Abstract
Veno-arterial extracorporeal membrane oxygenation (ECMO) is increasingly being deployed for selected patients in cardiac arrest who do not attain a native circulation with conventional CPR (ECPR). This ELSO guideline is intended to be a practical guide to implementing ECPR and the early management following establishment of ECMO support. Where a paucity of high-quality evidence exists, a consensus has been reached amongst the authors to provide guidance to the clinician. This guideline will be updated as further evidence in this field becomes available.
Keywords: ECPR, ECMO, Extracorporeal Membrane Oxygenation, CPR, Resuscitation
INTRODUCTION
Extracorporeal cardiopulmonary resuscitation (ECPR) is the application of extracorporeal membrane oxygenation (ECMO) in patients where conventional cardiopulmonary resuscitation (CCPR) measures are unsuccessful in achieving a sustained return of spontaneous circulation (ROSC)1.1 The primary purpose of ECPR is to restore the circulation and gas exchange. By providing organ perfusion, it provides time for the delivery of interventions necessary to regain an adequate native circulation. These may include percutaneous coronary intervention (PCI) and recovery from myocardial stunning, pulmonary thrombectomy, rewarming, or toxin clearance.
ECPR is a time-sensitive, complex intervention that requires teamwork, clearly defined roles, and well trained health care providers.2 ECPR can be deployed both for patients with in-hospital cardiac arrest (IHCA) and out of hospital cardiac arrest (OHCA). ECPR should be considered after 10–15 minutes of unsuccessful conventional resuscitation efforts,2 because organisation and preparation for ECPR will take some time and it has been clearly shown that time to ECMO correlates with neurological outcome.3,4
Currently, there are no published randomized controlled trials comparing outcomes of ECPR to CCPR. Observational studies comparing ECPR to historical controls and case matched controls have demonstrated favorable results for ECPR.5–9 However, these studies are heterogeneous and survival ranges from 15–50%. Among adult ECPR patients recorded in the international ELSO dataset, survival to hospital discharge is 29%.10
At the time being we do not know whether the number of neurological injured patients will increase with growing use of ECPR. A major task for the future will be to develop better neuro-prognostication tools. In the current observational studies in selected populations,5,8–10 >85% of survivors of cardiac arrest treated with ECPR had neurological outcomes fall into favorable neurologic performance categories (cerebral performance categories (CPC) 1 or 2).5,8,9 Future trials involving ECPR should endeavor to report neurological outcomes as well as mortality.
This document contains numerous additional literature references, organized by topic, found in the Supplemental Content.
Patient selection
Robust data to identify those who may benefit from ECPR are lacking. Protocols and guidelines strive to identify cases most likely to survive with favorable neurologic outcome—such as those patients who are witnessed to arrest and in whom high quality CPR was initiated rapidly, in addition to cardiac arrests with a presumed reversible pathology, such as acute coronary occlusions.1,11–13
We recommend that locally agreed inclusion criteria be formulated to guide clinicians on balancing the wise use of resources amongst patients who are thought to have an improved chance of survival following cardiac arrest (TABLE 1).
Table 1 -.
Example of inclusion criteria for ECPR
| • Age <7040 |
| • Witnessed arrest |
| • Arrest to first CPR (“No-Flow Interval”) <5 mins (i.e. Bystander CPR) |
| • Initial cardiac rhythm of VF/pVT/PEA |
| • Arrest to ECMO flow <60 minutes “Low Flow Interval”** |
| • ETCO2 > 10 mmHg (1.3 kPa) during CCPR prior to cannulation for ECMO |
| • Intermittent ROSC and/or recurrent VF |
| • “Signs of life” during conventional CPR may be a positive predictive factor for survival. |
| • Absence of previously known life limiting comorbidities (eg end stage heart failure / Chronic Obstructive Pulmonary Disease / End Stage Renal Failure / Liver failure / Terminal illness) and consistent with patient’s goals of care. |
| • No known aortic valve incompetence (>mild aortic valve incompetence should be excluded) |
(unless other favorable prognostic features are present: e.g. periods of intermittent ROSC / hypothermia pre-arrest / young age / signs of life during CPR)
Decision making for ECPR is often time critical and relies on incomplete information. As such, it may be reasonable to start ECPR as a bridge-to-seeking further information regarding appropriateness of ongoing support. Institutions may choose to use alternative inclusion criteria for OHCA vs IHCA given differences in etiology and expected survival.
Timing
The optimal time before initiation of ECPR in cases of refractory cardiac arrest is not well defined. If initiated too early, ROSC may have been gained with CCPR avoiding the additional risks of V-A ECMO. If initiated too late, the risk of hypoxic ischemic injury to the brain and other organs increases significantly; resuscitation related organ trauma and coagulation disorders may also increase. Among observational studies, shorter intervals between the cardiac arrest and ECPR initiation correlate with improved survival.4,14,15
The time required to establish ECMO support is highly dependent upon the capabilities of the resuscitation team and patient factors. It may be achieved in as little as 10 minutes but may take longer.9 We therefore advise early assessment for ECPR candidacy. It is reasonable to consider commencing cannulation after 10–20 minutes of failed resuscitation efforts. Beyond 20 minutes of refractory arrest, the probability of ROSC and survival with CCPR is <5%;16,17 thus, the risks of V-A ECMO and ECPR at this point, with appropriately selected patients and providers, may be justified.
