Abstract
Aims
Cisplatin-based chemotherapy (CBCT) is essential in the treatment of metastatic testicular cancer (TC) but has been associated with long-term risk of cardiovascular morbidity and mortality. Furthermore, cisplatin can be detected in the body decades after treatment. We aimed to evaluate the long-term impact of CBCT on cardiac function and morphology in TC survivors 30 years after treatment.
Methods and results
TC survivors treated with CBCT (1980–94) were recruited from the longitudinal Norwegian Cancer Study in Testicular Cancer Survivors and compared with a control group matched for sex, age, smoking status, and heredity for coronary artery disease. All participants underwent laboratory tests, blood pressure measurement, and 2D and 3D echocardiography including 2D speckle-tracking strain analyses. Ninety-four TC survivors, on average 60 ± 9 years old, received a median cumulative cisplatin dose of 780 mg (IQR 600–800). Compared with controls, TC survivors more frequently used anti-hypertensive (55% vs. 24%, P < 0.001) and lipid-lowering medication (44% vs. 18%, P < 0.001). TC survivors had worse diastolic function parameters with higher E/e′-ratio (9.8 ± 3.2 vs. 7.7 ± 2.5, P < 0.001), longer mitral deceleration time (221 ± 69 vs. 196 ± 57ms, P < 0.01), and higher maximal tricuspid regurgitation velocity (25 ± 7 vs. 21 ± 4 m/s, P = 0.001). The groups did not differ in left or right ventricular systolic function, prevalence of arrhythmias, or valvular heart disease. Cumulative cisplatin dose did not correlate with cardiac parameters.
Conclusion
No signs of overt or subclinical reduction in systolic function were identified. Long-term cardiovascular adverse effects three decades after CBCT may be limited to metabolic dysfunction and worse diastolic function in TC survivors.
Keywords: cancer survivorship, testicular cancer, echocardiography, cardiotoxicity, cisplatin
Graphical Abstract
Introduction
Since its introduction in the late 1970s, cisplatin-based chemotherapy (CBCT) has revolutionized the treatment of metastatic testicular cancer (TC) and dramatically increased survival rates.1 Today, cancer-specific survival is nearly universal in localized disease and ∼90% in metastatic disease.2,3 Since TC primarily affects young men, with a close to normal life expectancy after successful treatment, evaluation of treatment-related adverse health outcomes are of great importance.
With increasing survival rates during the past decades, more attention has been directed to the short- and long-term health effects of CBCT. Platinum-based compounds are strongly nephrotoxic, neurotoxic, and ototoxic.4 They have also been implicated in the development of the metabolic syndrome, vascular damage leading to hypertension, increased incidence of thromboembolic events, and a wide array of adverse cardiac effects.5,6 The latter group includes both indirect effects from hypertension and hyperlipidaemia and the direct effects of myocardial damage through mitochondrial dysfunction, overproduction of reactive oxygen species, and even necrosis and apoptosis of cardiomyocytes.5 Exploring these effects becomes especially pertinent as TC primarily affects young men and circulating serum-platinum (s-Pt) can be detected decades after cessation of treatment, possibly exposing cardiomyocytes to continuous long-term toxicity.7
Several studies have examined the link between CBCT and long-term cardiac morbidity and mortality, but few have evaluated cardiac function directly. Both van Schinkel et al.8 and Altena et al.9,10 found evidence of worse diastolic function in TC survivors, but were not able to show systolic impairment. However, these studies are limited by short follow-up, small sample size, and did not use deformation imaging to assess myocardial function.
This study aims to assess the impact of CBCT on cardiac risk factors, cardiac morphology, and left and right ventricular (LV and RV) function in long-term TC survivors and evaluate whether this population is at risk for chemotherapy-induced heart failure, similar to that seen after treatment with anthracyclines.11 Furthermore, we aimed to evaluate the impact of cumulative cisplatin dose on the same parameters in this population.
