Abstract
Objectives
To evaluate effect of rectal ozone in severe COVID-19 pneumonia and to compare it to standard of care (SOC).
Material and Methods
In a case-control study, 14 patients with severe bilateral COVID-19 pneumonia (positive RT-PCR), treated with SOC and rectal ozone, were evaluated before-and-after treatment and compared with SOC (14 patients) in a 10-day follow-up period. Ozone protocol consisted of 8 sessions (1 session/day) of intra-rectal ozone (150 mL volume, 35 μg/mL concentration [5.25mg total dose]). The SOC protocol included O2 supply, antivirals (Remdesivir), corticosteroids (Dexamethasone/Metilprednisolone), monoclonal antibodies (Anakinra/Tocilizumab), antibiotics (Azytromicine), and anticoagulants (Enoxaparine). Primary outcome variables were the following: (a) clinical (O2 saturation and O2 supply); (b) biochemical (lymphocyte count, fibrinogen, D-dimer, urea, ferritin, LDH, IL-6, and CRP); (c) radiological Taylor’s scale. Secondary outcome variables were the following: (a) hospitalization length of stay, (b) mortality rate.
Results
At baseline, ozone/SOC groups were not different on age, comorbidities, O2 saturation, and O2 supply. Patients in the ozone group improved O2 saturation and decrease O2 supply. SOC maintained O2 saturation and required more O2 supply. Lymphocyte count improved only in the ozone group and with statistical difference (p<0.05). Biomarkers of inflammation (fibrinogen, D-dimer, urea, LDH, CRP, and IL-6) decreased in both groups, but only significantly in favor of the ozone group (p<0.05). Ferritin showed a significant decrease in the ozone group but an increase on the SOC group. Radiological pneumonitis decreased on both groups but the decrease was only significant in the ozone group (p<0.0001). Mortality and length of stay, although not significant, were inferior in the ozone group.
Conclusion
Compassionate use of rectal ozone improved O2 saturation, reduced O2 supply, decreased inflammation biomarkers, and improved Taylor’s radiological scale significantly when compared to the SOC group. Mortality and length of stay were inferior in the ozone group, but this difference was not significant.
Keywords: Ozone, Ozone therapy, Pneumonia, COVID-19, SARS-Cov-2, Rectal insufflation
Introduction
After the discovery of a new coronavirus in December 2019 on Wuhan, province of China, by the 3rd of March 2020, the World Health Organization (WHO) has declared an exceptional situation of pandemic due to the new SARS-CoV-2 or COVID-19 virus (https://www.who.int/es/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19%2D%2D-11-march-2020).
Nowadays, there is no effective treatment for the management of SARS-CoV-2 infection or COVID-19 disease. The Spanish Ministry of Health literally states that at the moment there is no evidence from controlled clinical trials to recommend a specific treatment for the SARS-CoV-2 infection in patients with suspected/confirmed COVID-19. However, there are several ongoing clinical trials, which could modify this situation on the short-mid-term (https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Protocolo_manejo_clinico_ah_COVID-19.pdf).
In Spain, the pandemic situation constitutes a real risk of saturation of the health system, and there may be a need to reorganize material and human resources because of the shortage of such professionals and materials (https://www.eldiario.es/sociedad/coronavirus-sobrecarga-sanidad-Comunidad-Madrid_0_1004050083.html).
Currently, since there is no definite cure for COVID [1], there are 8 clinical trials (CT) that postulate the potential use of ozonized autohemotherapy on the management of COVID-19 disease (1 CT from Turkey, 2 CT from Italy, 2 CT from Spain, and 3 CT from China) but the results, except the CT from Italy, are still unreported [2]. As far as we know, there is only one CT from Cuba which considers rectal ozone as an alternative for the management of COVID-19 infection, but the study is still in phase of recruiting (https://rpcec.sld.cu/ensayos/RPCEC00000320-Sp).
The actual standard of care for COVID-19 is supportive, and respiratory failure is the main cause of mortality secondary to acute respiratory distress syndrome (ARDS). A small percentage of patients (15%) with severe COVID-19 could develop a “cytokine storm” or hyper inflammation syndrome [3]. Early identification and treatment of hyper inflammation will result in a decrease of mortality rate; therefore, any therapies with acceptable safety profiles and capable of decreasing/modulating inflammation (as corticosteroids, monoclonal antibodies and ozone, to date some) are indicated at this stage [4].
There is growing medicine-based evidence that comes from countries such as Cuba, Italy, Germany, Russia, and Spain that states that ozone (O3) is capable of modulating pain and inflammation; and recognized bactericidal, fungicidal, virucidal, and anti-parasitic properties are attributed to ozone [5, 6]. The germicidal effect of ozone is such that many of the water purification plants worldwide use ozone with great results [5]. Fernández-Cuadros et al. have postulated ozone as an alternative therapy for the management of the present SARS-CoV-2 pandemic [7]. Virucidal, immunomodulatory, and vasodilator properties that favor O2 transport to hypoxemic tissues are the main features to postulate ozone as a promising alternative in COVID-19 [7, 8].
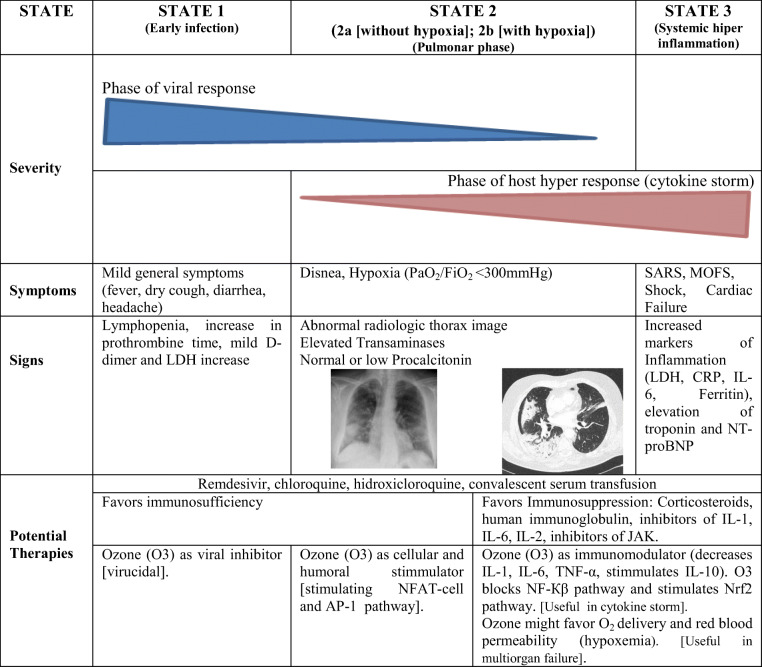
Three evolutionary stages are recognized in the SARS-CoV-2 infection: (a) stage 1 (early infection); (b) stages 2a and b (normoxic and hypoxic lung phase); and (c) stage 3 (systemic hyper inflammation or “cytokine storm”) [7]. In this scenario, Fernández-Cuadros et al. consider 4 biological properties of ozone (O3) that could act on the different phases of SARS-CoV-2 infection. (A) Ozone could inactivate the virus by direct (O3) or indirect oxidation (ROS [reactive oxygen species] and LOPs [lipid oxidative products]). (B) Ozone could stimulate the humoral and cellular immune system. Properties A and B could be useful on early COVID-19 infection phase (stages 1 and 2a). (C) Ozone reduces inflammation and modulates the antioxidant system, making it useful in the hyper inflammation or “cytokine storm” phase. (D) Ozone improves gas exchange. Properties C and D make ozone useful in the hypoxemia and/or multi-organ failure phase (stage 2b and stage 3) (Table 1) [7].
Table 1.
Severity of SARS-Cov-2 infection by stages, signs, symptoms, potential therapies, and ozone therapy proposal according to properties/evolution of the disease (modified from Mehta et al. [4], published by Fernández-Cuadros et al. [7])
SARS, severe acute respiratory syndrome; MOFS, multiorganic failure syndrome; CRP, C-reactive protein; LDH, lactate dehydrogenase; NT-proBNP, N-terminal pro-brain natriuretic peptide; IL, interleukin; NFAT-cell, nuclear factor activated T-cell; JAK, Janus kinase; NF-Кβ, nuclear factor-Кβ; AP-1, activated protein-1; Nrf2, nuclear eritroid factor 2
Recently, our study group has presented the preliminary results of 4 mild-severe COVID-19 patients treated by rectal ozone, with very promising results on the management of SARS-Cov-2 infection [9].
