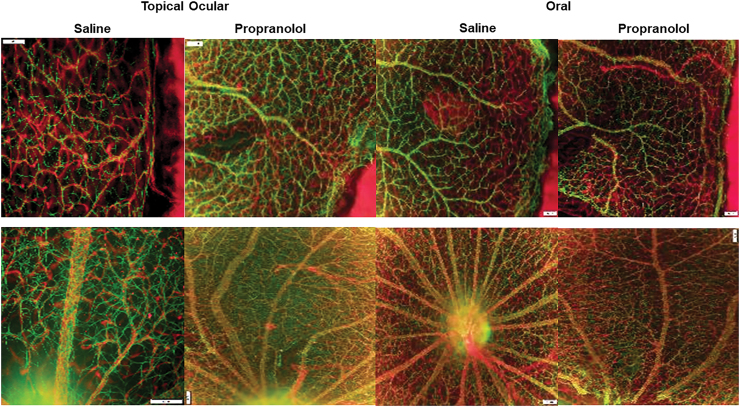
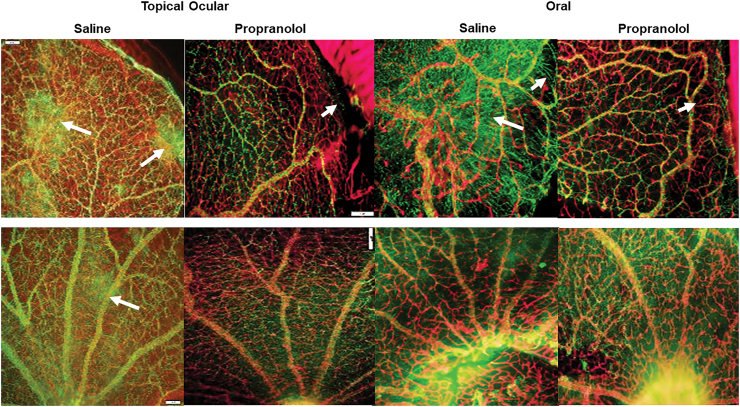
Astrocyte integrity
GFAP is glial fibrillary acidic protein, the chief constituent in astrocytes, and one of the markers of Müller cells and activated glial cells. Astrocytes are found in NFL/GCL, and they provide structure for the retinal vessels, damages to astrocytes can lead to disruption in retinal vasculature. Isolectin B4 is a biomarker of blood vessels and is found in ECs. The RA images are presented in Fig. 3 and the IH images in Fig. 4 for the P21 rats. The upper panels represent the peripheral vessels and the lower panels represent the vessels at the optic disk. In RA, flatmounts show normal astrocytic templates (green, GFAP) interlaced with normal retinal vasculature (red, Isolectin B4) at the periphery and optic disk. In IH conditions (Fig. 4), there were major disturbances in the astrocytic template in the groups treated with topical ocular and oral saline (arrows), with activated Müller cells and significant uptake of GFAP (reactive gliosis). Under normal conditions, Muller cells are not positive for GFAP, but they do so during injury. Pups exposed to IH conditions and treated with propranolol, on the other hand, had less evidence of astrocytic damage and gliosis (evident by lower uptake of GFAP), although there was some loss of astrocytes at the periphery (arrows).
FIG. 3.
Representative GFAP (green)/isolectin B (red)-stained retinas from rats raised in RA at postnatal day 21 (P21) and treated with topical ocular or oral saline or propranolol. The upper panels are peripheral vessels, and the lower panels are vessels at the optic disk. Pups were administered a single daily treatment of topical ocular or oral propranolol or saline occurred from P5 to P14. Images are 10 × magnification, scale bar is 100 μm. GFAP, glial fibrillary acid protein. Color images are available online.
FIG. 4.
Representative GFAP (green)/isolectin B (red)-stained retinas from rats exposed to neonatal IH at postnatal day 21 (P21) and treated with topical ocular or oral saline or propranolol. The upper panels are peripheral vessels, and the lower panels are vessels at the optic disk. Pups were administered a single daily treatment of topical ocular or oral propranolol or saline occurred from P5 to P14. Saline treatment in neonatal IH caused astrocyte damage and gliosis (arrows) which was prevented with propranolol treatment. Images are 10 × magnification, scale bar is 100 μm. Color images are available online.
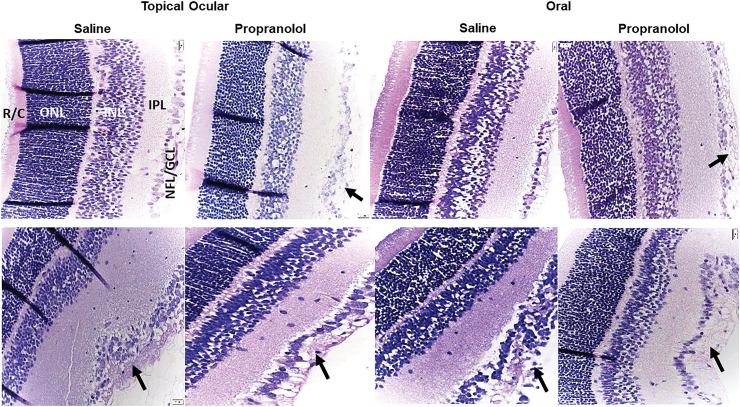
Retinal histopathology
Figure 5 shows representative images of the H&E-stained retinal layers at P21. The upper panels represent the RA groups, and the lower panels represent the IH groups. Images at P14 are not shown, however, at P14, under RA conditions, the pups exposed to topical propranolol had mild retinal changes, such as separation in NFL/GCL, and infiltration of vitreous. Under IH conditions, NFL/GCL changes were noticeable in saline groups. Topical propranolol showed some retinal folding and increased thickness. Retinal layers are identified in the retinas from pups raised in RA, which showed normal retinal layers. Retinas from pups receiving topical ocular propranolol in RA showed increased EC numbers and widening of the NFL/GCL (arrow). Similarly, oral saline treatment in RA showed normal retinal layers, but retinas from pups treated with oral propranolol showed widening of the NFL/GCL (arrow). At P21, increased retinal thickness was noticeable in all groups. Retinas from pups treated with saline and exposed to IH conditions showed increased EC numbers migrating into the vitreous fluid, which resulted in widening of the NFL/GCL (arrow). This response was also evident in the saline oral group exposed to IH (arrow). Retinas from pups treated with either topical ocular or oral propranolol in IH showed similar characteristics, suggesting no therapeutic benefits (arrows). Tables 5 and 6 shows retinal layer thickness at P14 and P21, respectively. At P14, topical ocular propranolol resulted in a significantly higher number of cells in the NFL/GCL and increased total retinal, IPL, INL, and ONL thickness compared with topical ocular saline in IH. An opposite effect was seen with oral propranolol.
