Summary
Background
The approval of anti-programmed death ligand 1 (PD-L1) and anti-programmed death 1 agents has expanded treatment options for patients with locally advanced or metastatic urothelial carcinoma. Avelumab, a human monoclonal anti-PD-L1 antibody, has shown promising antitumour activity and safety in this disease. We aimed to assess the safety profile in patients (both post-platinum therapy and cisplatin-naive) treated with avelumab and to assess antitumour activity of this drug in post-platinum patients.
Methods
In this pooled analysis of two cohorts from the phase 1 dose-expansion JAVELIN Solid Tumor study, patients aged 18 years and older with histologically or cytologically confirmed locally advanced or metastatic urothelial carcinoma that had progressed after at least one previous platinum-based chemotherapy were enrolled from 80 cancer treatment centres or hospitals in the USA, Europe, and Asia. Eligible patients had adequate end-organ function, an Eastern Cooperative Oncology Group performance status of 0 or 1, life expectancy of at least 3 months, and at least one measurable lesion. Cisplatin-ineligible patients who might have been previously treated in the perioperative setting, including platinum-naive patients, were also eligible. Patients unselected for PD-L1 expression received avelumab (10 mg/kg, 1 h intravenous infusion) every 2 weeks until confirmed disease progression, unacceptable toxicity, or other criterion for withdrawal. The primary endpoint for this efficacy expansion cohort was confirmed best overall response (according to RECIST version 1.1), adjudicated by independent review. Safety analysis was done in all patients who received at least one dose of avelumab. Antitumour activity was assessed in post-platinum patients who received at least one dose of avelumab. This trial is registered with ClinicalTrials.gov, number NCT01772004; enrolment in this cohort of patients with metastatic urothelial carcinoma is closed and the trial is ongoing.
Findings
Between Sept 3, 2014, and March 15, 2016, 329 patients with advanced metastatic urothelial carcinoma were screened for enrolment into this study; 249 patients were eligible and received treatment with avelumab for a median of 12 weeks (IQR 6·0–19·7) and followed up for a median of 9·9 months (4·3–12·1). Safety and antitumour activity were evaluated at data cutoff on June 9, 2016. In 161 post-platinum patients with at least 6 months of follow-up, a best overall response of complete or partial response was recorded in 27 patients (17%; 95% CI 11–24), including nine (6%) complete responses and 18 (11%) partial responses. The most frequent treatment-related adverse events (any grade in ≥10% patients) were infusion-related reaction (73 [29%]; all grade 1–2) and fatigue (40 [16%]). Grade 3 or worse treatment-related adverse events occurred in 21 (8%) of 249 patients, the most common of which were fatigue (four [2%]), and asthenia, elevated lipase, hypophosphataemia, and pneumonitis in two (1%) patients each. 19 (8%) of 249 patients had a serious adverse event related to treatment with avelumab, and one treatment-related death occurred (pneumonitis).
Interpretation
Avelumab showed antitumour activity in the treatment of patients with platinum-refractory metastatic urothelial carcinoma; a manageable safety profile was reported in all avelumab-treated patients. These data provide the rationale for therapeutic use of avelumab in metastatic urothelial carcinoma and it has received accelerated US FDA approval in this setting on this basis.
Introduction
Urothelial carcinoma is the ninth leading cause of cancer-related deaths worldwide, occurring mainly in patients older than 55 years.1 Around 77 000 cases are diagnosed in the USA every year. Although only 4% of patients present with metastatic disease, 25% of those initially presenting with muscle-invasive bladder cancer require a radical cystectomy and nearly half of these patients develop metastatic disease and die within 2 years.2,3 The 5-year relative overall survival for patients diagnosed with metastatic urothelial carcinoma is 5%.2 Factors predictive of shorter survival include abnormal albumin and low haemoglobin concentrations, reduced performance status, and presence of liver metastasis.4
Cisplatin-based combinations, including dose-dense regimens, are the standard of care for untreated patients with inoperable or advanced metastatic urothelial carcinoma,5 and are associated with around 40–50% of patients achieving an objective response and a median overall survival of roughly 14–15 months;6–8 however, responses to these chemotherapy regimens are not often durable, with 5-year overall survival of about 15%.9 However, around a third of patients with metastatic urothelial carcinoma are ineligible for cisplatin-based therapy because of renal impairment or other comorbidities; these patients might instead receive first-line treatment with carboplatin-based regimens,5,10 which are associated with shorter overall survival than cisplatin-based regimens.10 Vinflunine has been approved for second-line treatment in Europe where it provided an overall survival benefit of 2·6 months relative to best supportive care;11 in the USA, patients with recurrent or resistant disease may receive paclitaxel, docetaxel, gemcitabine, pemetrexed, or other monotherapy and combination chemotherapy.11–14 Median overall survival with second-line chemotherapy ranges from 5 to 7 months with only around 9% of patients achieving an objective response,11 highlighting the need for alternative treatment options.
Antibodies targeting the programmed death ligand 1 (PD-L1) or programmed death-1 (PD-1) axis can enhance T-cell-mediated antitumour immunity by blocking inhibitory signals generated by these immune checkpoint proteins.15 Anti-PD-L1 and anti-PD-1 antibodies have been associated with antitumour responses in patients with metastatic urothelial carcinoma,16–23 showing the therapeutic potential of these agents. Within this class, atezolizumab, durvalumab, and avelumab (anti-PD-L1), and nivolumab (anti-PD-1) have received accelerated approval in the USA for the treatment of platinum-refractory metastatic urothelial carcinoma; pembrolizumab (anti-PD-1) has received full approval in this treatment setting.5 Atezolizumab and pembrolizumab have also received accelerated approval for the treatment of cisplatin-ineligible patients with metastatic urothelial carcinoma.5 PD-L1 is widely expressed on urothelial carcinoma cells and has been associated with higher tumour grade and stage and shorter overall survival in non-muscle-invasive and muscle-invasive bladder cancers.24,25 In patients with metastatic urothelial carcinoma treated with systemic chemotherapy, tumour PD-L1 positivity did not correlate with overall survival, but PD-L1 on tumour-infiltrating mononuclear cells was associated with longer overall survival.26 Although PD-L1 expression on tumour cells and infiltrating immune cells predicts response to anti-PD-1 or anti-PD-L1 treatment in some cancers,27 in urothelial carcinoma, its predictive ability has been inconsistent across trials.16,18,19,21–23
Avelumab is a human anti-PD-L1 IgG1 antibody that binds PD-L1 and inhibits the interactions of PD-L1 with PD-1 and B7.1, leaving PD-1–PD-L2 interactions intact.28 In preclinical and in-vitro studies, avelumab induced antibody-dependent cell-mediated cytotoxicity against tumour cells,29,30 although this activity cannot be measured directly in vivo. These preclinical and in-vitro findings suggest that avelumab has a second mechanism of action, in addition to PD-1 pathway blockade. In a large phase 1 study (JAVELIN Solid Tumor), avelumab-treated patients with various advanced solid tumours had manageable safety and promising objective responses and disease stabilisation.28,31,32 Avelumab showed encouraging antitumour activity and safety in an initial cohort of 44 patients with metastatic urothelial carcinoma that had progressed after platinum-based treatment.19 We describe a planned pooled interim analysis of two patient cohorts with metastatic urothelial carcinoma from the JAVELIN Solid Tumor trial, further characterising antitumour activity and safety of avelumab in this disease setting.
Methods
Study design and participants
JAVELIN Solid Tumor is a phase 1, open-label, international, multicentre dose-expansion trial to investigate the biological and clinical activity, safety and tolerability, and pharmacokinetics of avelumab in patients with metastatic solid tumours, with expansion in selected tumour types, enrolled at 80 cancer treatment centres or hospitals in the USA, Europe, and Asia (appendix p 3). Patients with metastatic urothelial carcinoma were enrolled in two sequential cohorts: an initial cohort and an efficacy expansion cohort.
Eligible patients were aged 18 years and older and had histologically or cytologically confirmed locally advanced or metastatic urothelial carcinoma that had progressed after at least one previous platinum-based chemotherapy. Enrolment of cisplatin-ineligible patients who were previously treated in the perioperative setting, or who were platinum-naive, was permitted in the initial cohort after a protocol amendment on Nov 19, 2014, and was permitted from the start of enrolment in the expansion cohort. All eligible patients had adequate end-organ function, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, life expectancy of at least 3 months, and at least one measurable lesion (per Response Evaluation Criteria In Solid Tumors [RECIST] version 1.1). Eligible patients had no active or history of metastases of the CNS, and had adequate haematological, hepatic, and renal function (defined by the following laboratory values: white blood cell count of ≥3 × 109 cells per L with an absolute neutrophil count ≥1·5 × 109 cells per L, lymphocyte count ≥0·5 × 109 cells/L, platelet count ≥100 × 109 platelets per L, haemoglobin ≥9 g/dL, total bilirubin concentration of ≤1·5 × the upper limit of normal [ULN] range, aspartate aminotransferase and alanine aminotransferase concentrations of ≤2·5 × ULN, and an estimated creatinine clearance >50 mL per min based on the Cockcroft-Gault formula). Availability of a fresh biopsy or baseline formalin-fixed paraffin-embedded tumour archival sample was required for PD-L1 analyses, although patients were not preselected based on tumour PD-L1 expression level or other biomarkers. Principle exclusion criteria included concurrent anticancer treatment, immunosuppressive or hormonal agents, and previous treatment with any drug targeting T-cell co-regulatory proteins (see appendix p 5 for full eligibility criteria).