The objective of this article is to show the updated results of the effectiveness on the compassionate use of rectal ozone (O3) in a series of COVID-19 patients with severe bilateral pneumonia, and compare them with a series of patients treated by standard of care, in terms of clinical, biochemical, and radiological variables (primary outcomes). Mortality and hospitalization length of stay were also compared between groups (secondary outcomes).
Material and Methods
A prospective, before-and-after, non-profit, case-control study was performed. The study included 28 severe COVID-19 patients admitted at Hospital Universitario Santa Cristina, with clinical symptoms and RT-PCR (reverse transcriptase polymerase change reaction) positive for SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). The study run from August to November 2020 and the Health Care Ethics Committee (Report 15/4/2020) of Santa Cristina’s Hospital and the Ethics Committee for Medical Investigation of La Princesa’s Hospital (ACTA CEIm 12/20, 28/5/20) authorized the study and ozone treatment for compassionate use.
Inclusion criteria were the following: (1) man or woman, 18 years and older; (2) positive result on new coronavirus nucleic acid test (RT-PCR SARS-CoV-2); (3) moderate-severe pneumonia (SpO2 <93% or PaO2/FiO2 <300 mmHg, fever or moderate/severe respiratory symptoms; (4) bilateral “ground glass image” (compatible with lung lesions) on chest X-ray (according to Taylor’s scale) [10]; (5) hospitalized patients due to moderate or severe respiratory symptoms; (6) O2 supply with non-mechanical ventilation; (7) the patient/legal representative must be willing to be given informed consent to participate in the trial.
Exclusion criteria were the following: (1) pregnancy or breast feeding; (2) glucose 6-phosphate dehydrogenase (G6PD) deficiency (favism); (3) patients enrolled in other clinical studies.
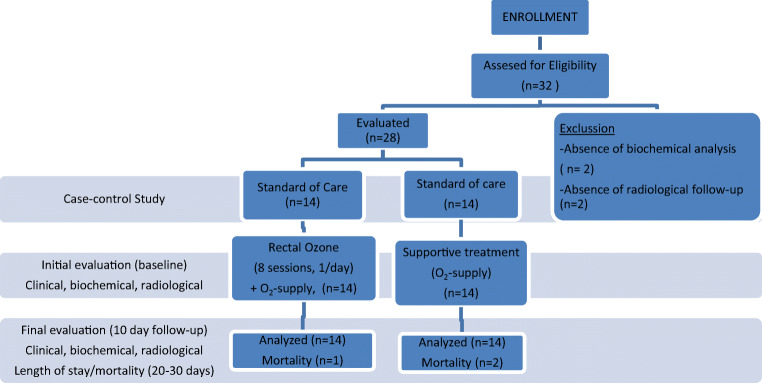
In the initial evaluation, after standard of care (SOC) was completed in all patients, the treating physician considered that ozone could be prescribed as last alternative and for compassionate use in COVID-19 treatment, because of stability/ceiling effect or even deterioration after SOC; the procedure/indications/contraindications were explained to the patients and/or legal representative, the initial biochemical evaluation (leucocyte and lymphocyte count, ferritin, D-dimer, fibrinogen, CRP [C-reactive protein], and IL-6) and the initial radiography of the chest were performed, and informed consent was signed (Fig. 1).
Fig. 1.
Study design. Case-control study on 14 patients in ozone group and 14 patients in standard of care group
The standard of care protocol included O2 supply, antivirals (Remdesivir [200 mg/1 day, the first day and 100 mg/day for 4 days]), corticosteroids [Dexamethasone 6 mg/day for 7 days] or Metilprednisolone [40 mg/day for 7 days]), antibiotics (Azytromicine [500mg/day for 5 days]), and anticoagulants (Enoxaparine [40 mg SC/day, all hospitalization period], anti-IL-6 (Tocilizumab 8 mg/kg IV twice with an interval of 12 h, and up to a maximum of 800 mg per dose]) or anti-IL-1 (Anakinra 100 mg, single dose) (Fig. 1).
In the ozone group (after standard of care was administered), the proposed technique, according to the Madrid International Ozone Therapy Declaration, was to administer intra-rectally a dose of 5.25mg of ozone (insufflation of a volume of 150 mL at a concentration of 35 μg/mL for 5 to 10 days), according to the severity of the patients (Fig. 1).
The supplies needed to perform the technique were the following: (a) Ozonosan α-Plus® [Ozone Generator]; (b) rectal probe; and (c) three silicone syringes of 50 mL capacity.
For administration, the patient was placed in the supine (for sedated patients) or lateral decubitus position (for collaborative patients) with the lower limbs flexed. Three 50-mL silicone syringes of ozone were loaded with the corresponding concentration (35 μg/mL), and were slowly injected rectally through a 14 French rectal probe, after lubrication with medical gel-type solution. The insufflation time will be a few minutes, at an administration rate of 1 mL/s.
After 10 sessions of the ozone protocol (O3), the final evaluation was performed, clinical and biochemical analysis and chest radiographies were performed and evaluated, and adverse effects (if any) were recorded. The same analysis was performed on the standard of care group (including best and worst value of variables during hospitalization period), in a 10-day follow-up period. Mortality and hospitalization length of stay were compared between both groups, at 20–30 days (before discharge) (Fig. 1).
Chest radiography was used to confirm diagnosis and to grade severity. Taylor has proposed a Severity Scale for (SARS) Severe Acute Respiratory Infection, ranging from 1 to 5 degrees. Grade 1 is considered normal. Grade 2 shows patchy atelectasis or hyper inflammation or thickening of the bronchial wall. Grade 3 includes focal alveolar consolidation but without involving more than one segment or lobe. Grade 4 shows multifocal consolidation and grade 5 includes diffuse alveolar consolidation [10].
Statistical analysis was performed using SPSS® (Statistical Package for Social Sciences, IL, USA) version 20.0. Frequencies and percentages were used to evaluate qualitative variables, while for the evaluation of quantitative variables, means and standard deviation were used. The Student T-test was the statistical tool used to evaluate a change before-and-after treatment in quantitative variables. The level of significance was 95% (p <0.05).
Results
We present the results of a series of severe COVID-19 pneumonia patients, confirmed with (+) RT-PCR for SARS-CoV-2, and with clinical and radiological signs of bilateral pneumonitis, who received standard of care (Azithromycin [500 mg/day for 5 days], corticotherapy as Dexamethasone [6 mg/day for 7 days] or Methylprednisolone [40 mg/day for 7 days], monoclonal antibodies such as Anakinra [anti-IL-1, 100 mg, single dose] or Tocilizumab [anti-IL-6, 800 mg c/12 h, up to 2 doses]), who despite this persisted with dyspnea, requiring high flow O2 supply.
At this point, and by decision of the treatment physician, based on stability/ceiling effect or even deterioration after SOC, one group of patients was asked to be treated by rectal ozone (ozone group), as compassionate use, while the other patients continued with O2 supply as needed (standard of care group) (Fig. 1).
Baseline Characteristics
Mean age of ozone group was 84.35 ± 9.52 years (range from 57 to 98) years). The male to female ratio was 3:1. The mean number of sessions was 7.83 ± 2.4 (range from 5 to 10 sessions) (Table 2). Mean age of the standard of care group was 83 ± 12.55 years (range from 60 to 104 years). The male to female ratio was 1.7:1. No difference in age was observed between both groups (p=0.7566).
Table 2.