Methods
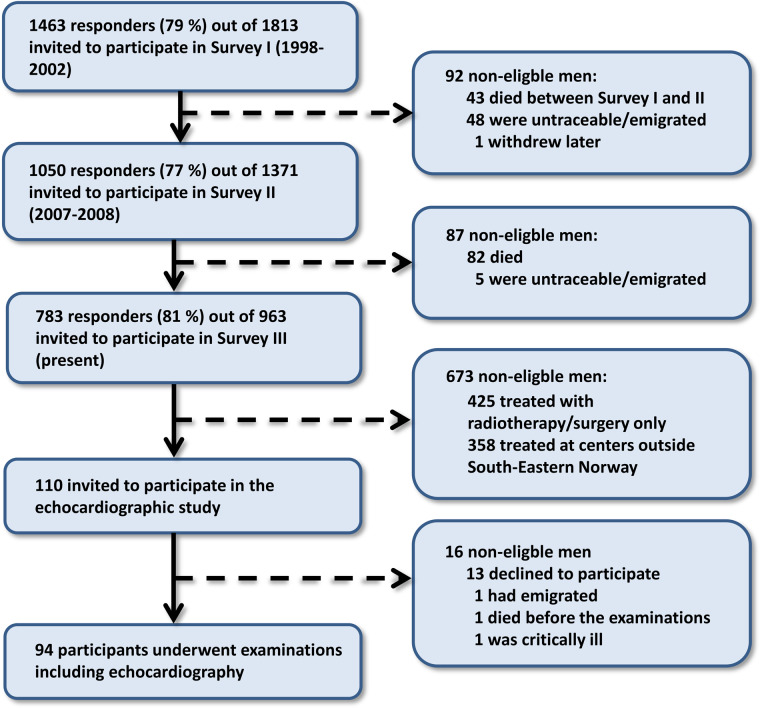
From April 2017 to May 2018, survivors of unilateral germ cell TC who had been treated between 1980 and 1994 and who participated in the third survey in the National study on TC survivors were invited to the present study (Figure 1). Only survivors living in the south-east of Norway treated with CBCT without concurrent mediastinal radiotherapy were eligible for inclusion.
Figure 1.
Study inclusion—flowchart of recruitment of participants from the Norwegian national study in testicular cancer survivors.
A total of 110 TC survivors of the 783 responders were both residents in the South-East of Norway and had received CBCT without radiotherapy. Blood samples were collected and analysed during the third survey in the National study on TC survivors in 2015–16.12 All other data, including a comprehensive echocardiography, blood pressure measurements, and a self-reported questionnaire on cardiac risk factors, medications, and cardiac morbidity were collected in 2017–18.
All patients had undergone orchiectomy. Subsequent treatment followed the protocols of the European Organization for Research and Treatment of Cancer Genito-Urinary Group or the Swedish-Norwegian Testicular Cancer Project.13–15 The majority of patients with disseminated disease received routinely four cycles of cisplatin, vinblastine, and bleomycin (CVB) until 1986, later three to four cycles of bleomycin, etoposide, and cisplatin (BEP). For patients undergoing primary removal of retroperitoneal lymph node metastases, two cycles of CBCT was given as adjuvant therapy.16
From February to June 2019, a control group of males was recruited from a database set up from the ‘Lier registry’, a population health study in conjunction with the Norwegian municipality of Lier.17 The control group was matched for age, smoking status, and heredity for coronary artery disease (CAD).
Written informed consent was given by all study participants. The study complies with the Declaration of Helsinki and was approved by the Regional Committee for Medical Research Ethics (ref. 2011/659 S-08702d).
Transthoracic echocardiography
All participants underwent an echocardiographic study (Vivid E9/E95, GE, Vingmed, Horten, Norway). Standard echocardiographic images were obtained from the parasternal long-axis, short axis, apical four-, three-, and two-chamber and subcostal views. All measurements were analysed post hoc using dedicated software (EchoPac, GE, Vingmed). All measurements were performed by a single observer. Diastolic function was assessed by two-dimensional echocardiography (2DE). Right atrial (RA) and RV dimensions were measured in the apical four-chamber view. Left atrial (LA) volume was measured using the biplane method in the four- and two-chamber apical views. Diastolic function was assessed using LA volume, Doppler-derived velocities across the mitral valve, tissue Doppler velocities, and tricuspid regurgitation velocity. All measurements were performed in accordance with recommendations from the European Association of Cardiovascular Imaging (EACVI).18,19 Diastolic dysfunction was considered present if more than half of the following criteria were met: average E/e′ >14, septal e′ <7 cm/s or lateral e′ <10 cm/s, TR velocity > 2.8 m/s, LA indexed volume >34 mL/m2.19 Heart failure with reduced ejection fraction (HFrEF) was considered present if the measured ejection fraction was ≤40% and the patient experienced symptoms of heart failure.20 Heart failure with preserved ejection fraction (HFpEF) was considered present if the Heart Failure Association Pre-test assessment, Echocardiography and natriuretic peptide, Functional testing, Final aetiology (HFA-PEFF) score was equal or greater than five, in accordance with the 2019 Heart Failure Association/European Society of Cardiology consensus recommendation.21 All 2D and 3D measurements were indexed to body surface area (BSA). LV dimensions, mass, ejection fraction, and stroke volume were measured in 3D using a semi-automatic technique.