The study protocol was approved by the institutional review board or independent ethics committee at each study centre. Patients were enrolled in accordance with international standards of good clinical practice and institutional safety monitoring. Written informed consent was provided by patients or their representatives before study entry, and all investigators signed Good Clinical Practice compliance forms.
Procedures
Patients received avelumab (EMD Serono Research & Development Institute, Inc, Billerica, MA, USA, a business of Merck KGaA, Darmstadt, Germany) at a dose of 10 mg/kg by 1 h intravenous infusion every 2 weeks until confirmed disease progression, unacceptable toxicity, or other protocol-specified criteria for withdrawal occurred (reported previously).32 Tumour burden at baseline was assessed by a CT scan or MRI of the chest, abdomen, and pelvis within 18 days before the start of avelumab treatment using RECIST (version 1.1) for target and non-target lesions. CT of the chest was mandatory when MRI was used. Tumour assessments were done every 6 weeks for the first 12 months, then every 12 weeks until end of treatment or until documented disease progression. Antitumour activity (according to RECIST version 1.1) was assessed by an independent endpoint review committee based in Valbonne, France, to establish the best overall response (best response obtained among all tumour assessments after the start of treatment with avelumab until documented disease progression) and duration of response. Patients without progressive disease at the end-of-treatment visit were followed up for disease progression (CT or MRI scans every 12 weeks) for up to 1 year. In the case of a partial or complete response, a confirmatory CT or MRI scan was done no sooner than 28 days and preferably at the scheduled 6-week interval. Change in the sum of target lesion diameters from baseline was evaluated in patients with baseline tumour assessments and at least one post-baseline assessment, based on independent review.
Safety was assessed at every clinical visit and was based on review of adverse events, laboratory values, changes in vital signs, electrocardiograms, and bodyweight. Immune-related or infusion-related adverse events were of special interest and were coded per the Medical Dictionary for Regulatory Activities terminology. Severity of adverse events was classified per the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 4.0). Premedication with diphenhydramine and acetaminophen was required before all infusions to mitigate possible infusion-related reactions; preliminary analyses from the JAVELIN Solid Tumor trial suggested that premedication was associated with a reduced severity of infusion-related reactions.31 Permanent discontinuation of treatment was specified for any grade 3 or worse adverse events (exceptions included single laboratory values out of normal range that were unrelated to trial treatment according to the investigator, that did not have clinical correlates, and that resolved within 7 days with adequate medical intervention; transient [≤6 h] flu-like symptoms or fever controlled with medical management; fatigue, local infusion-related reaction, headache, nausea, or emesis that resolved to grade ≤1 within 24 h; tumour flare, defined as local pain, irritation, or rash localised at sites of known or suspected malignant tissue); any grade 3 or worse drug-related amylase or lipase abnormality that was not associated with symptoms or clinical manifestations of pancreatitis that did not require dose delay, or recurring grade 2 treatment-related adverse events. Dose reductions were not permitted, but dose delays were required for grade 2 adverse events that did not resolve to grade 1 or lower by the last day of the current treatment cycle. Permanent discontinuation from treatment was required for unresolved or recurring grade 2 adverse events (appendix p 6).
Tumour PD-L1 expression level was assessed independently and masked to clinical data by immunohistochemistry using the proprietary Dako PD-L1 IHC73–10 pharmDx assay (Dako, Carpinteria, CA, USA) based on an anti-PD-L1 rabbit monoclonal antibody (clone 73–10; Merck KGaA). PD-L1 status was based on numbers of tumour cells with plasma membrane PD-L1 expression at any intensity using a staining cutoff of 5% or higher.
Mutational load was established by RNASeq (Asuragen, Austin, TX, USA) of RNA extracted from archival slides. Tumour-specific mutations were identified by combining tumour RNASeq data with germline non-tumour whole exome sequencing. Resulting mutations were analysed with NetMHCPan33 to predict peptide binding to major histocompatibility complex molecules of known sequences. Potential neoantigens were weighted based on expression level. Per-sample values were normalised across the dataset based on numbers of reads mapped to genes for each sample.
Outcomes
The primary study objectives were to ascertain the safety and tolerability of avelumab by assessing dose-limiting toxicities during the first 3 weeks of treatment in the dose-escalation part of the study, and to assess best overall response per RECIST version 1.1, adjudicated by an independent endpoint review committee, in efficacy expansion cohorts. Data from the efficacy expansion cohort were pooled with the initial cohort for analysis. Secondary endpoints for expansion cohorts included: safety, best overall response per investigator assessment using modified immune-related RECIST and RECIST version 1.1 (defined as best response obtained among all tumour assessments after the start of the trial until documented disease progression); progression-free survival (defined as first avelumab infusion until documented progressive disease or death, whichever occurred first; patients with no events at the time of analysis were censored at the time of last tumour assessment) and duration of response (defined as the time from the first confirmed response until documented disease progression or death, whichever occurred first; patients with no events at the time of analysis were censored at the time of last tumour assessment), by investigator assessment (per modified immune-related RECIST and RECIST version 1.1) and independent review, overall survival (defined as the time from first avelumab treatment to death; patients still alive, or who were lost to follow-up, were censored at the time of last tumour assessment), and tumour PD-L1 expression level based on the primary staining cutoff threshold of 5% or higher expression.
Safety assessments included incidence and severity of adverse events (per NCI-CTCAE, defined as events with onset dates during the avelumab on-treatment period, or which worsened during the on-treatment period; treatment-related adverse events were defined as possibly being related to avelumab treatment). Additional secondary endpoints were pharmacokinetic and pharmacodynamic profiling and assessment of avelumab immunogenicity; these have been analysed across several cohorts of the JAVELIN Solid Tumor phase 1 study of avelumab and have been reported previously.28
Statistical analyses
After the enrolment of the initial cohort with 44 patients, the planned sample size of 200 patients in the efficacy expansion cohort was chosen to provide an estimate of the proportion of patients achieving an objective response, defined as a complete or partial response (per RECIST version 1.1) in patient subgroups defined by tumour PD-L1 expression status, and was based on the assumption that 30–35% of tumour samples would be PD-L1-positve. For this pre-specified interim analysis, we assessed safety in all patients receiving at least one dose of avelumab and efficacy in the subset of patients with at least 6 months of follow-up, defined for each patient as the time from start of study treatment to analysis cutoff date. Patients with no post-baseline assessments due to discontinuation or death within the first 6 weeks were not evaluable for a confirmed best overall response. Efficacy analyses included the estimated proportion of patients achieving an objective response (a best overall response of complete or partial response based on independent endpoint review committee assessments), with 95% Clopper-Pearson CIs. We deemed that if at least 10% of patients had an objective response this would be indicative of clinical benefit. The association between PD-L1 expression status and response was assessed using Fisher’s exact test. Time-to-event outcomes, such as duration of response, progression-free survival, and overall survival, were estimated using Kaplan-Meier methods, and corresponding 95% CIs were calculated using the Brookmeyer-Crowley method. Best overall response, duration of response, and progression-free survival per investigator assessment were also evaluated. The association between tumour mutational load and response (complete response or partial response vs progressive disease) was assessed using the two-sample Wilcoxon rank sum test. Responses to avelumab per independent review in clinical subgroups were examined as part of a post-hoc exploratory analysis. Data were analysed with SAS (version 9.2) and sample size calculations were done with R (version 2.15.0).
This trial is registered with ClinicalTrials.gov, number NCT01772004.
Role of the funding source
The funder of the study provided the study drug and worked with investigators to design the study; collect, analyse, and interpret the data; and prepare the manuscript. All authors had full access to all data in the study and contributed to the writing, review, and submission of the manuscript. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Between Sept 3, 2014, and March 15, 2016, 329 patients with advanced metastatic urothelial carcinoma were screened for enrolment into this study; 249 eligible patients were enrolled (figure 1, table 1, appendix pp 3, 4). Excluded patients were those who did not meet eligibility criteria (n=67), withdrew consent before starting treatment (n=6), were excluded for other reasons (n=6), or for reasons not reported (n=1). Median age was 68 years (IQR 63–76), and most patients had visceral non-lymph node metastasis at baseline. At enrolment, 13 (5%) of 249 patients, six of whom had been previously treated with cisplatin-based therapy, were considered cisplatin-ineligible because of impaired renal function (six patients [2%]), hearing loss (three [1%]), peripheral neuropathy (three [1%]), and other reasons (two [1%]). Of the 249 patients, seven cisplatin-ineligible patients had never previously received platinum-based treatment; they were included in safety analyses but not in antitumour response analyses. Previous anticancer treatment for metastatic disease included at least two previous lines in 124 (50%) patients (table 1). Tumour specimens from 206 (83%) of 249 patients were evaluable for PD-L1 status (table 1). Of the patients with evaluable samples, 82 (33%) had PD-L1-positive tumours and 124 (50%) had PD-L1-negative tumours. Appendix pp 7–9 provides additional patient demographic data and details about previous anticancer treatments.