Baseline characteristics of ozone group and standard of care group
| Variable | Ozone | Standard of care | p |
|---|---|---|---|
| Age, years | 84.35 ± 9.52 | 83 ± 12.55 | 0.7566 |
| Charlson Index (comorbidities) | 4 ± 1.7 | 4.42 ± 1.57 | 0.4431 |
| O2 saturation % | 94.3 ± 0.94 | 92.96 ± 4.2 | 0.1129 |
| O2 supply L/min | 7.1 ± 6.31 | 4.4 ± 5.15 | 0.2192 |
| Hospitalization length of stay, days | 28.58± 16.97 | 35.67 ± 21.04 | 0.1736 |
| Male, % | 85.7 | 50 | NR |
NR, not referenced. p, statistical Student T-test, significant if p<0.05
Charlson Index, O2 saturation, and O2 supply were similar between both groups, making groups comparable (Table 2). No difference in comorbidities (p=0.4431), O2 saturation (p=0.1129), and O2 supply (p=0.2192) was observed between both groups.
Primary Outcomes (Clinical, Biochemical, and Radiological Variables)
Clinical Variables
In ozone group, clinical variables (O2 saturation and O2 supply) improved in all patients. Ozone saturation improved from 94.3 to 94.5% (p=0.6682), and O2 supply decreased from 7.1 to 3.5 L/min (p=0.09) (Table 3, Fig. 2).
Table 3.
Clinical, biochemical, and radiological variables before-and-after treatment in ozone group and standard of care group
| Variables | Ozone before (n=14) | Ozone after (n=14) | p | Standard of care before (n=14) | Standard of care after (n=14) | p |
|---|---|---|---|---|---|---|
| Clinical variables | ||||||
| O2 saturation % | 94.3 ± 0.94 | 94.5 ± 2.09 | 0.6682 | 92.96 ± 0.42 | 92.9 ± 0.12 | 0.9389 |
| O2 supply L/min | 7.1 ± 6.31 | 3.5 ± 2.3 | 0.0930 | 4.4 ± 5.1 | 5.04 ± 6.1 | 0.7920 |
| Biochemical variables | ||||||
| Leucocytes cells/mL | 8602 ± 3676 | 7823 ± 2568 | 0.4165 | 6791 ± 3252 | 6058 ± 1950 | 0.5478 |
| Lymphocytes cells/mL | 985 ± 484 | 1278 ± 583 | 0.0403* | 1616 ± 2350 | 1158 ± 503 | 0.5104 |
| Fibrinogen mg/dL | 713 ± 112 | 572 ± 163 | 0.0107* | 602 ± 160 | 528 ± 149 | 0.3659 |
| D-Dimer ng/mL | 3240 ± 2484 | 1343 ± 1320 | 0.0110* | 1153 ± 595 | 853 ± 330 | 0.0251* |
| Urea mg/dL | 67 ± 41 | 55 ± 24 | 0.1089 | 73 ± 47 | 69 ± 41 | 0.5997 |
| Ferritin ng/mL | 989 ± 799 | 840 ± 1060 | 0.6043 | 861 ± 806 | 1028 ± 1219 | 0.3379 |
| LDH U/L | 329 ± 111 | 241 ± 89 | 0.0209* | 262 ± 128 | 242 ± 84 | 0.0570 |
| CRP mg/mL | 8.9 ± 6.14 | 2.46 ± 3.78 | 0.0040* | 6.5 ± 6.6 | 1.91 ± 2.3 | 0.0525 |
| IL-6 pg/mL | 85.07 ± 50.5 | 30.48 ± 38.1 | 0.0048* | 44.2 ± 23.2 | 23.3 ± 17.3 | 0.2365 |
| Radiological variables | ||||||
| Taylor scale | 4.78 ± 0.42 | 3 ± 0.78 | 0.0000* | 4.25 ± 0.75 | 3. .75 ± 0.96 | 0.3145 |
p, statistical Student T-test; L/min, liters per minute; LDH, lactate dehydrogenase; CRP, C-reactive protein. *p<0.05
Fig. 2.
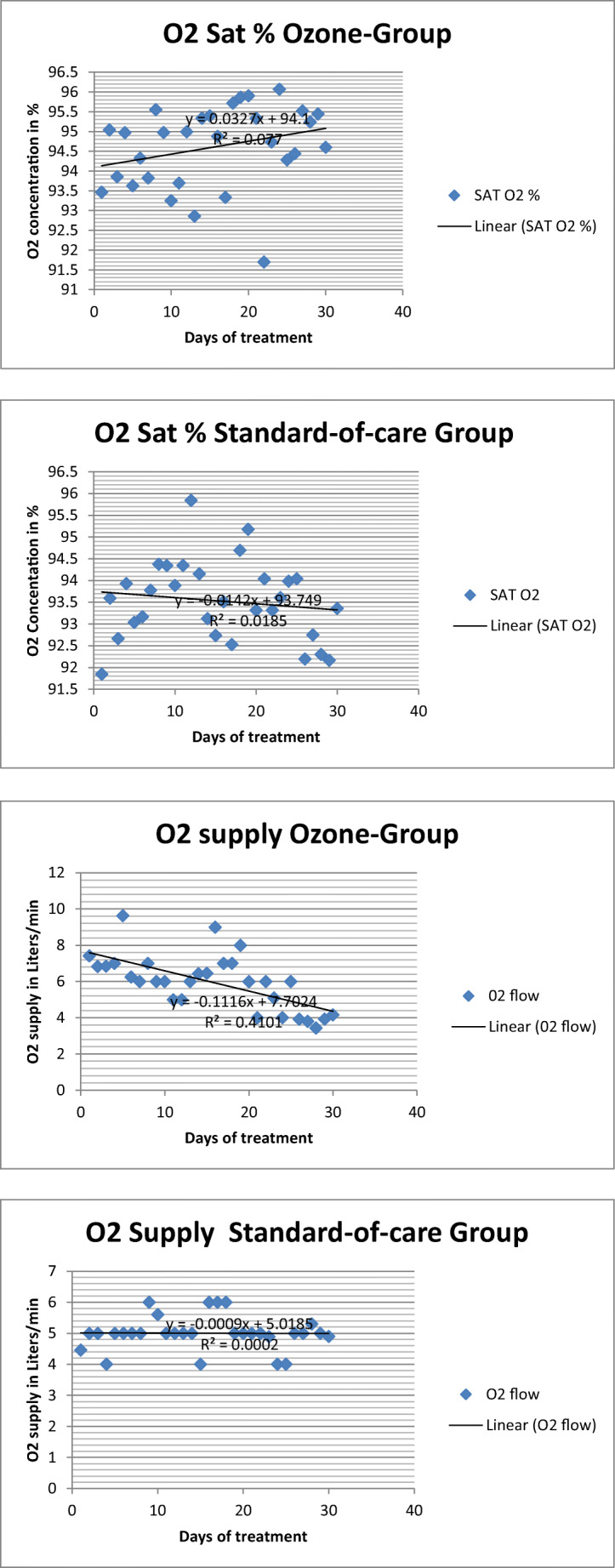
Tendency of rectal ozone group (n=14) compared to standard of care group (n=14) evaluated by dispersion curve on O2 saturation and O2 supply
In the standard of care group, clinical variables (O2 saturation and O2 supply) did not improved at all. Oxygen saturation slightly decreased from 92.96 to 92.9% (p=0.9389), and O2 supply worsened from 4.4 to 5.04 L/min (p=0.7920) (Table 3, Fig. 2).