A maximum arrest duration before ECPR becomes futile has also not been well defined. Neurologically intact survival has occurred following over 3 hours of mechanical CPR before ECPR in the context of a hypothermic arrest. In contrast, among non-hypothermic arrests, the majority of survivors were established on ECMO support in < 60 minutes from onset of cardiac arrest. In one center, ECPR survival after 90 minutes of CCPR was 14%.18 Until there are more robust data to the contrary, we recommend that the goal of ECPR is to establish adequate ECMO flow within 60 minutes of onset of cardiac arrest.
Location
The optimal location to provide safe and timely cannulation for ECPR in a hospital may differ between institutions depending on infrastructure, staffing, and other logistical considerations. Deployment of ECPR does not require an operating theatre. Some centers have proven the feasibility of providing prehospital ECPR to minimize low flow time.6 It is uncertain at present if provision of a prehospital ECPR system rather than transport of the OHCA patient to a center that provides ECPR leads to improved outcomes.
In the absence of definitive evidence, it is recommended that patients in refractory cardiac arrest who are suitable for ECPR should be transported to the nearest hospital which can provide this support as fast and as safely as possible. Early notification of a potential ECPR case is reasonable to gain time for hospital staff to mobilize the required team. If transport to a hospital-based ECPR setting is undertaken, EMS systems should strive to minimize interruptions to high-quality CPR during the extrication, transport, and hospital hand over. Automated mechanical compression devices may facilitate this.
Before further data become available, at the present time it is recommended to reserve out of hospital ECPR to highly specialized teams, possibly in the setting of controlled clinical trials.6
Mode of Support: Rationale for V-A ECMO
The goal of ECPR is the rapid restoration of adequate organ perfusion with cardiopulmonary support utilizing V-A ECMO.
Cannulation Phase
The cannulation phase commences with skin preparation to the femoral areas with antiseptic solution at which point modification to the ACLS algorithm should occur. Concurrently the following processes must occur:
External Cardiac Massage
External cardiac massage must continue throughout the cannulation phase, which may be assisted by application of an automated mechanical compression device. Studies comparing the mechanical chest compression devices with human-performed chest compression during CCPR have shown similar survival outcomes. We, therefore, extrapolate that a mechanical chest compression device may offer advantages during a prolonged resuscitation attempt during ECPR by reducing staff physical fatigue, providing more space around the patient and minimizing body movements during the cannulation.8,19
Some mechanical chest compression devices require careful positioning over the lower third of the sternum in the midline and must be checked regularly throughout application. Migration towards the upper abdomen reduces the effectiveness of compressions and is potentially harmful.20
Cannulation
Percutaneous ECMO cannulation has been shown to be effectively performed by providers from many disciplines, including surgeons,19,21 intensivists,22 cardiologists,9 and emergency physicians.19,21,23 However, those performing this technically challenging procedure must have the requisite training to develop the required skills, and sufficient volume of experience to maintain competency. Ideally, cannulators should be performing conventional vascular access procedures regularly. We advise that the decision to initiate cannulation occur after a risk/benefit assessment of the most skilled immediately available provider, within the context of patient specific cannulation complexities and the risk of continued CCPR.
Cannula Insertion
Percutaneous
The common femoral artery and vein may be accessed using a modified Seldinger technique.22 The femoral vessels should be imaged with ultrasound in real time to increase first-pass success rates. In suspected pulmonary embolism as the cause for arrest, ultrasound visualisation of the vessels is particularly important to exclude inadvertent cannulation of a thrombosed vein. Chest compression pauses, if required, should be kept to a minimum during cannulation. The first cannula may be flushed with heparinized saline and/or periodically back-flushed, to prevent clot formation whilst the second cannula is inserted. This step is not required for the second cannula as connection to the pre-primed circuit is imminent.
There is no strong preference regarding the side and, in case of difficulty, unilateral cannulation of both femoral vessels is acceptable. Time to support takes precedence over these other considerations. If two trained operators are available, contralateral cannulation may be faster.
The venous and arterial guidewires should be imaged prior to cannula insertion to confirm correct location and avoid inadvertent non-physiological support.24 This may be performed with bedside vascular ultrasound in combination with transthoracic or transesophageal echocardiography (for imaging of wires in the hepatic IVC and abdominal aorta) or with fluoroscopy.24 If available, fluoroscopy offers advantages in that it can evaluate the course and size of the vasculature. However, cannulator preference with alternative methodologies are acceptable; minimizing time to support remains the priority. Intra-arrest transesophageal echocardiography, in the hands of experienced providers, can facilitate placement and is supported by specialty guidelines.25
Distal Perfusion Cannula
Observational series suggest that ultrasound-guided placement of a smaller distal antegrade perfusion cannula (sometimes called distal limb perfusion cannula) in the ipsilateral superficial femoral artery, perfused off the side port on the arterial cannula, is associated with reduced critical limb ischemia.26 Leg perfusion could also be via retrograde flow via cannulation of the dorsalis pedis or posterior tibial arteries. This distal perfusion cannula is not required for initial deployment—priority should be given to therapies such as coronary catheterization—but should be sited ideally within 4 hours to reduce the risk of limb ischemia and subsequent need for fasciotomy or limb amputation.27 The use of calf tissue oxygen saturation with NIRS may provide monitoring of leg perfusion and onset of ischaemia.