FIG. 5.
Representative H&E-stained retinas from rats raised in RA (upper panels) or neonatal IH (lower panels) at postnatal day 21 (P21) and treated with topical ocular or oral saline or propranolol. Pups were administered a single daily treatment of topical ocular or oral propranolol or saline occurred from P5 to P14. Saline treatment in neonatal IH increased NFL/GCL layer and number of endothelial cells, as did propranolol treatment in RA and neonatal IH (arrows). Images are 40 × magnification, scale bar is 20 μm. H&E, Hematoxylin and Eosin; INL, inner nuclear layer; IPL, inner plexiform layer; NFL/GCL, nerve fiber layer/ganglion cell layer; ONL, outer nuclear layer; R/C, rods and cones. Color images are available online.
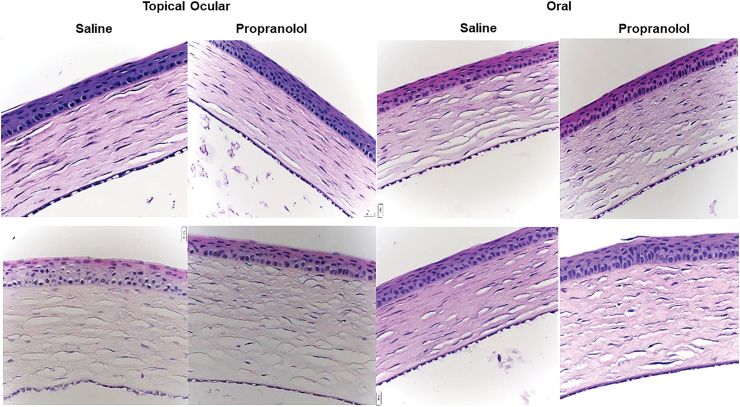
Corneal thickness
Since topical ocular administration occurred when the eyes were fused, we examined corneal integrity at the central cornea. Increased central corneal thickening may implicate intraocular pressure and edema. Imaging and image analysis were conducted in a masked manner. Figure 6 shows corneal stains at P21. Corneal thickening occurred in the topical ocular groups exposed to IH and in the oral propranolol groups. Corresponding measurements are presented in Tables 5 and 6. At P14 (Table 5) ocular administration in RA and IH caused significant thickening of the cornea compared with oral administration. In addition, we found lower numbers of corneal epithelial cells in the topical ocular saline group exposed to neonatal IH, compared with RA. Propranolol treatment in RA and IH decreased the number of corneal epithelial cells regardless of mode of administration, compared with the saline counterparts. At P21 (Table 6), topical ocular propranolol resulted in higher corneal thickness in RA and IH, whereas thickness was decreased when administered through oral in RA but not in IH. Topical ocular and oral saline treatment in IH increased corneal epithelial cells compared with RA. Both topical ocular and oral propranolol treatment in RA increased the number of epithelial cells compared with saline, but an opposite response was seen in IH. Furthermore, treatment with oral propranolol decreased the number of corneal epithelial cells compared with topical ocular propranolol treatment in neonatal IH.
FIG. 6.
Representative H&E-stained corneas from rats raised in RA (upper panels) or neonatal IH (lower panels) at postnatal day 21 (P21) and treated with topical ocular or oral saline or propranolol. Pups were administered a single daily treatment of topical ocular or oral propranolol or saline occurred from P5 to P14. Images are 40 × magnification, scale bar is 20 μm. Color images are available online.