Two-dimensional echocardiography acquisitions were used for speckle-tracking strain analysis. Global longitudinal strain (GLS) was calculated from peak systolic longitudinal strain in 16 segments using the apical four-, three-, and two-chamber views. The frame rate was 63 ± 11 Hz.
Significant valve pathology was considered present if the patients had at least one of the following: moderate or severe mitral or aortic valve insufficiency, any aortic, or mitral valve stenosis. Classification of valve disease was performed according to the guidelines.22
Blood sampling, blood pressure, and definitions
Blood samples were obtained from all participants at Oslo University Hospital. In all patients, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides, C-reactive protein (CRP), and N-terminal pro-brain natriuretic peptide (NT-proBNP) were analysed on Cobas 6000 and 8000 (Roche Diagnostics, Mannheim, Germany).
Blood pressure was measured in the supine position before and after the echocardiography. The average of the two measurements was used. Weight and height were self-reported. Metabolic syndrome and obesity were defined in accordance with guidelines from the American Heart Association.23 Hypercholesterolaemia was defined as the use of lipid-lowering medication, total cholesterol of >7 mmol/L, or LDL ≥ 3.6 mmol/L. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or the use of antihypertensive medication.24
Statistical analysis
We conducted an a priori power analysis to test the difference in GLS between two independent groups using a two-tailed test. We used an effect size of 1% and an alpha of 0.05. Results showed that two equally sized groups of 84 were required to achieve a power of 0.90.
Analyses were carried out using Stata 15.0 (StataCorp LLC, Texas, USA). Data were presented as mean ± SD or numbers and percentages. The χ2 test (categorical variables) and the Student’s t-test (continuous variables) were used to determine differences between groups. For non-parametric continuous parameters, the Mann–Whitney U test was used. Correlation between two continuous variables were analysed by means of linear regression and expressed by the Pearson's r coefficient. Multiple regression analysis adjusted for hypertension and dyslipidaemia were performed for intergroup comparison of functional parameters. Two-sided P-values <0.05 were considered significant. Reproducibility was expressed as intraclass correlation coefficient.
Results
In total, 94 (85%) of the 110 eligible TC survivors participated in this study (Figure 1). Age at diagnosis was 30.3 ± 8.8 years. Observation time from treatment to echocardiography was 29.8 ± 4.1 years. Cancer classification and treatment regimens are summarized in Table 1.
Table 1.
Classification and treatment in TC survivors
| Histology | |
| Seminoma | 12 (13) |
| Non-seminoma | 82 (87) |
| Modified Royal Marsden classification | |
| Stage 1 | 29 (31) |
| Stage 1 marker positive/stage 2 | 44 (47) |
| Stage 3 | 4 (4) |
| Stage 4 | 17 (18) |
| Treatment regime | |
| BEP/EP | 45 (48) |
| CVB | 33 (35) |
| BEP + CVB | 5 (5) |
| Other | 11 (12) |
| Total number of CBCT cycles | |
| 1–2 | 12 (13) |
| 3–4 | 68 (72) |
| >4 | 14 (15) |
Data are expressed as n (%).
BEP, bleomycin, etoposide, cisplatin; CBCT, cisplatin-based chemotherapy; CVB, cisplatin, vinblastine, bleomycin; EP, etoposide, cisplatin.
Thirteen participants (7%) reported established CAD. There was a trend towards more CAD amongst the TC survivors, but it was not statistically significant (11% vs. 4%, P = 0.09). Twenty-one participants reported atrial fibrillation, with no difference between TC survivors and controls (11% vs. 12%, P = 0.93).
Risk factors and biochemistry
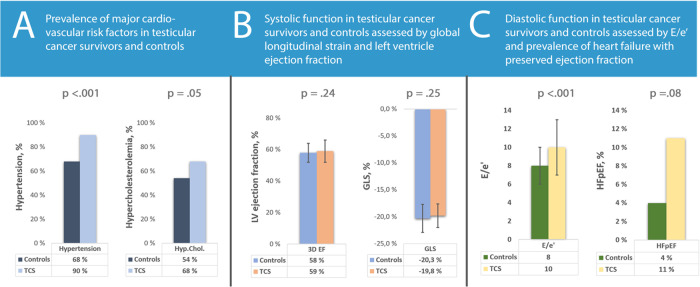
There was no difference in age, smoking status, or heredity for CAD between the TC survivors and the controls (Table 2). While measured blood pressure did not show any intergroup difference, TC survivors were more frequently treated with anti-hypertensive medication.
Table 2.
