Abstract
OBJECTIVE.
The purposes of this study were to assess correlation of apparent diffusion coefficient (ADC) and normalized ADC (ratio of tumor to nontumor tissue) with the Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) and updated International Society of Urological Pathology (ISUP) categories and to determine how to optimally use ADC metrics for objective assistance in categorizing lesions within PI-RADSv2 guidelines.
MATERIALS AND METHODS.
In this retrospective study, 100 patients (median age, 62 years; range, 44–75 years; prostate-specific antigen level, 7.18 ng/mL; range, 1.70–84.56 ng/mL) underwent 3-T multiparametric MRI of the prostate with an endorectal coil. Mean ADC was extracted from ROIs based on subsequent prostatectomy specimens. Histopathologic analysis revealed 172 lesions (113 peripheral, 59 transition zone). Two radiologists blinded to histopathologic outcome assigned PI-RADSv2 categories. Kendall tau was used to correlate ADC metrics with PI-RADSv2 and ISUP categories. ROC curves were used to assess the utility of ADC metrics in differentiating each reader’s PI-RADSv2 DWI category 4 or 5 assessment in the whole prostate and by zone.
RESULTS.
ADC metrics negatively correlated with ISUP category in the whole prostate (ADC, τ = −0.21, p = 0.0002; normalized ADC, τ = −0.21, p = 0.0001). Moderate negative correlation was found in expert PI-RADSv2 DWI categories (ADC, τ = −0.34; normalized ADC, τ = −0.31; each p < 0.0001) maintained across zones. In the whole prostate, AUCs of ADC and normalized ADC were 87% and 82% for predicting expert PI-RADSv2 DWI category 4 or 5. A derived optimal cutoff ADC less than 1061 and normalized ADC less than 0.65 achieved positive predictive values of 83% and 84% for correct classification of PI-RADSv2 DWI category 4 or 5 by an expert reader. Consistent relations and predictive values were found by an independent novice reader.
CONCLUSION.
ADC and normalized ADC inversely correlate with PI-RADSv2 and ISUP categories and can serve as quantitative metrics to assist with assigning PI-RADSv2 DWI category 4 or 5.
Keywords: apparent diffusion coefficient, International Society of Urological Pathology, multiparametric MRI, PI-RADS, prostate
Multiparametric MRI (mpMRI) of the prostate has played a large role in prostate cancer diagnosis and motivated reporting standardization [1–4]. In the Prostate Imaging Reporting and Data System version 2 (PI-RADSv2), which was introduced in 2015, a zone-based dominant sequence classification system is used to assign MRI-visible lesions a final category ranging from 1 to 5 that represents the likelihood that the lesion is clinically significant prostate cancer. In the peripheral zone (PZ), DWI is used as the dominant sequence; in the transition zone (TZ), T2-weighted MRI is dominant [5]. Although PI-RADSv2 allows critical standardization of mpMRI interpretation, it has limitations. Specifically, its language contains subjective terms, and interpretation remains subject to interreader variability, because all scoring criteria rely on qualitative evaluation, which can be difficult for radiologists to agree on and for novice readers to learn [6–9]. Specifically, studies have shown consistent challenges in differentiating PI-RADSv2 category 3 from PI-RADSv2 category 4, specifically in the PZ [9–14]. Quantitative parameters could improve the reproducibility of PI-RADSv2.
The apparent diffusion coefficient (ADC) derived from DWI is an objective measure of suspicious MRI lesions. Previous studies have shown that ADC values correlate inversely with Gleason score and can elucidate cellular proliferative activity [15–19]. In clinical imaging, ADC values are readily available, and the ADC ratio of tumor to normal tissue may further improve the reliability of ADC measurements by normalizing values to background tissue [20]. However, ADC values have not been incorporated into PI-RADSv2, largely over concern that different MRI units may produce different ADC values. As a result, guidance regarding DWI in PI-RADSv2 is subjective.
The International Society of Urological Pathology (ISUP) scoring system for prostate cancer is based on the Gleason scoring system [21]. One major contribution of the ISUP system is differentiating Gleason 3 + 4 (ISUP 2) from Gleason 4 + 3 (ISUP 3) tumors, which were formerly categorized collectively as Gleason 7 tumors. ADC values have not yet been correlated with this new pathologic categorization.
Although ADC metrics have been previously correlated with Gleason score, their correlation with the new PI-RADSv2 and ISUP categorizations remains unknown. These relations would subsequently support the addition of ADC as a quantitative parameter for categorization, and this remains to be explored. The goals of this study were to assess the correlation of quantitative ADC measurements with PI-RADSv2 and ISUP categories and to determine how to optimally use ADC metrics for objective assistance in categorizing lesions within existing PI-RADSv2 guidelines.
Materials and Methods
This single-institution retrospective study received institutional review board approval. Written informed consent was obtained from all patients. Between April 2012 and June 2015, 179 consecutively registered patients underwent 3-T mpMRI of the prostate with an endorectal coil followed by radical prostatectomy (median time between MRI and radical prostatectomy, 4 months; range, 0–20 months). Among the 179, 105 patients had the histopathologic features of their prostate cancer mapped on whole-mount prostate specimens and assessed by ISUP categories. Patients were excluded because of missing pathologic data (n = 74), hip prosthesis artifacts on MR images (n = 3), or inability to detect all pathologically proven lesions with MRI (n = 2) as assessed by a highly experienced genitourinary radiologist. A total of 100 patients were included in the final study. The final study population consisted of 100 patients with 172 MRI-visible lesions confirmed as tumor-positive by whole-mount histopathologic analysis. Patient and lesion characteristics are reported in Table 1. Most of the of lesions (113/172 [66%]) were in the PZ.