Biochemical Variables
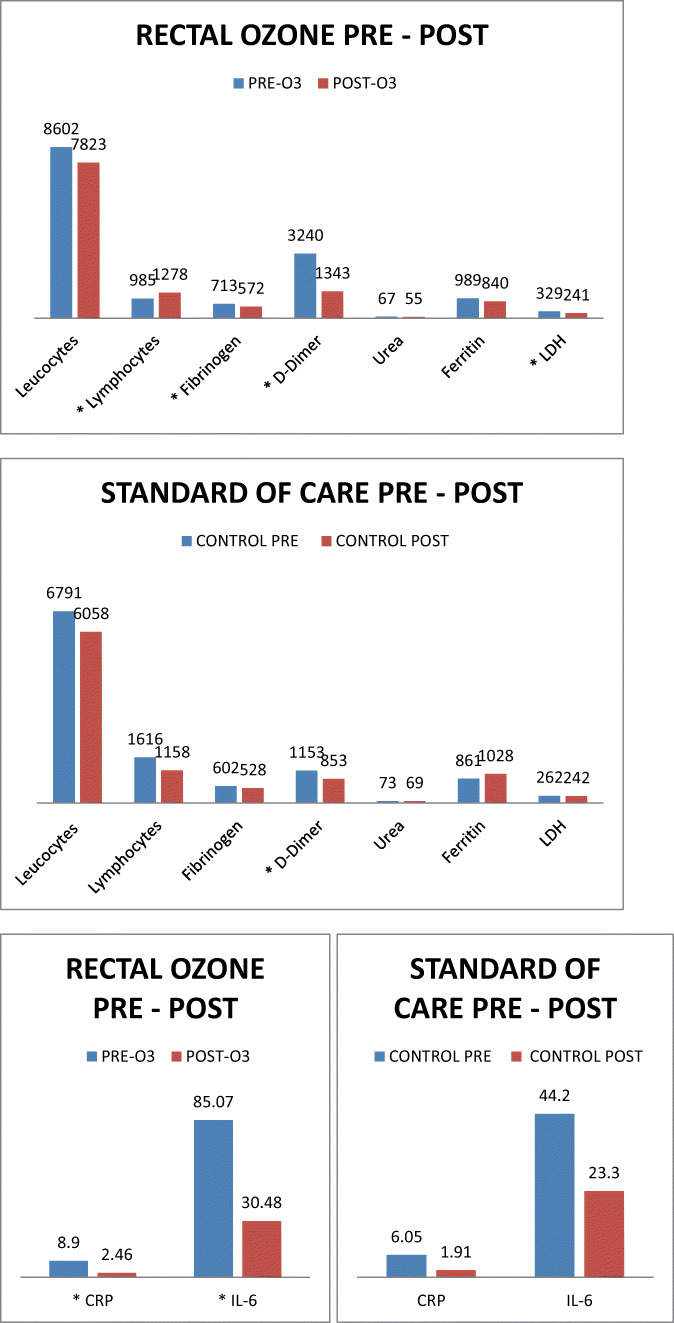
In the ozone group, biochemical variables of inflammation, leucocyte count, and lymphocyte count improved significantly in an overall view. Except from leucocyte count, ferritin, and urea, all changes were significant (p<0.05). Leucocytes decreased from 8.6 × 103/mL to 7.82 × 103/mL (p=0.4165); Lymphocytes improved from 0.98 × 103 to 1.27 × 103/mL (p=0.0403). Fibrinogen ameliorated from 713 to 572 mg/dL (p=0.0107). D-Dimer lowered from 3240 to 1343 ng/mL (p=0.0060). Urea improved slightly its value from 67 to 55 mg/dL (p=0.1089). Ferritin decreased its value from 989 to 840 ng/mL (p=0.6043). LDH ameliorated from 329 to 241 U/L (p=0.0209). CRP diminished values from 8.9 to 2.46 mg/mL (p=0.0040). IL-6 improved from 85.07 to 30.48 pg/mL (p=0.0048) (Table 3, Fig. 3).
Fig. 3.
Biochemical variables’ analysis (before-and-after treatment) in ozone group compared to standard of care group (control group). LDH, lactate dehydrogenase; CRP, C-reactive protein. *p<0.05
In the standard of care group, except for D-dimer, all biochemical variables of inflammation, leucocyte count, and lymphocyte count also improved in an overall view, but in a non-significant manner (p>0.05). Ferritin was the only variable that actually worsened after standard of care. All the changes in the variables analyzed were not significant (p>0.05). Leucocytes decreased from 6.79 × 103 to 6.05 × 103/mL (p=0.5478); lymphocytes decreased from 1.61 × 103 to 1.15 × 103/mL (p=0.5104). Fibrinogen ameliorated from 602 to 528 mg/dL (p=0.3659). D-Dimer lowered from 1153 to 853 ng/mL (p=0.0251). Urea improved slightly its value from 73 to 69 mg/dL (p=0.5997). Ferritin worsened its value from 861 to 1028 ng/mL (p=0.3379). LDH ameliorated from 262 to 242 U/L (p=0.0570). CRP diminished its value from 6.5 to 1.91 mg/mL (p=0.0525). IL-6 improved from 44.2 to 23.3 pg/mL (p=0.2365) (Table 3, Fig. 3).
Radiological Variables
According to Taylor’s scale, patients in the ozone group improved significantly from a 4.78 to a 3 grade (p=0.0000) (Table 3). According to Taylor’s scale, patients in the standard of care group improved from a 4.25 to a 3.75 grade (p=0.3145) (Table 3). The improvement was significant in favor of the ozone group.
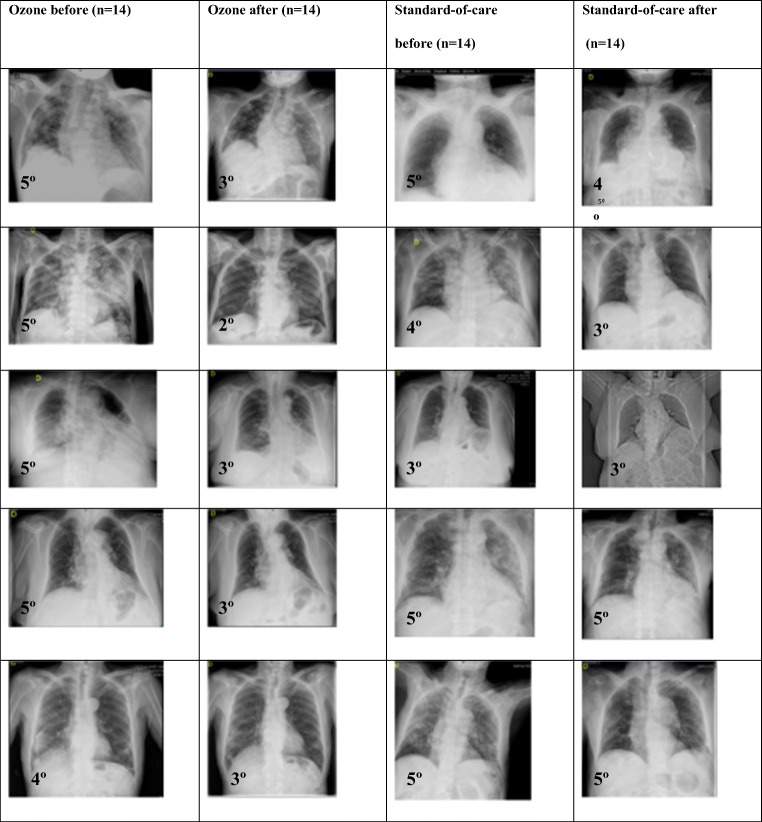
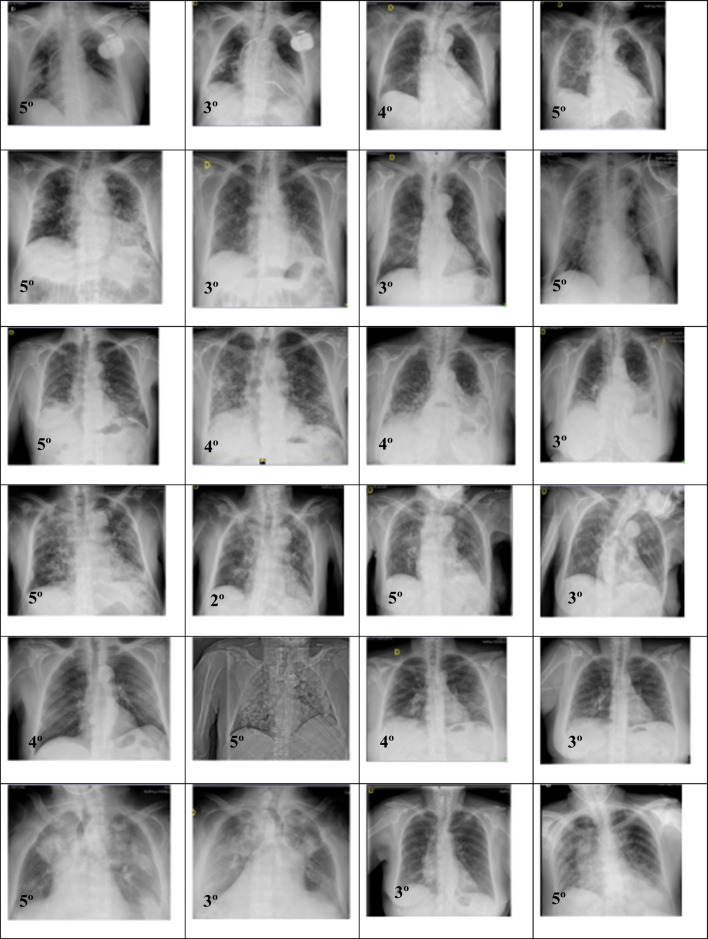
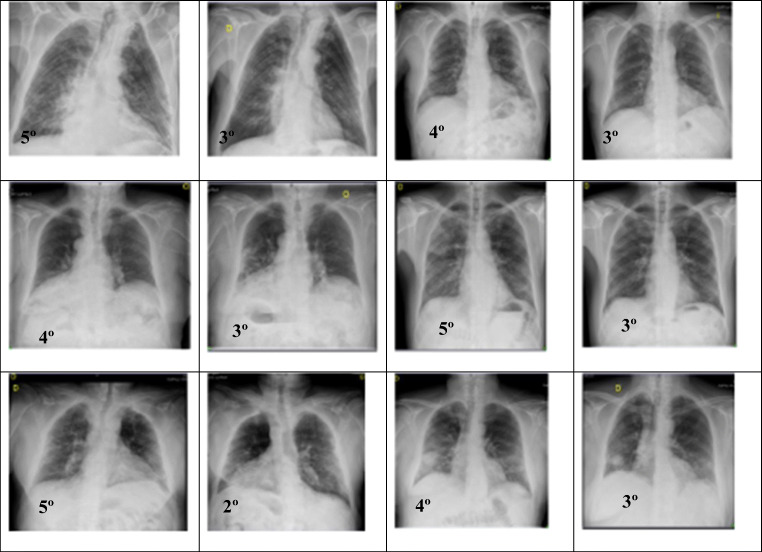
A resume of the radiographic evolution between the ozone group and standard of care group is presented in Fig. 4.