Risk factors for coronary artery disease and laboratory measurements
| TC survivors (n = 94) | Controls (n = 94) | P-value | |
|---|---|---|---|
| Risk factors for coronary artery disease | |||
| Age (years) | 60 ± 9 | 60 ± 9 | 0.96 |
| BMI (kg/m2) | 27.4 ± 4.1 | 26.7 ± 3.8 | 0.21 |
| Smoking, n (%) | 36 (41) | 39 (41) | 0.94 |
| Heredity for CAD, n (%) | 15 (17) | 17 (18) | 0.85 |
| Systolic blood pressure (mmHg) | 144 ± 18 | 144 ± 17 | 0.82 |
| Diastolic blood pressure (mmHg) | 84 ± 10 | 82 ± 9 | 0.33 |
| High blood pressure, n (%) | 74 (90) | 60 (68) | <0.001 |
| High measured blood pressure | 58 (71) | 55 (63) | 0.26 |
| Antihypertensive medication | 52 (55) | 23 (24) | <0.001 |
| High cholesterol, n (%) | 62 (68) | 51 (54) | 0.05 |
| High measured cholesterol | 28 (31) | 35 (37) | 0.38 |
| Lipid-lowering medication | 41 (44) | 17 (18) | <0.001 |
| Obesity, n (%) | 22 (23) | 17 (18) | 0.37 |
| Diabetes mellitus, n (%) | 10 (11) | 4 (4) | 0.07 |
| Laboratory measurements | |||
| Total cholesterol (mmol/L) | 5.3 ± 1.2 | 4.8 ± 0.9 | <0.01 |
| Low-density lipoprotein (mmol/L) | 3.2 ± 1.0 | 3.3 ± 0.9 | 0.48 |
| High-density lipoprotein (mmol/L) | 1.3 ± 0.3 | 1.3 ± 0.4 | 0.52 |
| Triglycerides (mmol/L) | 1.9 ± 1.3 | 1.5 ± 0.8 | <0.05 |
| C-reactive protein (mg/L) | 2.6 ± 4.8 | 1.5 ± 1.5 | 0.15 |
| NT-proBNP (ng/L) | 25 (0–61) | 52 (0–67) | 0.62 |
| Creatinine (µmol/L) | 95 ± 23 | — | — |
Data are expressed as mean ± SD for all parameters except NT-proBNP which is expressed as median (95% confidence interval). P-values derived from the Student’s t-test for continuous parameters and the χ2 test for categorical parameters. For non-parametric variables, the Mann–Whitney U test was used. Parameters with statistically significant differences between groups are highlighted in boldface.
BMI, body mass index; CAD, coronary artery disease; NT-proBNP, N-terminal pro-brain natriuretic peptide; TC, testicular cancer.
The TC survivors had comparable prevalence of dyslipidaemia to controls but were more frequently treated with lipid-lowering medication. Total cholesterol and triglycerides were higher in the TC survivors than in the controls. LDL, HDL, CRP, and NT-proBNP did not differ (Table 2).
There was a trend towards more Type 2 diabetes in the TC survivors, but it was not significant (P = 0.07). BMI did not differ between the two groups.
LDL and s-creatinine showed weak, significant correlations to cumulative cisplatin dose (R = 0.24, P < 0.05 and R = 0.25, P < 0.05). No other biochemical parameters were associated with cumulative cisplatin dose.
Cardiac function
Systolic function did not differ between the groups in either ventricle (Table 3). Four participants fulfilled the criteria for HFrEF, two in each group.
Table 3.
Echocardiographic data
| TC survivors (n = 94) | Controls (n = 94) | P-value | |
|---|---|---|---|
| Morphology | |||
| LA volume (mL/m2) | 31 ± 9 | 31 ± 9 | 0.77 |
| IVSd (mm) | 11 ± 2 | 10 ± 2 | 0.12 |
| LVIDd (mm) | 52 ± 6 | 51 ± 5 | 0.20 |
| LVPWd (mm) | 8.6 ± 1.4 | 9.2 ± 1.7 | <0.01 |
| LV mass (g/m2) | 70 ± 15 | 77 ± 13 | <0.01 |
| 3D LV EDV (mL/m2) | 64 ± 14 | 67 ± 10 | 0.11 |
| 3D LV ESV (mL/m2) | 27 ± 11 | 28 ± 5 | 0.22 |
| RA area (cm2/m2) | 9 ± 2 | 9 ± 2 | 0.71 |
| RV end-diastolic area (cm2/m2) | 11 ± 2 | 10 ± 2 | <0.01 |
| RV end-systolic area (cm2/m2) | 7 ± 1 | 6 ± 1 | <0.01 |
| Systolic function | |||
| 3D LV EF (%) | 59 ± 7 | 58 ± 5 | 0.24 |
| 3D LV SV (mL) | 77 ± 17 | 80 ± 15 | 0.20 |
| LV GLS (%) | 19.8 ± 2.6 | 20.3 ± 2.2 | 0.25 |
| RV FAC (%) | 41 ± 7 | 42 ± 5 | 0.15 |
| TAPSE (mm) | 23 ± 4 | 23 ± 4 | 0.23 |
| Diastolic function | |||
| Mitral E velocity (cm/s) | 69 ± 15 | 60 ± 13 | <0.001 |
| Mitral A velocity (cm/s) | 75 ± 18 | 55 ± 14 | <0.001 |
| Mitral E/A-ratio | 1.0 ± 0.4 | 1.1 ± 0.4 | <0.05 |
| Mitral DT (ms) | 221 ± 69 | 196 ± 57 | <0.01 |
| E/e′-ratio | 10 ± 3 | 8 ± 2 | <0.001 |
| TR velocity (m/s) | 2.5 ± 0.7 | 2.1 ± 0.4 | <0.001 |
Data are expressed as mean ± SD. P-values derived from the Student’s t-test for continuous parameters and the χ2 test for categorical parameters.