Surgical
Arterial (antegrade and retrograde) and venous cannulation can also be acquired via surgical cut down techniques. The main advantage of cut down technique is direct visualization of the vessels while cannulating. This can be used to salvage a failed percutaneous access attempt. Cut down approaches may increase subsequent bleeding or infection from cannulation sites.
Cannula Choice
Cannula size should be guided by the relative balance between vessel size and the anticipated need for flow. Some data suggest increased limb ischemia with larger cannula size28 though this has not been confirmed in other studies. Arterial 15–17 Fr and 19–25 Fr multi-stage venous cannula provide satisfactory blood flow,29 though smaller cannulae may be acceptable for small patients.28 A single stage drainage cannula is also acceptable. Most circuits can deliver >4 L/min blood flow with a 15 Fr arterial return cannula. For large males, a 17–19 Fr arterial return cannula may be used. Significant arterial vasospasm may be seen in prolonged cardiac arrest after multiple doses of IV adrenaline, which may impede percutaneous cannulation.
ACLS Modifications
Standard ACLS therapies should be applied throughout the resuscitation until the cannulation procedure commences. The code leader should not be engaged in the ECMO cannulation process, but should provide oversight of the parallel conventional and ECMO resuscitation. Extreme caution should be applied when defibrillating the patient once guide wire insertion commences, due to the risk of electrocution of the cannulators. It may be reasonable to suspend further defibrillation attempts from this point until the patient is established on V-A ECMO support. Brief rhythm and pulse checks may still occur at the discretion of the code leader, as ROSC may allow the cannulation team more time for cannulation. End tidal CO2 and tissue oxygenation monitoring may help in assessing CCPR quality and ROSC detection.30–32
Pauses to chest compressions for any reason should be kept to a minimum. Adrenaline, and other drug administration may continue through the cannulation phase as directed by the code leader. When V-A ECMO support is imminent, as the circuit is being connected to the cannulae, adrenaline boluses should be discontinued as there is a risk of significant hypertension on establishment of a circulation with V-A ECMO blood flow.
Connection and Establishment of ECMO Support
The primed circuit is connected to the inserted ECMO cannulae using a technique to purge all air from the lines, thereby avoiding risk of air embolism. There is no need to warm the saline prime in the circuit prior to V-A ECMO support in ECPR (FIGURE 1).
FIGURE 1:
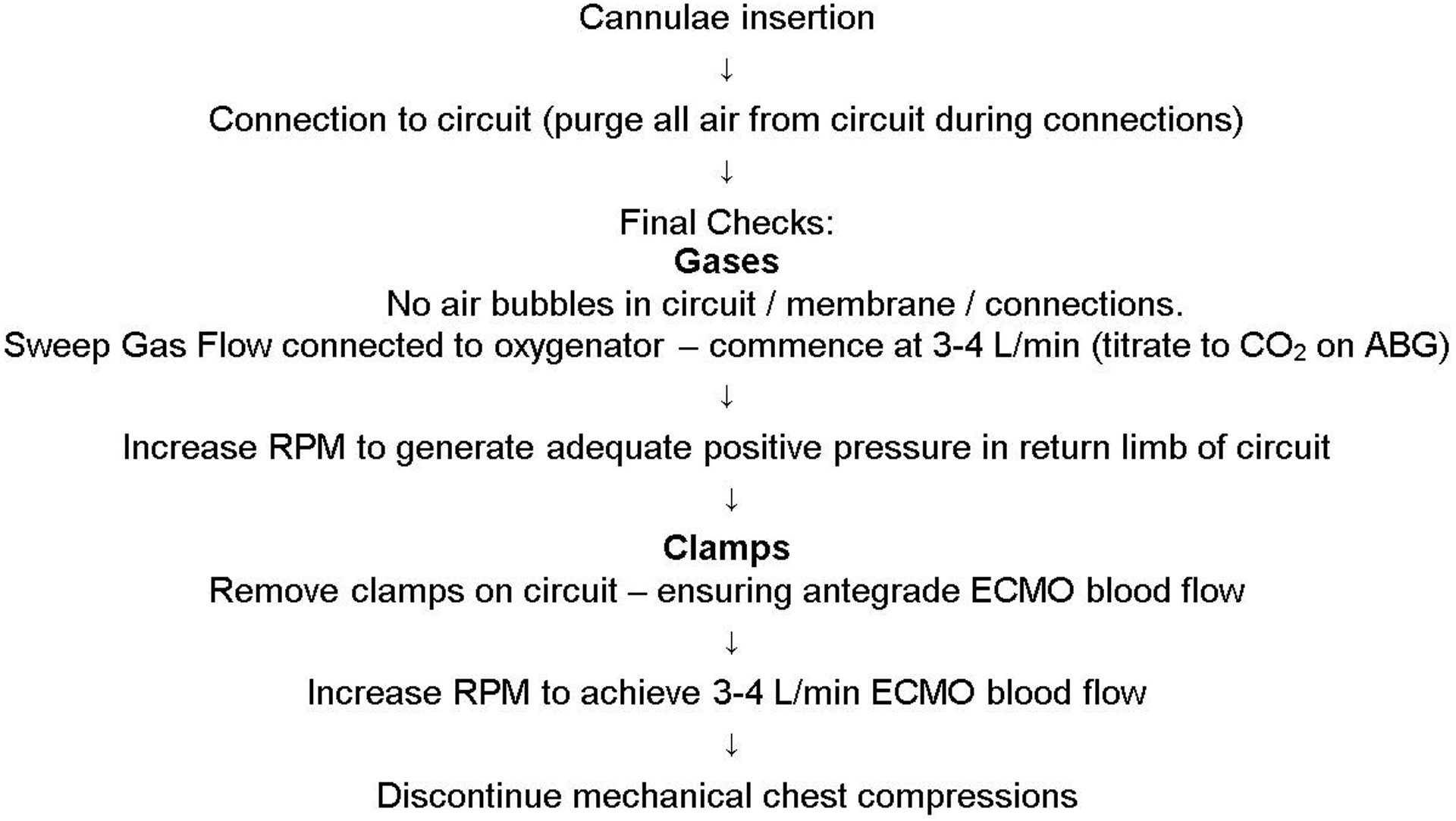
Cannulation sequence of events
The console operator should increase pump RPM to generate positive pressure in the return limb of the circuit prior to releasing the final clamp, this ensures antegrade circuit flow. Gradual increases in V-A ECMO support should occur over 20 seconds, aiming for a blood flow of 3–4 L/min. The goal of ECMO support is to halt ongoing global ischemia. The minimum flow rate required to achieve this goal is uncertain. Serial measurement of lactate to assess clearance and monitoring of organ function may be used to guide support.33