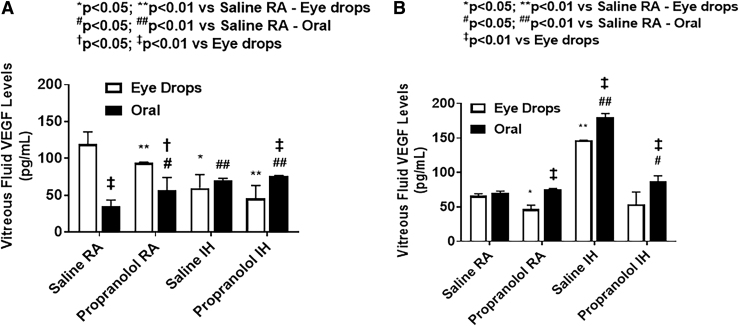
Ocular VEGF levels
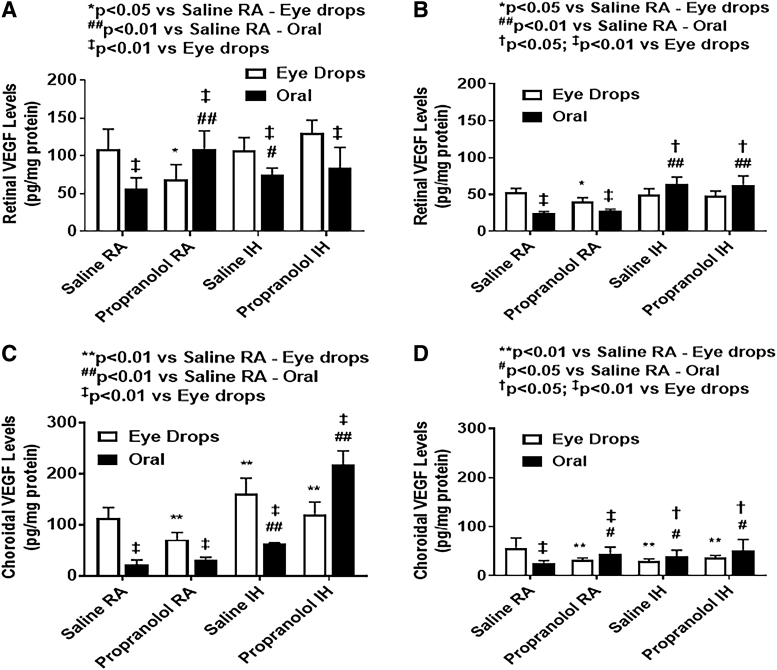
Vitreous fluid VEGF levels are presented in Fig. 7. At P14, pups treated with topical ocular propranolol had lower VEGF levels in the vitreous compared with pups receiving topical saline. This was the opposite for pups treated with oral propranolol, were pups had higher VEGF levels in the vitreous compared with their control pups treated with oral saline. This was the case whether these pups were exposed to IH or RA conditions. At P21, the pups treated with saline in IH conditions had the highest VEGF levels, and their levels seemed to go down with propranolol (both topical and oral). Retinal and choroidal VEGF levels are presented in Fig. 8.
FIG. 7.
Effects of topical ocular or oral propranolol or placebo saline on VEGF levels in the vitreous fluid at P14 (A) and P21 (B). The open bars represent the topical ocular administration and the solid bars represent oral administration. Data are expressed as mean ± SD (n = 4 samples/group). SD, standard deviation; VEGF, vascular endothelial growth factor.
FIG. 8.
Effects of topical ocular or oral propranolol or placebo saline on VEGF levels in the retina at P14 (A) and P21 (B) and in the choroid at P14 (C) and P21 (D). Levels were standardized using total cellular protein levels. The open bar represents the RA groups and the solid bar represents the IH groups. Data are expressed as mean ± SD (n = 6 samples/group).
At P14, one of the most interesting findings is the differences in ocular VEGF levels based on the route of propranolol administration. For pups exposed to IH conditions, VEGF levels at P14 were lower with oral propranolol in the retina compared with topical propranolol. Whereas the choroid produced an opposite response, with lower VEGF levels in the topical propranolol versus oral propranolol route. Oral saline administration pups had lower VEGF levels in comparison to the topical ocular group pups in both RA and IH conditions at P14. At P21, VEGF levels are generally lower than P14 in both retina and choroid.
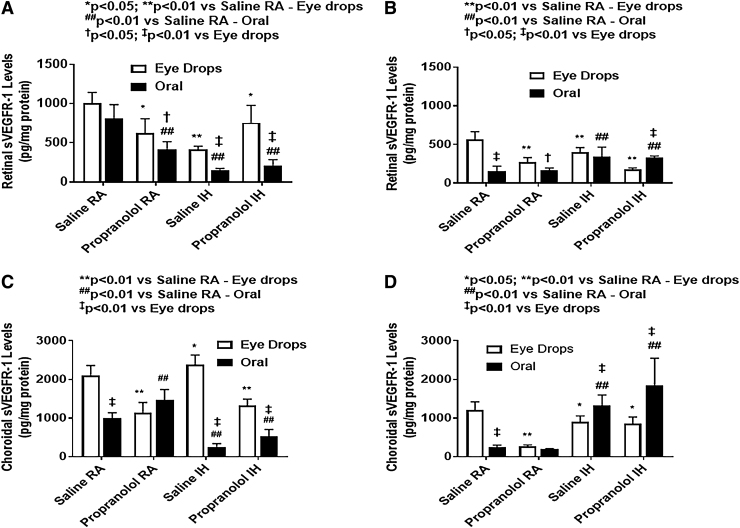
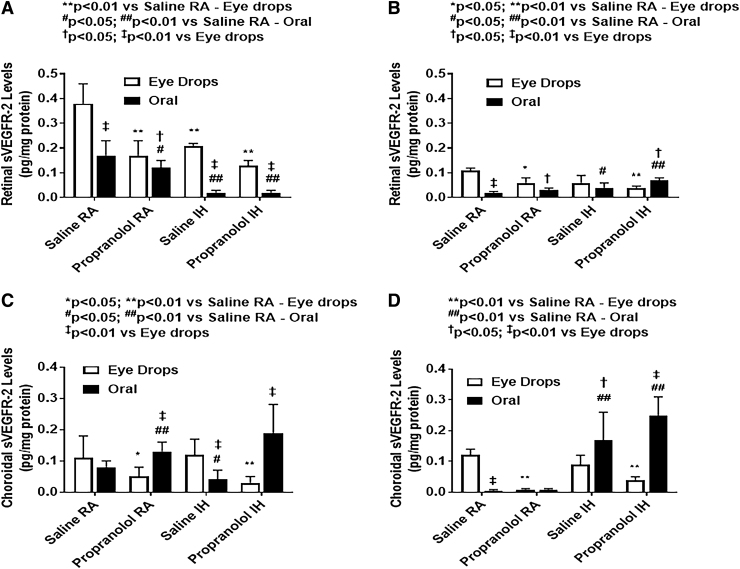
Ocular sVEGFR-1 and sVEGFR-2 levels
Ocular sVEGFR-1 and sVEGR-2 at P14 and P21 are presented in Figs. 9 and 10, respectively. At P14, in RA, both c groups (topical and oral) had lower retinal sVEGFR-1 than saline controls. Under IH conditions, pups receiving oral propranolol had significantly lower sVEGFR-1 levels compared with those receiving topical propranolol. At P21, Propranolol is not as effective in lowering sVEGFR-1 levels. Both groups (oral propranolol and oral saline) in IH conditions had significantly lower retinal sVEGFR-2 levels compared with their topical counterparts. Pups treated with topical propranolol had lower sVEGFR-2 levels compared with saline controls under RA and IH conditions at P14. At P21, sVEGFR-2 was decreased significantly in all groups, and oral propranolol was associated with higher sVEGFR-2 levels under IH conditions when compared with saline. In the choroid, sVEGFR-1 levels were generally higher than sVEGFR-2. Oral propranolol and saline have similar effect on sVEGFR-1 in the choroid in comparison to their topical counterparts, under IH conditions. At P14, oral propranolol-treated pups showed lower sVEGFR-1 levels compared with pups who received topical propranolol under IH conditions. The opposite occurred to sVEGFR-2 levels, which were lower in pups treated with topical propranolol. Overall, there was a noticeable reduction in the VEGF soluble receptors in all groups under IH condition at P14, but at P21, the levels increased following recovery/reoxygenation in RA.