DT, deceleration time; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; FAC, fractional area change; GLS, global longitudinal strain; IVSd, interventricular septum in end-diastole; LA, left atrium; LV, left ventricle; LVIDd, left ventricular internal diameter in end-diastole; LVPWd, left ventricular posterior wall in end-diastole; RA, right atrium; RV, right ventricle; RWT, relative wall thickness; SV, stroke volume; TAPSE, tricuspid annular plane systolic excursion; TC, testicular cancer; TR velocity, tricuspid regurgitation velocity. Parameters with statistically significant differences between groups are highlighted in boldface.
The TC survivors had slightly worse diastolic function parameters with greater mitral E and A velocities, longer mitral deceleration time (DT), higher E/e′-ratio, and higher tricuspid regurgitation maximal velocity (TR max) (Table 3).
When adjusted for hypertension and dyslipidaemia in a multiple regression analysis, mitral E velocity (0.71 ± 0.03 vs. 0.62 ± 0.03 m/s, P ≤ 0.001), mitral A velocity (0.65 ± 0.03 vs. 0.48 ± 0.03 m/s, P ≤ 0.001), mitral DT (212 ± 15 vs. 187 ± 12 ms, P ≤ 0.05), E/e′-ratio (9.3 ± 0.7 vs. 7.3 ± 0.5, P ≤ 0.001), and TR max (2.5 ± 0.2 vs. 2.0 ± 0.1 m/s, P ≤ 0.01) remained significantly higher in TC survivors than controls.
Fourteen (8%) participants fulfilled the criteria for heart failure with preserved ejection fraction (HFpEF). There was a trend towards more HFpEF amongst the TC survivors, but it was not statistically significant [10 (11%) vs. 4 (4%), P = 0.08]. We found no significant correlations between cumulative cisplatin dose and any functional parameters among TC survivors treated with CBCT.
Diastolic dysfunction was found in 18 (10%) of the participants, with no statistically significant difference between TC survivors and controls (12% vs. 7%, P = 0.19). Significant valve pathology was found in 15 (8%) of the participants. The prevalence of valve pathology did not differ significantly between TC survivors and controls (11% vs. 5%, P = 0.18).
Cardiac morphology
Compared with controls, the TC survivors had thinner LV posterior wall and lower indexed LV mass (Table 3). The TC survivors also had slightly larger RV end-diastolic area and RV end-systolic area. For the TC survivors, there were no significant correlations between cumulative cisplatin dose and any morphological parameter.
Intra- and inter-observer intraclass correlations for diastolic parameters were performed in eight participants and were 0.98 and 0.97 for LA volume, 0.97 and 0.96 for mitral E velocity, 0.97 and 0.91 for E/e′-ratio, and 0.99 and 0.97 for TR max, respectively. We have performed intra- and inter-observer variability analysis for morphological and functional parameters in earlier studies.25
Discussion
In the present study with median 30 years of follow-up, TC survivors had worse diastolic function and a trend towards more HFpEF and CAD than controls. They were also more frequently treated for hypertension and dyslipidaemia. Importantly, our cohort did not have higher prevalence of HFrEF or reduced systolic function assessed by strain echocardiography, nor did they have more atrial fibrillation or valve disease than the controls.
Cardiac function
This is the first study to assess systolic function by GLS in TC survivors treated with CBCT. Compared with EF, GLS is more sensitive to discrete systolic impairment and detection of early cancer treatment induced myocardial dysfunction and is currently recommended for the management of patients undergoing cardiotoxic treatment.26
We found no reduction in systolic function in the TC survivors compared with controls. Nor did we find any impact of cumulative cisplatin dose on functional parameters. This supports the notion that CBCT does not affect systolic cardiac function in long-term TC survivors.