Once V-A ECMO blood flow is ≥3 L/min, mechanical compressions should be discontinued as an adequate circulation has been established. Vasopressor and inotrope infusions, if running, may need to be weaned rapidly.
ECPR Specific Immediate Post Cardiac Arrest Care Management
The access cannula position should be confirmed with fluoroscopy or echocardiography prior to cannula securing and dressing. The tip of the drainage cannula should be positioned in the Right Atrium. Both cannulas should be secured once an acceptable position is achieved.
Mean Arterial Pressure measurement: This is best achieved with a right-sided upper limb arterial line (radial or brachial). Right upper limb blood gas sampling gives the easiest indicator of native circulation PaO2 which may represent the cerebral oxygen delivery depending on the mixing point in the aorta between native circulation and V-A ECMO blood flow.34 Until an arterial line is placed, non-invasive measurement using a Doppler and a cuff will assist with initial vasopressor dosing.
An optimal MAP target following ECPR has not been identified. We recommend titrating vasopressors to MAP target ≥60 mmHg for organ perfusion pressure and <80 mmHg to minimize risk of LV distension. Animal data suggest higher MAP targets during initial reperfusion may improve neurological outcome.
Titrate sweep gas flow mechanical ventilation if ROSC has occurred by frequent ABGs monitoring to avoid hypocarbia
Address potential drainage insufficiency (drainage line chatter) with fluids / transfusion / weaning of V-A ECMO blood flow if excess to requirement; total circulation is made up of native cardiac output, if present, plus ECMO blood flow.
Ensure correct placement of endotracheal tube.
Central venous access.
Sedation & analgesia.
Perform bedside ultrasound: cardiac, thoracic, and abdominal imaging to identify possible complications (pneumothorax, thoracic/abdominal bleeding) and assess valve competence and LV distension.
Temperature monitoring and control
12 lead ECG
Chest x-ray
Estalish end-tidal CO2 monitoring to assess native cardiopulmonary circulation.
Formal laboratory bloods including cross match.
Further Care Within the First Few Hours
Check circuit blood flow stability. Unstable (falling) ECMO circuit blood flow should prompt a search for intra-abdominal, including retroperitoneal, and thoracic sources of hemorrhage, cardiac tamponade (associated with prolonged chest compression or trauma), or ECMO driven left ventricular distension with pulmonary congestion.
Restoration of a potentially perfusing rhythm increases likelihood of native circulation with left ventricular (LV) ejection and thus reduces risks of LV distension, valvular regurgitation, pulmonary edema and intracardiac thrombus formation. Patients in refractory arrhythmias should have another attempt at electrical cardioversion after several minutes of extracorporeal support. The restoration of coronary perfusion pressure with the V-A ECMO along with improving acid base status may lead to a successful cardioversion at this point. However, coronary ischemia and other reversible factors need to be addressed. Therefore, multiple attempts should be avoided unless these factors are resolved.