Figure 1:
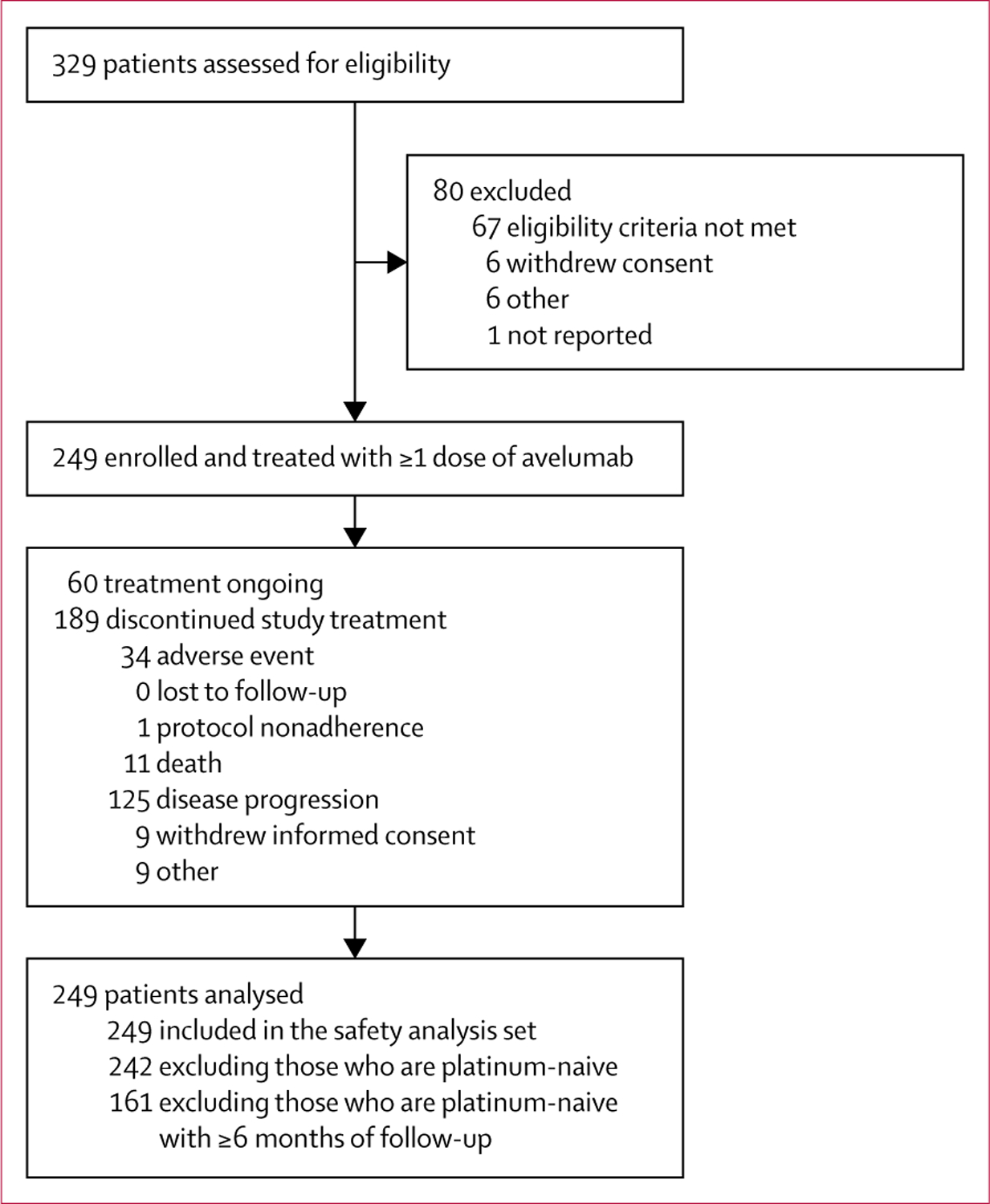
Trial profile
Table 1:
Baseline characteristics
| All participants (n=249) | |
|---|---|
| Age (years) | 68 (63–76) |
| Age category | |
| <65 years | 78 (31%) |
| ≥65 years | 171 (69%) |
| Sex | |
| Male | 178 (72%) |
| Female | 71 (29%) |
| Race or ethnic group | |
| White | 195 (78%) |
| Black or African American | 11 (4%) |
| Asian | 17 (7%) |
| Native Hawaiian or other Pacific Islander | 2 (1%) |
| Other | 24 (10%) |
| Geographical region | |
| USA | 174 (70%) |
| Europe | 63 (25%) |
| Asia | 12 (5%) |
| ECOG performance status | |
| 0 | 88 (35%) |
| 1 | 161 (65%) |
| Subsite of tumour | |
| Upper tract (renal pelvis or ureter) | 58 (23%) |
| Lower tract (bladder or urethra) | 191 (77%) |
| Visceral metastasis | |
| Present | 210 (84%) |
| Absent | 39 (16%) |
| Albumin concentration* | |
| <35 g/L | 45 (18%) |
| ≥35 g/L | 197 (79%) |
| Haemoglobin concentration* | |
| <100 g/L | 41 (16%) |
| ≥100 g/L | 201 (81%) |
| Smoking history | |
| Never smoked | 88 (35%) |
| Ever smoked | 161 (65%) |
| Time since first diagnosis (months) | 20 4 (1 9–289 2) |
| Time since diagnosis of metastatic disease (months) | 11 8 (0 6–70 7) |
| Number of previous anticancer lines for locally advanced or metastatic disease | |
| ≤1 | 119 (48%) |
| 2 | 72 (29%) |
| ≥3 | 52 (21%) |
| Median | 2 0 (1 0–2 0) |
| PD-L1 expression status, ≥5% cutoff† | |
| PD-L1-positive | 82 (33%) |
| PD-L1-negative | 124 (50%) |
| Bellmunt risk score‡ | |
| 0 | 58 (23%) |
| 1 | 114 (46%) |
| 2 | 59 (24%) |
| 3 | 18 (7%) |
| Eligibility status for platinum-based therapy | |
| Yes | 236 (95%) |
| No | 13 (5%) |
Data are median (IQR) or n (%). ECOG= Eastern Cooperative Oncology Group. PD-L1=programmed death ligand 1.
Baseline albumin and haemoglobin concentrations are reported for 242 post-platinum patients.
206 patients were evaluable for PD-L1 expression level; non-evaluable samples (n=43) were those that were missing, of poor quality due to insufficient tumour content or cellular preservation, or otherwise not available to provide results.
Risk group 0 represents patients without any adverse prognostic factors (ie, ECOG performance status >0, haemoglobin concentration <100 g/L, and the presence of liver metastasis); risk groups 1–3 represent the presence of one, two, and three prognostic factors, respectively.4
At data cutoff on June 9, 2016, patients had received a median of six avelumab doses (IQR 3–9) with dosing delays in 66 (27%) of 249 patients. Seven (3%) patients had a dose reduction (defined as <90% of the planned dose administered), and no patient had more than one dose reduction. Reasons for dose reductions included discontinuation of an infusion due to the occurrence of an infusion-related reaction or a dose not adjusted as a result of a patient’s increased bodyweight. Median duration of treatment was 12·0 weeks (IQR 6·0–19·7) and median follow-up was 9·9 months (4·3–12·1). 60 (24%) of 249 patients remained on avelumab at the time of analysis. Of 189 (76%) patients who discontinued study treatment, the most common reason was disease progression, recorded in 125 (50%) patients (figure 1). 50 (20%) of 249 patients received anticancer treatment after discontinuing avelumab, 43 (17%) of whom were given a subsequent drug; this included cytotoxic chemotherapy in 39 (16%) patients, kinase inhibitor in five (2%), antibody therapy in five (2%), and hormonal therapy in one (<1%).
In 161 post-platinum patients with at least 6 months of follow-up, a confirmed objective response (a best overall response of complete or partial response based on independent endpoint review committee assessments) was recorded in 27 patients (17%; 95% CI 11–24), including nine (6%) complete responses and 18 (11%) partial responses per independent review (table 2, figure 2A). The disease control rate (defined as the proportion of patients with a best overall response of complete response, partial response or stable disease) per independent review was 40% (64 of 161 patients), including 37 patients who had stable disease as their best response. 35 (22%) of 161 patients with at least 6 months of follow-up had tumour size reduction of 30% or more from baseline (figure 2B, 2C). The proportion of patients who achieved an objective response per investigator assessment was 26 (16%) of 161 (95% CI 11–23), with five complete and 21 partial responses. The proportion of patients achieving an objective response by immune-related response criteria was 17% (28 of 161, 95% CI 12–24). In patients with a confirmed response per independent review, median time to response was 11·4 weeks (IQR 5·9–17·4) and median duration of response was not reached by data cutoff (95% CI 42·1 weeks to not estimable; figure 2A). Responses were durable and the estimated proportion of responses lasting at least 24 weeks was 96% (95% CI 75–99). Based on immune-related response criteria, the duration of response was not reached by data cutoff (95% CI 42·1 weeks to not estimable), and the estimated proportion of responses lasting at least 24 weeks was 96% (95% CI 73–99). Responses were ongoing at data cutoff in 22 (82%) of 27 patients (range 6·1–75·6+ weeks), including in eight of nine patients with a complete response (figure 2A).