TABLE 1:
Baseline Characteristics of Patients and Lesions
| Characteristic | Result |
|---|---|
| No. of patients | 100 |
| Age (y) | 62 (44–75) |
| Prostate-specific antigen level (ng/mL) | 7.18 (1.70–84.56) |
| Time between MRI and robot-assisted radical prostatectomy (mo) | 4 (0–20) |
| International Society of Urological Pathology category | |
| Peripheral zone lesions | |
| 1 | 12 |
| 2 | 58 |
| 3 | 14 |
| 4 | 24 |
| 5 | 5 |
| Subtotal | 113 |
| Transition zone lesions | |
| 1 | 4 |
| 2 | 31 |
| 3 | 2 |
| 4 | 21 |
| 5 | 1 |
| Subtotal | 59 |
| All lesions | |
| 1 | 16 |
| 2 | 89 |
| 3 | 16 |
| 4 | 45 |
| 5 | 6 |
| Total | 172 |
Note—Values are medians with range in parentheses or number.
MRI Technique
MR images were acquired with a 3-T system (Achieva 3 T-TX, Philips Healthcare) and the combination of the anterior half of a 32-channel cardiac sensitivity-encoding coil (Invivo, Philips Healthcare) and an endorectal coil (BPX-30, Medrad) filled with 45 mL of perfluorocarbon-based fluid (Fluorinert, 3M). The mpMRI protocol included T2-weighted imaging (axial, coronal, sagittal), DWI (b = 2000 s/mm2), and dynamic contrast-enhanced MRI. DW images consisted of a high-b-value (2000 s/mm2) sequence and ADC map (from five evenly spaced b values of 0–750 s/mm2), which was the main sequence used for analysis. ADC maps were automatically calculated by the MRI unit software by means of a mono-exponential decay model fitted to data from images obtained at the five evenly spaced b values. The following model equation was used: S(b) = S(0) × exp(−b × ADC), where b is the gradient created by any one of the five b values used. The full mpMRI acquisition parameters are shown in Table 2.
TABLE 2:
Multiparametric MRI Acquisition Parameters at 3 T
| Parameter | T2-Weighted | DWIa | High-b-Value DWIb | DCE-MRIc |
|---|---|---|---|---|
| FOV (mm) | 140 × 140 | 140 × 140 | 140 × 140 | 262 × 262 |
| Acquisition matrix | 304 × 234 | 112 × 109 | 76 × 78 | 188 × 96 |
| TR (ms) | 4434 | 4986 | 6987 | 3.7 |
| TE (ms) | 120 | 54 | 52 | 2.3 |
| Flip angle (°) | 90 | 90 | 90 | 8.5 |
| Section thickness (mm), no gap | 3 | 3 | 3 | 3 |
| Image reconstruction matrix | 512 × 512 | 256 × 256 | 256 × 256 | 256 × 256 |
| Reconstruction voxel imaging resolution (mm/pixel) | 0.27 × 0.27 × 3.00 | 0.55 × 0.55 × 2.73 | 0.55 × 0.55 × 2.73 | 1.02 × 1.02 × 3.00 |
| Time for acquisition (min:s) | 2:48 | 4:54 | 3:50 | 5:16 |
Note—Multiparametric MRI parameters are given for prostate imaging at 3 T with use of an endorectal coil. DCE = dynamic contrast-enhanced.
For apparent diffusion coefficient map calculation. Five evenly spaced b values (0–750 s/mm2) were used.
b = 2000 s/mm2.
Images obtained before, during, and after administration of single dose of gadopentetate dimeglumine 0.1 mmol/kg at 3 mL/s. Acquisitions performed at 5.6-second intervals.
Whole-Mount Histopathologic Correlation
Soon after MRI, all patients underwent robotic-assisted radical prostatectomy. The median time between MRI and radical prostatectomy was 4 months (range, 0–20 months). Wholemount prostate specimens were processed with patient-specific MRI-based 3D-printed molds to allow optimal imaging-pathologic correlation [22]. Specimens were analyzed in 6-mm-thick sections and mapped by one highly experienced genitourinary pathologist. Mapping included visual delineation of lesion locations, note of extraprostatic extension and seminal vesical invasion, and provision of lesion-specific primary and secondary Gleason scores, which were converted to ISUP category based on the new guidelines [21]. Once histopathologic categorization was determined, the lesions were classified as clinically significant (Gleason score ≥ 7, including 3 + 4 with prominent but not predominant Gleason 4 component; volume ≥ 0.5 cm3; extraprostatic extension; or a combination of these features) versus lower grade according to PI-RADSv2 guidelines [5, 23, 24]. A postdoctoral research fellow focused on prostate mpMRI performed MRI-pathologic correlation using visible prostate landmarks in addition to lesion morphologic features and relative lesion location.
Apparent Diffusion Coefficient Measurements
All ADC measurements were performed manually by a postdoctoral research fellow focused on prostate mpMRI and confirmed by a highly experienced genitourinary radiologist (> 8000 scans read in past 10 years) blinded to the histopathologic results. Measurements were performed within the image-viewing software (Vue PACS, Carestream Health). ROIs were drawn on each ADC map outlining mpMRI-visible lesions with the histopathologic examination as ground truth. A second ROI was drawn on the same MRI slice contralateral to an identified lesion to include nontumor tissue within the same prostate zone in the same relative location (0.4 cm2 or smaller if less nontumor tissue was present) (Fig. 1). Of note, nontumor tissue included prostatic hyperplasia if this was present on the contralateral side in the same zone and relative location; a smaller area was used only if the location within the zone was not large enough to accommodate the full 0.4 cm2. The mean ADC of each ROI was calculated within the image-viewing software. Normalized ADC values were calculated by dividing the absolute ADC value of tumor tissue (ADC) by the mean ADC value of contralateral nontumor prostate tissue (ADCN). The following equation was used: normalized ADC = ADC / ADCN, consistent with the method in previous studies validating normalized ADC use for improving reproducibility and decreasing variability in measurement [20, 25, 26]. Mean ADC, normalized ADC, ADC for nontumor prostate, and area of tumor ROI were reported for each lesion.