Peripheral V-A ECMO may increase LV afterload. If LV distension and pulmonary congestion occur, consideration to LV venting should be given. Options include: an intra-aortic balloon pump, direct LV vent, Impella® or atrial septostomy.35
Consider mechanical ventilation with PEEP ≥ 10 cmH2O for reducing LV afterload and preventing or treating pulmonary oedema. Reduce minute ventilation, due to reduced pulmonary blood flow, to achieve low normal etCO2. Lack of etCO2 may indicate absent native circulation.
Hyperoxia may be associated with worse neurologic outcome after cardiac arrest, including in the ECPR population.36 Avoidance of hyperoxia can be achieved through careful blending of ECMO fresh gas flow with an air and oxygen mix. We recommend targeting a patient arterial oxygen saturation of 92–97%.
Carbon dioxide (CO2) targets after resuscitation from cardiac arrest are conflicting, but a predominance of studies demonstrates increasing mortality with initial hypocarbia.37 There is insufficient data among ECPR specific populations to provide further recommendations.
Targeted Temperature Management (TTM) can be precisely achieved using the heat exchanger on the ECMO oxygenator. Based on protocols with best outcomes and consensus, we advise on 33–36 for 24 hours, then gradual rewarming to 37C.8,9
Siting a distal limb perfusion cannula is known to be associated with decreased limb ischemia in V-A ECMO.26 Accordingly, we recommend a distal perfusion cannula should be sited on the side of arterial cannulation. This should be performed within 4 hours of cannulation, though this should not delay cardiac reperfusion or urgent diagnostic imaging for bleeding or pulmonary embolism if these are required.27
Coronary Angiography/PCI
Studies suggest improved outcomes in cardiac arrest patients treated with percutaneous coronary interventions. ECPR series targeting cardiac arrest believed to be due to acute coronary etiologies, that utilized protocolised catheterization post cannulation are associated with increased survival.8,9,19 Accordingly, we recommend emergent coronary angiography for all ECPR patients without an obvious alternate non-cardiac cause, independent of age and presenting rhythm.
The cardiology team should be informed at the time of set-up for all ECPRs and be presented with the history and 12-lead ECG post cannulation for assessment.
Imaging
In adults, as well as initial ultrasound, we recommend routine CT imaging is performed in all ECPR cases as soon as practical. If the cause of the cardiac arrest is unclear, or if there are signs for significant internal haemorrhage, CT should take place immediately after the cannulation, otherwise after coronary angiography.
If the cause of the arrest is unclear, consider:
CT brain
CT pulmonary angiography (timing of contrast administration and ECMO blood flow may need to be adjusted to improve image quality)
CT abdomen/pelvis
If the cause for cardiac arrest is identified in the cath lab, consider imaging:
CT brain
If there is evidence falling ECMO blood flows or drainage insufficiency (drainage line chatter), these additional scans may be useful:
CT abdomen/pelvis
CT chest
The rationale for routine CT imaging is to identify causes of the cardiac arrest, early identification of catastrophic brain injuries and solid organ bleeding from prolonged mechanical chest compressions. If patient has drainage insufficiency and an abdominal ultrasound scan is suggestive of free fluid, arterial phase CT scan of abdomen and pelvis should be considered to rule out injury to liver and spleen. This often occurs early and may present with falling ECMO blood flow or drainage insufficiency. Liver and splenic lacerations are commonly amenable to embolization with interventional radiology.
Early echocardiography to assess bi-ventricular and valvular function is helpful in predicting complications of ECMO support. The presence of greater than mild aortic regurgitation dramatically increases the likelihood of catastrophic LV distension and pulmonary oedema.
Weaning off ECMO
Once the etiology of the cardiac arrest is addressed, native cardiac function may return and be sufficient for separating from V-A ECMO support after a period of recovery. There is a lack of consensus on the timing of weaning and decannulation for V-A ECMO, though a period of 3 to 4 days ECMO support is typically seen.10,33 Premature decannulation results in haemodynamic deterioration and either urgent re-cannulation for support or cardiovascular collapse and possible death, whilst prolonging ECMO support unnecessarily may lead to significant morbidity and mortality.
V-A ECMO weaning usually consists of serial reductions in blood flow until a flow of 0.5–1.0 L/min is achieved, with serial echocardiographic and haemodynamic assessment at each stage. Even with this minimal ECMO flow, right ventricular (RV) preload is reduced and, hence, right heart function is not tested under fully unsupported, loading conditions.
Trial off support, either by using an arteriovenous bridge within the circuit, or by clamping, allows temporary separation of ECMO from the patient. However, it requires intermittent circuit clamping and carries a significant risk of circuit thrombosis.
Once a successful weaning study has been completed, flow should be increased to 2 L/min until time of decannulation to minimize the risk of circuit thrombosis.
Some patients will survive ECPR neurologically intact, but fail to recover sufficient myocardial function for successful weaning and decannulation; for these, long term mechanical cardiac support such as ventricular assist device (VAD) and/or cardiac transplantation may be the only option for survival.33,38 We recommend early discussion with a referral center that offers durable VAD and cardiac transplantation for these patients.