Table 2:
Confirmed best overall response by independent review in patients with ≥6 months of follow-up
| Overall (n=161) | PD-L1-positive patients (≥5%; n=63) | PD-L1-negative patients (<5%; n=76) | |
|---|---|---|---|
| Complete response | 9 (6%) | 6 (10%) | 2 (3%) |
| Partial response | 18 (11%) | 9 (14%) | 8 (11%) |
| Stable disease | 37 (23%) | 18 (29%) | 15 (20%) |
| Non-complete response or non-progressive disease | 1 (1%) | 0 | 1 (1%) |
| Progressive disease | 67 (42%) | 18 (29%) | 38 (50%) |
| Non-evaluable* | 29 (18%) | 12 (19%) | 12 (16%) |
| Confirmed proportion of patients with an objective response | 17% (11–24) | 24% (14–36) | 13% (7–23) |
| Proportion of patients with disease control | 40% | 52% | 33% |
Data are n (%) or % (95% CI). PD-L1=programmed death ligand 1.
Missing or not assessable information: 24 patients had no post-baseline tumour assessment (21 patients died within 6 weeks of enrolment and three withdrew from the study), one patient had post-baseline assessments with an overall response of not evaluable, three patients started new anticancer therapy before the first post-baseline assessment, and one patient had stable disease of insufficient duration.
Figure 2: Antitumour activity of avelumab.
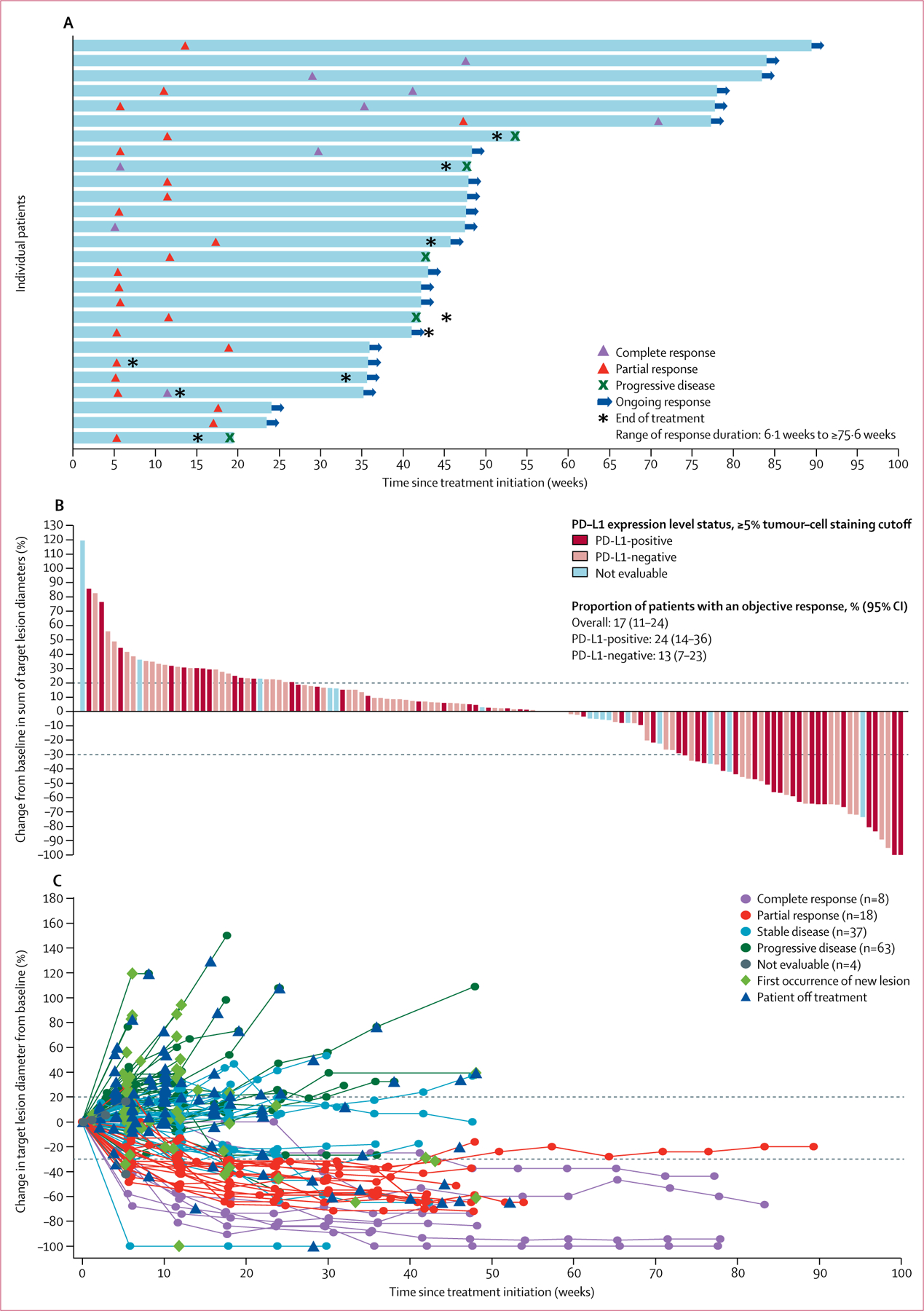
(A) Time and duration of confirmed responses in patients with at least 6 months of follow-up (27 confirmed responses as of data cutoff). (B) Change in size of target lesions from baseline in evaluable patients with at least 6 months of follow-up (n=130) with programmed death ligand 1 (PD-L1) expression status indicated (based on a ≥5% expression staining threshold on tumour cells; non-evaluable specimens [n=16] included those that were missing, of poor quality, or otherwise not available to provide results). The upper dotted line represents progression at 20% increase in size of target lesions and the lower dotted line represents the Response Evaluation Criteria In Solid Tumors (RECIST) boundary for complete response or partial response at 30% decrease in size of target lesions. (C) Percentage change in sum of target lesion diameters from baseline over time for all assessable patients with at least 6 months of follow-up (n=130), defined as those patients with baseline tumour assessments and at least one post-baseline assessment. The upper dotted line represents progression at 20% increase in size of target lesions and the lower dotted line represents the RECIST boundary for complete response or partial response at 30% decrease in size of target lesions.
Responses to avelumab occurred in all clinical subgroups assessed, except the subgroup with a Bellmunt score of 3; this included patients with poor-prognosis factors such as upper tract disease, ECOG performance status of 1, visceral metastasis at baseline, and low baseline albumin and haemoglobin concentrations (figure 3 A). B ased o n 2 9 s amples evaluable for mutational load, an exploratory post-hoc analysis of association between increased mutational load and improved antitumour response did not reach statistical significance ( p=0·076, W ilcoxon r ank s um test; figure 3B).
Figure 3: Subgroup analyses of responses based on patient and disease characteristics at baseline.
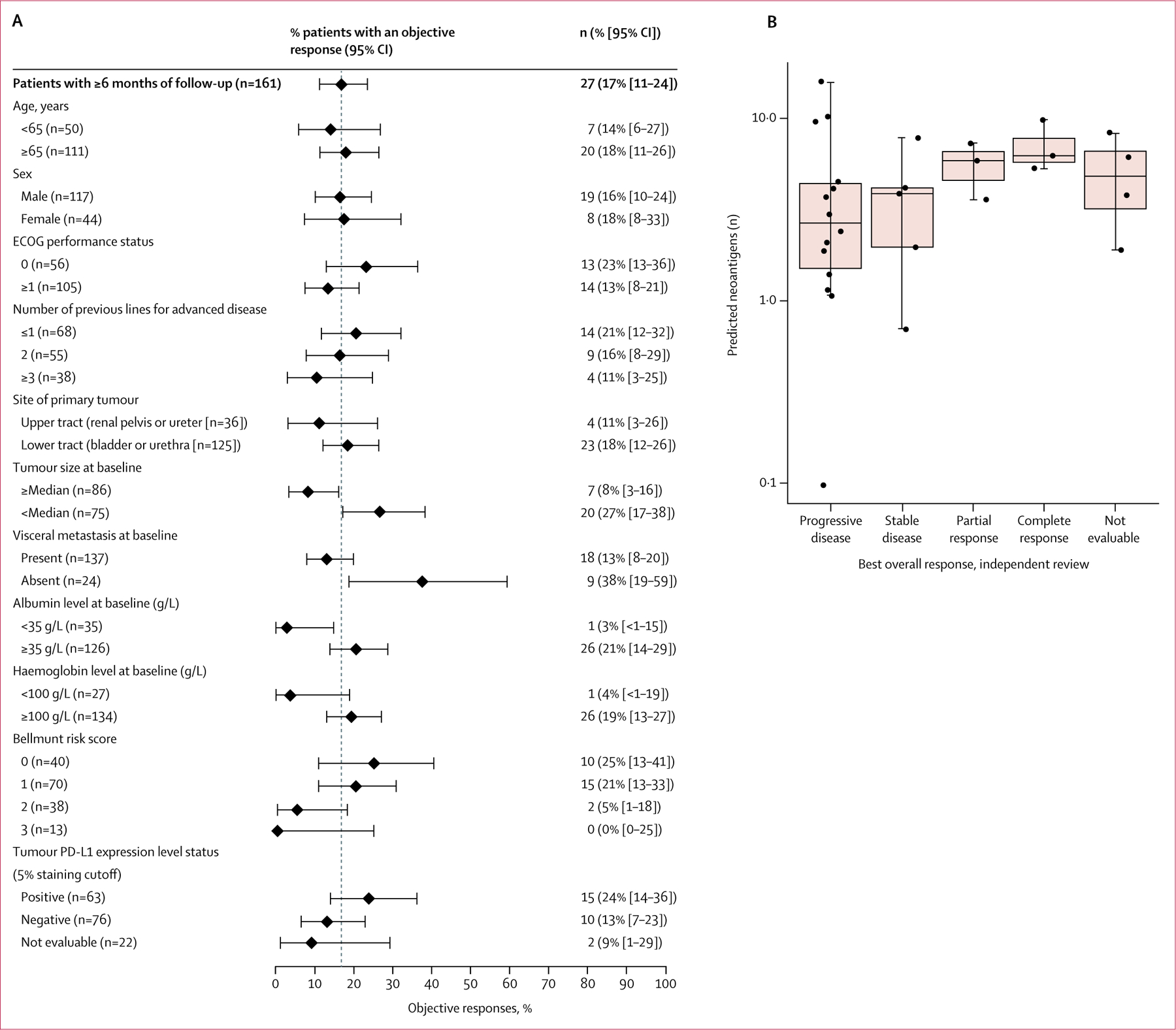
(A) Objective responses by subgroup for select patient characteristics in patients with at least 6 months of follow-up. Plotted points represent % objective responses in each patient subgroup; error bars show 95% CIs. Vertical dashed line represents response rate in the n=161 population (17%). (B) Association of best overall response with tumour mutation based on RNAseq in patients with evaluable samples (n=29). ECOG=Eastern Cooperative Oncology Group. PD-L1=programmed death ligand 1.