Fig. 1–
65-year-old biopsy-naive man with prostate-specific antigen value of 5.9 ng/mL who underwent radical prostatectomy after multiparametric MRI and MRI–transrectal ultrasound fusion-guided biopsy. Subsequent histopathologic mapping revealed prostatic adenocarcinoma with International Society of Urological Pathology score of 3. Method of determining ROIs for patient’s right-sided apical peripheral zone cancer is shown. Photomicrograph and MR image show whole-mount histopathologic specimen used as ground truth for correlation with MRI-visible lesions. ROI B17 encompasses lesion visualized at pathologic examination. This was transferred to most likely MRI lesion. Mean intensity value (average [AV], 1425.6 μm2/s) was used as apparent diffusion coefficient (ADC) measurement of tumor tissue. Second ROI (B16) was drawn contralaterally to encompass 0.4 cm2 of normal tissue. Mean intensity of this ROI (AV, 1731.3 μm2/s) was used as nontumor ADC, eventually incorporated in calculation of normalized ADC value. For this lesion, normalized ADC = 1425.6 / 1731.3 = 0.82. AR = area.
Prostate Imaging Reporting and Data System Version 2 Score Assignment
MRI-visible lesions were validated in histopathologic analysis, and overall PI-RADSv2 categories were assigned for each lesion by a highly experienced prostate-focused genitourinary radiologist blinded to ISUP scores (> 8000 scans read in past 10 years) [5]. PI-RADSv2 categorization by the first reader was performed prior to histopathologic correlation. A second radiologist with novice-level experience in prostate MRI (< 200 scans read in the past 1 year with < 6 months of experience) also independently assigned PI-RADSv2 categories to each lesion, blinded to results of the first reader and to any pathologic evaluation.
Statistical Analysis
Differences in lesion and nonlesion DWI characteristics were evaluated for mean ADC and mean normalized ADC measurements from each tissue type using a linear mixed-effects model to account for interlesion correlation on the patient level. Similarly, a linear mixed-effects model was used to evaluate differences in DWI characteristics across PI-RADSv2 categories from each reader and clinical ISUP risk categories. Correlation between DWI characteristics with PI-RADSv2 categories from each reader and ISUP categories was performed by Kendall tau estimation accounting for clustered data [27]. All analysis was completed for the whole prostate, that is, regardless of location, and for zone-specific subsets in the PZ and the TZ. Association of ADC metrics (mean ADC and normalized ADC) with PI-RADSv2 classification from each reader was evaluated for both PI-RADSv2 DWI scores and PI-RADSv2 overall scores. All tests were two-sided, and p < 0.05 was considered statistically significant.
ROC were used to assess the utility of quantitative metrics (mean ADC, normalized ADC, and ROI area) for discriminating between lesions categorized as PI-RADSv2 1–3 (low-risk, equivocal imaging findings) versus PI-RADSv2 4 and 5 (high-risk based on imaging findings), evaluated for both PI-RADSv2 DWI scores and PI-RADSv2 overall scores [5]. Bivariate logistic regression models with generalized estimating equations to account for interlesion correlation on the patient level were developed by use of combinations of uncorrelated imaging characteristics to evaluate the combined association for discriminating PI-RADSv2 categories 1–3 from categories 4 and 5.
Two combinations of metrics were considered: ADC + ROI area and normalized ADC + ROI area. AUCs were reported for both univariate and bivariate analyses. An optimal cutpoint on the ROC curve was defined as the point at which the sum of sensitivity and specificity was maximized. Predictive performance was measured by sensitivity, specificity, and positive predictive value (PPV) for accurate classification of PI-RADSv2 categories 4 and 5. Standard error of the prediction performance metrics and 95% CIs were calculated from 2000 bootstrap samples by randomly sampling patients with replacement. All analysis was completed for categoric assignments from each reader separately.
Results
Quantitative Lesion Characteristics
Comparison between tumor and nontumor tissue in the PZ, TZ, and whole prostate revealed significantly lower ADC values for tumor in all zones (p < 0.0001) (Table 3). Among 172 lesions, 119 (69%) were clinically significant. Using PI-RADSv2 DWI category 4 or 5, the expert reader detected 64% (48/75) of clinically significant lesions in the PZ and 84% (37/44) in the TZ. The novice reader detected 80% (60/75) of clinically significant lesions in the PZ and 89% (39/44) in the TZ. Using a PI-RADSv2 overall score of 4 or 5, the expert reader detected 84% (63/75) of clinically significant lesions in the PZ and 80% (35/44) in the TZ. The novice reader detected 84% (63/75) of lesions in the PZ and 93% (41/44) in the TZ.
TABLE 3:
Mean and Normalized Apparent Diffusion Coefficient (ADCs) Values by Prostate Zone
| Region | Mean Raw ADC (μm2/s) |
Mean Normalized ADC |
||||
|---|---|---|---|---|---|---|
| Whole Gland | Peripheral Zone | Transition Zone | Whole Gland | Peripheral Zone | Transition Zone | |
| Tumor | 1004.0 ± 201.9 | 1021.0 ± 213.1 | 971.5 ± 175.8 | 0.636 ± 0.137 | 0.637 ± 0.147 | 0.635 ± 0.118 |
| Normal tissue | 1598.6 ± 210.7 | 1626.9 ± 214.1 | 1544.3 ± 194.3 | |||
| p | < 0.0001 | < 0.0001 | < 0.0001 | |||
Note—Mean raw ADC values measured from tumor ROIs and nontumor ROIs are significantly different according to ANOVA. Normalized ADC calculated as ADC / ADCN, where ADCN is the mean raw ADC for normal tissue.
