Abstract
Bacteriophage λ of Escherichia coli has two alternative life cycles after infection–host survival with lysogen formation, or host lysis and phage production. In a lysogen, CI represses the two lytic promoters, pR and pL, and activates its own transcription from the auto-regulated pRM promoter. During induction from the lysogenic to lytic state, CI is inactivated, and the two lytic promoters are de-repressed allowing for expression of Cro from pR. Cro is known to repress transcription of CI from pRM to prevent lysogeny. We show here that when Cro and CI are both present but at low levels, the low level of Cro initially stimulates the lytic promoters while CI repressor is still present, stimulating the level of Cro to a concentration required for pRM repression. Cro has no stimulatory effect without the presence of CI. We propose that this early auto-activating role of Cro at lower concentrations is essential in the developmental switch to lytic growth, whereas pRM repression by Cro at relatively higher concentrations avoids restoring lysogeny.
Keywords: bacteriophage, lambda, induction, CI, Cro
Introduction
Temperate bacteriophage λ, which infects Escherichia coli, is studied as a model system for developmental decision-making processes because of its dual life cycles [1–3]. Once inside the host, the phage DNA either replicates, eventually lysing the host and producing progeny phage particles (lytic cycle), or integrates into the host chromosome as a prophage, remaining dormant (lysogenic cycle). Under DNA-damaging conditions that induce SOS response, for example, treatment of lysogenic cells with UV or mitomycin C, the prophage DNA is induced from dormancy and is excised from the chromosome; λ switches from the lysogenic to the lytic cycle [4]. This developmental process was termed “the genetic switch” [1].
λ’s so-called genetic switch is directly determined by the interplay of two regulatory proteins (CI and Cro), three promoters (pR, pL, and pRM), and six nearly homologous operators (oR1, oR2, and oR3 adjacent to pR and oL1, oL2 and oL3 adjacent to pL), all encoded in a 5.4-kb DNA segment of the phage chromosome called the immunity region (Fig. 1a). A λcI− mutant phage cannot maintain the lysogenic pathway [5], and a λcro− mutant cannot maintain lytic growth [6].
Fig. 1.
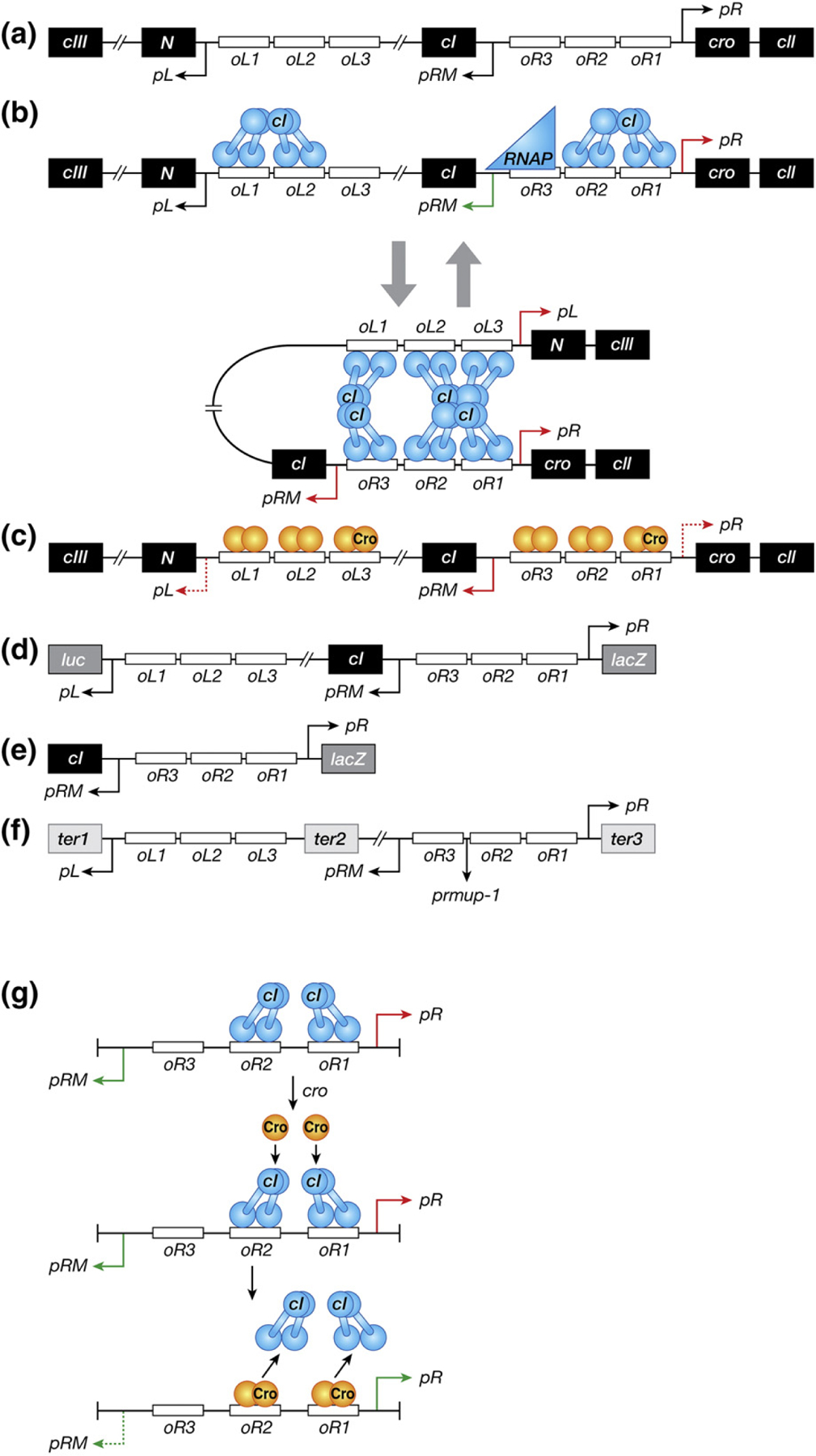
(a) Schematic representation of the regulatory region of bacteriophage λ that controls the switch from the lysogenic to lytic growth. Genes and operators are marked in black and white boxes, respectively. Promoters are represented as arrows whose direction corresponds to the direction of the transcription initiation from the promoters. The relevant genes and sites are discussed in the text. (b) DNA–CI interactions in the lysogenic state. CI molecules and RNA polymerase are shown as blue dumbbell shapes and triangle, respectively. Green arrows associated with promoters symbolize positive transcription, whereas red arrows signify repression. All the other symbols are the same as in panel A. (c) DNA–Cro interactions in the lytic state. Cro molecules bound to high-affinity operators (oR3 and oL3) are shown as dark orange ovals, whereas those in the low-affinity operators (the other oR and oL operators) are signified as light orange ovals. Red dashed arrows represent partial repression of corresponding promoters. All the other symbols are the same as in panel B. (d) A double-reporter system of λ prophage for measuring activities of pR and pL fused to lacZ and luc genes, respectively. (e) A reporter system to assay the activity of pR fused to lacZ in the absence of DNA looping. (f) The plasmid DNA template (pDL985) used for in vitro transcription assays [37]. Transcriptions from pL, pRM and pR stop at engineered terminators marked with black boxes, ter1 (timm of λ), ter2 (t1 t2 of rrnB operon), and ter3 (trpoC of rpo operon), respectively. The prmup-1 mutation is shown between oR2 and oR3. Genes located between oL3 and oR3 (cI, rexA, and rexB) are partially or completely deleted, resulting in the distance of 392 bp between oL3 and oR3 in this plasmid DNA [37]. All the other symbols are the same as in panel A. (g) Model of the de-repression of pR by Cro in the presence of CI. The details of the model depicted here are described in Discussion. All the symbols are the same as in panels B and C.