Median progression-free survival per independent review was 6·3 weeks (95% CI 6·0–10·1; figure 4A), and the proportion of patients who were progression free at 24 weeks was 23% (95% CI 17–30). At the time of analysis, 124 (77%) of 161 patients had experienced an event, which was disease progression in 94 (58%) patients and death in 30 (19%). Figure 4A shows progression-free survival in patients with PD-L1-positive and PD-L1-negative tumours according to independent review.
Figure 4: Progression-free survival (A) and overall survival (B) in patients with at least 6 months of follow-up (n=161) and according to PD-L1 expression status.
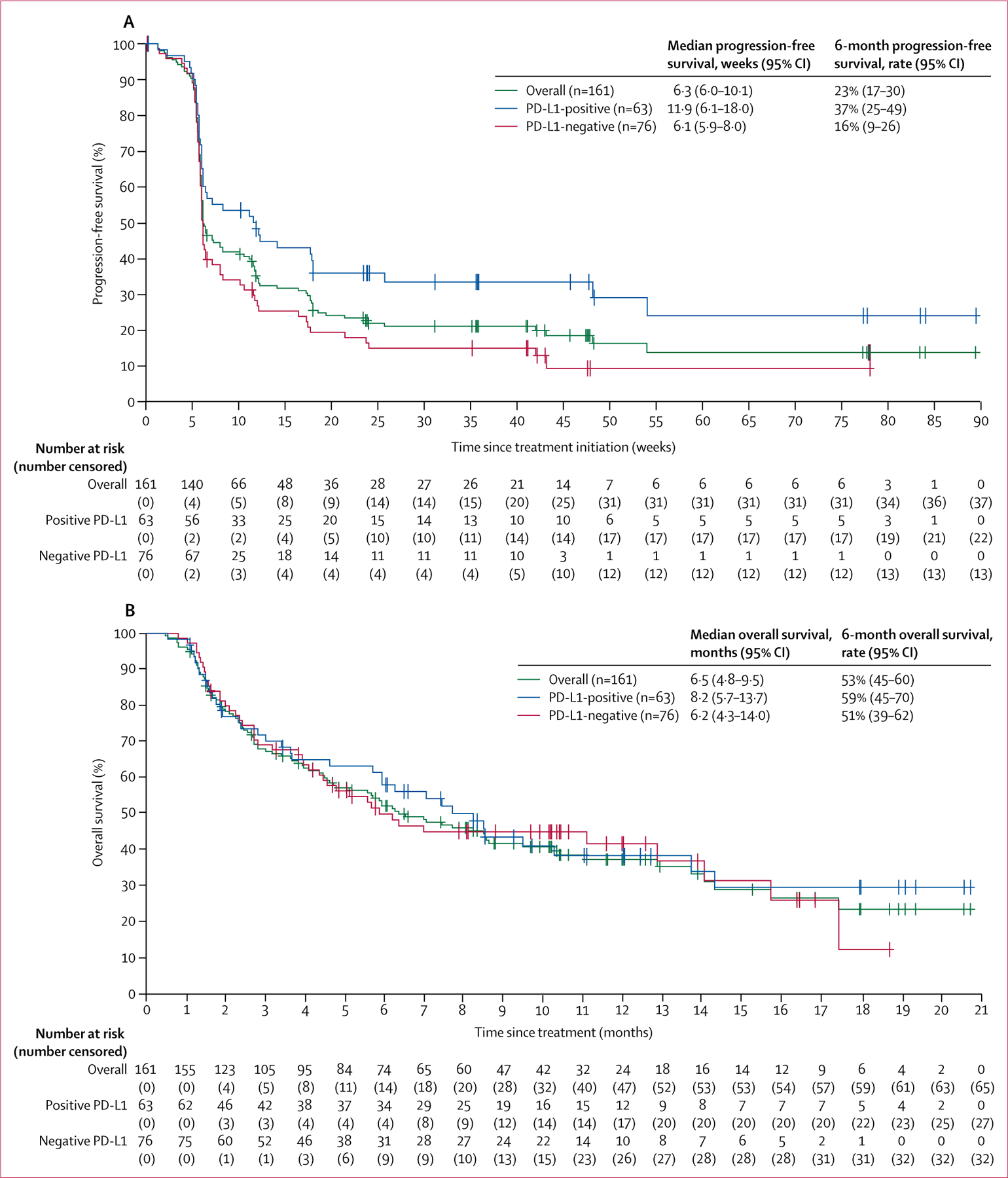
PD-L1 expression based on a 5% expression staining threshold (n=139 evaluable). PD-L1=programmed death ligand 1.
Median progression-free survival based on investigator assessment was 6·6 weeks (95% CI 6·1–11·4), with 24% (18–31) of patients progression free at 24 weeks. Median progression-free survival according to investigator-assessed immune-related response criteria was 11·6 weeks (95% CI 8·0–17·1), with 34% (26–42) of patients progression free at 24 weeks.
Median overall survival was 6·5 months (95% CI 4·8–9·5), and the 6-month overall survival rate was 53% (45–60; figure 4 B); 96 ( 60%) of 161 patients had died at data cutoff. Figure 4B shows overall survival in patients with PD-L1-positive and PD-L1-negative tumours.
In a post-hoc exploratory analysis of progression-free survival by patient subgroups (ECOG performance status, albumin and haemoglobin concentrations, and baseline metastasis), median progression-free survival was 8·3 weeks (95% CI 6·1–18·0) in patients with ECOG performance status 0 versus 6·1 weeks (5·9–8·0) in patients with ECOG performance status 1 or higher; 6·4 weeks (95% CI 6·0–11·6) in patients with albumin concentrations of 35 g/L or higher versus 6·1 weeks (5·4–11·1) in those with concentrations lower than 35 g/L; 6·4 weeks (6·0–11·1) in patients with haemoglobin concentrations 100 g/L or higher versus 6·1 weeks (5·0–12·1) in those with concentrations lower than 100 g/L; and 6·1 weeks (95% CI 6·0–8·0) in patients with baseline visceral metastasis versus 23·7 weeks (5·9–54·0) in those with lymph node-only metastasis.
Additionally, we did a post-hoc exploratory analysis of overall survival by the same clinical subgroups. Median overall survival was not estimable (95% CI 11·1 to not estimable) in patients with ECOG performance status 0 versus 4·3 months (2·7–6·2) in patients with ECOG performance status of 1 or higher; 8·5 months (95% CI 5·9–11·1) in patients with albumin concentrations of 35 g/L or higher versus 4·1 months (2·1–5·9) in those with albumin concentrations lower than 35 g/L; 7·0 months (95% CI 5·1–10·4) in patients with haemoglobin concentrations 100 g/L or higher versus 4·1 months (2·3–13·7) in those with haemoglobin concentrations below 100 g/L; and 5·9 months (95% CI 4·1–8·5) in those with visceral metastases at baseline versus 17·4 months (6·3 to not estimable) in patients with lymph node-only metastasis.