For those patients that are not weanable and who are not considered candidates for durable VAD or cardiac transplantation, terminal decannulation with palliation may have to be considered.
Brain death is a common mode of death following ECPR and organ donation may be considered in this circumstance. Declaration of brain death has to follow national guidelines and may need an adjusted protocol with on-going ECMO support. Organ donation is also possible following circulatory death after withdrawal of ECMO support.
Consent
Consent for ECPR therapy from those receiving CCPR is not possible. Further, it is unlikely that an appropriate consent process can take place with next of kin during this time. Whereas ELSO Guidelines for ECMO initiation recommend consent prior to initiation of ECMO, with a clear treatment plan including the rationale for withdrawing care due to lack of recovery or ineligibility for long-term mechanical support, this is usually not possible among those considered for ECPR. Patients are initiated on ECPR based on the presumption that they would want all efforts pursued.
We recommend that institutions offering ECPR develop a guideline for ECPR treatment which includes eligibility, goals of treatment, and a timeline with conditions for stopping ECMO in those without neurological recovery, or in those ineligible for long term mechanical cardiac support due to insufficient cardiac recovery. After ECPR initiation an immediate meeting should take place with the next of kin to explain the basics of ECPR, the institutional guidelines of care for ECPR, and obtain consent for continued treatment.
PROGRAM DEVELOPMENT AND LOGISTICS
Program Development
The provision of ECPR should not be first entertained during CPR, but rather requires careful organizational consideration. An institution wanting to provide ECPR should engage in a multi-disciplinary discernment process of clinicians and administrators to include: a needs assessment, program feasibility and sustainability, expectations, and resource availability.19 Program development should consider human resources, infrastructure already in place and the resources required to maintain competency. Eligibility criteria need to be developed which are clear and reproducible.
Training and Maintenance of Competency
Given the complexities of instituting ECPR and its infrequent usage even in large centers, we advocate for regular system and team-based simulation with simulators that can be used to practice cannulations with ongoing resuscitation measures. This training should be the cornerstone for any ECPR program development to aid in the delivery of consistent and safe care.
Quality Improvement
A quality improvement strategy needs to be developed to monitor process metrics and outcomes. With the added complexities of ECPR initiation, there is risk to the quality of CCPR. Quality of care metrics for both CCPR and ECPR processes should be identified and monitored. Case reviews should be performed for every ECPR case, identifying areas for improvement and reporting institutional goal performance metrics. Goal metrics and outcomes should be monitored and reported.
Governance
We recommend each institution has a robust process for clinical governance of their ECPR program with multi-disciplinary input and review. Demographic, outcome, and complication data should be collected and reported to the institution providing oversight of the service and consideration given to contributing to an international dataset, such as ELSO, to allow for benchmarking and research opportunities.
Additional Complexities for OHCA
Providing ECPR for those with OHCA poses additional logistical obstacles. Integrated prehospital protocols are required to identify appropriate patients early, provide pre-arrival notification to hospital teams and facilitate timely transport to hospital with continued high quality resuscitative efforts. Prehospital teams should identify methods to mitigate the risk to CCPR quality during extrication and transport, and should participate in ECPR simulation exercises.
Regardless of specific details of protocol, only a very small proportion of patients with OHCA will ultimately be considered eligible for ECPR.39 Thus, prehospital resuscitation for the remaining patients with OHCA should ideally not be altered, which poses the risk of worsening CCPR quality in the majority of arrests, which could decrease overall survival. Thus, clear collaboration of hospital and prehospital systems is required in order to identify ECPR-candidates in the prehospital setting to achieve early transport to hospital.
Supplementary Material
Conflicts of interest and source of funding:
Dr. Tonna was supported by a career development award (K23HL141596) from the National Heart, Lung, And Blood Institute (NHLBI) of the National Institutes of Health (NIH). Dr. Tonna received speakers fees for LivaNova related to cardiac arrest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. None of the funding sources were involved in the design or conduct of the study, collection, management, analysis or interpretation of the data, or preparation, review, or approval of the manuscript. None of the authors report any conflicts of interest related to this manuscript.
Footnotes
Sustained ROSC is deemed to have occurred when chest compressions are not required for 20 consecutive minutes following cardiac arrest.