FIG. 9.
Effects of topical ocular or oral propranolol or placebo saline on sVEGFR-1 levels in the retina at P14 (A) and P21 (B) and in the choroid at P14 (C) and P21 (D). Levels were standardized using total cellular protein levels. The open bar represents the RA groups and the solid bar represents the IH groups. Data are expressed as mean ± SD (n = 6 samples/group). sVEGFR, soluble vascular endothelial growth factor receptor.
FIG. 10.
Effects of topical ocular or oral propranolol or placebo saline on sVEGFR-2 levels in the retina at P14 (A) and P21 (B) and in the choroid at P14 (C) and P21 (D). Levels were standardized using total cellular protein levels. The open bar represents the RA groups and the solid bar represents the IH groups. Data are expressed as mean ± SD (n = 6 samples/group).
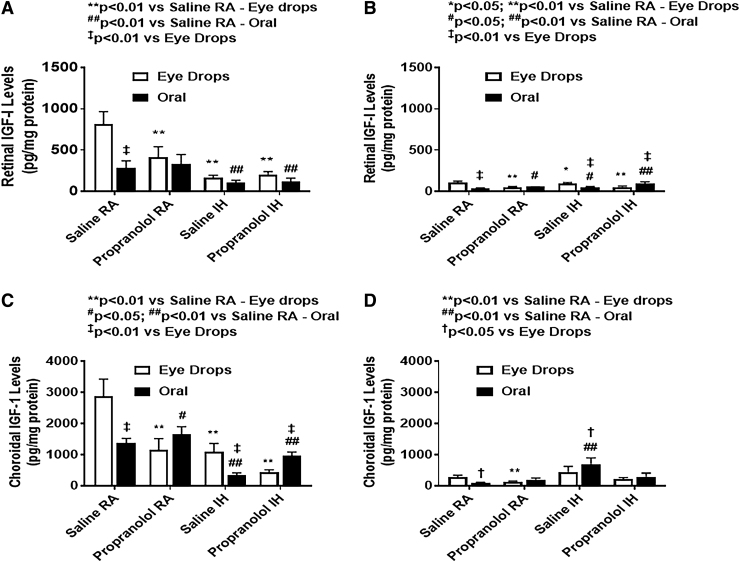
IGF-I in the retina and choroid
Retinal and choroidal IGF-I levels are presented in Fig. 11.
FIG. 11.
Effects of topical ocular or oral propranolol or placebo saline on IGF-I levels in the retina at P14 (A) and P21 (B) and in the choroid at P14 (C) and P21 (D). Levels were standardized using total cellular protein levels. The open bar represents the RA groups and the solid bar represents the IH groups. Data are expressed as mean ± SD (n = 4 samples/group). IGF, insulin-like growth factor.
At P14, IH resulted in lower retinal IGF-I levels compared with RA. Propranolol did not affect IGF-I levels in IH groups whether administered topically or orally. At P21, IGF-I levels increased in all groups. In the choroid, at P14, topical propranolol pups had lower IGF-I levels compared with saline under RA and IH conditions. At P21, IH propranolol groups maintained similar levels of IGF-I as P14.
Discussion
Laser phototherapy continues to be the gold standard treatment for ROP, despite its long-term side effects, such as loss of peripheral vision, and requirement of adjunctive therapy such as intravitreal bevacizumab. In our search for a noninvasive treatment for ROP, propranolol seemed very promising. The major findings of this study are: (1) poor growth accretion during treatment with propranolol, particularly in the topical ocular groups, which demonstrated catch-up growth during recovery/reoxygenation; (2) sustained impact on organ/body weight ratios with propranolol treatment in IH, which may indicate toxicity; (3) elevated systemic VEGF and reduced sVEGFR-1 levels during recovery/reoxygenation postpropranolol treatment in IH; (4) propranolol improved, but did not prevent, IH-induced retinal vascular damage; (5) propranolol reversed or prevented neonatal IH-induced astrocyte injury; and (6) propranolol had no beneficial effects on retinal thickness or EC abundance in the NFL/GCL layer. These data may provide support for the clinical trial findings30 but also demonstrate that while propranolol at the doses used, reduced the severity of neonatal IH-induced OIR, it does not fully prevent it. This was evidenced by persistence of hemorrhage, vascular tufts, and vascular abundance during recovery/reoxygenation. It is important to point out that our study differs from the clinical trial30 in the timing of treatment. We administered propranolol prophylactically because previous studies in our laboratory showed that in neonatal IH, retinal damage is evident as early as postnatal day 341 suggesting that early intervention may be more efficacious than rescue treatment when mechanisms involved in severe OIR are already activated. Regardless, the most important benefit of propranolol was preservation of the astrocytic template postneonatal IH. This finding is novel. While the exact mechanism of propranolol effect on astrocytes remain unclear, it may involve prevention of the complex metabolic pathways involved in aerobic glycolysis42,43 which is activated during neonatal IH. Alternatively, since astrocytes are one of the main sources of VEGF in the retina, it is likely that propranolol's suppressive effect on VEGF secretion by hypoxic astrocytes reduced their hyperproliferation and prevented gliosis. Further studies are needed to confirm this.