Adverse events of any grade occurred in 244 (98%) of 249 patients, including in 166 (67%) who had a treatment-related adverse event (table 3, appendix p 10). The most frequent treatment-related adverse events of any grade (occurring in ≥10% of 249 patients) were infusion-related reaction, reported in 73 (29%) patients (all grade ≤2 and occurred mostly during the first or second infusions), and fatigue in 40 (16%) patients. 21 (8%) of 249 patients had a grade 3 or worse treatment-related adverse event, the most common of which were fatigue (four [2%]), and asthenia, elevated lipase, hypophosphataemia, and pneumonitis in two (1%) patients each. There were three grade 4 treatment-related adverse events (elevated lipase and creatine phosphokinase concentrations, and hyperkalaemia), and one treatment-related death, due to pneumonitis in a patient with ongoing treatment-unrelated Clostridium difficile colitis and diverticulitis. Adverse events of any cause leading to death occurred in 46 (19%) of 249 patients: disease progression (n=32), sepsis (n=2), and abdominal pain, cerebrovascular accident, deterioration of health, gastrointestinal haemorrhage, intestinal perforation, malignant neoplasm progression, peritoneal metastasis, pneumonitis, respiratory failure, septic shock, unknown causes, and urosepsis (one patient each). Serious adverse events, irrespective of causality, occurred in 117 (47%) of 249 patients; the most commonly reported serious adverse event was disease progression in 40 (16%) patients. Other serious adverse events reported in 2% or more of patients were acute kidney injury and sepsis (six patients [2%] each) and abdominal pain, back pain, dehydration, haematuria, pyrexia, and urinary tract infection (five p atients [ 2%] each). Serious adverse events judged to be related to treatment with avelumab occurred in 19 (8%) of 249 patients, of which infusion-related reaction, diarrhoea, and pneumonitis were reported in more than one patient (three, two, and two patients, respectively). 34 (14%) of 249 patients had immune-related adverse events, mostly rash (n=12; 10%) and hypothyroidism (n=9; 4%). Avelumab was permanently discontinued after a treatment-related adverse event in 14 (6%) patients: infusion-related reaction and pneumonitis (in two [1%] patients each), and adrenal insufficiency, acute kidney injury, arthralgia, diarrhoea, raised concentrations of alkaline phosphatase, aspartate aminotransferase, gamma-glutamyl transferase and lipase, enterocolitis, fatigue, general physical health deterioration, Guillain-Barré syndrome, and rash in one (<1%) patient each.
Table 3:
Treatment-related adverse events and immune-related adverse events
| Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|
| Any event | 145 (58%) | 17 (7%) | 3 (1%) | 1 (<1%) |
| Infusion-related reaction* | 73 (29%) | 0 | 0 | 0 |
| Fatigue | 36 (14%) | 4 (2%) | 0 | 0 |
| Rash† | 36 (14%) | 1 (<1%) | 0 | 0 |
| Diarrhoea‡ | 14 (6%) | 1 (<1%) | 0 | 0 |
| Asthenia | 11 (4%) | 2 (1%) | 0 | 0 |
| Decreased appetite | 10 (4%) | 1 (<1%) | 0 | 0 |
| Hypothyroidism‡ | 10 (4%) | 0 | 0 | 0 |
| Pneumonitis‡ | 4 (2%) | 1 (<1%) | 0 | 1 (<1%) |
| Elevated lipase | 1 (<1%) | 1 (<1%) | 1 (<1%) | 0 |
| Hypophosphataemia | 1 (<1%) | 2 (1%) | 0 | 0 |
| Back pain | 1 (<1%) | 1 (<1%) | 0 | 0 |
| Dehydration | 1 (<1%) | 1 (<1%) | 0 | 0 |
| Elevated ALP | 1 (<1%) | 1 (<1%) | 0 | 0 |
| Elevated AST‡ | 1 (<1%) | 1 (<1%) | 0 | 0 |
| Elevated GGT | 1 (<1%) | 1 (<1%) | 0 | 0 |
| Hyponatraemia | 1 (<1%) | 1 (<1%) | 0 | 0 |
| Hyperthyroidism‡ | 2 (1%) | 0 | 0 | 0 |
| Acute kidney injury | 0 | 1 (<1%) | 0 | 0 |
| Adrenal insufficiency | 0 | 1 (<1%) | 0 | 0 |
| Autoimmune hepatitis‡ | 1 (<1%) | 0 | 0 | 0 |
| Elevated ALT‡ | 1 (<1%) | 0 | 0 | 0 |
| Elevated CPK‡ | 0 | 0 | 1 (<1%) | 0 |
| Enterocolitis‡ | 1 (<1%) | 0 | 0 | 0 |
| General physical health deterioration | 0 | 1 (<1) | 0 | 0 |
| Guillain-Barré syndrome‡ | 0 | 1 (<1%) | 0 | 0 |
| Hyperkalaemia | 0 | 0 | 1 (<1%) | 0 |
| Leucocytosis | 0 | 1 (<1%) | 0 | 0 |
| Rheumatoid arthritis‡ | 1 (<1%) | 0 | 0 | 0 |
| Uveitis‡ | 1 (<1%) | 0 | 0 | 0 |
Data are n (%). The table shows adverse events of any grade in at least 10% of patients or any grade 3 or worse adverse event based on the worst grade per patient, and any immune-related adverse events. The overall summary of safety is shown in appendix p 10.
Infusion-related reaction events occurring on the day of, or day after, infusion included events reported as infusion-related reactions, drug hypersensitivity, or hypersensitivity. Includes signs and symptoms of an infusion-related reaction that resolved within two days of infusion.
Rash includes preferred terms dermatitis exfoliative, erythema, erythema multiforme, pemphigoid, pruritus, pruritus generalised, rash, rash erythematous, rash generalised, rash macular, rash maculopapular, rash papular, and rash pruritic.
Adverse event types considered immune-related. Immune-related adverse events were identified via an expanded adverse event list and medical review. Rash was classified as immune-related by medical review in 12 patients; other adverse events classified as immune-related were: hypothyroidism (n=9), pneumonitis (n=3), and diarrhoea (n=1). All other adverse events marked with the double dagger symbol were classified as immune-related for all cases.
ALP=alkaline phosphatase. ALT=alanine aminotransferase. AST=aspartate aminotransferase. CPK=creatine phosphokinase. GGT=γ-glutamyl transferase.
Discussion
Avelumab monotherapy showed encouraging antitumour activity and a generally manageable safety profile in a large group of patients with platinum-refractory metastatic urothelial carcinoma enrolled in this phase 1 trial. This study had the longest follow-up for avelumab in this expanded patient population from the JAVELIN Solid Tumor study and data formed the basis for the accelerated approval of avelumab by the US Food and Drug Administration, on May 9, 2017, in this indication. Early and durable responses were recorded in 161 patients with at least 6 months of follow-up. The median duration of response was not reached (compared with around 6–7 months with second-line chemotherapy11,34) and the lower limit of the 95% CI of the confirmed proportion of patients achieving an objective response in this study (11%) exceeded a historical chemotherapy control of 10%.11
Responses were recorded across most clinical subgroups assessed, including patients with or without adverse prognostic factors such as metastasis to visceral sites. Exploratory subgroup analyses suggested that the proportion of patients achieving an objective response seemed to be higher in those with a lower disease burden or with no established poor prognostic factors, such as visceral metastasis or low albumin or haemoglobin concentrations, but the small size of these subgroup analyses preclude robust conclusions. Another prognostic factor that may have provided additional insight into antitumour activity of avelumab relative to aggressiveness of disease is the treatment-free interval before starting avelumab treatment; however, a limitation of this analysis is that data for treatment-free interval are not available.
In this study, the first t umour e valuations o ccurred 6 weeks following avelumab infusion; median progression-free survival was 6·3 weeks with a median follow-up of 9·9 months. Median progression-free survival reported in patients receiving other anti-PD-L1 or anti-PD-1 antibodies is roughly 2 months.17,18,21–23 Median overall survival was 6·5 months and the 6-month overall survival was 53%. Preliminary overall survival analyses by clinical subgroup are limited by the shorter follow-up duration, relative to that previously reported for the initial small cohort of patients. Although the short follow-up time and non-randomised study design are limitations of this report, longer-term analysis of response durability and survival is ongoing. Although no direct comparisons of antitumour activity with other anti-PD-L1 or anti-PD-1 agents can be made because no head-to-head trials exist, and study designs, treatment settings, and patient populations (including PD-L1 enrichment) differ a cross trials, antitumour responses to avelumab are similar to those reported for other US FDA-approved anti-PD-L1 and anti-PD-1 monoclonal antibodies—namely, atezolizumab, durvalumab, nivolumab, and pembrolizumab.16–18,21–23 Although in-vitro studies have shown antibody-dependent cellular cytotoxicity induced by avelumab, clinical antibody-dependent cellular cytotoxicity activity has so far not been established in avelumab-treated patients.29,30
Antitumour responses of avelumab in the pooled cohorts of patients with metastatic urothelial carcinoma were derived from a modest median 9·9 months of follow-up; however, the encouraging antitumour responses reported in the previous cohort of 44 patients with metastatic urothelial carcinoma were based on the substantially longer follow-up time of at least 15 months.19 Analysis of overall survival at 12 months in this pooled population of patients with metastatic urothelial carcinoma is planned.