Apparent Diffusion Coefficient Association With International Society of Urological Pathology and Prostate Imaging Reporting and Data System Version 2 Categories
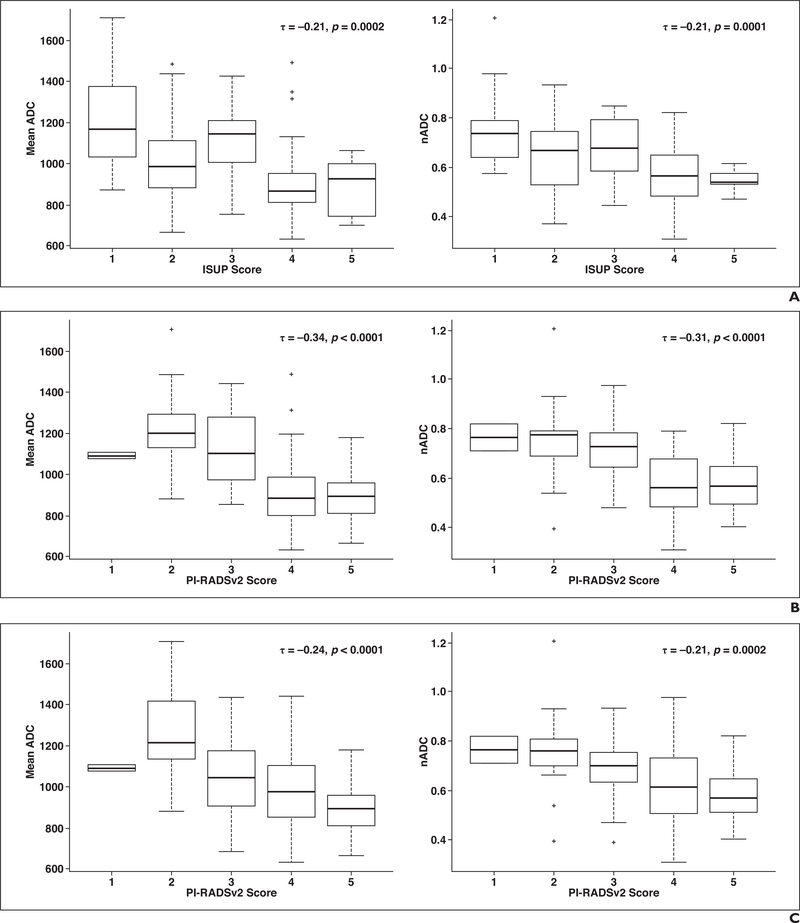
Inverse relations were found between ADC metrics (ADC and normalized ADC) with ISUP (Fig. 2) and both PI-RADSv2 DWI and overall categories for the expert reader (Fig. 2) and the novice reader (Fig. S1, which can be viewed in the AJR electronic supplement to this article, available at www.ajronline.org). For the whole prostate, a modest negative correlation was found between ADC (τ = −0.21, p = 0.0002) and normalized ADC values (τ = −0.21, p = 0.0001) and ISUP category. This was strengthened in the PZ for ADC (τ = −0.30, p = 0.0001) and normalized ADC (τ = −0.27, p = 0.0001). No significant correlation was found between ADC metrics and ISUP category in the TZ.
Fig. 2–
Correlation between apparent diffusion coefficient (ADC) metrics in whole prostate and results with scoring systems for expert reader. Lines in middle of boxes represent median, and boxes represent interquartile range (IQR). Whiskers are defined on basis of IQR and are calculated as 1.5 × IQR of lower and upper limits of IQR: upper whisker = third quartile (75th percentile) + 1.5 × IQR; lower whisker = first quartile (25th percentile) − 1.5 × IQR. τ = Kendall tau correlation coefficient; nADC = normalized apparent diffusion coefficient; + = outliers, defined as data points outside of whiskers.
A, Plot shows correlation between International Society of Urological Pathology (ISUP) score and ADC (left) and normalized ADC (right).
B, Plot shows correlation between Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) DWI category and ADC (left) and normalized ADC (right).
C, Plot shows correlation between PI-RADSv2 overall score and ADC (left) and normalized ADC (right).
ADC metric correlation with PI-RADSv2 DWI categories assigned by the expert was modest for all lesions (ADC, τ = −0.34; normalized ADC, τ = −0.31; p < 0.0001), dominated by a moderate relationship in the PZ (ADC, τ = −0.41; normalized ADC, τ = −0.38; p < 0.0001), and modest in the TZ (ADC, τ = −0.24; normalized ADC, τ = −0.25; p = 0.01). These correlations were consistent though slightly decreased for the novice reader’s PI-RADSv2 DWI categorization (τ = −0.27 to −0.33; all p < 0.0001). Moderate yet statistically significant negative correlation between ADC and normalized ADC characteristics with the expert’s overall PI-RADSv2 categories was observed in the whole prostate and the PZ (τ = −0.21 to −0.29; all p < 0.001) with weak but insignificant inverse correlation in the TZ. Modest correlation was observed for the correlation between the novice reader’s PI-RADSv2 overall categorization and DWI characteristics in the whole prostate and the PZ (τ = −0.17 to −0.21; p = 0.05), and TZ association was found to be similarly insignificant.