Fig. 4.
Radiographic evolution before and after ozone (n=14) and standard of care (n=14) is observed in both groups, based on Taylor’s scale
Secondary Outcomes
Hospitalization Length of Stay and Mortality Rate
In the ozone group, hospitalization length of stay was 28.58 ± 16.97 days. On the contrary, in the standard of care group, hospitalization length of stay was 35.67 ± 21.04 days. Even in the case that ozone was administered after standard of care was completed, there was a difference of 7.09 days in favor of the ozone group, although that difference was not statistically significant (p=0.1736) (Table 3).
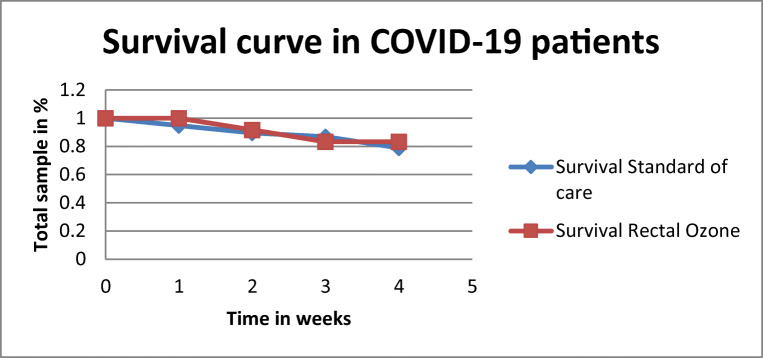
In the ozone group, mortality rate was 8.3% (n=1), while on the standard of care group, mortality rate was 16.6% (n=2) (Fig. 5).
Fig. 5.
Kaplan-Meier survival curve of ozone group (n=14) compared to standard of care group (n=14) in the case-control study
Adverse Events in Ozone Protocol
After a mean of 7.83 ± 2.4 sessions of rectal ozone treatment (150 mL of ozone at 35 μg/mL, total dose 5.25 mg) in the ozone group (n=14), clinical, biochemical, and radiological improvement was observed. After rectal insufflation, no side effect was observed, except slight meteorism and a feeling of bloating, which subsided spontaneously.
Discussion
To the best of our knowledge, this is the first report on the effectiveness of rectal ozone (for compassionate use) in a series of severe COVID-19 pneumonic patients and it was compared with a series of patients treated by standard of care, in the light of this new SARS-CoV-2 pandemic. Rectal ozone improved clinical, biochemical, and radiological variables and in a significant manner (p<0.05); on the contrary, the improvement observed in the standard of care group was not significant (p>0.05).
To date, despite the several clinical trials performed and updated (more than 1661 clinical trials registered in the International Clinical Trial Registry Platform Database) [11], there is no pharmacological therapy that has demonstrated effectivity in the management of SARS-CoV-2 pandemic and COVID-19 infection [1, 11]. There are 8 clinical trials (CT) that postulate ozone as a biologically effective therapy [1, 2]. From these 8 trials, 7 CT are concerned to ozone autohemotherapy, and one CT to rectal ozone [6, 7]. Only one CT has published its results (ozone autohemotherapy), while the others are still recruiting patients [1, 2]. There subsides the importance of the study; there is no report on the literature of the effectiveness of rectal ozone, apart from our preliminary results recently published (four cases) [9]. This study presents the largest sample of COVID-19 patients treated by rectal ozone insufflation in a case-control design.
Our study group identified up to 4 properties that could cope with the complications derived from this COVID-19 infection (antiviral, antioxidant, anti-inflammatory, and O2 delivery enhancer) (Table 1) [7].
The clinical improvement observed in our preliminary results [9] and now in this case-control study confirms the promising utility of ozone in the management of COVID-19 pandemic, as stated by some other authors [7, 12–14].
There are several reasons that justify ozone use for COVID-19 management. Ozone produces antioxidant response elements (super oxide dismutase [SOD], catalase [CAT], glutathione peroxidase [GPx], hemo-oxygenase 1 [HO-1], HSP-1 [heat shock protein-1]). Ozone reduces iron overload, reducing ferritin and oxidative stress produced by viral infection. Ozone increases 2,3DPG (diphosphoglycerate), shifting the hemoglobin curve to the right, improving blood rheology and permeability, increasing blood flow and oxygenation by the delivery of NO (nitric oxide). Ozone modulates interferons and cytokines; therefore, it may counteract hyperinflammation, cytokine storm, and oxidative stress in COVID-19 patients. Ozone has anti-platelet effect (by increasing prostacyclin or prostaglandin I2 [PGI2]), leading to vasodilation. Ozone releases NO, producing also vasodilation. Ozone modulates antithrombin III, reducing fibrinogen. Therefore, ozone could decrease hypercoagulation state in COVID-19 patients [15], as it was observed in our case-control study.
Ayanian et al. have identified five biomarkers capable of predicting clinical course in COVID-19 patients, and besides, they consider these biomarkers could inform of therapeutic interventions rather than simply demonstrate a consequence of disease. Elevated levels of D-dimer, CRP, IL-6, ferritin, and LDH have been related to ICU (intensive care unit) admission, intubation, and death. On the contrary, lower levels of such biomarkers were related to survival and positive clinical outcomes [16]. The fact that in our case-control study, ozone was capable of decreasing such biomarkers, apart from improving O2 saturation and radiologic amelioration, is a demonstration of the effectiveness of ozone on COVID-19 patients, based on clinical, biochemical, and radiologic variables.
Cattel states that ozone is antiviral and might inactivate the virus and inhibit its viral replication. Ozone could reduce inflammation and lung damage. Ozone might favor immunity and oxygenation, and decrease oxygen support. It is expected that ozone could increase lymphocyte count, decrease inflammation biomarkers (CRP, IL-6, ferritin, D-dimer, and LDH), improve O2 saturation, and decrease in O2 supply, and finally get a negativization of RT-PCR SARS-Cov-2 test [17]. As Cattel hypothesized, we have observed all these effects in our case-control study. Ozone improved all variables and in a significant manner (p<0.05); on the contrary, the change was not significant in the standard of care group (p>0.05). These observations would be a confirmation of the biological properties of ozone and this study might serve as a proof-of-concept of rectal ozone in SARS-Cov-2-infected patients.