The association between PD-L1 status and anti-PD-L1 or anti-PD-1 treatment activity in urothelial carcinoma is still uncertain. The use of PD-L1 as a biomarker could be complicated by several pre-analytical and analytical factors, including patient populations enrolled (cisplatin-ineligible, platinum-naive, or post-platinum), spatial heterogeneity of PD-L1 expression within tumours, variability in tissue collection between trials (eg, fresh vs archival samples, and tissue fixation procedures), antibody and assay used for staining, definition of PD-L1 positivity and associated scoring algorithm, use of non-standardised test designs, and the assessment of PD-L1 expression on tumour-infiltrating lymphocytes or other immune cells in the tumour microenvironment compared with expression on tumour cells.35 Data from other clinical trials of anti-PD-L1 and anti-PD-1 antibodies in metastatic urothelial carcinoma show variable results in terms of associations between response and level of PD-L1 expression.16–18,20–23 In this study, which used a PD-L1 expression cutoff of at least 5%, the association between PD-L1 expression level on tumour cells and avelumab antitumour activity was weaker than had been recorded in an earlier analysis of 44 patients with metastatic urothelial carcinoma treated with avelumab,19 the results of which were used to inform the PD-L1 cutoff selection for assay development in this pooled analysis. Furthermore, the proprietary Dako PD-L1 IHC 73–10 pharmDx assay that was used in this pooled analysis has been previously shown in non-small-cell lung cancer samples to have greater sensitivity at a low frequency of tumour PD-L1 expression relative to another immunohistochemistry assay used in trials of an anti-PD-1 inhibitor.36 Other ongoing analyses will assess the predictive value of PD-L1 expression levels on tumour-infiltrating immune cells in patients with metastatic urothelial carcinoma treated with avelumab.
The relatively small number of evaluable samples available for mutational load analysis is a limitation of this study. Mutational load was analysed using a previously unpublished method that can be done when biological samples are few in number; a separate techniques manuscript on this method is planned. An association between increased mutational load and improved outcome has been reported in similar analyses with other anti-PD-L1 or anti-PD-1 inhibitors;17,20,22,23 however, our similar analysis in a small subset of patients did not reach statistical significance. Assessment of antitumour activity related to mutational load and PD-L1 expression as a combined measure could provide further insights into the possible predictive role of these biomarkers; however, this analysis could not be done because of the small number of evaluable samples available. Other disease-associated molecular and genetic signatures—such as immune gene expression on effector T cells, molecular subtyping based on The Cancer Genome Atlas analysis or luminal or basal subgroups, and mutational load profiling—also show promise as diagnostic biomarkers of response in urothelial carcinoma.23 Additional investigations of biomarkers for urothelial carcinoma and other avelumab-treated patient populations, including tumour mutational burden, gene-expression signatures, and tumour-infiltrating lymphocytes in the tumour microenvironment, are ongoing. Efforts to cross-validate and standardise these diagnostic assays in urothelial carcinoma and other cancers are underway.27,37
The safety profile of avelumab was consistent with reports of avelumab in other tumour types28,31,32,38 and was generally similar to other anti-PD-L1 and anti-PD-1 antibodies in urothelial carcinoma.16–18,20–23 Treatment with avelumab was well tolerated for prolonged treatment duration, with few permanent treatment-related discontinuations. Treatment-related infusion-related reactions, based on prespecified analyses of fever, chills, and rigors occurring after infusion on the day of administration or the following day, were mild (all grade 1 or 2), occurred mostly at the time of the first or second infusion, and rarely led to treatment discontinuation. Pooled clinical findings across multiple cohorts of the JAVELIN Solid Tumor trial suggest that premedication with diphenhydramine and acetaminophen decreases the severity of infusion-related reactions;31 however, no formal statistical analyses have been done, and this finding was not assessed in the present analysis. The frequencies of grade 3 or worse treatment-related adverse events and immune-related adverse events were low. The favourable tolerability of avelumab and similar agents might enable treatment of a wider population of patients with metastatic urothelial carcinoma, many of whom present with factors such as advanced age, impaired renal function, cardiovascular disease, neuropathy, and hearing loss, which limit use of chemotherapy.10
Durable and complete responses following first-line chemotherapy in patients with metastatic urothelial carcinoma are rare. Given avelumab’s encouraging antitumour activity in post-platinum patients and safety profile in post-platinum and cisplatin-naive patients with metastatic urothelial carcinoma, an ongoing phase 3 trial will assess the antitumour activity of maintenance treatment with avelumab plus best supportive care compared with best supportive care alone in patients with metastatic urothelial carcinoma whose disease has not progressed after completion of first-line chemotherapy with a platinum-based regimen (NCT02603432). Overall, our findings suggest that avelumab is generally well tolerated and shows promising antitumour activity in patients with platinum-refractory metastatic urothelial carcinoma.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed and selected websites of annual congress abstracts to find articles published between Jan 1, 2011, and May 20, 2017. Search terms queried included “PD-L1” and “PD-1” and relevant generic and investigational drug names of immune checkpoint inhibitors. Additional search terms were “bladder,” “urothelial,” “carcinoma,” and “cancer.” Although our search included descriptions of studies in all lines of treatment, we focused on those articles specific to treatment of patients in post-platinum settings. We identified a rapidly expanding therapeutic landscape for treatment of patients with urothelial carcinoma whose disease is refractory to or progressive after platinum-based therapy and for whom there have been few treatment options with durable responses. Between 2016 and 2017, several phase 1 and 2 trials of anti-programmed death ligand 1 (PD-L1) and anti-programmed death-1 (PD-1) monoclonal antibodies reported encouraging antitumour activity and safety for treatment of advanced or metastatic urothelial carcinoma. The US Food and Drug Administration (FDA) subsequently approved use of five anti-PD-L1 and anti-PD-1 monoclonal antibodies for treatment of this disease, including four on an accelerated basis (atezolizumab, nivolumab, avelumab, and durvalumab); full approval of pembrolizumab, a fifth member of this class of drugs, was announced after positive results from a phase 3 trial. Despite these advances, urothelial carcinoma remains an area of great unmet medical need, with standard second-line chemotherapy regimens resulting in poor antitumour responses and safety. Avelumab, a human IgG1 monoclonal antibody that inhibits PD-L1, has shown promising antitumour activity and safety in patients with metastatic urothelial carcinoma previously treated with platinum-based chemotherapy regimens, providing a rationale for extended pooled analyses of avelumab in two cohorts of patients with metastatic urothelial carcinoma refractory to or who have progressed following platinum-based chemotherapy and in patients who are cisplatin-naive.
Added value of this study
After encouraging results from a smaller cohort of patients, this study is the largest study so far to report the activity and safety of avelumab in patients with metastatic, chemotherapy-refractory urothelial carcinoma. The pooled analysis presented in this report constituted the basis for the US FDA accelerated approval of avelumab for patients with locally advanced or metastatic urothelial carcinoma whose disease has recurred following platinum-containing chemotherapy given for first-line metastatic disease, or within 12 months of neoadjuvant or adjuvant chemotherapy for muscle-invasive bladder cancer. Avelumab monotherapy resulted in confirmed objective responses in patients irrespective of their PD-L1 expression status, and resulted in an acceptable and tolerable safety profile with few grade 3 or worse treatment-related adverse events. Based on the findings from initial and expanded efficacy cohorts of patients with locally advanced or metastatic urothelial carcinoma refractory to or progressed following platinum-based chemotherapy, the US FDA recently granted accelerated approval of avelumab for the treatment of patients in this disease setting.
Implications of all the available evidence
Our findings and the results from previous trials with other anti-PD-L1 and anti-PD-1 monoclonal antibodies suggest that these agents exert antitumour activity and are safe in patients with advanced disease, including those with factors known for poor prognoses. Antitumour activity across the different anti-PD-L1 and anti-PD-1 agents studied so far seems to be similar in populations unselected for PD-L1 status. The antitumour activity and safety profiles of anti-PD-L1 or PD-1 agents as monotherapy in post-platinum settings provide precedence for the study of these agents in earlier disease settings.
Acknowledgments
We thank the patients and their families, investigators, co-investigators, and the study teams at each of the participating centres and at Merck KGaA, Darmstadt, Germany, and EMD Serono, Billerica, MA, USA (a business unit of Merck KGaA, Darmstadt, Germany); and Alexander Rolfe for his assistance in the analysis of antitumour activity based on tumour mutational load. This trial was funded by Merck KGaA, Darmstadt, Germany, and is part of an alliance between Merck KGaA and Pfizer, Inc, New York, NY, USA. Medical writing support was provided by ClinicalThinking Inc, Hamilton, NJ, USA, and funded by Merck KGaA and Pfizer, Inc.