Similarly to earlier studies, we found that TC survivors had worse diastolic function than controls.8–10 There was also a non-significant trend towards more HFpEF in the TC survivors, although the numbers were low for both groups. Hypertension is a major risk factor for the development of both diastolic dysfunction and heart failure.27 Both the present study and earlier studies have shown increased prevalence of hypertension in patients treated with CBCT.8,28 However, diastolic function remained worse in TC survivors after controlling for hypertension. Furthermore, there were no morphological signs of long-standing hypertension in the TC survivors, who in fact had lower LV mass than controls. It is likely that metabolic changes in TC survivors are more complex than hypertension and dyslipidaemia. A recent study found epigenetic changes in CBCT-treated TC survivors associated with a host of metabolic processes.2
Although it is not unreasonable to ascribe some of the reduction in diastolic function to metabolic factors, we cannot rule out a direct toxic effect of cisplatin leading to worse diastolic function. Both van Schinkel et al.8 and Altena et al.9,10 identified decline in diastolic function at 10 and 3 months after initiation of CBCT, respectively. It is unlikely that hypertension alone could explain an acute impairment of diastolic function. It should be mentioned, however, that the prevalence of TC survivors who fulfilled task force criteria for diastolic dysfunction remained low, both in the present study and earlier studies.
Cardiac morphology
TC survivors had slightly lower LV mass and greater RV areas than controls. The difference in RV area is likely secondary to greater RV afterload in the TC survivors, as evident by a ∼20% higher TR regurgitation velocity. Etiologically, cisplatin has been associated with increased incidence of pulmonary arterial hypertension in humans.29 Furthermore, CBCT has been implicated in endothelial cell damage causing increased vessel stiffness and impaired endothelial function.30 While no prior study have assessed LV mass or RV areas in TC survivors, van Schinkel et al.8 found a small reduction in both LV and RV volumes on CMR 3 months after initiation of CBCT. This could indicate that the higher RV areas found in the present study are secondary to a long-term increase in RV afterload, rather than an acute effect of CBCT.
While we did not find increased systemic blood pressures in TC survivors, they were much more frequently treated with antihypertensive drugs. It could very well be that more frequent medical check-ups and attention to hypertension in TC survivors leads to earlier and better treatment of systemic hypertension, causing a paradoxical drop in LV afterload, and less concentric remodelling.
Risk factors for CAD
Hypertension is an important risk factor for CAD, and one in which the prevalence greatly increases with increasing age.24 Although generally well treated, hypertension was nearly universal in the TC survivors. Earlier studies in younger TC survivors with shorter follow-up times have found lower prevalence in absolute numbers, but still a significantly increased age-adjusted risk for hypertension.5,31 Similarly, use of lipid-lowering medication was more than twice as common in the TC survivors. While HDL and LDL did not differ between the groups, triglycerides and total cholesterol were higher in the TC survivors. The difference in total cholesterol is likely explained by greater concentrations of very low-density lipoproteins (VLDL) carrying triglycerides. Although, we did not measure VLDL directly, increase in VLDL has previously been linked to cisplatin-treatment.32
A previous study in this cohort found no pre-treatment differences in hypertension between TC survivors treated with CBCT and TC survivors treated with only surgery. However, a decade later, those treated with CBCT were much more likely to suffer from hypertension.33
Haugnes et al.5 found a hazard ratio of 5.7 for CAD in TC survivors treated with CBCT and assessed after a median of 19 years. However, recent studies have suggested that the majority of CAD events after CBCT occurs during the second decade after treatment, and there is evidence that patients surviving past those two decades have a substantially lower hazard ratio for CAD.34 While the present study was not powered to assess prevalence of CAD, we did find a non-significant trend towards more CAD in the TC survivors.
Limitations
It could be that after three decades, most TC survivors who were to suffer adverse cardiac effects of their CBCT would already be deceased. We cannot rule out a survival bias. Similarly, a recent paper assessed the risk of positive selection bias in long-running longitudinal studies using this cohort of TC survivors, and found that those who failed, for various reasons, to participate in the follow-ups are at higher risk for death.12 As such, the applicability of our findings is likely limited in patients recently treated with CBCT. However, as TC mostly affects young male with a low baseline risk of cardiovascular disease, it is the long-time survivors that make up most patients in a cardiological practice or hospital setting.
The cohort of patients in this study has all been a part of the Norwegian national study on TC survivors. As a result, they have received closer and more frequent medical attention than their peers. It is therefore likely that conditions like hypertension and dyslipidaemia have been identified and treated earlier than would otherwise be the case. This could potentially reduce the generalizability of these results to non-study TC survivor populations. Conversely, it suggests that the adverse effects of CBCT can be mitigated by close medical surveillance and early treatment of cardiovascular risk factors.