Our findings of the negative impact of topical ocular and oral propranolol on growth suggests that the topical ocular route is not completely safe. This is a major concern since ELGANs are already growth suppressed and vital organs, such as lungs and brain are still maturing. The sustained effects of propranolol on organ/body weight ratios are equally concerning because organ/body weight ratios are important indices of drug toxicity.37 There has been a recent interest in postnatal weight gain and a link to ROP.44 Pups from large litters are at risk for lower postnatal weight gain, lower IGF-I levels, and more severe ROP.34 By expanding the litter size, we were able to mimic the relative malnutrition of ELGANs, and thus predispose the pups to a greater risk for OIR. It was interesting that topical ocular propranolol also resulted in lower weight accretion and body length. This is likely due to leakage of propranolol into the systemic circulation under neonatal IH conditions, as weight gain was lower in neonatal IH than RA. Vascular leakage in neonatal IH is caused by IH induction of VEGF, which increases vessel permeability. Nevertheless, it was reassuring that the animals exhibited catchup growth post-treatment and neonatal IH and even surpassed that of the placebo littermates. This raises questions regarding propranolol's half-life and the longevity of its effect. Smits and Struyker-Boudier45 have shown that propranolol is cleared from rat bodies within hours and does not accumulate in tissues. However, while there are no studies examining the effect of neonatal IH on propranolol pharmacokinetics, studies show that the metabolism is slower in newborns than adults.46 Whether the excessive body weight seen in the propranolol group during recovery/reoxygenation is due to increased adiposity, which is linked to later onset of metabolic disorders, remains to be determined.
Retinal damage, including hemorrhage, vascular tufts, neovascularization, enlarged vessels, are characteristic features associated the recovery/reoxygenation phase (vasoproliferation) and are associated with overproduction of VEGF, a vascular permeability factor. Indeed, our model produced many of these features evidenced in the retinal flatmounts from the placebo group. In particular, retinal hemorrhage was pervasive predominantly at the periphery, which is consistent with vascular damage and leakage. Propranolol treatment successfully reduced the occurrence of retinal hemorrhage and severity of IH-induced OIR during the recovery/reoxygenation phase, but did not fully prevent it. This may be due to rebound elevations in VEGF levels and reductions in sVEGFR-1 levels, which is an endogenous inhibitor of VEGF action. Rebound VEGF levels during the recovery/reoxygenation period may be due to the drug's clearance within hours,45 as treatment occurred only during neonatal IH and not during the recovery/reoxygenation period. In this regard, preventing neonatal IH or apnea of prematurity, with the use of drugs such as caffeine citrate, may potentiate propranolol's efficacy. Further studies are needed to test this hypothesis.
It was surprising that topical ocular propranolol resulted in lower serum VEGF levels than oral administration under IH conditions. This could be explained by the first pass effect on oral propranolol, as it undergoes extensive metabolism by the liver, decreasing its bioavailability.47 With IH, an increased leakiness of retinal vessels due to VEGF upregulation, it is likely that more propranolol leaked into the systemic circulation from the topical ocular route. VEGF exerts is angiogenic action through 2 main receptors: VEGFR-1 and VEGFR-2, both of which have soluble variants, which act like as endogenous VEGF traps to inhibit angiogenesis. At P14, sVEGFR-1 levels were lower in IH than in RA, which is reasonable, as IH upregulates VEGF, so it would be counterintuitive for it to increase the VEGF trap levels. However, sVEGFR-1 levels decreased at P21 as a rebound recovery. Conversely, both orally administered saline and propranolol treatment in neonatal IH increased sVEGFR-2 levels compared with RA. These findings are consistent with previous reports, which showed that higher sVEGFR-2 was associated with increased plasma leakage.48 Whether sVEGFR-2 levels can serve as a biologic marker for vascular leakage in preterm infants at risk for ROP remain to be determined. Oral propranolol resulted in lower sVEGFR-1 and sVEGFR-2 retinal levels under IH conditions. Suppression of sVEGFR-1 allows more angiogenesis due to increased availability of VEGF. Rota et al.49 proved that injection of sVEGFR-1 into the eye of rats exposed to alternating cycles of high and low O2 decreased neovascularization by up to 97.5%. Vitreous fluid VEGF levels followed the same pattern as in the serum at P14. It is easier for propranolol to reach the vitreous through the systemic circulation than locally bypassing the anterior segment of the eye. Retinal VEGF levels were lower with oral propranolol under IH conditions compared with topical ocular propranolol. This finding may be due to the retina's anatomic location posteriorly, which makes it less accessible to topical ocular medications. In rats, subconjunctival injections of propranolol resulted in lower concentrations in retina compared with anterior eye segment.50 Another factor that could affect propranolol's penetration to retina was the hydrophilic vehicle we used to administer propranolol (saline). Main et al.s’ work51 showed that lipophilic solutions achieve better concentrations in the choroid and retina when applied locally.