The maintenance of the lysogenic state by repression of pR and pL is achieved by characteristic binding of CI dimers to the operators with different affinities (Fig. 1b) [7–12]. The intrinsic affinities of CI vary with binding to oR1 > oR2 > oR3 and binding to oL1 > oL3 > oL2 [12–15]. CI bound to oR1 or oR2 represses pR, and CI bound at oL1, oL2, or oL3 represses pL. Furthermore, binding of CI dimers to oR1 and oR2 and to oL1 and oL2 is cooperative, strengthening repression of pR and pL. The cooperativity is enhanced by DNA supercoiling [12,16]. CI also auto-stimulates its own synthesis. When CI is bound at oR2, it induces RNA polymerase to bind to pRM, thus increasing cellular CI concentration. At high concentrations, CI binds to oR3 that represses pRM. These two auto-regulatory mechanisms maintain a steady level of CI in the lysogenic cell [17,33]. The regulation of the lysogenic state also involves further fine tuning by DNA looping between the oL and oR operator DNA. Looping is forged by interactions between CI tetramers bound to oL and oR. This looping further stabilizes the CI regulation of pR, pL, and pRM (Fig. 1b) [12,16,18–21].
Cro plays a critical role during induction from the lysogenic to the lytic state. Unlike CI, Cro is known to control pR, pL, and pRM only negatively [6,10,22–25]. Cro dimers bind to the same oR and oL operators as CI but with the following relative affinities: oR3 > oR1 > oR2 and oL1 > oL2 > oL3 (Fig. 1c) [15,26,27]. After inactivation of CI by SOS-induced cleavage, pR and pL are de-repressed; Cro produced from pR binds to its strongest site oR3 and turns off further CI synthesis, thus ensuring a lysogenic-to-lytic transition [11,28,29]. This role of Cro switching off pRM (CI synthesis) was first realized genetically in a strain where Cro protein was constitutively present. There, λ phage only followed the lytic cycle because Cro already present in the cell shut down CI repressor synthesis from the incoming phage [6].
During induction, CI is cleaved and inactivated but over a rather long period of time by a RecA-mediated process (see references 28, 30–32). (i) The fraction of CI cleaved per unit time decreases at higher CI concentrations [33]. (ii) CI is preferentially cleaved as a monomer over unbound and DNA-bound dimers. (iii) Moreover, the SOS-induced cleavage activity is not high and persistent enough to cleave all CI molecules present in a lysogen [30]. We argue that CI inactivation is rate limiting during de-repression of the lytic promoters and does not result in the synthesis in a sufficient amount for Cro to repress pRM. Thus, a new presumptive role of Cro at lower concentrations would be to de-repress pR from repression by CI.
Interestingly, a few experiments showed that Cro in fact stimulates pR transcription in the presence of low-level CI [23,34]. In this study, we demonstrate both in vivo and in vitro a stimulatory effect of Cro on transcription from both pR and pL. This only occurs in the presence of low levels of CI and Cro. As expected, Cro blocks transcription from pRM, pR, and pL at higher concentrations. We discuss how Cro acts as an anti-repressor at pR and pL promoters in the developmental switch.
Results
De-repression of lytic pR promoter by Cro in vivo
We used the double-reporter E. coli strains (LEE1, LEE2, LEE19, and LEE20) in which pR and pL promoters are fused to lacZ and luc reporter genes, respectively, and the fusions were present in a prophage (Fig. 1d and Table 1) [35]. The strains were deleted for the prophage cro gene as well as most of the phage replication and morphogenetic genes but contained a plasmid bearing cro gene under the arabinose-inducible promoter (pBAD24-cro+) (LEE2 and 20) or the vector plasmid lacking cro (LEE1 and LEE19). We studied the effect of Cro provided in trans from the plasmid on pR and pL expression in the prophage. We presume that Cro produced in trans would not be any different from Cro originating in cis as in the wild-type situation.
Table 1.
List of bacteria, phages, and plasmids
| Name | Features | Source |
|---|---|---|
| <Bacteria> | ||
| NC417 | W3110 lacIΔ− kanR–luc-pLoL rexB rexA cI857 pRoR cro27 cII′<> lacZYA | Svenningsen et al., [35] |
| LEE1 | NC417 harboring pBAD24 | This study |
| LEE2 | NC417 harboring pBAD24-cro+ | This study |
| LT1056 | Double-reporter fusion similar to NC417 containing cI+ | Lynn Thomason |
| LEE19 | LT1056 harboring pBAD24 | This study |
| LEE20 | LT1056 harboring pBAD24-cro+ | This study |
| NC415 | NC417 without rexAB, pL, and oL (no DNA looping) | Svenningsen et al., [35] |
| LEE17 | NC415 harboring pBAD24 | This study |
| LEE18 | NC415 harboring pBAD24-cro+ | This study |
| MG1655 | Standard wild-type E. coli K-12 | NIH collection |
| LEE5 | MG1655 (W1102) lysogen | This study |
| JW3996 | F− Δ(araD–araB)567, ΔlacZ4787(::rrnB-3), λ−, rph-1, Δ(rhaD–rhaB)568, ΔlamB732::kan, hsdR514 | Baba et al., [46] |
| LEE44 | LEE5 ΔlamB732::kan | This study |
| LEE58 | LEE44 harboring pBAD24 | This study |
| LEE59 | LEE44 harboring pBAD24-cro+ | This study |
| <Phages> | ||
| W1102 | Wild-type λ::cam | This study |
| P1 vir | NIH collection | |
| <Plasmids> | ||
| pDL944 | oL + oR +, 392-bp spacer between oL3 and oR3 | Lewis et al., [37] |
| pDL985 | pDL944 with prmup-1 in pRM | Lewis et al., [37] |
pR expression was monitored by β-galactosidase levels, first in strains LEE1 and LEE2 harboring the temperature-sensitive prophage gene (cI857) that encodes a CI variant whose activity is inactivated at temperatures > 42 °C [36]. β-Galactosidase activities were measured semi-quantitatively using disk-diffusion plate assays as described in Materials and Methods. The plates were incubated at different temperatures: 32 °C, 34 °C, 37 °C (Fig. 2a), and 42 °C (data not shown). Increasing temperatures with the cI857 mutant reduces active CI, permitting in vivo study of the effect of Cro on pR at varying concentrations of CI. lacZ expression from pR is indicated by the red color zone on the MacConkey lactose plates resulting from lactose fermentation by β-galactosidase. At 32 °C and 34 °C where CI repressor activity is high, red color was only visible near the arabinose-filter paper and only in the strain harboring the cro+ plasmid (LEE2); no color change was observed in the vector plasmid strain (LEE1). Thus, Cro expressed from the plasmid increased pR-lacZ expression in the presence of CI. At temperatures of 37 °C (Fig. 2a) and 42 °C (data not shown), pR-lacZ expression turned the entire plate red masking any effects of Cro on pR (Fig. 2a).