While this is the largest echocardiographic study to date to assess long-term TC survivors, it is not powered to evaluate minor increases in risk for rare outcomes. Our data does not show an increased prevalence in heart failure or valve pathology, but we cannot rule out minor increases in risk.
Although we did not find any correlation between cumulative cisplatin dose and risk factors or echocardiographic parameters, most TC survivors in the present study were treated with similar regimes and received comparable cumulative cisplatin doses. This reduces statistical power and could potentially mask a dose-dependent effect.
While the number of participants experiencing symptoms due to cardiac disease was low, we did not systematically assess symptomatology.
Clinical implications
The number of TC survivors treated decades ago with CBCT is growing rapidly, and the survivors are reaching an age where cardiovascular disease is common.5 Therefore, it is essential to know if increased cardiovascular risk could be attributed to CBCT to provide the proper level of surveillance and follow-up for these patients.
We did not find any signs of reduced systolic function, either symptomatic in the setting of heart failure or discrete subclinical alterations in cardiac function. While we did find slightly worse diastolic function in the TC survivors, the effect size was small with limited clinical consequence. Careful monitoring regarding CAD risk factors and perhaps a more aggressive stance in their treatment might be warranted. However, the results from this study suggest that long-term follow-up with echocardiography in asymptomatic TC survivors treated with CBCT is in most cases not necessary.
Conclusions
Thirty years after cessation of treatment, TC survivors were more likely to use antihypertensive and lipid-lowering medication, and diastolic function was slightly worse than controls. No overt or subclinical reduction in systolic function could be identified in the TC survivors, suggesting that the risk of chemotherapy-induced heart failure decades after treatment remains low. While these patients should be closely monitored for CAD and risk factors for CAD, this study does not support routine follow-up with echocardiography in TC survivors.
Conflict of interest: No conflict of interest have been declared by any of the co-authors.
Data availability
The clinical data underlying this article cannot be shared publicly due to limitations placed by the Regional Committee for Medical Research Ethics. Anonymized data will be shared on reasonable request to the corresponding author.
References
- 1. Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 2007;7:573–84. [DOI] [PubMed] [Google Scholar]
- 2. Bucher-Johannessen C, Page CM, Haugen TB, Wojewodzic MW, Fossa SD, Grotmol T et al. Cisplatin treatment of testicular cancer patients introduces long-term changes in the epigenome. Clin Epigenet 2019;11:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fankhauser CD, Sander S, Roth L, Beyer J, Hermanns T. Improved survival in metastatic germ-cell cancer. Ann Oncol 2018;29:347–51. [DOI] [PubMed] [Google Scholar]
- 4. Chovanec M, Abu Zaid M, Hanna N, El-Kouri N, Einhorn LH, Albany C. Long-term toxicity of cisplatin in germ-cell tumor survivors. Ann Oncol 2017;28:2670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haugnes HS, Wethal T, Aass N, Dahl O, Klepp O, Langberg CW et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol 2010;28:4649–57. [DOI] [PubMed] [Google Scholar]
- 6. Haugnes HS, Aass N, Fossa SD, Dahl O, Klepp O, Wist EA et al. Components of the metabolic syndrome in long-term survivors of testicular cancer. Ann Oncol 2007;18:241–8. [DOI] [PubMed] [Google Scholar]
- 7. Boer H, Proost JH, Nuver J, Bunskoek S, Gietema JQ, Geubels BM et al. Long-term exposure to circulating platinum is associated with late effects of treatment in testicular cancer survivors. Ann Oncol 2015;26:2305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Schinkel LD, Willemse PM, van der Meer RW, Burggraaf J, van Elderen SG, Smit JW et al. Chemotherapy for testicular cancer induces acute alterations in diastolic heart function. Br J Cancer 2013;109:891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Altena R, Hummel YM, Nuver J, Smit AJ, Lefrandt JD, de Boer RA et al. Longitudinal changes in cardiac function after cisplatin-based chemotherapy for testicular cancer. Ann Oncol 2011;22:2286–93. [DOI] [PubMed] [Google Scholar]