Regarding age and comorbidities (Charlson Index) in our study, we have treated patients older than 80 years (84.3 years in ozone group, 83 years in standard of care group). Their comorbidities were similar in both groups (4 in ozone group and 4.42 in standard of care group). The present study differs in the form of ozone administration, age, and comorbidities from the studies of Franzini [18], Tascini [19], Araimo [2], Schwartz [20], and Hernández [21] (Table 4). In the previous studies referenced, there is a clear association between age and number of comorbidities. The older the age, the higher the Charlson Index. Our study has the oldest patients and the greatest comorbidities. Even in such a case, rectal ozone results were promising. It is expected that older patients show worst clinical outcomes, but ozone was effective even in older COVID-19 patients [7]. As far as we know, our study has treated the oldest patients, if compared with other ozone studies (Table 4) [18–21].
Table 4.
Main differences between recent published ozone trials on the management of COVID-19 disease when compared to our study
| Variables | Studies | |||||
|---|---|---|---|---|---|---|
| Fernandez-Cuadros et al. [22] | Franzini et al. [18] | Tascini et al. [19] | Araimo et al. [2] | Schwartz et al. [20] | Hernández et al. [21] | |
| Ozone application | Rectal insufflation | Auto hemotherapy | Auto hemotherapy | Auto hemotherapy | Ozonized saline solution | Auto hemotherapy |
| Study design | Case control | Before and after | Case control | Randomized control trial | Before and after | Case control |
| Age ozone group (years) | 84.3 | 75 | 57 | 63 | 55 | 64 |
| Comorbidities ozone group | 4 | 2.5 | 1 | 2.8 | 0.84 | 0.77 |
| Age Control Group (years) | 83 | NR | 65 | 60 | NR | 71 |
| Comorbidities control group | 4.42 | NR | 2 | 2.6 | NR | 1.2 |
| Ozone effect (stimulation) | Lymphocyte count | Leucocyte count | NR | NR | NR | NR |
| Ozone effect (down regulation) | CRP, IL-6, LDH, ferritin, fibrinogen, D-dimer | CRP, IL-6, LDH, D-dimer | CRP, IL-6 | CRP, IL-6, LDH, D-dimer | CRP, LDH, ferritin, D-dimer | CRP, LDH, ferritin, D-dimer |
| Length of stay ozone group (days) | 28.58 | 13.45 | NR | NR | 14 | 8 |
| Length of stay control group (days) | 35.67 | 22.15 | NR | NR | 68 | 28 |
| Mortality ozone group (%) | 8.3 | NR | 0 | NR | 0 | 11 |
| Mortality control group (%) | 16.6 | NR | 7 | NR | 20.7 | 22 |
LDH, lactate dehydrogenase; CRP, C-reactive protein; NR, not referenced
With regard to clinical variables, in our study ozone group improved O2 saturation (from 94.3 to 94.5%) and reduced O2 supply (from 7.1 to 3.5 L/min). It means that severe COVID-19 patients improved their clinical state decreasing O2 supply. In the case of standard of care group, O2 saturation decreased slightly (from 92.96 to 92.90%) but more O2 supply was needed (4.4 to 5.04 L/min). Ozone has demonstrated to be more effective than standard of care in improving respiratory parameters in severe COVID-19 patients. This observation comes in line with what was reported by Franzini et al. [18]. In Franzini’s study, O2 saturation improved from 85 to 95% (p<0.0001) after 8.6 ± 1.4 days of treatment [18]. In Araimo’s study, it was observed that ozone group moderately reduced the need for ventilatory support (reduced use of CPAP [continuous positive air pressure], high flow nasal cannula, or venturi mask) [2]. In Schwartz’ study, patients that required supplemental O2 decreased from 68 to 24% [20]. From the cited articles, it could be inferred that ozone (by rectal, autohemotherapy or by ozonized saline solution application) is capable of improving ventilatory indexes, mainly O2 saturation and O2 supply, as it was observed in our study.
Henry et al. have stated that many proinflammatory biomarkers such as CRP, IL-6, ferritin, and even ESR (erythrocyte sedimentation rate) are considerably increased over the upper limit in COVID-19 patients [23]. In the same line, Webb et al. have considered a hyperinflammatory COVID-19 score based on different parameters: (a) ferritin >700 ng/mL, (b) LDH >400 U/L, (c) D-dimer >1500 ng/mL, (d) CRP >15 mg/mL, (e) IL-6 >15 pg/mL [24]. Ayanian et al. have observed a cut-point in the levels of biomarkers with good and bad clinical outcomes in COVID-19 patients. In COVID-19 patients with no need for ICU admission, no intubation, and good clinical outcomes (survivors), the range of biomarkers was the following: (a) ferritin 340–370 μg/L, (b) LDH 863–915 U/L, (c) D-dimer 1600–1700 ng/mL, (d) CRP 6.8–7.8 mg/mL, (e) IL-6 50–60 pg/mL. On the contrary, in patients with need to ICU admission, intubation, mechanical ventilation, and death, the range of biomarkers was greater: (a) ferritin 1320-1575 μg/L, (b) LDH 1478–2050 U/L, (c) D-dimer 5800–7800 ng/mL, (d) CRP 29–33.3 mg/mL, (e) IL-6 188–266 pg/mL [2]. With regard to inflammatory biomarkers, the patients in our study presented moderate and severe pneumonia but were not critical; therefore, the levels of inflammatory markers were over the upper limit, as Ayanian, Henry, and Webb have previously stated [2, 23, 24].
As Menendez-Cepero stated, ozone is capable of modulating interferons and cytokines, decreasing inflammation biomarkers [15]. Bocci has also stated that ozone is capable of stimulating stem cells, improving differentiation of white cells and platelets [25]. This would explain why in our study ozone improved lymphocyte count, ameliorated inflammation biomarkers (CRP, IL-6, ferritin, and LDH), and decreased coagulation parameters (fibrinogen and D-fimer). In Franzini’s study, ozone decreased inflammation biomarkers (CRP, IL-6), thromboembolic biomarkers (D-dimer), LDH, and improved leucocyte count [18]. In Tascini’s study, ozone ameliorated CRP and IL-6 [19], and this comes in line with Clavo et al., who stated that ozone effect is based on oxidative preconditioning, reducing IL-1β and IL-6 [26]. This would explain the decreasing of inflammation biomarkers (IL-6 and CRP) observed in Tascini’s [19] and in our present study. Schwartz et al. have stated that ozonized saline solution was capable of decreasing inflammation biomarkers (ferritin, LDH, D-dimer, and CRP) from baseline to the end of treatment. In fact, by the10th day of treatment, fibrinogen and LDH were on normal ranges in all COVID-19 patients [20]. Hernandez et al. have stated that ozone autohemotherapy decreased inflammatory biomarkers (ferritin, LDH, D-dimer, and CRP) significantly at 7 days after treatment started [21]. Although Franzini’s, Tascini’s, and Hernandez’s studies used ozone autohemotherapy and Schwartz’ study used ozonized saline solution, their results were similar to our approach (rectal ozone insufflation); that is, ozone in its different administration techniques was capable of decreasing such biomarkers of inflammation (Table 4).
The decreasing of inflammation biomarkers observed in our study is similar to Araimo’s study [2]. In that study, all inflammation biomarkers in the ozone group ameliorated (ferritin, D-dimer, CRP, and IL-6). On the contrary, D-dimer, ferritin, and IL-6 worsened in the standard of care group [2]. Coincidentally, it was also observed in our study that ferritine was the variable that worsened in the standard of care group similarly as in Araimo’s study [2]. Ferritin is a biomarker of viral inflammation and ozone is largely recognized as an antiviral agent [7, 25]. This would explain why ferritin decreased in the ozone group but increased in standard of care groups, as observed in Araimo’s study [2] and in ours.