Apparent Diffusion Coefficient Metrics in Prostate Imaging Reporting and Data System Version 2 DWI Category Assessment
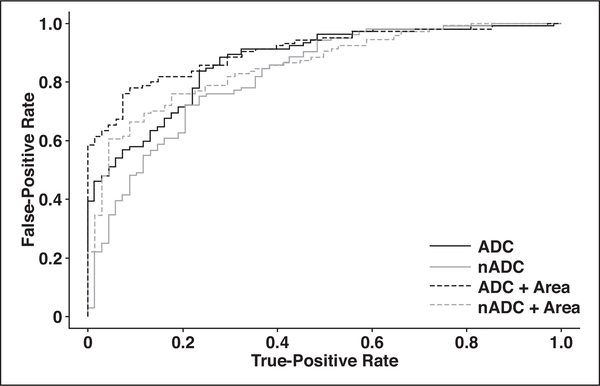
Both mean ADC and normalized ADC had strong utility for differentiating the expert’s assignment of PI-RADSv2 DWI category 4 or 5. They had AUCs of 87% and 82%, respectively, for the expert reader (Table 4) and 89% and 85% for the novice reader (Tables S2 and S3, which can be viewed in the AJR electronic supplement to this article, available at www.ajronline.org). Optimal cutoff values from ROC analysis showed high PPVs: ADC < 1061 μm2/s had an 83% PPV, and normalized ADC < 0.65 had an 84% PPV. Combining ADC metrics with ROI area improved predictability in classifying lesions to the expert’s PI-RADSv2 DWI category 4 or 5 (Fig. 3), achieving 93% PPV in the combined model of ADC and area (Table 4). Optimal cutoffs from ROC analysis for discriminating novice reader PI-RADSv2 DWI category 4 or 5 were highly concordant with results modeling the expert reader (Table S3). An ADC cutoff of 1056 μm2/s or less achieved 98% PPV, and normalized ADC ≤ 0.69 achieved 95% PPV for predicting the novice reader’s categorizations.
TABLE 4:
Univariate and Multivariate Analysis for Prostate Imaging Reporting and Data System Version 2 DWI Category 4 or 5 Classification by the Expert Reader
| Metric | ROC Analysis AUC | Prediction Performance for Score Classification 1–3 Versus 4 and 5 |
|||
|---|---|---|---|---|---|
| Optimal Cutoffa | Sensitivity at Cutoff | Specificity at Cutoff | PPV at Cutoff | ||
| Whole prostate | |||||
| Mean ADC | 0.87 (0.82–0.92) | < 1061 (1074–950) | 0.88 (0.73–0.95) | 0.72 (0.64–0.89) | 0.83 (0.77–0.93) |
| Normalized ADC | 0.82 (0.76–0.88) | < 0.65 (0.74–0.58) | 0.72 (0.62–0.96) | 0.79 (0.53–0.90) | 0.84 (0.74–0.92) |
| Area | 0.65 (0.56–0.73) | ≥ 0.54 (0.20–0.90) | 0.37 (0.23–0.82) | 0.87 (0.44–0.97) | 0.81 (0.66–0.95) |
| Mean ADC and area | 0.91 (0.87–0.94) | ≥ 0.72 (0.50–0.80) | 0.78 (0.68–0.91) | 0.91 (0.77–0.98) | 0.93 (0.85–0.99) |
| Normalized ADC and area | 0.86 (0.81–0.90) | ≥ 0.63 (0.48–0.81) | 0.76 (0.60–0.87) | 0.82 (0.72–0.98) | 0.87 (0.80–0.98) |
| Peripheral zone | |||||
| Mean ADC | 0.87 (0.81–0.92) | ≤ 1045 (1074–910) | 0.87 (0.61–0.97) | 0.71 (0.61–0.96) | 0.78 (0.70–0.95) |
| Normalized ADC | 0.82 (0.75–0.88) | ≤ 0.65 (0.72–0.56) | 0.77 (0.59–0.93) | 0.75 (0.59–0.94) | 0.78 (0.68–0.93) |
| Area | 0.60 (0.50–0.70) | ≥ 0.70 (0.20–1.10) | 0.23 (0.13–0.81) | 0.94 (0.39–1.00) | 0.82 (0.55–1.00) |
| Mean ADC and area | 0.91 (0.83–0.94) | ≥ 0.71 (0.39–0.80) | 0.69 (0.61–0.92) | 0.94 (0.73–1.00) | 0.93 (0.77–1.00) |
| Normalized ADC and area | 0.84 (0.77–0.90) | ≥ 0.69 (0.40–0.77) | 0.67 (0.59–0.90) | 0.90 (0.69–0.98) | 0.89 (0.73–0.97) |
| Transition zone | |||||
| Mean ADC | 0.89 (0.80–0.97) | ≤ 1024 (1068–896) | 0.86 (0.57–0.98) | 0.81 (0.67–1.00) | 0.93 (0.86–1.00) |
| Normalized ADC | 0.86 (0.74–0.96) | ≤ 0.64 (0.76–0.57) | 0.70 (0.56–1.00) | 0.88 (0.52–1.00) | 0.94 (0.81–1.00) |
| Area | 0.66 (0.50–0.80) | ≥ 0.90 (0.30–1.20) | 0.40 (0.26–0.91) | 0.94 (0.43–1.00) | 0.94 (0.78–1.00) |
| Mean ADC and area | 0.93 (0.86–0.99) | ≥ 0.77 (0.55–0.91) | 0.84 (0.68–0.98) | 0.94 (0.78–1.00) | 0.97 (0.91–1.00) |
| Normalized ADC and area | 0.89 (0.80–0.97) | ≥ 0.76 (0.37–0.92) | 0.79 (0.57–1.00) | 0.81 (0.67–1.00) | 0.92 (0.86–1.00) |
Note—Values in parentheses are 95% CI. ADC = apparent diffusion coefficient. Mean ADC is measured in square micrometers per second.