- 10. Altena R, de Haas EC, Nuver J, Brouwer CA, van den Berg MP, Smit AJ et al. Evaluation of sub-acute changes in cardiac function after cisplatin-based combination chemotherapy for testicular cancer. Br J Cancer 2009;100:1861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lipshultz SE, Landy DC, Lopez-Mitnik G, Lipsitz SR, Hinkle AS, Constine LS et al. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol 2012;30:1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fosså SD, Dahl AA, Myklebust T, Kiserud CE, Nome R, Klepp OH et al. Risk of positive selection bias in longitudinal surveys among cancer survivors: lessons learnt from the national Norwegian Testicular Cancer Survivor Study. Cancer Epidemiol 2020;67:101744. [DOI] [PubMed] [Google Scholar]
- 13. Fossa SD, Droz JP, Stoter G, Kaye SB, Vermeylen K, Cisplatin SR. vincristine and ifosphamide combination chemotherapy of metastatic seminoma: results of EORTC trial 30874. EORTC GU Group. Br J Cancer 1995;71:619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fossa SD, Paluchowska B, Horwich A, Kaiser G, de Mulder PH, Koriakine O et al. ; the EORTC GU Group. Intensive induction chemotherapy with C-BOP/BEP for intermediate- and poor-risk metastatic germ cell tumours (EORTC trial 30948). Br J Cancer 2005;93:1209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tandstad T, Stahl O, Hakansson U, Wahlqvist R, Klepp O, Cavallin-Stahl E et al. The SWENOTECA group: a good example of continuous binational and multidisciplinary collaboration for patients with testicular cancer in Sweden and Norway. Scand J Urol 2016;50:9–13. [DOI] [PubMed] [Google Scholar]
- 16. Hollender A, Stenwig EA, Ous S, Fossa SD. Survival of patients with viable malignant non-seminomatous germ cell tumour persistent after cisplatin-based induction chemotherapy. Eur Urol 1997;31:141–7. [DOI] [PubMed] [Google Scholar]
- 17. Bjerring IT, Lier registeret. Lier Kommune. Lier kommune;2019. https://www.lier.kommune.no/lire (04 September 2020, date last accessed).
- 18. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 19. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–60. [DOI] [PubMed] [Google Scholar]
- 20. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 21. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E et al. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297–317. [DOI] [PubMed] [Google Scholar]
- 22. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ et al. ; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–91. [DOI] [PubMed] [Google Scholar]
- 23. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–52. [DOI] [PubMed] [Google Scholar]
- 24. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- 25. Bjerring AW Landgraff HE Stokke TM Murbræch K Leirstein S Aaeng A et al. The developing athlete's heart: a cohort study in young athletes transitioning through adolescence. Eur J Prev Cardiol. 2019;26:2001–8. [DOI] [PubMed] [Google Scholar]
- 26. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Authors/Task Force Members et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail 2017;19:9–42. [DOI] [PubMed] [Google Scholar]
- 27. Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res 2019;124:1598–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Haas EC, Altena R, Boezen HM, Zwart N, Smit AJ, Bakker SJ et al. Early development of the metabolic syndrome after chemotherapy for testicular cancer. Ann Oncol 2013;24:749–55. [DOI] [PubMed] [Google Scholar]
- 29. Ranchoux B, Gunther S, Quarck R, Chaumais MC, Dorfmuller P, Antigny F et al. Chemotherapy-induced pulmonary hypertension: role of alkylating agents. Am J Pathol 2015;185:356–71. [DOI] [PubMed] [Google Scholar]
- 30. Nuver J, Smit AJ, Sleijfer DT, van Gessel AI, van Roon AM, van der Meer J et al. Microalbuminuria, decreased fibrinolysis, and inflammation as early signs of atherosclerosis in long-term survivors of disseminated testicular cancer. Eur J Cancer 2004;40:701–6. [DOI] [PubMed] [Google Scholar]
- 31. Fung C, Dinh P Jr, Ardeshir-Rouhani-Fard S, Schaffer K, Fossa SD, Travis LB. Toxicities associated with cisplatin-based chemotherapy and radiotherapy in long-term testicular cancer survivors. Adv Urol 2018;2018:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abdel-Gayoum AA, El-Jenjan KB, Ghwarsha KA. Hyperlipidaemia in cisplatin-induced nephrotic rats. Hum Exp Toxicol 1999;18:454–9. [DOI] [PubMed] [Google Scholar]
- 33. Sagstuen H, Aass N, Fosså SD, Dahl O, Klepp O, Wist EA et al. Blood pressure and body mass index in long-term survivors of testicular cancer. J Clin Oncol 2005;23:4980–90. [DOI] [PubMed] [Google Scholar]
- 34. Lauritsen J, Hansen MK, Bandak M, Kreiberg MB, Skøtt JW, Wagner T et al. Cardiovascular risk factors and disease after male germ cell cancer. J Clin Oncol 2020;38:584–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The clinical data underlying this article cannot be shared publicly due to limitations placed by the Regional Committee for Medical Research Ethics. Anonymized data will be shared on reasonable request to the corresponding author.