Declaration of interests
MA holds stock and other ownership interests in Caremission and WCCT Global. CDB has received institutional grant support from EMD Serono during the conduct of the study and grants from Five Prime Therapeutics, Pfizer, Merck, and Roche outside of the submitted work. CDB has received housing and travel accommodations from Five Prime Therapeutics and Pfizer outside of the submitted work. K-WL has received institutional grant support from Merck KGaA during the conduct of the study, and grants from Ono Pharmaceutical, AstraZeneca, and MSD outside of the submitted work. MT has received honoraria from and is a member of the advisory board of Eisai Inc, Bristol-Myers Squibb, Blue Print Medicines, and Trillium Pharma for work performed outside this submission. MT has delivered non-promotional/unbranded talks at Eisai Inc and Bristol-Myers Squibb for work outside of this submission. PS has received institutional funding from Bayer, Blueprint Medicines, CoBioRes NV, Exelixis, GSK, Novartis, and Plexxikon. PS has received honoraria from Daiichi Sankyo, Eisai, Eli Lilly, Medpace, Novartis, and Swedish Orphan Biovitrium. PS has a consulting or advisory role for 6th Element Capital, Adaptimmune, Amcure, AstraZeneca, Bayer, Blueprint Medicines, Bristol-Myers Squibb, Boehringer Ingelheim, Cristal Therapeutics, Daiichi Sankyo Eisai, Eli Lilly, Epizyme, Genzyme, Ipsen, Loxo Oncology, Medpace, Nektar, Novartis, Philogen, Piqur Therapeutics, and Plexxikon. PS is on the speaker bureaus of Bayer, Eisai, Eli Lilly, GSK, Novartis, PharmaMar, and Swedish Orphan Biovitrium. PS has received funding for travel and accommodations from 6th Element Capital, Adaptimmune, Amcure, AstraZeneca, Bayer, Blueprint Medicines, Bristol-Myers Squibb, Boehringer Ingelheim, Cristal Therapeutics, Daiichi Sankyo, Eisai, Eli Lilly, Epizyme, Genzyme, GSK, Ipsen, Loxo Oncology, Medpace, Nektar, Novartis, PharmaMar, Philogen, Piqur Therapeutics, Plexxikon, and Swedish Orphan Biovitrium. During the conduct of the study, AR has received institutional grant support from Pfizer, and funding for travel and accommodations from Pfizer, Novartis, Bristol-Myers Squibb, AstraZeneca, Roche, and MSD. AR also reports grants and funding for travel and accommodation from Ipsen outside of the submitted work. JLG’s institution has a cooperative research and development agreement with, and has received funding for drug development from Merck KGaA, Darmstadt, Germany. ABG, JX, and GR are employees of EMD Serono Research & Development Institute, Inc, a business of Merck KGaA, Darmstadt, Germany, that, together with Alliance partner Pfizer, Inc, sponsors the avelumab clinical trial programme. All other authors declare no competing interests.
Contributor Information
Manish R Patel, Florida Cancer Specialists/Sarah Cannon Research Institute, Sarasota, FL, USA.
John Ellerton, Nevada Cancer Research Foundation, Las Vegas, NV, USA.
Jeffrey R Infante, Sarah Cannon Research Institute/Tennessee Oncology, Nashville, TN, USA.
Manish Agrawal, Associates in Oncology, Rockville, MD, USA.
Michael Gordon, Pinnacle Oncology Hematology, A Division of Arizona Center for Cancer Care, HonorHealth Research Institute Clinical Trials Program at the Virginia G Piper Cancer Center, University of Arizona College of Medicine, Phoenix, Scottsdale, AZ, USA.
Raid Aljumaily, Oklahoma University Medical Center, Oklahoma City, OK, USA.
Carolyn D Britten, Medical University of South Carolina, Division of Hematology/Oncology, Charleston, SC, USA.
Luc Dirix, Sint-Augustinus Hospital, Oncology Center, Medical Oncology, Antwerpen, Belgium.
Keun-Wook Lee, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, South Korea.
Mathew Taylor, Oregon Health and Science University, Knight Cancer Institute, Portland, OR, USA.
Patrick Schöffski, Department of General Medical Oncology, Leuven Cancer Institute, University Hospitals Leuven, Leuven, Belgium.
Ding Wang, Henry Ford Hospital, Detroit, MI, USA.
Alain Ravaud, Groupe Hospitalier Saint Andr, Hpital Saint Andr, CHU de Bordeaux, Bordeaux Cedex, France.
Arnold B Gelb, EMD Serono Research & Development Institute, Inc, Billerica, MA, USA.
Junyuan Xiong, EMD Serono Research & Development Institute, Inc, Billerica, MA, USA.
Galit Rosen, EMD Serono Research & Development Institute, Inc, Billerica, MA, USA.
James L Gulley, Genitourinary Malignancies Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA, and Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Andrea B Apolo, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, Magnuson Clinical Center, Bethesda, MD, USA.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortalitiy worldwide: IARC CancerBase no. 11. Lyon, France: International Agency for Research on Cancer. 2013. http://globocan.iarc.fr (accessed July 6, 2017). [Google Scholar]
- 2.National Cancer Institute, Surveillance, Epidemiology, and End Results Program. SEER stat fact sheets: bladder cancer. 2016. https://seer.cancer.gov/statfacts/html/urinb.html (accessed July 6, 2017).
- 3.Chism DD, Apolo AB, Milowsky MI. Chemotherapy for metastatic bladder cancer. In: Xylinas E, Shariat SF, eds. Advances in bladder cancer management. London, UK: Future Science Group, 2015: 206–20. [Google Scholar]
- 4.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 2010; 28: 1850–55. [DOI] [PubMed] [Google Scholar]
- 5.NCCN. NCCN clinical practice guidelines in oncology (NCCN guidelines) for bladder cancer. 2017. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf (accessed June 6, 2017).
- 6.Kaufman D, Raghavan D, Carducci M, et al. Phase II trial of gemcitabine plus cisplatin in patients with metastatic urothelial cancer. J Clin Oncol 2000; 18: 1921–27. [DOI] [PubMed] [Google Scholar]
- 7.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000; 18: 3068–77. [DOI] [PubMed] [Google Scholar]
- 8.Sternberg CN, de Mulder PH, Schornagel JH, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer protocol no. 30924. J Clin Oncol 2001; 19: 2638–46. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Gill D, Poole A, Agarwal N. Systemic immunotherapy for urothelial cancer: Current trends and future directions. Cancers 2017; 10.3390/cancers9020015. [DOI] [PMC free article] [PubMed]
- 10.Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol 2011; 29: 2432–38. [DOI] [PubMed] [Google Scholar]
- 11.Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009; 27: 4454–61. [DOI] [PubMed] [Google Scholar]
- 12.Vaughn DJ, Broome CM, Hussain M, Gutheil JC, Markowitz AB. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol 2002; 20: 937–40. [DOI] [PubMed] [Google Scholar]
- 13.Galsky MD, Mironov S, Iasonos A, Scattergood J, Boyle MG, Bajorin DF. Phase II trial of pemetrexed as second-line therapy in patients with metastatic urothelial carcinoma. Invest New Drugs 2007; 25: 265–70. [DOI] [PubMed] [Google Scholar]
- 14.McCaffrey JA, Hilton S, Mazumdar M, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol 1997; 15: 1853–57. [DOI] [PubMed] [Google Scholar]
- 15.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016; 39: 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn N, Powles T, Massard C, et al. Updated efficacy and tolerability of durvalumab in locally advanced or metastatic urothelial carcinoma (UC). Proc Am Soc Clin Oncol 2017; 35: (abstr 4525). [Google Scholar]
- 17.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017; 18: 312–22. [DOI] [PubMed] [Google Scholar]
- 18.Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol 2017; 18: 212–20. [DOI] [PubMed] [Google Scholar]
- 19.Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase 1b study. J Clin Oncol 2017; 35: 2117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017; 389: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajorin DF, de Wit R, Vaugh DJ, et al. Planned survival analysis from KEYNOTE-045: Phase 3, open-label study of pembrolizumab (pembro) versus paclitaxel, docetaxel, or vinflunine in recurrent, advanced urothelial cancer (UC). J Clin Oncol 2017; 35: abstr 4501. [Google Scholar]
- 22.Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol 2016; 17: 1590–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387: 1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother 2007; 56: 1173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boorjian SA, Sheinin Y, Crispen PL, et al. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res 2008; 14: 4800–08. [DOI] [PubMed] [Google Scholar]
- 26.Bellmunt J, Mullane SA, Werner L, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol 2015; 26: 812–17. [DOI] [PubMed] [Google Scholar]
- 27.Scheel AH, Dietel M, Heukamp LC, et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol 2016; 29: 1165–72. [DOI] [PubMed] [Google Scholar]
- 28.Heery CR, O’Sullivan-Coyne G, Madan RA, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol 2017; 18: 587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyerinas B, Jochems C, Fantini M, et al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res 2015; 3: 1148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujii R, Friedman ER, Richards J, et al. Enhanced killing of chordoma cells by antibody-dependent cell-mediated cytotoxicity employing the novel anti-PD-L1 antibody avelumab. Oncotarget 2016; 7: 33498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly K, Infante JR, Taylor MH, et al. Safety profile of avelumab in patients with advanced solid tumors: A JAVELIN pooled analysis of phase 1 and 2 data. Proc Am Soc Clin Oncol 2017; 35 (abstr 3059). [Google Scholar]
- 32.Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017; 18: 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen M, Lundegaard C, Blicher T, et al. NetMHCpan, a method for quantitative predictions of peptide binding to any HLA-A and -B locus protein of known sequence. PLoS One 2007; 2: e796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaughn DJ, Srinivas S, Stadler WM, et al. Vinflunine in platinum-pretreated patients with locally advanced or metastatic urothelial carcinoma: Results of a large phase 2 study. Cancer 2009; 115: 4110–17. [DOI] [PubMed] [Google Scholar]
- 35.Novotny JF Jr, Cogswell J, Inzunza H, Harbison C, Horak C, Averbuch S. Establishing a complementary diagnostic for anti-PD-1 immune checkpoint inhibitor therapy. Ann Oncol 2016; 27: 1966–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng Z, Schlichting M, Helwig C, et al. Comparative study of two PD-L1 expression assays in patients with non-small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol 2017; 35 (abstr e20581). [Google Scholar]
- 37.Sweis RF, Galsky MD. Emerging role of immunotherapy in urothelial carcinoma-immunobiology/biomarkers. Urol Oncol 2016; 34: 556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 2016; 17: 1374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