It was interesting to note that oral propranolol achieved a better control of VEGF levels in the retina, whereas topical ocular propranolol was more effective in the choroid. This is not surprising because the choroid is continuous with the ciliary bodies, having direct access to the anterior segment of the eye,52 hence the better penetration by topical ocular than oral propranolol, and may have had a greater influence on the astrocyte outcomes. Unlike Müller cells, which have their end feet in NFL/GCL and extend from the inner limiting membrane to the outer limiting membrane, astrocytes are found primarily in the NFL/GCL layer, and reacts with GFAP. They are major sources of VEGF and form a template for the retinal vasculature to promote normal angiogenesis.53 On the other hand, Müller cells are not generally reactive to GFAP, except when injured.54 Beharry et al.16,41 have shown that neonatal IH-induced retinal damage is associated with degeneration of photoreceptors and activation of Müller cells, indicating gliosis. It was reassuring that propranolol was beneficial for astrocyte preservation during IH. Weidemann et al.55 argued that astrocytes' role might be more important in pathological than developmental angiogenesis. Dal Monte et al.23 showed that propranolol (2% eye drops) decreased angiogenesis in superficial vascular plexus of retinas of mice exposed to the OIR model. An effect that was demonstrated at P17 lasted to P21. Our study provides some support for those findings. However, there are several reasons for the lack of complete protection in our study. First, the model is different. Mice are generally exposed to 75% O2 from P7 to P12, not neonatal IH. Second, the effect of hyperoxia in mice produces vaso-obliteration in the central vessels, not in the periphery, as seen in human neonates, and in our model. Third, regression/normalization of neovascularization in mice occurs spontaneously at around P21.56 In human neonates, and in our model, the damage worsens, if left untreated. Fourth, propranolol was applied 4 times daily D12–16 during recovery/reoxygenation in RA, whereas in our study, the rats were exposed to neonatal IH from P0 to P14 and treatment occurred during neonatal IH, not during recovery/reoxygenation. Retinal changes noted on H&E-stained retinal layers in IH were consistent with our previous findings of increased total retinal, NFL/GCL, and IPL, and ONL thickness, as well as increased EC abundance violating the vitreous fluid.16 These changes persisted despite treatment with propranolol. Increased retinal thickness has been shown to predict severe ROP using spectral domain optical coherence tomography.57 While the present study has important clinical implications, there are limitations. Topical ocular administration began at P5. This was due to loosening of the skin at that age and fear of damaging the eyes while treating with subpalpebral injections. Subpalpebral injections may cause increased ocular pressure, which may account for the differences in the outcomes between oral and topical ocular. Subpalpebral injections are not consistent with general topical ocular administration of drugs. Our neonatal IH model produces retinal damage as early as P3 and may be irreversible.41 Furthermore, we used a hydrophilic vehicle to deliver propranolol topically, limiting its availability to the retina. Whether lipophilic preparations and earlier treatment will provide better outcomes remain to be determined. We administered treatment or placebo to both eyes, and did not use one eye as placebo, since we have proven, ocular drugs can leak into the contralateral eye under neonatal IH conditions (likely due to vessel damage and leakage), and into the systemic circulation, affecting the outcomes.15
Conclusions
Apnea of prematurity with brief, alternating, frequent episodes of arterial oxygen desaturations occurs in 100% of neonates born <28 weeks gestation.58 The present study is the first to compare the beneficial effects of topical ocular versus oral propranolol in neonatal IH. We tested the hypothesis that topical ocular propranolol at the currently proposed dose for ROP, is safe and effective for reducing the severity of IH-induced OIR. Our findings led us to partially accept the hypothesis. Regarding safety, the effects on growth and organ/body weight ratios are concerning. Regarding efficacy, propranolol reduced in neonatal IH-induced OIR, but did not prevent it, as many unwanted characteristics consistent with OIR persisted. On the other hand, propranolol was effective for preserving astrocyte integrity. Given the importance of astrocytes in retinal and brain development, it is possible that propranolol at the appropriate doses may be neuroprotective, but the mechanism underlying its beneficial effect in the setting of neonatal IH remains to be determined.
Author Contributions
Conceptualization: A.Q., J.V.A, and K.D.B. Methodology: A.Q., C.L.C., K.D., F.S., and K.D.B. Validation: J.V.A. and K.D.B. Formal analysis: A.Q., C.L.C., K.D., F.S., and K.D.B. Investigation: A.Q., C.L.C., K.D., F.S., and K.D.B. Resources: J.V.A. Data curation: K.D.B. Writing—original draft preparation: A.Q. Writing—review and editing: A.Q. C.L.C., K.D., F.S., and J.V.A. Visualization: A.Q., C.L.C., and K.D.B. Supervision: J.V.A. and K.D.B. Project administration: J.V.A. and K.D.B. Funding acquisition: J.V.A.
Author Disclosure Statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the article; or in the decision to publish the results.
Funding Information
This work was made possible through the Eunice Kennedy Shriver National Institute of Child Health & Human Development Grant #U54HD071594 and the Alicia and Madu Rao Translational Research Foundation Grant EIN 262145011.