REFERENCES
- 1.Jacobs I, Nadkarni V, Outcomes tITFoCAaCR, et al. : Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: Update and Simplification of the Utstein Templates for Resuscitation Registries: A Statement for Healthcare Professionals From a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa). Circulation 110 (21): 3385–3397, 2004. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 2.Soar J, Maconochie I, Wyckoff MH, et al. : 2019 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations: Summary From the Basic Life Support; Advanced Life Support; Pediatric Life Support; Neonatal Life Support; Education, Implementation, and Teams; and First Aid Task Forces. Circulation 140 (24): e826–e880, 2019. doi: 10.1161/CIR.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 3.Bougouin W, Dumas F, Lamhaut L, et al. : Extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a registry study. Eur Heart J, 2019. doi: 10.1093/eurheartj/ehz753. [DOI] [PubMed] [Google Scholar]
- 4.Debaty G, Babaz V, Durand M, et al. : Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation 112: 1–10, 2017. doi: 10.1016/j.resuscitation.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y-S, Lin J-W, Yu H-Y, et al. : Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. The Lancet 372 (9638): 554–561, 2008. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 6.Lamhaut L, Hutin A, Puymirat E, et al. : A Pre-Hospital Extracorporeal Cardio Pulmonary Resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: An observational study and propensity analysis. Resuscitation 117: 109–117, 2017. doi: 10.1016/j.resuscitation.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Sakamoto T, Morimura N, Nagao K, et al. : Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: A prospective observational study. Resuscitation 85 (6): 762–768, 2014. doi: 10.1016/j.resuscitation.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Stub D, Bernard S, Pellegrino V, et al. : Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation 86: 88–94, 2015. doi: 10.1016/j.resuscitation.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Yannopoulos D, Bartos JA, Raveendran G, et al. : Coronary Artery Disease in Patients With Out-of-Hospital Refractory Ventricular Fibrillation Cardiac Arrest. J Am Coll Cardiol 70 (9): 1109–1117, 2017. doi: 10.1016/j.jacc.2017.06.059. [DOI] [PubMed] [Google Scholar]
- 10.Richardson AS, Schmidt M, Bailey M, Pellegrino VA, Rycus PT, Pilcher DV: ECMO Cardio-Pulmonary Resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12-years. Resuscitation 112: 34–40, 2017. doi: 10.1016/j.resuscitation.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Donnino M, Andersen L, Deakin C, et al. : Extracorporeal Cardiopulmonary Resuscitation (ECPR) for Cardiac Arrest – Adults Consensus on Science with Treatment Recommendations [DRAFT], International Liaison Committee on Resuscitation (ILCOR) Advanced Life Support Task Force, 2018. [Google Scholar]
- 12.Neumar RW, Shuster M, Callaway CW, et al. : Part 1: Executive Summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 132 (18 suppl 2): S315–S367, 2015. doi: 10.1161/CIR.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 13.Yannopoulos D, Bartos JA, Aufderheide TP, et al. : The Evolving Role of the Cardiac Catheterization Laboratory in the Management of Patients With Out-of-Hospital Cardiac Arrest: A Scientific Statement From the American Heart Association. Circulation 139 (12), 2019 doi: 10.1161/CIR.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 14.D’Arrigo S, Cacciola S, Dennis M, et al. : Predictors of favourable outcome after in-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: A systematic review and meta-analysis. Resuscitation 121: 62–70, 2017. doi: 10.1016/j.resuscitation.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Wengenmayer T, Rombach S, Ramshorn F, et al. : Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR). Crit Care 21 (1): 157, 2017. doi: 10.1186/s13054-017-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds JC, Frisch A, Rittenberger JC, Callaway CW: Duration of resuscitation efforts and functional outcome after out-of-hospital cardiac arrest: when should we change to novel therapies? Circulation [Multicenter Study] 128 (23): 2488–94, 2013. doi: 10.1161/CIRCULATIONAHA.113.002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto Y, Funada A, Goto Y: Relationship Between the Duration of Cardiopulmonary Resuscitation and Favorable Neurological Outcomes After Out-of-Hospital Cardiac Arrest: A Prospective, Nationwide, Population-Based Cohort Study. Journal of the American Heart Association 5 (3): e002819, 2016. doi: 10.1161/JAHA.115.002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartos JA, Grunau B, Carlson C, al. e: Improved survival with extracorporeal life support despite progressive metabolic derangement associated with prolonged resuscitation. Journal of the American College of Cardiology, 2019. [Google Scholar]
- 19.Tonna JE, Selzman CH, Mallin MP, et al. : Development and Implementation of a Comprehensive, Multidisciplinary Emergency Department Extracorporeal Membrane Oxygenation Program. Ann Emerg Med 70 (1): 32–40, 2017. doi: 10.1016/j.annemergmed.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Koster RW, Beenen LF, van der Boom EB, et al. : Safety of mechanical chest compression devices AutoPulse and LUCAS in cardiac arrest: a randomized clinical trial for non-inferiority. European Heart Journal 38 (40): 3006–3013, 2017. doi: 10.1093/eurheartj/ehx318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mooney MR, Arom KV, Joyce LD, et al. : Emergency cardiopulmonary bypass support in patients with cardiac arrest. The Journal of Thoracic and Cardiovascular Surgery 101 (3): 450–454, 1991. [PubMed] [Google Scholar]