Fig. 2.
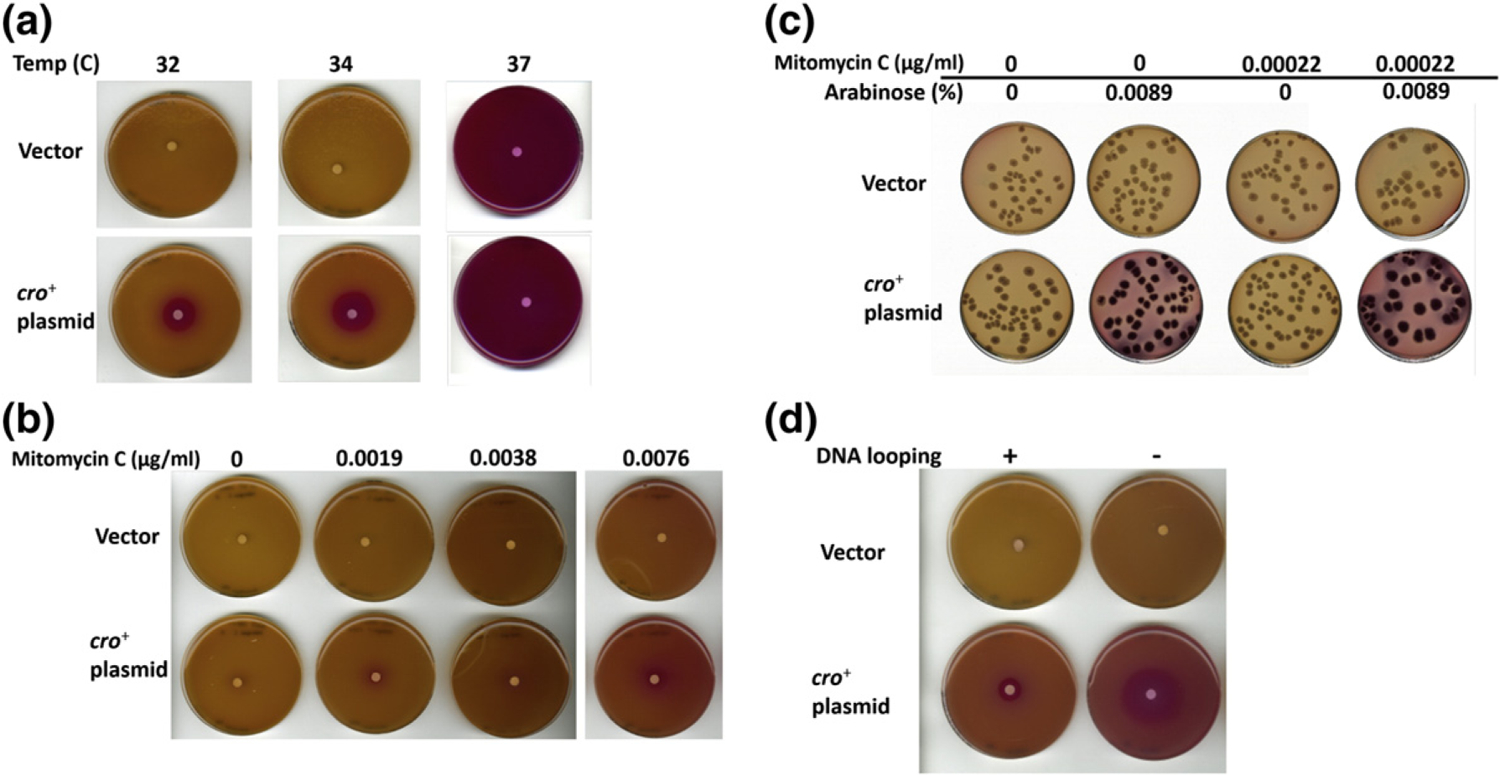
Qualitative examination of in vivo pR expression with color change of MacConkey lactose plates. (a) Disk diffusion assay of the double-reporter system strains bearing temperature-sensitive cIts gene (cI857) (LEE1 and LEE2), which harbor vector or cro-containing plasmid, respectively. Cells were grown at different temperatures (32 °C, 34 °C, and 37 °C). (b) Disk diffusion assay of the double-reporter system strains containing cI+ gene (LEE19 and LEE20), which were grown at 34 °C. The strains contain the vector or cro+ plasmid, respectively. The assays were conducted as described for the cI857 strains except that mitomycin C was added to soft agar so that the final mitomycin C concentration in the plates reaches 0, 0.0019, 0.0038, and 0.0076 μg/ml, respectively. (c) Single colonies of the strains in panel B grown at 34 °C on the MacConkey lactose plates supplemented without or with mitomycin C (0.00022 μg/ml) and arabinose (0.0089%). (d) Disk diffusion assay of the cIts reporter system strains with (LEE1 and LEE2) and without DNA looping (LEE17 and LEE18). These strains contain vector (LEE1 and LEE17) or cro+ plasmid (LEE2 and LEE18) and were grown at 34C.
To test whether Cro also positively regulates pR when CI is wild-type CI, similar experiments were conducted with the equivalent strains but harboring wild-type cI+ gene in place of the temperature-sensitive cI857 gene (LEE19 and LEE20). To decrease the cellular concentration of CI in cI+ strains, the MacConkey lactose plates were supplemented with different concentrations of mitomycin C. The red color around the arabinose-filter paper of the cro+ plasmid strain (LEE20) increased in intensity with increasing concentrations of mitomycin C, suggesting that the stimulatory effect by Cro on pR increases as CI concentration decreases (Fig. 2b). Note that the concentration range of mitomycin C was well below the concentration (0.1 μg/ml) needed for full inactivation of CI and where pR is fully de-repressed and red color becomes so intense that it prevents observation of any Cro-dependent de-repression (data not shown).
Enhancement of pR activity by Cro in cI+ strains was further examined by investigating the color of single colonies spread on MacConkey lactose plates supplemented with arabinose (0.0089%) and/or mitomycin C (0.00022 μg/ml; Fig. 2c). Under these conditions, the cro+ plasmid strain (LEE20) displayed bright red colonies in contrast to no color change of colonies carrying the vector plasmid in strain (LEE19).
Quantitative assays of pR expression were performed by measuring β-galactosidase activities in cI+ strains grown in liquid cultures in the presence of mitomycin C (0.0223 μg/ml) and/or arabinose (0.00893% and 0.0893%) at 34 °C. The cro+ plasmid strain (LEE20) exhibited a noticeable increase in β-galactosidase activity compared with the vector plasmid strain (LEE19) when grown in the presence of mitomycin C at both concentrations of arabinose (Fig. 3a). The increase in β-galactosidase activities was not observed in cells grown in the presence of the same level of mitomycin C but without arabinose. Similar results were obtained for temperature-dependent cI857 strains. The cro+ plasmid-containing strain displayed markedly higher β-galactosidase activities than the vector plasmid strain when grown in the presence of arabinose (0.05%) at 34 °C (data not shown). The β-galactosidase activities were similar between the cro+ plasmid strain and the vector plasmid strain grown without arabinose.
Fig. 3.