References
- 1. Gilbert, C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum. Dev. 84:77–82, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Smith, L. Pathogenesis of retinopathy of prematurity. Growth Horm. IGF Res. 14:S140–S144, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Di Fiore, J.M., Bloom, J.N., Orge, F., et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr. 157:69–73, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di Fiore, J.M., Kaffashi, F., Loparo, K., et al. The relationship between patterns of intermittent hypoxia and retinopathy of prematurity in preterm infants. Pediatr. Res. 72:606–612, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin, R.J., Di Fiore, J.M., Macfarlane, P.M., and Wilson, C.G.. Physiologic basis for intermittent hypoxic episodes in preterm infants. Adv. Exp. Med. Biol. 758:351–358, 2012 [DOI] [PubMed] [Google Scholar]
- 6. Martin, R.J., Wang, K., Köroğlu, O., Di Fiore, J., and Kc, P.. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology. 100:303–310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cunningham, S., Fleck, B.W., Elton, R.A., et al. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 346:1464–1465, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Penn, J.S., Madan, A., Caldwell, R.B., Bartoli, M., Caldwell, R.W., and Hartnett, M.E.. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 27:331–371, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen, J., and Smith, L.E.. Retinopathy of prematurity. Angiogenesis. 10:133–14, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Lalwani, G.A., Berrocal, A.M., Murray, T.G., et al. Off-label use of intravitreal bevacizumab (Avastin) for salvage treatment in progressive threshold retinopathy of prematurity. Retina. 28: S13–S18, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Mintz-Hittner, H.A., Kennedy, K.A., and Chuang, A.Z; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 364:603–615, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morin, J., Luu, T.M., Superstein, R., et al. Canadian Neonatal Network and the Canadian Neonatal Follow-Up Network Investigators. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics. 137:e20153218, 2016 [DOI] [PubMed] [Google Scholar]
- 13. Theophanous, C., and Schechet, S.Rodriguez, S.H. and Blair, M.. Bilateral vitreous hemorrhage following bilateral intravitreal injections of bevacizumab in an infant with retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retina. 49:893–896, 2018 [DOI] [PubMed] [Google Scholar]
- 14. Seto, R., Yamada, H., Wada, H., Osawa, M., Nagao, T., and Nakano, Y.. Diffuse alveolar haemorrhage may be associated with intravitreal injection of bevacizumab in a patient with systemic risk factors. BMJ Case Rep. 2011:bcr0820103224, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan, J., Cai, C.L., Shrier, E.M., et al. Ocular adverse effects of intravitreal bevacizumab are potentiated by intermittent hypoxia in a rat model of oxygen-induced retinopathy. J. Ophthalmol. 2017:4353129, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beharry, K.D., Cai, C.L., Skelton, J., et al. Oxygen-induced retinopathy from recurrent intermittent hypoxia is not dependent on resolution with room air or oxygen, in neonatal rats. Int. J. Mol. Sci. 19:1337, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aranda, J.V., Cai, C.L., Ahmad, T., et al. Pharmacologic synergism of ocular ketorolac and systemic caffeine citrate in rat oxygen-induced retinopathy. Pediatr. Res. 80:554–565, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanghvi, K.P., Kabra, N.S., Padhi, P., Singh, U., Dash, S.K., and Avasthi, B.S.. Prophylactic propranolol for prevention of ROP and visual outcome at 1 year (PreROP trial). Arch. Dis. Child. Fetal Neonatal Ed. 2017; 102:F389–F394 [DOI] [PubMed] [Google Scholar]
- 19. Filippi, L., Cavallaro, G., Bagnoli, P., Dal Monte, M., and Mosca, F.. Propranolol 0.1% eye micro-drops in newborns with retinopathy of prematurity: a pilot clinical trial. Pediatr. Res. 81:307–314, 2017 [DOI] [PubMed] [Google Scholar]
- 20. Korkmaz, L., Baştuğ, O., Ozdemir, A., et al. The efficacy of propranolol in retinopathy of prematurity and its correlation with the platelet mass index. Curr. Eye Res. 42:88–97, 2017 [DOI] [PubMed] [Google Scholar]
- 21. Bancalari, A., Schade, R., Muñoz, T., Lazcano, C., Parada, R., and Peña, R.. Oral propranolol in early stages of retinopathy of prematurity. J. Perinat. Med. 44:499–503, 2016 [DOI] [PubMed] [Google Scholar]
- 22. Bührer, C., Erdeve, Ö., Bassler, D., and Bar-Oz, B.. Oral propranolol for prevention of threshold retinopathy of prematurity (ROPROP): protocol of a randomised controlled trial. BMJ Open. 8:e021749, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dal Monte, M., Casini, G., la Marca, G., Isacchi, B., Filippi, L., and Bagnoli, P.. Eye drop propranolol administration promotes the recovery of oxygen-induced retinopathy in mice. Exp. Eye Res. 111:27–35, 2013 [DOI] [PubMed] [Google Scholar]
- 24. Ristori, C., Filippi, L., Dal Monte, M., et al. Role of the adrenergic system in a mouse model of oxygen-induced retinopathy: antiangiogenic effects of beta-adrenoreceptor blockade. Invest. Ophthalmol. Vis. Sci. 52:155–170, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Pandey, A., Singh, A., Ali, W., et al. Evaluation of effect of propranolol on serum vascular endothelial growth factor and tissue inhibitor of metalloproteinase-2 levels in infantile hemangioma. J. Indian Assoc. Pediatr. Surg. 25:96–102, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hyland, R.M., Komlósi, K., Alleman, B.W., et al. Infantile hemangiomas and retinopathy of prematurity: clues to the regulation of vasculogenesis. Eur. J. Pediatrics 172:803–809, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Léauté-Labrèze, C., Hoeger, P., Mazereeuw-Hautier, J., et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N. Engl. J. Med. 372:735–746, 2015 [DOI] [PubMed] [Google Scholar]
- 28. Stritzke, A., Kabra, N., Kaur, S., Robertson, H.L., and Lodha, A.. Oral propranolol in prevention of severe retinopathy of prematurity: a systematic review and meta-analysis. J. Perinatol. 39:1584–1594, 2019 [DOI] [PubMed] [Google Scholar]
- 29. Filippi, L., Cavallaro, G., Bagnoli, P., et al. Oral propranolol for retinopathy of prematurity: risks, safety concerns, and perspectives. J. Pediatr. 163:1570–1577, 2013 [DOI] [PubMed] [Google Scholar]