With use of the optimal cutoffs, the sensitivity, specificity, and positive predictive value (PPV) shown can be achieved. Univariate cutoffs refer to actual value of the variable; bivariate cutoffs refer to risk calculated with the given logistic regression model.
Fig. 3–
ROC curves for classifying Prostate Imaging Reporting and Data System version 2 DWI category 4 or 5. ADC = apparent diffusion coefficient, nADC = normalized ADC.
ADC metrics showed moderate utility in differentiating PI-RADSv2 overall category 1–3 from PI-RADSv2 overall category 4 or 5 scored by both readers. ADC and normalized ADC both yielded an AUC of 72% for the expert reader and 77% and 72%, respectively, for the novice reader. The multivariate model combining ADC with ROI area improved the AUC to 79–80% for both readers (Tables S2 and S3).
In a zone-based analysis, the utility of ADC metrics in differentiating expert-scored PI-RADSv2 DWI category 1–3 from category 4 or 5 in the PZ was nearly identical to results for the whole prostate. The AUC and optimal cutoffs were the same, but the PPV was lower (78%) for both metrics (Table 4). Combining ADC with area achieved the highest discriminative ability in the PZ. The AUCs were 91% for the expert reader and 80% for the novice reader, and the optimal cutoffs achieved PPVs of 93% for the expert reader and 100% for the novice reader. In the TZ, ADC metrics showed higher discrimination of PI-RADSv2 DWI category 4 or 5 compared with whole prostate and PZ (Table 4), with AUCs of 89% and 86% for ADC and normalized ADC for the expert reader. Prediction was improved when ADC metrics were combined with area (expert AUC, 93%; novice AUC, 89%). These results similarly reflected prediction of PI-RADSv2 overall scores by the novice reader in both the PZ and the TZ (Table S3).
Discussion
In the current method of mpMRI evaluation of localized prostate cancer, PI-RADSv2 recommendations for DWI assessment mainly rely on the radiologist’s subjective interpretation of qualitative lesion intensity characteristics. The ADC map and high-b-value images are characterized with terms such as “moderately hypointense” and “markedly hyperintense” [5, 9]. PI-RADSv2 documentation provides only a general recommendation that ADC values less than 750–900 μm2/s suggest malignant tissue in the PZ, though this recommendation is based only on expert consensus; no guidelines are given for implementation [5, 28].
In the current study, we established an inverse relation between ADC values and PI-RADSv2 categories and reaffirmed the inverse correlation between ADC values and histopathologic categorization. With mean ADC less than 1061 μm2/s and normalized ADC less than 0.65, high PPVs for assigning expert-based PI-RADSv2 DWI category 4 or 5 in the whole prostate and PZ were achieved. These results successfully extended to classification of scores assigned by a novice radiologist. As we look ahead to improved versions of this standardization system, a more quantitative measure of DWI could assist radiologists in assigning PI-RADS categories more reliably in the future.
We observed a significant negative correlation between ADC values and ISUP categories in both the whole prostate and the PZ. The major motivation behind the new ISUP categorization is to more accurately convey prognosis of the various grades of prostate cancer [29]. For example, a change brought about by the new ISUP classification is that Gleason 3 + 4 (corresponding to ISUP 2) and Gleason 4 + 3 (corresponding to ISUP 3) are not clustered together as Gleason 7 but are categorized separately [21]. The correlation that we observed between ADC and normalized ADC with ISUP categories was modest in the whole gland but was stronger in the PZ. This inverse relation with histopathologic grading is consistent with results of multiple previous studies performed with traditional Gleason score categories. Those studies showed moderately negative correlation in the PZ (ρ = −0.39 to −0.60; p < 0.05) that was stronger than the negative correlation seen in the whole gland [15, 17–19, 30]. The weaker correlation in the whole gland could possibly be attributed to regions of increased cellularity in the TZ due to benign prostatic hypertrophy [31].
Correlation of ADC metrics and PI-RADSv2 classification has received less attention in the literature than has ISUP correlation, but the guidelines suggest increasing PI-RADSv2 category with more distinct ADC map hypointensity. This reflects an expected decreasing trend in ADC value. Tissues with higher cellular density, such as rapidly dividing tumors, are expected to have lower ADC values and lower signal intensity on images. Our moderate negative correlations between ADC and normalized ADC for PI-RADSv2 DWI categories in the whole gland maintained across readers with different experience levels are consistent with this expectation. This result was consistent across prostatic zones and maintained, albeit more weakly, in PI-RADSv2 overall category assignments from both readers. Both the expert’s and the novice’s PI-RADSv2 DWI and overall categories in the current study captured most of the aggressive lesions, establishing a possible positive relation between PI-RADSv2 category and clinically significant cancer in this population. This relation, in addition to the correlations, suggests that ADC metrics may provide quantitative information to supplement PI-RADSv2 in detection and categorization of clinically significant cancers.
Currently, PI-RADSv2 suffers from a high discordance rate for category assignment. Agreement between highly experienced readers has been reported to be as low as 51% and between moderately experienced readers as low as 53% [12, 28]. Use of a quantitative parameter to guide mpMRI interpretations may help to improve consistency in category assignment. The goal of this study was not to assess reader agreement in PI-RADSv2 scoring but to assess the stability of quantitative metric correlation with scoring by readers of different experiences. Both ADC and normalized ADC were found to improve the PPV of PI-RADSv2 DWI category 4 or 5; optimal cutoffs achieved PPVs of 83–97% in the whole gland and across zones for both readers.