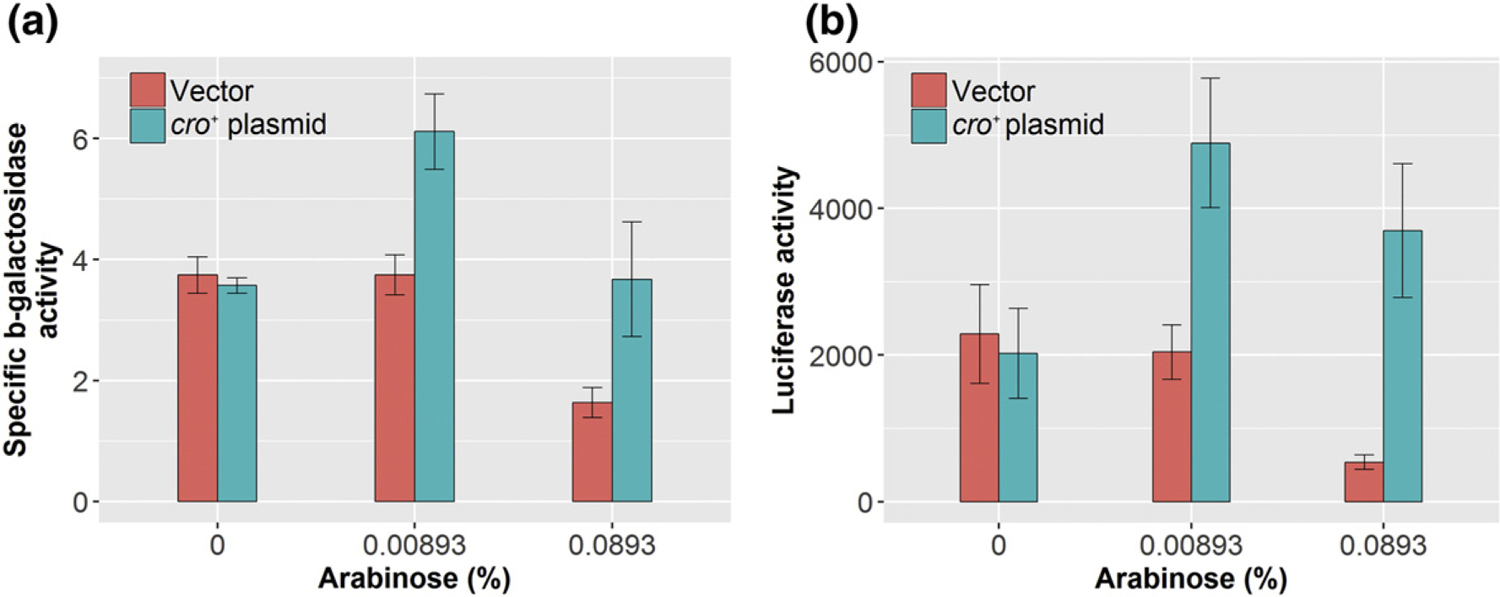
Quantitative assessment of in vivo pR and pL expression using the β-galactosidase assay of the cI+ double-reporter system strains (LEE19 and LEE20) harboring the vector or cro+ plasmid, respectively. The cells were grown at 34 °C in the presence of mitomycin C (0.0223 μg/ml). (a) pR expression assessment by β-galactosidase assays. The assays were repeated four times and error bars are shown. (b) pL expression assessment by luciferase assays. Three independent experiments were performed with error bars shown.
We also tested the effect of DNA looping on the Cro-mediated de-repression of pR using a cI857 strain in which all the oL elements were deleted to prevent DNA looping (Fig. 2d). The results show that Cro de-repressed pR more in the non-looping strain than in the looping-proficient strain at 34 °C.
Cro de-represses the prophage pL promoter
pL expression was quantitatively measured by luciferase assays in liquid cultures using the double-reporter strains that bear the wild-type cI+ gene (LEE19 and LEE20). These strains were grown at 34 °C in the presence of mitomycin C (0.0223 μg/ml) and/or arabinose (0.00893% and 0.0893%). Luciferase activities increased considerably in the cro+ plasmid strain compared with the vector when grown in the presence of mitomycin C at either level of arabinose (Fig. 3b). At the same mitomycin C concentration, no increase was observed when cells were grown without arabinose, that is, without Cro.
The temperature-sensitive cI857 mutant cells grown in the presence of arabinose (0.05%) at 34 °C gave similar results (data not shown). In the arabinose-supplemented media, the luciferase activity in the cro+ plasmid strain was much higher than the vector strain; this was not observed in the cultures grown without arabinose (data not shown).
Effect of Cro on pR and pL promoter expression in the presence of CI in vitro
We investigated the Cro-mediated de-repression of pR and pL in the presence of CI in vitro by transcription assays using a DNA template (pDL985) that contains the three promoters (pR, pL, and pRM) and their cognate operators (oR and oL) (Fig. 1f) [37] The gel results are shown in Fig. 4a and their quantifications in Fig. 4b–d. The results show that CI negatively regulates pR [concentration at half-maximal repression or activation (C0.5) = 54.7 nM] and pL (C0.5 = 52.5 nM) and activates pRM (C0.5 = 86.0 nM). CI represses pRM at higher concentrations (C0.5 = 452.9 nM) (Fig. 4b). The Cro titration experiments, as expected, showed that Cro represses all three promoters with C0.5 of 123.7 nM for pRM, 181.7 nM for pR, and 150.4 nM for pL (Fig. 4c). These results are in complete agreement with that of Johnson et al. [23], Meyer et al. [10], and Lewis et al. [37] However, when the Cro experiment was conducted in the presence of 120 nM CI, a concentration that alone reduces ~ 90% of pR and pL transcription, Cro at lower than 120-nM concentrations (6.25–75 nM) increased pR and pL transcription up to ~ 3.5-fold above the CI-only control (Fig. 4d). At higher Cro concentrations (> 150 nM), pR and pL become repressed again. The Cro-mediated de-repression of the lytic promoters was observed only at the intermediate concentration ranges of CI; at lower CI levels, there is not sufficient repression of pR by CI to notice any de-repression by Cro, while at very high concentrations, when repression of pR becomes too tight due to co-operative binding of CI, there is also no Cro effect (experiments leading to the results shown in Fig. 4d). Similar results were obtained with plasmid (pDL944) DNA without the prmup-1 mutation (data not shown).
Fig. 4.