- 30. Filippi, L., Cavallaro, G., Berti, E., et al. Propranolol 0.2% eye micro-drops for retinopathy of prematurity: a prospective phase IIB study. Front. Pediatr. 7:180, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Binnes, K.E., and Salt, T.E.. Post eye-opening maturation of visual receptive field diameters in superior colliculus of normal- and dark-reared rats. Dev. Brain Res. 99:263–266, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Zieske J.D. Corneal development associated with eyelid opening. Int. J. Dev. Biol. 48:903–911, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Robinson, S.R., and Dreher, B.. The visual pathways of eutherian mammals and marsupials develop according to a common timetable. Brain Behav. Evol. 36:177–195, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Beharry, K.D., Cai, C.L., Sharma, P., et al. Hydrogen peroxide accumulation in the choroid during intermittent hypoxia increases risk of severe oxygen-induced retinopathy in neonatal rats. Invest. Ophthalmol. Vis. Sci. 54:7644–7657, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lundgren, P., Hård, A.L., Wilde, Å., Löfqvist, C., Smith, L.E.H., and Hellström. A.. Implementing higher oxygen saturation targets reduced the impact of poor weight gain as a predictor for retinopathy of prematurity. Acta Paediatr. 107:767–773, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu, C., Vanderveen, D.K., Hellström, A., Löfqvist, C., and Smith, L.E.. Longitudinal postnatal weight measurements for the prediction of retinopathy of prematurity. Arch. Ophthalmol. 128:443–447, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bailey, S.A., Zidell, R.H., and Perry, R.W.. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol. Pathol. 2004; 32:448–466 [DOI] [PubMed] [Google Scholar]
- 38. Shibuya, M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various disease. J. Biochem. 153:13–19, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hellström, A., Engström, E., Hård, A.L., et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 112:1016–1020, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Smith, L.E. IGF-1 and retinopathy of prematurity in the preterm infant. Biol. Neonate. 88:237–244, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Beharry, K.D., Cai, C.L., Ahmad, T., Guzel, S., Valencia, G.B., and Aranda, J.V.. Impact of chronic neonatal intermittent hypoxia on severity of retinal damage in a rat model of oxygen-induced retinopathy. J. Nat. Sci. 4:e488, 2018 [PMC free article] [PubMed] [Google Scholar]
- 42. Dembinska, O. Graded contribution of retinal maturation to the development of oxygen-induced retinopathy in rats. Invest. Ophthalmol. Vis. Sci. 42:1111–1118, 2001 [PubMed] [Google Scholar]
- 43. Dienel, G.A., and Cruz, N.F.. Aerobic glycolysis during brain activation: adrenergic regulation and influence of norepinephrine on astrocytic metabolism. J. Neurochem. 138:14–52, 2016 [DOI] [PubMed] [Google Scholar]
- 44. Binenbaum, G. Algorithms for prediction of retinopathy of prematurity based upon postnatal weight gain. Clin. Perinatol. 40:261–270, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smits, J.M., and Struyker-Boudier, H.A.. Propranolol in conscious spontaneously hypertensive rats. II. Disposition after subcutaneous and intracerebroventricular administration. Naunyn Schmiedebergs Arch. Pharmacol. 1979; 309:19–24 [DOI] [PubMed] [Google Scholar]
- 46. Filippi, L., Cavallaro, G., Fiorini, P., et al. Propranolol concentrations after oral administration in term and preterm neonates. J. Matern. Fetal Neonatal Med. 26:833–840, 2013 [DOI] [PubMed] [Google Scholar]
- 47. Anderson, B.D., Chu, W.W., and Galinsky, R.E.. Reduction of first-pass metabolism of propranolol after oral administration of ester prodrugs. Int. J. Pharmaceutics. 43:261–265, 1988 [Google Scholar]
- 48. van de Weg, C.A., Pannuti, C.S., van den Ham, H.J., et al. Serum angiopoietin-2 and soluble VEGF receptor 2 are surrogate markers for plasma leakage in patients with acute dengue virus infection. J. Clin. Virol. 60:328–335, 2014 [DOI] [PubMed] [Google Scholar]
- 49. Rota, R., Riccioni, T., Zaccarini, M., et al. Marked inhibition of retinal neovascularization in rats following soluble-flt-1 gene transfer. J Gene Med. 6:992–1002, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Kadam, R.S., and Kompella, U.B.. Influence of lipophilicity on drug partitioning into sclera, choroid-retinal pigment epithelium, retina, trabecular meshwork, and optic nerve. J. Pharmacol. Exp. Ther. 332:1107e1120, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51. Mains, J., Tan, L.E., Wilson, C., and Urquhart, A.. A pharmacokinetic study of a combination of beta adrenoreceptor antagonists—in the isolated perfused ovine eye. Eur. J. Pharm. Biopharm. 80:393–401, 2012 [DOI] [PubMed] [Google Scholar]
- 52. Del Amo, E.M., Rimpelä, A.K., Heikkinen, E., et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 57:134–185, 2017 [DOI] [PubMed] [Google Scholar]
- 53. Chan-Ling, T., McLeod, D.S., Hughes, S., et al. Astrocyte-endothelial cell relationships during human retinal vascular development. Invest. Ophthalmol. Vis. Sci. 45:2020–2032, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Luna, G., Keeley, P.W., Reese, B.E., Linberg, K.A., Lewis, G.P., and Fisher, S.K.. Astrocyte structural reactivity and plasticity in models of retinal detachment. Exp. Eye Res. 150:4–21, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weidemann, A., Krohne, T.U., Aguilar, E., et al. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 58:1177–1185, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Connor, K.M., Krah, N.M., Dennison, R.J., et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 4:1565–1573, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maldonado, R.S., O'Connell, R., Ascher, S.B., et al. Spectral-domain optical coherence tomographic assessment of severity of cystoid macular edema in retinopathy of prematurity Arch. Ophthalmol. 130:569-578, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Du, L., Tong, X., Chen, C., et al. Caffeine citrate for apnea of prematurity: a prospective, open-label, single-arm study in Chinese neonates. Front. Pediatr. 8:76, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]