- 22.Conrad SA, Grier LR, Scott LK, Green R, Jordan M: Percutaneous Cannulation for Extracorporeal Membrane Oxygenation by Intensivists: A Retrospective Single-Institution Case Series*. Critical Care Medicine 43 (5): 1010–1015, 2015. doi: 10.1097/CCM.0000000000000883. [DOI] [PubMed] [Google Scholar]
- 23.Bellezzo JM, Shinar Z, Davis DP, et al. : Emergency physician-initiated extracorporeal cardiopulmonary resuscitation. Resuscitation 83 (8): 966–970, 2012. doi: 10.1016/j.resuscitation.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Fair J, Tonna J, Ockerse P, et al. : Emergency physician-performed transesophageal echocardiography for extracorporeal life support vascular cannula placement. Am J Emerg Med 34 (8): 1637–9, 2016. doi: 10.1016/j.ajem.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 25.Guidelines for the Use of Transesophageal Echocardiography (TEE) in the ED for Cardiac Arrest. Ann Emerg Med 70 (3): 442–445, 2017. doi: 10.1016/j.annemergmed.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 26.Juo Y-Y, Skancke M, Sanaiha Y, Mantha A, Jimenez JC, Benharash P: Efficacy of Distal Perfusion Cannulae in Preventing Limb Ischemia During Extracorporeal Membrane Oxygenation: A Systematic Review and Meta-Analysis: EFFICACY OF DPC IN PREVENTING LIMB ISCHEMIA DURING ECMO. Artificial organs 41 (11): E263–E273, 2017. doi: 10.1111/aor.12942. [DOI] [PubMed] [Google Scholar]
- 27.Kaufeld T, Beckmann E, Ius F, et al. : Risk factors for critical limb ischemia in patients undergoing femoral cannulation for venoarterial extracorporeal membrane oxygenation: Is distal limb perfusion a mandatory approach? Perfusion: 267659119827231, 2019 doi: 10.1177/0267659119827231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Cho YH, Sung K, et al. : Impact of Cannula Size on Clinical Outcomes in Peripheral Venoarterial Extracorporeal Membrane Oxygenation:. ASAIO Journal: 1, 2018 doi: 10.1097/MAT.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 29.Burkhoff D, Sayer G, Doshi D, Uriel N: Hemodynamics of Mechanical Circulatory Support. J Am Coll Cardiol 66 (23): 2663–2674, 2015. doi: 10.1016/j.jacc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Ibrahim AW, Trammell AR, Austin H, et al. : Cerebral Oximetry as a Real-Time Monitoring Tool to Assess Quality of In-Hospital Cardiopulmonary Resuscitation and Post Cardiac Arrest Care. J Am Heart Assoc 4 (8): e001859, 2015. doi: 10.1161/JAHA.115.001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy RA, Bobrow BJ, Spaite DW, Hu C, McDannold R, Vadeboncoeur TF: Association between Prehospital CPR Quality and End-Tidal Carbon Dioxide Levels in Out-of-Hospital Cardiac Arrest. Prehosp Emerg Care 20 (3): 369–77, 2016. doi: 10.3109/10903127.2015.1115929. [DOI] [PubMed] [Google Scholar]
- 32.Sheak KR, Wiebe DJ, Leary M, et al. : Quantitative relationship between end-tidal carbon dioxide and CPR quality during both in-hospital and out-of-hospital cardiac arrest. Resuscitation 89: 149–54, 2015. doi: 10.1016/j.resuscitation.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 33.Bartos JA, Carlson K, Carlson C, et al. : Surviving refractory out-of-hospital ventricular fibrillation cardiac arrest: Critical care and extracorporeal membrane oxygenation management. Resuscitation 132: 47–55, 2018. doi: 10.1016/j.resuscitation.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 34.Abrams D, Combes A, Brodie D: Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 63 (25 Pt A): 2769–78, 2014. doi: 10.1016/j.jacc.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 35.Patel SM, Lipinski J, Al-Kindi SG, et al. : Simultaneous Venoarterial Extracorporeal Membrane Oxygenation and Percutaneous Left Ventricular Decompression Therapy with Impella Is Associated with Improved Outcomes in Refractory Cardiogenic Shock. ASAIO J, 2018. doi: 10.1097/MAT.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 36.Munshi L, Kiss A, Cypel M, Keshavjee S, Ferguson ND, Fan E: Oxygen Thresholds and Mortality During Extracorporeal Life Support in Adult Patients*:. Critical Care Medicine 45 (12): 1997–2005, 2017. doi: 10.1097/CCM.0000000000002643. [DOI] [PubMed] [Google Scholar]
- 37.Schneider AG, Eastwood GM, Bellomo R, et al. : Arterial carbon dioxide tension and outcome in patients admitted to the intensive care unit after cardiac arrest. Resuscitation 84 (7): 927–934, 2013. doi: 10.1016/j.resuscitation.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Chen YS, Chao A, Yu HY, et al. : Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol 41 (2): 197–203, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Grunau B, Scheuermeyer FX, Stub D, et al. : Potential Candidates for a Structured Canadian ECPR Program for Out-of-Hospital Cardiac Arrest. CJEM : Canadian journal of emergency medical care = JCMU : journal canadien de soins médicaux d'urgence: 1–8, 2016. doi: 10.1017/cem.2016.8. [DOI] [PubMed] [Google Scholar]
- 40.Goto T, Morita S, Kitamura T, et al. : Impact of extracorporeal cardiopulmonary resuscitation on outcomes of elderly patients who had out-of-hospital cardiac arrests: a single-centre retrospective analysis. BMJ Open 8 (5): e019811, 2018. doi: 10.1136/bmjopen-2017-019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