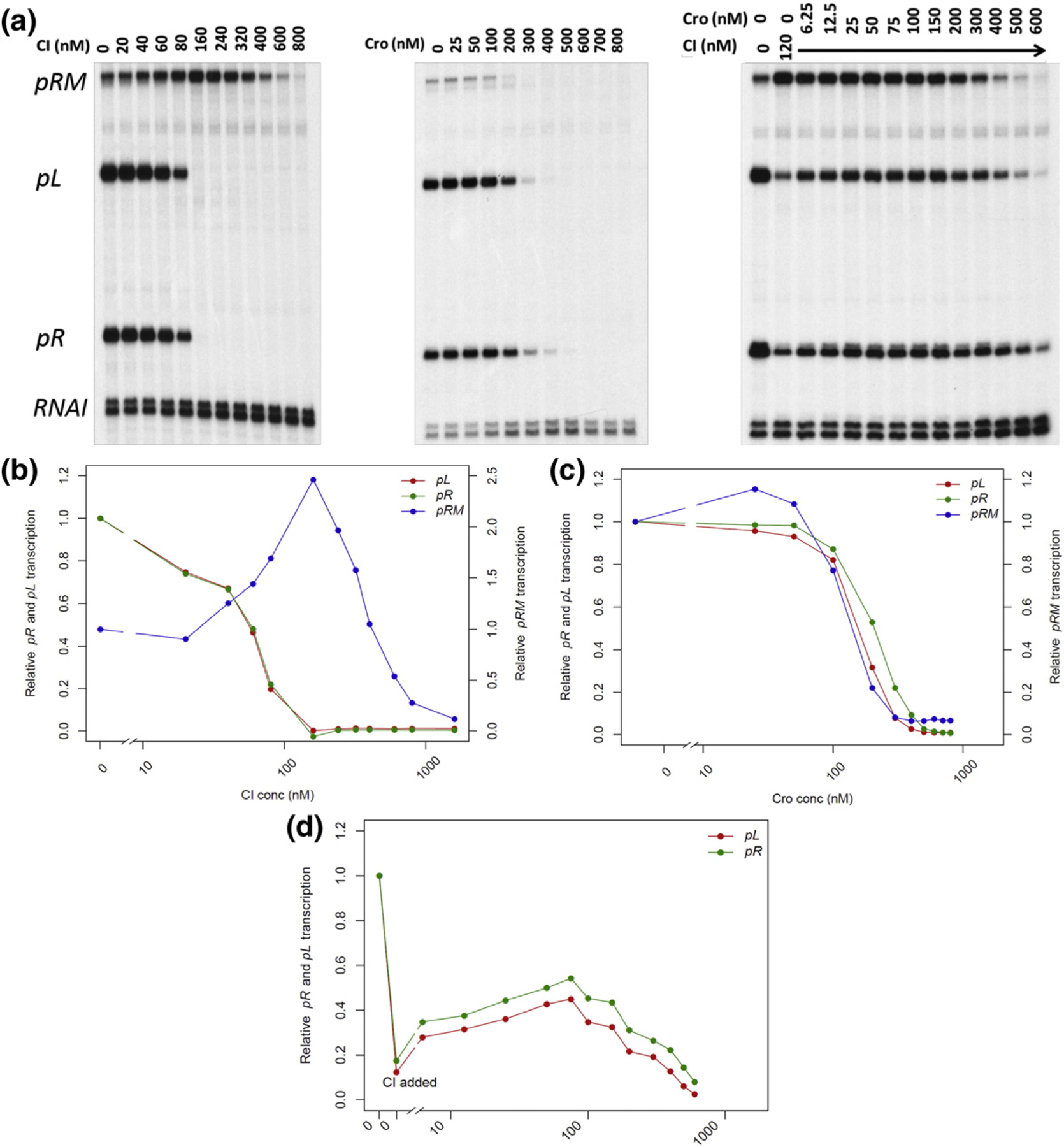
In vitro transcription assay results. All transcription assays were repeated 2–4 times with similar results. (a) Results of a typical in vitro transcription assay. The transcripts are identified in left margin. (b–d) Quantification of the representative in vitro transcription assays in panel A. The right y-axis represents the relative pR and pL transcription levels against no-protein control, whereas the left y-axis shows that of pRM. The x-axis is shown in log scale. CI titration (B), Cro titration (C), and in vitro transcription assay (D) with increasing amounts of Cro and a fixed amount of CI (120 nM).
Increase in prophage production by additional Cro supplied in trans
We hypothesized that the increase in expression of pL and pR by Cro would lead to increased phage production from a lysogen in which a controlled amount of Cro is made available while the prophage Cro is suppressed. This hypothesis was tested by growing λ lysogens harboring the cro+ plasmid in the absence and presence of mitomycin C (0.00125 μg/ml) and/or arabinose (0.05%) and assaying phage yields in the cell supernatants as described in Materials and Methods.
More phage particles were detected in the presence of the cro+ plasmid strain (~ 37-fold) than the vector plasmid strain when cultures were incubated with arabinose in the absence of mitomycin C (Fig. 5). The fold difference in phage titer between the cro+ and vector plasmid strains was slightly decreased (22-fold increase in phage yield) when mitomycin C was present. Without extra Cro (no arabinose), the phage titers from both strains were comparable without mitomycin C or slightly increased with mitomycin C.
Fig. 5.
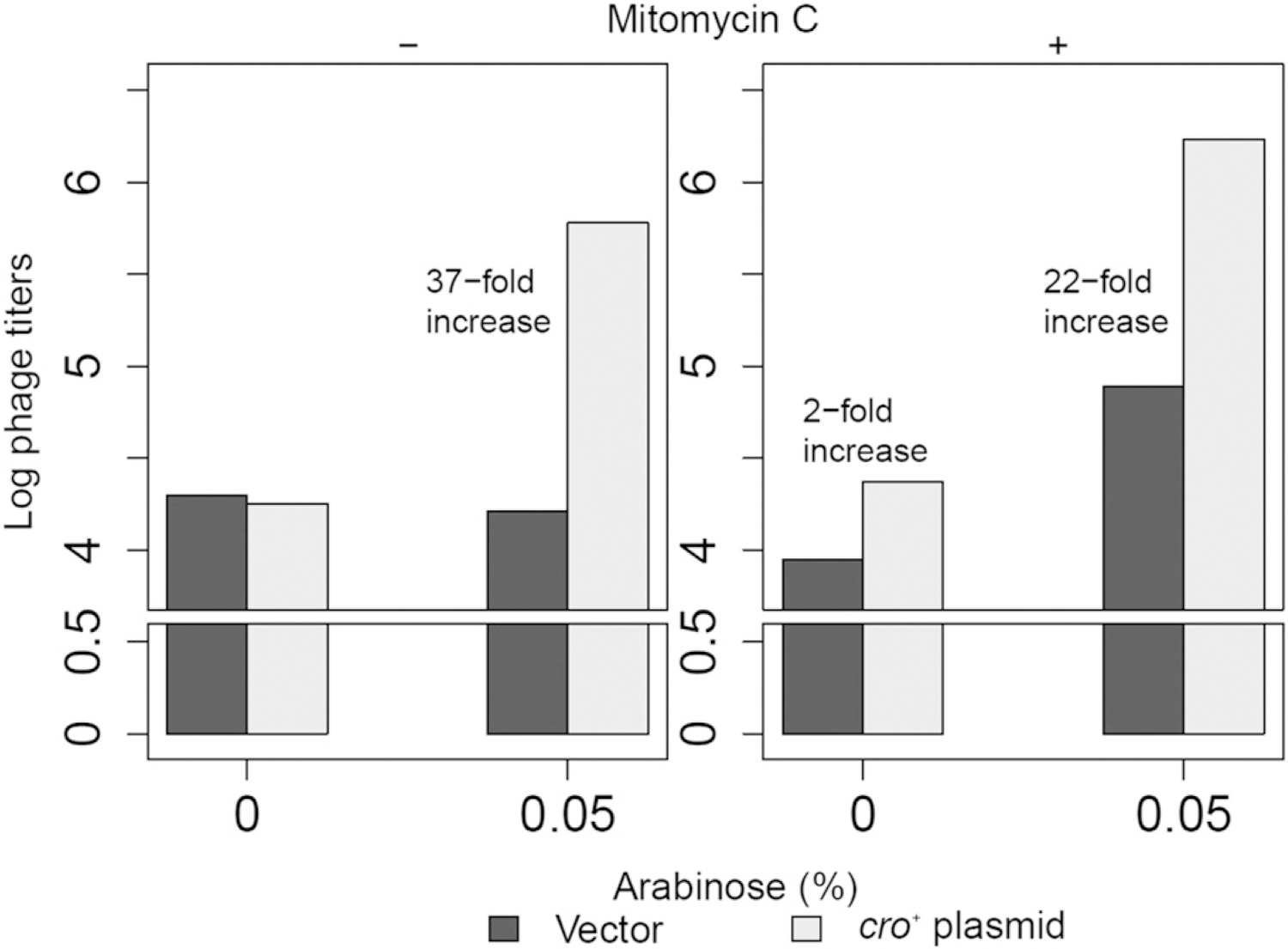
Phage production assays of the λ lysogens (LEE58 and LEE59) harboring vector or cro+ plasmid, respectively, with or without mitomycin C (0.00125 μg/ml) or/and arabinose (0.05%). Plaque-forming units per milliliter were normalized against OD600 and converted to log scale. The average of three experiments is shown in this figure. When no phage was detected, it was designated as 0 in the log-transformed data.