In the present study, bilateral radiographic pneumonia improved from 4.78 to 3 (on Taylor’s radiologic scale) in ozone group (p=0.0000), while in the standard of care group, improvement was just moderate (from 4.25 to 3.75) according to Taylor’s scale (p=0.3145). There is a clear improvement in bilateral pneumonia in favor of ozone treatment, as evidenced by improvement in Taylor’s radiologic scale. Our findings correlate with Schwartz’ study, in which radiologic signs of pneumonia changed from 60% lung affection to 24% lung affection; and the improvement was observed at 3–5 days of ozone treatment [20]. The great improvement on Taylor radiological scale observed in the ozone group would explain why ozone patients improved in O2 saturation and decreased in O2 supply. On the contrary, the slight increased observed in Taylor’s scale in standard of care group would explain why these patients had only a slight improvement on O2 saturation and therefore needed even more O2 supply, as it was observed in our study (Figs. 2 and 4).
In the present case-control study, although ozone treatment (compassionate use) started after standard of care was provided, hospitalization length of stay was inferior in ozone group if compared to standard of care (28.58 days vs 35.67 days). In the studies of Franzini [18], Schwartz [20], and Hernandez [21], length of stay was shorter in the ozone group if compared to the control group (Table 4). Despite the fact that in our study ozone treatment started as compassionate when no longer improvement was observed once standard of care treatment finished, all referenced studies including ours state that hospitalization period is shorter in ozone groups [18, 20, 21].
In our study, mortality rate in the ozone group was 8.3% whereas in the standard of care group was 16.6%. Hernandez et al. have reported a mortality rate of 11% for the ozone group, and 22% for the standard of care group, a rate very similar to ours [21]. Tascini et al. have stated that poor clinical outcome was inferior in ozone (7%) that in standard of care (17%), and the mortality rate was 0% in the ozone group and 7% in the standard of care group [19]. Schwartz has reported no mortality in the ozone group, but a 20.7–21.1% mortality rate in homogeneous groups treated by standard of care [20]. All previous studies stated a lower mortality for the ozone group than for the standard of care group. The expected mortality in severe COVID-19 cases is 18% and in the moderate cases is 5% [19]. From the previous results, it can be inferred that severe cases treated by ozone therapy reduced its mortality rate to the mortality expected in moderate COVID-19 cases [19–21]. This suggests that ozone has an impact on mortality rate.
Finally, in our study, we have observed no severe events after rectal ozone insufflation except slight meteorism and bloating, which subside in minutes after procedure. Ozone is very safe, to the point that only 0.7 adverse events in 100,000 treatments have been reported in literature [27].
As a summary, the spread of COVID-19 pandemic has led to the need to determine standardized treatment for the management of SARS-Cov-2 infection. Unfortunately, no specific drug or drug regimen has been approved for COVID-19. In the pathogenesis of COVID-19, two clinical presentations are the most observed: (a) respiratory failure and (b) systemic coagulopathy secondary to hyper activation of complement cascade and exacerbation of cytokine cascade. As a result, hyper production of interleukins and hypercoagulability with diffuse thrombosis in the circulation are observed. Since there is no proven efficacy of antivirals in treating COVID-19 by themselves, it is reasonable to treat COVID-19 with multimodal therapies [11]. Ozone is a multi-target drug with proven biological properties: (a) antiviral, (b) modulation of inflammatory interleukins (IL-1, IL-6, TNF-α), (c) antioxidant (via Nrf2 pathway), (d) anti-inflammatory (blocking inflammasome NRLP3), (e) anticoagulant (antithrombin III effect), and (f) vasodilation effect (NO release) [7, 28–31]. The multi-target profile of ozone would explain the good clinical outcomes observed in the present study, and in the articles referenced and published in the management of COVID-19 [2, 18–21, 32, 33].
The number of sessions in autohemotherapy varied from 3 to 7 sessions [2, 18, 19, 21], in ozonized saline solution was 10 sessions [20] and in rectal ozone the sessions were 7.8 on average.
A limitation of this case-control study is the small sample size analyzed. However, despite the number of patients evaluated, the fact that the ozone group and standard of care group were homogeneous has made them comparable, and important conclusions can be obtained from this case-control study. Although the specific variant treated in this case-control study was not verified, it is expected that the antiviral, antioxidant, and anti-inflammatory properties of ozone that acted on former SARS-CoV-2 strain could similarly act on latter SARS-CoV-2 strains (British, Brazilian, or South African mutations), because of its biological properties on S-spike, viral capside, and COVID-19 pathophysiology [7, 9].
This is the first study that reports the effectiveness of rectal ozone in the management of this new pandemic situation, so it constitutes a first proof-of-concept study. The prospective nature of this study shows the pragmatic real-world COVID-19 population. Another strength of the study is the use of objective and standardized clinical, radiological, and biochemical variables to evaluate the effect of rectal ozone in the face of this new pandemic situation.
Finally, ozone is an anti-inflammatory therapy capable of modulating inflammation biomarkers, ozone is cheaper and safer if compared to biological treatments (monoclonal antibodies) or antivirals (Remdesivir), and O3 might be an alternative for low-middle income countries, where patients have to pay for their medical bills, there is scarcity of economic resources, and the health systems have limited resources (expensive drugs and trained personnel) [11, 13]. A RCT (randomized controlled trial) is necessary to validate and reproduce the promising results observed in this proof-of-concept study.
Conclusion
Compassionate use of rectal ozone improved O2 saturation, reduced O2 supply, decreased inflammation biomarkers, and improved Taylor’s radiological scale with statistical significant difference when compared to standard of care, in patients with severe COVID-19 pneumonia. Mortality and days of hospitalization were inferior in the ozone group, but this difference was not significant.
Rectal ozone is a costless, safe, effective, and easy-to-perform alternative for the management of SARS-Cov-2 infection and it is presented as an adjunctive therapeutic option to consider as a compassionate use in severe bilateral COVID-19 pneumonia.
Acknowledgements
The librarian Saturnino Díaz Trujillo is acknowledged for the bibliographic search for the preparation of this Study.
Declarations
Conflict of Interest
The authors declare no competing interest.
Ethical Approval
The Study Protocol has been approved by the Ethical Committee of the Santa Cristina’s Hospital (15th April 2020) and by Ethics Committee for Medical Investigation of La Princesa’s Hospital (ACTA CEIm 12/20, 28/5/20)
Informed Consent
For the treatment used in this Research Article (1) informed consent was obtained from the patient/legal representative included in the study and (2) the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethical Committee of the Hospital.
Footnotes
This article is part of the Topical Collection on COVID-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Coronavirus disease (COVID-2019) situation reports. 2020.
- 2.Araimo F, Imperiale C, Tordiglione P, Ceccarelli G, Borrazzo C, Alessandri F, et al. Ozone as Adjuvant Support in the Treatment of COVID-19: A Preliminary Report of Probiozovid Trial. J Med Virol. 2020;93:2210. doi: 10.1002/jmv.26636. [DOI] [PubMed] [Google Scholar]
- 3.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B, von Bergwelt-Baildon MS. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta P, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1022–1034. doi: 10.1016/S0140-6736(20)30630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández-Cuadros ME, Pérez-Moro OS, Albaladejo-Florín MJ. Ozone fundamentals and effectiveness in knee pain: Chondromalacia and knee osteoarthritis. Saarbrücken: Lambert Academic Publishing; 2016. [Google Scholar]
- 6.Fernández-Cuadros ME, Pérez-Moro OS, Albaladejo-Florin MJ, Álava-Rabasa S. El ozono intraarticular modula la inflamación, mejora el dolor, la rigidez, la función y tiene un efecto anabólico sobre la artrosis de rodilla: estudio cuasi-experimental prospectivo tipo antes-después, 115 pacientes. Intra-articular Ozone modulates inflammation, ameliorates pain and rigidity, improves function and has anabolic effect on knee osteoarthritis: a prospective quasi-experimental before-and-after study, 115 patients. Rev Soc Esp Dolor. 2020;27.2:78–88. 10.20986/resed.2020.3775/2019.