Mean ADC and normalized ADC have previously been proposed as quantitative metrics for objective classification of prostate cancer lesions. Our results show broad agreement with results of a study by Park et al. [32] on the use of ADC ratio (normalized ADC) for interpretation of DWI in prostate cancer. Those authors reported that normalized ADC < 0.77 achieved 92–100% PPV for differentiating PI-RADSv2 DWI categories 1–3 from categories 4 and 5. ROI-derived mean ADC has shown consistent quantitative accuracy in test-retest settings [33]. We consistently found that use of normalized ADC did not compromise predictive ability, which is clinically useful because normalized ADC has been found to be more reproducible across patients and MRI systems [20, 25, 26, 34]. Each of these metrics was enhanced when combined with lesion area to predict PI-RADSv2 DWI category 4 or 5. We additionally observed that ADC metrics could be used to differentiate PI-RADSv2 overall category 4 or 5 with 72–79% AUC. Because DWI sequences only represent one aspect of PI-RADSv2 overall scoring with mpMRI, this relation substantiates the strength of DWI within the overall scoring paradigm.
Interobserver reproducibility of PI-RADSv2 interpretation has been reported to differ across zones, there being poorer agreement on PI-RADSv2 classification of TZ versus PZ lesions [12, 28]. Interestingly, ADC metrics improved DWI classification performance in the TZ, both alone and when combined with lesion area (PPV, 92–97%). In PI-RADSv2 recommendations, DWI is generally not used for lesion identification in the TZ because of confounding ADC hypointensities from prostatic hyperplasia. Instead, T2-weighted imaging serves as the dominant scoring sequence, and the score can be upgraded only from particularly distinct DWI findings. Results of a 2017 study by Rosenkrantz et al. [14], however, suggested that the inclusion of DWI in the categorization of TZ tumors may help differentiate PI-RADSv2 category 3 from PI-RADSv2 category 4 in the TZ. An objective measure, such as ADC or normalized ADC in this setting, could improve the reproducibility of PI-RADSv2 scoring.
Limitations
Our study had several limitations. First, all lesions were tumor positive, and therefore our data did not include false-positive MRI lesions. Thus, specificity for tumor detection was not evaluated in this study [22]. The correlations and model presented achieved reasonably high detection of clinically significant disease, presenting a best-case scenario for PI-RADSv2 reading. The application of our models and analyses can be further tested for clinical significance in a more general population that includes true- and false-positive MRI lesions.
Second, PI-RADSv2 scoring is subject to interreader variability, which we did not address. We found, however, that ADC metrics correlated well with PI-RADSv2 DWI and overall categorization in two independent interpretations.
Third, although ADC metrics have strong predictive utility in the TZ for discriminating PI-RADSv2 DWI category, we found a poor ISUP correlation with ADC metrics in the TZ because of the fairly small number of lesions. Therefore, although correlations between ADC metrics and ISUP and between ADC metrics and PI-RADSv2 were seen in the whole prostate and PZ, the correlation was less convincing in the TZ. However, objective assistance of PI-RADSv2 categorization was still observed in the TZ with the objective of improving reader performance and concordance.
Finally, all of our measurements were performed manually, and ROI areas and average intensities might have been subjective. Future applications of this work would benefit from prospective multicenter validation for reproducibility across readers and sites.
Conclusion
ADC metrics are inversely related to ISUP and PI-RADSv2 categories. Both alone and combined with ROI area, ADC metrics show reasonable utility for assisting PI-RADSv2 DWI classification with possible future application in improving reader agreement and clinically significant tumor detection when PI-RADSv2 is used.
Supplementary Material
Acknowledgments
Supported in whole or in part by federal funds from the National Cancer Institute, National Institutes of Health (contract HHSN261200800001E). The National Institutes of Health (NIH) Medical Research Scholars Program is a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the National Institutes of Health from the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, and alumni of student research programs and other individual supporters via contributions to the Foundation for the National Institutes of Health.
Footnotes
B. J. Wood has a fancial interest in Philips Healthcare Invivo, and P. A. Pinto has a financial interest in Philips Healthcare.
WEB
This is a web exclusive article.
Supplemental Data
Available online at www.ajronline.org.