Discussion
The mechanisms of maintenance of prophage λ and its induction to lytic growth involve two regulatory proteins CI and Cro, respectively [1,2,24]. Interestingly, CI and Cro act by binding to the same DNA binding sites but with opposite outcomes. The devil lies in the details. The two outcomes originate from different affinities of the two proteins to the operator sites. Lysogeny and its maintenance are initially achieved by CI binding to its strongest binding sites (oR1, oR2 and oL1, oL2), thereby repressing the lytic promoters pL and pR and activating its own expression from the pRM promoter. As repressor levels increase, CI binds to weaker sites oR3 and oL3, leading to auto-repression of pRM to modulate a constant level of CI in the cell.
Induction allows the lytic cycle to ensue with Cro binding to its highest affinity sites, oR3 and oL3, thereby preventing further CI expression. More Cro can then bind to oR1, oR2 and oL1, oL2 to reduce expression of pL and pR. This reduces expression of CIII and CII (other lysogenic factors), which are transcribed from pL and pR, respectively. This would also facilitate lytic growth and block any chance of restoring lysogeny. The major role of Cro in the switch to lytic growth has been emphasized to be its interaction with oR3 and repression of pRM to shut off CI synthesis. Consistently, a prophage carrying mutation(s) in oR3 that restricts Cro binding is non-inducible [24,38]. However, whether Cro repression of pRM is the critical element in the genetic switch has not been rigorously tested. A predicted phenotype of a λcro− lysogen is its non-inducibility. A cro mutant can exist as a lysogen but is defective in lytic growth of challenge phage [6,22], However, non-inducibility from the prophage state, like that observed for oR3, has not been confirmed. Also, the properties of the Cro protein in the presence of CI in the cell, a situation that must exist during early stages of SOS induction of a prophage, have not been properly investigated. In the current study, we investigated an up-regulatory role of Cro, which has not been explored previously; a small amount of Cro de-represses the pL and pR promoters being repressed by CI, although the affinity of Cro for the cognate operators is less than that of CI. The current model for the genetic switch does not explain this property of Cro. We argue that the positive effect of Cro on pR and pL is more critical during the transition from lysogenic to lytic growth than the role of Cro as was suggested previously, that is, turning-off of CI synthesis at pRM [1]. Our finding that a small amount of extra Cro supplied in trans increases phage production in a lysogen (Fig. 5) is consistent with the idea that transient Cro-mediated up-regulation of pR and pL ultimately leads to its own increased expression and to a successful switch to lytic growth.
The in vitro demonstration of the de-repression of the lytic promoters helped our quantitative and mechanistic understanding of the stimulatory effect of Cro on pR and pL in the switch. First, the in vitro confirmation of de-repression of pR and pL by Cro in the presence of CI showed that the effect is direct and not through an unknown factor. Second, we showed that Cro alone does not stimulate pR and pL promoters; it only enhances pR and pL when lower levels of CI are also present. Thus, in de-repressing pR and pL, Cro is acting as an anti-repressor that somehow neutralizes the repressive effect of CI on the lytic promoters. The stimulation of pR and pL by Cro under these conditions is partial; at higher concentrations, Cro represses the lytic promoters in the presence of CI just as it does when present alone.
Since SOS-mediated CI degradation in prophage induction for lytic growth of λ is a slow process [28,30], we argue that the ensuing de-repression of pR and pL and synthesis of Cro might be slow as well. Our finding that the absence of DNA looping enhances the Cro-mediated de-repression suggests that tighter DNA binding of CI mediated by DNA looping prevents Cro action. Cooperative CI binding also does not allow for Cro stimulation of lytic promoters in our in vitro assay.
We propose the following mechanism of how Cro antagonizes CI-mediated pR repression (Fig. 1g). At the initial stages of SOS induction, the CI concentration in a lysogen is somewhat less than needed for cooperative binding of CI to oR1 and oR2 (tight repression of pR and pL). CI occupies oR1 and oR2 with their intrinsic affinities, albeit less frequently. We propose that at this stage, Cro removes CI at oR1/oR2, thus removing the repressive effects of CI on pR. Cro may or may not replace CI at oR1/oR2. During these events, Cro acts at concentrations lower than its intrinsic binding affinities to any of the operators. However, Cro may be recruited to the operators by CI itself. A potential CI–Cro interaction, although not tested, has been previously suggested to play a role in a different context [17]. Interestingly, a cooperative binding of Cro to oR1 and oR2, which may be part of the model, has been shown under some conditions [27]. At the final stage, the auto-stimulated Cro binds to oR3, turning off CI synthesis, and to oR1/oR2, turning down CII and CIII levels from pR and pL, respectively, which is needed for continuance of the lytic cycle [2,22,23,34,35]. We are currently investigating this model and exploring how a low-affinity protein (Cro) modulates the function of a high-affinity protein (CI) at their common DNA binding sites.
In summary, a Cro-mediated de-repression of the lytic promoters from repression by CI at an early stage of prophage induction is the critical component of the switch from lysogenic to lytic state of prophage λ; repression of CI synthesis from pRM by Cro at a later stage of the switch may be an auxiliary event. It has even been suggested that pRM repression by Cro may not even be critical for the switch [39], although evidence has been provided to demonstrate that Cro-mediated repression of CI synthesis certainly reinforces the commitment to lysis [24].
Materials and Methods
Bacterial strains and plasmids
Bacterial strains used in this study are listed in Table 1. Cells were grown at 30 °C or 37 °C on LB liquid or agar media. When necessary, the plates or broth cultures were supplemented with an appropriate antibiotic. Standard methods were used for microbiological manipulations and assays [40,41]. Bacterial cells were stored in the long term at −80 °C with ~ 20% glycerol.
To conduct in vivo experiments, we transformed various strains with the plasmid vector pBAD24 [42] and pBAD24-cro+; the latter was constructed and kindly provided by Dr. Francisco Malagon ((Naval Medical Research Center, Frederick, MD). The plasmid pBAD24-cro+ expresses the cro gene from the pBAD promoter dependent on the presence of arabinose in the media. The plasmids were confirmed by PCR using appropriate primers as well as by DNA sequencing.