- 7.Fernández-Cuadros, Peña-Lora, Albaladejo-Florín, Álava-Rabasa, Pérez-Moro Ozone (O3) and SARS-CoV-2: physiological bases and their therapeutic possibilities according to COVID-19 evolutionary stage. SN Compr Clin Med. 2020;2:1–9. doi: 10.1007/s42399-020-003287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavazza A, Marchegiani A, Rossi G, Franzini M, Spaterna A, Mangiaterra S, Cerquetella M. Ozone Therapy as a Possible Option in COVID-19 Management. Front Public Health. 2020;8:417. doi: 10.3389/fpubh.2020.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández-Cuadros ME, Albaladejo-Florín MJ, Álava-Rabasa S, Usandizaga-Elio I, Jimenez DMQ, Peña-Lora D, et al. Effect of Rectal Ozone (O 3) in Severe COVID-19 Pneumonia: Preliminary Results. SN Compr Clin Med. 2020;2.9:1328–1336. doi: 10.1007/s42399-020-00374-1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor E, Haven K, Reed P, Bissielo A, Harvey D, McArthur C, Wilson F. A chest radiograph scoring system in patients with severe acute respiratory infection: a validation study. BMC Med Imaging. 2015;15.1:61. doi: 10.1186/s12880-015-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Franco S, Alfieri A, Petrou S, Damiani G, Passavanti MB, Pace MC, et al. Current status of COVID-19 treatment: An opinion review. World J Virol. 2020;9.3:27. doi: 10.5501/wjv.v9.i3.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-Sánchez G, Schwartz A, Donna VD. Potential Cytoprotective Activity of Ozone Therapy in SARS-CoV-2/COVID-19. Antioxidants (Basel) 2020;9.5:389. doi: 10.3390/antiox9050389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conti P, Gallenga CE, Tetè G, Caraffa A, Ronconi G, Younes A, et al. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS- CoV-2) infection and lung inflammation mediated by IL-1. J Biol Regul Homeost Agents. 2020;34.2. 10.23812/Editorial-Conti-2. [DOI] [PubMed]
- 14.Marini S, Maggiorotti M, Dardes N, Bonetti M, Martinelli M, Re L, et al. Oxygen-ozone therapy as adjuvant in the current emergency in SARS-COV-2 infection: a clinical study. J Biol Regul Homeost Agents. 2020;34.3. 10.23812/20-250-E-56. [DOI] [PubMed]
- 15.Menendez-Cepero S, Marques-Magallanes-Regojo JA, Hernandez-Martinez A, Tallón FJH, Baeza-Noci J. Therapeutic effects of Ozone therapy that justifies its use for the treatment of COVID-19. Res Open J Neurol. 2020;3.1:1–6. doi: 10.31038/JNNC.2020314. [DOI] [Google Scholar]
- 16.Ayanian S, Reyes J, Lynn L, Teufel K. The association between biomarkers and clinical outcomes in novel coronavirus pneumonia in a US cohort. Biomark Med. 2020;14.12:1091–1097. doi: 10.2217/bmm-2020-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cattel F, Giordano S, Bertiond C, Lupia T, Corcione S, Scaldaferri M, et al. Ozone Therapy in COVID-19: a Narrative Review. Virus Res. 2021;291:198207. doi: 10.1016/j.virusres.2020.198207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franzini M, Valdenassi L, Ricevuti G, Chirumbolo S, Depfenhart M, Bertossi D, Tirelli U. Oxygen-ozone (O2-O3) immunoceutical therapy for patients with COVID-19. Preliminary evidence reported. Int Immunopharmacol. 2020;88:106879. doi: 10.1016/j.intimp.2020.106879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tascini C, Sermann G, Pagotto A, Sozio E, De Carlo C, Giacinta A, et al. Blood ozonization in patients with mild to moderate COVID-19 pneumonia: a single centre experience. Intern Emerg Med. 2020;2020:1–7. doi: 10.1007/s11739-020-02542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz A, Martínez-Sánchez G, de Lucía AM, Viana SM, Mita CA. Complementary application of the ozonized saline solution in mild and severe patients with pneumonia COVID-19: A non-randomized pilot study. J Pharm Pharmacogn Res. 2021;9(2):126–142. [Google Scholar]
- 21.Hernández A, Viñals M, Pablos A, Vilás F, Papadakos PJ, Wijeysundera D, et al. Ozone therapy for patients with COVID-19 pneumonia: preliminary report of a prospective case-control study. Int Immunopharmacol. 2021;90:107261. doi: 10.1016/j.intimp.2020.107261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-Cuadros ME, Albaladejo-Florín MJ, Alava-Rabasa S, Gallego-Galiana J, Pérez-Cruz GF, Usandizaga-Elio I, et al. Compassionate use of rectal Ozone (O3) in severe COVID-19 pneumonia: a case-control study. PREPRINT. Res Square. 2021. 10.21203/rs.3.rs-231696/v1. [DOI] [PMC free article] [PubMed]
- 23.Henry BM, De Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med (CCLM) 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 24.Webb BJ, Peltan ID, Jensen P, Hoda D, Hunter B, Silver A, et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2.12:e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bocci V. Is it true that ozone is always toxic? The end of a dogma. Toxicol Appl Pharmacol. 2006;216.3:493–504. doi: 10.1016/j.taap.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Clavo B, Rodríguez-Esparragón F, Rodríguez-Abreu D, Martínez-Sánchez G, Llontop P, Aguiar-Bujanda D, Fernández-Pérez L, Santana-Rodríguez N. Modulation of Oxidative Stress by Ozone Therapy in the Prevention and Treatment of Chemotherapy-Induced Toxicity: Review and Prospects. Antioxidants (Basel) 2019;8.12:588. doi: 10.3390/antiox8120588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valdenassi L, Franzini M, Ricevuti G, Rinaldi L, Galoforo AC, Tirelli U. Potential mechanisms by which the oxygen-ozone (O2-O3) therapy could contribute to the treatment against the coronavirus COVID-19. Eur Rev Med Pharmacol Sci. 2020;24.8:4059–4061. doi: 10.26355/eurrev_202004_20976. [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Cuadros ME, Perez-Moro OS, Mirón-Canelo JA. Could ozone be used as a feasible future treatment in osteoarthritis of the knee. Divers Equal Health Care. 2016;13.3:232–239. doi: 10.21767/2049-5471.100057. [DOI] [Google Scholar]
- 29.Fernández-Cuadros ME, Pérez-Moro OS, Albaladejo-Florín MJ, Álava-Rabasa S, Tobar-Izquierdo M, Rodriguez-de-Cía J. A New Paradigm for the Management of Knee Osteoarthritis: The Role of Hyaluronic Acid, Platelet-Rich Plasma (PRP) and Ozone in the Modulation of Inflammation: A Review. Sci Rep. 2020). In press. 10.31487/j.JSR.2020.01.01.
- 30.Fernandez-Cuadros ME, Perez-Moro OS, Albaladejo-Florin MJ, Algarra-Lopez R. Ozone decreases biomarkers of inflamation (C-reactive protein and erytrocyte sedimentation rate) and improves pain, function and quality of life in knee osteoarthrtitis patients: A before-and-after study and review of the literature. Middle East J Rehabil Health Stud. 2018;5(2):e64507. doi: 10.5812/mejrh.64507. [DOI] [Google Scholar]
- 31.Fernandez-Cuadros ME, Perez-Moro OS, Albaladejo-Florin MJ, Algarra-Lopez R. Intra Articular Ozone Reduces Serum Uric Acid and Improves Pain, Function and Quality of Life in Knee Osteoarthritis Patients: A Before-and-After Study. Middle East J Rehabil Health Stud. 2018;5.3:e68599. doi: 10.5812/mejrh.68599. [DOI] [Google Scholar]
- 32.Peña-Lora D, Albaladejo-Florín MJ, Fernández-Cuadros ME. Uso de ozonoterapia en paciente anciana con neumonía grave por COVID-19. Revista Espanola de Geriatria y Gerontologia. 2020;55(6):362–364. doi: 10.1016/j.regg.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elvis AM, Ekta JS. Ozone therapy: a clinical review. J Nat Sci Biol Med. 2011;2:66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]