References
- 1.Sala E, Akin O, Moskowitz CS, et al. Endorectal MR imaging in the evaluation of seminal vesicle invasion: diagnostic accuracy and multivariate feature analysis. Radiology 2006; 238:929–937 [DOI] [PubMed] [Google Scholar]
- 2.Rørvik J, Halvorsen OJ, Albrektsen G, Ersland L, Daehlin L, Haukaas S. MRI with an endorectal coil for staging of clinically localised prostate cancer prior to radical prostatectomy. Eur Radiol 1999; 9:29–34 [DOI] [PubMed] [Google Scholar]
- 3.Lawrence EM, Gallagher FA, Barrett T, et al. Preoperative 3-T diffusion-weighted MRI for the qualitative and quantitative assessment of extracapsular extension in patients with intermediate- or high-risk prostate cancer. AJR 2014; 203:[web] W280–W286 [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313:390–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging Reporting and Data System: 2015, version 2. Eur Urol 2016; 69:16–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruprecht O, Weisser P, Bodelle B, Ackermann H, Vogl TJ. MRI of the prostate: interobserver agreement compared with histopathologic outcome after radical prostatectomy. Eur J Radiol 2012; 81:456–460 [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Reyes K, Passoni NM, Palmeri ML, et al. Detection of prostate cancer with multiparametric MRI (mpMRI): effect of dedicated reader education on accuracy and confidence of index and anterior cancer diagnosis. Abdom Imaging 2015; 40:134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenkrantz AB, Ayoola A, Hoffman D, et al. The learning curve in prostate MRI interpretation: self-directed learning versus continual reader feedback. AJR 2017; 208:[web]W92–W100 [DOI] [PubMed] [Google Scholar]
- 9.Rosenkrantz AB, Oto A, Turkbey B, Westphalen AC. Prostate Imaging Reporting and Data System (PI-RADS), version 2: a critical look. AJR 2016; 206:1179–1183 [DOI] [PubMed] [Google Scholar]
- 10.Woo S, Kim SY, Lee J, Kim SH, Cho JY. PI-RADS version 2 for prediction of pathological downgrading after radical prostatectomy: a preliminary study in patients with biopsy-proven Gleason score 7 (3+4) prostate cancer. Eur Radiol 2016; 26:3580–3587 [DOI] [PubMed] [Google Scholar]
- 11.Mehralivand S, Bednarova S, Shih JH, et al. Prospective evaluation of Prostate Imaging Reporting and Data System™ version 2 using the International Society of Urological Pathology prostate cancer grade group system. J Urol 2017; 198:583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greer MD, Brown AM, Shih JH, et al. Accuracy and agreement of PIRADSv2 for prostate cancer mpMRI: a multireader study. J Magn Reson Imaging 2017; 45:579–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mertan FV, Berman R, Szajek K, Pinto PA, Choyke PL, Turkbey B. Evaluating the role of mpMRI in prostate cancer assessment. Expert Rev Med Devices 2016; 13:129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenkrantz AB, Babb JS, Taneja SS, Ream JM. Proposed adjustments to PI-RADS version 2 decision rules: impact on prostate cancer detection. Radiology 2017; 283:119–129 [DOI] [PubMed] [Google Scholar]
- 15.Wang XZ, Wang B, Gao ZQ, et al. Diffusion-weighted imaging of prostate cancer: correlation between apparent diffusion coefficient values and tumor proliferation. J Magn Reson Imaging 2009; 29:1360–1366 [DOI] [PubMed] [Google Scholar]
- 16.Zelhof B, Pickles M, Liney G, et al. Correlation of diffusion-weighted magnetic resonance data with cellularity in prostate cancer. BJU Int 2009; 103:883–888 [DOI] [PubMed] [Google Scholar]
- 17.Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology 2011; 258:488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamada T, Sone T, Jo Y, et al. Apparent diffusion coefficient values in peripheral and transition zones of the prostate: comparison between normal and malignant prostatic tissues and correlation with histologic grade. J Magn Reson Imaging 2008; 28:720–726 [DOI] [PubMed] [Google Scholar]
- 19.Hambrock T, Somford DM, Huisman HJ, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 2011; 259:453–461 [DOI] [PubMed] [Google Scholar]
- 20.Barrett T, Priest AN, Lawrence EM, et al. Ratio of tumor to normal prostate tissue apparent diffusion coefficient as a method for quantifying DWI of the prostate. AJR 2015; 205:[web]W585–W593 [DOI] [PubMed] [Google Scholar]
- 21.Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 2016; 40:244–252 [DOI] [PubMed] [Google Scholar]
- 22.Shah V, Pohida T, Turkbey B, et al. A method for correlating in vivo prostate magnetic resonance imaging and histopathology using individualized magnetic resonance-based molds. Rev Sci Instrum 2009; 80:104301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM. Biological determinants of cancer progression in men with prostate cancer. JAMA 1999; 281:1395–1400 [DOI] [PubMed] [Google Scholar]
- 24.Turkbey B, Mani H, Aras O, et al. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology 2013; 268:144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebovici A, Sfrangeu SA, Feier D, et al. Evaluation of the normal-to-diseased apparent diffusion coefficient ratio as an indicator of prostate cancer aggressiveness. BMC Med Imaging 2014; 14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litjens GJ, Hambrock T, Hulsbergen-van de Kaa C, Barentsz JO, Huisman HJ. Interpatient variation in normal peripheral zone apparent diffusion coefficient: effect on the prediction of prostate cancer aggressiveness. Radiology 2012; 265:260–266 [DOI] [PubMed] [Google Scholar]
- 27.Shih JH, Fay MP. Pearson’s chi-square test and rank correlation inferences for clustered data. Biometrics 2017; 73:822–834 [DOI] [PubMed] [Google Scholar]
- 28.Rosenkrantz AB, Ginocchio LA, Cornfeld D, et al. Interobserver reproducibility of the PI-RADS version 2 lexicon: a multicenter study of six experienced prostate radiologists. Radiology 2016; 280:793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epstein JI, Amin MB, Reuter VE, Humphrey PA. Contemporary Gleason grading of prostatic carcinoma: an update with discussion on practical issues to implement the 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol 2017; 41:e1–e7 [DOI] [PubMed] [Google Scholar]
- 30.Verma S, Rajesh A, Morales H, et al. Assessment of aggressiveness of prostate cancer: correlation of apparent diffusion coefficient with histologic grade after radical prostatectomy. AJR 2011; 196:374–381 [DOI] [PubMed] [Google Scholar]
- 31.McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol 1978; 15:340–345 [PubMed] [Google Scholar]
- 32.Park SY, Shin SJ, Jung DC, et al. PI-RADS version 2: quantitative analysis aids reliable interpretation of diffusion-weighted imaging for prostate cancer. Eur Radiol 2017; 27:2776–2783 [DOI] [PubMed] [Google Scholar]
- 33.Fedorov A, Vangel MG, Tempany CM, Fennessy FM. Multiparametric magnetic resonance imaging of the prostate: repeatability of volume and apparent diffusion coefficient quantification. Invest Radiol 2017; 52:538–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaish H, Kang SK, Rosenkrantz AB. The utility of quantitative ADC values for differentiating high-risk from low-risk prostate cancer: a systematic review and meta-analysis. Abdom Radiol (NY) 2017; 42:260–270 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.