Measurement of in vivo pR expression
Bacterial strains containing a pR ~ lacZ fusion in a cryptic prophage (Table 1) were used to semi-quantitatively examine the expression from pR by observing color changes due to lactose fermentation of the strains on MacConkey lactose-ampicillin agar plates. For the agar plate experiments, 200 μl of overnight cultures was mixed with 5 ml of soft agar (0.7%) containing 25 ng/μl ampicillin (half the ampicillin concentration of the MacConkey lactose-ampicillin plates) and poured onto MacConkey lactose-ampicillin plates. Sterile paper disks (6.5 mm in diameter) (GE Healthcare, Marlborough, MA) were placed in the middle of plates after soft agar was solidified. Five microliters of 4% arabinose was spotted on top of the filter paper disk and let sit for 5 min before incubating overnight at different temperatures (32 °C, 34 °C, 37 °C, and 42 °C). Color changes, if any, around the disks were visually examined. These disk diffusion assays were conducted with the pR ~ lacZ fusion strains bearing both temperature-sensitive cIts (cI857) (LEE1 and LEE2) or wild-type cI+ genes (LEE19 and LEE20).
For strains harboring the cI+ gene, different concentrations of the prophage-inducing agent (mitomycin C; Sigma, St. Louis, MO) were also added with the soft agar to decrease the cellular CI level. pR expression of the cI+ strains was also examined in a modified manner in which the diluted culture was plated for single colonies on the MacConkey plates supplemented with mitomycin C and/or arabinose.
The pR expression in liquid cultures grown under different conditions was measured by assaying β-galactosidase. Log phase cultures of the cI+ double-reporter strains grown to an OD600 of ~ 0.3 at 34 °C were treated with different concentrations of mitomycin C and/or arabinose, and further incubated at 34 °C for overnight. β-Galactosidase activities were assayed on SpectraMax Plus 96-well microtiter plate reader (Molecular Devices, Caesarea, Israel) as described previously [43,44]. The β-galactosidase-specific activities were determined by the rate of OD420 increase divided by the cell OD600 at the time of measurements.
Measurement of in vivo pL expression
The in vivo expression level of pL was assessed by assaying luciferase activities in the pL~ luc reporter strains as described previously [35]. cI+ strains were grown and collected as described for the β-galactosidase assays above. Luminescence was read with the Infinite M200 pro luminometer (Tecan, Morrisville, NC) or VICTOR3 luminometer (PerkinElmer, Waltham, MA). Luciferase activities were calculated by subtracting the luminescence reading of the control (LB) from those of the samples and dividing by the cell OD600.
Purification of CI and Cro
CI protein was purified and stored as described before [37]. Cro protein was purified using the IMPACT (Intein Mediated Purification with an Affinity Chitin-binding Tag) system (New England BioLabs, Ipswich, MA). The purified protein was dialyzed against the 2 × storage buffer [20 mM Tris–HCl (pH 7.5), 0.2 mM EDTA, and 400 mM KCl] and the equal volume of 80% glycerol was added [45]. The protein was run on the SDS–polyacrylamide gel to check the purity and stored at −80 °C.
In vitro transcription assays
In vitro transcription assays were conducted as described by Lewis et al. [37] except that 2 nM DNA template, instead of 4 nM, was used. The plasmid templates pDL944 and pDL985 used for in vitro transcription assays contain the oR and oL regions with a 392-bp spacer between oL3 and oR3 (Fig. 1f) [37]. An additional mutation (prmup-1) was added in pRM in plasmid pDL985 to increase the basal level of pRM transcription for easy quantification (Fig. 4a and Table 1) [10,37]. In vitro transcription assay signals were visualized with STORM 860 (Molecular Dynamics) and quantified with Image-Quant™ TL software (GE Healthcare).
Prophage induction
E. coli strain MG1655 was lysogenized with a λ derivative carrying a chloramphenicol resistance marker at a nonessential DNA region of the phage genome. This lysogenic strain LEE5 was rendered resistant to λ adsorption by deleting the λ receptor gene lamB using P1vir grown on a ΔlamB::kanR strain (from Keio collection) [46]. The new strain LEE44 was confirmed to be inactive for lamB by its resistance to λ infection.
The phage production experiments were conducted with lysogens LEE58 and LEE59 (LEE44 derivatives harboring plasmids pBAD24 or pBAD24-cro+, respectively) (Table 1). Overnight cultures were diluted 200-fold in fresh LB supplemented with kanamycin and ampicillin (both 50 μg/ml) and grown at 37 °C until OD600 reached ~ 0.1. Different concentrations of mitomycin C and/or arabinose were added to induce phage and Cro production, respectively. After growth to stationary phase, free phages were assayed in the supernatants after chloroform treatments.
Data analysis
All visualizations and calculations were conducted with Python† and R‡ (Python Software Foundation; R Core Team, 2014).
Acknowledgements
This work was funded by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. We thank Dr. Lynn Thomason for gift of valuable strains and helpful discussions. We are grateful to our colleagues in the laboratory, especially Dr. Amlanjyoti Dhar, for various help and discussions. We also thank Drs. Donald L. Court and Dhruba K. Chattoraj for critical reading of the manuscript and invaluable suggestions.
Abbreviations used:
- C0.5
concentration at half-maximal repression or activation
Footnotes
Conflict of Interest: All authors declare no conflicts of interest.
References
- [1].Ptashne M, A Genetic Switch, Phage Lambda Revisited, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 2004. [Google Scholar]
- [2].Oppenheim AB, Kobiler O, Stavans J, Court DL, Adhya S, Switches in bacteriophage lambda development, Annu. Rev. Genet 39 (2005) 409–429. [DOI] [PubMed] [Google Scholar]
- [3].Casjens SR, Hendrix RW, Bacteriophage lambda: early pioneer and still relevant, Virology 479–480 (2015) 310–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hershey A, Dove W, Introduction to lambda, Cold Spring Harb. Monogr. Arch 13 (1983). [Google Scholar]
- [5].Kaiser AD, Mutations in a temperate bacteriophage affecting its ability to lysogenize Escherichia coli, Virology 3 (1957) 42–61. [DOI] [PubMed] [Google Scholar]
- [6].Eisen H, Brachet P, Pereira da Silva L, Jacob F, Regulation of repressor expression in lambda, Proc. Natl. Acad. Sci. U. S. A 66 (1970) 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maniatis T, Ptashne M, Multiple repressor binding at the operators in bacteriophage lambda, Proc. Natl. Acad. Sci. U. S. A 70 (1973) 1531–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Maurer R, Meyer B, Ptashne M, Gene regulation at the right operator (OR) bacteriophage lambda. I. OR3 and autogenous negative control by repressor, J. Mol. Biol 139 (1980) 147–161. [DOI] [PubMed] [Google Scholar]
- [9].Meyer BJ, Ptashne M, Gene regulation at the right operator (OR) of bacteriophage lambda. III. Lambda repressor directly activates gene transcription, J. Mol. Biol 139 (1980) 195–205. [DOI] [PubMed] [Google Scholar]
- [10].Meyer BJ, Maurer R, Ptashne M, Gene regulation at the right operator (OR) of bacteriophage lambda. II. OR1, OR2, and OR3: their roles in mediating the effects of repressor and cro, J. Mol. Biol 139 (1980) 163–194. [DOI] [PubMed] [Google Scholar]
- [11].Ptashne M, Jeffrey A, Johnson AD, Maurer R, Meyer BJ, Pabo CO, Roberts TM, Sauer RT, How the lambda repressor and cro work, Cell 19 (1980) 1–11. [DOI] [PubMed] [Google Scholar]
- [12].Lewis DE, Gussin GN, Adhya S, New insights into the phage genetic switch: effects of bacteriophage lambda operator mutations on DNA looping and regulation of PR, PL, and PRM, J. Mol. Biol 428 (2016) 4438–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ackers GK, Shea MA, Smith FR, Free energy coupling within macromolecules, J. Mol. Biol 170 (1983) 223–242. [DOI] [PubMed] [Google Scholar]
- [14].Sarai A, Takeda Y, Lambda repressor recognizes the approximately 2-fold symmetric half-operator sequences asymmetrically, Proc. Natl. Acad. Sci. U. S. A 86 (1989) 6513–6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Takeda Y, Sarai A, Rivera VM, Analysis of the sequence-specific interactions between Cro repressor and operator DNA by systematic base substitution experiments, Proc. Natl. Acad. Sci. U. S. A 86 (1989) 439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Norregaard K, Andersson M, Sneppen K, Nielsen PE, Brown S, Oddershede LB, DNA supercoiling enhances cooperativity and efficiency of an epigenetic switch, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 17386–17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aurell E, Brown S, Johanson J, Sneppen K, Stability puzzles in phage lambda, Phys. Rev. E Stat. Nonlinear Soft Matter Phys 65 (2002), 051914. [DOI] [PubMed] [Google Scholar]
- [18].Dodd IB, Shearwin KE, Perkins AJ, Burr T, Hochschild A, Egan JB, Cooperativity in long-range gene regulation by the lambda CI repressor, Genes Dev 18 (2004) 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Anderson LM, Yang H, DNA looping can enhance lysogenic CI transcription in phage lambda, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 5827–5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cui L, Murchland I, Shearwin KE, Dodd IB, Enhancer-like long-range transcriptional activation by lambda CI-mediated DNA looping, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 2922–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Michalowski CB, Little JW, Role of cis-acting sites in stimulation of the phage λPRM promoter by CI-mediated looping, J. Bacteriol 195 (2013) 3401–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Folkmanis A, Maltzman W, Mellon P, Skalka A, Echols H, The essential role of the cro gene in lytic development by bacteriophage lambda, Virology 81 (1977) 352–362. [DOI] [PubMed] [Google Scholar]
- [23].Johnson A, Meyer BJ, Ptashne M, Mechanism of action of the cro protein of bacteriophage lambda, Proc. Natl. Acad. Sci. U. S. A 75 (1978) 1783–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schubert RA, Dodd IB, Egan JB, Shearwin KE, Cro’s role in the CI Cro bistable switch is critical for λ’s transition from lysogeny to lytic development, Genes Dev 21 (2007) 2461–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Takeda Y, Folkmanis A, Echols H, Cro regulatory protein specified by bacteriophage lambda. Structure, DNA-binding, and repression of RNA synthesis, J. Biol. Chem 252 (1977) 6177–6183. [PubMed] [Google Scholar]
- [26].Kim JG, Takeda Y, Matthews BW, Anderson WF, Kinetic studies on Cro repressor–operator DNA interaction, J. Mol. Biol 196 (1987) 149–158. [DOI] [PubMed] [Google Scholar]
- [27].Darling PJ, Holt JM, Ackers GK, Coupled energetics of lambda cro repressor self-assembly and site-specific DNA operator binding II: cooperative interactions of cro dimers, J. Mol. Biol 302 (2000) 625–638. [DOI] [PubMed] [Google Scholar]
- [28].Roberts J, Devoret R, Lysogenic induction, Cold Spring Harbor Monograph Archive 13, 1983. [Google Scholar]
- [29].Ptashne M, Lambda’s switch: lessons from a module swap, Curr. Biol 16 (2006) R459–62. [DOI] [PubMed] [Google Scholar]
- [30].Bailone A, Levine A, Devoret R, Inactivation of prophage lambda repressor in vivo, J. Mol. Biol 131 (1979) 553–572. [DOI] [PubMed] [Google Scholar]
- [31].Cohen S, Knoll BJ, Little JW, Mount DW, Preferential cleavage of phage lambda repressor monomers by recA protease, Nature 294 (1981) 182–184. [DOI] [PubMed] [Google Scholar]
- [32].Pal A, Chattopadhyaya R, RecA-mediated cleavage of λ cI repressor accepts repressor dimers: probable role of prolyl cis-trans isomerization and catalytic involvement of H163, K177, and K232 of RecA, J. Biomol. Struct. Dyn 27 (2009) 221–233. [DOI] [PubMed] [Google Scholar]
- [33].Phizicky EM, Roberts JW, Kinetics of RecA protein-directed inactivation of repressors of phage lambda and phage P22, J. Mol. Biol 139 (1980) 319–328. [DOI] [PubMed] [Google Scholar]
- [34].Takeda Y, Specific repression of in vitro transcription by the Cro repressor of bacteriophage lambda, J. Mol. Biol 127 (1979) 177–189. [DOI] [PubMed] [Google Scholar]
- [35].Svenningsen SL, Costantino N, Court DL, Adhya S, On the role of Cro in lambda prophage induction, Proc. Natl. Acad. Sci. U. S. A 102 (2005) 4465–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sussman R, Jacob F, On a thermosensitive repression system in the Escherichia coli lambda bacteriophage, C. R. Hebd. Seances Acad. Sci 254 (1962) 1517–1519. [PubMed] [Google Scholar]
- [37].Lewis D, Le P, Zurla C, Finzi L, Adhya S, Multilevel autoregulation of lambda repressor protein CI by DNA looping in vitro, Proc. Natl. Acad. Sci. U. S. A 108 (2011) 14807–14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Johnson AD, Mechanism of Action of the λ cro Protein. (Ph.D. thesis) Harvard University, Cambridge, MA, 1980. [Google Scholar]
- [39].Atsumi S, Little JW, A synthetic phage λ regulatory circuit, Proc. Natl. Acad. Sci. U. S. A 103 (2006) 19045–19050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Miller JH, Experiments in Molecular Genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, N. Y., 1972 [Google Scholar]
- [41].Maniatis T, Fritsch EF, Sambrook J, Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, New York, 1982. [Google Scholar]
- [42].Guzman LM, Belin D, Carson MJ, Beckwith J, Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter, J. Bacteriol 177 (1995) 4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhou Y, Gottesman S, Regulation of proteolysis of the stationary-phase sigma factor RpoS, J. Bacteriol 180 (1998) 1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S, DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription, Proc. Natl. Acad. Sci. U. S. A 95 (1998) 12462–12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Darling PJ, Holt JM, Ackers GK, Coupled energetics of lambda cro repressor self-assembly and site-specific DNA operator binding I: analysis of cro dimerization from nanomolar to micromolar concentrations, Biochemistry 39 (2000) 11500–11507. [DOI] [PubMed] [Google Scholar]
- [46].Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H, Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection, Mol. Syst. Biol 2 (2006) 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]


